Abstract
Antibiotic resistance in microorganisms is an important problem of modern medicine which can be solved by searching for antimicrobial preparations of the new generation. Nanoparticles (NPs) of metals and their oxides are the most promising candidates for the role of such preparations. In the last few years, the number of studies devoted to the antimicrobial properties of silver oxide NPs have been actively growing. Although the total number of such studies is still not very high, it is quickly increasing. Advantages of silver oxide NPs are the relative easiness of production, low cost, high antibacterial and antifungal activities and low cytotoxicity to eukaryotic cells. This review intends to provide readers with the latest information about the antimicrobial properties of silver oxide NPs: sensitive organisms, mechanisms of action on microorganisms and further prospects for improving the antimicrobial properties.
1. Introduction
Since the moment of their discovery, antibiotics have been the “golden standard” in the treatment of many bacterial infections [1,2]. Unfortunately, the uncontrolled use of over-the-counter (OTC) antibiotics available without prescription has led to the emergence of new antibiotic-resistant bacterial strains. Diseases caused by such bacteria are not amenable to treatment. This phenomenon is called antibiotic resistance [3,4,5]. The development of antibiotic resistance in bacteria led to a new wave of growth in the number of infectious diseases and the necessity to search for new antimicrobial agents [6]. One of the ways to overcome antibiotic resistance in bacteria is the use of metal and metal oxide nanoparticles (NPs) [7]. Fungal diseases are a multi-national problem. More than 150 million people in the world have severe fungal diseases. More than 1.5 million cases of fungal diseases have a lethal outcome [8]. The problem is exacerbated by the development of fungal resistance to antifungal drugs [9]. There are reports about the antifungal properties of metal oxide NPs [10,11]. Since the beginning of the COVID-19 pandemic, special attention has been given to the search for inexpensive and effective antiviral agents [12,13].
The antimicrobial properties of silver and its compounds have been known since ancient times. The first references to the use of silver are dated back to 3500–1000 B.C. In particular, silver was used for dishware production and water storage; later on, there were attempts to use silver powder to treat various diseases [14,15,16]. It has been shown many times in the literature that nanoparticles (NPs) of silver and its compounds have significant bactericidal, fungicidal and antiviral activities [17,18,19]. Ag2O NPs have attracted particular attention of researchers in the field of nanomaterials because of their unique properties that ensure multiple functions and a wide field of application. The most significant applications of Ag2O NPs are the production of catalyzers, chemical sensors, optoelectronic devices and systems of targeted delivery of drugs in vivo [20,21,22,23,24]. Ag2O NPs also have significant antimicrobial potential [25,26,27]. Silver oxide is used as an antimicrobial agent in the creation of biocompatible materials when developing bone implants [28]. Biomedical applications also include cancer therapy, wound treatment, tissue protection from oxidative stress, therapy of stomach ulcer, etc. [29,30,31]. An important application at the interface of biomedicine and ecology is the use of Ag2O NPs for photocatalytic destruction of pharmaceutical micro-pollutants [32].
The aim of this review is to provide readers with methods for Ag2O NP production, a range of sensitive microorganisms, mechanisms of the antimicrobial activity and some ways for improving their antimicrobial properties.
2. Sensitive Microorganisms
There are data in the literature about the antimicrobial activity of Ag2O NPs against, at least, 53 microbial species (Table 1), including 21 species of Gram-negative bacteria, 15 species of Gram-positive bacteria and 17 fungal species (Figure 1a). Among the most often mentioned organisms are Gram-negative bacteria Escherichia coli, Pseudomonas aeruginosa and Klebsiella pneumoniae; Gram-positive bacteria Staphylococcus aureus and Bacillus subtilis; and fungi Aspergillus and Candida albicans. All mentioned microorganisms have epidemiological significance. Antibiotic-resistant strains are most often found among Escherichia coli and Staphylococcus aureus [27,33,34,35,36]. We expected that the antimicrobial activity of Ag2O NPs against bacteria with different structures of cell wall (Gram-negative and Gram-positive) will greatly differ. An approximately equal amount (~20) of species of Gram-negative bacteria and Gram-positive bacteria sensitive to Ag2O NP was observed. This fact suggests the universality of the mechanisms of the antibacterial activity of Ag2O NPs. Ag2O NPs not only effectively inhibited bacterial growth, but also killed them. Therefore, Ag2O NPs are a perfect candidate for the role of a therapeutic agent against nosocomial bacterial infections [37].

Table 1.
Antimicrobial properties of Polymers/Ag2O nanocomposites.
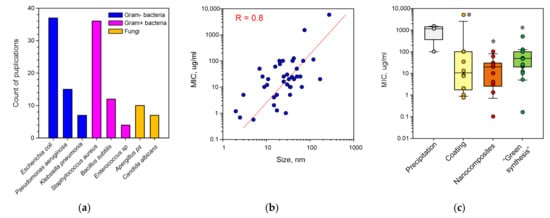
Figure 1.
Results of the data analysis regarding antimicrobial properties of Ag2O NPs: (a) microorganisms, against which the inhibitory activity of NPs was shown most often; (b) dependence of MIC against E. coli on NP sizes. R—value of the correlation coefficient; (c) dependence of MIC on a method of NP generation. *—p < 0.05, a significant difference from the precipitation variant using the Mann–Whitney test. Each dot represents a mention in one publication. The data are presented as medians, percentiles (10, 25, 75 and 90%).
When assessing a ratio of reports about the bactericidal and bacteriostatic activity of Ag2O NPs (Table 1), we found that bacteriostatic activity was described in about 75% of studies and bactericidal activity in 25% of studies. It is worth noting that the ratio of reports about the bactericidal and bacteriostatic activity of Ag2O NPs (equal to 1:3) is comparable to other widely used metal oxide NPs with antimicrobial activities, for example, iron oxides or ZnO NPs [7,88]. Iron oxides or ZnO NPs demonstrated high cytotoxicity in contradistinction to Ag2O NPs [89,90,91]. Having the same antimicrobial activity with other metal oxide NPs and low cytotoxicity makes Ag2O NPs an interesting candidate for the role of new generation antiseptic. For antifungal activities, the ratio shifted towards a reduction of the fungicidal activity. Only 15% of studies indicate the presence of the fungicidal effect and 85% contain data about the fungistatic effect. Therefore, fungi have higher resistance to Ag2O NPs compared to bacteria. This effect can be explained by the higher resistance of eukaryotic cells to the genotoxic effect of metal ions compared to prokaryotes, in particular, due to differences in the structure of the genetic apparatus and function of the reparation systems [92,93,94].
3. Synthesis Methods
Methods for the synthesis of Ag2O nanoparticles can be divided into physical, chemical and biological, otherwise referred to as “green synthesis” [95].
Chemical methods include various types of precipitation. The simplest method is realized when mixing AgNO3 with NaOH at high temperatures [13,58,75,96].
In this case, NP synthesis occurs in two stages described by the reaction equations:
AgNO3 + NaOH → AgOH + Na+ + NO3−
2AgOH → Ag2O + H2O (pK = 2.875)
Modifications of the method are possible: the addition of strong oxidizers, for example, K2S2O4, and KOH as a base [19,50]. Sometimes AgNO3 is obtained directly at the moment of synthesis upon the oxidation of silver foil with nitric acid; then, precipitation with alkali described above is performed [77]. To prevent the premature aggregation of synthesized Ag2O NPs, a surfactant—for example, citrate, polyethylene glycol, triethylene glycol, chitosan, urea and other compounds—can be added to the reaction mixture [40,82,96,97,98,99]. Another method for Ag2O NP production is the reduction of AgNO3 using organic acids citrate, acetate and oleic acid [45,53,56]. In the literature, this method is sometimes called the sol-gel method [100]. A method of Ag2O production upon the reduction of complex compounds, for example, ammoniate [Ag(NH3)2]x, is described [59,101]. To obtain NPs with a complex chemical composition, the drying of metal oxide NPs in the AgNO3 solution is used, as in the case of TiO2/Ag2O NPs [47].
The electrochemical synthesis (anode oxidation of metal silver) [102], precipitation upon ultrasound treatment [63], boiling [67,78], treatment with microwave radiation [22,78], evaporation of metal silver under the action of plasma [81] and laser ablation in water [52,53] can be assigned to physical methods.
Chemical and physical methods used today for NP synthesis can be expensive, require high temperatures and pressure or lead to the generation of waste that is hazardous for the environment [103]. Therefore, biological methods for the synthesis of nanomaterials, the so-called “green synthesis”, are preferable [26,104]. Moreover, silver oxide NPs obtained using biological methods have several advantages: low cost of synthesis, high antimicrobial activity, low cytotoxicity to mammalian cells and the possibility to use in pharmacology and biomedicine, like for NPs obtained by classical methods [105]. Similar to Ag NPs, “green” synthesis using extracts of medicinal plants is one of the methods for improving the antimicrobial properties of Ag2O NPs [106].
“Green synthesis” of Ag2O NPs consists of, as a rule, the reduction of water-soluble salt AgNO3 in an extract of medicinal plants or cultural liquid of non-pathogenic/weakly pathogenic microorganisms [107,108,109].
However, cases of real biosynthesis of Ag2O NPs are described, for example, synthesis by bacteria isolated from seeds of agricultural crops and cultivated in medium with the addition of AgNO3 [110,111] and soil bacteria Nitrobacter sp. [61]. In addition, methods for synthesis of Ag/Ag2O NPs by silver reduction in the medium of Fusarium oxysporum mycelium or dead biomass of yeasts [56,80] were described.
4. Methods for Studying Ag2O NPs
Dozens of methods have been applied to describe the parameters of Ag2O NPs. These methods are commonly used to study other Me/MexOy NPs [26]. To determine the size and shape of Ag2O NPs, various microscopic methods are used: atomic force microscopy (AFM) [112], scanning tunneling microscopy (STM) [113], scanning electron microscopy (SEM) [114] and transmission electron microscopy (TEM). The indicated methods allow us to image dry NPs and assess their size, shape, distribution on the surface of composite materials. To assess the elementary composition, proportion of organic impurities and conjugates, the following methods are used: UV–vis spectroscopy [115], Fourier transform infrared spectroscopy (FT-IR) [116,117], energy dispersive spectroscopy (EDX) [118], X-ray photoelectron spectroscopy (XPS) [119] and thermal gravimetric analysis (TGA) [120].
To determine the crystalline structure of NPs, the X-ray diffraction (XRD) method is applied [121,122]. To assess the hydrodynamic radius of NPs and stability of NP colloids in solvents, the dynamic light scattering (DLS) method and measurement of zeta potential, respectively, are used [123]. Assessment of the NP surface area and rheological properties of obtained nanomaterials is carried out by differential scanning calorimetry (DSC) and the Brunauer–Emmett–Teller (BET) method, respectively [124,125]. In the case of NP embedding into a polymeric material, it is possible to assess NP spatial distribution inside a polymeric matrix using modulation interference microscopy (MIM) [126].
5. Mechanisms of the Antimicrobial Activity
Antimicrobial properties of NPs are conditioned, first of all, by the antimicrobial properties of elements being their constituents. Silver ions show high toxicity to microorganisms. For example, Ag+ causes the death of Aspergillus niger spores at a concentration of 5.5 × 10−5 M (0.00006% w/w) and higher [127]. Ag NPs exert a significant antibacterial effect beginning from a concentration of 20 µg/mL [128,129]. It is shown that silver can be accumulated in microorganisms as Ag0, Ag2O or Ag+ [130]. Five mechanisms (as a minimum) of the antibacterial activity are described for these forms (Figure 2) [131].
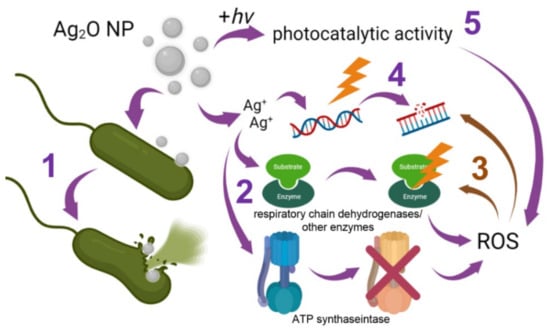
Figure 2.
Schematic representation of mechanisms of the antibacterial activity of Ag2O NPs (explanations are given in the text).
The first mechanism is binding to the bacterial cell wall and disruption of the cell wall integrity, resulting in direct damage of the cell envelope and cytoplasmic components [96,97,100]. It is assumed that after Ag2O NP penetration into a bacterial cell, the release of Ag0 and/or Ag+ having the bactericidal activity according to the mechanisms described below takes place [132,133].
The second mechanisms of toxicity is binding to SH-groups of proteins with the subsequent disorder of their function [134]. Silver-induced inactivation of bacterial enzymes, in particular, dehydrogenases of the respiratory chain, is described [110]. This, in turn, inhibits ATP synthesis, disturbs the energy balance in cells, enhances an intracellular ROS production and causes oxidative stress [110,135]. Moreover, Ag2O NPs are able to release O2, which can also exert antibacterial activity [96].
The third mechanism is the oxidative stress described above. ROS cause protein modifications and exert a genotoxic effect [136,137,138]. An increase in ROS generation leads to the destruction of the cell wall and biofilms of both Gram-positive and Gram-negative bacteria [123].
The fourth mechanism of the antibacterial activity of Ag2O NPs is the genotoxic activity of Ag compounds, which after penetration inside a bacterial cell interact not only with proteins but also with phosphoric acid residues in DNA molecules [59,139].
It is assumed that silver compounds from Ag2O NPs and Ag NPs are also capable of binding to the N7 atom of guanine in DNA, therefore disturbing the process of its replication, inhibiting cell division [139].
The fifth mechanism is photocatalytic activity. The addition of Ag2O NPs can enhance the photocatalytic properties of other metal NPs. In particular, composites of Ag2O/TiO2 NPs and Ag2O/ZnO NPs demonstrate enhanced photocatalytic activity compared to TiO2 or ZnO NPs [140,141,142]. Furthermore, photocatalytic activity of Ag2O NPs was demonstrated. It is interesting that the photocatalytic activity of Ag2O NPs enhanced after the conjugation of Ag2O NPs with certain pharmaceutical agents, for example, moxifloxacin [48,62].
It is notable that Ag2O NPs possess high toxicity to pathogenic microorganisms and low toxicity to soil microorganisms. In particular, soil Nitrobacter sp., Bacillus sp. and Pseudomonas strains are able to synthesize Ag2O NPs from AgNO3 in amounts sufficient for the growth inhibition of pathogenic microorganisms of the human oral cavity [49,54,61,78,143]. Specific Ag2O NP cytotoxicity to pathogenic microorganisms is an attractive feature for the creation of eco-friendly antimicrobial materials and preparations.
6. Methods for Improving Antimicrobial Properties
In meta-analysis, we found a dependence of the bacteriostatic activity (expressed in MIC) on NP size (Figure 1b). When a NP’s size decreases, an increase in its toxicity to microbes is observed. This dependence corresponds to the literature data about NPs of other metal oxides [7,144], and can be explained by a growth in the release of Ag+, Ag0 and Ag2O from NPs into the surrounding solution due to an increase in the area to volume ratio.
Antimicrobial properties of Ag2O NPs can be improved at the initial stage of NP synthesis: precipitation of Ag2O NPs. For example, precipitation of Ag2O NPs in medium with low (10 mM) or high (100 mM) concentration of AgNO3 lead to obtaining cubic or octahedral Ag2O NPs, respectively [74]. Cubic Ag2O NPs showed more pronounced bacteriostatic effects compared to octahedral [74].
The most common other modifications of Ag2O NP synthesis are NP coating with polymers, Ag2O NP inclusion into other nanocomposites or fusion with NPs of oxides of other elements and NP synthesis in the medium of a substrate of the biological origin—most often an extract of plant leaves (Figure 1c) [34,47,118].
Coatings can be conditionally divided into two large groups. The first group includes organic polymers: chitosan, polyethersulfone, cellulose acetate, polyvinyl alcohol, polyethylene terephthalate and starch [41,42,43,57,96]. This modification commonly had bacteriostatic and fungistatic activity [39,43]. Pharmaceutical preparations, in particular, aspirin and moxifloxacin, can be assigned to the second group [43,62]. For example, Ag2O NP coating with aspirin increased their bacteriostatic and fungistatic activity by 50% compared to non-conjugated NPs. In the case of Ag2O NP conjugation with moxifloxacin, a more pronounced increase in the bacteriostatic and fungistatic activity of Ag2O NPs (by 2–3 times) was shown [62]. Ag2O NP coating with chitosan allows practically 100% inhibition of the bacterial growth to be achieved irrespective of their Gram stain group [40]. An opportunity to use conjugates chitosan/Ag2O NPs for the creation of fabrics and cloths with the bacteriostatic properties is shown [40,41].
Examples of nanocomposites with Ag2O NPs are relatively rare. Among them, composites with ZrO2, TiO2 NPs, H2Ti3O7·2H2O and graphene oxide can be highlighted [60,122,123]. The addition of graphene oxide resulted in a dose-dependent increase in the antibacterial properties of Ag2O NPs. It is notable that in the case of graphene oxide, an enhancement of the bacteriostatic properties against Gram-negative bacteria was more pronounced [46].
The most common modification of Ag2O NP synthesis is the so-called “green synthesis”. There are reports about the use of extracts of plants Abroma augusta, Lawsonia inermis, Ficus benghal, Lippia citriodora, Eupatorium odoratum, Cleome gynandra, Aloe vera, Vaccinium arctostaphylos, Coleus aromaticus, Rhamnus virgate, Cyathea nilgiriensis, Centella Asiatica, Tridax sp., Hylocereus undatus, Paeonia emodi, Pinus longifolia and Telfairia occidentalis Telfairia occidentalis [33,34,35,36,37,51,60,64,65,66,69,73,76,83,84,85]; fungi Fusarium oxysporum, Kitasatospora albolonga, Rhodotorula mucilaginosa and Aspergillus terreus VIT 2013 [27,78,79,135]; and culture media of bacteria Bacillus paramycoides, Bacillus thuringiensis SSV1, Nitrobacter sp. (strain NCIM 5067) and Pseudomonas aeruginosa M6 [63,77,113,114]. “Green synthesis” enables Ag/Ag2O NPs to be obtained from wastes of silver mines, which may increase the production of silver mines and decrease environmental pollution [145]. “Green synthesized” Ag2O NP had not only bacteriostatic activity, but also fungicidal activity [37,79,125].
It is worth noting that all modifications of Ag2O NP synthesis enhance their antimicrobial properties compared to the chemical synthesis methods, in particular, precipitation (Figure 1c). Therefore, the selection of the conditions of Ag2O NP synthesis can make it possible to obtain NPs with high antimicrobial activity against antibiotic resistance bacteria. There are data that show that a synergetic effect is possible due to the use of several methods to improve the bacteriostatic activity of Ag2O NPs [75], for example, the synthesis of complex composites Cu·PES/CA/Ag2O NPs. This composite had more pronounced bacteriostatic properties compared to PES/CA/Ag2O NPs [42].
A growth in the studies devoted to the creation of various composites with the addition of Ag/Ag2O NPs (Table 1) allows us to suggest that the development of new composite materials with Ag2O NP introduction and, as a consequence, the extension of application fields for Ag2O NP-based nanomaterials will be promising investigations in this field [60,118,122,123].
7. Cytotoxicity to Human Cells
Data on Ag2O NP cytotoxicity are ambiguous and constantly being enriched. There are data about the toxicity of Ag2O NPs/Aspergillus terreus to Dalton’s lymphoma ascites (DLA) cells, which enables the use of Ag2O NPs in the therapy of oncological diseases [36]. High cytotoxicity of Ag2O/Ag NPs reported against breast cancer cell line MCF-7 and lung cancer cell line A549. Mechanisms of toxicity are genotoxic effects and ROS overproduction and membrane disruption [146]. Cytotoxicity of Ag NPs and consequently Ag2O NPs against eukaryotic cells is actively studied. Induction of apoptosis and necrosis by Ag2O/Ag NPs was shown on lung cells lines A549, MRC-5, bronchial cells BEAS-2B and NIH3T3, 3D-cultures of human primary small airway epithelial cell, etc. [147,148,149,150,151]. The ways to increase the cytotoxicity of Ag NPs against cancer and decrease against normal cells have been researched [152]. An interesting approach is using different coating agents; for example, Ag NP cytotoxicity increases in range “PVP > citrate > plant extracts > without coating”, but in the case of PVP and citrate, increased predominantly anticancer activity [153].
However, many studies report the low cytotoxicity of Ag2O NPs to eukaryotic cells. For example, Ag2O NPs did not affect the survival and migration of 3T3 fibroblast cells [63]. It was shown for Ag/Ag2O NPs/R. mucilaginosa that the cytotoxic action against eukaryotic cells was realized at concentrations 4–10 times higher than the cytotoxic action against bacteria and fungi [80]. For nanocomposites based on borosiloxane and PLGA and Ag2O NPs, the high bactericidal activity was found at Ag2O NP concentrations from 1 μg/ml; with that, the survival and the proliferation rate of eukaryotic cells on the above mentioned composites was comparable to these parameters obtained on the culture plastic [52,53]. Low cytotoxicity allows Ag2O NPs to be used for wound healing [37].
We assume that the cause of high biocompatibility with eukaryotic cells in the majority of studies is the use of Ag2O NP conjugates and composites instead of “pure” Ag2O NPs. We also proposed that Ag2O is more biologically invert compared to pure Ag.
Metal oxide NPs were potential drug delivery systems. The moderate/low cytotoxicity of Ag2O/Ag NPs makes them a perfect candidate for drug delivery systems [154,155,156]. Ag2O/Ag NPs can be used in anticancer and antiviral therapy [157,158,159]. Ag2O/Ag NPs can also be used as a photoactivated drug delivery unit, for example, in the localized induction of bone regeneration [160].
8. Conclusions
A search for antimicrobial agents of the new generation that allow us to overcome bacterial antibiotic resistance is an important task for world public health. Candidates for such agents are Ag2O NPs. Over the last three years, the interest of researchers in Ag2O NPs has increased manifold. The reason for this is the high toxicity to Gram-positive and Gram-negative bacteria, including antibiotic resistance, as well as fungi having epidemiological significance. Moreover, Ag2O NPs are inexpensive and easy to produce, and the field of their possible application includes regenerative medicine, prosthetics, therapy of oncological diseases, as well as the development of a wide spectrum of materials with antimicrobial properties (textile and construction). Ag2O NP cytotoxicity to eukaryotic cells and nonpathogenic microorganisms is significantly lower than against human pathogens, which makes Ag2O NPs an attractive candidate for the role of an antimicrobial agent safe for humans and the environment. Extension of the list of composite materials with the addition of Ag2O NPs and, as a consequence, an increase in the number of application fields for Ag2O NP-based nanomaterials can be considered the expected outcomes of investigations in this field.
Author Contributions
Conceptualization, S.V.G. and D.A.S.; writing—original draft preparation, S.V.G. and D.A.S.; writing—review and editing S.V.G. and A.A.S.; visualization, D.A.S. and M.E.A.; funding acquisition, A.B.L. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Ministry of Science and Education of the Russian Federation (Grant Agreement 075-15-2020-775).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data sharing not applicable.
Acknowledgments
The authors are grateful to the Center for the collective use of the GPI RAS and to the heavy metal band Aria for what they are.
Conflicts of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Davies, J.; Davies, D. Origins and evolution of antibiotic resistance. Microbiol. Mol. Biol. Rev. 2010, 74, 417–433. [Google Scholar] [CrossRef] [PubMed]
- Cavalieri, F.; Tortora, M.; Stringaro, A.; Colone, M.; Baldassarri, L. Nanomedicines for antimicrobial interventions. J. Hosp. Infect. 2014, 88, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Devi, L.S.; Joshi, S.R. Evaluation of the antimicrobial potency of silver nanoparticles biosynthesized by using an endophytic fungus Cryptosporiopsis ericae PS4. J. Microbiol. 2014, 52, 667–674. [Google Scholar] [CrossRef] [PubMed]
- Almatar, M.; Makky, E.A.; Var, I.; Koksal, F. The role of nanoparticles in the inhibition of multidrug-resistant bacteria and biofilms. Curr. Drug Deliv. 2018, 15, 470–484. [Google Scholar] [CrossRef]
- Solberg, C.O. Spread of Staphylococcus aureus in Hospitals: Causes and Prevention. Scand. J. Infect. Dis. 2000, 32, 587–595. [Google Scholar] [CrossRef]
- Gajbhiye, M.; Kesharwani, J.; Ingle, A.; Gade, A.; Rai, M. Fungus-mediated synthesis of silver nanoparticles and their activity against pathogenic fungi in combination with fluconazole. Nanomed. Nanotechnol. Biol. Med. 2009, 5, 382–386. [Google Scholar] [CrossRef]
- Gudkov, S.V.; Burmistrov, D.E.; Serov, D.A.; Rebezov, M.B.; Semenova, A.A.; Lisitsyn, A.B. A Mini Review of Antibacterial Properties of ZnO Nanoparticles. Front. Phys. 2021, 9, 641481. [Google Scholar] [CrossRef]
- Bongomin, F.; Gago, S.; Oladele, R.O.; Denning, D.W. Global and Multi-National Prevalence of Fungal Diseases—Estimate Precision. J. Fungi 2017, 3, 57. [Google Scholar] [CrossRef]
- Ben-Ami, R.; Kontoyiannis, D.P. Resistance to Antifungal Drugs. Infect. Dis. Clin. N. Am. 2021, 35, 279–311. [Google Scholar] [CrossRef]
- Du, W.; Gao, Y.; Liu, L.; Sai, S.; Ding, C. Striking Back against Fungal Infections: The Utilization of Nanosystems for Antifungal Strategies. Int. J. Mol. Sci. 2021, 22, 10104. [Google Scholar] [CrossRef]
- Khalil, N.M.; Abd El-Ghany, M.N.; Rodríguez-Couto, S. Antifungal and anti-mycotoxin efficacy of biogenic silver nanoparticles produced by Fusarium chlamydosporum and Penicillium chrysogenum at non-cytotoxic doses. Chemosphere 2019, 218, 477–486. [Google Scholar] [CrossRef]
- Coutard, B.; Valle, C.; De Lamballerie, X.; Canard, B.; Seidah, N.; Decroly, E. The Spike Glycoprotein of The New Coronavirus 2019-nCoV Contains A Furin-Like Cleavage Site Absent in Cov of The Same Clade. Antivir. Res. 2020, 176, 104742. [Google Scholar] [CrossRef]
- Hosseini, M.; Chin, A.W.H.; Williams, M.D.; Behzadinasab, S.; Falkinham, J.O.; Poon, L.L.M.; Ducker, W.A. Transparent Anti-SARS-CoV-2 and Antibacterial Silver Oxide Coatings. ACS Appl. Mater. Interfaces 2022, 14, 8718–8727. [Google Scholar] [CrossRef]
- Fong, J. The use of silver products in the management of burn wounds: Change in practice for the burn unit at Royal Perth Hospital. Prim. Intent. Aust. J. Wound Manag. 2005, 13, 16–22. [Google Scholar]
- Russell, F.R.; Pathm, W.B.; Hugo, A.D. Antimicrobial activity and action of silver. Prog. Med. Chem. 1994, 31, 351–371. [Google Scholar] [CrossRef]
- Uttayarat, P.; Eamsiri, J.; Tangthong, T.; Suwanmala, P. Radiolytic synthesis of colloidal silver nanoparticles for antibacterial wound dressings. Adv. Mater. Sci. Eng. 2015, 2015, 376082. [Google Scholar] [CrossRef]
- Kim, J.S.; Kuk, E.; Yu, K.N.; Kim, J.H.; Park, S.J.; Lee, H.J.; Kim, S.H.; Park, Y.K.; Park, Y.H.; Hwang, C.Y.; et al. Antimicrobial effects of silver nanoparticles. Nanomed. Nanotechnol. Biol. Med. 2007, 3, 95–101. [Google Scholar] [CrossRef]
- Chen, X.; Schluesener, H.J. Nanosilver: A nanoproduct in medical application. Toxicol. Lett. 2008, 176, 1–12. [Google Scholar] [CrossRef]
- Shen, W.; Li, P.; Feng, H.; Ge, Y.; Liu, Z.; Feng, L. The bactericidal mechanism of action against Staphylococcus aureus for AgO nanoparticles. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 75, 610–619. [Google Scholar] [CrossRef]
- Jain, S.; Mehata, M.S. Medicinal Plant Leaf Extract and Pure Flavonoid Mediated Green Synthesis of Silver Nanoparticles and their Enhanced Antibacterial Property. Sci. Rep. 2017, 7, 15867. [Google Scholar] [CrossRef]
- Ravichandran, S.; Paluri, V.; Kumar, G.; Loganathan, K.; Kokati Venkata, B.R. A novel approach for the biosynthesis of silver oxide nanoparticles using aqueous leaf extract of Callistemon lanceolatus (Myrtaceae) and their therapeutic potential. J. Exp. Nanosci. 2016, 11, 445–458. [Google Scholar] [CrossRef]
- Chakraborty, U.; Garg, P.; Bhanjana, G.; Kaur, G.; Kaushik, A.; Chaudhary, G.R. Spherical silver oxide nanoparticles for fabrication of electrochemical sensor for efficient 4-Nitrotoluene detection and assessment of their antimicrobial activity. Sci. Total Environ. 2022, 808, 152179. [Google Scholar] [CrossRef]
- Rahman, M.; Khan, S.; Jamal, A.; Faisal, M.; Asiri, A.M. Highly Sensitive Methanol Chemical Sensor Based on Undoped Silver Oxide Nanoparticles Prepared by a Solution Method. Microchim. Acta 2012, 178, 99–106. [Google Scholar] [CrossRef]
- Zhou, X.; Lu, Y.; Zhai, L.-L.; Zhao, Y.; Liu, Q.; Sun, W.-Y. Propargylamines formed from three-component coupling reactions catalyzed by silver oxide nanoparticles. RSC Adv. 2013, 3, 1732–1734. [Google Scholar] [CrossRef]
- Konop, M.; Damps, T.; Misicka, A.; Rudnicka, L. Certain Aspects of Silver and Silver Nanoparticles in Wound Care: A Minireview. J. Nanomater. 2016, 2016, 7614753. [Google Scholar] [CrossRef]
- Ghotekar, S.; Dabhane, H.; Pansambal, S.; Oza, R.; Tambade, P.; Medhane, V. A Review on Biomimetic Synthesis of Ag2O Nanoparticles using Plant Extract, Characterization and its Recent Applications. Adv. J. Chem. Sect. B 2020, 2, 102–111. [Google Scholar] [CrossRef]
- Sangappa, M.; Thiagarajan, P. Combating drug resistant pathogenic bacteria isolated from clinical infections, with silver oxide nanoparticles. Indian J. Pharm. Sci. 2015, 77, 151–155. [Google Scholar] [CrossRef]
- Ni, S.; Li, X.; Yang, P.; Ni, S.; Hong, F.; Webster, T.J. Enhanced apatite-forming ability and antibacterial activity of porous anodic alumina embedded with CaO-SiO2-Ag2O bioactive materials. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 58, 700–708. [Google Scholar] [CrossRef]
- Borges Rosa De Moura, F.; Antonio Ferreira, B.; Helena Muniz, E.; Benatti Justino, A.; Gabriela Silva, A.; De Azambuja Ribeiro, R.I.M.; Oliveira Dantas, N.; Lisboa Ribeiro, D.; De Assis Araújo, F.; Salmen Espindola, F.; et al. Antioxidant, anti-inflammatory, and wound healing effects of topical silver-doped zinc oxide and silver oxide nanocomposites. Int. J. Pharm. 2022, 617, 121620. [Google Scholar] [CrossRef]
- Iqbal, S.; Fakhar-E-Alam, M.; Akbar, F.; Shafiq, M.; Atif, M.; Amin, N.; Ismail, M.; Hanif, A.; Farooq, W.A. Application of silver oxide nanoparticles for the treatment of cancer. J. Mol. Struct. 2019, 1189, 203–209. [Google Scholar] [CrossRef]
- Salem, N.A.; Wahba, M.A.; Eisa, W.H.; El-Shamarka, M.; Khalil, W. Silver oxide nanoparticles alleviate indomethacin-induced gastric injury: A novel antiulcer agent. Inflammopharmacology 2018, 26, 1025–1035. [Google Scholar] [CrossRef] [PubMed]
- Pu, S.; Yang, Z.; Tang, J.; Ma, H.; Xue, S.; Bai, Y. Plasmonic silver/silver oxide nanoparticles anchored bismuth vanadate as a novel visible-light ternary photocatalyst for degrading pharmaceutical micropollutants. J. Environ. Sci. 2020, 96, 21–32. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, B.A.; Iqbal, J.; Nasir, J.A.; Zahra, S.A.; Shahbaz, A.; Uddin, S.; Hameed, S.; Gul, F.; Kanwal, S.; Mahmood, T. Environmentally friendly green approach for the fabrication of silver oxide nanoparticles: Characterization and diverse biomedical applications. Microsc. Res. Tech. 2020, 83, 1308–1320. [Google Scholar] [CrossRef] [PubMed]
- Khatun, Z.; Lawrence, R.S.; Jalees, M.; Lawerence, K. Green synthesis and Anti-bacterial activity of Silver Oxide nanoparticles prepared from Pinus longifolia leaves extract. Int. J. Adv. Res. 2015, 3, 337–343. [Google Scholar]
- Shah, A.; Haq, S.; Rehman, W.; Waseem, M.; Shoukat, S.; Rehman, M.-U. Photocatalytic and antibacterial activities of paeonia emodi mediated silver oxide nanoparticles. Mater. Res. Express 2019, 6, 045045. [Google Scholar] [CrossRef]
- Pradheesh, G.; Suresh, S.; Suresh, J.; Alexramani, V. Antimicrobial and anticancer activity studies on green synthesized silver oxide nanoparticles from the medicinal plant cyathea nilgiriensis holttum. Int. J. Pharm. Investig. 2020, 10, 146–150. [Google Scholar] [CrossRef]
- Roy, A.; Srivastava, S.K.; Shrivastava, S.L.; Mandal, A.K. Hierarchical Assembly of Nanodimensional Silver–Silver Oxide Physical Gels Controlling Nosocomial Infections. ACS Omega 2020, 5, 32617–32631. [Google Scholar] [CrossRef]
- Jin, Y.; Dong, S. One-Pot Synthesis and Characterization of Novel Silver−Gold Bimetallic Nanostructures with Hollow Interiors and Bearing Nanospikes. J. Phys. Chem. B 2003, 107, 12902–12905. [Google Scholar] [CrossRef]
- Tripathi, S.; Mehrotra, G.K.; Dutta, P.K. Chitosan–silver oxide nanocomposite film: Preparation and antimicrobial activity. Bull. Mater. Sci. 2011, 34, 29–35. [Google Scholar] [CrossRef]
- Hu, Z.; Zhang, J.; Chan, W.L.; Szeto, Y.S. Suspension of Silver Oxide Nanoparticles in Chitosan Solution and its Antibacterial Activity in Cotton Fabrics. MRS Online Proc. Libr. 2006, 920, 203. [Google Scholar] [CrossRef]
- Hu, Z.; Chan, W.L.; Szeto, Y.S. Nanocomposite of chitosan and silver oxide and its antibacterial property. J. Appl. Polym. Sci. 2008, 108, 52–56. [Google Scholar] [CrossRef]
- Gul, S.; Rehan, Z.A.; Khan, S.A.; Akhtar, K.; Khan, M.A.; Khan, M.I.; Rashid, M.I.; Asiri, A.M.; Khan, S.B. Antibacterial PES-CA-Ag2O nanocomposite supported Cu nanoparticles membrane toward ultrafiltration, BSA rejection and reduction of nitrophenol. J. Mol. Liq. 2017, 230, 616–624. [Google Scholar] [CrossRef]
- Kakakhel, S.A.; Rashid, H.; Jalil, Q.; Munir, S.; Barkatullah, B.; Khan, S.; Ullah, R.; Shahat, A.; Mahmood, H.; A-Mishari, A.; et al. Polymers Encapsulated Aspirin Loaded Silver Oxide Nanoparticles: Synthesis, Characterization and its Bio-Applications. Sains Malays. 2019, 48, 1887–1897. [Google Scholar] [CrossRef]
- Aazem, I.; Rathinam, P.; Pillai, S.; Honey, G.; Vengellur, A.; Bhat, S.G.; Sailaja, G.S. Active bayerite underpinned Ag2O/Ag: An efficient antibacterial nanohybrid combating microbial contamination. Metallomics 2021, 13, mfab049. [Google Scholar] [CrossRef]
- Sajjad, S.; Arshad, F.; Uzair, B.; Leghari, S.A.K.; Noor, S.; Maaza, M. GO/Ag2O Composite Nanostructure as an Effective Antibacterial Agent. ChemistrySelect 2019, 4, 10365–10371. [Google Scholar] [CrossRef]
- Rajabi, A.; Ghazali, M.J.; Mahmoudi, E.; Baghdadi, A.H.; Mohammad, A.W.; Mustafah, N.M.; Ohnmar, H.; Naicker, A.S. Synthesis, Characterization, and Antibacterial Activity of Ag₂O-Loaded Polyethylene Terephthalate Fabric via Ultrasonic Method. Nanomaterials 2019, 9, 450. [Google Scholar] [CrossRef]
- Sboui, M.; Lachheb, H.; Bouattour, S.; Gruttadauria, M.; La Parola, V.; Liotta, L.F.; Boufi, S. TiO2/Ag2O immobilized on cellulose paper: A new floating system for enhanced photocatalytic and antibacterial activities. Environ. Res. 2021, 198, 111257. [Google Scholar] [CrossRef]
- Lin, Z.; Lu, Y.; Huang, J. A hierarchical Ag2O-nanoparticle/TiO2-nanotube composite derived from natural cellulose substance with enhanced photocatalytic performance. Cellulose 2019, 26, 6683–6700. [Google Scholar] [CrossRef]
- Dharmaraj, D.; Krishnamoorthy, M.; Rajendran, K.; Karuppiah, K.; Annamalai, J.; Durairaj, K.R.; Santhiyagu, P.; Ethiraj, K. Antibacterial and cytotoxicity activities of biosynthesized silver oxide (Ag2O) nanoparticles using Bacillus paramycoides. J. Drug Deliv. Sci. Technol. 2021, 61, 102111. [Google Scholar] [CrossRef]
- Li, D.; Chen, S.; Zhang, K.; Gao, N.; Zhang, M.; Albasher, G.; Shi, J.; Wang, C. The interaction of Ag2O nanoparticles with Escherichia coli: Inhibition–sterilization process. Sci. Rep. 2021, 11, 1703. [Google Scholar] [CrossRef]
- Fayyadh, A.A.; Jaduaa Alzubaidy, M.H. Green-synthesis of Ag2O nanoparticles for antimicrobial assays**. J. Mech. Behav. Mater. 2021, 30, 228–236. [Google Scholar] [CrossRef]
- Chausov, D.N.; Smirnova, V.V.; Burmistrov, D.E.; Sarimov, R.M.; Kurilov, A.D.; Astashev, M.E.; Uvarov, O.V.; Dubinin, M.V.; Kozlov, V.A.; Vedunova, M.V.; et al. Synthesis of a Novel, Biocompatible and Bacteriostatic Borosiloxane Composition with Silver Oxide Nanoparticles. Materials 2022, 15, 2. [Google Scholar] [CrossRef]
- Smirnova, V.V.; Chausov, D.N.; Serov, D.A.; Kozlov, V.A.; Ivashkin, P.I.; Pishchalnikov, R.Y.; Uvarov, O.V.; Vedunova, M.V.; Semenova, A.A.; Lisitsyn, A.B.; et al. A Novel Biodegradable Composite Polymer Material Based on PLGA and Silver Oxide Nanoparticles with Unique Physicochemical Properties and Biocompatibility with Mammalian Cells. Materials 2021, 14, 22. [Google Scholar] [CrossRef]
- Karunagaran, V.; Rajendran, K.; Sen, S. Antimicrobial Activity of Biosynthesized Silver Oxide Nanoparticles. J. Pure Appl. Microbiol. 2014, 4, 3263–3268. [Google Scholar]
- Ayanwale, A.P.; Ruíz-Baltazar, A.D.J.; Espinoza-Cristóbal, L.; Reyes-López, S.Y. Bactericidal Activity Study of ZrO2-Ag2O Nanoparticles. Dose-Response 2020, 18, 1559325820941374. [Google Scholar] [CrossRef]
- Islam, S.N.; Naqvi, S.M.A.; Parveen, S.; Ahmad, A. Application of mycogenic silver/silver oxide nanoparticles in electrochemical glucose sensing; alongside their catalytic and antimicrobial activity. 3 Biotech 2021, 11, 342. [Google Scholar] [CrossRef]
- Rokade, A.A.; Patil, M.P.; Yoo, S.I.; Lee, W.K.; Park, S.S. Pure green chemical approach for synthesis of Ag2O nanoparticles. Green Chem. Lett. Rev. 2016, 9, 216–222. [Google Scholar] [CrossRef]
- Shaffiey, S.R.; Shaffiey, S. Synthesis and evaluation of bactericidal properties of Ag2O nanoparticles against Aeromonashydrophila. Int. J. Nano Dimens. 2014, 6, 263–269. [Google Scholar] [CrossRef]
- Panáček, A.; Kvítek, L.; Prucek, R.; Kolář, M.; Večeřová, R.; Pizúrová, N.; Sharma, V.K.; Nevěčná, T.J.; Zbořil, R. Silver Colloid Nanoparticles: Synthesis, Characterization, and Their Antibacterial Activity. J. Phys. Chem. B 2006, 110, 16248–16253. [Google Scholar] [CrossRef]
- Manikandan, V.; Velmurugan, P.; Park, J.H.; Chang, W.S.; Park, Y.J.; Jayanthi, P.; Cho, M.; Oh, B.T. Green synthesis of silver oxide nanoparticles and its antibacterial activity against dental pathogens. 3 Biotech 2017, 7, 72. [Google Scholar] [CrossRef]
- Karunakaran, G.; Jagathambal, M.; Gusev, A.; Minh, N.V.; Kolesnikov, E.; Mandal, A.R.; Kuznetsov, D. Nitrobacter sp. extract mediated biosynthesis of Ag(2)O NPs with excellent antioxidant and antibacterial potential for biomedical application. IET Nanobiotechnol. 2016, 10, 425–430. [Google Scholar] [CrossRef] [PubMed]
- Haq, S.; Rehman, W.; Waseem, M.; Meynen, V.; Awan, S.U.; Saeed, S.; Iqbal, N. Fabrication of pure and moxifloxacin functionalized silver oxide nanoparticles for photocatalytic and antimicrobial activity. J. Photochem. Photobiol. B Biol. 2018, 186, 116–124. [Google Scholar] [CrossRef] [PubMed]
- Babu, P.J.; Doble, M.; Raichur, A.M. Silver oxide nanoparticles embedded silk fibroin spuns: Microwave mediated preparation, characterization and their synergistic wound healing and anti-bacterial activity. J. Colloid Interface Sci. 2018, 513, 62–71. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Chen, Z.; Ren, N.; Wang, Y.; Wang, Y.; Yu, F. Biosynthesis of silver oxide nanoparticles and their photocatalytic and antimicrobial activity evaluation for wound healing applications in nursing care. J. Photochem. Photobiol. B Biol. 2019, 199, 111593. [Google Scholar] [CrossRef]
- Elemike, E.E.; Onwudiwe, D.C.; Ekennia, A.C.; Sonde, C.U.; Ehiri, R.C. Green Synthesis of Ag/Ag₂O Nanoparticles Using Aqueous Leaf Extract of Eupatorium odoratum and Its Antimicrobial and Mosquito Larvicidal Activities. Molecules 2017, 22, 674. [Google Scholar] [CrossRef]
- Mani, M.; Harikrishnan, R.; Purushothaman, P.; Pavithra, S.; Rajkumar, P.; Kumaresan, S.; Al Farraj, D.A.; Elshikh, M.S.; Balasubramanian, B.; Kaviyarasu, K. Systematic green synthesis of silver oxide nanoparticles for antimicrobial activity. Environ. Res. 2021, 202, 111627. [Google Scholar] [CrossRef]
- Hoque, M.I.U.; Chowdhury, A.N.; Islam, M.T.; Firoz, S.H.; Luba, U.; Alowasheeir, A.; Rahman, M.M.; Rehman, A.U.; Ahmad, S.H.A.; Holze, R.; et al. Fabrication of highly and poorly oxidized silver oxide/silver/tin(IV) oxide nanocomposites and their comparative anti-pathogenic properties towards hazardous food pathogens. J. Hazard. Mater. 2021, 408, 124896. [Google Scholar] [CrossRef]
- Negi, H.; Rathinavelu Saravanan, P.; Agarwal, T.; Ghulam Haider Zaidi, M.; Goel, R. In vitro assessment of Ag2O nanoparticles toxicity against Gram-positive and Gram-negative bacteria. J. Gen. Appl. Microbiol. 2013, 59, 83–88. [Google Scholar] [CrossRef]
- Flores-Lopez, N.S.; Cervantes-Chávez, J.A.; Téllez De Jesús, D.G.; Cortez-Valadez, M.; Estévez-González, M.; Esparza, R. Bactericidal and fungicidal capacity of Ag(2)O/Ag nanoparticles synthesized with Aloe vera extract. J. Environ. Sci. Health. Part A Toxic/Hazard. Subst. Environ. Eng. 2021, 56, 762–768. [Google Scholar] [CrossRef]
- Chen, Y.; Gao, A.; Bai, L.; Wang, Y.; Wang, X.; Zhang, X.; Huang, X.; Hang, R.; Tang, B.; Chu, P.K. Antibacterial, osteogenic, and angiogenic activities of SrTiO(3) nanotubes embedded with Ag(2)O nanoparticles. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 75, 1049–1058. [Google Scholar] [CrossRef]
- Jin, Y.; Dai, Z.; Liu, F.; Kim, H.; Tong, M.; Hou, Y. Bactericidal mechanisms of Ag₂O/TNBs under both dark and light conditions. Water Res. 2013, 47, 1837–1847. [Google Scholar] [CrossRef]
- Gao, A.; Hang, R.; Huang, X.; Zhao, L.; Zhang, X.; Wang, L.; Tang, B.; Ma, S.; Chu, P.K. The effects of titania nanotubes with embedded silver oxide nanoparticles on bacteria and osteoblasts. Biomaterials 2014, 35, 4223–4235. [Google Scholar] [CrossRef]
- Khodadadi, S.; Mahdinezhad, N.; Fazeli-Nasab, B.; Heidari, M.J.; Fakheri, B.; Miri, A. Investigating the Possibility of Green Synthesis of Silver Nanoparticles Using Vaccinium arctostaphlyos Extract and Evaluating Its Antibacterial Properties. BioMed Res. Int. 2021, 2021, 5572252. [Google Scholar] [CrossRef]
- Wang, X.; Wu, H.F.; Kuang, Q.; Huang, R.B.; Xie, Z.X.; Zheng, L.S. Shape-dependent antibacterial activities of Ag2O polyhedral particles. Langmuir ACS J. Surf. Colloids 2010, 26, 2774–2778. [Google Scholar] [CrossRef]
- Kundu, S.; Sain, S.; Choudhury, P.; Sarkar, S.; Das, P.K.; Pradhan, S.K. Microstructure characterization of biocompatible heterojunction hydrogen titanate-Ag(2)O nanocomposites for superior visible light photocatalysis and antibacterial activity. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 99, 374–386. [Google Scholar] [CrossRef]
- Lekshmi, G.S.; Tamilselvi, R.; Geethalakshmi, R.; Kirupha, S.D.; Bazaka, O.; Levchenko, I.; Bazaka, K.; Mandhakini, M. Multifunctional oil-produced reduced graphene oxide - Silver oxide composites with photocatalytic, antioxidant, and antibacterial activities. J. Colloid Interface Sci. 2022, 608, 294–305. [Google Scholar] [CrossRef]
- Sajjad, S.; Uzair, B.; Shaukat, A.; Jamshed, M.; Leghari, S.A.K.; Ismail, M.; Mansoor, Q. Synergistic evaluation of AgO(2) nanoparticles with ceftriaxone against CTXM and blaSHV genes positive ESBL producing clinical strains of Uro-pathogenic E. coli. IET Nanobiotechnol. 2019, 13, 435–440. [Google Scholar] [CrossRef]
- Boopathi, S.; Gopinath, S.; Boopathi, T.; Balamurugan, V.; Rajeshkumar, R.; Sundararaman, M. Characterization and Antimicrobial Properties of Silver and Silver Oxide Nanoparticles Synthesized by Cell-Free Extract of a Mangrove-Associated Pseudomonas aeruginosa M6 Using Two Different Thermal Treatments. Ind. Eng. Chem. Res. 2012, 51, 5976–5985. [Google Scholar] [CrossRef]
- D’lima, L.; Phadke, M.; Ashok, V.D. Biogenic silver and silver oxide hybrid nanoparticles: A potential antimicrobial against multi drug-resistant Pseudomonas aeruginosa. New. J. Chem. 2020, 44, 4935–4941. [Google Scholar] [CrossRef]
- Salvadori, M.; Monezi, T.; Mehnert, D.; Corrêa, B. Antimicrobial Activity of Ag/Ag2O Nanoparticles Synthesized by Dead Biomass of Yeast and their Biocompatibility with Mammalian Cell Lines. Int. J. Res. Stud. Microbiol. Biotechnol. 2019, 5, 2454–9428. [Google Scholar] [CrossRef]
- Kayed, K.; Mansour, G. The Antimicrobial Activity of Silver Nanoparticles in Ag/Ag2O Composites Synthesized by Oxygen Plasma Treatment of Silver Thin Films. Curr. Appl. Sci. Technol. 2022, 22, 9. [Google Scholar] [CrossRef]
- Akbari, Z.; Rashidi Ranjbar, Z.; Khaleghi, M. Synthesis, characterization, and antibacterial activities of Ag2O nanoparticle and silver (I) nano-rod complex. Nanochemistry Res. 2020, 5, 233–240. [Google Scholar]
- Rashmi, B.N.; Harlapur, S.F.; Avinash, B.; Ravikumar, C.R.; Nagaswarupa, H.P.; Anil Kumar, M.R.; Gurushantha, K.; Santosh, M.S. Facile green synthesis of silver oxide nanoparticles and their electrochemical, photocatalytic and biological studies. Inorg. Chem. Commun. 2020, 111, 107580. [Google Scholar] [CrossRef]
- Phongtongpasuk, S.; Poadang, S.; Yongvanich, N. Environmental-friendly Method for Synthesis of Silver Nanoparticles from Dragon Fruit Peel Extract and their Antibacterial Activities. Energy Procedia 2016, 89, 239–247. [Google Scholar] [CrossRef]
- Aisida, S.; Ugwu, K.; Nwanya, A.; Bashir, A.K.H.; Nwankwo, U.; Ahmed, I.; Ezema, F. Biosynthesis of silver oxide nanoparticles using leave extract of Telfairia Occidentalis and its antibacterial activity. Mater. Today Proc. 2021, 36, 208–213. [Google Scholar] [CrossRef]
- Kiani, F.A.; Shamraiz, U.; Badshah, A.; Tabassum, S.; Ambreen, M.; Patujo, J.A. Optimization of Ag2O nanostructures with strontium for biological and therapeutic potential. Artif. Cells Nanomed. Biotechnol. 2018, 46 (Suppl. S3), S1083–S1091. [Google Scholar] [CrossRef]
- Elyamny, S.; Eltarahony, M.; Abu-Serie, M.; Nabil, M.M.; Kashyout, A.E.-H.B. One-pot fabrication of Ag @Ag2O core–shell nanostructures for biosafe antimicrobial and antibiofilm applications. Sci. Rep. 2021, 11, 22543. [Google Scholar] [CrossRef]
- Gudkov, S.V.; Burmistrov, D.E.; Serov, D.A.; Rebezov, M.B.; Semenova, A.A.; Lisitsyn, A.B. Do Iron Oxide Nanoparticles Have Significant Antibacterial Properties? Antibiotics 2021, 10, 884. [Google Scholar] [CrossRef] [PubMed]
- Arias, L.S.; Pessan, J.P.; Vieira, A.P.M.; Lima, T.M.T.D.; Delbem, A.C.B.; Monteiro, D.R. Iron oxide nanoparticles for biomedical applications: A perspective on synthesis, drugs, antimicrobial activity, and toxicity. Antibiotics 2018, 7, 46. [Google Scholar] [CrossRef] [PubMed]
- Apopa, P.L.; Qian, Y.; Shao, R.; Guo, N.L.; Schwegler-Berry, D.; Pacurari, M.; Porter, D.; Shi, X.; Vallyathan, V.; Castranova, V. Iron oxide nanoparticles induce human microvascular endothelial cell permeability through reactive oxygen species production and microtubule remodeling. Part. Fibre Toxicol. 2009, 6, 1. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.; Ji, Y.; Liu, F.; Li, J.; Cao, Y. Cytotoxicity, oxidative stress and inflammation induced by ZnO nanoparticles in endothelial cells: Interaction with palmitate or lipopolysaccharide. J. Appl. Toxicol. 2017, 37, 895–901. [Google Scholar] [CrossRef]
- Chatterjee, N.; Walker, G.C. Mechanisms of DNA damage, repair, and mutagenesis. Env. Mol Mutagen 2017, 58, 235–263. [Google Scholar] [CrossRef]
- Doolittle, W.F. A paradigm gets shifty. Nature 1998, 392, 15–16. [Google Scholar] [CrossRef]
- Wigley, D.B. Bacterial DNA repair: Recent insights into the mechanism of RecBCD, AddAB and AdnAB. Nat. Rev. Microbiol. 2013, 11, 9–13. [Google Scholar] [CrossRef]
- Torabi, S.; Mansoorkhani, M.J.K.; Majedi, A.; Motevalli, S. REVIEW: Synthesis, Medical and Photocatalyst Applications of Nano-Ag2O. J. Coord. Chem. 2020, 73, 1861–1880. [Google Scholar] [CrossRef]
- Sullivan, K.T.; Wu, C.; Piekiel, N.W.; Gaskell, K.; Zachariah, M.R. Synthesis and reactivity of nano-Ag2O as an oxidizer for energetic systems yielding antimicrobial products. Combust. Flame 2013, 160, 438–446. [Google Scholar] [CrossRef]
- Phan, C.M.; Nguyen, H.M. Role of Capping Agent in Wet Synthesis of Nanoparticles. J. Phys. Chem. A 2017, 121, 3213–3219. [Google Scholar] [CrossRef]
- Yong, N.L.; Ahmad, A.; Mohammad, A.W. Synthesis and characterization of silver oxide nanoparticles by a novel method. Int. J. Sci. Eng. Res. 2013, 4, 155–158. [Google Scholar]
- Jeung, D.-G.; Lee, M.; Paek, S.-M.; Oh, J.-M. Controlled Growth of Silver Oxide Nanoparticles on the Surface of Citrate Anion Intercalated Layered Double Hydroxide. Nanomaterials 2021, 11, 455. [Google Scholar] [CrossRef]
- Harish, K.; Manisha, K. Synthesis and characterization of silveroxide nanoparticles by sol-gel method. Int. J. Adv. Res. Sci. Eng. 2018, 7, 632–637. [Google Scholar]
- Wang, G.; Ma, X.; Huang, B.; Cheng, H.; Wang, Z.; Zhan, J.; Qin, X.; Zhang, X.; Dai, Y. Controlled synthesis of Ag 2 O microcrystals with facet-dependent photocatalytic activities. J. Mater. Chem. 2012, 22, 21189–21194. [Google Scholar] [CrossRef]
- Murray, B.; Li, Q.; Newberg, J.; Menke, E.; Hemminger, J.; Penner, R. Shape-and size-selective electrochemical synthesis of dispersed silver (I) oxide colloids. Nano Lett. 2005, 5, 2319–2324. [Google Scholar] [CrossRef]
- Saha, S.; Chattopadhyay, D.; Acharya, K. Preparation of silver nanoparticles by bio-reduction using nigrospora oryzae culture filtrate and its antimicrobial activity. Dig. J. Nanomater. Biostructures (DJNB) 2011, 6, 1842–3582. [Google Scholar]
- Ponnuchamy, K.; Selvi, S.; Prabha, L.; Premkumar, K.; Ganeshkumar, R.; Munisamy, G. Synthesis of Silver Nanoparticles from Sargassum Tenerrimum and Screening Phytochemicals for Its Antibacterial Activity. Nano Biomed. Eng. 2012, 4, 12–16. [Google Scholar] [CrossRef]
- Maiti, S.; Krishnan, D.; Barman, G.; Ghosh, S.K.; Laha, J.K. Antimicrobial activities of silver nanoparticles synthesized from Lycopersicon esculentum extract. J. Anal. Sci. Technol. 2014, 5, 40. [Google Scholar] [CrossRef]
- Antony, E.; Sathiavelu, M.; Arunachalam, S. Synthesis of silver nanoparticles from the medicinal plant bauhinia acuminata and biophytum sensitivum–a comparative study of its biological activities with plant extract. Int. J. Appl. Pharm. 2016, 9, 22. [Google Scholar] [CrossRef]
- Mohamed, H.E.A.; Afridi, S.; Khalil, A.T.; Zia, D.; Iqbal, J.; Ullah, I.; Shinwari, Z.K.; Maaza, M. Biosynthesis of silver nanoparticles from Hyphaene thebaica fruits and their in vitro pharmacognostic potential. Mater. Res. Express 2019, 6, 1050c9. [Google Scholar] [CrossRef]
- Kahsay, M.H.; Ramadevi, D.; Kumar, Y.P.; Mohan, B.S.; Tadesse, A.; Battu, G.; Basavaiah, K. Synthesis of silver nanoparticles using aqueous extract of Dolichos lablab for reduction of 4-Nitrophenol, antimicrobial and anticancer activities. OpenNano 2018, 3, 28–37. [Google Scholar] [CrossRef]
- Laouini, S.E.; Bouafia, A.; Soldatov, A.V.; Algarni, H.; Tedjani, M.L.; Ali, G.A.M.; Barhoum, A. Green Synthesized of Ag/Ag(2)O Nanoparticles Using Aqueous Leaves Extracts of Phoenix dactylifera L. and Their Azo Dye Photodegradation. Membranes 2021, 11, 468. [Google Scholar] [CrossRef]
- Gomaa, E.Z. Silver nanoparticles as an antimicrobial agent: A case study on Staphylococcus aureus and Escherichia coli as models for Gram-positive and Gram-negative bacteria. J. Gen. Appl. Microbiol. 2017, 63, 36–43. [Google Scholar] [CrossRef]
- Gurunathan, S.; Kalishwaralal, K.; Vaidyanathan, R.; Venkataraman, D.; Pandian, S.R.K.; Muniyandi, J.; Hariharan, N.; Eom, S.H. Biosynthesis, purification and characterization of silver nanoparticles using Escherichia coli. Colloids Surf. B Biointerfaces 2009, 74, 328–335. [Google Scholar] [CrossRef] [PubMed]
- Rao, K.; Imran, M.; Jabri, T.; Ali, I.; Perveen, S.; Shafiullah; Ahmed, S.; Shah, M.R. Gum tragacanth stabilized green gold nanoparticles as cargos for Naringin loading: A morphological investigation through AFM. Carbohydr. Polym. 2017, 174, 243–252. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.; Hrbek, J.; Osgood, R. Formation of TiO2 nanoparticles by reactive-layer-assisted deposition and characterization by XPS and STM. Nano Lett. 2005, 5, 1327–1332. [Google Scholar] [CrossRef] [PubMed]
- Nagajyothi, P.C.; Sreekanth, T.V.; Tettey, C.O.; Jun, Y.I.; Mook, S.H. Characterization, antibacterial, antioxidant, and cytotoxic activities of ZnO nanoparticles using Coptidis Rhizoma. Bioorganic Med. Chem. Lett. 2014, 24, 4298–4303. [Google Scholar] [CrossRef]
- Mittal, A.K.; Chisti, Y.; Banerjee, U.C. Synthesis of metallic nanoparticles using plant extracts. Biotechnol. Adv. 2013, 31, 346–356. [Google Scholar] [CrossRef]
- Wang, T.; Lin, J.; Chen, Z.; Megharaj, M.; Naidu, R. Green synthesized iron nanoparticles by green tea and eucalyptus leaves extracts used for removal of nitrate in aqueous solution. J. Clean. Prod. 2014, 83, 413–419. [Google Scholar] [CrossRef]
- Rauwel, P.; Küünal, S.; Ferdov, S.; Rauwel, E. A Review on the Green Synthesis of Silver Nanoparticles and Their Morphologies Studied via TEM. Adv. Mater. Sci. Eng. 2015, 2015, 682749. [Google Scholar] [CrossRef]
- Singh, V.; Shrivastava, A.; Wahi, N. Biosynthesis of silver nanoparticles by plants crude extracts and their characterization using UV, XRD, TEM and EDX. Afr. J. Biotechnol. 2015, 14, 2554–2567. [Google Scholar] [CrossRef]
- Naraginti, S.; Li, Y. Preliminary investigation of catalytic, antioxidant, anticancer and bactericidal activity of green synthesized silver and gold nanoparticles using Actinidia deliciosa. J. Photochem. Photobiol. B Biol. 2017, 170, 225–234. [Google Scholar] [CrossRef]
- Sana, S.S.; Dogiparthi, L.K. Green synthesis of silver nanoparticles using Givotia moluccana leaf extract and evaluation of their antimicrobial activity. Mater. Lett. 2018, 226, 47–51. [Google Scholar] [CrossRef]
- Monshi, A.; Foroughi, M.; Monshi, M. Modified Scherrer Equation to Estimate More Accurately Nano-Crystallite Size Using XRD. World J. Nano Sci. Eng. 2012, 2, 154–160. [Google Scholar] [CrossRef]
- Zad, Z.R.; Davarani, S.S.H.; Taheri, A.; Bide, Y. A yolk shell Fe3O4@ PA-Ni@ Pd/Chitosan nanocomposite-modified carbon ionic liquid electrode as a new sensor for the sensitive determination of fluconazole in pharmaceutical preparations and biological fluids. J. Mol. Liq. 2018, 253, 233–240. [Google Scholar] [CrossRef]
- Das, B.; Dash, S.K.; Mandal, D.; Ghosh, T.; Chattopadhyay, S.; Tripathy, S.; Das, S.; Dey, S.K.; Das, D.; Roy, S. Green synthesized silver nanoparticles destroy multidrug resistant bacteria via reactive oxygen species mediated membrane damage. Arab. J. Chem. 2017, 10, 862–876. [Google Scholar] [CrossRef]
- Surma, N.; Ijuo, G.; Ogoh-Orch, B. Fuel Gases from Waste High Density Polyethylene (Hdpe) Via Low Temperature Catalytic Pyrolysis. Prog. Chem. Biochem. Res. 2020, 3, 20–30. [Google Scholar] [CrossRef]
- Venkateswarlu, S.; Natesh Kumar, B.; Prasad, C.H.; Venkateswarlu, P.; Jyothi, N.V.V. Bio-inspired green synthesis of Fe3O4 spherical magnetic nanoparticles using Syzygium cumini seed extract. Phys. B Condens. Matter 2014, 449, 67–71. [Google Scholar] [CrossRef]
- Astashev, M.E.; Sarimov, R.M.; Serov, D.A.; Matveeva, T.A.; Simakin, A.V.; Ignatenko, D.N.; Burmistrov, D.E.; Smirnova, V.V.; Kurilov, A.D.; Mashchenko, V.I.; et al. Antibacterial behavior of organosilicon composite with nano aluminum oxide without influencing animal cells. React. Funct. Polym. 2022, 170, 105143. [Google Scholar] [CrossRef]
- Von Naegelli, V. Silver nitrate: A very effective antimicrobial agent. Deut. Schr. Schweiz Nat. Ges 1893, 33, 174–182. [Google Scholar]
- Raffi, M.; Hussain, F.; Bhatti, T.; Akhter, J.; Hameed, A.; Hasan, M. Antibacterial characterization of silver nanoparticles against E. coli ATCC-15224. J. Mater. Sci. Technol. 2008, 24, 192–196. [Google Scholar]
- Sondi, I.; Salopek-Sondi, B. Silver nanoparticles as antimicrobial agent: A case study on E. coli as a model for Gram-negative bacteria. J. Colloid Interface Sci. 2004, 275, 177–182. [Google Scholar] [CrossRef]
- Belly, R.T.; Kydd, G.C. Silver resistance in microorganisms. Dev. Ind. Microbiol. 1982, 23, 567–578. [Google Scholar]
- Grigor’eva, A.; Saranina, I.; Tikunova, N.; Safonov, A.; Timoshenko, N.; Rebrov, A.; Ryabchikova, E. Fine mechanisms of the interaction of silver nanoparticles with the cells of Salmonella typhimurium and Staphylococcus aureus. Biometals Int. J. Role Met. Ions Biol. Biochem. Med. 2013, 26, 479–488. [Google Scholar] [CrossRef]
- Song, H.; Ko, K.; Oh, L.; Lee, B. Fabrication of silver nanoparticles and their antimicrobial mechanisms. Eur. Cells Mater. 2006, 11 (Suppl. S1), 58. [Google Scholar]
- Sambhy, V.; Macbride, M.M.; Peterson, B.R.; Sen, A. Silver bromide nanoparticle/polymer composites: Dual action tunable antimicrobial materials. J. Am. Chem. Soc. 2006, 128, 9798–9808. [Google Scholar] [CrossRef]
- Russell, A.D.; Hugo, W.B. 7 Antimicrobial Activity and Action of Silver Periodical 7 Antimicrobial Activity and Action of Silver [Online]. 1994, pp. 351–370. Available online: https://www.sciencedirect.com/science/article/pii/S0079646808700249 (accessed on 25 April 2022). [CrossRef]
- Xia, T.; Kovochich, M.; Brant, J.; Hotze, M.; Sempf, J.; Oberley, T.; Sioutas, C.; Yeh, J.I.; Wiesner, M.R.; Nel, A.E. Comparison of the abilities of ambient and manufactured nanoparticles to induce cellular toxicity according to an oxidative stress paradigm. Nano Lett. 2006, 6, 1794–1807. [Google Scholar] [CrossRef]
- Bruskov, V.I.; Karp, O.E.; Garmash, S.A.; Shtarkman, I.N.; Chernikov, A.V.; Gudkov, S.V. Prolongation of oxidative stress by long-lived reactive protein species induced by X-ray radiation and their genotoxic action. Free Radic. Res. 2012, 46, 1280–1290. [Google Scholar] [CrossRef]
- Bruskov, V.I.; Malakhova, L.V.; Masalimov, Z.K.; Chernikov, A.V. Heat-induced formation of reactive oxygen species and 8-oxoguanine, a biomarker of damage to DNA. Nucleic Acids Res. 2002, 30, 1354–1363. [Google Scholar] [CrossRef]
- Burmistrov, D.E.; Simakin, A.V.; Smirnova, V.V.; Uvarov, O.V.; Ivashkin, P.I.; Kucherov, R.N.; Ivanov, V.E.; Bruskov, V.I.; Sevostyanov, M.A.; Baikin, A.S.; et al. Bacteriostatic and Cytotoxic Properties of Composite Material Based on ZnO Nanoparticles in PLGA Obtained by Low Temperature Method. Polymers 2022, 14, 49. [Google Scholar] [CrossRef]
- Ocsoy, I.; Paret, M.L.; Ocsoy, M.A.; Kunwar, S.; Chen, T.; You, M.; Tan, W. Nanotechnology in Plant Disease Management: DNA-Directed Silver Nanoparticles on Graphene Oxide as an Antibacterial against Xanthomonas perforans. ACS Nano 2013, 7, 8972–8980. [Google Scholar] [CrossRef]
- Allahverdiyev, A.M.; Abamor, E.S.; Bagirova, M.; Rafailovich, M. Antimicrobial effects of TiO2 and Ag2O nanoparticles against drug-resistant bacteria and leishmania parasites. Future Microbiol. 2011, 6, 933–940. [Google Scholar] [CrossRef]
- Hua, H.; Xi, Y.; Zhao, Z.; Xie, X.; Hu, C.; Liu, H. Gram-scale wet chemical synthesis of Ag2O/TiO2 aggregated sphere heterostructure with high photocatalytic activity. Mater. Lett. 2013, 91, 81–83. [Google Scholar] [CrossRef]
- Wu, C.; Shen, L.; Cai Zhang, Y.; Huang, Q. Solvothermal synthesis of Ag/ZnO nanocomposite with enhanced photocatalytic activity. Mater. Lett. 2013, 106, 104–106. [Google Scholar] [CrossRef]
- Fernandez, C.; Thomas, A.; M, S. Green synthesis of silver oxide nanoparticle and its antimicrobial activity against organisms causing Dental plaques. Int. J. Pharma. Bio. Sci. 2016, 7, 14–19. [Google Scholar] [CrossRef]
- Bai, X.; Li, L.; Liu, H.; Tan, L.; Liu, T.; Meng, X. Solvothermal Synthesis of ZnO Nanoparticles and Anti-Infection Application in Vivo. ACS Appl. Mater. Interfaces 2015, 7, 1308–1317. [Google Scholar] [CrossRef] [PubMed]
- Salvadori, M.R.; Ando, R.A.; Nascimento, C.A.O.; Corrêa, B. Dead biomass of Amazon yeast: A new insight into bioremediation and recovery of silver by intracellular synthesis of nanoparticles. J. Environ. Sci. Health Part A 2017, 52, 1112–1120. [Google Scholar] [CrossRef]
- Vinay, S.P.; Udayabhanu; Nagaraju, G.; Chandrappa, C.P.; Chandrasekhar, N. Rauvolfia tetraphylla (Devil Pepper)-Mediated Green Synthesis of Ag Nanoparticles: Applications to Anticancer, Antioxidant and Antimitotic. J. Clust. Sci. 2019, 30, 1545–1564. [Google Scholar] [CrossRef]
- Fard, N.N.; Noorbazargan, H.; Mirzaie, A.; Hedayati Ch, M.; Moghimiyan, Z.; Rahimi, A. Biogenic synthesis of AgNPs using Artemisia oliveriana extract and their biological activities for an effective treatment of lung cancer. Artif. Cells Nanomed. Biotechnol. 2018, 46 (Suppl. S3), S1047–S1058. [Google Scholar] [CrossRef]
- Schlinkert, P.; Casals, E.; Boyles, M.; Tischler, U.; Hornig, E.; Tran, N.; Zhao, J.; Himly, M.; Riediker, M.; Oostingh, G.J.; et al. The oxidative potential of differently charged silver and gold nanoparticles on three human lung epithelial cell types. J. Nanobiotechnol. 2015, 13, 1. [Google Scholar] [CrossRef]
- Guo, C.; Buckley, A.; Marczylo, T.; Seiffert, J.; Römer, I.; Warren, J.; Hodgson, A.; Chung, K.F.; Gant, T.W.; Smith, R.; et al. The small airway epithelium as a target for the adverse pulmonary effects of silver nanoparticle inhalation. Nanotoxicology 2018, 12, 539–553. [Google Scholar] [CrossRef]
- Yeasmin, S.; Datta, H.K.; Chaudhuri, S.; Malik, D.; Bandyopadhyay, A. In-vitro anti-cancer activity of shape controlled silver nanoparticles (AgNPs) in various organ specific cell lines. J. Mol. Liq. 2017, 242, 757–766. [Google Scholar] [CrossRef]
- Miyayama, T.; Fujiki, K.; Matsuoka, M. Silver nanoparticles induce lysosomal-autophagic defects and decreased expression of transcription factor EB in A549 human lung adenocarcinoma cells. Toxicol. Vitr. Int. J. Publ. Assoc. BIBRA 2018, 46, 148–154. [Google Scholar] [CrossRef]
- Akter, M.; Sikder, M.T.; Rahman, M.M.; Ullah, A.; Hossain, K.F.B.; Banik, S.; Hosokawa, T.; Saito, T.; Kurasaki, M. A systematic review on silver nanoparticles-induced cytotoxicity: Physicochemical properties and perspectives. J. Adv. Res. 2018, 9, 1–16. [Google Scholar] [CrossRef]
- González-Vega, J.G.; García-Ramos, J.C.; Chavez-Santoscoy, R.A.; Castillo-Quiñones, J.E.; Arellano-Garcia, M.E.; Toledano-Magaña, Y. Lung Models to Evaluate Silver Nanoparticles’ Toxicity and Their Impact on Human Health. Nanomaterials 2022, 12, 2316. [Google Scholar] [CrossRef]
- García, M.C.; Torres, J.; Dan Córdoba, A.V.; Longhi, M.; Uberman, P.M. Drug delivery using metal oxide nanoparticles Periodical Drug delivery using metal oxide nanoparticles [Online]. 2022, pp. 35–83. Available online: https://www.sciencedirect.com/science/article/pii/B9780128230336000296 (accessed on 8 May 2022). [CrossRef]
- Qureshi, A.T. Silver Nanoparticles as Drug Delivery Systems. LSU Dr. Diss. 2013, 1069. [Google Scholar] [CrossRef]
- Ghiuță, I.; Cristea, D. Silver nanoparticles for delivery purposes. In Nanoengineered Biomaterials for Advanced Drug Delivery; Mozafari, M., Ed.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 347–371. [Google Scholar] [CrossRef]
- Zhang, X.F.; Gurunathan, S. Combination of salinomycin and silver nanoparticles enhances apoptosis and autophagy in human ovarian cancer cells: An effective anticancer therapy. Int. J. Nanomed. 2016, 11, 3655–3675. [Google Scholar] [CrossRef]
- Yuan, Y.G.; Peng, Q.L.; Gurunathan, S. Silver nanoparticles enhance the apoptotic potential of gemcitabine in human ovarian cancer cells: Combination therapy for effective cancer treatment. Int. J. Nanomed. 2017, 12, 6487–6502. [Google Scholar] [CrossRef]
- Baram-Pinto, D.; Shukla, S.; Perkas, N.; Gedanken, A.; Sarid, R. Inhibition of herpes simplex virus type 1 infection by silver nanoparticles capped with mercaptoethane sulfonate. Bioconjugate Chem. 2009, 20, 1497–1502. [Google Scholar] [CrossRef]
- Ivanova, N.; Gugleva, V.; Dobreva, M.; Pehlivanov, I.; Stefanov, S.; Andonova, V. Silver Nanoparticles as Multi-Functional Drug Delivery Systems; IntechOpen: London, UK, 2019; pp. 71–91. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).