Role of Drug Transporters in Elucidating Inter-Individual Variability in Pediatric Chemotherapy-Related Toxicities and Response
Abstract
:1. Introduction
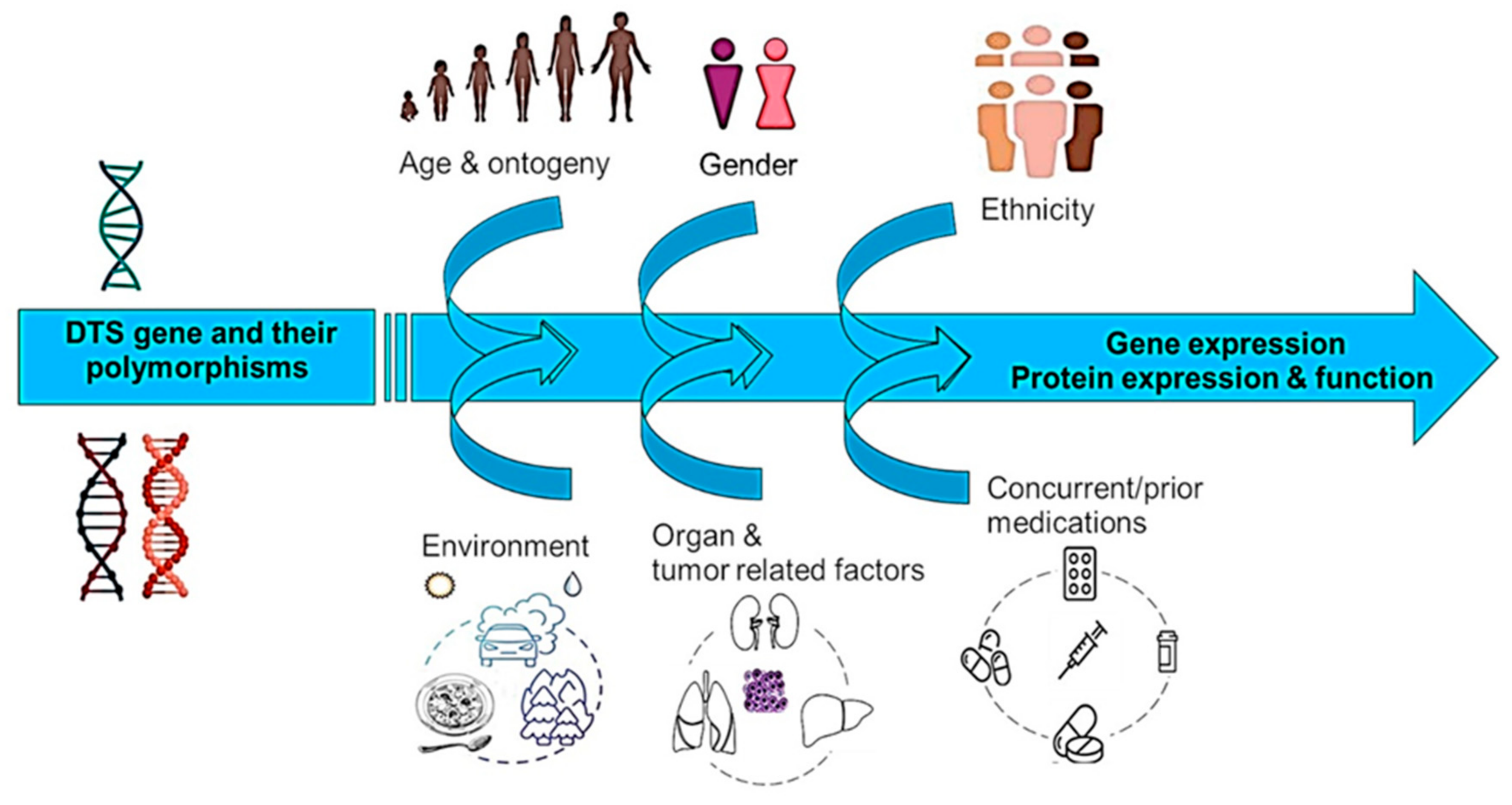
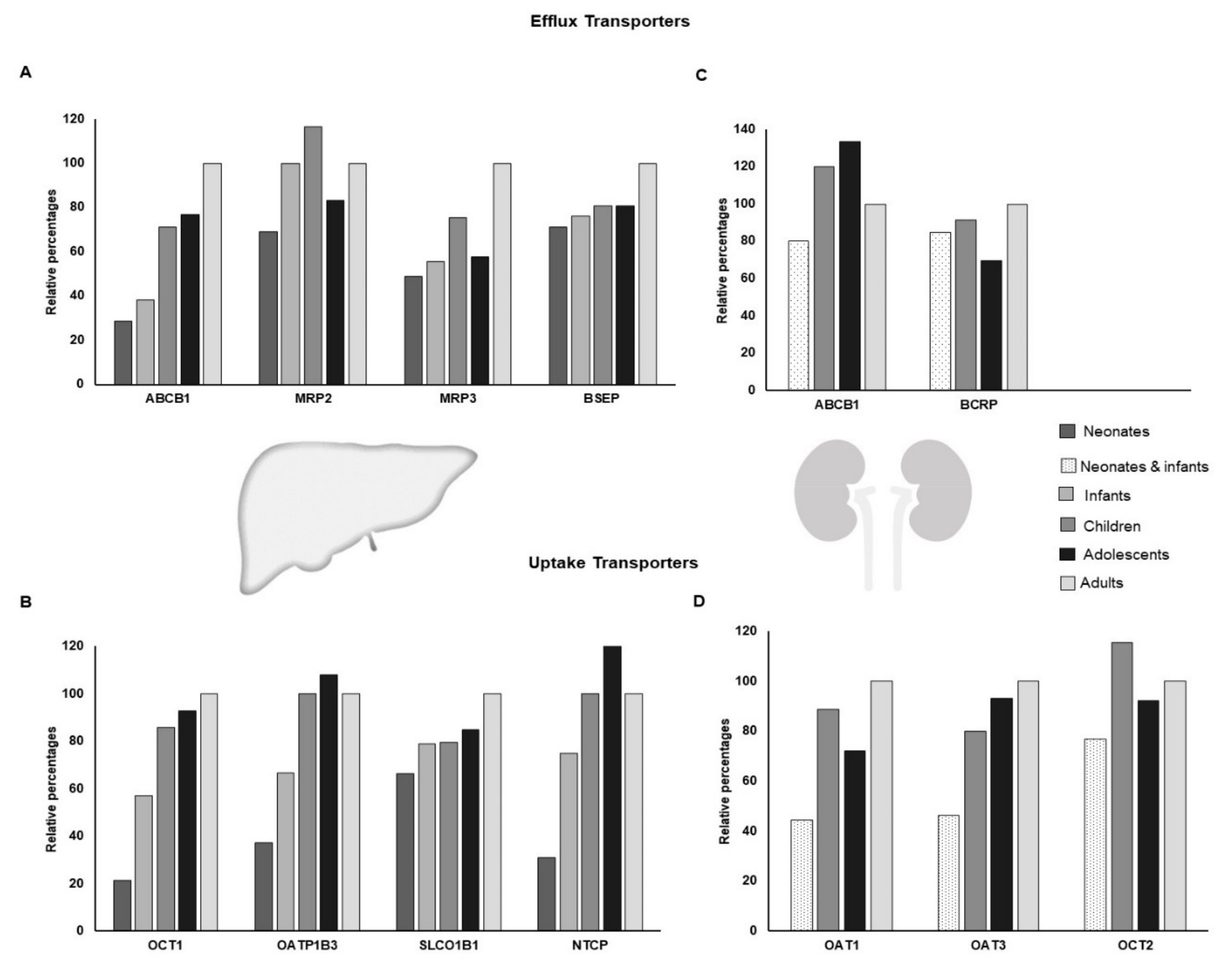
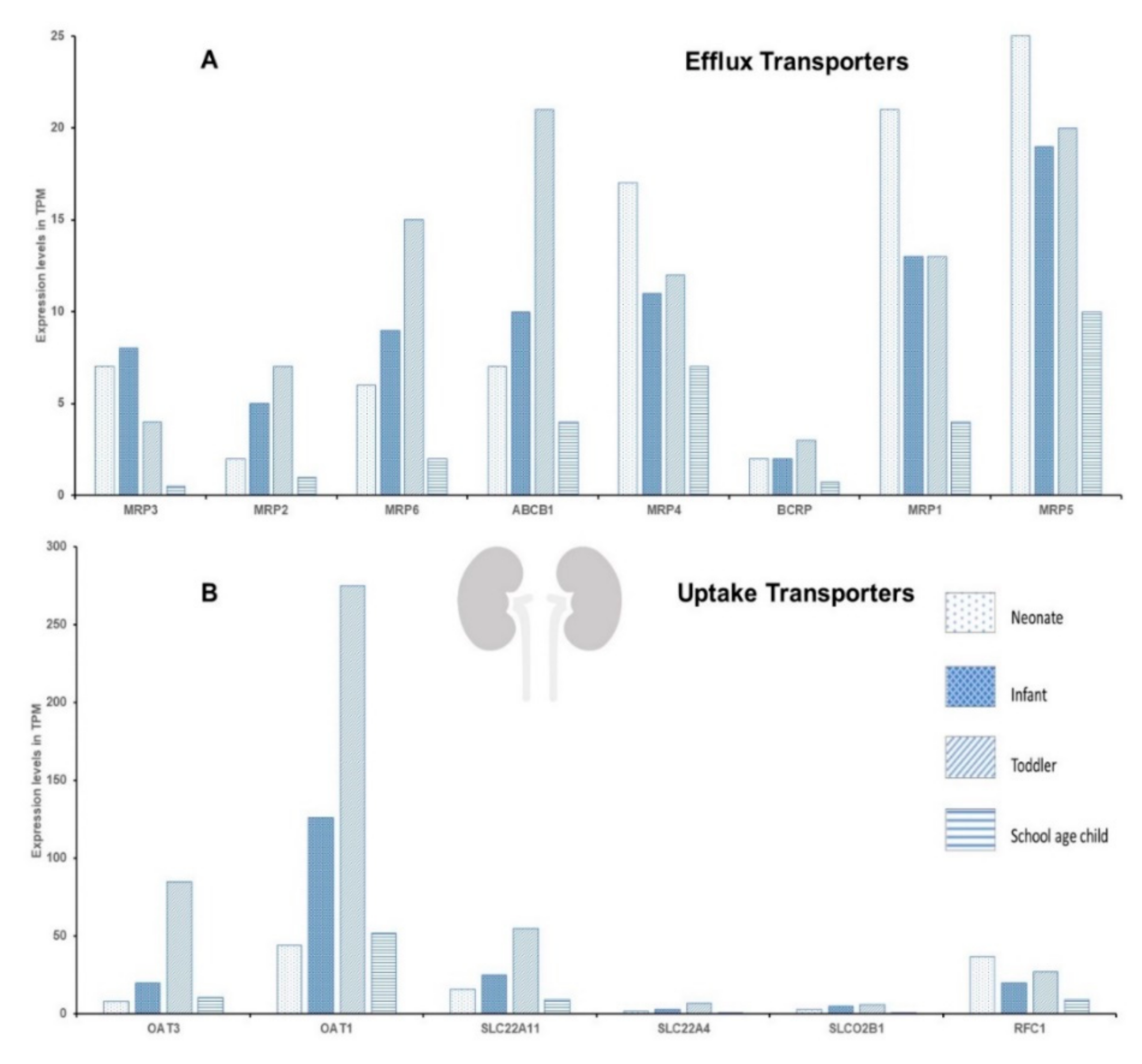
2. Ontogeny in DTS and Possible Consequences for Pediatric Chemotherapy
3. Role of DTS in Chemotherapy-Related Toxicities and Response in Pediatric Oncology
4. Enzymes
Asparaginase (Asparaginase Erwinia Chrysanthemi, Calaspargase Pegol-mknl, and Pegaspargase)
5. Purine Nucleoside Analogues
5.1. Clofarabine
5.2. 6-Mercaptopurine (6-MP)/Azathioprine
5.3. Nelarabine
6. Pyrimidine Nucleoside Analogues
Cytarabine
7. Folate Antagonist
Methotrexate
8. Microtubule Inhibitors
Vincristine
9. Alkylating Agents
Cyclophosphamide
10. Topoisomerase Inhibitors & Transcription Inhibitor
Doxorubicin, Daunorubicin (Anthracyclines), and Actinomycin-D
11. Kinase Inhibitors
11.1. Imatinib
11.2. Nilotinib
11.3. Dasatinib
11.4. Crizotinib
11.5. Entrectinib
11.6. Larotrectinib
11.7. Selumetinib
11.8. Everolimus
12. CD33-Directed Antibody-Drug Conjugate
Gemtuzumab Ozogamycin
13. Future Perspectives and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2016. CA Cancer J. Clin. 2016, 66, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Seibel, N.L.; Janeway, K.; Allen, C.E.; Chi, S.N.; Cho, Y.-J.; Glade Bender, J.L.; Kim, A.; Laetsch, T.W.; Irwin, M.S.; Takebe, N.; et al. Pediatric oncology enters an era of precision medicine. Curr. Probl. Cancer 2017, 41, 194–200. [Google Scholar] [CrossRef] [PubMed]
- Mlakar, V.; Huezo-Diaz Curtis, P.; Satyanarayana Uppugunduri, C.R.; Krajinovic, M.; Ansari, M. Pharmacogenomics in Pediatric Oncology: Review of Gene—Drug Associations for Clinical Use. Int. J. Mol. Sci. 2016, 17, 1502. [Google Scholar] [CrossRef] [PubMed]
- DeGorter, M.K.; Xia, C.Q.; Yang, J.J.; Kim, R.B. Drug transporters in drug efficacy and toxicity. Annu. Rev. Pharm. Toxicol. 2012, 52, 249–273. [Google Scholar] [CrossRef] [PubMed]
- Lozano, E.; Briz, O.; Macias, R.I.R.; Serrano, M.A.; Marin, J.J.G.; Herraez, E. Genetic Heterogeneity of SLC22 Family of Transporters in Drug Disposition. J. Pers. Med. 2018, 8, E14. [Google Scholar] [CrossRef] [PubMed]
- Li, L.-T.; Li, Z.-D.; Yang, Y.; Lu, Y.; Xie, X.-B.; Chen, L.; Feng, J.-Y.; Knisely, A.S.; Wang, J.-S. ABCB11 deficiency presenting as transient neonatal cholestasis: Correlation with genotypes and BSEP expression. Liver Int. 2020, 40, 2788–2796. [Google Scholar] [CrossRef] [PubMed]
- Joseph, S.; Nicolson, T.J.; Hammons, G.; Word, B.; Green-Knox, B.; Lyn-Cook, B. Expression of drug transporters in human kidney: Impact of sex, age, and ethnicity. Biol. Sex Differ. 2015, 6, 4. [Google Scholar] [CrossRef]
- Yang, L.; Li, Y.; Hong, H.; Chang, C.-W.; Guo, L.-W.; Lyn-Cook, B.; Shi, L.; Ning, B. Sex Differences in the Expression of Drug-Metabolizing and Transporter Genes in Human Liver. J. Drug Metab. Toxicol. 2012, 3, 1000119. [Google Scholar] [CrossRef]
- Iskar, M.; Campillos, M.; Kuhn, M.; Jensen, L.J.; van Noort, V.; Bork, P. Drug-induced regulation of target expression. PLoS Comput. Biol. 2010, 6, e1000925. [Google Scholar] [CrossRef]
- Sissung, T.M.; Huang, P.A.; Hauke, R.J.; McCrea, E.M.; Peer, C.J.; Barbier, R.H.; Strope, J.D.; Ley, A.M.; Zhang, M.; Hong, J.A.; et al. Severe Hepatotoxicity of Mithramycin Therapy Caused by Altered Expression of Hepatocellular Bile Transporters. Mol. Pharm. 2019, 96, 158–167. [Google Scholar] [CrossRef]
- Boscaini, S.; Cabrera-Rubio, R.; Speakman, J.R.; Cotter, P.D.; Cryan, J.F.; Nilaweera, K.N. Dietary α-lactalbumin alters energy balance, gut microbiota composition and intestinal nutrient transporter expression in high-fat diet-fed mice. Br. J. Nutr. 2019, 121, 1097–1107. [Google Scholar] [CrossRef]
- Oyola, M.G.; Johnson, R.C.; Bauman, B.M.; Frey, K.G.; Russell, A.L.; Cho-Clark, M.; Buban, K.N.; Bishop-Lilly, K.A.; Merrell, D.S.; Handa, R.J.; et al. Gut microbiota and metabolic marker alteration following dietary isoflavone-photoperiod interaction. Endocrinol. Diabetes Metab. 2021, 4, e00190. [Google Scholar] [CrossRef]
- Gao, Q.; Li, X.-X.; Xu, Y.-M.; Zhang, J.-Z.; Rong, S.; Qin, Y.-Q.; Fang, J. IRE1α-targeting downregulates ABC transporters and overcomes drug resistance of colon cancer cells. Cancer Lett. 2020, 476, 67–74. [Google Scholar] [CrossRef]
- Norris, M.D.; Bordow, S.B.; Marshall, G.M.; Haber, P.S.; Cohn, S.L.; Haber, M. Expression of the gene for multidrug-resistance-associated protein and outcome in patients with neuroblastoma. N. Engl. J. Med. 1996, 334, 231–238. [Google Scholar] [CrossRef]
- Haber, M.; Bordow, S.B.; Haber, P.S.; Marshall, G.M.; Stewart, B.W.; Norris, M.D. The prognostic value of MDR1 gene expression in primary untreated neuroblastoma. Eur. J. Cancer 1997, 33, 2031–2036. [Google Scholar] [CrossRef]
- Burkhart, C.A.; Watt, F.; Murray, J.; Pajic, M.; Prokvolit, A.; Xue, C.; Flemming, C.; Smith, J.; Purmal, A.; Isachenko, N.; et al. Small-molecule multidrug resistance-associated protein 1 inhibitor reversan increases the therapeutic index of chemotherapy in mouse models of neuroblastoma. Cancer Res. 2009, 69, 6573–6580. [Google Scholar] [CrossRef]
- Vander Borght, S.; van Pelt, J.; van Malenstein, H.; Cassiman, D.; Renard, M.; Verslype, C.; Libbrecht, L.; Roskams, T.A. Up-regulation of breast cancer resistance protein expression in hepatoblastoma following chemotherapy: A study in patients and in vitro. Hepatol. Res. 2008, 38, 1112–1121. [Google Scholar] [CrossRef]
- Veringa, S.J.E.; Biesmans, D.; van Vuurden, D.G.; Jansen, M.H.A.; Wedekind, L.E.; Horsman, I.; Wesseling, P.; Vandertop, W.P.; Noske, D.P.; Kaspers, G.J.L.; et al. In vitro drug response and efflux transporters associated with drug resistance in pediatric high grade glioma and diffuse intrinsic pontine glioma. PLoS ONE 2013, 8, e61512. [Google Scholar] [CrossRef]
- Sadhasivam, S.; Chidambaran, V.; Zhang, X.; Meller, J.; Esslinger, H.; Zhang, K.; Martin, L.J.; McAuliffe, J. Opioid-induced respiratory depression: ABCB1 transporter pharmacogenetics. Pharm. J. 2015, 15, 119–126. [Google Scholar] [CrossRef]
- Stage, T.B.; Hu, S.; Sparreboom, A.; Kroetz, D.L. Role for Drug Transporters in Chemotherapy-Induced Peripheral Neuropathy. Clin. Transl. Sci. 2021, 14, 460–467. [Google Scholar] [CrossRef]
- Xiao, H.; Zheng, Y.; Ma, L.; Tian, L.; Sun, Q. Clinically-Relevant ABC Transporter for Anti-Cancer Drug Resistance. Front. Pharm. 2021, 12, 648407. [Google Scholar] [CrossRef]
- Tzvetkov, M.V.; Saadatmand, A.R.; Bokelmann, K.; Meineke, I.; Kaiser, R.; Brockmöller, J. Effects of OCT1 polymorphisms on the cellular uptake, plasma concentrations and efficacy of the 5-HT(3) antagonists tropisetron and ondansetron. Pharm. J. 2012, 12, 22–29. [Google Scholar] [CrossRef]
- Brouwer, K.L.R.; Aleksunes, L.M.; Brandys, B.; Giacoia, G.P.; Knipp, G.; Lukacova, V.; Meibohm, B.; Nigam, S.K.; Rieder, M.; de Wildt, S.N.; et al. Human Ontogeny of Drug Transporters: Review and Recommendations of the Pediatric Transporter Working Group. Clin. Pharm. 2015, 98, 266–287. [Google Scholar] [CrossRef]
- Rodieux, F.; Gotta, V.; Pfister, M.; van den Anker, J.N. Causes and Consequences of Variability in Drug Transporter Activity in Pediatric Drug Therapy. J. Clin. Pharm. 2016, 56 (Suppl. S7), S173–S192. [Google Scholar] [CrossRef]
- Streekstra, E.J.; Russel, F.G.M.; van de Steeg, E.; de Wildt, S.N. Application of proteomics to understand maturation of drug metabolizing enzymes and transporters for the optimization of pediatric drug therapy. Drug Discov. Today Technol. 2021, 39, 31–48. [Google Scholar] [CrossRef]
- Hines, R.N. Developmental expression of drug metabolizing enzymes: Impact on disposition in neonates and young children. Int. J. Pharm. 2013, 452, 3–7. [Google Scholar] [CrossRef]
- Maier, T.; Güell, M.; Serrano, L. Correlation of mRNA and protein in complex biological samples. FEBS Lett. 2009, 583, 3966–3973. [Google Scholar] [CrossRef]
- Home <Expression Atlas <EMBL-EBI. Available online: https://www.ebi.ac.uk/gxa/home (accessed on 27 June 2022).
- Papatheodorou, I.; Moreno, P.; Manning, J.; Fuentes, A.M.-P.; George, N.; Fexova, S.; Fonseca, N.A.; Füllgrabe, A.; Green, M.; Huang, N.; et al. Expression Atlas update: From tissues to single cells. Nucleic Acids Res. 2020, 48, D77–D83. [Google Scholar] [CrossRef]
- Kodidela Developmental Pattern of Hepatic Drug-Metabolizing Enzymes in Pediatric Population and Its Role in Optimal Drug Treatment. Available online: https://www.amhsjournal.org/article.asp?issn=2321-4848;year=2017;volume=5;issue=1;spage=115;epage=122;aulast=Kodidela (accessed on 22 May 2022).
- Momper, J.D.; Tsunoda, S.M.; Ma, J.D. Evaluation of Proposed In Vivo Probe Substrates and Inhibitors for Phenotyping Transporter Activity in Humans. J. Clin. Pharmacol. 2016, 56, S82–S98. [Google Scholar] [CrossRef]
- Hillgren, K.M.; Keppler, D.; Zur, A.A.; Giacomini, K.M.; Stieger, B.; Cass, C.E.; Zhang, L. International Transporter Consortium Emerging transporters of clinical importance: An update from the International Transporter Consortium. Clin. Pharm. 2013, 94, 52–63. [Google Scholar] [CrossRef]
- Prasad, B.; Gaedigk, A.; Vrana, M.; Gaedigk, R.; Leeder, J.S.; Salphati, L.; Chu, X.; Xiao, G.; Hop, C.E.C.A.; Evers, R.; et al. Ontogeny of Hepatic Drug Transporters as Quantified by LC-MS/MS Proteomics. Clin. Pharm. 2016, 100, 362–370. [Google Scholar] [CrossRef]
- Cheung, K.W.K.; van Groen, B.D.; Spaans, E.; van Borselen, M.D.; de Bruijn, A.C.J.M.; Simons-Oosterhuis, Y.; Tibboel, D.; Samsom, J.N.; Verdijk, R.M.; Smeets, B.; et al. A Comprehensive Analysis of Ontogeny of Renal Drug Transporters: mRNA Analyses, Quantitative Proteomics, and Localization. Clin. Pharm. 2019, 106, 1083–1092. [Google Scholar] [CrossRef]
- Mizuno, T.; Fukuda, T.; Masuda, S.; Uemoto, S.; Matsubara, K.; Inui, K.-I.; Vinks, A.A. Developmental trajectory of intestinal MDR1/ABCB1 mRNA expression in children. Br. J. Clin. Pharm. 2014, 77, 910–912. [Google Scholar] [CrossRef]
- Mooij, M.G.; Schwarz, U.I.; de Koning, B.A.E.; Leeder, J.S.; Gaedigk, R.; Samsom, J.N.; Spaans, E.; van Goudoever, J.B.; Tibboel, D.; Kim, R.B.; et al. Ontogeny of human hepatic and intestinal transporter gene expression during childhood: Age matters. Drug Metab. Dispos. 2014, 42, 1268–1274. [Google Scholar] [CrossRef]
- Fakhoury, M.; Litalien, C.; Medard, Y.; Cavé, H.; Ezzahir, N.; Peuchmaur, M.; Jacqz-Aigrain, E. Localization and mRNA expression of CYP3A and P-glycoprotein in human duodenum as a function of age. Drug Metab. Dispos. 2005, 33, 1603–1607. [Google Scholar] [CrossRef]
- Van Kalken, C.K.; Giaccone, G.; van der Valk, P.; Kuiper, C.M.; Hadisaputro, M.M.; Bosma, S.A.; Scheper, R.J.; Meijer, C.J.; Pinedo, H.M. Multidrug resistance gene (P-glycoprotein) expression in the human fetus. Am. J. Pathol. 1992, 141, 1063–1072. [Google Scholar]
- Fakhoury, M.; Lecordier, J.; Medard, Y.; Peuchmaur, M.; Jacqz-Agrain, E. Impact of inflammation on the duodenal mRNA expression of CYP3A and P-glycoprotein in children with Crohn’s disease. Inflamm. Bowel Dis. 2006, 12, 745–749. [Google Scholar] [CrossRef]
- Konieczna, A.; Erdösová, B.; Lichnovská, R.; Jandl, M.; Cížková, K.; Ehrmann, J. Differential expression of ABC transporters (MDR1, MRP1, BCRP) in developing human embryos. J. Mol. Histol. 2011, 42, 567–574. [Google Scholar] [CrossRef]
- Van Groen, B.D.; van de Steeg, E.; Mooij, M.G.; van Lipzig, M.M.H.; de Koning, B.A.E.; Verdijk, R.M.; Wortelboer, H.M.; Gaedigk, R.; Bi, C.; Leeder, J.S.; et al. Proteomics of human liver membrane transporters: A focus on fetuses and newborn infants. Eur. J. Pharm. Sci. 2018, 124, 217–227. [Google Scholar] [CrossRef]
- Li, C.Y.; Hosey-Cojocari, C.; Basit, A.; Unadkat, J.D.; Leeder, J.S.; Prasad, B. Optimized Renal Transporter Quantification by Using Aquaporin 1 and Aquaporin 2 as Anatomical Markers: Application in Characterizing the Ontogeny of Renal Transporters and Its Correlation with Hepatic Transporters in Paired Human Samples. AAPS J. 2019, 21, 88. [Google Scholar] [CrossRef]
- Hao, C.; Ma, X.; Wang, L.; Zhang, W.; Hu, J.; Huang, J.; Yang, W. Predicting the presence and mechanism of busulfan drug-drug interactions in hematopoietic stem cell transplantation using pharmacokinetic interaction network-based molecular structure similarity and network pharmacology. Eur. J. Clin. Pharm. 2021, 77, 595–605. [Google Scholar] [CrossRef]
- Myers, A.L.; Kawedia, J.D.; Champlin, R.E.; Kramer, M.A.; Nieto, Y.; Ghose, R.; Andersson, B.S. Clarifying busulfan metabolism and drug interactions to support new therapeutic drug monitoring strategies: A comprehensive review. Expert Opin. Drug Metab. Toxicol. 2017, 13, 901–923. [Google Scholar] [CrossRef]
- Billington, S.; Salphati, L.; Hop, C.E.C.A.; Chu, X.; Evers, R.; Burdette, D.; Rowbottom, C.; Lai, Y.; Xiao, G.; Humphreys, W.G.; et al. Interindividual and Regional Variability in Drug Transporter Abundance at the Human Blood-Brain Barrier Measured by Quantitative Targeted Proteomics. Clin. Pharm. 2019, 106, 228–237. [Google Scholar] [CrossRef]
- Sasongko, L.; Link, J.M.; Muzi, M.; Mankoff, D.A.; Yang, X.; Collier, A.C.; Shoner, S.C.; Unadkat, J.D. Imaging P-glycoprotein Transport Activity at the Human Blood-brain Barrier with Positron Emission Tomography. Clin. Pharmacol. Ther. 2005, 77, 503–514. [Google Scholar] [CrossRef]
- Toornvliet, R.; van Berckel, B.N.M.; Luurtsema, G.; Lubberink, M.; Geldof, A.A.; Bosch, T.M.; Oerlemans, R.; Lammertsma, A.A.; Franssen, E.J.F. Effect of age on functional P-glycoprotein in the blood-brain barrier measured by use of (R)-[(11)C]verapamil and positron emission tomography. Clin. Pharm. 2006, 79, 540–548. [Google Scholar] [CrossRef]
- Drugs Approved for Childhood Cancers—NCI. Available online: https://www.cancer.gov/about-cancer/treatment/drugs/childhood-cancer-fda-approved-drugs (accessed on 22 May 2022).
- Palmerini, E.; Meazza, C.; Tamburini, A.; Bisogno, G.; Ferraresi, V.; Asaftei, S.D.; Milano, G.M.; Coccoli, L.; Manzitti, C.; Luksch, R.; et al. Phase 2 study for nonmetastatic extremity high-grade osteosarcoma in pediatric and adolescent and young adult patients with a risk-adapted strategy based on ABCB1/P-glycoprotein expression: An Italian Sarcoma Group trial (ISG/OS-2). Cancer 2022, 128, 1958–1966. [Google Scholar] [CrossRef]
- Black, J.L.; Litzow, M.R.; Hogan, W.J.; O’Kane, D.J.; Walker, D.L.; Lesnick, T.G.; Kremers, W.K.; Avula, R.; Ketterling, R.P. Correlation of CYP2B6, CYP2C19, ABCC4 and SOD2 genotype with outcomes in allogeneic blood and marrow transplant patients. Leuk. Res. 2012, 36, 59–66. [Google Scholar] [CrossRef]
- Kim, I.-W.; Yun, H.; Choi, B.; Han, N.; Kim, M.G.; Park, S.; Oh, J.M. Population pharmacokinetics analysis of cyclophosphamide with genetic effects in patients undergoing hematopoietic stem cell transplantation. Eur. J. Clin. Pharm. 2013, 69, 1543–1551. [Google Scholar] [CrossRef]
- Kalra, S.; Kaur, R.P.; Ludhiadch, A.; Shafi, G.; Vashista, R.; Kumar, R.; Munshi, A. Association of CYP2C19*2 and ALDH1A1*1/*2 variants with disease outcome in breast cancer patients: Results of a global screening array. Eur. J. Clin. Pharm. 2018, 74, 1291–1298. [Google Scholar] [CrossRef]
- Steinbach, D.; Sell, W.; Voigt, A.; Hermann, J.; Zintl, F.; Sauerbrey, A. BCRP gene expression is associated with a poor response to remission induction therapy in childhood acute myeloid leukemia. Leukemia 2002, 16, 1443–1447. [Google Scholar] [CrossRef]
- Armenian, S.H.; Ding, Y.; Mills, G.; Sun, C.; Venkataraman, K.; Wong, F.L.; Neuhausen, S.L.; Senitzer, D.; Wang, S.; Forman, S.J.; et al. Genetic susceptibility to anthracycline-related congestive heart failure in survivors of haematopoietic cell transplantation. Br. J. Haematol. 2013, 163, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Gregers, J.; Gréen, H.; Christensen, I.J.; Dalhoff, K.; Schroeder, H.; Carlsen, N.; Rosthoej, S.; Lausen, B.; Schmiegelow, K.; Peterson, C. Polymorphisms in the ABCB1 gene and effect on outcome and toxicity in childhood acute lymphoblastic leukemia. Pharm. J. 2015, 15, 372–379. [Google Scholar] [CrossRef] [PubMed]
- Hattinger, C.M.; Biason, P.; Iacoboni, E.; Gagno, S.; Fanelli, M.; Tavanti, E.; Vella, S.; Ferrari, S.; Roli, A.; Roncato, R.; et al. Candidate germline polymorphisms of genes belonging to the pathways of four drugs used in osteosarcoma standard chemotherapy associated with risk, survival and toxicity in non-metastatic high-grade osteosarcoma. Oncotarget 2016, 7, 61970–61987. [Google Scholar] [CrossRef]
- Krajinovic, M.; Elbared, J.; Drouin, S.; Bertout, L.; Rezgui, A.; Ansari, M.; Raboisson, M.-J.; Lipshultz, S.E.; Silverman, L.B.; Sallan, S.E.; et al. Polymorphisms of ABCC5 and NOS3 genes influence doxorubicin cardiotoxicity in survivors of childhood acute lymphoblastic leukemia. Pharm. J. 2016, 16, 530–535. [Google Scholar] [CrossRef] [PubMed]
- Hontecillas-Prieto, L.; Garcia-Dominguez, D.J.; Vaca, D.P.; Garcia-Mejias, R.; Marcilla, D.; Ramirez-Villar, G.L.; Saez, C.; de Álava, E. Multidrug resistance transporter profile reveals MDR3 as a marker for stratification of blastemal Wilms tumour patients. Oncotarget 2017, 8, 11173–11186. [Google Scholar] [CrossRef] [PubMed]
- Drenberg, C.D.; Paugh, S.W.; Pounds, S.B.; Shi, L.; Orwick, S.J.; Li, L.; Hu, S.; Gibson, A.A.; Ribeiro, R.C.; Rubnitz, J.E.; et al. Inherited variation in OATP1B1 is associated with treatment outcome in acute myeloid leukemia. Clin. Pharm. 2016, 99, 651–660. [Google Scholar] [CrossRef] [PubMed]
- Mehrvar, N.; Abolghasemi, H.; Rezvany, M.R.; Akbari, M.E.; Saberynejad, J.; Mehrvar, A.; Movafagh, A. Characterizing Iranian Pediatric Patients with Relapsed Acute Lymphoblastic Leukemia Through Gene Expression Profiling of Common ATP Binding Cassette Transporters Subfamily C. J. Pediatr. Hematol. Oncol. 2020, 42, 41–45. [Google Scholar] [CrossRef] [PubMed]
- Drugs@FDA: FDA-Approved Drugs. Available online: https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&ApplNo=101063 (accessed on 22 May 2022).
- Hill, C.R.; Cole, M.; Errington, J.; Malik, G.; Boddy, A.V.; Veal, G.J. Characterisation of the clinical pharmacokinetics of actinomycin D and the influence of ABCB1 pharmacogenetic variation on actinomycin D disposition in children with cancer. Clin. Pharm. 2014, 53, 741–751. [Google Scholar] [CrossRef]
- Baskin, Y.; Amirfallah, A.; Calibasi, G.; Olgun, N. Hepatopathy-Thrombocytopenia Syndrome During Actinomycin D Treatment May Be Related to MDR1 (ABCB1) Gene Polymorphisms. Am. J. 2016, 23, e594–e596. [Google Scholar] [CrossRef]
- Kim, H.Y.; Veal, G.J.; Zhou, F.; Boddy, A.V. The role of solute carrier (SLC) transporters in actinomycin D pharmacokinetics in paediatric cancer patients. Eur. J. Clin. Pharm. 2018, 74, 1575–1584. [Google Scholar] [CrossRef]
- BLINCYTO® (Blinatumomab) for Injection 40. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/125557s013lbl.pdf (accessed on 27 June 2022).
- KYMRIAH (Tisagenlecleucel)|FDA. Available online: https://www.fda.gov/vaccines-blood-biologics/cellular-gene-therapy-products/kymriah-tisagenlecleucel (accessed on 27 March 2022).
- Walter, R.B.; Raden, B.W.; Hong, T.C.; Flowers, D.A.; Bernstein, I.D.; Linenberger, M.L. Multidrug resistance protein attenuates gemtuzumab ozogamicin-induced cytotoxicity in acute myeloid leukemia cells. Blood 2003, 102, 1466–1473. [Google Scholar] [CrossRef]
- Boyer, T.; Gonzales, F.; Barthélémy, A.; Marceau-Renaut, A.; Peyrouze, P.; Guihard, S.; Lepelley, P.; Plesa, A.; Nibourel, O.; Delattre, C.; et al. Clinical Significance of ABCB1 in Acute Myeloid Leukemia: A Comprehensive Study. Cancers 2019, 11, 1323. [Google Scholar] [CrossRef]
- Rafiee, R.; Chauhan, L.; Alonzo, T.A.; Wang, Y.-C.; Elmasry, A.; Loken, M.R.; Pollard, J.; Aplenc, R.; Raimondi, S.; Hirsch, B.A.; et al. ABCB1 SNP predicts outcome in patients with acute myeloid leukemia treated with Gemtuzumab ozogamicin: A report from Children’s Oncology Group AAML0531 Trial. Blood Cancer J. 2019, 9, 51. [Google Scholar] [CrossRef]
- Uwai, Y.; Taniguchi, R.; Motohashi, H.; Saito, H.; Okuda, M.; Inui, K. Methotrexate-loxoprofen interaction: Involvement of human organic anion transporters hOAT1 and hOAT3. Drug Metab. Pharm. 2004, 19, 369–374. [Google Scholar] [CrossRef]
- Hegyi, M.; Arany, A.; Semsei, A.F.; Csordas, K.; Eipel, O.; Gezsi, A.; Kutszegi, N.; Csoka, M.; Muller, J.; Erdelyi, D.J.; et al. Pharmacogenetic analysis of high-dose methotrexate treatment in children with osteosarcoma. Oncotarget 2017, 8, 9388–9398. [Google Scholar] [CrossRef]
- Wang, S.-M.; Zeng, W.-X.; Wu, W.-S.; Sun, L.-L.; Yan, D. Association between a microRNA binding site polymorphism in SLCO1A2 and the risk of delayed methotrexate elimination in Chinese children with acute lymphoblastic leukemia. Leuk. Res. 2018, 65, 61–66. [Google Scholar] [CrossRef]
- Ramsey, L.B.; Mizuno, T.; Vinks, A.A.; O’Brien, M.M. Delayed methotrexate clearance in patients with acute lymphoblastic leukemia concurrently receiving dasatinib. Pediatr. Blood Cancer 2019, 66, e27618. [Google Scholar] [CrossRef]
- Huang, C.; Xia, F.; Xue, L.; Liu, L.; Bian, Y.; Jin, Z.; Miao, L. Coadministration of vindesine with high-dose methotrexate therapy increases acute kidney injury via BCRP, MRP2, and OAT1/OAT3. Cancer Chemother. Pharm. 2020, 85, 433–441. [Google Scholar] [CrossRef]
- Lopez-Lopez, E.; Autry, R.J.; Smith, C.; Yang, W.; Paugh, S.W.; Panetta, J.C.; Crews, K.R.; Bonten, E.J.; Smart, B.; Pei, D.; et al. Pharmacogenomics of intracellular methotrexate polyglutamates in patients’ leukemia cells in vivo. J. Clin. Investig. 2020, 130, 6600–6615. [Google Scholar] [CrossRef]
- Qarziba (Dinutuximab Beta)—Summary of Product Characteristics (SmPC)—(emc). Available online: https://www.medicines.org.uk/emc/product/9441/smpc#gref (accessed on 27 March 2022).
- Merchant, M.S.; Wright, M.; Baird, K.; Wexler, L.H.; Rodriguez-Galindo, C.; Bernstein, D.; Delbrook, C.; Lodish, M.; Bishop, R.; Wolchok, J.D.; et al. Phase I Clinical Trial of Ipilimumab in Pediatric Patients with Advanced Solid Tumors. Clin. Cancer Res. 2016, 22, 1364–1370. [Google Scholar] [CrossRef]
- Drake, K.J.; Singhal, J.; Yadav, S.; Nadkar, A.; Pungaliya, C.; Singhal, S.S.; Awasthi, S. RALBP1/RLIP76 mediates multidrug resistance. Int. J. Oncol. 2007, 30, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Hopper-Borge, E.; Xu, X.; Shen, T.; Shi, Z.; Chen, Z.-S.; Kruh, G.D. Human multidrug resistance protein 7 (ABCC10) is a resistance factor for nucleoside analogues and epothilone B. Cancer Res. 2009, 69, 178–184. [Google Scholar] [CrossRef] [PubMed]
- Caronia, D.; Patiño-Garcia, A.; Peréz-Martínez, A.; Pita, G.; Moreno, L.T.; Zalacain-Díez, M.; Molina, B.; Colmenero, I.; Sierrasesúmaga, L.; Benítez, J.; et al. Effect of ABCB1 and ABCC3 polymorphisms on osteosarcoma survival after chemotherapy: A pharmacogenetic study. PLoS ONE 2011, 6, e26091. [Google Scholar] [CrossRef] [PubMed]
- Guilhaumou, R.; Simon, N.; Quaranta, S.; Verschuur, A.; Lacarelle, B.; Andre, N.; Solas, C. Population pharmacokinetics and pharmacogenetics of vincristine in paediatric patients treated for solid tumour diseases. Cancer Chemother. Pharm. 2011, 68, 1191–1198. [Google Scholar] [CrossRef]
- Ceppi, F.; Langlois-Pelletier, C.; Gagné, V.; Rousseau, J.; Ciolino, C.; De Lorenzo, S.; Kevin, K.M.; Cijov, D.; Sallan, S.E.; Silverman, L.B.; et al. Polymorphisms of the vincristine pathway and response to treatment in children with childhood acute lymphoblastic leukemia. Pharmacogenomics 2014, 15, 1105–1116. [Google Scholar] [CrossRef]
- Zgheib, N.K.; Ghanem, K.M.; Tamim, H.; Aridi, C.; Shahine, R.; Tarek, N.; Saab, R.; Abboud, M.R.; El-Solh, H.; Muwakkit, S.A. Genetic polymorphisms in candidate genes are not associated with increased vincristine-related peripheral neuropathy in Arab children treated for acute childhood leukemia: A single institution study. Pharm. Genom. 2018, 28, 189–195. [Google Scholar] [CrossRef]
- Hegedus, C.; Ozvegy-Laczka, C.; Apáti, A.; Magócsi, M.; Német, K.; Orfi, L.; Kéri, G.; Katona, M.; Takáts, Z.; Váradi, A.; et al. Interaction of nilotinib, dasatinib and bosutinib with ABCB1 and ABCG2: Implications for altered anti-cancer effects and pharmacological properties. Br. J. Pharm. 2009, 158, 1153–1164. [Google Scholar] [CrossRef]
- Eadie, L.N.; Hughes, T.P.; White, D.L. Interaction of the efflux transporters ABCB1 and ABCG2 with imatinib, nilotinib, and dasatinib. Clin. Pharm. 2014, 95, 294–306. [Google Scholar] [CrossRef]
- White, D.L.; Saunders, V.A.; Dang, P.; Engler, J.; Zannettino, A.C.W.; Cambareri, A.C.; Quinn, S.R.; Manley, P.W.; Hughes, T.P. OCT-1-mediated influx is a key determinant of the intracellular uptake of imatinib but not nilotinib (AMN107): Reduced OCT-1 activity is the cause of low in vitro sensitivity to imatinib. Blood 2006, 108, 697–704. [Google Scholar] [CrossRef]
- Singh, O.; Chan, J.Y.; Lin, K.; Heng, C.C.T.; Chowbay, B. SLC22A1-ABCB1 haplotype profiles predict imatinib pharmacokinetics in Asian patients with chronic myeloid leukemia. PLoS ONE 2012, 7, e51771. [Google Scholar] [CrossRef]
- Zheng, Q.; Wu, H.; Yu, Q.; Kim, D.H.D.; Lipton, J.H.; Angelini, S.; Soverini, S.; Vivona, D.; Takahashi, N.; Cao, J. ABCB1 polymorphisms predict imatinib response in chronic myeloid leukemia patients: A systematic review and meta-analysis. Pharm. J. 2015, 15, 127–134. [Google Scholar] [CrossRef]
- Eadie, L.N.; Dang, P.; Saunders, V.A.; Yeung, D.T.; Osborn, M.P.; Grigg, A.P.; Hughes, T.P.; White, D.L. The clinical significance of ABCB1 overexpression in predicting outcome of CML patients undergoing first-line imatinib treatment. Leukemia 2017, 31, 75–82. [Google Scholar] [CrossRef]
- Dessilly, G.; Elens, L.; Panin, N.; Karmani, L.; Demoulin, J.-B.; Haufroid, V. ABCB1 1199G>A polymorphism (rs2229109) affects the transport of imatinib, nilotinib and dasatinib. Pharmacogenomics 2016, 17, 883–890. [Google Scholar] [CrossRef]
- Eadie, L.N.; Dang, P.; Goyne, J.M.; Hughes, T.P.; White, D.L. ABCC6 plays a significant role in the transport of nilotinib and dasatinib, and contributes to TKI resistance in vitro, in both cell lines and primary patient mononuclear cells. PLoS ONE 2018, 13, e0192180. [Google Scholar] [CrossRef]
- Zhou, W.; Zhang, X.; Cheng, C.; Wang, F.; Wang, X.; Liang, Y.; To, K.K.W.; Zhou, W.; Huang, H.; Fu, L. Crizotinib (PF-02341066) reverses multidrug resistance in cancer cells by inhibiting the function of P-glycoprotein. Br. J. Pharm. 2012, 166, 1669–1683. [Google Scholar] [CrossRef]
- Fujiwara, Y.; Hamada, A.; Mizugaki, H.; Aikawa, H.; Hata, T.; Horinouchi, H.; Kanda, S.; Goto, Y.; Itahashi, K.; Nokihara, H.; et al. Pharmacokinetic profiles of significant adverse events with crizotinib in Japanese patients with ABCB1 polymorphism. Cancer Sci. 2016, 107, 1117–1123. [Google Scholar] [CrossRef]
- Vagiannis, D.; Yu, Z.; Novotna, E.; Morell, A.; Hofman, J. Entrectinib reverses cytostatic resistance through the inhibition of ABCB1 efflux transporter, but not the CYP3A4 drug-metabolizing enzyme. Biochem. Pharm. 2020, 178, 114061. [Google Scholar] [CrossRef]
- Wang, Y.; Sparidans, R.W.; Li, W.; Lebre, M.C.; Beijnen, J.H.; Schinkel, A.H. OATP1A/1B, CYP3A, ABCB1, and ABCG2 limit oral availability of the NTRK inhibitor larotrectinib, while ABCB1 and ABCG2 also restrict its brain accumulation. Br. J. Pharm. 2020, 177, 3060–3074. [Google Scholar] [CrossRef]
- Dymond, A.W.; Elks, C.; Martin, P.; Carlile, D.J.; Mariani, G.; Lovick, S.; Huang, Y.; Lorch, U.; Brown, H.; So, K. Pharmacokinetics and pharmacogenetics of the MEK1/2 inhibitor, selumetinib, in Asian and Western healthy subjects: A pooled analysis. Eur. J. Clin. Pharm. 2017, 73, 717–726. [Google Scholar] [CrossRef]
- De Gooijer, M.C.; Zhang, P.; Weijer, R.; Buil, L.C.M.; Beijnen, J.H.; van Tellingen, O. The impact of P-glycoprotein and breast cancer resistance protein on the brain pharmacokinetics and pharmacodynamics of a panel of MEK inhibitors. Int. J. Cancer 2018, 142, 381–391. [Google Scholar] [CrossRef]
- Pascual, T.; Apellániz-Ruiz, M.; Pernaut, C.; Cueto-Felgueroso, C.; Villalba, P.; Álvarez, C.; Manso, L.; Inglada-Pérez, L.; Robledo, M.; Rodríguez-Antona, C.; et al. Polymorphisms associated with everolimus pharmacokinetics, toxicity and survival in metastatic breast cancer. PLoS ONE 2017, 12, e0180192. [Google Scholar] [CrossRef]
- De Wolf, C.; Jansen, R.; Yamaguchi, H.; de Haas, M.; van de Wetering, K.; Wijnholds, J.; Beijnen, J.; Borst, P. Contribution of the drug transporter ABCG2 (breast cancer resistance protein) to resistance against anticancer nucleosides. Mol. Cancer 2008, 7, 3092–3102. [Google Scholar] [CrossRef]
- Nagai, S.; Takenaka, K.; Nachagari, D.; Rose, C.; Domoney, K.; Sun, D.; Sparreboom, A.; Schuetz, J.D. Deoxycytidine kinase modulates the impact of the ABC transporter ABCG2 on clofarabine cytotoxicity. Cancer Res. 2011, 71, 1781–1791. [Google Scholar] [CrossRef]
- Matimba, A.; Li, F.; Livshits, A.; Cartwright, C.S.; Scully, S.; Fridley, B.L.; Jenkins, G.; Batzler, A.; Wang, L.; Weinshilboum, R.; et al. Thiopurine pharmacogenomics: Association of SNPs with clinical response and functional validation of candidate genes. Pharmacogenomics 2014, 15, 433–447. [Google Scholar] [CrossRef]
- Gervasini, G.; de Murillo, S.G.; Jiménez, M.; de la Maya, M.D.; Vagace, J.M. Effect of polymorphisms in transporter genes on dosing, efficacy and toxicity of maintenance therapy in children with acute lymphoblastic leukemia. Gene 2017, 628, 72–77. [Google Scholar] [CrossRef]
- Homminga, I.; Zwaan, C.M.; Manz, C.Y.; Parker, C.; Bantia, S.; Smits, W.K.; Higginbotham, F.; Pieters, R.; Meijerink, J.P.P. In vitro efficacy of forodesine and nelarabine (ara-G) in pediatric leukemia. Blood 2011, 118, 2184–2190. [Google Scholar] [CrossRef]
- Akahane, K.; Murakami, Y.; Kagami, K.; Abe, M.; Harama, D.; Shinohara, T.; Watanabe, A.; Goi, K.; Nishi, R.; Yamauchi, T.; et al. High ENT1 and DCK gene expression levels are a potential biomarker to predict favorable response to nelarabine therapy in T-cell acute lymphoblastic leukemia. Hematol. Oncol. 2019, 37, 516–519. [Google Scholar] [CrossRef] [PubMed]
- Stam, R.W.; den Boer, M.L.; Meijerink, J.P.P.; Ebus, M.E.G.; Peters, G.J.; Noordhuis, P.; Janka-Schaub, G.E.; Armstrong, S.A.; Korsmeyer, S.J.; Pieters, R. Differential mRNA expression of Ara-C-metabolizing enzymes explains Ara-C sensitivity in MLL gene-rearranged infant acute lymphoblastic leukemia. Blood 2003, 101, 1270–1276. [Google Scholar] [CrossRef] [PubMed]
- Yee, S.W.; Mefford, J.A.; Singh, N.; Percival, M.-E.; Stecula, A.; Yang, K.; Witte, J.S.; Takahashi, A.; Kubo, M.; Matsuda, K.; et al. Impact of polymorphisms in drug pathway genes on disease-free survival in adults with acute myeloid leukemia. J. Hum. Genet. 2013, 58, 353–361. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Yin, J.; Li, X.; Zhang, Y.; Xu, X.; Zhai, M.; Chen, J.; Qian, C.; Zhou, H.; Liu, Z. Association of ABCB1 polymorphisms with prognostic outcomes of anthracycline and cytarabine in Chinese patients with acute myeloid leukemia. Eur. J. Clin. Pharm. 2015, 71, 293–302. [Google Scholar] [CrossRef] [PubMed]
- Megías-Vericat, J.E.; Rojas, L.; Herrero, M.J.; Bosó, V.; Montesinos, P.; Moscardó, F.; Poveda, J.L.; Sanz, M.Á.; Aliño, S.F. Influence of ABCB1 polymorphisms upon the effectiveness of standard treatment for acute myeloid leukemia: A systematic review and meta-analysis of observational studies. Pharm. J. 2015, 15, 109–118. [Google Scholar] [CrossRef]
- Drenberg, C.D.; Hu, S.; Li, L.; Buelow, D.R.; Orwick, S.J.; Gibson, A.A.; Schuetz, J.D.; Sparreboom, A.; Baker, S.D. ABCC4 Is a Determinant of Cytarabine-Induced Cytotoxicity and Myelosuppression. Clin. Transl. Sci. 2016, 9, 51–59. [Google Scholar] [CrossRef]
- Drenberg, C.D.; Gibson, A.A.; Pounds, S.B.; Shi, L.; Rhinehart, D.P.; Li, L.; Hu, S.; Du, G.; Nies, A.T.; Schwab, M.; et al. OCTN1 Is a High-Affinity Carrier of Nucleoside Analogues. Cancer Res. 2017, 77, 2102–2111. [Google Scholar] [CrossRef]
- Fine, B.M.; Kaspers, G.J.L.; Ho, M.; Loonen, A.H.; Boxer, L.M. A genome-wide view of the in vitro response to l-asparaginase in acute lymphoblastic leukemia. Cancer Res. 2005, 65, 291–299. [Google Scholar] [CrossRef]
- Sun, J.; Nagel, R.; Zaal, E.A.; Ugalde, A.P.; Han, R.; Proost, N.; Song, J.-Y.; Pataskar, A.; Burylo, A.; Fu, H.; et al. SLC1A3 contributes to L-asparaginase resistance in solid tumors. EMBO J. 2019, 38, e102147. [Google Scholar] [CrossRef]
- Kanerva, J.; Tiirikainen, M.; Mäkipernaa, A.; Riikonen, P.; Möttönen, M.; Salmi, T.T.; Krusius, T.; Saarinen-Pihkala, U.M. Multiple drug resistance mediated by P-glycoprotein is not a major factor in a slow response to therapy in childhood ALL. Pediatr. Hematol. Oncol. 1998, 15, 11–21. [Google Scholar] [CrossRef]
- Svirnovski, A.I.; Shman, T.V.; Serhiyenka, T.F.; Savitski, V.P.; Smolnikova, V.V.; Fedasenka, U.U. ABCB1 and ABCG2 proteins, their functional activity and gene expression in concert with drug sensitivity of leukemia cells. Hematology 2009, 14, 204–212. [Google Scholar] [CrossRef]
- Cai, J.; Damaraju, V.L.; Groulx, N.; Mowles, D.; Peng, Y.; Robins, M.J.; Cass, C.E.; Gros, P. Two distinct molecular mechanisms underlying cytarabine resistance in human leukemic cells. Cancer Res. 2008, 68, 2349–2357. [Google Scholar] [CrossRef]
- King, K.M.; Damaraju, V.L.; Vickers, M.F.; Yao, S.Y.; Lang, T.; Tackaberry, T.E.; Mowles, D.A.; Ng, A.M.L.; Young, J.D.; Cass, C.E. A comparison of the transportability, and its role in cytotoxicity, of clofarabine, cladribine, and fludarabine by recombinant human nucleoside transporters produced in three model expression systems. Mol. Pharm. 2006, 69, 346–353. [Google Scholar] [CrossRef]
- Hijiya, N.; Thomson, B.; Isakoff, M.S.; Silverman, L.B.; Steinherz, P.G.; Borowitz, M.J.; Kadota, R.; Cooper, T.; Shen, V.; Dahl, G.; et al. Phase 2 trial of clofarabine in combination with etoposide and cyclophosphamide in pediatric patients with refractory or relapsed acute lymphoblastic leukemia. Blood 2011, 118, 6043–6049. [Google Scholar] [CrossRef]
- Pastor-Anglada, M.; Pérez-Torras, S. Nucleoside transporter proteins as biomarkers of drug responsiveness and drug targets. Front Pharm. 2015, 6, 13. [Google Scholar] [CrossRef]
- Hucke, A.; Ciarimboli, G. The Role of Transporters in the Toxicity of Chemotherapeutic Drugs: Focus on Transporters for Organic Cations. J. Clin. Pharm. 2016, 56 (Suppl. S7), S157–S172. [Google Scholar] [CrossRef]
- Elwi, A.N.; Damaraju, V.L.; Baldwin, S.A.; Young, J.D.; Sawyer, M.B.; Cass, C.E. Renal nucleoside transporters: Physiological and clinical implications. Biochem. Cell Biol. 2006, 84, 844–858. [Google Scholar] [CrossRef]
- Fukuda, Y.; Schuetz, J.D. ABC transporters and their role in nucleoside and nucleotide drug resistance. Biochem. Pharm. 2012, 83, 1073–1083. [Google Scholar] [CrossRef]
- Bonate, P.L.; Cunningham, C.C.; Gaynon, P.; Jeha, S.; Kadota, R.; Lam, G.N.; Razzouk, B.; Rytting, M.; Steinherz, P.; Weitman, S. Population pharmacokinetics of clofarabine and its metabolite 6-ketoclofarabine in adult and pediatric patients with cancer. Cancer Chemother. Pharm. 2011, 67, 875–890. [Google Scholar] [CrossRef]
- Choi, R.; Sohn, I.; Kim, M.-J.; Woo, H.I.; Lee, J.W.; Ma, Y.; Yi, E.S.; Koo, H.H.; Lee, S.-Y. Pathway genes and metabolites in thiopurine therapy in Korean children with acute lymphoblastic leukaemia. Br. J. Clin. Pharm. 2019, 85, 1585–1597. [Google Scholar] [CrossRef]
- Eldem, İ.; Yavuz, D.; Cumaoğullari, Ö.; İleri, T.; Ünal İnce, E.; Ertem, M.; Doğanay Erdoğan, B.; Bindak, R.; Özdağ, H.; Şatiroğlu-Tufan, N.L.; et al. SLCO1B1 Polymorphisms are Associated with Drug Intolerance in Childhood Leukemia Maintenance Therapy. J. Pediatr. Hematol. Oncol. 2018, 40, e289–e294. [Google Scholar] [CrossRef]
- Tanaka, Y. Susceptibility to 6-mercaptopurine toxicity related with NUDT15 and ABCC4 variants in Japanese childhood acute lymphoblastic leukemia. Rinsho Ketsueki 2017, 58, 950–956. [Google Scholar] [CrossRef]
- Kim, H.; Seo, H.; Park, Y.; Min, B.-J.; Seo, M.-E.; Park, K.D.; Shin, H.Y.; Kim, J.H.; Kang, H.J. APEX1 Polymorphism and Mercaptopurine-Related Early Onset Neutropenia in Pediatric Acute Lymphoblastic Leukemia. Cancer Res. Treat. 2018, 50, 823–834. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, Y.; Nakadate, H.; Kondoh, K.; Nakamura, K.; Koh, K.; Manabe, A. Interaction between NUDT15 and ABCC4 variants enhances intolerability of 6-mercaptopurine in Japanese patients with childhood acute lymphoblastic leukemia. Pharm. J. 2018, 18, 275–280. [Google Scholar] [CrossRef] [PubMed]
- Hareedy, M.S.; El Desoky, E.S.; Woillard, J.-B.; Thabet, R.H.; Ali, A.M.; Marquet, P.; Picard, N. Genetic variants in 6-mercaptopurine pathway as potential factors of hematological toxicity in acute lymphoblastic leukemia patients. Pharmacogenomics 2015, 16, 1119–1134. [Google Scholar] [CrossRef] [PubMed]
- Zaza, G.; Cheok, M.; Yang, W.; Panetta, J.C.; Pui, C.-H.; Relling, M.V.; Evans, W.E. Gene expression and thioguanine nucleotide disposition in acute lymphoblastic leukemia after in vivo mercaptopurine treatment. Blood 2005, 106, 1778–1785. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.-X.; Shi, Z.; Damaraju, V.L.; Huang, X.-C.; Kruh, G.D.; Wu, H.-C.; Zhou, Y.; Tiwari, A.; Fu, L.; Cass, C.E.; et al. Up-regulation of MRP4 and down-regulation of influx transporters in human leukemic cells with acquired resistance to 6-mercaptopurine. Leuk. Res. 2008, 32, 799–809. [Google Scholar] [CrossRef] [PubMed]
- Kurtzberg, J.; Ernst, T.J.; Keating, M.J.; Gandhi, V.; Hodge, J.P.; Kisor, D.F.; Lager, J.J.; Stephens, C.; Levin, J.; Krenitsky, T.; et al. Phase I study of 506U78 administered on a consecutive 5-day schedule in children and adults with refractory hematologic malignancies. J. Clin. Oncol. 2005, 23, 3396–3403. [Google Scholar] [CrossRef]
- Guo, Y.; Köck, K.; Ritter, C.A.; Chen, Z.-S.; Grube, M.; Jedlitschky, G.; Illmer, T.; Ayres, M.; Beck, J.F.; Siegmund, W.; et al. Expression of ABCC-type nucleotide exporters in blasts of adult acute myeloid leukemia: Relation to long-term survival. Clin. Cancer Res. 2009, 15, 1762–1769. [Google Scholar] [CrossRef]
- Becton, D.; Dahl, G.V.; Ravindranath, Y.; Chang, M.N.; Behm, F.G.; Raimondi, S.C.; Head, D.R.; Stine, K.C.; Lacayo, N.J.; Sikic, B.I.; et al. Randomized use of cyclosporin A (CsA) to modulate P-glycoprotein in children with AML in remission: Pediatric Oncology Group Study 9421. Blood 2006, 107, 1315–1324. [Google Scholar] [CrossRef]
- Wiley, J.S.; Jones, S.P.; Sawyer, W.H.; Paterson, A.R. Cytosine arabinoside influx and nucleoside transport sites in acute leukemia. J. Clin. Investig. 1982, 69, 479–489. [Google Scholar] [CrossRef]
- Català, A.; Pastor-Anglada, M.; Caviedes-Cárdenas, L.; Malatesta, R.; Rives, S.; Vega-García, N.; Camós, M.; Fernández-Calotti, P. FLT3 is implicated in cytarabine transport by human equilibrative nucleoside transporter 1 in pediatric acute leukemia. Oncotarget 2016, 7, 49786–49799. [Google Scholar] [CrossRef]
- Kim, J.-H.; Lee, C.; Cheong, H.S.; Koh, Y.; Ahn, K.-S.; Kim, H.-L.; Shin, H.D.; Yoon, S.-S. SLC29A1 (ENT1) polymorphisms and outcome of complete remission in acute myeloid leukemia. Cancer Chemother. Pharm. 2016, 78, 533–540. [Google Scholar] [CrossRef]
- Jaramillo, A.C.; Cloos, J.; Lemos, C.; Stam, R.W.; Kaspers, G.J.L.; Jansen, G.; Peters, G.J. Ex vivo resistance in childhood acute lymphoblastic leukemia: Correlations between BCRP, MRP1, MRP4 and MRP5 ABC transporter expression and intracellular methotrexate polyglutamate accumulation. Leuk. Res. 2019, 79, 45–51. [Google Scholar] [CrossRef]
- Treviño, L.R.; Shimasaki, N.; Yang, W.; Panetta, J.C.; Cheng, C.; Pei, D.; Chan, D.; Sparreboom, A.; Giacomini, K.M.; Pui, C.-H.; et al. Germline genetic variation in an organic anion transporter polypeptide associated with methotrexate pharmacokinetics and clinical effects. J. Clin. Oncol. 2009, 27, 5972–5978. [Google Scholar] [CrossRef]
- Lopez-Lopez, E.; Ballesteros, J.; Piñan, M.A.; Sanchez de Toledo, J.; Garcia de Andoin, N.; Garcia-Miguel, P.; Navajas, A.; Garcia-Orad, A. Polymorphisms in the methotrexate transport pathway: A new tool for MTX plasma level prediction in pediatric acute lymphoblastic leukemia. Pharm. Genom. 2013, 23, 53–61. [Google Scholar] [CrossRef]
- Zgheib, N.K.; Akra-Ismail, M.; Aridi, C.; Mahfouz, R.; Abboud, M.R.; Solh, H.; Muwakkit, S.A. Genetic polymorphisms in candidate genes predict increased toxicity with methotrexate therapy in Lebanese children with acute lymphoblastic leukemia. Pharm. Genom. 2014, 24, 387–396. [Google Scholar] [CrossRef]
- Den Hoed, M.A.H.; Lopez-Lopez, E.; te Winkel, M.L.; Tissing, W.; de Rooij, J.D.E.; Gutierrez-Camino, A.; Garcia-Orad, A.; den Boer, E.; Pieters, R.; Pluijm, S.M.F.; et al. Genetic and metabolic determinants of methotrexate-induced mucositis in pediatric acute lymphoblastic leukemia. Pharm. J. 2015, 15, 248–254. [Google Scholar] [CrossRef]
- Zaruma-Torres, F.; Lares-Asseff, I.; Reyes-Espinoza, A.; Loera-Castañeda, V.; Chairez-Hernández, I.; Sosa-Macías, M.; Galaviz-Hernández, C.; Almanza-Reyes, H. Association of ABCB1, ABCC5 and xanthine oxidase genetic polymorphisms with methotrexate adverse reactions in Mexican pediatric patients with ALL. Drug Metab. Pers. 2015, 30, 195–201. [Google Scholar] [CrossRef]
- Rajšić, I.; Lazarević, S.; Đanić, M.; Al-Salami, H.; Mooranian, A.; Vukmirović, S.; Mikov, M.; Goločorbin-Kon, S. Plasma Distribution of Methotrexate and Its Polyglutamates in Pediatric Acute Lymphoblastic Leukemia: Preliminary Insights. Eur. J. Drug Metab. Pharm. 2022, 47, 127–134. [Google Scholar] [CrossRef]
- Lee, C.M.; Zane, N.R.; Veal, G.; Thakker, D.R. Physiologically Based Pharmacokinetic Models for Adults and Children Reveal a Role of Intracellular Tubulin Binding in Vincristine Disposition. CPT Pharmacomet. Syst. Pharm. 2019, 8, 759–768. [Google Scholar] [CrossRef]
- Lopez-Lopez, E.; Gutierrez-Camino, A.; Astigarraga, I.; Navajas, A.; Echebarria-Barona, A.; Garcia-Miguel, P.; Garcia de Andoin, N.; Lobo, C.; Guerra-Merino, I.; Martin-Guerrero, I.; et al. Vincristine pharmacokinetics pathway and neurotoxicity during early phases of treatment in pediatric acute lymphoblastic leukemia. Pharmacogenomics 2016, 17, 731–741. [Google Scholar] [CrossRef]
- Wright, G.E.B.; Amstutz, U.; Drögemöller, B.I.; Shih, J.; Rassekh, S.R.; Hayden, M.R.; Carleton, B.C.; Ross, C.J.D. Pharmacogenomics of Vincristine-Induced Peripheral Neuropathy Implicates Pharmacokinetic and Inherited Neuropathy Genes. Clin. Pharm. 2019, 105, 402–410. [Google Scholar] [CrossRef]
- Gutierrez-Camino, Á.; Umerez, M.; Martin-Guerrero, I.; García de Andoin, N.; Santos, B.; Sastre, A.; Echebarria-Barona, A.; Astigarraga, I.; Navajas, A.; Garcia-Orad, A. Mir-pharmacogenetics of Vincristine and peripheral neurotoxicity in childhood B-cell acute lymphoblastic leukemia. Pharm. J. 2018, 18, 704–712. [Google Scholar] [CrossRef]
- Low, S.-K.; Kiyotani, K.; Mushiroda, T.; Daigo, Y.; Nakamura, Y.; Zembutsu, H. Association study of genetic polymorphism in ABCC4 with cyclophosphamide-induced adverse drug reactions in breast cancer patients. J. Hum. Genet. 2009, 54, 564–571. [Google Scholar] [CrossRef] [PubMed]
- Theis, J.G.; Chan, H.S.; Greenberg, M.L.; Malkin, D.; Karaskov, V.; Moncica, I.; Koren, G.; Doyle, J. Assessment of systemic toxicity in children receiving chemotherapy with cyclosporine for sarcoma. Med. Pediatr. Oncol. 2000, 34, 242–249. [Google Scholar] [CrossRef]
- Xiao, Q.; Zhou, Y.; Lauschke, V.M. Impact of variants in ATP-binding cassette transporters on breast cancer treatment. Pharmacogenomics 2020, 21, 1299–1310. [Google Scholar] [CrossRef] [PubMed]
- Samuel, L.; Cummings, J.; Shaw, P. Daunorubicin cardiotoxicity in childhood cancer. Lancet 1998, 352, 1150. [Google Scholar] [CrossRef]
- Loscocco, F.; Visani, G.; Ruzzo, A.; Bagaloni, I.; Fuligni, F.; Galimberti, S.; Di Paolo, A.; Stagno, F.; Pregno, P.; Annunziata, M.; et al. Clinical Relevance of ABCB1, ABCG2, and ABCC2 Gene Polymorphisms in Chronic Myeloid Leukemia Patients Treated With Nilotinib. Front Oncol. 2021, 11, 672287. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Peñafiel, C.; Olarte-Carrillo, I.; Maldonado, R.C.; de la Cruz Rosas, A.; Collazo-Jaloma, J.; Martínez-Tovar, A. Association of three factors (ABCB1 gene expression, steroid response, early response at day + 8) on the response to induction in patients with acute lymphoblastic leukemia. Ann. Hematol. 2020, 99, 2629–2637. [Google Scholar] [CrossRef]
- Varatharajan, S.; Panetta, J.C.; Abraham, A.; Karathedath, S.; Mohanan, E.; Lakshmi, K.M.; Arthur, N.; Srivastava, V.M.; Nemani, S.; George, B.; et al. Population pharmacokinetics of Daunorubicin in adult patients with acute myeloid leukemia. Cancer Chemother. Pharm. 2016, 78, 1051–1058. [Google Scholar] [CrossRef]
- Cotteret, C.; Pham, Y.-V.; Marcais, A.; Driessen, M.; Cisternino, S.; Schlatter, J. Maternal ABVD chemotherapy for Hodgkin lymphoma in a dichorionic diamniotic pregnancy: A case report. BMC Pregnancy Childbirth 2020, 20, 231. [Google Scholar] [CrossRef]
- Davies, A.; Rodriguez-Vicente, A.E.; Austin, G.; Loaiza, S.; Foroni, L.; Clark, R.E.; Pirmohamed, M. Serotonin re-uptake transporter gene polymorphisms are associated with imatinib-induced diarrhoea in chronic myeloid leukaemia patients. Sci. Rep. 2020, 10, 8394. [Google Scholar] [CrossRef]
- Ben Hassine, I.; Gharbi, H.; Soltani, I.; Teber, M.; Farrah, A.; Ben Hadj Othman, H.; Amouri, H.; Bellaaj, H.; Lakhal, R.B.; Romdhane, N.B.; et al. hOCT1 gene expression predict for optimal response to Imatinib in Tunisian patients with chronic myeloid leukemia. Cancer Chemother. Pharm. 2017, 79, 737–745. [Google Scholar] [CrossRef]
- Nambu, T.; Hamada, A.; Nakashima, R.; Yuki, M.; Kawaguchi, T.; Mitsuya, H.; Saito, H. Association of SLCO1B3 polymorphism with intracellular accumulation of imatinib in leukocytes in patients with chronic myeloid leukemia. Biol. Pharm. Bull. 2011, 34, 114–119. [Google Scholar] [CrossRef]
- Yamakawa, Y.; Hamada, A.; Nakashima, R.; Yuki, M.; Hirayama, C.; Kawaguchi, T.; Saito, H. Association of genetic polymorphisms in the influx transporter SLCO1B3 and the efflux transporter ABCB1 with imatinib pharmacokinetics in patients with chronic myeloid leukemia. Drug Monit. 2011, 33, 244–250. [Google Scholar] [CrossRef]
- Zhao, P.; Huang, J.; Zhang, D.; Zhang, D.; Wang, F.; Qu, Y.; Guo, T.; Qin, Y.; Wei, J.; Niu, T.; et al. SLC2A5 overexpression in childhood philadelphia chromosome-positive acute lymphoblastic leukaemia. Br. J. Haematol. 2018, 183, 242–250. [Google Scholar] [CrossRef]
- Adeagbo, B.A.; Olugbade, T.A.; Durosinmi, M.A.; Bolarinwa, R.A.; Ogungbenro, K.; Bolaji, O.O. Population Pharmacokinetics of Imatinib in Nigerians with Chronic Myeloid Leukemia: Clinical Implications for Dosing and Resistance. J. Clin. Pharm. 2017, 57, 1554–1563. [Google Scholar] [CrossRef]
- Gardner, E.R.; Burger, H.; van Schaik, R.H.; van Oosterom, A.T.; de Bruijn, E.A.; Guetens, G.; Prenen, H.; de Jong, F.A.; Baker, S.D.; Bates, S.E.; et al. Association of enzyme and transporter genotypes with the pharmacokinetics of imatinib. Clin. Pharm. 2006, 80, 192–201. [Google Scholar] [CrossRef]
- Gurney, H.; Wong, M.; Balleine, R.L.; Rivory, L.P.; McLachlan, A.J.; Hoskins, J.M.; Wilcken, N.; Clarke, C.L.; Mann, G.J.; Collins, M.; et al. Imatinib disposition and ABCB1 (MDR1, P-glycoprotein) genotype. Clin. Pharm. 2007, 82, 33–40. [Google Scholar] [CrossRef]
- Dulucq, S.; Bouchet, S.; Turcq, B.; Lippert, E.; Etienne, G.; Reiffers, J.; Molimard, M.; Krajinovic, M.; Mahon, F.-X. Multidrug resistance gene (MDR1) polymorphisms are associated with major molecular responses to standard-dose imatinib in chronic myeloid leukemia. Blood 2008, 112, 2024–2027. [Google Scholar] [CrossRef]
- Takahashi, N.; Miura, M.; Scott, S.A.; Kagaya, H.; Kameoka, Y.; Tagawa, H.; Saitoh, H.; Fujishima, N.; Yoshioka, T.; Hirokawa, M.; et al. Influence of CYP3A5 and drug transporter polymorphisms on imatinib trough concentration and clinical response among patients with chronic phase chronic myeloid leukemia. J. Hum. Genet. 2010, 55, 731–737. [Google Scholar] [CrossRef]
- Seong, S.J.; Lim, M.; Sohn, S.K.; Moon, J.H.; Oh, S.-J.; Kim, B.S.; Ryoo, H.M.; Chung, J.S.; Joo, Y.D.; Bang, S.M.; et al. Influence of enzyme and transporter polymorphisms on trough imatinib concentration and clinical response in chronic myeloid leukemia patients. Ann. Oncol. 2013, 24, 756–760. [Google Scholar] [CrossRef]
- Francis, J.; Dubashi, B.; Sundaram, R.; Pradhan, S.C.; Chandrasekaran, A. A study to explore the correlation of ABCB1, ABCG2, OCT1 genetic polymorphisms and trough level concentration with imatinib mesylate-induced thrombocytopenia in chronic myeloid leukemia patients. Cancer Chemother. Pharm. 2015, 76, 1185–1189. [Google Scholar] [CrossRef]
- Hijiya, N.; Maschan, A.; Rizzari, C.; Shimada, H.; Dufour, C.; Goto, H.; Kang, H.J.; Guinipero, T.; Karakas, Z.; Bautista, F.; et al. Phase 2 study of nilotinib in pediatric patients with Philadelphia chromosome-positive chronic myeloid leukemia. Blood 2019, 134, 2036–2045. [Google Scholar] [CrossRef]
- Maia, R.C.; Vasconcelos, F.C.; Souza, P.S.; Rumjanek, V.M. Towards Comprehension of the ABCB1/P-Glycoprotein Role in Chronic Myeloid Leukemia. Molecules 2018, 23, 119. [Google Scholar] [CrossRef]
- Trojani, A.; Pungolino, E.; Dal Molin, A.; Lodola, M.; Rossi, G.; D’Adda, M.; Perego, A.; Elena, C.; Turrini, M.; Borin, L.; et al. Nilotinib interferes with cell cycle, ABC transporters and JAK-STAT signaling pathway in CD34+/lin- cells of patients with chronic phase chronic myeloid leukemia after 12 months of treatment. PLoS ONE 2019, 14, e0218444. [Google Scholar] [CrossRef]
- Johnson, T.R.; Tan, W.; Goulet, L.; Smith, E.B.; Yamazaki, S.; Walker, G.S.; O’Gorman, M.T.; Bedarida, G.; Zou, H.Y.; Christensen, J.G.; et al. Metabolism, excretion and pharmacokinetics of [14C]crizotinib following oral administration to healthy subjects. Xenobiotica 2015, 45, 45–59. [Google Scholar] [CrossRef]
- Sato, T.; Mishima, E.; Mano, N.; Abe, T.; Yamaguchi, H. Potential Drug Interactions Mediated by Renal Organic Anion Transporter OATP4C1. J. Pharm. Exp. 2017, 362, 271–277. [Google Scholar] [CrossRef]
- Zhao, D.; Chen, J.; Chu, M.; Long, X.; Wang, J. Pharmacokinetic-Based Drug-Drug Interactions with Anaplastic Lymphoma Kinase Inhibitors: A Review. Drug Des. Devel. 2020, 14, 1663–1681. [Google Scholar] [CrossRef]
- Shu, W.; Ma, L.; Hu, X.; Zhang, M.; Chen, W.; Ma, W.; Huang, J.; Li, J. Drug-drug interaction between crizotinib and entecavir via renal secretory transporter OCT2. Eur. J. Pharm. Sci. 2020, 142, 105153. [Google Scholar] [CrossRef] [PubMed]
- Arakawa, H.; Omote, S.; Tamai, I. Inhibitory Effect of Crizotinib on Creatinine Uptake by Renal Secretory Transporter OCT2. J. Pharm. Sci. 2017, 106, 2899–2903. [Google Scholar] [CrossRef] [PubMed]
- Omote, S.; Matsuoka, N.; Arakawa, H.; Nakanishi, T.; Tamai, I. Effect of tyrosine kinase inhibitors on renal handling of creatinine by MATE1. Sci. Rep. 2018, 8, 9237. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.; Ito, H.; Hirata, A.; Abe, T.; Mano, N.; Yamaguchi, H. Interactions of crizotinib and gefitinib with organic anion-transporting polypeptides (OATP)1B1, OATP1B3 and OATP2B1: Gefitinib shows contradictory interaction with OATP1B3. Xenobiotica 2018, 48, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Combes, F.P.; Einolf, H.J.; Coello, N.; Heimbach, T.; He, H.; Grosch, K. Model-Informed Drug Development for Everolimus Dosing Selection in Pediatric Infant Patients. CPT Pharmacomet. Syst. Pharm. 2020, 9, 230–237. [Google Scholar] [CrossRef]
- Picard, N.; Levoir, L.; Lamoureux, F.; Yee, S.W.; Giacomini, K.M.; Marquet, P. Interaction of sirolimus and everolimus with hepatic and intestinal organic anion-transporting polypeptide transporters. Xenobiotica 2011, 41, 752–757. [Google Scholar] [CrossRef]
- Sievers, E.L.; Appelbaum, F.R.; Spielberger, R.T.; Forman, S.J.; Flowers, D.; Smith, F.O.; Shannon-Dorcy, K.; Berger, M.S.; Bernstein, I.D. Selective ablation of acute myeloid leukemia using antibody-targeted chemotherapy: A phase I study of an anti-CD33 calicheamicin immunoconjugate. Blood 1999, 93, 3678–3684. [Google Scholar] [CrossRef]
- Aplenc, R.; Alonzo, T.A.; Gerbing, R.B.; Lange, B.J.; Hurwitz, C.A.; Wells, R.J.; Bernstein, I.; Buckley, P.; Krimmel, K.; Smith, F.O.; et al. Safety and efficacy of gemtuzumab ozogamicin in combination with chemotherapy for pediatric acute myeloid leukemia: A report from the Children’s Oncology Group. J. Clin. Oncol. 2008, 26, 2390–3295. [Google Scholar] [CrossRef] [PubMed]
- Cooper, T.M.; Franklin, J.; Gerbing, R.B.; Alonzo, T.A.; Hurwitz, C.; Raimondi, S.C.; Hirsch, B.; Smith, F.O.; Mathew, P.; Arceci, R.J.; et al. AAML03P1, a pilot study of the safety of gemtuzumab ozogamicin in combination with chemotherapy for newly diagnosed childhood acute myeloid leukemia: A report from the Children’s Oncology Group. Cancer 2012, 118, 761–769. [Google Scholar] [CrossRef]
- Gamis, A.S.; Alonzo, T.A.; Meshinchi, S.; Sung, L.; Gerbing, R.B.; Raimondi, S.C.; Hirsch, B.A.; Kahwash, S.B.; Heerema-McKenney, A.; Winter, L.; et al. Gemtuzumab ozogamicin in children and adolescents with de novo acute myeloid leukemia improves event-free survival by reducing relapse risk: Results from the randomized phase III Children’s Oncology Group trial AAML0531. J. Clin. Oncol. 2014, 32, 3021–3032. [Google Scholar] [CrossRef]
- Iacobucci, I.; Lonetti, A.; Candoni, A.; Sazzini, M.; Papayannidis, C.; Formica, S.; Ottaviani, E.; Ferrari, A.; Michelutti, A.; Simeone, E.; et al. Profiling of drug-metabolizing enzymes/transporters in CD33+ acute myeloid leukemia patients treated with Gemtuzumab-Ozogamicin and Fludarabine, Cytarabine and Idarubicin. Pharm. J. 2013, 13, 335–341. [Google Scholar] [CrossRef]
- Matsui, H.; Takeshita, A.; Naito, K.; Shinjo, K.; Shigeno, K.; Maekawa, M.; Yamakawa, Y.; Tanimoto, M.; Kobayashi, M.; Ohnishi, K.; et al. Reduced effect of gemtuzumab ozogamicin (CMA-676) on P-glycoprotein and/or CD34-positive leukemia cells and its restoration by multidrug resistance modifiers. Leukemia 2002, 16, 813–819. [Google Scholar] [CrossRef]
- Walter, R.B.; Gooley, T.A.; van der Velden, V.H.J.; Loken, M.R.; van Dongen, J.J.M.; Flowers, D.A.; Bernstein, I.D.; Appelbaum, F.R. CD33 expression and P-glycoprotein-mediated drug efflux inversely correlate and predict clinical outcome in patients with acute myeloid leukemia treated with gemtuzumab ozogamicin monotherapy. Blood 2007, 109, 4168–4170. [Google Scholar] [CrossRef]
- Fenwarth, L.; Fournier, E.; Cheok, M.; Boyer, T.; Gonzales, F.; Castaigne, S.; Boissel, N.; Lambert, J.; Dombret, H.; Preudhomme, C.; et al. Biomarkers of Gemtuzumab Ozogamicin Response for Acute Myeloid Leukemia Treatment. Int. J. Mol. Sci. 2020, 21, 65626. [Google Scholar] [CrossRef]
- Foster, M.C.; Kumar, P.; Walko, C.M.; Ivanova, A.; Ewesuedo, R.; Van Deventer, H.; Garcia, R.; Park, S.I.; Shea, T.C. Pharmacokinetic study of clofarabine: Oral bioavailability and the effect of cimetidine on renal clearance. JCO 2012, 30, e13074. [Google Scholar] [CrossRef]
- Zhang, Y.; Sun, L.; Chen, X.; Zhao, L.; Wang, X.; Zhao, Z.; Mei, S. A Systematic Review of Population Pharmacokinetic Models of Methotrexate. Eur. J. Drug Metab. Pharm. 2022, 47, 143–164. [Google Scholar] [CrossRef] [PubMed]
- Ishii, Y.; Ito, Y.; Matsuki, S.; Sanpei, K.; Ogawa, O.; Takeda, K.; Schuck, E.L.; Uemura, N. Clinical Drug-Drug Interaction Potential of BFE1224, Prodrug of Antifungal Ravuconazole, Using Two Types of Cocktails in Healthy Subjects. Clin. Transl. Sci. 2018, 11, 477–486. [Google Scholar] [CrossRef] [PubMed]
- Garimella, T.; Tao, X.; Sims, K.; Chang, Y.-T.; Rana, J.; Myers, E.; Wind-Rotolo, M.; Bhatnagar, R.; Eley, T.; LaCreta, F.; et al. Effects of a Fixed-Dose Co-Formulation of Daclatasvir, Asunaprevir, and Beclabuvir on the Pharmacokinetics of a Cocktail of Cytochrome P450 and Drug Transporter Substrates in Healthy Subjects. Drugs R D 2018, 18, 55–65. [Google Scholar] [CrossRef]
- Keppler, D. The roles of MRP2, MRP3, OATP1B1, and OATP1B3 in conjugated hyperbilirubinemia. Drug Metab. Dispos. 2014, 42, 561–565. [Google Scholar] [CrossRef]
- Takehara, I.; Yoshikado, T.; Ishigame, K.; Mori, D.; Furihata, K.-I.; Watanabe, N.; Ando, O.; Maeda, K.; Sugiyama, Y.; Kusuhara, H. Comparative Study of the Dose-Dependence of OATP1B Inhibition by Rifampicin Using Probe Drugs and Endogenous Substrates in Healthy Volunteers. Pharm. Res. 2018, 35, 138. [Google Scholar] [CrossRef]
- Tsuruya, Y.; Kato, K.; Sano, Y.; Imamura, Y.; Maeda, K.; Kumagai, Y.; Sugiyama, Y.; Kusuhara, H. Investigation of Endogenous Compounds Applicable to Drug-Drug Interaction Studies Involving the Renal Organic Anion Transporters, OAT1 and OAT3, in Humans. Drug Metab. Dispos. 2016, 44, 1925–1933. [Google Scholar] [CrossRef]
- Yoshikado, T.; Maeda, K.; Furihata, S.; Terashima, H.; Nakayama, T.; Ishigame, K.; Tsunemoto, K.; Kusuhara, H.; Furihata, K.-I.; Sugiyama, Y. A Clinical Cassette Dosing Study for Evaluating the Contribution of Hepatic OATPs and CYP3A to Drug-Drug Interactions. Pharm. Res. 2017, 34, 1570–1583. [Google Scholar] [CrossRef]

| Mechanism of Action | Drug Name | Labelled Indications | Important Adverse Effects | Uptake Transporter/s | Efflux Transporter/s | References |
|---|---|---|---|---|---|---|
| Alkylating drug | Cyclophosphamide | ALL | Cytopenia, hemorrhagic cystitis, cardiotoxicity, hepatic veno-occlusive disease | NA | MRP4, MDR1, MRP2 | [50,51,52] |
| Topoisomerase inhibitors | Doxorubicin hydrochloride | WTCKC | Cardiomyopathy, myelosuppression, secondary malignancy | NA | MDR1, MDR3, MRP1, MRP2, MRP5 BRCP | [53,54,55,56,57,58] |
| Daunorubicin hydrochloride | ALL | Myocardial toxicity, myelosuppression | OATP1B1 | MRP2–6 | [59,60] | |
| Enzymes | Asparaginase Erwinia chrysanthemi, Calaspargase Pegol-mknl, Pegaspargase | ALL | Hemorrhagic or thrombotic events; pancreatitis; hypersensitivity reaction; diabetic ketoacidosis; posterior reversible encephalopathy syndrome | NA | NA | [61] |
| Transcription inhibitor | Dactinomycin | RMS, WTCKC, NHL, ES | Veno-occlusive disease, myelosuppression, secondary malignancy | OAT4, PEPT2 | MDR1 | [62,63,64] |
| Bispecific CD19-directed CD3 T-cell engager | Blinatumomab | ALL | Cytokine release syndrome, neurological toxicity | NA | NA | [65] |
| CD19-directed genetically modified autologous T-cell immunotherapy | Tisagenlecleucel | ALL | Cytokine release syndrome, neurological toxicity | NA | NA | [66] |
| CD33-directed antibody-drug conjugate | Gemtuzumab ozogamicin | AML | Hepatotoxicity, infusion-related reactions, thrombocytopenia, neutropenia | NA | NA | [67,68,69] |
| Folate antagonist | Methotrexate Sodium | ALL | Bone marrow suppression, impaired renal function, hepatotoxicity, penumonitis | RFC1, OATP1B1, OATP1A2, OAT1, OAT3 | MDR1, BRCP, MRP1–5, MRP7 | [70,71,72,73,74,75] |
| GD2-binding monoclonal antibody | Dinutuximab | NB | Infusion reactions, neuropathy | NA | NA | [76] |
| Naxitamab-gqgk | NB | Infusion reactions, neurotoxicity | NA | NA | [61] | |
| Human cytotoxic T-lymphocyte antigen 4 (CTLA-4)-blocking antibody | Ipilimumab | Melanoma, CRC | Immune-mediated adverse reactions | NA | NA | [77] |
| Microtubule inhibitor | Vincristine Sulfate | ALL, AML, NB, NHL, RMS, WTCKC | Neuropathy, hepatic veno-occlusive disease | NA | MDR1, MRP1–3, MRP7, RLIP1 | [78,79,80,81,82,83] |
| Kinase inhibitors | Dasatinib | ALL, CML | Myelosuppression, hemorrhage, fluid retention, cardiac dysfunction | NA | MDR1, BRCP | [84,85] |
| Imatinib Mesylate | ALL, CML | Cytopenias, congestive heart failure, hepatotoxicity, hemorrhage | OCT1 | MDR1 | [86,87,88,89] | |
| Nilotinib | CML | Myelosuppression, QT prolongation, electrolyte abnormalities, pancreatitis, hepatotoxicity | NA | MDR1, BRCP, MRP6 | [84,90,91] | |
| Crizotinib | NHL | Ocular toxicity, hepatotoxicity, interstitial lung disease | OATPB1/3, OATP2B1 | MDR1 | [92,93] | |
| Entrectinib | ST | Congestive heart failure, CNS adverse effects, fracture, hepatotoxicity, QT prolongation, vision disorders | NA | MDR1, BRCP | [94] | |
| Larotrectinib Sulfate | ST | Neurotoxicity, hepatotoxicity | OATP1 | MDR1, BRCP | [95] | |
| Selumetinib Sulfate | NF Type 1 | Cardiomyopathy, ocular toxicity, skin rash, diarrhoea, rhabdomyolysis | NA | MDR1, BRCP1 | [96,97] | |
| Everolimus | GCA | Pneumonitis, infections | NA | MDR1 | [98] | |
| Programmed death ligand-1 (PD-L1) blocking antibody | Avelumab | MCC | Immune-mediated reactions | NA | NA | [61] |
| Programmed death receptor-1 (PD-1)-blocking antibody | Pembrolizumab | ST, HL, MCC, NHL | Immune-mediated reactions | NA | NA | [61] |
| Nivolumab | CRC | Immune-mediated reactions | NA | NA | [61] | |
| Purine nucleoside metabolic inhibitor | Clofarabine | ALL | Bone marrow suppression, infectious complications, tumor lysis syndrome, systemic inflammatory response syndrome | OAT1, OAT3, OCT1 | BRCP, MRP4, MRP5 | [99,100] |
| Mercaptopurine | ALL | Bone marrow suppression, immunosuppression, hepatotoxicity | CNT2, CNT3, ENT1 | MRP4, MRP5, MDR1 | [95,101,102] | |
| Nelarabine | ALL, NHL | Myelosuppression, neurological toxicity | ENT1, ENT2 | NA | [103,104] | |
| Pyrimidine nucleoside metabolic inhibitor | Cytarabine | ALL, CML | Bone marrow suppression, cytarabine syndrome, cerebral and cerebellar dysfunction, bowel necrosis, pulmonary edema, cardiomyopathy | ENT1, OCTN1, CNT3 | MRP1, MRP3, MRP4, MDR1 | [105,106,107,108,109,110] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kamath, A.; Srinivasamurthy, S.K.; Chowta, M.N.; Ullal, S.D.; Daali, Y.; Chakradhara Rao, U.S. Role of Drug Transporters in Elucidating Inter-Individual Variability in Pediatric Chemotherapy-Related Toxicities and Response. Pharmaceuticals 2022, 15, 990. https://doi.org/10.3390/ph15080990
Kamath A, Srinivasamurthy SK, Chowta MN, Ullal SD, Daali Y, Chakradhara Rao US. Role of Drug Transporters in Elucidating Inter-Individual Variability in Pediatric Chemotherapy-Related Toxicities and Response. Pharmaceuticals. 2022; 15(8):990. https://doi.org/10.3390/ph15080990
Chicago/Turabian StyleKamath, Ashwin, Suresh Kumar Srinivasamurthy, Mukta N. Chowta, Sheetal D. Ullal, Youssef Daali, and Uppugunduri S. Chakradhara Rao. 2022. "Role of Drug Transporters in Elucidating Inter-Individual Variability in Pediatric Chemotherapy-Related Toxicities and Response" Pharmaceuticals 15, no. 8: 990. https://doi.org/10.3390/ph15080990
APA StyleKamath, A., Srinivasamurthy, S. K., Chowta, M. N., Ullal, S. D., Daali, Y., & Chakradhara Rao, U. S. (2022). Role of Drug Transporters in Elucidating Inter-Individual Variability in Pediatric Chemotherapy-Related Toxicities and Response. Pharmaceuticals, 15(8), 990. https://doi.org/10.3390/ph15080990







