Abstract
Severe infections such as viral, bacterial, or fungal sepsis can cause an inflammatory response in the host, leading to organ failure and septic shock—phosphodiesterase-4 (PDE-4) inhibiting related agents from suppressing cyclic adenosine monophosphate (cAMP) degradation. Regulatory organisations have approved some substances in this category to reduce the risk of chronic obstructive pulmonary disease (COPD) exacerbations in patients with chronic bronchitis and a history of COPD exacerbations. Roflumilast has been shown to alleviate inflammatory responses, thus regulating airway inflammation. Additionally, roflumilast therapy dramatically enhanced B-cell lymphoma 2 (Bcl-2) expression, an anti-apoptotic marker lowered in septic animals. Previous research has indicated that roflumilast may help reverse sepsis-induced liver and lung harm, but whether it is also effective in reversing sepsis-induced renal impairment remains unknown. Therefore, this review determines whether roflumilast protects against renal dysfunction, inflammatory response, and apoptosis in sepsis-induced kidney damage. Additionally, we discussed the molecular mechanism through which roflumilast exerts its protective effect to uncover a possible treatment agent for sepsis-induced renal impairment.
1. Introduction
Phosphodiesterase-4 (PDEs), which break down cAMP by hydrolysing the phosphodiester link of cAMP to form AMP, and adenylate cyclases, which catalyse the cyclization of adenosine triphosphate (ATP) into cAMP, both play a role in tightly managing intracellular cAMP levels. Eleven people represent the PDE family. PDEs 4, 7, and 8 hydrolyze cAMP, whereas PDEs 1, 2, 3, 10, and 11 hydrolyze cAMP and cyclic guanosine monophosphate (cGMP). PDEs 5, 6, and 9 hydrolyze cGMP. The most prominent mammalian PDE family members, PDE4 A through D, are encoded by four genes, with more than 25 splice variants produced by combining the four genes. PDE4 isotypes are extensively distributed throughout the body, except in the heart, where they are absent or weakly expressed but are substantially expressed in skeletal muscle, nerve, and inflammatory cells [1,2,3,4].
Some of the substances from this category have been licenced by regulatory authorities to reduce the risk of COPD exacerbations in patients with chronic bronchitis and a history of COPD exacerbations [5,6,7]. PDE4 can be a therapeutic target for a wide range of diseases. Antidepressant benefits and improved cognition and memory have been demonstrated in animal models with Rolipram, the most extensively investigated generic PDE4 inhibitor [8,9]. In addition, the anti-inflammatory characteristics of PDE4 inhibitors benefit their use [10,11,12]. Since modifying the metabolic benefits of resveratrol, Rolipram can activate AMP-activated protein kinase (AMPK) and increase mitochondrial activity, thereby preventing obesity and improving glucose tolerance in mice fed high-fat diets, as demonstrated in this study [13,14,15]. Whether roflumilast’s capacity to activate AMPK and Sirtuin 1 (SIRT1) in the kidneys is specific to PDE4 inhibitors or not is unknown.
As part of our ongoing investigation into the cellular roles and activities of AMPK, PDE4, and Sirt1, many compounds have been examined for their capacity to inhibit PDE4. However, research has indicated that roflumilast may help reverse sepsis-induced liver and lung harm, but whether it effectively reverses sepsis-induced renal impairment remains unknown. Therefore, this review determines whether roflumilast protects against renal dysfunction, inflammatory response, and apoptosis in sepsis-induced kidney damage (Figure 1). Additionally, we discussed the molecular mechanism through which roflumilast exerts its protective effect to uncover a possible treatment agent for sepsis-induced renal impairment.
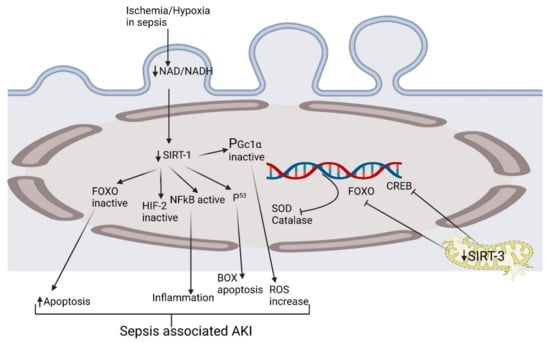
Figure 1.
Exploring the pathophysiological activation of various biomolecular pathways in septic acute kidney injury. CREB, cAMP-response element-binding protein; HIF-2, hypoxia-inducible factor-2; FOXO, forkhead box O; NAD, nicotinamide adenine dinucleotide; NF-κB, nuclear factor kappa-light-chain-enhancer of activated B cells; PGc1α, peroxisome proliferator-activated receptor-gamma coactivator (PGC)-1alpha; ROS, reactive oxygen species; SIRT1, Sirtuin 1; SODs, Ssuperoxide dismutases.
2. Selection of Literature Review
Medline, Mendeley, PubMed, ScienceDirect, SpringerLink, and Google Scholar were used to search for studies that could be relevant. We used various terms to perform the literature search, alone and in combination. As a part of our research, the following terms were used: ‘Definition sepsis-associated acute kidney injury, ‘Epidemiology S-AKI, ‘Early-stage sepsis-associated inflammation’, ‘Pathogenesis of S-AKI’, ‘Involvement of various biomarkers of S-AKI, ‘Cytokine activation mediated biological response in S-AKI, ‘Evidence-based roflumilast treatment in S-AKI’, ‘Role of kidney injury molecule (KIM) and N-acetyl-β-D-glucosidase (NAG) in kidney injury. Only works in English were considered for this investigation. The reference lists of the papers identified have also been checked for items not detected by an initial search method.
3. Clinical and Preclinical Events of Septic Acute Kidney Injury
Severe infections such as viral, bacterial, or fungal sepsis can cause an inflammatory response in the host that leads to organ failure and septic shock. “Life-threatening organ failure induced by an altered host immunological responsiveness to infection” was the new definition of sepsis in 2016 [16]. Septic shock is a kind of sepsis in which the underlying circulatory and cellular/metabolic abnormalities are severe enough to increase mortality. Throughout sepsis, the kidney is among the first organs to be affected. AKI and sepsis syndrome are complicated and varied illnesses with many risk factors. AKI is the primary cause of mortality and disability in ICUs in developed countries [17,18]. There is no one effective therapy for AKI, despite advancements in supportive care, such as infection control and the use of blood products. The mainstay of treatment for kidney disease is early identification and appropriate supportive and preventative maintenance. There have been various analyses of the epidemiological data of sepsis-related acute renal injury.
Furthermore, the death rate for adults with AKI during sepsis is 60% [19], and the figure for children ranges from 57% to 66% [20]. Sepsis-related AKI is more severe than nonseptic AKI and is linked with a higher fatality rate. S-AKI was found in 48.1 per cent of patients overall, with 59.2 per cent of those in the ICU and 31.6 per cent of those outside the ICU, according to a recent study published in dove press and a 55percent overall fatality incidence among S-AKI patient populations. In addition, AKI in sepsis is associated with the rapid decline in renal function resulting in nitrogenous waste substance retention, a common symptom of sepsis [21,22]. Even though AKI complicates the short-term treatment of patients with sepsis, it also increases the risk of long-term consequences, namely, the development of end-stage renal disease (ESRD), kidney failure with replacement therapy (KFRT), and short- and long-term fatalities [23,24,25,26]. As with sepsis, ongoing efforts to standardise the definition of AKI have resulted in the development of the following three major classification systems over the last two decades: the acute dialysis quality initiative (ADQI)—proposed Risk, Injury, Failure, Loss of kidney function, and RIFLE criteria, the AKIN criteria [27], and the most recent KDIGO criteria [28]. To determine the diagnosis of AKI, all three categorization systems rely on a rise in serum creatinine (sCr) and a reduction in urine output (UO). Despite this, current sepsis recommendations continue to propose using the sequential organ failure assessment (SOFA) score to identify AKI, which is problematic since SOFA makes no distinction between chronic and acute renal illness or takes demographic disparities in baseline sCr level into account.
In general, the previous murine model divides sepsis into an initial stage of hyperinflammatory/immune activation (around 0–6 h) and a late episode of immunological suppression (24–48 h) [29,30]. During the initial stages of sepsis, pro-inflammatory cytokines such as tumour necrosis factor α (TNFα), interleukin-1 (IL-1), IL-2, IL-6, IL-8, and interferon-gamma (IFN-γ) directly exacerbate the damage by attracting diverse leukocytic populations to the site of injury, resulting in a vicious cycle of the inflammatory process [31]. An increase in pro-inflammatory mediators released into the body, which are then filtered by the kidneys, leads to damage to the kidney’s proximal tubules and the loss of tubular epithelial cells, leading to metabolic irregularities and kidney injury as a result of this stimulus [32,33].
In the initial stages of S-AKI, the hyperinflammatory and active immunological responses, together with the oxidative load, promote further pathological alterations such as cellular proliferation, podocyte injury, extracellular matrix (ECM) deposition, and activation of pro-apoptotic pathways [34]. The immunosuppression seen in the late stages of sepsis compromises the body’s defences, leading to enhanced organ damage. Interestingly, prolonged inflammation and poor T cell response were observed in old septic mice and geriatric septic humans 24 h after infection (late-stage) and were linked to decreased life expectancy [35]. The increased number of monocytes and neutrophils found in the peripheral blood of old mice and humans throughout a comparable period suggests that the immune system is out of whack in the late stages of sepsis. Septic patients in the later or immunosuppressive period (three to five days) have been shown to have high CD10 and CD16 and altered chemotaxis of neutrophils in their blood, which raises the risk of mortality [36].
4. Recent Updates of Biomolecules in S-AKI
Galactin-3 (Gal-3), a soluble β-galactoside-binding substance, is extensively expressed in the cardiac, renal, hepatic, pulmonary tissues, and intestine and is particularly abundant in activated macrophages and mast cells and basophils [37]. The principal renal Gal-3 synthesis and secretion sources are inflammatory cells and macrophages. Gal-3 expression in the kidney is quickly increased in models of renal injury [38,39,40,41,42]. According to recent research, increased Gal-3 levels after ICU admission predicted S-AKI and death in sepsis patients, but Gal-3 inhibition in a CLP rat model dramatically decreased S-AKI and mortality. S-AKI and sepsis mortality may be linked to Gal-3’s participation in the aetiology of S-AKI [43].
To maintain renal homeostasis and sustain the metabolic pressure seen during sepsis, the existence of healthy mitochondria is required [44,45,46]. Renal ATP depletion and elevated production of reactive oxygen species (ROS) are caused by mitochondrial failure in sepsis and eventually result in cell homeostasis collapse and organ dysfunction [47]. When sepsis develops, inflammatory molecules such as TNF-α and IL-6 may bind to signal transducers and activators of transcription 3 (STAT3) due to early inflammation in the body. As a result, STAT-3 is activated, enhancing its transcriptional capacity for IL-6 and TNF-alpha. This may eventually convey pro-inflammatory signals and worsen inflammation. As indicated in muscle biopsies, sepsis reduces ATP levels and increases biomarkers for mitochondrial dysfunction. It reduces antioxidant defense in muscle samples from 16 critically ill patients compared to 10 healthy, age-matched people having elective hip surgery [48].
On the other hand, mitochondrial biogenesis is linked with an enhanced chance of longevity in septic shock, and pathological processes of sepsis-AKI indicate the crucial function of healthy mitochondria in renal recovery and lifespan [49,50,51,52,53]. In addition, participants with sepsis-AKI showed significantly lower mRNA signaling of SIRT1, which is implicated in regulating oxidative stress and biogenesis, than control participants [54]. In the case of pulmonary hypertension, activation of AMPK also slowed the growth of Gal-3-activated cells [55]. Additionally, when all these active biological components are connected, Sirtuin 1 can be activated indirectly via AMPK by increasing the availability of nicotinamide adenine dinucleotide (NAD+) in the setting of kidney injury. Protection against AMPK activation in the CLP model was related to enhanced sirtuin 1 expression [56,57], corroborating our findings. These studies consistently demonstrate that activating AMPK during sepsis protects against AKI and lowers mortality, independent of the underlying molecular mechanism [57]. Both AMPK and SIRT1 regulate each other and share several target molecules, and this review will assess the evidence that these similarities are due, at least in part, to reciprocal regulation (Figure 2).
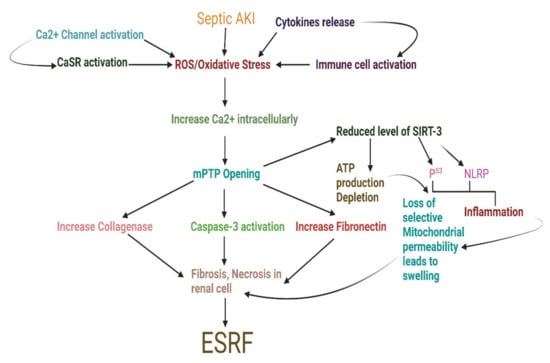
Figure 2.
Schematic diagram exploring sepsis-associated acute kidney injury progression through enhanced biomolecules burden to End-stage renal failure. AKI, acute kidney injury; ATP, adenosine triphosphate; CaSR, calcium-sensing receptor; ESRF, end-stage renal failure; mPTP, mitochondrial permeability transition pore; NLRP, NACHT, LRR, and PYD domains-containing protein; ROS, reactive oxygen species; SIRT3, sirtuin 3.
5. Evidence of Roflumilast at the Molecular Level for Acute Kidney Disease in Sepsis
The recruitment of inflammatory cells increases ROS generation at the damage site, aggravating renal impairment [58]. Free radicals activate inflammatory cells such as neutrophils and macrophages, promoting the generation of additional inflammatory mediators and uncontrolled ROS accumulation, thus establishing a vicious cycle of inflammatory responses and oxidative load via the nuclear factor kappa B (NF-κB) pathway [59]. Research indicated that the PDE-4 blocker roflumilast reduced lipopolysaccharides (LPS)-associated nitric oxide (NO), TNF-alpha, and IL-1beta expression in the macrophage cell line RAW 264.7 by lowering NFk-B stimulation, stress-activated protein kinases (SAPK)/Jun amino-terminal kinases (JNK), and p38 MAPK mechanisms [60]. Furthermore, the research results indicated that following administration of a PDE-4 inhibitor derivative, Sirt1, phosphoinositide 3-kinases (PI3Ks), and pAKT protein expression rise and renal function is effectively maintained. Injured neurons treated with PDE4 inhibitors exhibit increased Sirt1 expression, AKT phosphorylation, and decreased apoptosis [61]. Septic rats had a high bacterial load, myeloperoxidase activity, decreased cAMP concentration, increased vascular permeability, lowered Na + K + ATPase activity, increased caspase-3, excessive interstitial leukocyte infiltration, elevated renal expression of PDE-4B and 4D isoforms, and electrolyte imbalance in addition to histological changes. A large dosage of roflumilast pretreatment avoided all AKI-related symptoms in septic rats [62]. The activation of PDE-4 isoforms strongly contributes to the inflammatory response and the engagement of the immunological system. Inflammatory mediators such as TNF-α may be released by PDE-4B, one of the PDE-4 isoforms, on monocytes and neutrophils [63]. PDE-4B mutant mice could not secrete TNF-α from circulated leukocytes in response to LPS, revealing that this isoform plays a pivotal role in the pathogenesis of inflammatory disorders [64]. Additionally, suppression of PDE-4B rectified inflammatory cytokine generation and death in kidney tubular epithelial cells following cisplatin therapy. Other research reveals increased expression of PDE-4B and PDE-4D variants in the bronchiolar lavage fluid of treated animals with ovalbumin; however, inhibiting the PDE-4B subtype significantly cured rodent allergies [65]. Roflumilast at a large concentration significantly decreased the production of PDE-4B and PDE-4D subtypes, indicating that both variants significantly impact the sepsis AKI condition. A study found that activating the cAMP–CREB pathway decreased cytokine production and promoted inflammation [66]. These results demonstrate that silencing both variants was adequate for elevating cAMP concentrations in kidney homogenates and producing reno-protective benefits in the S-AKI model. Additionally, pre-treatment dosage decreased renal oxidative stress and inflammatory cytokine production in CLP rats’ renal homogenates. Additionally, this therapy inhibited the activity of myeloperoxidase (MPO) in kidney homogenates. MPO, a neutrophil-secreted enzyme, exhibits significant pro-oxidant and pro-inflammatory activity [67]. The MPO activity in the urine and plasma of S-AKI patients rose 24 h after neutrophil activation was boosted by IL-8, according to Törnblom and colleagues [68]. As a consequence of ROS overproduction, various cellular processes, including the polyol pathway, the MAPK, AMPK, and the NF-kB signaling pathways, as well as transcription factors such as activator protein 1 (AP-1), nuclear factor erythroid 2–related factor 2 (Nrf2), and forkhead box O (FOXO) are activated [69,70]. Our research also revealed that the serum of cecal ligation and puncture (CLP) septic rats had higher lactate dehydrogenase (LDH) activity, a sign of impaired aerobic respiration. Increased baseline lactate levels in sepsis were related to decreased renal microcirculation, hypoxia, and cellular dysmetabolism [71,72]. Sepsis causes an increase in anaerobic glycolysis and a buildup of lactate due to reduced renal perfusion and mitochondrial dysfunction [73]. This results in intracellular hypoxia, as most oxygen is used during anaerobic glycolysis to create ATP from lactate. Thus, renal tissue ATP levels fall short of supporting the activity of ATP-dependent transporters and enzymes in nephron segments. According to another study, CBU91 is a strong and selective PDE4 inhibitor that increases mitochondrial activity in myotubes while activating Sirt1 and AMPK [14]. Notably, AMPK and SIRT1 act synergistically and have comparable impacts on various biological functions, including DNA repair, cell proliferation, mitochondrial function, and cell metabolism [74]. For example, AMPK enhances SIRT1 activity by raising the intercellular NAD+ concentration [75]. Alternatively, SIRT1 deacetylates and activates liver kinase B1 (LKB1), AMPK’s upstream kinase, activating AMPK and suppressing ROS generation [76,77]. SIRT1 and AMPK have several shared targets, including FOXOs, the peroxisome proliferator-activated receptors (PPARs), and peroxisome proliferator-activated receptor-gamma coactivator (PGC). Joint activation of SIRT1 and AMPK can activate these downstream effectors independently or in combination, exerting antioxidant protective responses and alleviating oxidative stress [15]. In this experiment, pre-treatment with a PDE-4 inhibitor significantly reduced LDH levels, showing that this medication is beneficial in addressing metabolic imbalance and tissue hypoperfusion associated with septic AKI (Figure 3).
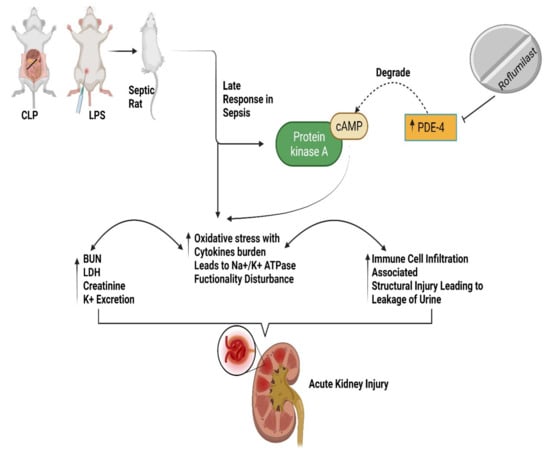
Figure 3.
Represents septic rats with PDE-4 inhibition leading to increased cAMP levels, alleviating renal impairment, oxidative and inflammatory stress, and decreasing immune cell infiltration and leakage in urine [15]. BUN, Blood urea nitrogen; cAMP, cyclic adenosine monophosphate; cecal ligation and puncture (CLP), LDH, lactate dehydrogenase; lipopolysaccharide (LPS); PDE-4, phosphodiesterase-4.
In prior research, roflumilast therapy ameliorated diabetic nephropathy in rats by restoring kidney function, reducing oxidative stress, and accumulating extracellular matrix, minimizing glomerular damage and apoptosis [15]. Furthermore, apoptosis, a controlled cell death, is critical for the damage and growth of multicellular organisms across various tissues [78]. A combination of inflammation and apoptosis may influence the development of AKI in sepsis. This study observed that CLP enhanced the number of apoptotic cells, but roflumilast therapy reversed this effect. Additionally, roflumilast treatment dramatically improved Bcl-2 expression, an anti-apoptotic marker lowered in septic animals [79].
On the other hand, roflumilast had the opposite effect on cleaved caspase-3, cleaved caspase-9, and Bax, all of which were increased in septic mice, indicating that roflumilast may protect animals against kidney damage in part by inhibiting cell death [80]. The p38 MAPK phosphorylation was likewise dramatically lowered by Roflumilast. The roflumilast intervention improved the tissue morphology of sepsis mice by reducing JAK and STAT-3 protein and mRNA expression [81] and, making things even more interesting, roflumilast significantly decreased the activity of STAT3, as well as Janus kinase 1 and Janus kinase 2, which are both downstream of STAT3. Furthermore, phosphorylation of p38 MAPK by interferon-gamma and TNF-alpha in HaCaT cells was dramatically inhibited by cAMP-dependent PKA activity [82,83]. It seems that roflumilast guards against sepsis by suppressing STAT3, MAPK, and NF-kB signalling pathways [67].
KIM1 and neutrophil gelatinase-associated lipocalin (NGAL) expression levels are the primary markers of early AKI, and their monitoring is critical for guiding AKI prognosis. KIM1, an extracellular and cytoplasmic domain, is expressed at deficient levels in normal kidneys [84]. Extracellular fragments may cleave and enter tubule lumens after kidney damage, which may be detected in the urine. A characteristic of renal tubular impairment is the presence of NGAL [85]. Severe sepsis patients may benefit from using NGAL as a diagnostic and predictive biomarker for AKI because of its high sensitivity and low negative predictive value [86,87]. Additionally, the study’s experimental results indicated that roflumilast could decrease the expression of KIM1 and NGAL, which were raised in sepsis-induced AKI, implying that roflumilast acted as a protective factor for kidney function. The pathological alterations associated with sepsis-induced AKI in the kidney are thought to be strongly connected to the course of the disease, as evidenced by the histological findings in various investigations [88].
For individuals with COPD, the recommended dose of roflumilast is 500 g/day. For the first time, roflumilast has been shown to protect mice against CLP-caused sepsis, with a statistically significant level of protection at 1.0 mg/kg. Roflumilast dosed at 1.0 mg/kg in mice is comparable to 0.08 mg/kg in humans, as determined by the FDA’s “Conversion of Animal Doses to Human Comparable Doses” [89,90,91]. Although roflumilast is often given in the range of 1–10 mg/kg in animal studies, it is worth noting that the study’s minimum effective dose of roflumilast is almost tenfold that of the recommended human dosage for COPD.
6. Conclusions
PDE-4 inhibitors and roflumilast decreased S-AKI by lowering immune cell infiltration into renal tissues and urine leakage, as observed by us. In addition, researchers found that Roflumilast influenced antioxidant and inflammation levels and Na + K + ATPase activity through the upregulation of cAMP levels in kidney tissue homogenates for these immunosuppressive effects. According to the reviewed literature, pre-treatment with Roflumilast enhanced kidney function and decreased histological deterioration in septic rats’ renal tubules. Roflumilast, a PDE-4 inhibitor, has been shown to defend against S-AKI in an animal model of septic shock, and this new information sheds light on how it works.
Author Contributions
I.K. designed the extensive review and prepared the initial draft, F.A.A.-A., M.A., M.S.N., H.N.A. and G.G. critically revised manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by Institutional Fund Projects under grant no. IFPRP: 264-130-1442.
Data Availability Statement
Data sharing not applicable.
Acknowledgments
This research work was funded by Institutional Fund Projects under grant no. IFPRP: 264-130-1442. Therefore, authors gratefully acknowledge technical and financial support from the Ministry of Education and King Abdulaziz University, DSR, Jeddah, Saudi Arabia.
Conflicts of Interest
The authors disclose that they have no conflicting interests.
References
- Berdeaux, R.; Stewart, R. cAMP signaling in skeletal muscle adaptation: Hypertrophy, metabolism, and regeneration. Am. J. Physiol. Endocrinol. Metab. 2012, 303, E1–E17. [Google Scholar] [CrossRef] [PubMed]
- Keravis, T.; Lugnier, C. Cyclic nucleotide phosphodiesterase (PDE) isozymes as targets of the intracellular signalling network: Benefits of PDE inhibitors in various diseases and perspectives for future therapeutic developments. J. Cereb. Blood Flow Metab. 2012, 165, 1288–1305. [Google Scholar] [CrossRef]
- Gancedo, J.M. Biological roles of cAMP: Variations on a theme in the different kingdoms of life. Biol. Rev. 2013, 88, 645–668. [Google Scholar] [CrossRef] [PubMed]
- Maurice, D.H.; Ke, H.; Ahmad, F.; Wang, Y.; Chung, J.; Manganiello, V.C. Advances in targeting cyclic nucleotide phosphodiesterases. Nat. Rev. Drug Discov. 2014, 13, 290–314. [Google Scholar] [CrossRef] [PubMed]
- Calverley, P.M.; Rabe, K.F.; Goehring, U.-M.; Kristiansen, S.; Fabbri, L.M.; Martinez, F.J. Roflumilast in symptomatic chronic obstructive pulmonary disease: Two randomised clinical trials. Lancet 2009, 374, 685–694. [Google Scholar] [CrossRef]
- Kim, S.; Kim, H.-J.; Ahn, H.-S.; Song, J.Y.; Um, T.-H.; Cho, C.-R.; Jung, H.; Koo, H.-K.; Park, J.H.; Lee, S.-S.; et al. Is plasma neutrophil gelatinase-associated lipocalin a predictive biomarker for acute kidney injury in sepsis patients? A systematic review and meta-analysis. J. Crit. Care 2016, 33, 213–223. [Google Scholar] [CrossRef]
- Martinez, F.J.; Rabe, K.F.; Sethi, S.; Pizzichini, E.; McIvor, A.; Anzueto, A.; Alagappan, V.K.; Siddiqui, S.; Rekeda, L.; Miller, C.J.; et al. Effect of Roflumilast and Inhaled Corticosteroid/Long-Acting β2-Agonist on Chronic Obstructive Pulmonary Disease Exacerbations (RE(2)SPOND). A Randomized Clinical Trial. Am. J. Respir. Crit. Care Med. 2016, 194, 559–567. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.-F.; Huang, Y.; Amsdell, S.L.; Xiao, L.; O’Donnell, J.M.; Zhang, H.-T. Antidepressant- and Anxiolytic-like Effects of the Phosphodiesterase-4 Inhibitor Rolipram on Behavior Depend on Cyclic AMP Response Element Binding Protein-Mediated Neurogenesis in the Hippocampus. Neuropsychopharmacology 2009, 34, 2404–2419. [Google Scholar] [CrossRef]
- Korhonen, R.; Hömmö, T.; Keränen, T.; Laavola, M.; Hämäläinen, M.; Vuolteenaho, K.; Lehtimäki, L.; Kankaanranta, H.; Moilanen, E. Attenuation of TNF production and experimentally induced inflammation by PDE4 inhibitor rolipram is mediated by MAPK phosphatase-1. Br. J. Pharmacol. 2013, 169, 1525–1536. [Google Scholar] [CrossRef]
- Field, S.K. Roflumilast, a Novel Phosphodiesterase 4 Inhibitor, for COPD Patients with a History of Exacerbations. Clin. Med. Insights: Circ. Respir. Pulm. Med. 2011, 5, 57–70. [Google Scholar] [CrossRef]
- Vollert, S.; Kaessner, N.; Heuser, A.; Hanauer, G.; Dieckmann, A.; Knaack, D.; Kley, H.P.; Beume, R.; Weiss-Haljiti, C. The glucose-lowering effects of the PDE4 inhibitors roflumilast and roflumilast-N-oxide in db/db mice. Diabetologia 2012, 55, 2779–2788. [Google Scholar] [CrossRef] [PubMed]
- Jensterle, M.; Kocjan, T.; Janez, A. Phosphodiesterase 4 Inhibition as a Potential New Therapeutic Target in Obese Women with Polycystic Ovary Syndrome. J. Clin. Endocrinol. Metab. 2014, 99, E1476–E1481. [Google Scholar] [CrossRef] [PubMed]
- Chung, J.H. Metabolic benefits of inhibiting cAMP-PDEs with resveratrol. Adipocyte 2012, 1, 256–258. [Google Scholar] [CrossRef] [PubMed]
- Park, S.-J.; Ahmad, F.; Bahde, R.J.; Philp, A.; Kim, J.; Huang, T.; Kim, M.K.; Trenkle, W.C.; Chung, J.H. Potent PDE4 inhibitor activates AMPK and Sirt1 to induce mitochondrial biogenesis. PLoS ONE 2021, 16, e0253269. [Google Scholar] [CrossRef]
- Tikoo, K.; Lodea, S.; Karpe, P.A.; Kumar, S. Calorie restriction mimicking effects of roflumilast prevents diabetic nephropathy. Biochem. Biophys. Res. Commun. 2014, 450, 1581–1586. [Google Scholar] [CrossRef]
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.-D.; Coopersmith, C.M.; et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef]
- Bagshaw, S.M.; George, C.; Bellomo, R.; The ANZICS Database Management Committee. Early acute kidney injury and sepsis: A multicentre evaluation. Crit. Care 2008, 12, R47. [Google Scholar] [CrossRef]
- Bagshaw, S.M.; Uchino, S.; Bellomo, R.; Morimatsu, H.; Morgera, S.; Schetz, M.; Tan, I.; Bouman, C.; Macedo, E.; Gibney, N.; et al. Septic acute kidney injury in critically ill patients: Clinical characteristics and outcomes. Clin. J. Am. Soc. Nephrol. CJASN 2007, 2, 431–439. [Google Scholar] [CrossRef]
- Barbar, S.D.; Binquet, C.; Monchi, M.; Bruyère, R.; Quenot, J.-P. Impact on mortality of the timing of renal replacement therapy in patients with severe acute kidney injury in septic shock: The IDEAL-ICU study (initiation of dialysis early versus delayed in the intensive care unit): Study protocol for a randomized controlled trial. Trials 2014, 15, 270. [Google Scholar] [CrossRef]
- Mickells, G.E.; Moga, M.-A.; Smith, C.M. Acute Kidney Injury in Pediatric Sepsis. Clin. Pediatr. Emerg. Med. 2014, 15, 185–192. [Google Scholar] [CrossRef]
- Alobaidi, R.; Basu, R.K.; Goldstein, S.L.; Bagshaw, S.M. Sepsis-associated acute kidney injury. Semin. Nephrol. 2015, 35, 2–11. [Google Scholar] [CrossRef]
- Peerapornratana, S.; Manrique-Caballero, C.L.; Gómez, H.; Kellum, J.A. Acute kidney injury from sepsis: Current concepts, epidemiology, pathophysiology, prevention and treatment. Kidney Int. 2019, 96, 1083–1099. [Google Scholar] [CrossRef]
- Coca, S.G.; Singanamala, S.; Parikh, C.R. Chronic kidney disease after acute kidney injury: A systematic review and meta-analysis. Kidney Int. 2012, 81, 442–448. [Google Scholar] [CrossRef]
- Gallagher, M.; Cass, A.; Bellomo, R.; Finfer, S.; Gattas, D.; Lee, J.; Lo, S.; McGuinness, S.; Myburgh, J.; Parke, R.; et al. Long-Term Survival and Dialysis Dependency Following Acute Kidney Injury in Intensive Care: Extended Follow-up of a Randomized Controlled Trial. PLOS Med. 2014, 11, e1001601. [Google Scholar] [CrossRef]
- Wald, R.; Shariff, S.Z.; Adhikari, N.K.; Bagshaw, S.M.; Burns, K.E.; Friedrich, J.O.; Garg, A.X.; Harel, Z.; Kitchlu, A.; Ray, J.G. The association between renal replacement therapy modality and long-term outcomes among critically ill adults with acute kidney injury: A retrospective cohort study. Crit. Care Med. 2014, 42, 868–877. [Google Scholar] [CrossRef]
- Godin, M.; Murray, P.; Mehta, R.L. Clinical Approach to the Patient with AKI and Sepsis. Semin. Nephrol. 2015, 35, 12–22. [Google Scholar] [CrossRef]
- Bellomo, R.; Ronco, C.; Kellum, J.A.; Mehta, R.L.; Palevsky, P.; Acute Dialysis Quality Initiative Workgroup. Acute renal failure—Definition, outcome measures, animal models, fluid therapy and information technology needs: The Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit. Care 2004, 8, R204–R212. [Google Scholar] [CrossRef] [PubMed]
- Khwaja, A. KDIGO Clinical Practice Guidelines for Acute Kidney Injury. Nephron Exp. Nephrol. 2012, 120, c179–c184. [Google Scholar] [CrossRef]
- Xiao, H.; Siddiqui, J.; Remick, D.G. Mechanisms of Mortality in Early and Late Sepsis. Infect. Immun. 2006, 74, 5227–5235. [Google Scholar] [CrossRef]
- Kim, J.-S.; Kim, S.-J.; Lee, S.-M. Genipin attenuates sepsis-induced immunosuppression through inhibition of T lymphocyte apoptosis. Int. Immunopharmacol. 2015, 27, 15–23. [Google Scholar] [CrossRef]
- Adib-Conquy, M.; Cavaillon, J.-M. Stress molecules in sepsis and systemic inflammatory response syndrome. FEBS Lett. 2007, 581, 3723–3733. [Google Scholar] [CrossRef] [PubMed]
- De Pablo, R.; Monserrat, J.; Prieto, A.; Álvarez-Mon, M. Role of Circulating Lymphocytes in Patients with Sepsis. BioMed Res. Int. 2014, 2014, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Zarbock, A.; Gomez, H.; Kellum, J.A. Sepsis-induced acute kidney injury revisited: Pathophysiology, prevention and future therapies. Curr. Opin. Crit. Care 2014, 20, 588–595. [Google Scholar] [CrossRef] [PubMed]
- Salvesen, Ø.; Reiten, M.R.; Kamstra, J.H.; Bakkebø, M.K.; Espenes, A.; Tranulis, M.A.; Ersdal, C. Goats without Prion Protein Display Enhanced Proinflammatory Pulmonary Signaling and Extracellular Matrix Remodeling upon Systemic Lipopolysaccharide Challenge. Front. Immunol. 2017, 8, 1722. [Google Scholar] [CrossRef]
- Osuchowski, M.F.; Welch, K.; Yang, H.; Siddiqui, J.; Remick, D.G. Chronic Sepsis Mortality Characterized by an Individualized Inflammatory Response. J. Immunol. 2007, 179, 623–630. [Google Scholar] [CrossRef]
- Demaret, J.; Venet, F.; Friggeri, A.; Cazalis, M.-A.; Plassais, J.; Jallades, L.; Malcus, C.; Poitevin-Later, F.; Textoris, J.; Lepape, A.; et al. Marked alterations of neutrophil functions during sepsis-induced immunosuppression. J. Leukoc. Biol. 2015, 98, 1081–1090. [Google Scholar] [CrossRef]
- De Boer, R.A.; Yu, L.; van Veldhuisen, D.J. Galectin-3 in Cardiac Remodeling and Heart Failure. Curr. Heart Fail. Rep. 2010, 7, 1–8. [Google Scholar] [CrossRef]
- Winyard, P.J.; Bao, Q.; Hughes, R.C.; Woolf, A. Epithelial galectin-3 during human nephrogenesis and childhood cystic diseases. J. Am. Soc. Nephrol. 1997, 8, 1647–1657. [Google Scholar] [CrossRef]
- Nishiyama, J.; Kobayashi, S.; Ishida, A.; Nakabayashi, I.; Tajima, O.; Miura, S.; Katayama, M.; Nogami, H. Up-Regulation of Galectin-3 in Acute Renal Failure of the Rat. Am. J. Pathol. 2000, 157, 815–823. [Google Scholar] [CrossRef]
- Bullock, S.; Johnson, T.M.; Bao, Q.; Hughes, R.C.; Winyard, P.J.D.; Woolf, A. Galectin-3 Modulates Ureteric Bud Branching in Organ Culture of the Developing Mouse Kidney. J. Am. Soc. Nephrol. 2001, 12, 515–523. [Google Scholar] [CrossRef]
- Henderson, N.C.; Mackinnon, A.C.; Farnworth, S.L.; Kipari, T.; Haslett, C.; Iredale, J.P.; Liu, F.-T.; Hughes, J.; Sethi, T. Galectin-3 Expression and Secretion Links Macrophages to the Promotion of Renal Fibrosis. Am. J. Pathol. 2008, 172, 288–298. [Google Scholar] [CrossRef]
- Okamura, D.M.; Pasichnyk, K.; Lopez-Guisa, J.M.; Collins, S.; Hsu, D.K.; Liu, F.-T.; Eddy, A.A. Galectin-3 preserves renal tubules and modulates extracellular matrix remodeling in progressive fibrosis. Am. J. Physiol. Physiol. 2011, 300, F245–F253. [Google Scholar] [CrossRef]
- Sun, H.; Jiang, H.; Eliaz, A.; Kellum, J.A.; Peng, Z.; Eliaz, I. Galectin-3 in septic acute kidney injury: A translational study. Crit. Care 2021, 25, 1–11. [Google Scholar] [CrossRef]
- Parikh, S.M.; Yang, Y.; He, L.; Tang, C.; Zhan, M.; Dong, Z. Mitochondrial Function and Disturbances in the Septic Kidney. Semin. Nephrol. 2015, 35, 108–119. [Google Scholar] [CrossRef]
- Emma, F.; Montini, G.; Parikh, S.M.; Salviati, L. Mitochondrial dysfunction in inherited renal disease and acute kidney injury. Nat. Rev. Nephrol. 2016, 12, 267–280. [Google Scholar] [CrossRef]
- Cecconi, M.; Evans, L.; Levy, M.; Rhodes, A. Sepsis and septic shock. Lancet 2018, 392, 75–87. [Google Scholar] [CrossRef]
- Brealey, D.; Brand, M.; Hargreaves, I.; Heales, S.; Land, J.; Smolenski, R.; Davies, N.A.; Cooper, C.E.; Singer, M. Association between mitochondrial dysfunction and severity and outcome of septic shock. Lancet 2002, 360, 219–223. [Google Scholar] [CrossRef]
- Carré, J.E.; Orban, J.-C.; Re, L.; Felsmann, K.; Iffert, W.; Bauer, M.; Suliman, H.B.; Piantadosi, C.A.; Mayhew, T.M.; Breen, P.; et al. Survival in Critical Illness Is Associated with Early Activation of Mitochondrial Biogenesis. Am. J. Respir. Crit. Care Med. 2010, 182, 745–751. [Google Scholar] [CrossRef]
- Tran, M.; Tam, D.; Bardia, A.; Bhasin, M.; Rowe, G.C.; Kher, A.; Zsengeller, Z.K.; Akhavan-Sharif, M.R.; Khankin, E.V.; Saintgeniez, M.; et al. PGC-1α promotes recovery after acute kidney injury during systemic inflammation in mice. J. Clin. Investig. 2011, 121, 4003–4014. [Google Scholar] [CrossRef]
- Tran, M.T.; Zsengeller, Z.K.; Berg, A.H.; Khankin, E.V.; Bhasin, M.K.; Kim, W.; Clish, C.B.; Stillman, I.E.; Karumanchi, S.A.; Rhee, E.P.; et al. PGC1α drives NAD biosynthesis linking oxidative metabolism to renal protection. Nature 2016, 531, 528–532. [Google Scholar] [CrossRef]
- Funk, J.A.; Schnellmann, R.G. Persistent disruption of mitochondrial homeostasis after acute kidney injury. Am. J. Physiol. Physiol. 2012, 302, F853–F864. [Google Scholar] [CrossRef]
- Gunst, J.; Derese, I.; Aertgeerts, A.; Ververs, E.J.; Wauters, A.; Van den Berghe, G.; Vanhorebeek, I. Insufficient autophagy contributes to mitochondrial dysfunction, organ failure, and adverse outcome in an animal model of critical illness. Crit. Care Med. 2013, 41, 182–194. [Google Scholar] [CrossRef]
- Stallons, L.J.; Funk, J.A.; Schnellmann, R.G. Mitochondrial Homeostasis in Acute Organ Failure. Curr. Pathobiol. Rep. 2013, 1, 169–177. [Google Scholar] [CrossRef]
- Van der Slikke, E.C.; Star, B.S.; van Meurs, M.; Henning, R.H.; Moser, J.; Bouma, H.R. Sepsis is associated with mitochondrial DNA damage and a reduced mitochondrial mass in the kidney of patients with sepsis-AKI. Crit. Care 2021, 25, 1–13. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, W.; Zhu, Y.; Wang, Q.; Zhai, C.; Shi, W.; Feng, W.; Wang, J.; Yan, X.; Chai, L.; et al. Activation of AMPK inhibits Galectin-3-induced pulmonary artery smooth muscle cells proliferation by upregulating hippo signaling effector YAP. Mol. Cell. Biochem. 2021, 476, 3037–3049. [Google Scholar] [CrossRef]
- Jin, K.; Ma, Y.; Manrique-Caballero, C.L.; Li, H.; Emlet, D.R.; Li, S.; Baty, C.J.; Wen, X.; Kim-Campbell, N.; Frank, A.; et al. Activation of AMP-activated protein kinase during sepsis/inflammation improves survival by preserving cellular metabolic fitness. FASEB J. 2020, 34, 7036–7057. [Google Scholar] [CrossRef]
- Tan, C.; Gu, J.; Li, T.; Chen, H.; Liu, K.; Liu, M.; Zhang, H.; Xiao, X. Inhibition of aerobic glycolysis alleviates sepsis-induced acute kidney injury by promoting lactate/Sirtuin 3/AMPK-regulated autophagy. Int. J. Mol. Med. 2021, 47, 19. [Google Scholar] [CrossRef]
- Mittal, M.; Siddiqui, M.R.; Tran, K.; Reddy, S.P.; Malik, A.B. Reactive Oxygen Species in Inflammation and Tissue Injury. Antioxid. Redox Signal. 2014, 20, 1126–1167. [Google Scholar] [CrossRef]
- Sanlioglu, S.; Williams, C.M.; Samavati, L.; Butler, N.S.; Wang, G.; McCray, P.B., Jr.; Ritchie, T.C.; Hunninghake, G.W.; Zandi, E.; Engelhardt, J.F. Lipopolysaccharide induces Rac1-dependent reactive oxygen species formation and coordinates tumor necrosis factor-alpha secretion through IKK regulation of NF-kappa B. J. Biol. Chem. 2001, 276, 30188–30198. [Google Scholar] [CrossRef]
- Kwak, H.J.; Song, J.S.; Heo, J.Y.; Yang, S.D.; Nam, J.Y.; Cheon, H.G. Roflumilast inhibits lipopolysaccharide-induced inflammatory mediators via suppression of nuclear factor-kappaB, p38 mitogen-activated protein kinase, and c-Jun NH2-terminal kinase activation. J. Pharmacol. Exp. Ther. 2005, 315, 1188–1195. [Google Scholar] [CrossRef]
- Xu, M.; Yu, X.; Meng, X.; Huang, S.; Zhang, Y.; Zhang, A.; Jia, Z. Inhibition of PDE4/PDE4B improves renal function and ameliorates inflammation in cisplatin-induced acute kidney injury. Am. J. Physiol. Ren. Physiol. 2020, 318, F576–F588. [Google Scholar] [CrossRef] [PubMed]
- Dua, K.; Gupta, G.; Awasthi, R.; Chellappan, D.K. Why is there an emerging need to look for a suitable drug delivery platform in targeting and regulating microbiota? Panminerva Med. 2018, 60, 136–137. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Wu, P.; Ohleth, K.M.; Egan, R.W.; Billah, M.M. Phosphodiesterase 4B2 Is the Predominant Phosphodiesterase Species and Undergoes Differential Regulation of Gene Expression in Human Monocytes and Neutrophils. Mol. Pharmacol. 1999, 56, 170–174. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.-L.C.; Conti, M. Induction of the cyclic nucleotide phosphodiesterase PDE4B is essential for LPS-activated TNF-α responses. Proc. Natl. Acad. Sci. USA 2002, 99, 7628–7633. [Google Scholar] [CrossRef]
- Zheng, X.Y.; Chen, J.C.; Xie, Q.M.; Chen, J.Q.; Tang, H.F. Anti-inflammatory effect of ciclamilast in an allergic model involving the expression of PDE4B. Mol. Med. Rep. 2019, 19, 1728–1738. [Google Scholar] [CrossRef]
- Raker, V.K.; Becker, C.; Steinbrink, K. The cAMP Pathway as Therapeutic Target in Autoimmune and Inflammatory Diseases. Front. Immunol. 2016, 7, 123. [Google Scholar] [CrossRef]
- Souto, F.O.; Alves-Filho, J.C.; Turato, W.M.; Auxiliadora-Martins, M.; Basile-Filho, A.; Cunha, F.Q. Essential Role of CCR2 in Neutrophil Tissue Infiltration and Multiple Organ Dysfunction in Sepsis. Am. J. Respir. Crit. Care Med. 2011, 183, 234–242. [Google Scholar] [CrossRef]
- Törnblom, S.; Nisula, S.; Vaara, S.T.; Poukkanen, M.; Andersson, S.; Pettilä, V.; Pesonen, E. Neutrophil activation in septic acute kidney injury: A post hoc analysis of the FINNAKI study. Acta Anaesthesiol. Scand. 2019, 63, 1390–1397. [Google Scholar] [CrossRef]
- Keane, K.N.; Cruzat, V.F.; Carlessi, R.; de Bittencourt, P.I., Jr.; Newsholme, P. Molecular Events Linking Oxidative Stress and Inflammation to Insulin Resistance and β-Cell Dysfunction. Oxidative Med. Cell. Longev. 2015, 2015, 181643. [Google Scholar] [CrossRef]
- Volpe, C.M.O.; Villar-Delfino, P.H.; Dos Anjos, P.M.F.; Nogueira-Machado, J.A. Cellular death, reactive oxygen species (ROS) and diabetic complications. Cell Death Dis. 2018, 9, 119. [Google Scholar] [CrossRef]
- Casserly, B.; Phillips, G.S.; Schorr, C.; Dellinger, R.P.; Townsend, S.R.; Osborn, T.M.; Reinhart, K.; Selvakumar, N.; Levy, M.M. Lactate measurements in sepsis-induced tissue hypoperfusion: Results from the Surviving Sepsis Campaign database. Crit. Care Med. 2015, 43, 567–573. [Google Scholar] [CrossRef]
- Bakker, J. Lactate levels and hemodynamic coherence in acute circulatory failure. Best Pract. Res. Clin. Anaesthesiol. 2016, 30, 523–530. [Google Scholar] [CrossRef]
- Van Wyngene, L.; Vandewalle, J.; Libert, C. Reprogramming of basic metabolic pathways in microbial sepsis: Therapeutic targets at last? EMBO Mol. Med. 2018, 10, e8712. [Google Scholar] [CrossRef]
- Yuan, Y.; Shi, M.; Li, L.; Liu, J.; Chen, B.; Chen, Y.; An, X.; Liu, S.; Luo, R.; Long, D.; et al. Mesenchymal stem cell-conditioned media ameliorate diabetic endothelial dysfunction by improving mitochondrial bioenergetics via the Sirt1/AMPK/PGC-1α pathway. Clin. Sci. 2016, 130, 2181–2198. [Google Scholar] [CrossRef]
- Ruderman, N.B.; Xu, X.J.; Nelson, L.; Cacicedo, J.M.; Saha, A.K.; Lan, F.; Ido, Y. AMPK and SIRT1: A long-standing partnership? Am. J. Physiol. Endocrinol. Metab. 2010, 298, E751–E760. [Google Scholar] [CrossRef]
- Chen, S.; Xiao, X.; Feng, X.; Li, W.; Zhou, N.; Zheng, L.; Sun, Y.; Zhang, Z.; Zhu, W. Resveratrol induces Sirt1-dependent apoptosis in 3T3-L1 preadipocytes by activating AMPK and suppressing AKT activity and survivin expression. J. Nutr. Biochem. 2011, 23, 1100–1112. [Google Scholar] [CrossRef]
- Zheng, Z.; Chen, H.; Li, J.; Li, T.; Zheng, B.; Zheng, Y.; Jin, H.; He, Y.; Gu, Q.; Xu, X. Sirtuin 1–Mediated Cellular Metabolic Memory of High Glucose Via the LKB1/AMPK/ROS Pathway and Therapeutic Effects of Metformin. Diabetes 2012, 61, 217–228. [Google Scholar] [CrossRef]
- Liu, W.; Dong, M.; Bo, L.; Li, C.; Liu, Q.; Li, Z.; Jin, F. Epigallocatechin-3-gallate suppresses alveolar epithelial cell apoptosis in seawater aspiration-induced acute lung injury via inhibiting STAT1-caspase-3/p21 associated pathway. Mol. Med. Rep. 2016, 13, 829–836. [Google Scholar] [CrossRef]
- Lin, Z.; Jin, J.; Shan, X. Fish oils protects against cecal ligation and puncture-induced septic acute kidney injury via the regulation of inflammation, oxidative stress and apoptosis. Int. J. Mol. Med. 2019, 44, 1771–1780. [Google Scholar] [CrossRef]
- Zhao, H.; Liu, Z.; Shen, H.; Jin, S.; Zhang, S. Glycyrrhizic acid pretreatment prevents sepsis-induced acute kidney injury via suppressing inflammation, apoptosis and oxidative stress. Eur. J. Pharmacol. 2016, 781, 92–99. [Google Scholar] [CrossRef]
- Chang, X.; Hu, L.-F.; Ma, X.-J.; Yin, J.; Liu, X.-Y.; Li, J.-B. Influence of roflumilast on sepsis mice through the JAK/STAT signaling pathway. Eur. Rev. Med Pharmacol. Sci. 2019, 23, 1335–1341. [Google Scholar]
- Qi, X.-F.; Kim, D.-H.; Yoon, Y.-S.; Li, J.-H.; Song, S.-B.; Jin, D.; Huang, X.-Z.; Teng, Y.-C.; Lee, K.-J. The adenylyl cyclase-cAMP system suppresses TARC/CCL17 and MDC/CCL22 production through p38 MAPK and NF-κB in HaCaT keratinocytes. Mol. Immunol. 2009, 46, 1925–1934. [Google Scholar] [CrossRef]
- Feng, H.; Chen, J.; Wang, H.; Cheng, Y.; Zou, Z.; Zhong, Q.; Xu, J. Roflumilast reverses polymicrobial sepsis-induced liver damage by inhibiting inflammation in mice. Lab. Investig. 2017, 97, 1008–1019. [Google Scholar] [CrossRef]
- Yin, C.; Wang, N. Kidney injury molecule-1 in kidney disease. Ren. Fail. 2016, 38, 1567–1573. [Google Scholar] [CrossRef]
- Abassi, Z.; Sagi, O.; Armaly, Z.; Bishara, B. Neutrophil gelatinase-associated lipocalin (NAGL): A novel biomarker for acute kidney injury. Harefuah 2011, 150, 111–116. [Google Scholar]
- Dai, X.; Zeng, Z.; Fu, C.; Zhang, S.; Cai, Y.; Chen, Z. Diagnostic value of neutrophil gelatinase-associated lipocalin, cystatin C, and soluble triggering receptor expressed on myeloid cells-1 in critically ill patients with sepsis-associated acute kidney injury. Crit. Care 2015, 19, 1–10. [Google Scholar] [CrossRef]
- Kim, S.W.; Kim, J.H.; Park, C.K.; Kim, T.J.; Lee, S.Y.; Kim, Y.K.; Kwon, S.S.; Rhee, C.K.; Yoon, H.K. Effect of roflumilast on airway remodelling in a murine model of chronic asthma. Clinical and experimental allergy. J. Br. Soc. Allergy Clin. Immunol. 2016, 46, 754–763. [Google Scholar] [CrossRef]
- Xu, X.; Liao, L.; Hu, B.; Jiang, H.; Tan, M. Roflumilast, a Phosphodiesterases-4 (PDE4) Inhibitor, Alleviates Sepsis-induced Acute Kidney Injury. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2020, 26, e921319. [Google Scholar] [CrossRef] [PubMed]
- Hatzelmann, A.; Morcillo, E.J.; Lungarella, G.; Adnot, S.; Sanjar, S.; Beume, R.; Schudt, C.; Tenor, H. The preclinical pharmacology of roflumilast—A selective, oral phosphodiesterase 4 inhibitor in development for chronic obstructive pulmonary disease. Pulm. Pharmacol. Ther. 2010, 23, 235–256. [Google Scholar] [CrossRef]
- Aldrich, A.; Bosch, M.E.; Fallet, R.; Odvody, J.; Burkovetskaya, M.; Rao, K.V.R.; Cooper, J.; Drack, A.; Kielian, T. Efficacy of phosphodiesterase-4 inhibitors in juvenile Batten disease (CLN3). Ann. Neurol. 2016, 80, 909–923. [Google Scholar] [CrossRef]
- Koga, H.; Recke, A.; Vidarsson, G.; Pas, H.; Jonkman, M.F.; Hashimoto, T.; Kasprick, A.; Ghorbanalipoor, S.; Tenor, H.; Zillikens, D.; et al. PDE4 Inhibition as Potential Treatment of Epidermolysis Bullosa Acquisita. J. Investig. Dermatol. 2016, 136, 2211–2220. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).