Pharmacovigilance of Herb-Drug Interactions: A Pharmacokinetic Study on the Combined Administration of Tripterygium Glycosides Tablets and Leflunomide Tablets in Rats by LC-MS/MS
Abstract
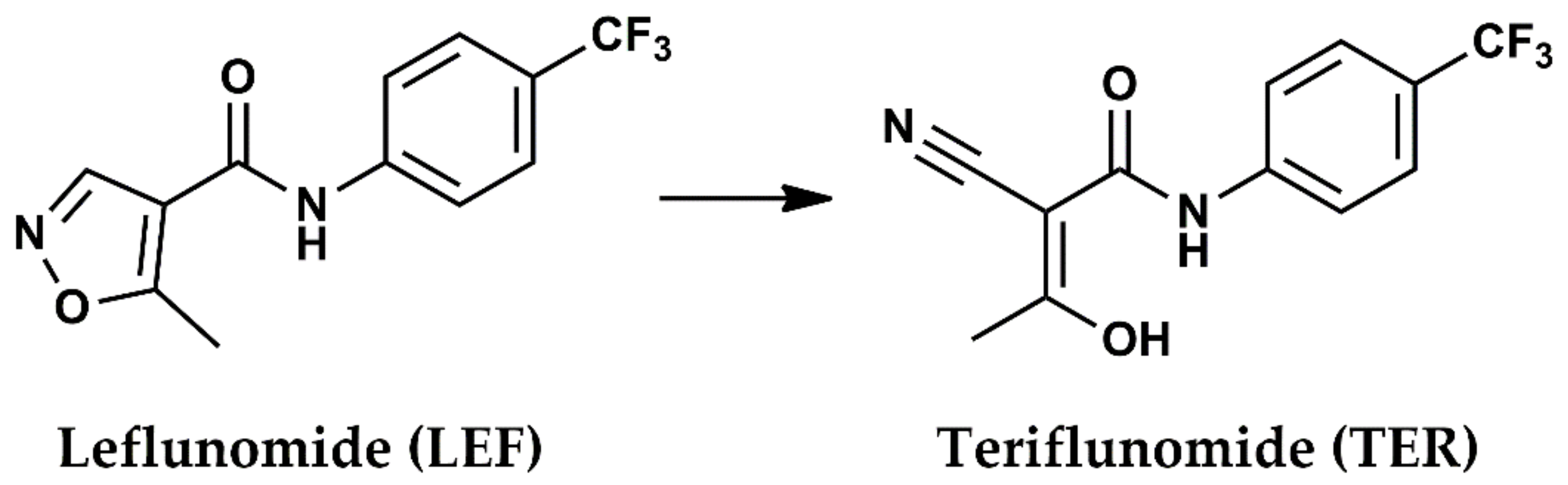
:1. Introduction
2. Results
2.1. Method Validation
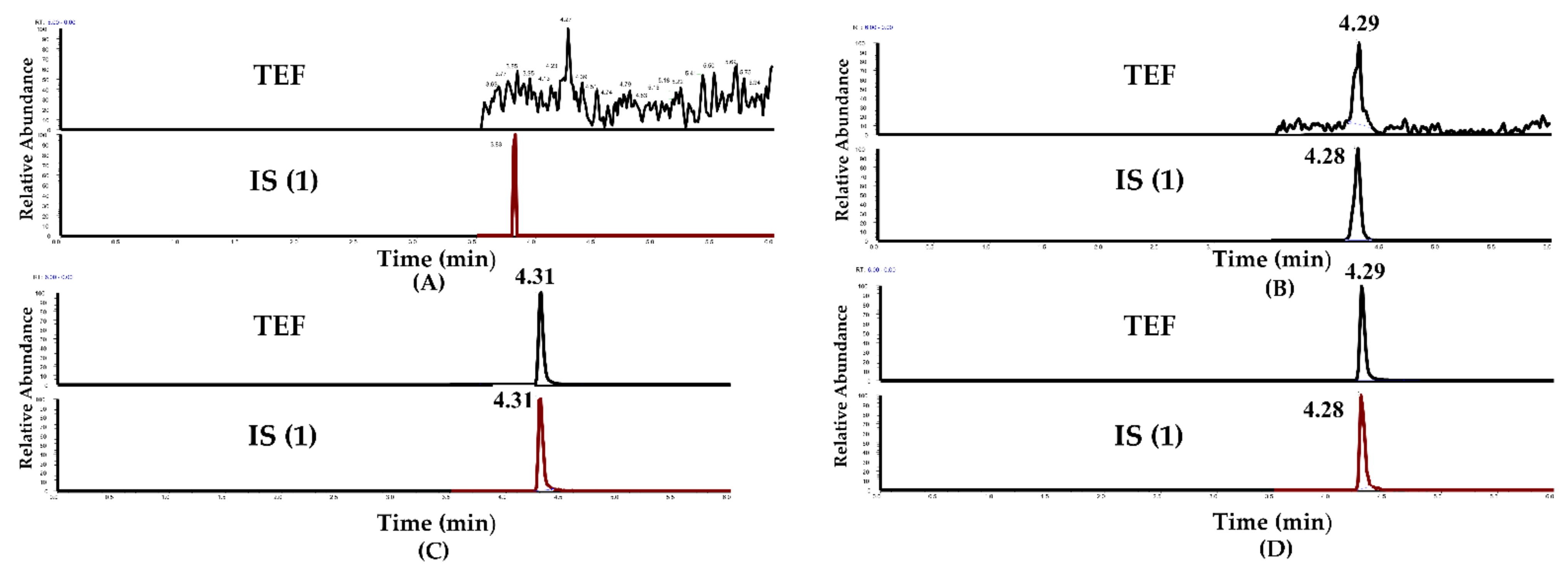
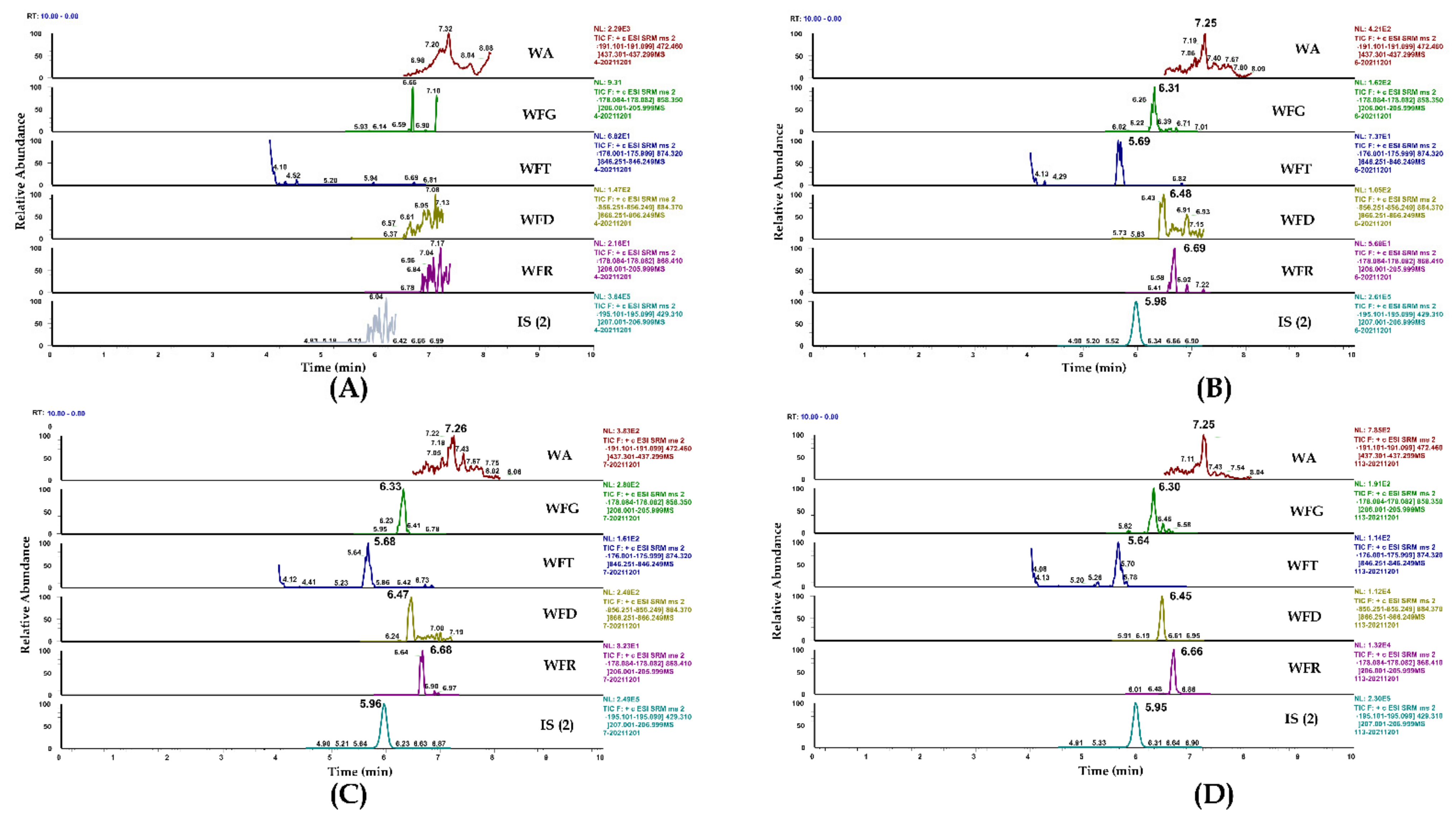
2.1.1. Selectivity
2.1.2. Linearity and Lower Limit of Quantification (LLOQ)
2.1.3. Precision and Accuracy
2.1.4. Extraction Recovery and Matrix Effect
2.1.5. Stability
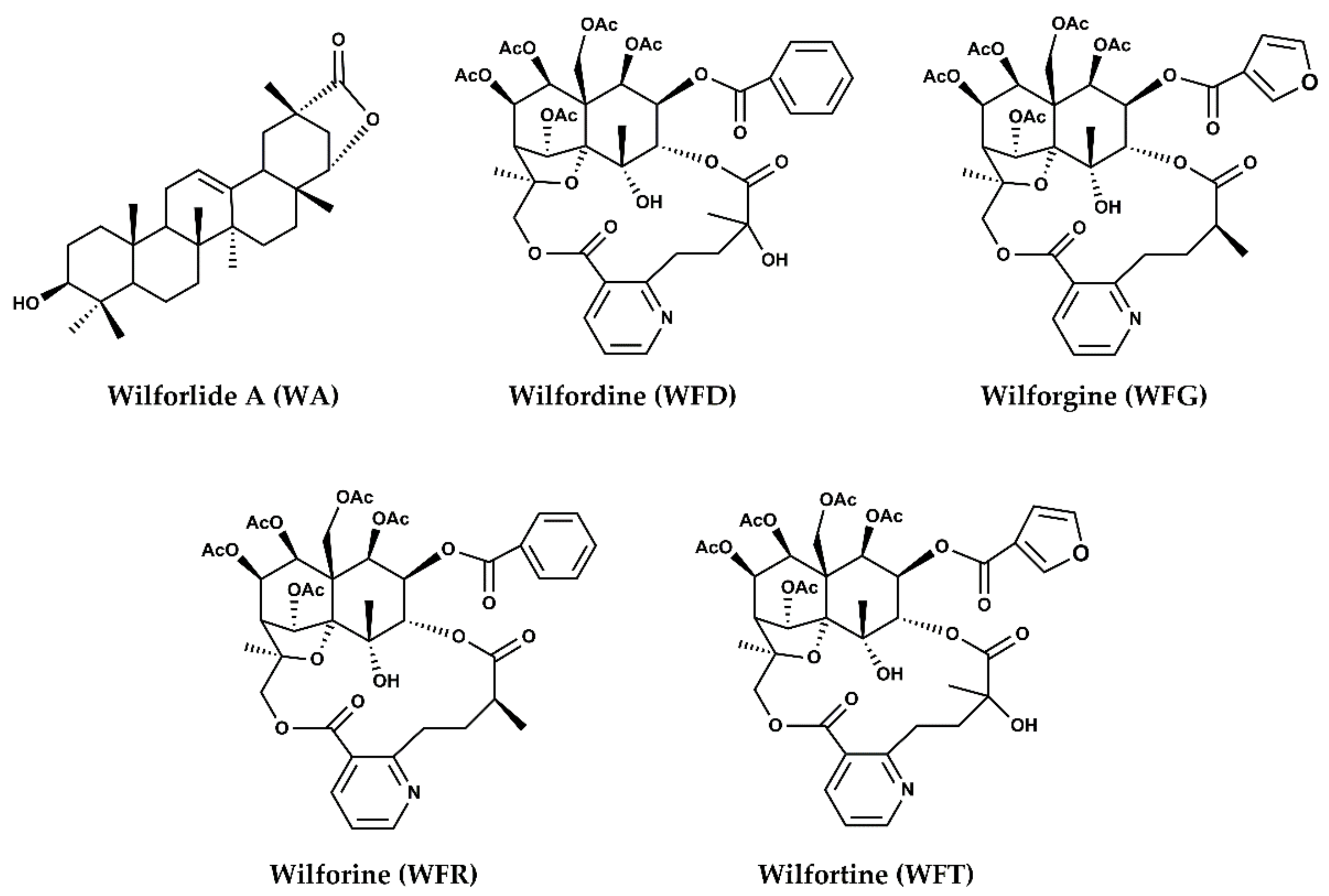
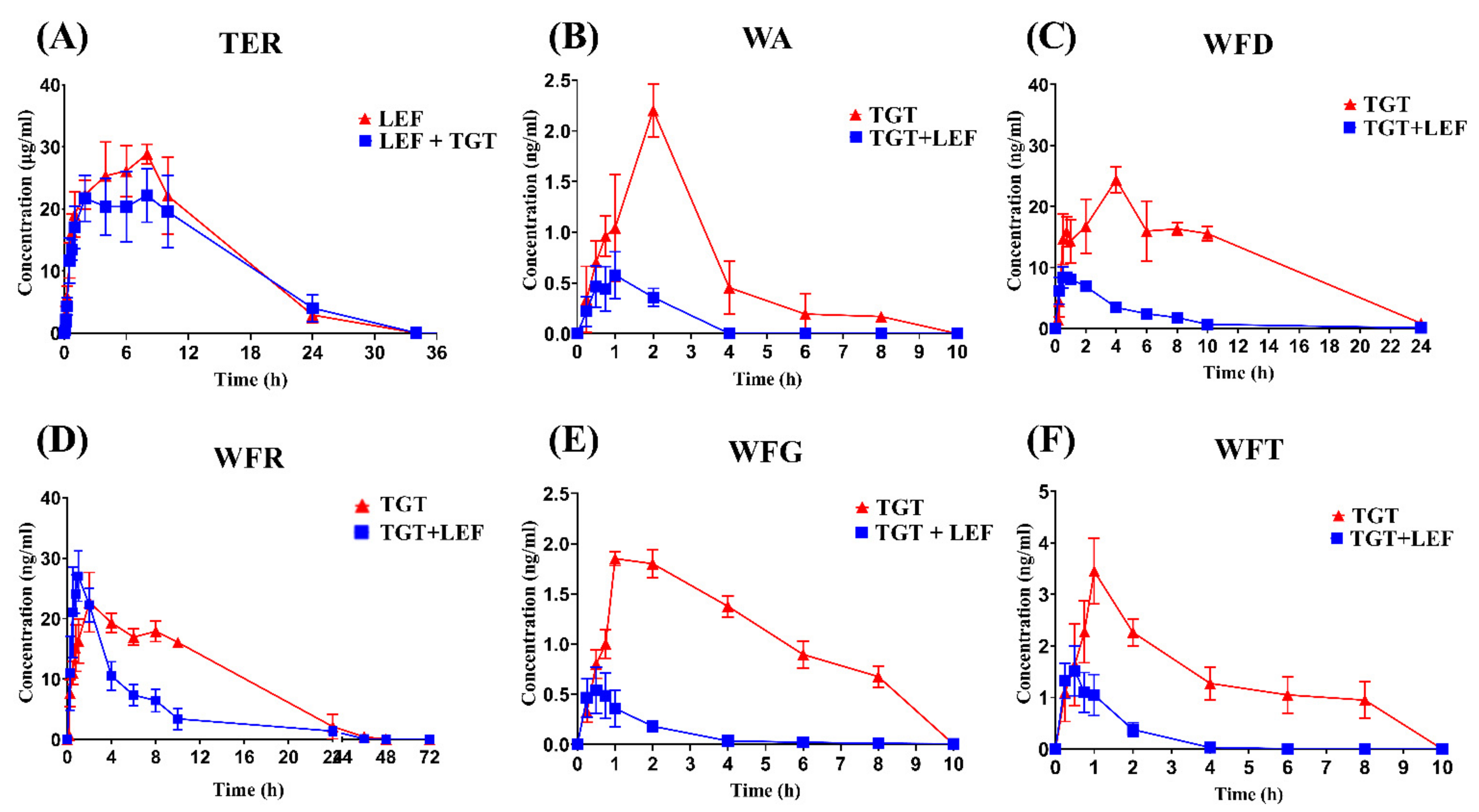
2.2. Herbal–Drug Interactions Study
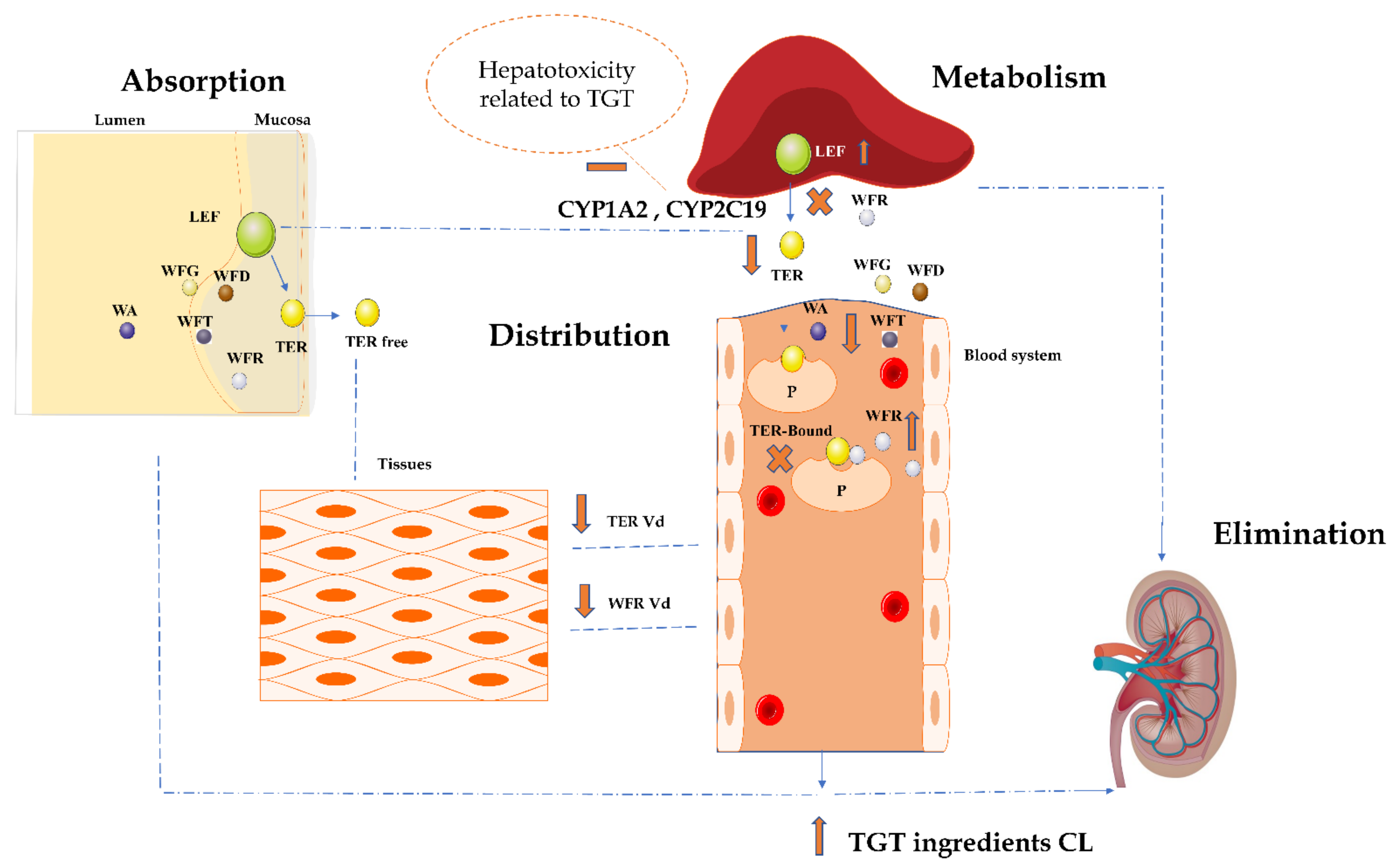
3. Discussion
4. Materials and Methods
4.1. Reagents and Materials
4.2. UHPLC-MS/MS Conditions
4.3. Pharmacokinetic and Herb-Drug Interaction Study Design
4.4. Sample Preparation
4.4.1. Calibration Standards
4.4.2. TER and TGT Constituents
4.5. Method Validation
4.5.1. Selectivity
4.5.2. Linearity and Lower Limits of Quantification (LLOQ)
4.5.3. Precision and Accuracy
4.5.4. Extraction Recovery and Matrix Effect
4.5.5. Stability
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kayhan, S.; Guzel, A.; Duran, L.; Tutuncu, S.; Guzel, A.; Gunaydin, M.; Salis, O.; Okuyucu, A.; Selcuk, M.Y. Effects of leflunomide on inflamation and fibrosis in bleomycine induced pulmonary fibrosis in wistar albino rats. J. Thorac. Dis. 2013, 5, 641–649. [Google Scholar] [CrossRef]
- Su, M.X.; Di Zhou, W.; Lan, J.; Di, B.; Hang, T.J. Rapid and sensitive analysis of multiple bioactive constituents in tripterygium glycosides tablets using liquidchromatography coupled with time-of-flight mass spectrometry. J. Sep. Sci. 2015, 38, 804–812. [Google Scholar] [CrossRef]
- Xiao, N.; Xiao, F.; Gao, J.; Xu, Z.; Wang, Q.; Kuai, J.; Wei, W.; Wang, C. Effects of paeoniflorin-6′-O-benzene sulfonate on the pharmacokinetics, excretion and tissue distribution of leflunomide in rats. Basic Clin. Pharmacol. Toxicol. 2021, 130, 364–374. [Google Scholar] [CrossRef]
- Fragoso, Y.D.; Brooks, J.B.B. Leflunomide and teriflunomide: Altering the metabolism of pyrimidines for the treatment of autoimmune diseases. Expert Rev. Clin. Pharmacol. 2015, 8, 315–320. [Google Scholar] [CrossRef]
- Chon, W.J.; Josephson, M.A. Leflunomide in renal transplantation. Expert Rev. Clin. Immunol. 2011, 7, 273–281. [Google Scholar] [CrossRef]
- Breedveld, F.C.; Dayer, J.M. Leflunomide: Mode of action in the treatment of rheumatoid arthritis. Ann. Rheum. Dis. 2000, 59, 841–849. [Google Scholar] [CrossRef]
- Fathy, N.; Furst, D.E. Combination therapy for autoimmune diseases: The rheumatoid arthritis model. Springer Semin. Immunopathol. 2001, 23, 5–26. [Google Scholar] [CrossRef]
- Van Roon, E.N.; Jansen, T.L.T.A.; Houtman, N.M.; Spoelstra, P.; Brouwers, J.R.B.J. Leflunomide for the treatment of rheumatoid arthritis in clinical practice: Incidence and severity of hepatotoxicity. Drug Saf. 2004, 27, 345–352. [Google Scholar] [CrossRef]
- Ng, J.C.Y.; Leung, M.; Wright, A.J.; Ensom, M.H.H. Clinical Pharmacokinetic Monitoring of Leflunomide in Renal Transplant Recipients with BK Virus Reactivation: A Review of the Literature. Clin. Pharmacokinet. 2017, 56, 1015–1031. [Google Scholar] [CrossRef]
- Levin, S.; Rimmer, K.; Vargas, W.S. Chapter 6—Neuroimmunologic disorders in pregnancy. In Neurology and Pregnancy: Neuro-Obstetric Disorders; Steegers, E.A.P., Cipolla, M.J., Miller, E.C., Eds.; Handbook of Clinical Neurology; Elsevier: Amsterdam, The Netherlands, 2020; Volume 172, pp. 105–123. [Google Scholar]
- Li, H.; Wang, Y.; Zhou, Z.; Tian, F.; Yang, H.; Yan, J. Combination of leflunomide and benazepril reduces renal injury of diabetic nephropathy rats and inhibits high-glucose induced cell apoptosis through regulation of NF-κB, TGF-β and TRPC6. Ren. Fail. 2019, 41, 899–906. [Google Scholar] [CrossRef]
- Korn, T.; Magnus, T.; Toyka, K.; Jung, S. Modulation of effector cell functions in experimental autoimmune encephalomyelitis by leflunomide- mechanisms independent of pyrimidine depletion. J. Leukoc. Biol. 2004, 76, 950–960. [Google Scholar] [CrossRef]
- Korn, T.; Toyka, K.; Hartung, H.P.; Jung, S. Suppression of experimental autoimmune neuritis by leflunomide. Brain 2001, 124, 1791–1802. [Google Scholar] [CrossRef]
- Gupta, R.; Bhatia, J.; Gupta, S.K. Risk of hepatotoxicity with add-on leflunomide in rheumatoid arthritis patients. Arzneimittelforschung 2011, 61, 312–316. [Google Scholar] [CrossRef]
- Lin, N.; Zhang, Y.Q.; Jiang, Q.; Liu, W.; Liu, J.; Huang, Q.C.; Wu, K.Y.; Tu, S.H.; Zhou, Z.S.; Chen, W.H.; et al. Clinical Practice Guideline for Tripterygium Glycosides/Tripterygium wilfordii Tablets in the Treatment of Rheumatoid Arthritis. Front. Pharmacol. 2021, 11, 608703. [Google Scholar] [CrossRef]
- Yang, Y.J.; Deng, Y.; Liao, L.L.; Peng, J.; Peng, Q.H.; Qin, Y.H. Tripterygium Glycosides Combined with Leflunomide for Rheumatoid Arthritis: A Systematic Review and Meta-Analysis. Evid.-Based Complement. Altern. Med. 2020, 2020, 1230320. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, X.; Jia, L.Y.; Liu, B.L.; Zhang, S.R.; Wang, G.Y.; Wang, L.S.; Liu, J.P. Tripterygium glycosides for treatment of nephrotic syndrome: A systematic review and meta-analysis of randomised controlled trials. Eur. J. Integr. Med. 2018, 20, 131–145. [Google Scholar] [CrossRef]
- Liu, R.; Li, X.; Huang, N.; Fan, M.; Sun, R. Chapter Eleven-Toxicity of traditional Chinese medicine herbal and mineral products. In Pharmacological Advances in Natural Product Drug Discovery; Du, G., Ed.; Advances in Pharmacology; Academic Press: Cambridge, MA, USA, 2020; Volume 87, pp. 301–346. [Google Scholar]
- Wang, M.; Huang, J.; Fan, H.; He, D.; Zhao, S.; Shu, Y.; Li, H.; Liu, L.; Lu, S.; Xiao, C.; et al. Treatment of rheumatoid arthritis using combination of methotrexate and tripterygium glycosides tablets-A quantitative plasma pharmacochemical and pseudotargeted metabolomic approach. Front. Pharmacol. 2018, 9, 1051. [Google Scholar] [CrossRef]
- Gao, C.; Huang, X.X.; Bai, M.; Wu, J.; Li, J.Y.; Liu, Q.B.; Li, L.Z.; Song, S.J. Anti-inflammatory sesquiterpene pyridine alkaloids from Tripterygium wilfordii. Fitoterapia 2015, 105, 49–54. [Google Scholar] [CrossRef]
- Li, X.X.; Du, F.Y.; Liu, H.X.; Ji, J.B.; Xing, J. Investigation of the active components in Tripterygium wilfordii leading to its acute hepatotoxicty and nephrotoxicity. J. Ethnopharmacol. 2015, 162, 238–243. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.G.; Zhao, Q.; Sun, W.; Zhang, H.L.; Li, M.; Wei, Q.X.; Wu, W.; Yue, L.J.; Wang, Q. Contrasting dose-effects of multi-glycoside of Tripterygium wilfordii Hook. f. on glomerular inflammation and hepatic damage in two types of anti-thy1.1 glomerulonephritis. J. Pharmacol. Sci. 2012, 118, 433–446. [Google Scholar] [CrossRef]
- Wu, W.; Cheng, R.; Boucetta, H.; Xu, L.; Pan, J.-R.; Song, M.; Lu, Y.-T.; Hang, T.-J. Differences in Multicomponent Pharmacokinetics, Tissue Distribution, and Excretion of Tripterygium Glycosides Tablets in Normal and Adriamycin-Induced Nephrotic Syndrome Rat Models and Correlations With Efficacy and Hepatotoxicity. Front. Pharmacol. 2022, 13, 910923. [Google Scholar] [CrossRef] [PubMed]
- Nair, A.; Jacob, S. A simple practice guide for dose conversion between animals and human. J. Basic Clin. Pharm. 2016, 7, 27–31. [Google Scholar] [CrossRef] [PubMed]
- Alamri, R.D.; Elmeligy, M.A.; Albalawi, G.A.; Alquayr, S.M.; Alsubhi, S.S.; El-Ghaiesh, S.H. Leflunomide an immunomodulator with antineoplastic and antiviral potentials but drug-induced liver injury: A comprehensive review. Int. Immunopharmacol. 2021, 93, 107398. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Zhou, X.; Shi, H.; Shi, W.; Ye, S.; Zhang, H. The effect of tripterygium glucoside tablet on pharmacokinetics of losartan and its metabolite EXP3174 in rats. Biomed. Chromatogr. 2017, 31, e3973. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Mo, L.; Ye, C.; Xun, T.; Wang, X.; Lv, B.; Zhan, X.; Liu, B.; Ding, Q.; Peng, J.; et al. Effect of total glucosides of paeony and Tripterygium wilfordii polyglycosides on erythrocyte methotrexate polyglutamates in rats, analysed using ultra-high-performance liquid chromatography-tandem mass spectrometry. J. Pharm. Pharmacol. 2021, 73, 1039–1048. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Du, X.; An, L.; Wang, Y.; Wang, L.; Wu, Z.; Huang, C.; He, X. Wilforine, the Q-marker and PK-maker of Tripterygium glycosides tablet: Based on preparation quantitative analysis and PK-PD study. Phytomedicine 2019, 54, 357–364. [Google Scholar] [CrossRef] [PubMed]

| Analytes | Regression Equation | Linear Range (ng/mL) | R2 | LLOQ (ng/mL) |
|---|---|---|---|---|
| TER | Y = 0.0148x + 0.2126 | 1–5000 # | 0.9997 | 1 |
| WA | Y = 0.004174 X − 0.001699 | 0.05–100 | 0.9986 | 0.05 |
| WFG | Y = 0.01266 X − 0.01096 | 0.02–100 | 0.9964 | 0.02 |
| WFT | Y = 0.008693 X − 0.007188 | 0.02–100 | 0.9956 | 0.02 |
| WFD | Y = 0.01460 X − 0.01277 | 0.02–100 | 0.9973 | 0.02 |
| WFR | Y = 0.003441 X − 0.003744 | 0.02–100 | 0.9935 | 0.02 |
| Analyte | Added Concentration (ng/mL) | Intra-Batch (n = 6) | Inter-Batch (n = 18) | ||
|---|---|---|---|---|---|
| RSD (%) | RE (%) | RSD (%) | RE (%) | ||
| 1 | 4.23 | 13.57 | 1.14 | 12.87 | |
| TER | 2.5 | 11.57 | −0.16 | 2.08 | 5.92 |
| 100 | 0.89 | −0.49 | 0.27 | −0.21 | |
| 2500 | 3.53 | 3.53 | 1.35 | 3.18 | |
| Analyte | Added Concentration (ng/mL) | Recovery | Matrix Effect | ||
|---|---|---|---|---|---|
| (Mean ± SD%) | RSD (%) | (Mean ± SD%) | RSD (%) | ||
| 1 | 90.5 ± 4.8 | 5.3 | 91.1 ± 6.2 | 6.82 | |
| TER | 2.5 | 96.0 ± 3.8 | 3.9 | 100.9 ± 2.7 | 2.68 |
| 100 | 98.4 ± 1.1 | 1.08 | 98.0 ± 3.6 | 3.7 | |
| 2500 | 96.1 ± 1.4 | 1.43 | 99.7 ± 2.2 | 2.17 | |
| TER-D4 (IS 1) | 1000 | 88.2 ± 3.9 | 4.42 | 100.8 ± 4.0 | 3.96 |
| Analyte | Added Concentration | Room Temperature for 4 h | Autosampler for 24 h (−4 °C) | Three Freeze-Thaw Cycles (3 Times, −80 °C) | −80 °C for 30 d | ||||
|---|---|---|---|---|---|---|---|---|---|
| (ng/mL) | RSD (%) | RE (%) | RSD (%) | RE (%) | RSD (%) | RE (%) | RSD (%) | RE (%) | |
| TER | 2.5 | 8.66 | 11.52 | 9.24 | 4.67 | 6.15 | −8.59 | 3.20 | −9.92 |
| 100 | 9.50 | 10.56 | 6.35 | −1.19 | 5.89 | −5.63 | 2.91 | −9.96 | |
| 2500 | 1.74 | 2.57 | 0.66 | −3.66 | 1.89 | −4.99 | 2.67 | −7.83 | |
| Plasma | TER # | WA | WFD | WFG | WFR | WFT | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LEF | LEF+TGT | TGT | TGT + LEF | TGT | TGT + LEF | TGT | TGT + LEF | TGT | TGT + LEF | TGT | TGT + LEF | |
| Cmax (ng/mL) | 30.17 ± 1.55 | 24.47 ± 2.50 *** | 1.3 ± 0.3 | 0.790 ± 0.478 * | 12.5 ± 2.01 | 9.01 ± 0.86 ** | 1.67 ± 0.78 | 0.57 ± 0.22 ** | 10.6 ± 1.52 | 27.73 ± 3.96 **** | 2.97 ± 1.06 | 1.60 ± 0.41 * |
| Tmax (h) | 6.67 ± 1.10 | 6 ± 1.63 | 1.57 ± 0.53 | 1.13 ± 0.74 | 1.43 ± 0.53 | 0.625 ± 0.137 ** | 1.57 ± 0.53 | 0.5 ± 0.2 ** | 1.86 ± 0.38 | 1.04 ± 0.51 * | 0.96 ± 0.09 | 0.56 ± 0.12 **** |
| AUC0-t (ng* h/mL) | 374.55 ± 15.54 | 336.94 ± 21.19 ** | 4.67 ± 0.26 | 1.0378 ± 0.34 **** | 137 ± 24.08 | 40.0 ± 4.50 **** | 5.6 ± 2.29 | 1.06 ± 0.31 *** | 137 ± 20.5 | 141.42 ± 23.22 | 8.64 ± 3.48 | 2.08 ± 0.75 ** |
| AUC0-∞ (ng* h/mL) | 374.74 ± 15.49 | 337.02 ± 21.17 ** | 4.92 ± 1.01 | 1.23 ± 0.27 **** | 138 ± 25.4 | 44.85 ± 5.07 **** | 6.52 ± 2.74 | 1.09 ± 0.33 *** | 141 ± 21.6 | 148.07 ± 14.60 | 8.75 ± 3.49 | 2.20 ± 0.66 ** |
| t1/2 (h) | 7.70 ± 0.73 | 5.26 ± 0.704 *** | 2.11± 0.62 | 1.05 ± 0.0175 ** | 8.24 ± 1.36 | 3.96 ± 0.96 **** | 3.92 ± 0.57 | 2.04 ± 0.64 *** | 14.4 ± 1.93 | 6.0 ± 1.21 **** | 2.57 ± 1.11 | 1.08 ± 0.39 * |
| MRT0-t (h) | 9.147 ± 0.909 | 9.86 ± 1.04 | 2.99 ± 0.48 | 1.36 ± 0.23 **** | 10.9 ± 2.01 | 4.43 ± 1.10 *** | 3.16 ± 0.18 | 1.78 ± 0.21 **** | 16.1 ± 1.78 | 7.37 ± 0.62 **** | 2.83 ± 0.62 | 1.09 ± 0.22 **** |
| MRT0-∞ (h) | 9.186 ± 0.918 | 9.88 ± 1.04 | 3.84 ± 0.75 | 1.78 ± 0.18 **** | 11.2 ± 2.29 | 5.97 ± 1.27 *** | 4.87 ± 0.39 | 2.08 ± 0.2 9 **** | 18.4 ± 2.18 | 7.48 ± 0.64 **** | 3.01 ± 0.59 | 1.26 ± 0.15 **** |
| Vd (L/kg) | 0.304 ± 0.06417 | 0.230 ± 0.04967 ** | 16.3 ± 6.1 | 23.22 ± 10.9 | 10.6 ± 1.49 | 13.81 ± 1.85 | 155 ± 83.3 | 268.39 ± 95.0 | 26.3 ± 5.96 | 9.72 ± 2.79 *** | 39.7 ± 17.2 | 52.22 ± 8.71 |
| CL (L/h/kg) | 0.02 ± 0.0015 | 0.030 ± 0.0027 * | 5.35 ± 1.12 | 20.57 ± 5.58 **** | 0.91 ± 0.11 | 2.75 ± 0.31 **** | 28 ± 15.4 | 126.19 ± 22.78 **** | 1.26 ± 0.19 | 1.19 ± 0.12 | 11.6 ± 5.5 | 31.44 ± 3.85 **** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boucetta, H.; Wu, W.; Hong, T.; Cheng, R.; Jiang, J.; Liu, C.; Song, M.; Hang, T. Pharmacovigilance of Herb-Drug Interactions: A Pharmacokinetic Study on the Combined Administration of Tripterygium Glycosides Tablets and Leflunomide Tablets in Rats by LC-MS/MS. Pharmaceuticals 2022, 15, 991. https://doi.org/10.3390/ph15080991
Boucetta H, Wu W, Hong T, Cheng R, Jiang J, Liu C, Song M, Hang T. Pharmacovigilance of Herb-Drug Interactions: A Pharmacokinetic Study on the Combined Administration of Tripterygium Glycosides Tablets and Leflunomide Tablets in Rats by LC-MS/MS. Pharmaceuticals. 2022; 15(8):991. https://doi.org/10.3390/ph15080991
Chicago/Turabian StyleBoucetta, Hamza, Wei Wu, Tao Hong, Rui Cheng, Jing Jiang, Chengxi Liu, Min Song, and Taijun Hang. 2022. "Pharmacovigilance of Herb-Drug Interactions: A Pharmacokinetic Study on the Combined Administration of Tripterygium Glycosides Tablets and Leflunomide Tablets in Rats by LC-MS/MS" Pharmaceuticals 15, no. 8: 991. https://doi.org/10.3390/ph15080991
APA StyleBoucetta, H., Wu, W., Hong, T., Cheng, R., Jiang, J., Liu, C., Song, M., & Hang, T. (2022). Pharmacovigilance of Herb-Drug Interactions: A Pharmacokinetic Study on the Combined Administration of Tripterygium Glycosides Tablets and Leflunomide Tablets in Rats by LC-MS/MS. Pharmaceuticals, 15(8), 991. https://doi.org/10.3390/ph15080991






