The Antioxidant Role of Hemp Phytocomplex in Cannabis Oil-Based Extracts
Abstract
:1. Introduction
2. Results and Discussion
2.1. Determination of Tocopherols and their Oxidized Forms
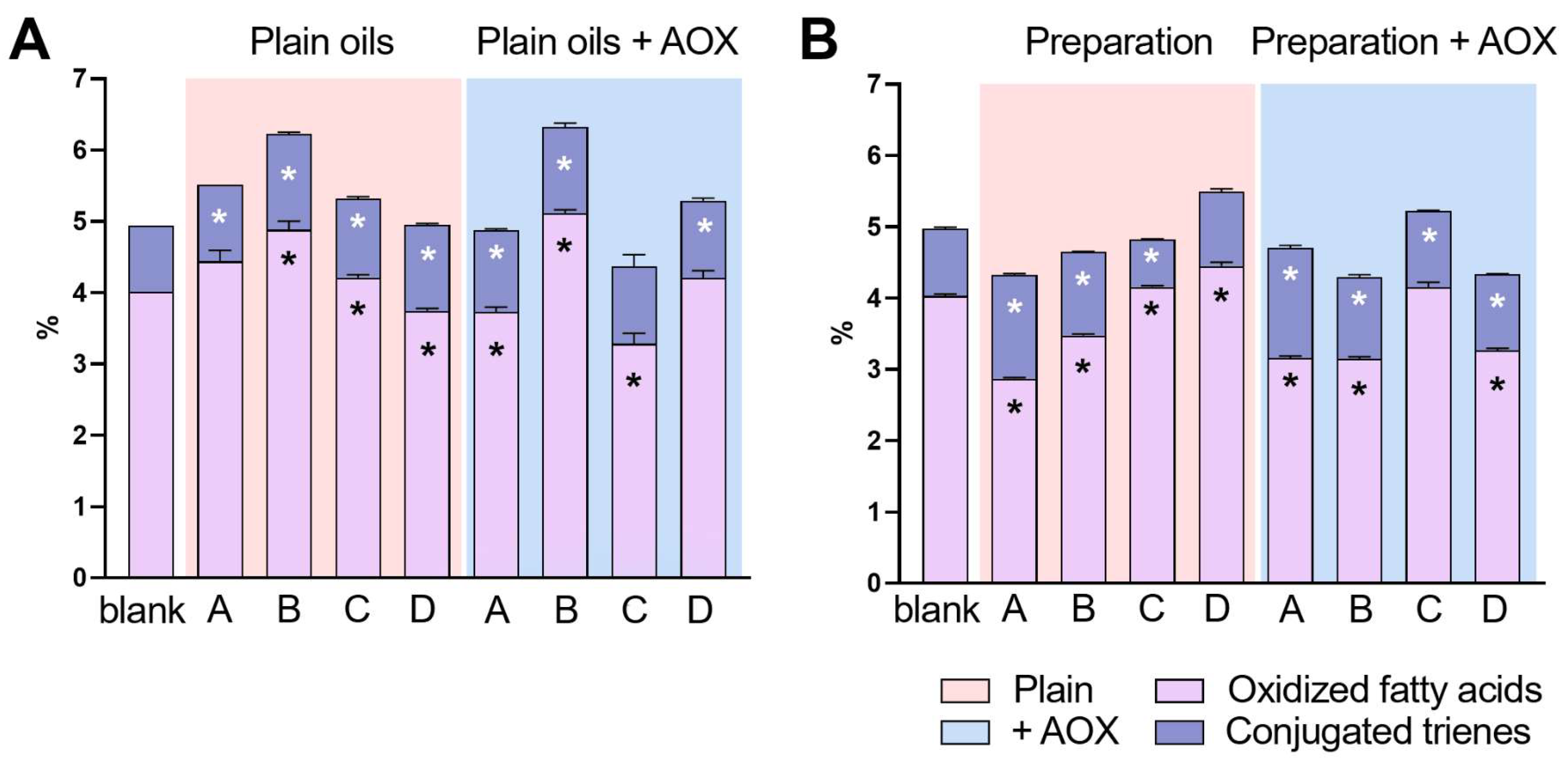
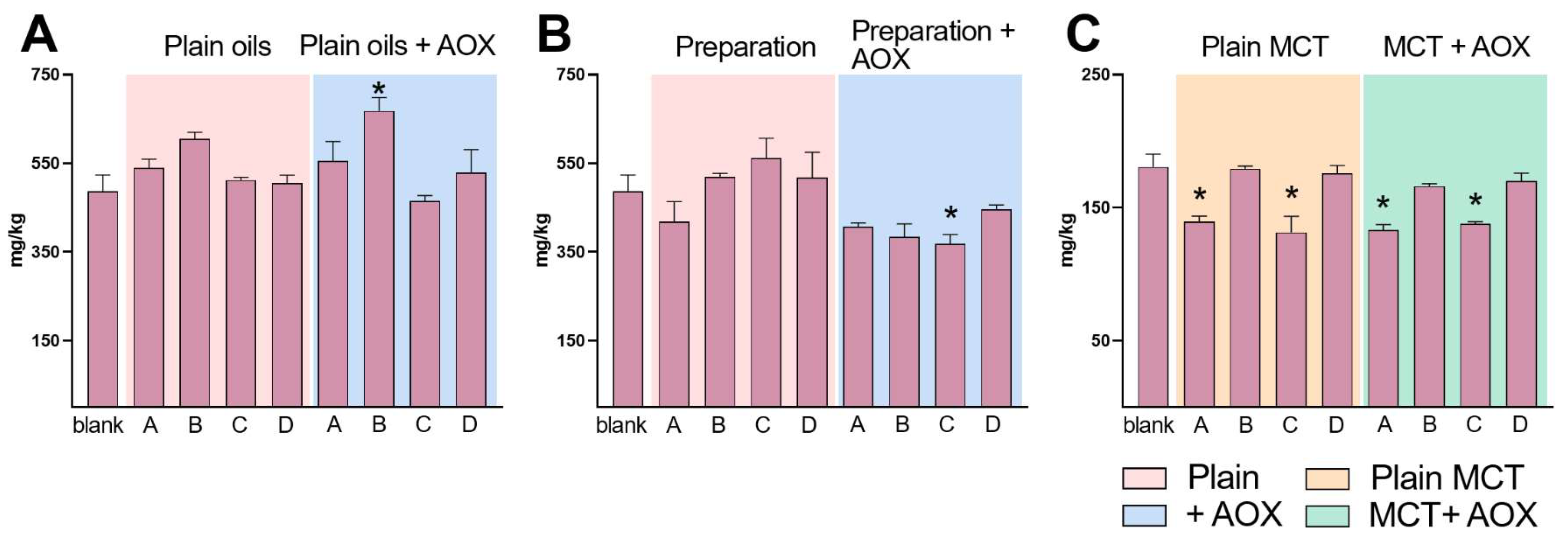
2.2. Determination of Oxidized and Conjugated Fatty Acids
2.3. Determination of Volatile Carbonyl Compounds (VCCs)
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Samples
3.3. Methods
3.3.1. Determination of Tocopherols and their Oxidized Forms
3.3.2. Determination of Oxidized and Conjugated Fatty Acids
3.3.3. Determination of Volatile Carbonyl Compounds (VCCs)
3.4. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Klumpers, L.E.; Thacker, D.L. A Brief Background on Cannabis: From Plant to Medical Indications. J. AOAC Int. 2019, 102, 412–420. [Google Scholar] [CrossRef] [PubMed]
- Borgelt, L.M.; Franson, K.L.; Nussbaum, A.M.; Wang, G.S. The Pharmacologic and Clinical Effects of Medical Cannabis. Pharmacotherapy 2013, 33, 195–209. [Google Scholar] [CrossRef] [PubMed]
- Baron, E.P. Medicinal Properties of Cannabinoids, Terpenes, and Flavonoids in Cannabis, and Benefits in Migraine, Headache, and Pain: An Update on Current Evidence and Cannabis Science. Headache 2018, 58, 1139–1186. [Google Scholar] [CrossRef] [PubMed]
- Thomas, B.; Elsohly, M. The Analytical Chemistry of Cannabis: Quality Assessment, Assurance, and Regulation of Medicinal Marijuana and Cannabinoid Preparations; Elsevier: Amsterdam, The Netherlands, 2015. [Google Scholar]
- Dei Cas, M.; Arnoldi, S.; Monguzzi, L.; Casagni, E.; Morano, C.; de Manincor, E.V.; Bolchi, C.; Pallavicini, M.; Gambaro, V.; Roda, G. Characterization of Chemotype-Dependent Terpenoids Profile in Cannabis by Headspace Gas-Chromatography Coupled to Time-of-Flight Mass Spectrometry. J. Pharm. Biomed. Anal. 2021, 203, 114180. [Google Scholar] [CrossRef] [PubMed]
- Pertwee, R.G. Pharmacological Actions of Cannabinoids. In Handbook of Experimental Pharmacology; Springer: Berlin/Heidelberg, Germany, 2005; Volume 168, pp. 1–51. [Google Scholar] [CrossRef]
- Russo, E.B. Cannabis Therapeutics and the Future of Neurology. Front. Integr. Neurosci. 2018, 12, 51. [Google Scholar] [CrossRef] [PubMed]
- Bolchi, C.; Pallavicini, M.; Casagni, E.; de Manincor, E.V.; Gambaro, V.; Dei Cas, M.; Roda, G. Development and Early Identification of Cannabis Chemotypes during the Plant Growth: Current Analytical and Chemometric Approaches. Anal. Sci. 2021, 37, 1665–1673. [Google Scholar] [CrossRef] [PubMed]
- Dei Cas, M.; Casagni, E.; Casiraghi, A.; Minghetti, P.; Fornasari, D.M.M.; Ferri, F.; Arnoldi, S.; Gambaro, V.; Roda, G. Phytocannabinoids Profile in Medicinal Cannabis Oils: The Impact of Plant Varieties and Preparation Methods. Front. Pharmacol. 2020, 11, 1752. [Google Scholar] [CrossRef] [PubMed]
- Società Italiana Farmacisti Preparatori SIFAP. Estratto Oleoso Di Infiorescenze Femminili Di Cannabis. 2016. Available online: https://www.sifap.org/procedure/estrazione-oleosa-di-infiorescenze-femminili-di-cannabi (accessed on 7 July 2022).
- Romano, L.L.; Hazekamp, A. Cannabis Oil: Chemical Evaluation of an Upcoming Cannabis-Based Medicine. Cannabinoids 2013, 1, 1–11. [Google Scholar]
- Citti, C.; Ciccarella, G.; Braghiroli, D.; Parenti, C.; Vandelli, M.A.; Cannazza, G. Medicinal Cannabis: Principal Cannabinoids Concentration and Their Stability Evaluated by a High Performance Liquid Chromatography Coupled to Diode Array and Quadrupole Time of Flight Mass Spectrometry Method. J. Pharm. Biomed. Anal. 2016, 128, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Calvi, L.; Pentimalli, D.; Panseri, S.; Giupponi, L.; Gelmini, F.; Beretta, G.; Vitali, D.; Bruno, M.; Zilio, E.; Pavlovic, R.; et al. Comprehensive Quality Evaluation of Medical Cannabis Sativa L. Inflorescence and Macerated Oils Based on HS-SPME Coupled to GC–MS and LC-HRMS (q-Exactive Orbitrap®) Approach. J. Pharm. Biomed. Anal. 2018, 150, 208–219. [Google Scholar] [CrossRef] [PubMed]
- Rovellini, P.; Cortesi, N.; Mattei, A.; Marotta, F. Composti Carbonilici Volatili Nell’aroma Dell’olio Vergine Di Oliva. Riv. Ital. Delle Sostanze Grasse 2002, 79, 429–438. [Google Scholar]
- Rovellini, P.; Cortesi, N. α-Tocopherol Oxidation Products. Riv. Ital. Delle Sostanze Grasse 2002, 79, 335–341. [Google Scholar]
- Rovellini, P.; Cortesi, N. Oxidative Status of Extra Virgin Olive Oils: HPLC Evaluation. Ital. J. Food Sci. 2004, 16, 335–344. [Google Scholar]

| Cannabis Varieties | Preparation Methods | THC tot (% w/w) | CBD tot (% w/w) |
|---|---|---|---|
| Bediol | A. Romano-Hazekamp | 0.48 | 0.65 |
| Bedrocan | B. Citti-Cannazza | 1.49 | - |
| Bedrocan | C. Sifap | 1.52 | - |
| Bedrolite | C. Sifap | - | 0.60 |
| Bedrocan | D. Calvi | 1.23 | - |
| Bediol | D. Calvi | 0.31 | 0.53 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morano, C.; Dei Cas, M.; Roda, G.; Fabbriconi, A.; Casagni, E.; Pallavicini, M.; Bolchi, C.; Pallotti, G.; Romaniello, F.; Rovellini, P. The Antioxidant Role of Hemp Phytocomplex in Cannabis Oil-Based Extracts. Pharmaceuticals 2022, 15, 1102. https://doi.org/10.3390/ph15091102
Morano C, Dei Cas M, Roda G, Fabbriconi A, Casagni E, Pallavicini M, Bolchi C, Pallotti G, Romaniello F, Rovellini P. The Antioxidant Role of Hemp Phytocomplex in Cannabis Oil-Based Extracts. Pharmaceuticals. 2022; 15(9):1102. https://doi.org/10.3390/ph15091102
Chicago/Turabian StyleMorano, Camillo, Michele Dei Cas, Gabriella Roda, Adalberto Fabbriconi, Eleonora Casagni, Marco Pallavicini, Cristiano Bolchi, Gloria Pallotti, Francesco Romaniello, and Pierangela Rovellini. 2022. "The Antioxidant Role of Hemp Phytocomplex in Cannabis Oil-Based Extracts" Pharmaceuticals 15, no. 9: 1102. https://doi.org/10.3390/ph15091102
APA StyleMorano, C., Dei Cas, M., Roda, G., Fabbriconi, A., Casagni, E., Pallavicini, M., Bolchi, C., Pallotti, G., Romaniello, F., & Rovellini, P. (2022). The Antioxidant Role of Hemp Phytocomplex in Cannabis Oil-Based Extracts. Pharmaceuticals, 15(9), 1102. https://doi.org/10.3390/ph15091102







