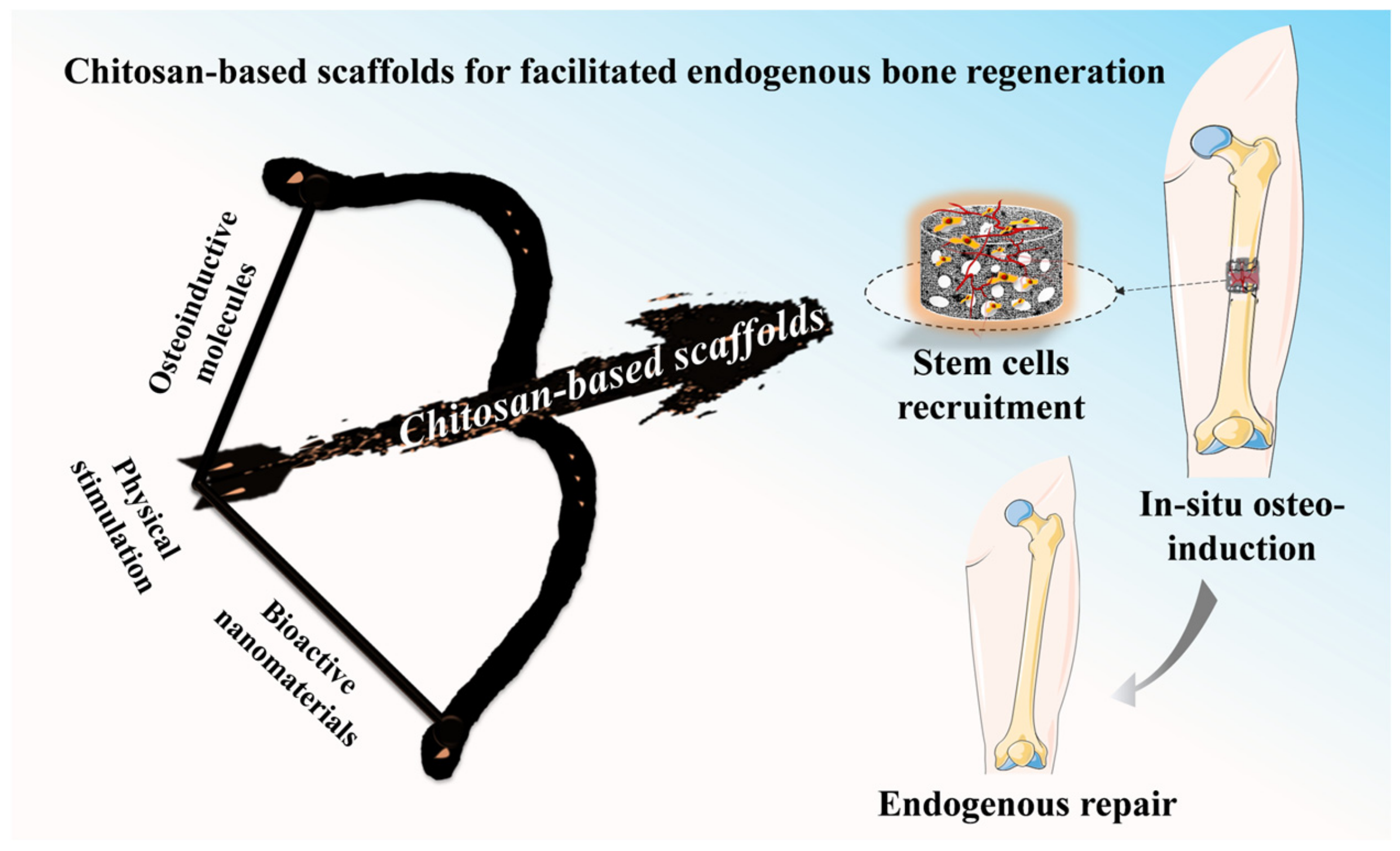
Chitosan-Based Scaffolds for Facilitated Endogenous Bone Re-Generation
Abstract
:1. Introduction
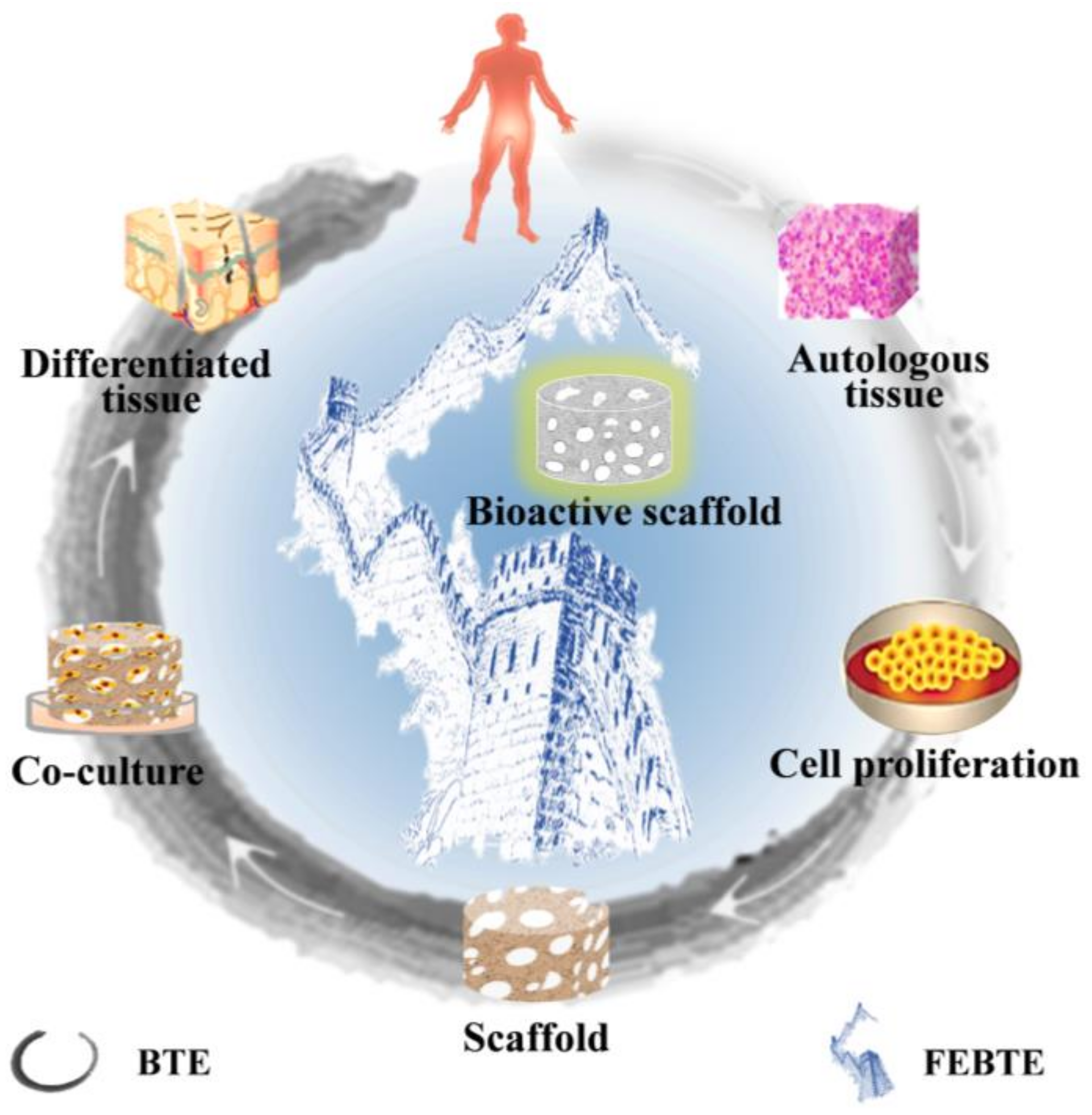
2. Bone Repair Strategies
2.1. Traditional Bone Tissue Engineering
2.2. Facilitated Endogenous Bone Tissue Engineering
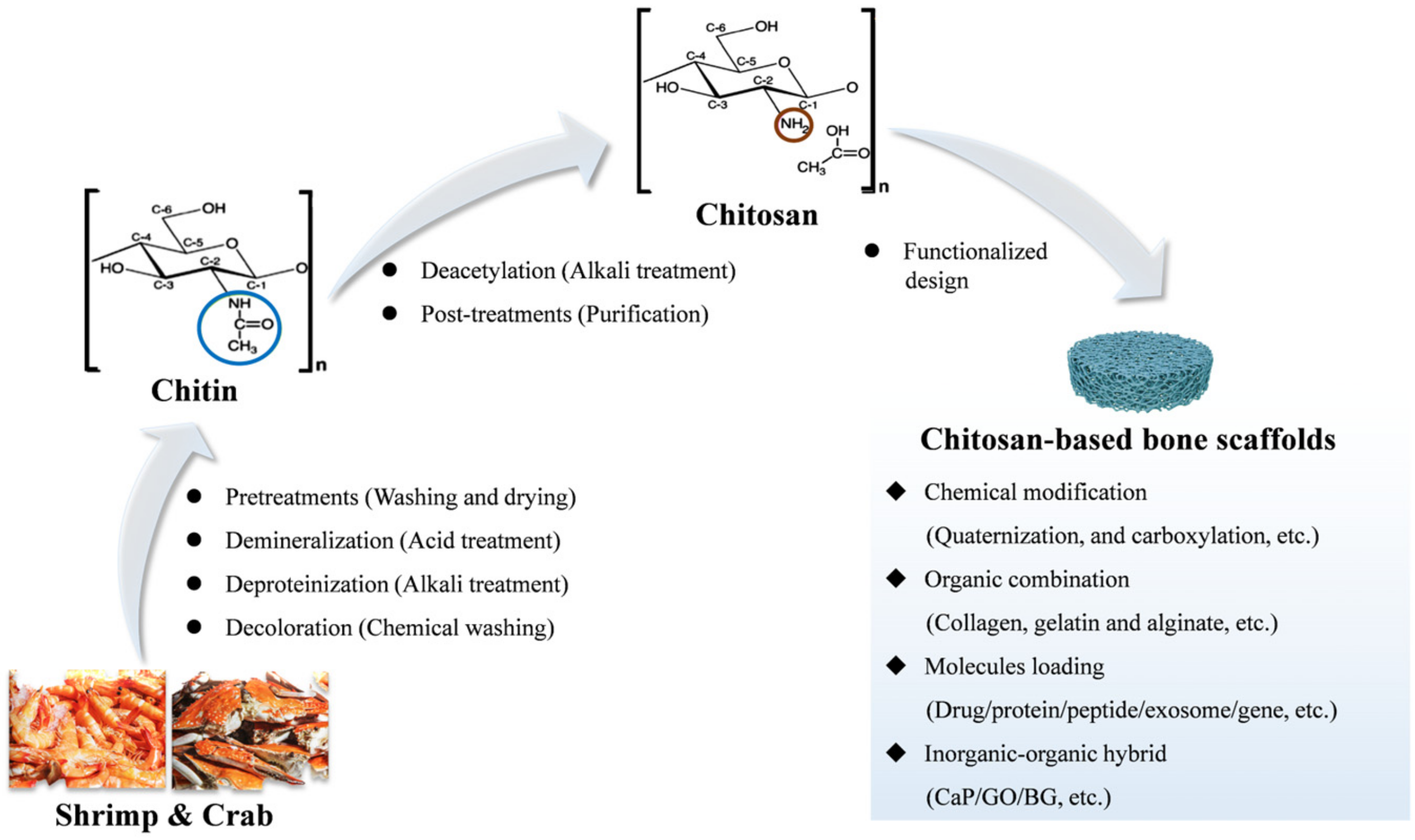
3. CS-Based Bone Repair Scaffolds and Their Fabrication Methods
3.1. Source of CS and CS-Based Bone Repair Scaffolds
3.2. Fabrication Methods
3.2.1. Freeze-Drying
3.2.2. Electrospinning
3.2.3. Three-Dimensional Printing
3.2.4. Sol-Gel Method
3.2.5. Others
4. Multifunctional Design of CS-Based Scaffolds in Bone Regenerations
4.1. CS-Based Scaffolds Integrate with Osteo-Inductive Molecules to Mediate Osteogenesis
4.1.1. Drugs
4.1.2. Proteins/Peptides
4.1.3. Exosomes
4.1.4. Genes
| Types | Molecules | Composite Matrix | Key Results | Ref. |
|---|---|---|---|---|
| Drugs | Icariin | Carboxymethyl CS/HAP/poly(lactide-co-glycolide) | Improved the adhesion, proliferation and differentiation of MC3T3-E1 and finally achieved the repair of bone defects. | [123] |
| Ursolic acid | MHAP/CS | Upregulated the expression of osteogenic-related genes through promoting the M2-type polarization of macrophages. | [122] | |
| Chrysin | CS/carboxymethyl Cellulose/HAP | Stimulated cell proliferation and promoted osteoblast differentiation. | [39] | |
| Proteins | VEGF, BMP-4 | Gelatin/CS | Induced bone regeneration by angiogenesis and osteogenesis. | [125] |
| BMP-2 | PCL/carboxymethyl chitosan | Supported the proliferation, differentiation and ossification of hBMSCs. | [148] | |
| BMP-2, insulin-like growth factor-1 | CS/gelatin | Significantly enhanced osteoblastic differentiation. | [149] | |
| Peptides | FRHRNRKGY (HVP), GRGDSPK (RGD) | CS | Increased osteoblast adhesion, proliferation differentiation and calcium deposition. | [43] |
| Parathyroid hormone-derived peptide | CS/HAP | Remarkably stimulated new bone formation in rabbit radial defects (size: 1.5 cm). | [72] | |
| Exosomes | Pulp stem cell-derived exosomes (DPSC-Exo) | CS | Greatly facilitated the repair of alveolar bone and treated the periodontitis. | [139] |
| Human umbilical cord mesenchymal stem cells-derived exosome | HAP/silk fibroin/glycol CS/polyethylene glycol | Effectively recruited stem cells, promoted their proliferation and osteogenic differentiation, and finally mediated bone repair. | [140] | |
| hMSCs-derived exosome | CS | Significantly increased osteogenic induction, promoted calvarial bone repair. | [138] | |
| Genes | microRNA (siFlt-1+siCkip-1) | CS | Enhanced the osteogenesis and angiogenesis, finally promoted new bone regeneration in vivo. | [146] |
| miR-24 | CS/gelatin | Promoted osteogenic differentiation and skull defect regeneration in vivo. | [150] | |
| miR-590-5p | CS/HAP/nano-ZrO2 | Upregulated osteogenic genes (RUNX2, COL I, ALP) expression and promoted osteoblast differentiation. | [151] |
4.2. CS-Based Scaffolds Functionalized with Bioactive Nanomaterials to Induce Osteogenesis
4.2.1. Calcium Phosphate
4.2.2. Bioactive Glass
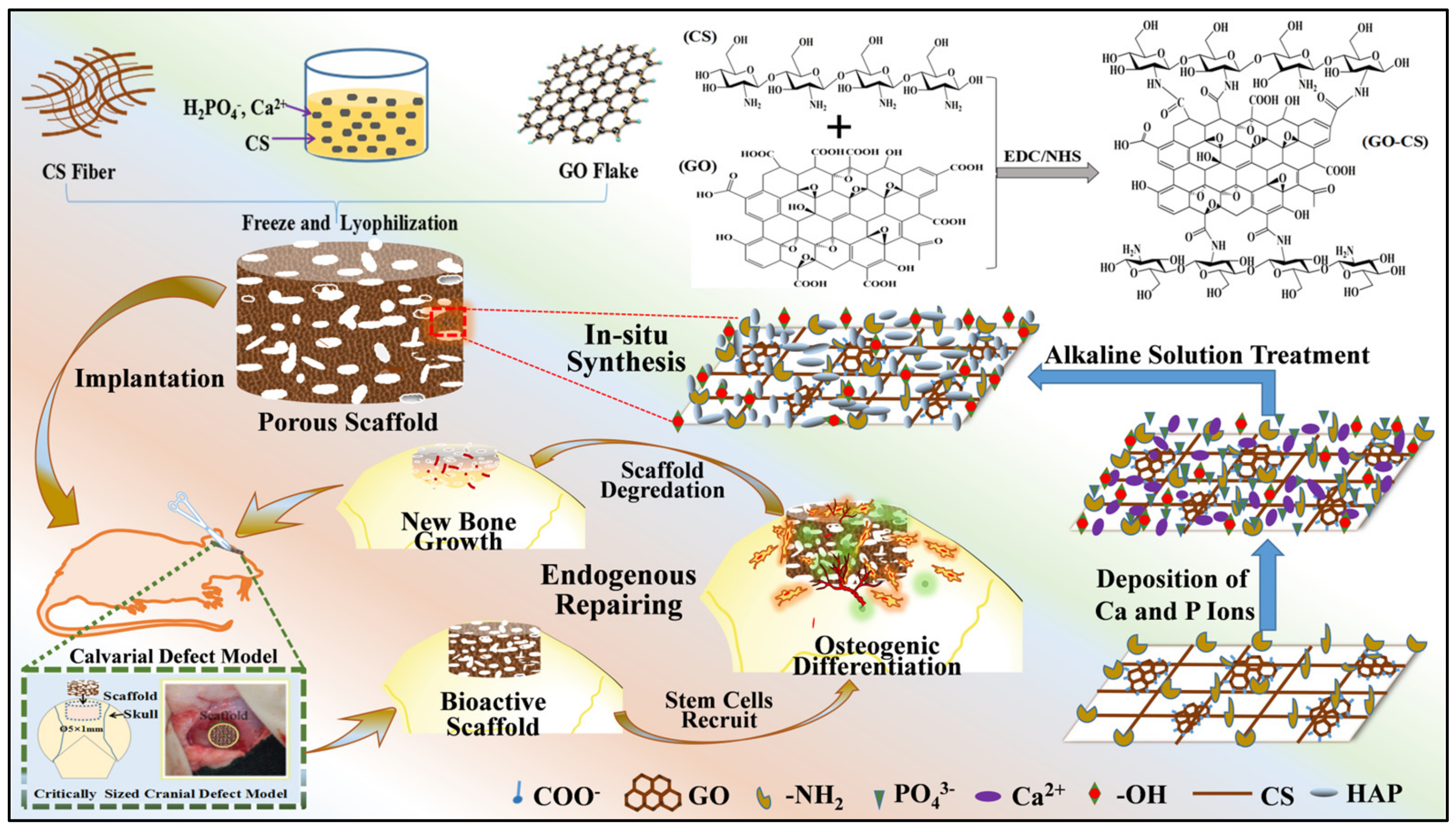
4.2.3. Carbon-Based Nanomaterials
4.2.4. Gadolinium Orthophosphate
4.2.5. Silica Minerals
4.3. CS-Based Scaffolds Synergize with Physical Stimulation to Promote Osteogenesis
4.3.1. Hyperthermia Stimulation
4.3.2. Magnetic Stimulation
4.3.3. Electrical Stimulation
4.3.4. Photobiomodulation
5. Conclusions and Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wegst, U.G.K.; Bai, H.; Saiz, E.; Tomsia, A.P.; Ritchie, R.O. Bioinspired structural materials. Nat. Mater. 2015, 14, 23–36. [Google Scholar] [CrossRef]
- Farokhi, M.; Mottaghitalab, F.; Samani, S.; Shokrgozar, M.A.; Kundu, S.C.; Reis, R.L.; Fatahi, Y.; Kaplan, D.L. Silk fibroin/hydroxyapatite composites for bone tissue engineering. Biotechnol. Adv. 2018, 36, 68–91. [Google Scholar] [CrossRef]
- Wu, M.; Zhao, Y.; Jiang, H.; Xu, X.; Wang, D.; Xu, X.; Zhou, Y.; Tan, H.; Ding, C.; Li, J. Self-Organized Spatiotemporal Mineralization of Hydrogel: A Simulant of Osteon. Small 2022, 18, e2106649. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, S.; Luo, D.; Xue, Z.; Yang, X.; Gu, L.; Zhou, Y.; Wang, T. Hierarchically Staggered Nanostructure of Mineralized Collagen as a Bone-Grafting Scaffold. Adv. Mater. 2016, 28, 8740–8748. [Google Scholar] [CrossRef]
- Li, X.; Wei, L.; Li, J.; Shao, J.; Yi, B.; Zhang, C.; Liu, H.; Ma, B.; Ge, S. Multifunctional SDF-1-loaded hydroxyapatite/polylactic acid membranes promote cell recruitment, immunomodulation, angiogenesis, and osteogenesis for biomimetic bone regeneration. Appl. Mater. Today 2021, 22, 100942. [Google Scholar] [CrossRef]
- Koons, G.L.; Diba, M.; Mikos, A.G. Materials design for bone-tissue engineering. Nat. Rev. Mater. 2020, 5, 584–603. [Google Scholar] [CrossRef]
- Qu, H.; Fu, H.; Han, Z.; Sun, Y. Biomaterials for bone tissue engineering scaffolds: A review. RSC Adv. 2019, 9, 26252–26262. [Google Scholar] [CrossRef]
- Oryan, A.; Alidadi, S.; Moshiri, A.; Maffulli, N. Bone regenerative medicine: Classic options, novel strategies, and future directions. J. Orthop. Surg. Res. 2014, 9, 18. [Google Scholar] [CrossRef]
- Bose, S.; Roy, M.; Bandyopadhyay, A. Recent advances in bone tissue engineering scaffolds. Trends Biotechnol. 2012, 30, 546–554. [Google Scholar] [CrossRef]
- Amini, A.R.; Laurencin, C.T.; Nukavarapu, S.P. Bone tissue engineering: Recent advances and challenges. Crit. Rev. Biomed. Eng. 2012, 40, 363–408. [Google Scholar] [CrossRef]
- Evans, C.H.; Palmer, G.D.; Pascher, A.; Porter, R.; Kwong, F.N.; Gouze, E.; Gouze, J.N.; Liu, F.; Steinert, A.; Betz, O.; et al. Facilitated endogenous repair: Making tissue engineering simple, practical, and economical. Tissue Eng. 2007, 13, 1987–1993. [Google Scholar] [CrossRef] [PubMed]
- Yao, Q.; Liu, Y.; Tao, J.; Baumgarten, K.M.; Sun, H. Hypoxia-Mimicking Nanofibrous Scaffolds Promote Endogenous Bone Regeneration. ACS Appl. Mater. Interfaces 2016, 8, 32450–32459. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhang, Y.; Pan, P.; Fan, T.; Chen, M.; Zhang, Q. In situ strategy for bone repair by facilitated endogenous tissue engineering. Colloids Surf. B Biointerfaces 2015, 135, 581–587. [Google Scholar] [CrossRef]
- Wang, Q.; Tang, Y.; Ke, Q.; Yin, W.; Zhang, C.; Guo, Y.; Guan, J. Magnetic lanthanum-doped hydroxyapatite/chitosan scaffolds with endogenous stem cell-recruiting and immunomodulatory properties for bone regeneration. J. Mater. Chem. B 2020, 8, 5280–5292. [Google Scholar] [CrossRef]
- Zhao, Y.; Chen, J.; Zou, L.; Xu, G.; Geng, Y. Facile one-step bioinspired mineralization by chitosan functionalized with graphene oxide to activate bone endogenous regeneration. Chem. Eng. J. 2019, 378, 122174. [Google Scholar] [CrossRef]
- Collins, M.N.; Ren, G.; Young, K.; Pina, S.; Reis, R.L.; Oliveira, J.M. Scaffold fabrication technologies and structure/function properties in bone tissue engineering. Adv. Funct. Mater. 2021, 31, 2010609. [Google Scholar] [CrossRef]
- Turnbull, G.; Clarke, J.; Picard, F.; Riches, P.; Jia, L.; Han, F.; Li, B.; Shu, W. 3D bioactive composite scaffolds for bone tissue engineering. Bioact. Mater. 2018, 3, 278–314. [Google Scholar] [CrossRef]
- Wang, S.; Yang, Y.; Zhao, Z.; Wang, X.; Mikos, A.G.; Qiu, Z.; Song, T.; Sun, X.; Zhao, L.; Zhang, C.; et al. Mineralized Collagen-Based Composite Bone Materials for Cranial Bone Regeneration in Developing Sheep. ACS Biomater. Sci. Eng. 2017, 3, 1092–1099. [Google Scholar] [CrossRef]
- Wang, S.; Zhao, Z.; Yang, Y.; Mikos, A.G.; Qiu, Z.; Song, T.; Cui, F.; Wang, X.; Zhang, C. A high-strength mineralized collagen bone scaffold for large-sized cranial bone defect repair in sheep. Regen. Biomater. 2018, 5, 283–292. [Google Scholar] [CrossRef]
- Torgbo, S.; Sukyai, P. Bacterial cellulose-based scaffold materials for bone tissue engineering. Appl. Mater. Today 2018, 11, 34–49. [Google Scholar] [CrossRef]
- Gambari, L.; Amore, E.; Raggio, R.; Bonani, W.; Barone, M.; Lisignoli, G.; Grigolo, B.; Motta, A.; Grassi, F. Hydrogen sulfide-releasing silk fibroin scaffold for bone tissue engineering. Mater. Sci. Eng. C 2019, 102, 471–482. [Google Scholar] [CrossRef]
- Park, S.H.; Park, J.Y.; Ji, Y.B.; Ju, H.J.; Min, B.H.; Kim, M.S. An injectable click-crosslinked hyaluronic acid hydrogel modified with a BMP-2 mimetic peptide as a bone tissue engineering scaffold. Acta Biomater. 2020, 117, 108–120. [Google Scholar] [CrossRef]
- Janmohammadi, M.; Nazemi, Z.; Salehi, A.O.M.; Seyfoori, A.; John, J.V.; Nourbakhsh, M.S.; Akbari, M. Cellulose-based composite scaffolds for bone tissue engineering and localized drug delivery. Bioact. Mater. 2023, 20, 137–163. [Google Scholar] [CrossRef] [PubMed]
- Deshpande, R.; Shukla, S.; Sayyad, R.; Salunke, S.; Nisal, A.; Venugopalan, P. Silk fibroin and ceramic scaffolds: Comparative in vitro studies for bone regeneration. Bioeng.Transl. Med. 2021, 6, e10221. [Google Scholar] [CrossRef]
- Abdallah, M.M.; Fernández, N.; Matias, A.A.; Bronze, M.d.R. Hyaluronic acid and Chondroitin sulfate from marine and terrestrial sources: Extraction and purification methods. Carbohydr. Polym. 2020, 243, 116441. [Google Scholar] [CrossRef]
- Pakizeh, M.; Moradi, A.; Ghassemi, T. Chemical extraction and modification of chitin and chitosan from shrimp shells. Eur. Polym. J. 2021, 159, 110709. [Google Scholar] [CrossRef]
- Hu, D.; Ren, Q.; Li, Z.; Zhang, L. Chitosan-based biomimetically mineralized composite materials in human hard tissue repair. Molecules 2020, 25, 4785. [Google Scholar] [CrossRef]
- Levengood, S.L.; Zhang, M. Chitosan-based scaffolds for bone tissue engineering. J. Mater. Chem. B 2014, 2, 3161–3184. [Google Scholar] [CrossRef]
- LogithKumar, R.; KeshavNarayan, A.; Dhivya, S.; Chawla, A.; Saravanan, S.; Selvamurugan, N. A review of chitosan and its derivatives in bone tissue engineering. Carbohydr. Polym. 2016, 151, 172–188. [Google Scholar] [CrossRef]
- Tian, Y.; Wu, D.; Wu, D.; Cui, Y.; Ren, G.; Wang, Y.; Wang, J.; Peng, C. Chitosan-Based Biomaterial Scaffolds for the Repair of Infected Bone Defects. Front. Bioeng. Biotechnol. 2022, 10, 899760. [Google Scholar] [CrossRef]
- Querido, D.; Vieira, T.; Ferreira, J.L.; Henriques, C.; Borges, J.P.; Silva, J.C. Study on the incorporation of chitosan flakes in electrospun polycaprolactone scaffolds. Polymers 2022, 14, 1496. [Google Scholar] [CrossRef]
- Xu, T.; Yang, H.; Yang, D.; Yu, Z.Z. Polylactic acid nanofiber scaffold decorated with chitosan islandlike topography for bone tissue engineering. ACS Appl. Mater. Interfaces 2017, 9, 21094–21104. [Google Scholar] [CrossRef]
- Amirian, J.; Tripathi, G.; Kang, H.-J.; Lee, B.-T. Porous BMP-2 immobilized PLGA/Glycol chitosan scaffold with enhanced hydrophilicity, mineralization and osteogenesis. Mater. Lett. 2022, 308, 131140. [Google Scholar] [CrossRef]
- Tao, F.; Cheng, Y.; Tao, H.; Jin, L.; Wan, Z.; Dai, F.; Xiang, W.; Deng, H. Carboxymethyl chitosan/sodium alginate-based micron-fibers fabricated by emulsion electrospinning for periosteal tissue engineering. Mater. Des. 2020, 194, 108849. [Google Scholar] [CrossRef]
- Yang, Y.; Ao, H.; Wang, Y.; Lin, W.; Yang, S.; Zhang, S.; Yu, Z.; Tang, T. Cytocompatibility with osteogenic cells and enhanced in vivo anti-infection potential of quaternized chitosan-loaded titania nanotubes. Bone Res. 2016, 4, 16027. [Google Scholar] [CrossRef]
- Cui, Z.-K.; Kim, S.; Baljon, J.J.; Wu, B.M.; Aghaloo, T.; Lee, M. Microporous methacrylated glycol chitosan-montmorillonite nanocomposite hydrogel for bone tissue engineering. Nat. Commun. 2019, 10, 3523. [Google Scholar] [CrossRef]
- Zhang, S.; Zhao, G.; Ma, W.; Song, Y.; Huang, C.; Xie, C.; Chen, K.; Li, X. The root-like chitosan nanofiber porous scaffold cross-linked by genipin with type I collagen and its osteoblast compatibility. Carbohydr. Polym. 2022, 285, 119255. [Google Scholar] [CrossRef]
- Menon, A.H.; Soundarya, S.P.; Sanjay, V.; Chandran, S.V.; Balagangadharan, K.; Selvamurugan, N. Sustained release of chrysin from chitosan-based scaffolds promotes mesenchymal stem cell proliferation and osteoblast differentiation. Carbohydr. Polym. 2018, 195, 356–367. [Google Scholar] [CrossRef]
- Stevanović, M.; Djošić, M.; Janković, A.; Kojić, V.; Vukašinović-Sekulić, M.; Stojanović, J.; Odović, J.; Crevar Sakač, M.; Kyong Yop, R.; Mišković-Stanković, V. Antibacterial graphene-based hydroxyapatite/chitosan coating with gentamicin for potential applications in bone tissue engineering. J. Biomed. Mater. Res. A 2020, 108, 2175–2189. [Google Scholar] [CrossRef]
- Ding, T.; Kang, W.; Li, J.; Yu, L.; Ge, S. An in situ tissue engineering scaffold with growth factors combining angiogenesis and osteoimmunomodulatory functions for advanced periodontal bone regeneration. J. Nanobiotechnol. 2021, 19, 247. [Google Scholar] [CrossRef]
- Zeng, R.; Tu, M.; Liu, H.; Zhao, J.; Zha, Z.; Zhou, C. Preparation, structure, drug release and bioinspired mineralization of chitosan-based nanocomplexes for bone tissue engineering. Carbohydr. Polym. 2009, 78, 107–111. [Google Scholar] [CrossRef]
- Brun, P.; Zamuner, A.; Battocchio, C.; Cassari, L.; Todesco, M.; Graziani, V.; Iucci, G.; Marsotto, M.; Tortora, L.; Secchi, V.; et al. Bio-Functionalized Chitosan for Bone Tissue Engineering. Int. J. Mol. Sci. 2021, 22, 5916. [Google Scholar] [CrossRef]
- Zhao, Y.; Fan, T.; Chen, J.; Su, J.; Zhi, X.; Pan, P.; Zou, L.; Zhang, Q. Magnetic bioinspired micro/nanostructured composite scaffold for bone regeneration. Colloids Surf. B 2019, 174, 70–79. [Google Scholar] [CrossRef]
- Patel, A.; Zaky, S.H.; Schoedel, K.; Li, H.; Sant, V.; Beniash, E.; Sfeir, C.; Stolz, D.B.; Sant, S. Design and evaluation of collagen-inspired mineral-hydrogel nanocomposites for bone regeneration. Acta Biomater. 2020, 112, 262–273. [Google Scholar] [CrossRef]
- Cao, S.; Zhao, Y.; Hu, Y.; Zou, L.; Chen, J. New perspectives: In-situ tissue engineering for bone repair scaffold. Compos. B Eng. 2020, 202, 108445. [Google Scholar] [CrossRef]
- Wang, S.; Yang, Y.; Koons, G.L.; Mikos, A.G.; Qiu, Z.; Song, T.; Cui, F.; Wang, X. Tuning pore features of mineralized collagen/PCL scaffolds for cranial bone regeneration in a rat model. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 106, 110186. [Google Scholar] [CrossRef]
- Crane, G.M.; Ishaug, S.L.; Mikos, A.G. Bone tissue engineering. Nat. Med. 1995, 1, 1322–1324. [Google Scholar] [CrossRef]
- Santin, M. 14—Bone tissue engineering. In Bone Repair Biomaterials; Planell, J.A., Best, S.M., Lacroix, D., Merolli, A., Eds.; Woodhead Publishing Series in Biomaterials: Brighton, UK, 2009; Volume 14, pp. 378–422. [Google Scholar]
- Brown, J.L.; Kumbar, S.G.; Laurencin, C.T. Chapter II.6.7—Bone tissue engineering. In Biomaterials Science, 3rd ed.; Ratner, B.D., Hoffman, A.S., Schoen, F.J., Lemons, J.E., Eds.; Academic Press: Cambridge, MA, USA, 2013; pp. 1194–1214. [Google Scholar]
- Datta, S.; Rameshbabu, A.P.; Bankoti, K.; Roy, M.; Gupta, C.; Jana, S.; Das, A.K.; Sen, R.; Dhara, S. Decellularized bone matrix/oleoyl chitosan derived supramolecular injectable hydrogel promotes efficient bone integration. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 119, 111604. [Google Scholar] [CrossRef]
- Bouchie, A. Tissue engineering firms go under. Nat. Biotechnol. 2002, 20, 1178–1179. [Google Scholar] [CrossRef]
- Yang, H.; Wang, S.; Bian, H.; Xing, X.; Yu, J.; Wu, X.; Zhang, L.; Liang, X.; Lu, A.; Huang, C. Extracellular matrix-mimicking nanofibrous chitosan microspheres as cell micro-ark for tissue engineering. Carbohydr. Polym. 2022, 292, 119693. [Google Scholar] [CrossRef]
- Raftery, R.M.; Woods, B.; Marques, A.L.P.; Moreira-Silva, J.; Silva, T.H.; Cryan, S.-A.; Reis, R.L.; O’Brien, F.J. Multifunctional biomaterials from the sea: Assessing the effects of chitosan incorporation into collagen scaffolds on mechanical and biological functionality. Acta Biomater. 2016, 43, 160–169. [Google Scholar] [CrossRef]
- Islam, S.; Bhuiyan, M.A.R.; Islam, M.N. Chitin and chitosan: Structure, properties and applications in biomedical engineering. J. Polym. Environ. 2017, 25, 854–866. [Google Scholar] [CrossRef]
- Horton, D.; Lineback, D.R. N-Deacetylation and depolymerization reactions of chitin/chitosan: Influence of the source of chitin. Carbohydr. Polym. 2005, 62, 316–320. [Google Scholar]
- Al Sagheer, F.A.; Al Sughayer, M.A.; Muslim, S.; Elsabee, M.Z. Application of spectroscopic methods for structural analysis of chitin and chitosan. Carbohydr. Polym. 2009, 77, 410–419. [Google Scholar]
- Alishahi, A.; Mirvaghefi, A.; Tehrani, M.R.; Farahmand, H.; Shojaosadati, S.A.; Dorkoosh, F.A.; Elsabee, M.Z. Enhancement and Characterization of Chitosan Extraction from the Wastes of Shrimp Packaging Plants. J. Polym. Environ. 2011, 19, 776–783. [Google Scholar] [CrossRef]
- Bajaj, M.; Winter, J.; Gallert, C. Effect of deproteination and deacetylation conditions on viscosity of chitin and chitosan extracted from Crangon crangon shrimp waste. Biochem. Eng. J. 2011, 56, 51–626. [Google Scholar] [CrossRef]
- Datta, P.; Dhara, S.; Chatterjee, J. Hydrogels and electrospun nanofibrous scaffolds of N-methylene phosphonic chitosan as bioinspired osteoconductive materials for bone grafting. Carbohydr. Polym. 2012, 87, 1354–1362. [Google Scholar] [CrossRef]
- Patel, D.K.; Dutta, S.D.; Hexiu, J.; Ganguly, K.; Lim, K.-T. 3D-printable chitosan/silk fibroin/cellulose nanoparticle scaffolds for bone regeneration via M2 macrophage polarization. Carbohydr. Polym. 2022, 281, 119077. [Google Scholar] [CrossRef]
- Ma, J.; Wang, Y.; Lu, R. Mechanism and application of chitosan and Its derivatives in promoting permeation in transdermal drug delivery systems: A review. Pharmaceuticals 2022, 15, 459. [Google Scholar] [CrossRef]
- Flórez, M.; Guerra-Rodríguez, E.; Cazón, P.; Vázquez, M. Chitosan for food packaging: Recent advances in active and intelligent films. Food Hydrocoll. 2022, 124, 107328. [Google Scholar] [CrossRef]
- Cao, Z.; Bai, X.; Wang, C.; Ren, L.; Ma, D. A simple polysaccharide based injectable hydrogel compositing nano-hydroxyapatite for bone tissue engineering. Mater. Lett. 2021, 293, 129755. [Google Scholar] [CrossRef]
- Jiang, T.; Abdel-Fattah, W.I.; Laurencin, C.T. In vitro evaluation of chitosan/poly(lactic acid-glycolic acid) sintered microsphere scaffolds for bone tissue engineering. Biomaterials 2006, 27, 4894–4903. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.B.; Xu, C.P.; Li, W.Q.; Meng, Q.J.; Qu, H.Z. Halloysites modified polyethylene glycol diacrylate/thiolated chitosan double network hydrogel combined with BMP-2 for rat skull regeneration. Artif. Cells Nanomed. Biotechnol. 2021, 49, 71–82. [Google Scholar] [CrossRef] [PubMed]
- Esmaeili, J.; Jadbabaee, S.; Far, F.M.; Lukolayeh, M.E.; Kırboğa, K.K.; Rezaei, F.S.; Barati, A. Decellularized Alstroemeria flower stem modified with chitosan for tissue engineering purposes: A cellulose/chitosan scaffold. Int. J. Biol. Macromol. 2022, 204, 321–332. [Google Scholar] [CrossRef] [PubMed]
- Lai, G.-J.; Shalumon, K.T.; Chen, S.-H.; Chen, J.-P. Composite chitosan/silk fibroin nanofibers for modulation of osteogenic differentiation and proliferation of human mesenchymal stem cells. Carbohydr. Polym. 2014, 111, 288–297. [Google Scholar] [CrossRef]
- Liu, L.; He, Y.; Shi, X.; Gao, H.; Wang, Y.; Lin, Z. Phosphocreatine-modified chitosan porous scaffolds promote mineralization and osteogenesis in vitro and in vivo. Appl. Mater. Today 2018, 12, 21–33. [Google Scholar] [CrossRef]
- Chen, Y.; Sheng, W.; Lin, J.; Fang, C.; Deng, J.; Zhang, P.; Zhou, M.; Liu, P.; Weng, J.; Yu, F.; et al. Magnesium oxide nanoparticle coordinated phosphate-functionalized chitosan injectable hydrogel for osteogenesis and angiogenesis in bone regeneration. ACS Appl. Mater. Interfaces 2022, 14, 7592–7608. [Google Scholar] [CrossRef]
- Yilgor, P.; Tuzlakoglu, K.; Reis, R.L.; Hasirci, N.; Hasirci, V. Incorporation of a sequential BMP-2/BMP-7 delivery system into chitosan-based scaffolds for bone tissue engineering. Biomaterials 2009, 30, 3551–3559. [Google Scholar] [CrossRef]
- Yang, L.; Huang, J.; Yang, S.; Cui, W.; Wang, J.; Zhang, Y.; Li, J.; Guo, X. Bone regeneration induced by local delivery of a modified PTH-derived peptide from nanohydroxyapatite/chitosan coated true bone ceramics. ACS Biomater. Sci. Eng. 2018, 4, 3246–3258. [Google Scholar] [CrossRef]
- Khoshakhlagh, P.; Rabiee, S.M.; Kiaee, G.; Heidari, P.; Miri, A.K.; Moradi, R.; Moztarzadeh, F.; Ravarian, R. Development and characterization of a bioglass/chitosan composite as an injectable bone substitute. Carbohydr. Polym. 2017, 157, 1261–1271. [Google Scholar] [CrossRef]
- Zhao, C.; Qazvini, N.T.; Sadati, M.; Zeng, Z.; Huang, S.; De La Lastra, A.L.; Zhang, L.; Feng, Y.; Liu, W.; Huang, B.; et al. A pH-triggered, self-assembled, and bioprintable hybrid hydrogel scaffold for mesenchymal stem cell based bone tissue engineering. ACS Appl. Mater. Interfaces 2019, 11, 8749–8762. [Google Scholar] [CrossRef] [PubMed]
- Ateş, G.B.; Ak, A.; Garipcan, B.; Gülsoy, M. Methylene blue mediated photobiomodulation on human osteoblast cells. Lasers Med. Sci. 2017, 32, 1847–1855. [Google Scholar] [CrossRef] [PubMed]
- Shaabani, A.; Sedghi, R. Preparation of chitosan biguanidine/PANI-containing self-healing semi-conductive waterborne scaffolds for bone tissue engineering. Carbohydr. Polym. 2021, 264, 118045. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, J.; Lin, S.; Dong, L.; Cheng, K.; Weng, W. Magnetically actuated mechanical stimuli on Fe3O4/mineralized collagen coatings to enhance osteogenic differentiation of the MC3T3-E1 cells. Acta Biomater. 2018, 71, 49–60. [Google Scholar] [CrossRef]
- Mahanta, A.K.; Patel, D.K.; Maiti, P. Nanohybrid scaffold of chitosan and functionalized graphene oxide for controlled drug delivery and bone regeneration. ACS Biomater. 2019, 5, 5139–5149. [Google Scholar] [CrossRef]
- Liu, Y.; Fang, N.; Liu, B.; Song, L.; Wen, B.; Yang, D. Aligned porous chitosan/graphene oxide scaffold for bone tissue engineering. Mater. Lett. 2018, 233, 78–81. [Google Scholar] [CrossRef]
- Shrestha, S.; Shrestha, B.K.; Ko, S.W.; Kandel, R.; Park, C.H.; Kim, C.S. Engineered cellular microenvironments from functionalized multiwalled carbon nanotubes integrating Zein/Chitosan @Polyurethane for bone cell regeneration. Carbohydr. Polym. 2021, 251, 117035. [Google Scholar] [CrossRef]
- Liu, H.; Peng, H.; Wu, Y.; Zhang, C.; Cai, Y.; Xu, G.; Li, Q.; Chen, X.; Ji, J.; Zhang, Y.; et al. The promotion of bone regeneration by nanofibrous hydroxyapatite/chitosan scaffolds by effects on integrin-BMP/Smad signaling pathway in BMSCs. Biomaterials 2013, 34, 4404–4417. [Google Scholar] [CrossRef]
- Januariyasa, I.K.; Ana, I.D.; Yusuf, Y. Nanofibrous poly(vinyl alcohol)/chitosan contained carbonated hydroxyapatite nanoparticles scaffold for bone tissue engineering. Mater. Sci. Eng. C 2020, 107, 110347. [Google Scholar] [CrossRef]
- Torres, P.M.C.; Ribeiro, N.; Nunes, C.M.M.; Rodrigues, A.F.M.; Sousa, A.; Olhero, S.M. Toughening robocast chitosan/biphasic calcium phosphate composite scaffolds with silk fibroin: Tuning printable inks and scaffold structure for bone regeneration. Biomater. Adv. 2022, 134, 112690. [Google Scholar] [CrossRef]
- Zafeiris, K.; Brasinika, D.; Karatza, A.; Koumoulos, E.; Karoussis, I.K.; Kyriakidou, K.; Charitidis, C.A. Additive manufacturing of hydroxyapatite-chitosan-genipin composite scaffolds for bone tissue engineering applications. Mater. Sci. Eng. C 2021, 119, 111639. [Google Scholar] [CrossRef]
- Ma, W.; Zhang, S.; Xie, C.; Wan, X.; Li, X.; Chen, K.; Zhao, G. Preparation of high mechanical strength chitosan nanofiber/NanoSiO2/PVA composite scaffolds for bone tissue engineering using Sol-gel method. Polymers 2022, 14, 2083. [Google Scholar] [CrossRef] [PubMed]
- Correia, C.O.; Leite, Á.J.; Mano, J.F. Chitosan/bioactive glass nanoparticles scaffolds with shape memory properties. Carbohydr. Polym. 2015, 123, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Kaynak Bayrak, G.; Demirtaş, T.T.; Gümüşderelioğlu, M. Microwave-induced biomimetic approach for hydroxyapatite coatings of chitosan scaffolds. Carbohydr. Polym. 2017, 157, 803–813. [Google Scholar] [CrossRef]
- Pitrolino, K.A.; Felfel, R.M.; Pellizzeri, L.M.; Mlaren, J.; Popov, A.A.; Sottile, V.; Scotchford, C.A.; Scammell, B.E.; Roberts, G.A.F.; Grant, D.M. Development and in vitro assessment of a bi-layered chitosan-nano-hydroxyapatite osteochondral scaffold. Carbohydr. Polym. 2022, 282, 119126. [Google Scholar] [CrossRef] [PubMed]
- Fereshteh, Z. 7—Freeze-drying technologies for 3D scaffold engineering. In Functional 3D Tissue Tngineering Scaffolds; Deng, Y., Kuiper, J., Eds.; Woodhead Publishing: Sawston, UK, 2018; pp. 151–174. [Google Scholar]
- Tang, X.; Pikal, M.J. Design of freeze-drying processes for pharmaceuticals: Practical advice. Pharm. Res. 2004, 21, 191–200. [Google Scholar] [CrossRef]
- Das, U.; Bordoloi, R.; Ganguly, S. Freeze-drying technique and its wide application in biomedical and pharmaceutical sciences. Res. Environ. Sci. 2014, 2, 1–4. [Google Scholar]
- Prosapio, V.; Lopez-Quiroga, E. Freeze-Drying Technology in Foods. Foods 2020, 9, 920. [Google Scholar] [CrossRef]
- Haugh, M.G.; Murphy, C.M.; O’Brien, F.J. Novel freeze-drying methods to produce a range of collagen-glycosaminoglycan scaffolds with tailored mean pore sizes. Tissue Eng. Part. C Methods 2010, 16, 887–894. [Google Scholar] [CrossRef]
- Loh, Q.L.; Choong, C. Three-dimensional scaffolds for tissue engineering applications: Role of porosity and pore size. Tissue Eng. Part. B Rev. 2013, 19, 485–502. [Google Scholar] [CrossRef]
- Whang, K.; Thomas, C.H.; Healy, K.E.; Nuber, G. A novel method to fabricate bioabsorbable scaffolds. Polymer 1995, 36, 837–842. [Google Scholar] [CrossRef]
- Garcia-Amezquita, L.E.; Welti-Chanes, J.; Vergara-Balderas, F.T.; Bermúdez-Aguirre, D. Freeze-drying: The basic process. In Encyclopedia of Food and Health; Caballero, B., Finglas, P.M., Toldrá, F., Eds.; Academic Press: Oxford, UK, 2016; pp. 104–109. [Google Scholar]
- Akdere, M.; Schneiders, T. 9—Modeling of the electrospinning process. In Advances in Modeling and Simulation in Textile Engineering; Akankwasa, N.T., Veit, D., Eds.; Woodhead Publishing: Sawston, UK, 2021; pp. 237–253. [Google Scholar]
- Lim, D.J. Bone mineralization in electrospun-based bone tissue engineering. Polymers 2022, 14, 2123. [Google Scholar] [CrossRef]
- Chen, S.; Nakamoto, T.; Kawazoe, N.; Chen, G. Engineering multi-layered skeletal muscle tissue by using 3D microgrooved collagen scaffolds. Biomaterials 2015, 73, 23–31. [Google Scholar] [CrossRef]
- Gu, Q.; Hao, J.; Lu, Y.; Wang, L.; Gordon, G.; Zhou, Q. Three-dimensional bio-printing. Sci. China Life Sci. 2015, 58, 411–419. [Google Scholar] [CrossRef]
- Bose, S.; Vahabzadeh, S.; Bandyopadhyay, A. Bone tissue engineering using 3D printing. Mater. Today 2013, 16, 496–504. [Google Scholar] [CrossRef]
- Vieira de Souza, T.; Malmonge, S.M.; Santos, A.R., Jr. Development of a chitosan and hyaluronic acid hydrogel with potential for bioprinting utilization: A preliminary study. J. Biomater. Appl. 2021, 36, 358–371. [Google Scholar] [CrossRef]
- Ma, H.; Feng, C.; Chang, J.; Wu, C. 3D-printed bioceramic scaffolds: From bone tissue engineering to tumor therapy. Acta Biomater. 2018, 79, 37–59. [Google Scholar] [CrossRef]
- Wang, C.; Huang, W.; Zhou, Y.; He, L.; He, Z.; Chen, Z.; He, X.; Tian, S.; Liao, J.; Lu, B.; et al. 3D printing of bone tissue engineering scaffolds. Bioact. Mater. 2020, 5, 82–91. [Google Scholar] [CrossRef]
- Zhang, C.; Chen, Z.; Liu, J.; Wu, M.; Yang, J.; Zhu, Y.; Lu, W.W.; Ruan, C. 3D-printed pre-tapped-hole scaffolds facilitate one-step surgery of predictable alveolar bone augmentation and simultaneous dental implantation. Compos. Part B Eng. 2022, 229, 109461. [Google Scholar] [CrossRef]
- Chi, C.-Y.; Chen, C.-Y.; Huang, J.-Y.; Kuan, C.-Y.; Lin, Y.-Y.; Li, C.-H.; Yang, C.-C.; Lin, F.-H. Preparation and in-vitro evaluation of Fe2O3-doped DP-bioglass in combination with 3D-printing and selective laser sintering process (3DP-SLS) for alveolar bone augmentation. Ceram. Int. 2021, 47, 12725–12734. [Google Scholar] [CrossRef]
- Zhu, N.; Li, M.G.; Cooper, D.; Chen, X.B. Development of novel hybrid poly(l-lactide)/chitosan scaffolds using the rapid freeze prototyping technique. Biofabrication 2011, 3, 034105. [Google Scholar] [CrossRef]
- Li, L.H.; Kommareddy, K.P.; Pilz, C.; Zhou, C.R.; Fratzl, P.; Manjubala, I. In vitro bioactivity of bioresorbable porous polymeric scaffolds incorporating hydroxyapatite microspheres. Acta Biomater. 2010, 6, 2525–2531. [Google Scholar] [CrossRef]
- Chen, X.; Lian, T.; Zhang, B.; Du, Y.; Du, K.; Xiang, N.; Jung, D.W.; Wang, G.; Osaka, A. Design and mechanical compatibility of nylon bionic cancellous bone fabricated by selective laser sintering. Materials 2021, 14, 1965. [Google Scholar] [CrossRef]
- Mancuso, E.; Alharbi, N.; Bretcanu, O.A.; Marshall, M.; Birch, M.A.; McCaskie, A.W.; Dalgarno, K.W. Three-dimensional printing of porous load-bearing bioceramic scaffolds. Proc. Inst. Mech. Eng. H 2017, 231, 575–585. [Google Scholar] [CrossRef]
- Marques, C.F.; Olhero, S.M.; Torres, P.M.C.; Abrantes, J.C.C.; Fateixa, S.; Nogueira, H.I.S.; Ribeiro, I.A.C.; Bettencourt, A.; Sousa, A.; Granja, P.L.; et al. Novel sintering-free scaffolds obtained by additive manufacturing for concurrent bone regeneration and drug delivery: Proof of concept. Mater. Sci. Eng C 2019, 94, 426–436. [Google Scholar] [CrossRef]
- Rodrigues, A.F.M.; Torres, P.M.C.; Barros, M.J.S.; Presa, R.; Ribeiro, N.; Abrantes, J.C.C.; Belo, J.H.; Amaral, J.S.; Amaral, V.S.; Bañobre-López, M.; et al. Effective production of multifunctional magnetic-sensitive biomaterial by an extrusion-based additive manufacturing technique. Biomed. Mater. 2020, 16, 015011. [Google Scholar] [CrossRef]
- Wang, X. Preparation, synthesis and application of sol-gel method. Vidyasirimedhi Inst. Sci. Technol. 2020, 25, 1–30. [Google Scholar]
- Pipattanawarothai, A.; Suksai, C.; Srisook, K.; Trakulsujaritchok, T. Non-cytotoxic hybrid bioscaffolds of chitosan-silica: Sol-gel synthesis, characterization and proposed application. Carbohydr. Polym. 2017, 178, 190–199. [Google Scholar] [CrossRef]
- Farazin, A.; Ghasemi, A.H. Design, synthesis, and fabrication of chitosan/hydroxyapatite composite scaffold for use as bone replacement tissue by sol–gel method. J. Inorg. Organomet. Polym. Mater. 2022. [Google Scholar] [CrossRef]
- Kazemi-Aghdam, F.; Jahed, V.; Dehghan-Niri, M.; Ganji, F.; Vasheghani-Farahani, E. Injectable chitosan hydrogel embedding modified halloysite nanotubes for bone tissue engineering. Carbohydr. Polym. 2021, 269, 118311. [Google Scholar] [CrossRef]
- Mooney, D.J.; Baldwin, D.F.; Suh, N.P.; Vacanti, J.P.; Langer, R. Novel approach to fabricate porous sponges of poly(d,l-lactic-co-glycolic acid) without the use of organic solvents. Biomaterials 1996, 17, 1417–1422. [Google Scholar] [CrossRef]
- Kim, H.J.; Park, I.K.; Kim, J.H.; Cho, C.S.; Kim, M.S. Gas foaming fabrication of porous biphasic calcium phosphate for bone regeneration. Tissue Eng. Regen. Med. 2012, 9, 63–68. [Google Scholar] [CrossRef]
- Leena, R.S.; Vairamani, M.; Selvamurugan, N. Alginate/gelatin scaffolds incorporated with silibinin-loaded chitosan nanoparticles for bone formation in vitro. Colloids Surf. B 2017, 158, 308–318. [Google Scholar] [CrossRef] [PubMed]
- Vargas, Z.; Luzardo-Álvarez, A.; Piñeiro, Y.; Vázquez-Vázquez, C.; Gomez, J.; Mendez, J.; Otero-Espinar, F.; Rivas, J. Three-dimensional hybrid mesoporous scaffolds for simvastatin sustained delivery with in vitro cell compatibility. ACS Omega 2019, 4, 5496–5508. [Google Scholar] [CrossRef]
- Xu, X.; Gu, Z.; Chen, X.; Shi, C.; Liu, C.; Liu, M.; Wang, L.; Sun, M.; Zhang, K.; Liu, Q.; et al. An injectable and thermosensitive hydrogel: Promoting periodontal regeneration by controlled-release of aspirin and erythropoietin. Acta Biomater. 2019, 86, 235–246. [Google Scholar] [CrossRef]
- Yu, X.; Wang, Y.; Liu, X.; Ge, Y.; Zhang, S. Ursolic Acid Loaded-Mesoporous hydroxylapatite/chitosan therapeutic scaffolds regulate bone regeneration ability by promoting the M2-type polarization of macrophages. Int. J. Nanomed. 2021, 16, 5301–5315. [Google Scholar] [CrossRef]
- Hu, Y.; Cao, S.; Chen, J.; Zhao, Y.; He, F.; Li, Q.; Zou, L.; Shi, C. Biomimetic fabrication of icariin loaded nano hydroxyapatite reinforced bioactive porous scaffolds for bone regeneration. Chem. Eng. J. 2020, 394, 124895. [Google Scholar] [CrossRef]
- Won, J.E.; Yun, Y.R.; Jang, J.H.; Yang, S.H.; Kim, J.H.; Chrzanowski, W.; Wall, I.B.; Knowles, J.C.; Kim, H.W. Multifunctional and stable bone mimic proteinaceous matrix for bone tissue engineering. Biomaterials 2015, 56, 46–57. [Google Scholar] [CrossRef]
- Lee, S.S.; Kim, J.H.; Jeong, J.; Kim, S.H.L.; Koh, R.H.; Kim, I.; Bae, S.; Lee, H.; Hwang, N.S. Sequential growth factor releasing double cryogel system for enhanced bone regeneration. Biomaterials 2020, 257, 120223. [Google Scholar] [CrossRef]
- Li, H.; Koenig, A.M.; Sloan, P.; Leipzig, N.D. In vivo assessment of guided neural stem cell differentiation in growth factor immobilized chitosan-based hydrogel scaffolds. Biomaterials 2014, 35, 9049–9057. [Google Scholar] [CrossRef]
- Saito, N.; Takaoka, K. New synthetic biodegradable polymers as BMP carriers for bone tissue engineering. Biomaterials 2003, 24, 2287–2293. [Google Scholar] [CrossRef]
- Wang, Y.; Peng, W.; Liu, X.; Zhu, M.; Sun, T.; Peng, Q.; Zeng, Y.; Feng, B.; Zhi, W.; Weng, J.; et al. Study of bilineage differentiation of human-bone-marrow-derived mesenchymal stem cells in oxidized sodium alginate/N-succinyl chitosan hydrogels and synergistic effects of RGD modification and low-intensity pulsed ultrasound. Acta Biomater. 2014, 10, 2518–2528. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Zhou, C.; Chen, J.; Luo, H.; Li, R.; Chen, D.; Zou, X.; Wang, W. Synergistic osteogenic and angiogenic effects of KP and QK peptides incorporated with an injectable and self-healing hydrogel for efficient bone regeneration. Bioact. Mater. 2022, 18, 267–283. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, D.; Liu, G.; Wang, J.; Luo, Z.; Peng, X.; Zeng, X.; Wang, X.; Tan, H.; Li, J. Bioinspired from mussel and salivary acquired pellicle: A universal dual-functional polypeptide coating for implant materials. Mater. Today Chem. 2019, 14, 100205. [Google Scholar] [CrossRef]
- Kim, S.; Cui, Z.K.; Fan, J.; Fartash, A.; Aghaloo, T.L.; Lee, M. Photocrosslinkable chitosan hydrogels functionalized with the RGD peptide and phosphoserine to enhance osteogenesis. J. Mater. Chem. B 2016, 4, 5289–5298. [Google Scholar] [CrossRef]
- Tsai, W.-B.; Chen, Y.-R.; Li, W.-T.; Lai, J.-Y.; Liu, H.-L. RGD-conjugated UV-crosslinked chitosan scaffolds inoculated with mesenchymal stem cells for bone tissue engineering. Carbohydr. Polym. 2012, 89, 379–387. [Google Scholar] [CrossRef]
- Bei, H.P.; Hung, P.M.; Yeung, H.L.; Wang, S.; Zhao, X. Bone-a-petite: Engineering exosomes towards bone, osteochondral, and cartilage repair. Small 2021, 17, e2101741. [Google Scholar] [CrossRef]
- Zhang, M.; Li, Y.; Feng, T.; Li, R.; Wang, Z.; Zhang, L.; Yin, P.; Tang, P. Bone engineering Scaffolds with exosomes: A promising strategy for bone defects repair. Front. Bioeng. Biotechnol. 2022, 10, 920378. [Google Scholar] [CrossRef]
- Li, W.; Liu, Y.; Zhang, P.; Tang, Y.; Zhou, M.; Jiang, W.; Zhang, X.; Wu, G.; Zhou, Y. Tissue-engineered bone immobilized with human adipose stem cells-derived exosomes promotes bone regeneration. ACS Appl. Mater. Interfaces 2018, 10, 5240–5254. [Google Scholar] [CrossRef]
- Gao, Y.; Yuan, Z.; Yuan, X.; Wan, Z.; Yu, Y.; Zhan, Q.; Zhao, Y.; Han, J.; Huang, J.; Xiong, C.; et al. Bioinspired porous microspheres for sustained hypoxic exosomes release and vascularized bone regeneration. Bioact. Mater. 2022, 14, 377–388. [Google Scholar] [CrossRef]
- Xie, Y.; Chen, Y.; Zhang, L.; Ge, W.; Tang, P. The roles of bone-derived exosomes and exosomal microRNAs in regulating bone remodelling. J. Cell. Mol. Med. 2017, 21, 1033–1041. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Lee, C.S.; Kim, S.; Chen, C.; Aghaloo, T.; Lee, M. Generation of small RNA-modulated exosome mimetics for bone regeneration. ACS Nano 2020, 14, 11973–11984. [Google Scholar] [CrossRef] [PubMed]
- Shen, Z.; Kuang, S.; Zhang, Y.; Yang, M.; Qin, W.; Shi, X.; Lin, Z. Chitosan hydrogel incorporated with dental pulp stem cell-derived exosomes alleviates periodontitis in mice via a macrophage-dependent mechanism. Bioact. Mater. 2020, 5, 1113–1126. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, J.; Zhou, X.; Sun, J.; Zhu, B.; Duan, C.; Chen, P.; Guo, X.; Zhang, T.; Guo, H. A new self-healing hydrogel containing hucMSC-derived exosomes promotes bone regeneration. Front. Bioeng. Biotechnol. 2020, 8, 564731. [Google Scholar] [CrossRef]
- Malek-Khatabi, A.; Javar, H.A.; Dashtimoghadam, E.; Ansari, S.; Hasani-Sadrabadi, M.M.; Moshaverinia, A. In situ bone tissue engineering using gene delivery nanocomplexes. Acta Biomater. 2020, 108, 326–336. [Google Scholar] [CrossRef]
- Collon, K.; Gallo, M.C.; Lieberman, J.R. Musculoskeletal tissue engineering: Regional gene therapy for bone repair. Biomaterials 2021, 275, 120901. [Google Scholar] [CrossRef]
- Damiati, L.A.; El-Messeiry, S. An overview of RNA-based scaffolds for osteogenesis. Front. Mol. Biosci. 2021, 8, 682581. [Google Scholar] [CrossRef]
- Wu, G.; Feng, C.; Hui, G.; Wang, Z.; Tan, J.; Luo, L.; Xue, P.; Wang, Q.; Chen, X. Improving the osteogenesis of rat mesenchymal stem cells by chitosan-based-microRNA nanoparticles. Carbohydr. Polym. 2016, 138, 49–58. [Google Scholar] [CrossRef]
- Grol, M.W.; Lee, B.H. Gene therapy for repair and regeneration of bone and cartilage. Curr. Opin. Pharmacol. 2018, 40, 59–66. [Google Scholar] [CrossRef]
- Jia, S.; Yang, X.; Song, W.; Wang, L.; Fang, K.; Hu, Z.; Yang, Z.; Shan, C.; Lei, D.; Lu, B. Incorporation of osteogenic and angiogenic small interfering RNAs into chitosan sponge for bone tissue engineering. Int. J. Nanomed. 2014, 9, 5307–5316. [Google Scholar]
- Meng, Y.; Liu, C.; Zhao, J.; Li, X.; Li, Z.; Wang, J.; Wang, R.; Liu, Y.; Yuan, X.; Cui, Z.; et al. An injectable miRNA-activated matrix for effective bone regeneration in vivo. J. Mater. Chem. B 2016, 4, 6942–6954. [Google Scholar] [CrossRef] [PubMed]
- Sharifi, F.; Atyabi, S.M.; Irani, S.; Bakhshi, H. Bone morphogenic protein-2 immobilization by cold atmospheric plasma to enhance the osteoinductivity of carboxymethyl chitosan-based nanofibers. Carbohydr. Polym. 2020, 231, 115681. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Kang, Y.; Krueger, C.A.; Sen, M.; Holcomb, J.B.; Chen, D.; Wenke, J.C.; Yang, Y. Sequential delivery of BMP-2 and IGF-1 using a chitosan gel with gelatin microspheres enhances early osteoblastic differentiation. Acta Biomater. 2012, 8, 1768–1777. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Deng, Y.; Li, T.; Zhu, F.; Zhou, X.; He, Y. The opposite functions of miR-24 in the osteogenesis and adipogenesis of adipose-derived mesenchymal stem cells are mediated by the HOXB7/β-catenin complex. FASEB J. 2020, 34, 9034–9050. [Google Scholar] [CrossRef]
- Balagangadharan, K.; Viji Chandran, S.; Arumugam, B.; Saravanan, S.; Devanand Venkatasubbu, G.; Selvamurugan, N. Chitosan/nano-hydroxyapatite/nano-zirconium dioxide scaffolds with miR-590-5p for bone regeneration. Int. J. Biol. Macromol. 2018, 111, 953–958. [Google Scholar] [CrossRef]
- Sukpaita, T.; Chirachanchai, S.; Pimkhaokham, A.; Ampornaramveth, R.S. Chitosan-based scaffold for mineralized tissues regeneration. Mar. Drugs 2021, 19, 551. [Google Scholar] [CrossRef]
- Pighinelli, L.; Kucharska, M. Chitosan–hydroxyapatite composites. Carbohydr. Polym. 2013, 93, 256–262. [Google Scholar] [CrossRef]
- Nitta, S.; Komatsu, A.; Ishii, T.; Ohnishi, M.; Inoue, A.; Iwamoto, H. Fabrication and characterization of water-dispersed chitosan nanofiber/poly(ethylene glycol) diacrylate/calcium phosphate-based porous composites. Carbohydr. Polym. 2017, 174, 1034–1040. [Google Scholar] [CrossRef]
- Lou, C.W.; Wen, S.P.; Chen, W.C.; Chen, Y.S.; Lin, J.H. The freeze-dry method and in vitro assay of chitosan/gelatin/hydroxyapatite artificial bone scaffolds. Appl. Mech. Mater. 2015, 749, 441–444. [Google Scholar] [CrossRef]
- Huang, C.; Fang, G.; Zhao, Y.; Bhagia, S.; Meng, X.; Yong, Q.; Ragauskas, A.J. Bio-inspired nanocomposite by layer-by-layer coating of chitosan/hyaluronic acid multilayers on a hard nanocellulose-hydroxyapatite matrix. Carbohydr. Polym. 2019, 222, 115036. [Google Scholar] [CrossRef]
- Frohbergh, M.E.; Katsman, A.; Botta, G.P.; Lazarovici, P.; Schauer, C.L.; Wegst, U.G.K.; Lelkes, P.I. Electrospun hydroxyapatite-containing chitosan nanofibers crosslinked with genipin for bone tissue engineering. Biomaterials 2012, 33, 9167–9178. [Google Scholar] [CrossRef] [PubMed]
- Postnova, I.; Silant’ev, V.; Sarin, S.; Shchipunov, Y. Chitosan hydrogels and bionanocomposites formed through the mineralization and regulated charging. Chem. Rec. 2018, 18, 1247–1260. [Google Scholar] [CrossRef]
- Schamel, M.; Groll, J.; Gbureck, U. Simultaneous formation and mineralization of star-P(EO-stat-PO) hydrogels. Mater. Sci. Eng. C Mater. Biol.Appl. 2017, 75, 471–477. [Google Scholar] [CrossRef] [PubMed]
- Teng, L.; Chen, Y.; Jin, M.; Jia, Y.; Wang, Y.; Ren, L. Weak hydrogen bonds lead to self-healable and bioadhesive hybrid polymeric hydrogels with mineralization-active functions. Biomacromolecules 2018, 19, 1939–1949. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Zhou, L.; Li, Q.; Zou, Q.; Du, C. Biomimetic mineralization of carboxymethyl chitosan nanofibers with improved osteogenic activity in vitro and in vivo. Carbohydr. Polym. 2018, 195, 225–234. [Google Scholar] [CrossRef]
- Park, H.; Choi, B.; Nguyen, J.; Fan, J.; Shafi, S.; Klokkevold, P.; Lee, M. Anionic carbohydrate-containing chitosan scaffolds for bone regeneration. Carbohydr. Polym. 2013, 97, 587–596. [Google Scholar] [CrossRef]
- Ding, C.; Chen, Z.; Li, J. From molecules to macrostructures: Recent development of bioinspired hard tissue repair. Biomater. Sci. 2017, 5, 1435–1449. [Google Scholar] [CrossRef]
- Hu, Y.; Chen, J.; Fan, T.; Zhang, Y.; Zhao, Y.; Shi, X.; Zhang, Q. Biomimetic mineralized hierarchical hybrid scaffolds based on in situ synthesis of nano-hydroxyapatite/chitosan/chondroitin sulfate/hyaluronic acid for bone tissue engineering. Colloids Surf. B 2017, 157, 93–100. [Google Scholar] [CrossRef]
- Rauner, N.; Meuris, M.; Zoric, M.; Tiller, J.C. Enzymatic mineralization generates ultrastiff and tough hydrogels with tunable mechanics. Nature 2017, 543, 407–410. [Google Scholar] [CrossRef]
- Chen, G.; Liang, X.; Zhang, P.; Lin, S.; Cai, C.; Yu, Z.; Liu, J. Bioinspired 3D Printing of Functional Materials by Harnessing Enzyme-Induced Biomineralization. Adv. Funct. Mater. 2022, 2113262. [Google Scholar] [CrossRef]
- Guo, R.; Hou, X.; Zhao, D.; Wang, H.; Shi, C.; Zhou, Y. Mechanical stability and biological activity of Mg–Sr co-doped bioactive glass/chitosan composite scaffolds. J. Non.-Cryst. Solids 2022, 583, 121481. [Google Scholar] [CrossRef]
- Nawaz, A.; Bano, S.; Yasir, M.; Wadood, A.; Ur Rehman, M.A. Ag and Mn-doped mesoporous bioactive glass nanoparticles incorporated into the chitosan/gelatin coatings deposited on PEEK/bioactive glass layers for favorable osteogenic differentiation and antibacterial activity. Mater. Adv. 2020, 1, 1273–1284. [Google Scholar] [CrossRef]
- Oudadesse, H.; Najem, S.; Mosbahi, S.; Rocton, N.; Refifi, J.; El Feki, H.; Lefeuvre, B. Development of hybrid scaffold: Bioactive glass nanoparticles/chitosan for tissue engineering applications. J. Biomed. Mater. Res. A 2021, 109, 590–599. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Zheng, K.; Huang, X.; Liu, J.; Liu, H.; Boccaccini, A.R.; Wan, Y.; Guo, X.; Shao, Z. Thermally triggered injectable chitosan/silk fibroin/bioactive glass nanoparticle hydrogels for in-situ bone formation in rat calvarial bone defects. Acta Biomater. 2019, 91, 60–71. [Google Scholar] [CrossRef] [PubMed]
- Geetha Bai, R.; Muthoosamy, K.; Manickam, S.; Hilal-Alnaqbi, A. Graphene-based 3D scaffolds in tissue engineering: Fabrication, applications, and future scope in liver tissue engineering. Int. J. Nanomed. 2019, 14, 5753–5783. [Google Scholar] [CrossRef]
- Peng, Z.; Zhao, T.; Zhou, Y.; Li, S.; Li, J.; Leblanc, R.M. Bone tissue engineering via carbon-based nanomaterials. Adv. Healthc. Mater. 2020, 9, e1901495. [Google Scholar] [CrossRef]
- Depan, D.; Girase, B.; Shah, J.S.; Misra, R.D. Structure-process-property relationship of the polar graphene oxide-mediated cellular response and stimulated growth of osteoblasts on hybrid chitosan network structure nanocomposite scaffolds. Acta Biomater. 2011, 7, 3432–3445. [Google Scholar] [CrossRef]
- Huang, Y.; Zhang, L.; Ji, Y.; Deng, H.; Long, M.; Ge, S.; Su, Y.; Chan, S.Y.; Loh, X.J.; Zhuang, A.; et al. A non-invasive smart scaffold for bone repair and monitoring. Bioact. Mater. 2023, 19, 499–510. [Google Scholar] [CrossRef]
- Kaur, K.; Paiva, S.S.; Caffrey, D.; Cavanagh, B.L.; Murphy, C.M. Injectable chitosan/collagen hydrogels nano-engineered with functionalized single wall carbon nanotubes for minimally invasive applications in bone. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 128, 112340. [Google Scholar] [CrossRef]
- Ruan, J.; Wang, X.; Yu, Z.; Wang, Z.; Xie, Q.; Zhang, D.; Huang, Y.; Zhou, H.; Bi, X.; Xiao, C.; et al. Enhanced physiochemical and mechanical performance of chitosan-grafted graphene oxide for superior osteoinductivity. Adv. Funct. Mater. 2016, 26, 1085–1097. [Google Scholar] [CrossRef]
- Keldani, Z.; Lord, M.L.; McNeill, F.E.; Chettle, D.R.; Gräfe, J.L. Coherent normalization for in vivoI measurements of gadolinium in bone. Physiol. Meas. 2017, 38, 1848–1858. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Li, L.; Wang, Y.; Huang, J.; Wang, Z.; Shi, X.; Zhang, P. An electrically and magnetically responsive nanocomposite of GdPO4·H2O/P3HT/PLGA with electrical stimulation for synergistically enhancing the proliferation and differentiation of pre-osteoblasts. New J. Chem. 2019, 43, 17315–17326. [Google Scholar] [CrossRef]
- Zhao, P.-P.; Hu, H.-R.; Liu, J.-Y.; Ke, Q.-F.; Peng, X.-Y.; Ding, H.; Guo, Y.-P. Gadolinium phosphate/chitosan scaffolds promote new bone regeneration via Smad/Runx2 pathway. Chem. Eng. J. 2019, 359, 1120–1129. [Google Scholar] [CrossRef]
- Zhao, P.-P.; Ge, Y.-W.; Liu, X.-L.; Ke, Q.-F.; Zhang, J.-W.; Zhu, Z.-A.; Guo, Y.-P. Ordered arrangement of hydrated GdPO4 nanorods in magnetic chitosan matrix promotes tumor photothermal therapy and bone regeneration against breast cancer bone metastases. Chem. Eng. J. 2020, 381, 122694. [Google Scholar] [CrossRef]
- Salama, A.; Hesemann, P. Synthesis and characterization of N-guanidinium chitosan/silica ionic hybrids as templates for calcium phosphate mineralization. Int. J. Biol. Macromol. 2020, 147, 276–283. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Xia, Y.; Cheng, X.; Wang, P.; Xie, Y.; Xu, S. Enhanced bone tissue regeneration by antibacterial and osteoinductive silica-HACC-zein composite scaffolds loaded with rhBMP-2. Biomaterials 2014, 35, 10033–10045. [Google Scholar] [CrossRef]
- Perez-Moreno, A.; Reyes-Peces, M.L.V.; de Los Santos, D.M.; Pinaglia-Tobaruela, G.; de la Orden, E.; Vilches-Pérez, J.I.; Salido, M.; Piñero, M.; de la Rosa-Fox, N. Hydroxyl groups induce bioactivity in silica/chitosan aerogels designed for bone tissue engineering. In vitro model for the assessment of osteoblasts behavior. Polymers 2020, 122, 2802. [Google Scholar] [CrossRef]
- Jaque, D.; Martínez Maestro, L.; del Rosal, B.; Haro-Gonzalez, P.; Benayas, A.; Plaza, J.L.; Martín Rodríguez, E.; García Solé, J. Nanoparticles for photothermal therapies. Nanoscale 2014, 6, 9494–9530. [Google Scholar] [CrossRef]
- Sheng, L.; Zhang, Z.; Zhang, Y.; Wang, E.; Ma, B.; Xu, Q.; Ma, L.; Zhang, M.; Pei, G.; Chang, J. A novel “hot spring”-mimetic hydrogel with excellent angiogenic properties for chronic wound healing. Biomaterials 2021, 264, 120414. [Google Scholar] [CrossRef]
- Shao, J.; Ruan, C.; Xie, H.; Li, Z.; Wang, H.; Chu, P.K.; Yu, X.-F. Black-phosphorus-incorporated hydrogel as a sprayable and biodegradable photothermal platform for postsurgical treatment of cancer. Adv. Sci. 2018, 5, 1700848. [Google Scholar] [CrossRef]
- Wu, Y.; Liao, Q.; Wu, L.; Luo, Y.; Zhang, W.; Guan, M.; Pan, H.; Tong, L.; Chu, P.K.; Wang, H. ZnL2-BPs integrated bone scaffold under sequential photothermal mediation: A win–win strategy delivering antibacterial therapy and fostering osteogenesis thereafter. ACS Nano 2021, 15, 17854–17869. [Google Scholar] [CrossRef] [PubMed]
- Tong, L.; Liao, Q.; Zhao, Y.; Huang, H.; Gao, A.; Zhang, W.; Gao, X.; Wei, W.; Guan, M.; Chu, P.K.; et al. Near-infrared light control of bone regeneration with biodegradable photothermal osteoimplant. Biomaterials 2019, 193, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Hu, P.; Jiang, H.; Zhao, J.; Tang, J.; Jiang, D.; Wang, J.; Shi, J.; Jia, W. Mild hyperthermia-mediated osteogenesis and angiogenesis play a critical role in magnetothermal composite-induced bone regeneration. Nano Today 2022, 43, 101401. [Google Scholar] [CrossRef]
- Tan, L.; Hu, Y.; Li, M.; Zhang, Y.; Xue, C.; Chen, M.; Luo, Z.; Cai, K. Remotely-activatable extracellular matrix-mimetic hydrogel promotes physiological bone mineralization for enhanced cranial defect healing. Chem. Eng. J. 2022, 431, 133382. [Google Scholar] [CrossRef]
- Ma, L.; Feng, X.; Liang, H.; Wang, K.; Song, Y.; Tan, L.; Wang, B.; Luo, R.; Liao, Z.; Li, G.; et al. A novel photothermally controlled multifunctional scaffold for clinical treatment of osteosarcoma and tissue regeneration. Materials Today 2020, 36, 48–62. [Google Scholar] [CrossRef]
- Meshkini, A.; Sistanipour, E.; Izadi, A. Mg.ATP-decorated ultrafine magnetic nanofibers: A bone scaffold with high osteogenic and antibacterial properties in the presence of an electromagnetic field. Colloids Surf. B 2022, 210, 112256. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, F.; Yan, Y.; Zhang, C.; Zhao, H.; Heng, B.C.; Huang, Y.; Shen, Y.; Zhang, J.; Chen, L.; et al. Remote tuning of built-in magnetoelectric microenvironment to promote bone regeneration by modulating cellular exposure to arginylglycylaspartic acid peptide. Adv. Funct. Mater. 2021, 31, 2006226. [Google Scholar] [CrossRef]
- Kotani, H.; Kawaguchi, H.; Shimoaka, T.; Iwasaka, M.; Ueno, S.; Ozawa, H.; Nakamura, K.; Hoshi, K. Strong static magnetic field stimulates bone formation to a definite orientation in vitro and in vivo. J. Bone Miner. Res. 2002, 17, 1814–1821. [Google Scholar] [CrossRef]
- Xia, Y.; Sun, J.; Zhao, L.; Zhang, F.; Liang, X.-J.; Guo, Y.; Weir, M.D.; Reynolds, M.A.; Gu, N.; Xu, H.H.K. Magnetic field and nano-scaffolds with stem cells to enhance bone regeneration. Biomaterials 2018, 183, 151–170. [Google Scholar] [CrossRef]
- Tang, Y.-Q.; Wang, Q.-Y.; Ke, Q.-F.; Zhang, C.-Q.; Guan, J.-J.; Guo, Y.-P. Mineralization of ytterbium-doped hydroxyapatite nanorod arrays in magnetic chitosan scaffolds improves osteogenic and angiogenic abilities for bone defect healing. Chem. Eng. J. 2020, 387, 124166. [Google Scholar] [CrossRef]
- Yu, B.; Qiao, Z.; Cui, J.; Lian, M.; Han, Y.; Zhang, X.; Wang, W.; Yu, X.; Yu, H.; Wang, X.; et al. A host-coupling bio-nanogenerator for electrically stimulated osteogenesis. Biomaterials 2021, 276, 120997. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.; Li, Y.; Huang, F.; Chen, Z.; Xie, J.; Ding, C.; Li, J. Promotion of the osteogenic activity of an antibacterial polyaniline coating by electrical stimulation. Biomater. Sci. 2019, 7, 4730–4737. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Zhang, F.; Zhangyang, Y.; Zhang, H.; Zhao, Y.; Xu, X.; Qin, M.; Ding, C.; Li, J. Electrically facilitated mineralization of osteoblasts and polypyrrole micro-bowl coatings for promotion of the osteogenic activity. Colloids Interface Sci. Commun. 2021, 43, 100450. [Google Scholar] [CrossRef]
- Yarmolenko, P.S.; Moon, E.J.; Landon, C.; Manzoor, A.; Hochman, D.W.; Viglianti, B.L.; Dewhirst, M.W. Thresholds for thermal damage to normal tissues: An update. Int. J. Hyperth. 2011, 27, 320–343. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Huang, Y.Y.; Wang, Y.; Lyu, P.; Hamblin, M.R. Photobiomodulation (blue and green light) encourages osteoblastic-differentiation of human adipose-derived stem cells: Role of intracellular calcium and light-gated ion channels. Sci. Rep. 2016, 6, 33719. [Google Scholar] [CrossRef]
- Hayashi, K.; Munar, M.L.; Ishikawa, K. Effects of macropore size in carbonate apatite honeycomb scaffolds on bone regeneration. Mater. Sci. Eng. C 2020, 111, 110848. [Google Scholar] [CrossRef]
- Wang, S.; Shi, Z.a.; Liu, L.; Huang, Z.; Li, Z.; Liu, J.; Hao, Y. Honeycomb structure is promising for the repair of human bone defects. Mater. Des. 2021, 207, 109832. [Google Scholar] [CrossRef]



| Fabrication Techniques | Composite | Important Properties | Ref. |
|---|---|---|---|
| Freeze drying | CS/graphene oxide/tetracycline hydrochloride | Controlled the drug release and promoted faster bone growth in rat femur defects. | [78] |
| CS/graphene oxide | Oriented pores enhanced the alignment of MC3T3-E1 cells, facilitated osteogenesis. | [79] | |
| Electrospinning | Zein/CS/polyurethane/carbon nanotubes | Facilitated cell proliferation, differentiation and upregulated the expression of osteogenic proteins. | [80] |
| CS/HAP | Supported cell adhesion and promoted bone regeneration by activating integrin-BMP/Smad signaling pathway. | [81] | |
| CS/poly (vinyl alcohol)/carbonated hydroxyapatite | Promoted cell adhesion, growth and osteogenesis. | [82] | |
| 3D printing | CS/silk fibroin/cellulose | Osteo-immunomodulatory effects, accelerated bone regeneration in rat calvaria defects. | [61] |
| Silk fibroin/CS/CaP | Enhanced the strength of scaffold, facilitated the proliferation and osteogenic differentiation. | [83] | |
| CS/HAP | Created a cell-friendly living environment, promoted cell adhesion, proliferation and osteogenesis. | [84] | |
| Sol-gel method | CS/polyvinyl alcohol/SiO2 | Excellent mechanical properties and osteogenic differentiation ability. | [85] |
| CS/bioactive glass | Good shape memory properties and geometrical accommodation in bone implantation. | [86] | |
| Gas foaming + microwave irradiation | CS/HAP | Scaffold with interconnective pores facilitated cells growth and upregulated osteogenic genes (RUNX2, OCN, COL I, ALP) expression. | [87] |
| Freeze drying + porogen-leaching out | CS/HAP | Scaffold with gradient pore and HAP composition implemented the bidirectional repair of osteochondral defects. | [88] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Y.; Zhao, S.; Ma, Z.; Ding, C.; Chen, J.; Li, J. Chitosan-Based Scaffolds for Facilitated Endogenous Bone Re-Generation. Pharmaceuticals 2022, 15, 1023. https://doi.org/10.3390/ph15081023
Zhao Y, Zhao S, Ma Z, Ding C, Chen J, Li J. Chitosan-Based Scaffolds for Facilitated Endogenous Bone Re-Generation. Pharmaceuticals. 2022; 15(8):1023. https://doi.org/10.3390/ph15081023
Chicago/Turabian StyleZhao, Yao, Sinuo Zhao, Zhengxin Ma, Chunmei Ding, Jingdi Chen, and Jianshu Li. 2022. "Chitosan-Based Scaffolds for Facilitated Endogenous Bone Re-Generation" Pharmaceuticals 15, no. 8: 1023. https://doi.org/10.3390/ph15081023
APA StyleZhao, Y., Zhao, S., Ma, Z., Ding, C., Chen, J., & Li, J. (2022). Chitosan-Based Scaffolds for Facilitated Endogenous Bone Re-Generation. Pharmaceuticals, 15(8), 1023. https://doi.org/10.3390/ph15081023






