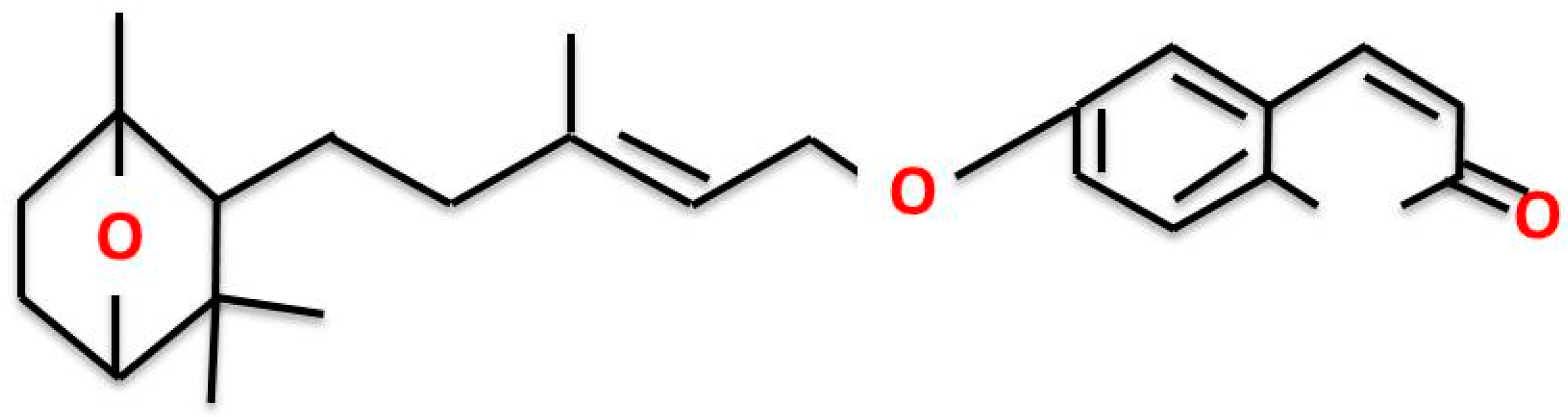
Farnesiferol C Exerts Antiproliferative Effects on Hepatocellular Carcinoma HepG2 Cells by Instigating ROS-Dependent Apoptotic Pathway
Abstract
:1. Introduction
2. Results
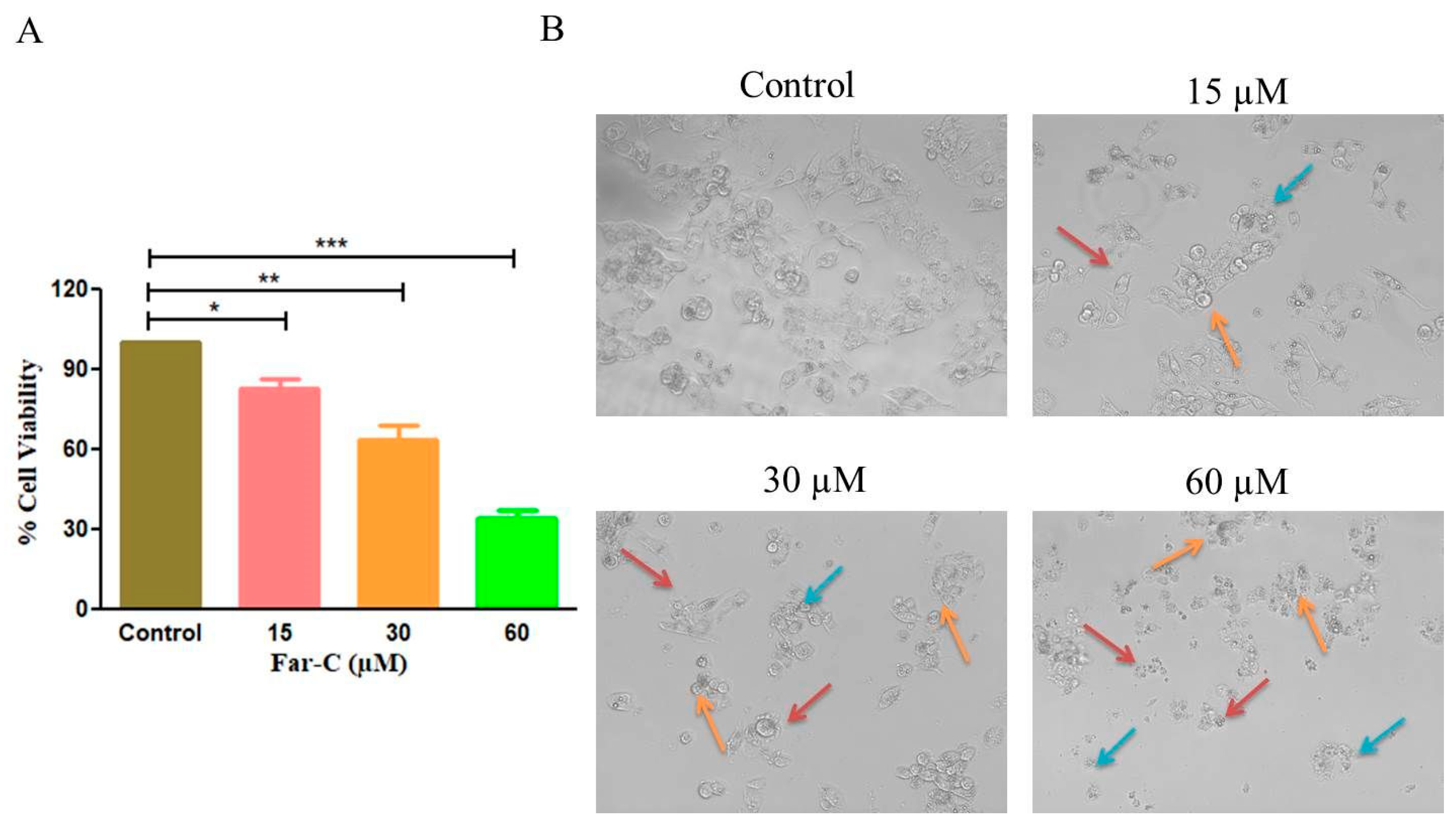
2.1. Far-C Exerted Cytotoxic Effects on Liver Cancer Cells
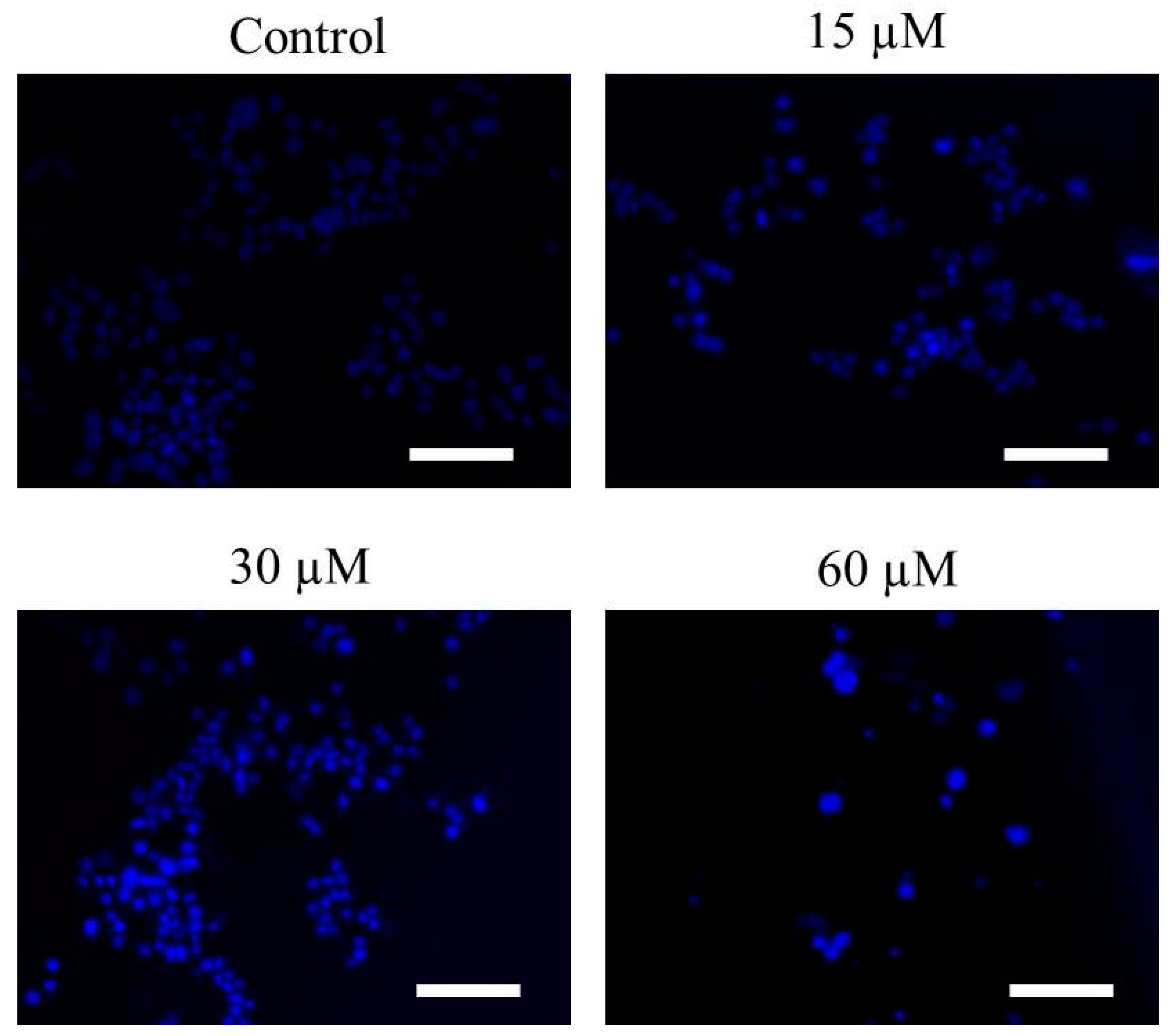
2.2. Far-C Induced Abruptions in Nuclear Morphology
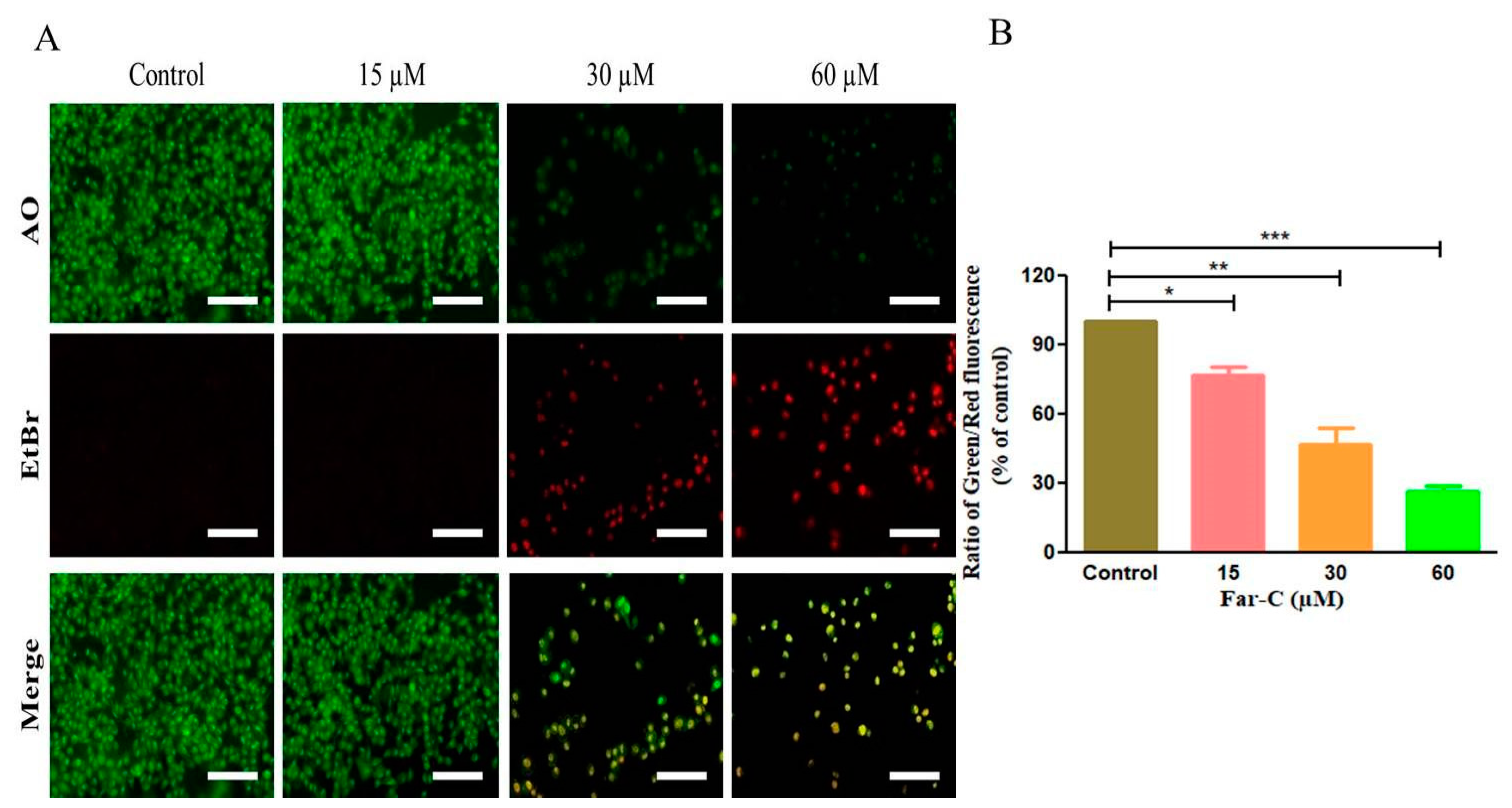
2.3. Far-C Exposure Induced Apoptosis within Liver Cancer HepG2 Cells
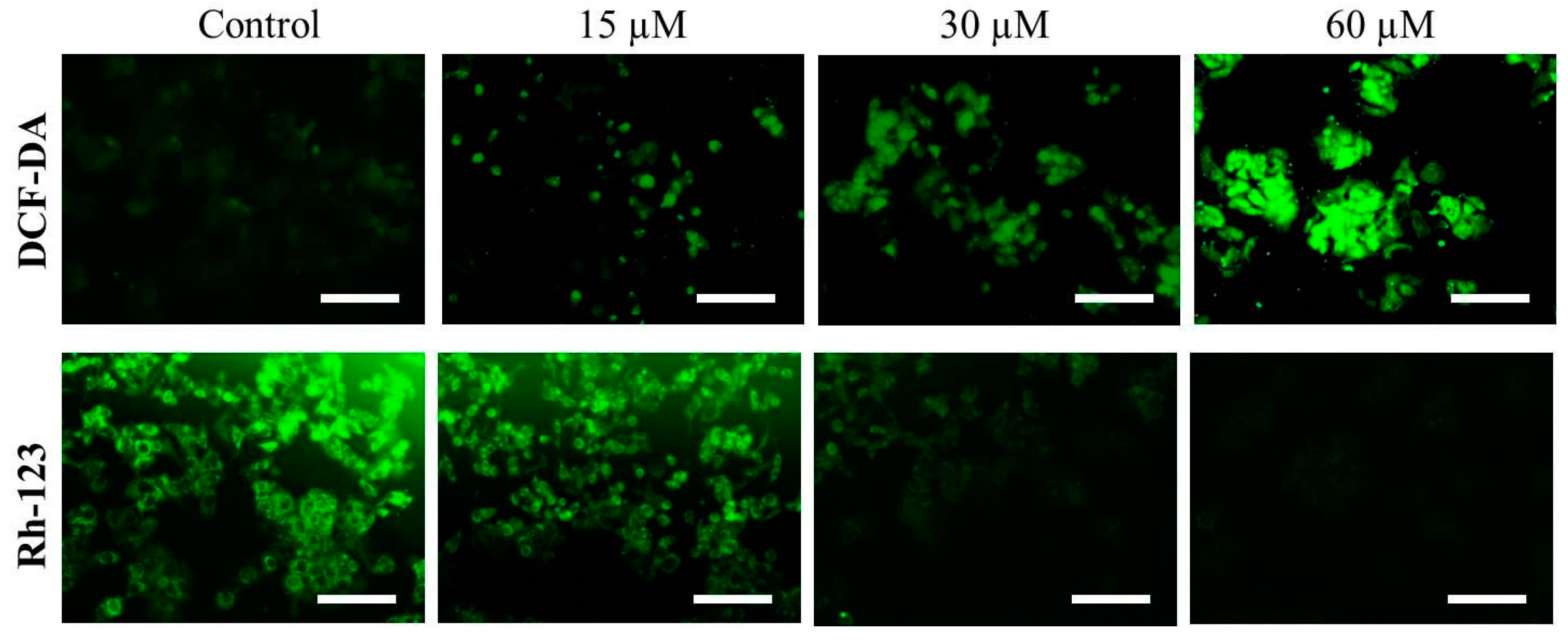
2.4. Far-C Instigated Intracellular ROS and Dissipation of ΔΨm
2.5. Far-C Exposure Activated Caspase-3 and -9
2.6. Caspase Inhibitors Ameliorated Far-C-Instigated Apoptosis in HepG2 Cells
2.7. Far-C Treatment Modulated the Expression of Apoptotic and Cell Cycle Regulatory Genes
3. Discussion
4. Materials and Methods
4.1. Cell Culture and Maintenance
4.2. Cytotoxicity and Morphological Evaluations
4.3. Assessment of Nuclear Morphology
4.4. Evaluation of Intracellular ROS
4.5. Evaluation of Mitochondrial Membrane Potential (ΔΨm)
4.6. AO/EtBr Dual Staining
4.7. Estimation of Caspase Activity
4.8. Effect of Caspase Inhibitor
4.9. Quantitative Real-Time PCR
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Global Cancer Observatory Report, Liver Cancer Factsheet, 2020. Last Accessed on 10 April 2022. Available online: https://gco.iarc.fr/today/data/factsheets/cancers/11-Liver-fact-sheet.pdf (accessed on 5 July 2022).
- Hin Tang, J.J.; Hao Thng, D.K.; Lim, J.J.; Toh, T.B. JAK/STAT signaling in hepatocellular carcinoma. Hepat Oncol. 2020, 7, HEP18. [Google Scholar] [CrossRef] [PubMed]
- McGlynn, K.A.; Petrick, J.L.; El-Serag, H.B. Epidemiology of hepatocellular carcinoma. Hepatology 2021, 73, 4–13. [Google Scholar] [CrossRef] [PubMed]
- Llovet, J.M.; Montal, R.; Sia, D.; Finn, R.S. Molecular therapies and precision medicine for hepatocellular carcinoma. Nat. Rev. Clin. Oncol. 2018, 15, 599–616. [Google Scholar] [CrossRef] [PubMed]
- European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J. Hepatol. 2018, 69, 182–236. [Google Scholar] [CrossRef] [PubMed]
- Bruix, J.; Qin, S.; Merle, P.; Granito, A.; Huang, Y.H.; Bodoky, G.; Pracht, M.; Yokosuka, O.; Rosmorduc, O.; Breder, V.; et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2017, 389, 56–66. [Google Scholar] [CrossRef]
- Zhu, A.X.; Kang, Y.K.; Yen, C.J.; Finn, R.S.; Galle, P.R.; Llovet, J.M.; Assenat, E.; Brandi, G.; Lim, H.Y.; Pracht, M.; et al. REACH-2: A randomized, double-blind, placebo-controlled phase 3 study of ramucirumab versus placebo as second-line treatment in patients with advanced hepatocellular carcinoma (HCC) and elevated baseline alpha-fetoprotein (AFP) following first-line sorafenib. J. Clin. Oncol. 2018, 36, 4003. [Google Scholar] [CrossRef]
- Cruz Martínez, C.; Diaz Gómez, M.; Oh, M.S. Use of traditional herbal medicine as an alternative in dental treatment in Mexican dentistry: A review. Pharm. Biol. 2017, 55, 1992–1998. [Google Scholar] [CrossRef]
- Venugopala, K.N.; Rashmi, V.; Odhav, B. Review on natural coumarin lead compounds for their pharmacological activity. BioMed Res. Int. 2013, 2013, 963248. [Google Scholar] [CrossRef]
- Jain, P.K.; Joshi, H. Coumarin: Chemical and pharmacological profile. J. Appl. Pharm. Sci. 2012, 2, 236–240. [Google Scholar] [CrossRef]
- Madari, H.; Panda, D.; Wilson, L.; Jacobs, R.S. Dicoumarol: A unique microtubule stabilizing natural product that is synergistic with Taxol. Cancer Res. 2003, 63, 1214–1220. [Google Scholar]
- Smith, G.F.; Neubauer, B.L.; Sundboom, J.L.; Best, K.L.; Goode, R.L.; Tanzer, L.R.; Merriman, R.L.; Frank, J.D.; Herrmann, R.G. Correlation of the in vivo anticoagulant, antithrombotic, and antimetastatic efficacy of warfarin in the rat. Thromb. Res. 1998, 50, 163–174. [Google Scholar] [CrossRef]
- Lewis, A.; Ough, M.; Li, L.; Hinkhouse, M.M.; Ritchie, J.M.; Spitz, D.R.; Cullen, J.J. Treatment of pancreatic cancer cells with dicumarol induces cytotoxicity and oxidative stress. Clin. Cancer Res. 2004, 10, 4550–4558. [Google Scholar] [CrossRef]
- Kasaian, J.; Mohammadi, A. Biological activities of farnesiferol C: A review. J. Asian Nat. Prod. Res. 2018, 20, 27–35. [Google Scholar] [CrossRef]
- Lee, J.H.; Choi, S.; Lee, Y.; Lee, H.J.; Kim, K.H.; Ahn, K.S.; Bae, H.; Lee, H.J.; Lee, E.O.; Ahn, K.S.; et al. Herbal compound farnesiferol C exerts antiangiogenic and antitumor activity and targets multiple aspects of VEGFR1 (Flt1) or VEGFR2 (Flk1) signaling cascades. Mol. Cancer Ther. 2010, 9, 389–399. [Google Scholar] [CrossRef]
- Ozben, T. Oxidative stress and apoptosis: Impact on cancer therapy. J. Pharm. Sci. 2007, 96, 2181–2196. [Google Scholar] [CrossRef]
- NavaneethaKrishnan, S.; Rosales, J.L.; Lee, K.Y. ROS-Mediated Cancer Cell Killing through Dietary Phytochemicals. Oxid. Med. Cell Longev. 2019, 2019, 9051542. [Google Scholar] [CrossRef]
- Llovet, J.M.; Kelley, R.K.; Villanueva, A.; Singal, A.G.; Pikarsky, E.; Roayaie, S.; Lencioni, R.; Koike, K.; Zucman-Rossi, J.; Finn, R.S. Hepatocellular carcinoma. Nat. Rev. Dis. Primers 2021, 7, 6. [Google Scholar] [CrossRef]
- Weber, U.S.; Steffen, B.; Siegers, C.P. Antitumor-activities of coumarin, 7-hydroxy-coumarin and its glucuronide in several human tumor cell lines. Res. Commun. Mol. Pathol. Pharmacol. 1998, 99, 193–206. [Google Scholar]
- Barry, M.A.; Behnke, C.A.; Eastman, A. Activation of programmed cell death (apoptosis) by cisplatin, other anticancer drugs, toxins and hyperthermia. Biochem. Pharmacol. 1990, 40, 2353–2362. [Google Scholar] [CrossRef]
- Richardson, J.S.M.; Aminudin, N.; Abd Malek, S.N. Chalepin: A Compound from Ruta angustifolia L. Pers Exhibits Cell Cycle Arrest at S phase, Suppresses Nuclear Factor-Kappa B (NF-κB) Pathway, Signal Transducer and Activation of Transcription 3 (STAT3) Phosphorylation and Extrinsic Apoptotic Pathway in Non-small Cell Lung Cancer Carcinoma (A549). Pharmacogn Mag. 2017, 13, S489–S498. [Google Scholar] [CrossRef]
- Ahmad, A.; Saeed, M.; Ansari, I.A. Molecular insights on chemopreventive and anticancer potential of carvacrol: Implications from solid carcinomas. J. Food Biochem. 2021, 45, e14010. [Google Scholar] [CrossRef] [PubMed]
- Taylor, R.C.; Cullen, S.P.; Martin, S.J. Apoptosis: Controlled demolition at the cellular level. Nat. Rev. Mol. Cell Biol. 2008, 9, 231–241. [Google Scholar] [CrossRef] [PubMed]
- Ying, T.H.; Yang, S.F.; Tsai, S.J.; Hsieh, S.C.; Huang, Y.C.; Bau, D.T.; Hsieh, Y.H. Fisetin induces apoptosis in human cervical cancer HeLa cells through ERK1/2-mediated activation of caspase-8-/caspase-3-dependent pathway. Arch. Toxicol. 2012, 86, 263–273. [Google Scholar] [CrossRef] [PubMed]
- Slee, E.A.; Adrain, C.; Martin, S.J. Executioner caspase-3, -6, and -7 perform distinct, non-redundant roles during the demolition phase of apoptosis. J. Biol. Chem. 2001, 276, 7320–7326. [Google Scholar] [CrossRef] [PubMed]
- Petrosillo, G.; Ruggiero, F.M.; Paradies, G. Role of reactive oxygen species and cardiolipin in the release of cytochrome c from mitochondria. FASEB J. 2003, 17, 2202–2208. [Google Scholar] [CrossRef] [PubMed]
- Qie, S.; Diehl, J.A. Cyclin D1, cancer progression, and opportunities in cancer treatment. J. Mol. Med. 2016, 94, 1313–1326. [Google Scholar] [CrossRef]
- Wei, M.; Liu, B.; Gu, Q.; Su, L.; Yu, Y.; Zhu, Z. Stat6 cooperates with Sp1 in controlling breast cancer cell proliferation by modulating the expression of p21(Cip1/WAF1) and p27 (Kip1). Cell Oncol. 2013, 36, 79–93. [Google Scholar] [CrossRef]
- Kale, J.; Osterlund, E.J.; Andrews, D.W. BCL-2 family proteins: Changing partners in the dance towards death. Cell Death Differ. 2018, 25, 65–80. [Google Scholar] [CrossRef]
- Alafnan, A.; Hussain, T.; Rizvi, S.; Moin, A.; Alamri, A. Prostate Apoptotic Induction and NFκB Suppression by Dammarolic Acid: Mechanistic Insight into Onco-Therapeutic Action of an Aglycone Asiaticoside. Curr. Issues Mol. Biol. 2021, 43, 932–940. [Google Scholar] [CrossRef]
- Crowley, L.C.; Marfell, B.J.; Waterhouse, N.J. Analyzing Cell Death by Nuclear Staining with Hoechst 33342. Cold Spring Harb Protoc. 2016, 2016, 778–781. [Google Scholar] [CrossRef]
- Gansukh, E.; Mya, K.K.; Jung, M.; Keum, Y.S.; Kim, D.H.; Saini, R.K. Lutein derived from marigold (Tagetes erecta) petals triggers ROS generation and activates Bax and caspase-3 mediated apoptosis of human cervical carcinoma (HeLa) cells. Food Chem Toxicol. 2019, 127, 11–18. [Google Scholar] [CrossRef]
- Tiwari, R.K.; Singh, S.; Gupta, C.L.; Pandey, P.; Singh, V.K.; Sayyed, U.; Shekh, R.; Bajpai, P. Repolarization of glioblastoma macrophage cells using non-agonistic Dectin-1 ligand encapsulating TLR-9 agonist: Plausible role in regenerative medicine against brain tumor. Int. J. Neurosci. 2021, 131, 591–598. [Google Scholar] [CrossRef]
- Elumalai, P.; Gunadharini, D.N.; Senthilkumar, K.; Banudevi, S.; Arunkumar, R.; Benson, C.S.; Sharmila, G.; Arunakaran, J. Induction of apoptosis in human breast cancer cells by nimbolide through extrinsic and intrinsic pathway. Toxicol Lett. 2012, 215, 131–142. [Google Scholar] [CrossRef]


| Gene Name | Forward Sequence | Reverse Sequence |
|---|---|---|
| GAPDH | CGACCACTTTGTCAAGCTCA | CCCCTCTTCAAGGGGTCTAC |
| Bax | GCTGGACATTGGACTTCCTC | CTCAGCCCATCTTCTTCCAG |
| Bad | CCTCAGGCCTATGCAAAAAG | AAACCCAAAACTTCCGATGG |
| Bcl2 | ATTGGGAAGTTTCAAATCAGC | TGCATTCTTGGACGAGGG |
| cyclinD1 | CTTCCTCTCCAAAATGCCAG | AGAGATGGAAGGGGGAAAGA |
| CDK4 | CCTGGCCAGAATCTACAGCTA | ACATCTCGAGGCCAGTCATC |
| p21Cip1 | TGTCCGTCAGAACCCATG | GTGGGAAGGTAGAGCTTGG |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alafnan, A.; Alamri, A.; Alanazi, J.; Hussain, T. Farnesiferol C Exerts Antiproliferative Effects on Hepatocellular Carcinoma HepG2 Cells by Instigating ROS-Dependent Apoptotic Pathway. Pharmaceuticals 2022, 15, 1070. https://doi.org/10.3390/ph15091070
Alafnan A, Alamri A, Alanazi J, Hussain T. Farnesiferol C Exerts Antiproliferative Effects on Hepatocellular Carcinoma HepG2 Cells by Instigating ROS-Dependent Apoptotic Pathway. Pharmaceuticals. 2022; 15(9):1070. https://doi.org/10.3390/ph15091070
Chicago/Turabian StyleAlafnan, Ahmed, Abdulwahab Alamri, Jowaher Alanazi, and Talib Hussain. 2022. "Farnesiferol C Exerts Antiproliferative Effects on Hepatocellular Carcinoma HepG2 Cells by Instigating ROS-Dependent Apoptotic Pathway" Pharmaceuticals 15, no. 9: 1070. https://doi.org/10.3390/ph15091070
APA StyleAlafnan, A., Alamri, A., Alanazi, J., & Hussain, T. (2022). Farnesiferol C Exerts Antiproliferative Effects on Hepatocellular Carcinoma HepG2 Cells by Instigating ROS-Dependent Apoptotic Pathway. Pharmaceuticals, 15(9), 1070. https://doi.org/10.3390/ph15091070







