Analgesic and Anesthetic Efficacy of Rocuronium/Sugammadex in Otorhinolaryngologic Surgery: A Propensity Score-Matched Analysis
Abstract
:1. Introduction
2. Results
3. Discussion
Limitations
4. Materials and Methods
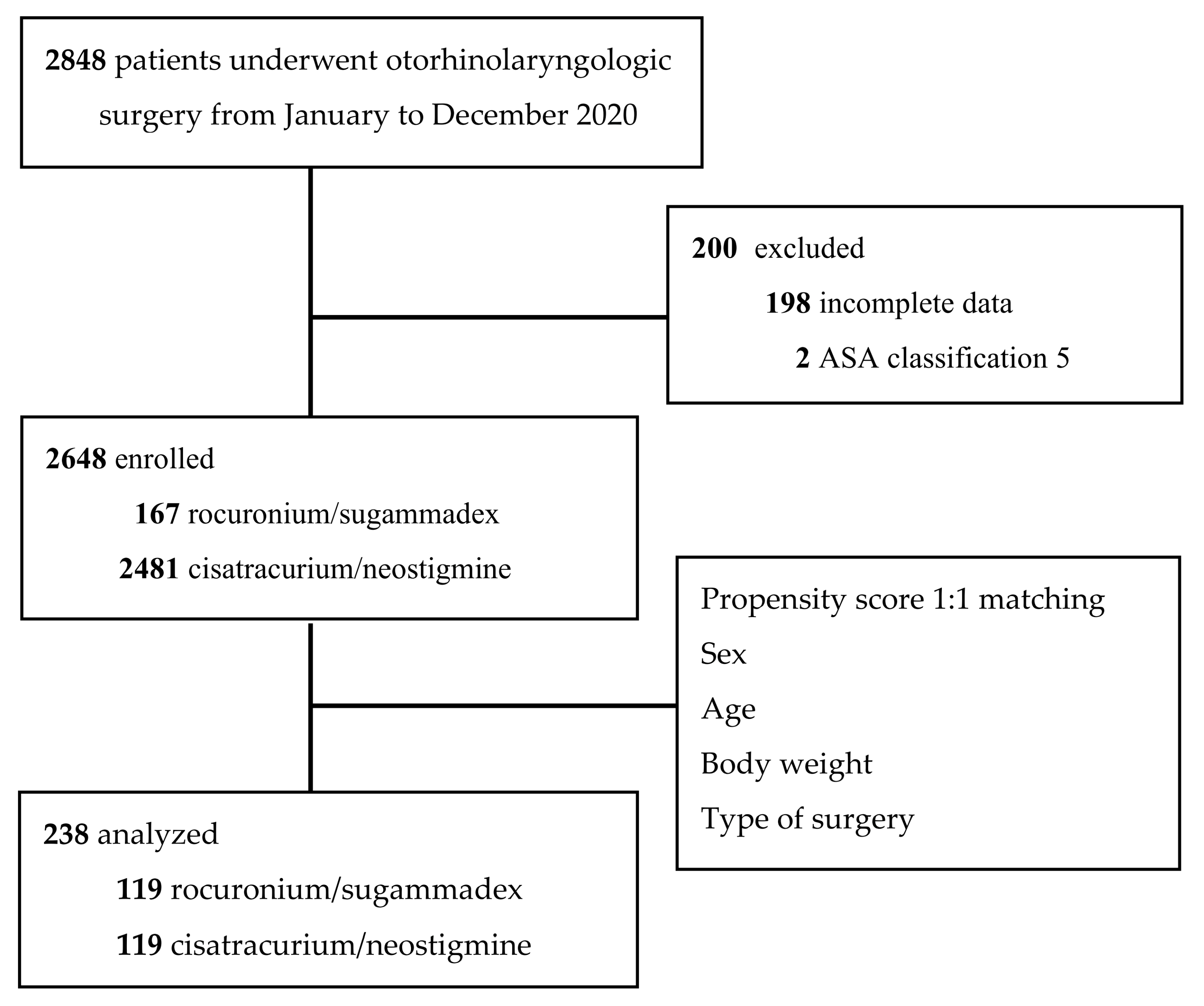
4.1. Data Collection and Study Design
4.2. Anesthesia Management
4.3. Primary and Secondary Outcomes
4.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kim, H.J.; Lee, K.; Park, W.K.; Lee, B.R.; Joo, H.M.; Koh, Y.W.; Seo, Y.W.; Kim, W.S.; Yoo, Y.C. Deep neuromuscular block improves the surgical conditions for laryngeal microsurgery. Br. J. Anaesth. 2015, 115, 867–872. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cedborg, A.I.; Sundman, E.; Boden, K.; Hedstrom, H.W.; Kuylenstierna, R.; Ekberg, O.; Eriksson, L.I. Pharyngeal function and breathing pattern during partial neuromuscular block in the elderly: Effects on airway protection. Anesthesiology 2014, 120, 312–325. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cammu, G. Residual neuromuscular blockade and postoperative pulmonary complications: What does the recent evidence demonstrate? Curr. Anesthesiol. Rep. 2020, 10, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Bulka, C.M.; Terekhov, M.A.; Martin, B.J.; Dmochowski, R.R.; Hayes, R.M.; Ehrenfeld, J.M. Nondepolarizing neuromuscular blocking agents, reversal, and risk of postoperative pneumonia. Anesthesiology 2016, 125, 647–655. [Google Scholar] [CrossRef]
- Togioka, B.M.; Yanez, D.; Aziz, M.F.; Higgins, J.R.; Tekkali, P.; Treggiari, M.M. Randomised controlled trial of sugammadex or neostigmine for reversal of neuromuscular block on the incidence of pulmonary complications in older adults undergoing prolonged surgery. Br. J. Anaesth. 2020, 124, 553–561. [Google Scholar] [CrossRef]
- Brull, S.J.; Kopman, A.F. Current status of neuromuscular reversal and monitoring: Challenges and opportunities. Anesthesiology 2017, 126, 173–190. [Google Scholar] [CrossRef]
- Alday, E.; Munoz, M.; Planas, A.; Mata, E.; Alvarez, C. Effects of neuromuscular block reversal with sugammadex versus neostigmine on postoperative respiratory outcomes after major abdominal surgery: A randomized-controlled trial. Can. J. Anaesth. 2019, 66, 1328–1337. [Google Scholar] [CrossRef]
- Taş, N.; Korkmaz, H.; Yağan, Ö.; Korkmaz, M. Effect of sugammadex on postoperative bleeding and coagulation parameters after septoplasty: A randomized prospective study. Med. Sci. Monit. 2015, 21, 2382–2386. [Google Scholar] [CrossRef] [Green Version]
- Kheterpal, S.; Vaughn, M.T.; Dubovoy, T.Z.; Shah, N.J.; Bash, L.D.; Colquhoun, D.A.; Shanks, A.M.; Mathis, M.R.; Soto, R.G.; Bardia, A.; et al. Sugammadex versus neostigmine for reversal of neuromuscular blockade and postoperative pulmonary complications (STRONGER): A multicenter matched cohort analysis. Anesthesiology 2020, 132, 1371–1381. [Google Scholar] [CrossRef]
- Choi, E.S.; Oh, A.Y.; Koo, B.W.; Hwang, J.W.; Han, J.W.; Seo, K.S.; Ahn, S.H.; Jeong, W.J. Comparison of reversal with neostigmine of low-dose rocuronium vs. reversal with sugammadex of high-dose rocuronium for a short procedure. Anaesthesia 2017, 72, 1185–1190. [Google Scholar] [CrossRef] [Green Version]
- Park, E.S.; Lim, B.G.; Lee, W.J.; Lee, I.O. Sugammadex facilitates early recovery after surgery even in the absence of neuromuscular monitoring in patients undergoing laryngeal microsurgery: A single-center retrospective study. BMC Anesthesiol. 2016, 16, 48. [Google Scholar] [CrossRef] [Green Version]
- Cappellini, I.; Ostento, D.; Loriga, B.; Tofani, L.; De Gaudio, A.R.; Adembri, C. Comparison of neostigmine vs. sugammadex for recovery of muscle function after neuromuscular block by means of diaphragm ultrasonography in microlaryngeal surgery: A randomised controlled trial. Eur. J. Anaesthesiol. 2020, 37, 44–51. [Google Scholar] [CrossRef]
- Ünal, D.Y.; Baran, İ.; Mutlu, M.; Ural, G.; Akkaya, T.; Özlü, O. Comparison of sugammadex versus neostigmine costs and respiratory complications in patients with obstructive sleep apnoea. Turk. J. Anaesthesiol. Reanim. 2015, 43, 387–395. [Google Scholar] [CrossRef]
- Korkmaz, M.O.; Sayhan, H.; Guven, M. Does sugammadex decrease the severity of agitation and complications in pediatric patients undergoing adenotonsillectomy? Saudi Med. J. 2019, 40, 907–913. [Google Scholar] [CrossRef]
- Palanca, B.J.A.; Avidan, M.S.; Mashour, G.A. Human neural correlates of sevoflurane-induced unconsciousness. Br. J. Anaesth. 2017, 119, 573–582. [Google Scholar] [CrossRef] [Green Version]
- Wulf, H.; Kahl, M.; Ledowski, T. Augmentation of the neuromuscular blocking effects of cisatracurium during desflurane, sevoflurane, isoflurane or total i.v. anaesthesia. Br. J. Anaesth. 1998, 80, 308–312. [Google Scholar] [CrossRef]
- Vanlinthout, L.E.; Booij, L.H.; van Egmond, J.; Robertson, E.N. Effect of isoflurane and sevoflurane on the magnitude and time course of neuromuscular block produced by vecuronium, pancuronium and atracurium. Br. J. Anaesth. 1996, 76, 389–395. [Google Scholar] [CrossRef] [Green Version]
- Bock, M.; Klippel, K.; Nitsche, B.; Bach, A.; Martin, E.; Motsch, J. Rocuronium potency and recovery characteristics during steady-state desflurane, sevoflurane, isoflurane or propofol anaesthesia. Br. J. Anaesth. 2000, 84, 43–47. [Google Scholar] [CrossRef]
- Bevan, J.C.; Reimer, E.J.; Smith, M.F.; Scheepers, L.D.; Bridge, H.S.; Martin, G.R.; Bevan, D.R. Decreased mivacurium requirements and delayed neuromuscular recovery during sevoflurane anesthesia in children and adults. Anesth. Analg. 1998, 87, 772–778. [Google Scholar] [CrossRef]
- Mekawy, N.; Ali, E. Improved recovery profiles in sinonasal surgery Sugammadex: Does it have a role? Egypt. J. Anaesth. 2012, 28, 175–178. [Google Scholar] [CrossRef] [Green Version]
- Yasuda, N.; Targ, A.G.; Eger, E.I., 2nd. Solubility of I-653, sevoflurane, isoflurane, and halothane in human tissues. Anesth. Analg. 1989, 69, 370–373. [Google Scholar] [CrossRef]
- Detsch, O.; Schneider, G.; Kochs, E.; Hapfelmeier, G.; Werner, C. Increasing isoflurane concentration may cause paradoxical increases in the EEG bispectral index in surgical patients. Br. J. Anaesth. 2000, 84, 33–37. [Google Scholar] [CrossRef]
- Kharasch, E.D.; Avram, M.J.; Clark, J.D. Rational perioperative opioid management in the era of the opioid crisis. Anesthesiology 2020, 132, 603–605. [Google Scholar] [CrossRef]
- Aragón-Benedí, C.; Visiedo-Sánchez, S.; Pascual-Bellosta, A.; Ortega-Lucea, S.; Fernández-Liesa, R.; Martínez-Ubieto, J. Study of rocuronium-sugammadex as an alternative to succinylcholine-cisatracurium in microlaryngeal surgery. Laryngoscope 2021, 131, E212–E218. [Google Scholar] [CrossRef]
- Ezri, T.; Evron, S.; Petrov, I.; Schachter, P.; Berlovitz, Y.; Shimonov, M. Residual curarization and postoperative respiratory complications following laparoscopic sleeve gastrectomy. The effect of reversal agents: Sugammadex vs. neostigmine. J. Crit. Care Med. 2015, 1, 61–67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gu, B.; Fang, J.; Lian, Y.; Zhou, X.; Xie, K.; Zhu, Y.; Yuan, J.; Jiang, H. Effect of deep versus moderate neuromuscular block on pain after laparoscopic colorectal surgery: A randomized clinical trial. Dis. Colon Rectum 2021, 64, 475–483. [Google Scholar] [CrossRef] [PubMed]
- Oh, T.K.; Ji, E.; Na, H.S. The effect of neuromuscular reversal agent on postoperative pain after laparoscopic gastric cancer surgery: Comparison between the neostigmine and sugammadex. Medicine 2019, 98, e16142. [Google Scholar] [CrossRef] [PubMed]
- O’Gara, B.P.; Gao, L.; Marcantonio, E.R.; Subramaniam, B. Sleep, Pain, and Cognition: Modifiable Targets for Optimal Perioperative Brain Health. Anesthesiology 2021, 135, 1132–1152. [Google Scholar] [CrossRef]
- Frauenknecht, J.; Kirkham, K.R.; Jacot-Guillarmod, A.; Albrecht, E. Analgesic impact of intra-operative opioids vs. opioid-free anaesthesia: A systematic review and meta-analysis. Anaesthesia 2019, 74, 651–662. [Google Scholar] [CrossRef]
- Colvin, L.A.; Bull, F.; Hales, T.G. Perioperative opioid analgesia-when is enough too much? A review of opioid-induced tolerance and hyperalgesia. Lancet 2019, 393, 1558–1568. [Google Scholar] [CrossRef] [Green Version]
- Gottschalk, A.; Sharma, S.; Ford, J.; Durieux, M.E.; Tiouririne, M. Review article: The role of the perioperative period in recurrence after cancer surgery. Anesth. Analg. 2010, 110, 1636–1643. [Google Scholar] [CrossRef]
- Fiore, J.F., Jr.; Olleik, G.; El-Kefraoui, C.; Verdolin, B.; Kouyoumdjian, A.; Alldrit, A.; Figueiredo, A.G.; Valanci, S.; Marquez-Gde, V.J.; Schulz, M.; et al. Preventing opioid prescription after major surgery: A scoping review of opioid-free analgesia. Br. J. Anaesth. 2019, 123, 627–636. [Google Scholar] [CrossRef]
- Olausson, A.; Svensson, C.J.; Andrell, P.; Jildenstal, P.; Thorn, S.E.; Wolf, A. Total opioid-free general anaesthesia can improve postoperative outcomes after surgery, without evidence of adverse effects on patient safety and pain management: A systematic review and meta-analysis. Acta Anaesthesiol. Scand. 2022, 66, 170–185. [Google Scholar] [CrossRef]
- Beloeil, H. Opioid-free anesthesia. Best Pract. Res. Clin. Anaesthesiol. 2019, 33, 353–360. [Google Scholar] [CrossRef]
- Gabriel, R.A.; Swisher, M.W.; Sztain, J.F.; Furnish, T.J.; Ilfeld, B.M.; Said, E.T. State of the art opioid-sparing strategies for post-operative pain in adult surgical patients. Expert Opin. Pharmacother. 2019, 20, 949–961. [Google Scholar] [CrossRef] [Green Version]
- Hristovska, A.M.; Duch, P.; Allingstrup, M.; Afshari, A. Efficacy and safety of sugammadex versus neostigmine in reversing neuromuscular blockade in adults. Cochrane Database Syst. Rev. 2017, 8, CD012763. [Google Scholar] [CrossRef]
- Hunter, J.M.; Flockton, E.A. The doughnut and the hole: A new pharmacological concept for anaesthetists. Br. J. Anaesth. 2006, 97, 123–126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krause, M.; McWilliams, S.K.; Bullard, K.J.; Mayes, L.M.; Jameson, L.C.; Mikulich-Gilbertson, S.K.; Fernandez-Bustamante, A.; Bartels, K. Neostigmine versus sugammadex for reversal of neuromuscular blockade and effects on reintubation for respiratory failure or newly initiated noninvasive ventilation: An interrupted time series design. Anesth. Analg. 2020, 131, 141–151. [Google Scholar] [CrossRef]
- Ledowski, T.; Szabó-Maák, Z.; Loh, P.S.; Turlach, B.A.; Yang, H.S.; de Boer, H.D.; Asztalos, L.; Shariffuddin, I.I.; Chan, L.; Fülesdi, B. Reversal of residual neuromuscular block with neostigmine or sugammadex and postoperative pulmonary complications: A prospective, randomised, double-blind trial in high-risk older patients. Br. J. Anaesth. 2021, 127, 316–323. [Google Scholar] [CrossRef]
- Habib, A.S.; Chen, Y.T.; Taguchi, A.; Hu, X.H.; Gan, T.J. Postoperative nausea and vomiting following inpatient surgeries in a teaching hospital: A retrospective database analysis. Curr. Med. Res. Opin. 2006, 22, 1093–1099. [Google Scholar] [CrossRef]
- Berger, E.R.; Huffman, K.M.; Fraker, T.; Petrick, A.T.; Brethauer, S.A.; Hall, B.L.; Ko, C.Y.; Morton, J.M. Prevalence and risk factors for bariatric surgery readmissions: Findings from 130,007 admissions in the metabolic and bariatric surgery accreditation and quality improvement program. Ann. Surg. 2018, 267, 122–131. [Google Scholar] [CrossRef] [PubMed]
- Myles, P.S.; Williams, D.L.; Hendrata, M.; Anderson, H.; Weeks, A.M. Patient satisfaction after anaesthesia and surgery: Results of a prospective survey of 10,811 patients. Br. J. Anaesth. 2000, 84, 6–10. [Google Scholar] [CrossRef] [PubMed]
- Awad, A.G.; Rabie, A.H.A.; Elshafie, M.A.A.; Youssef, G.F.K. A comparative study between sugammadex versus neostigmine as regards efficiency of reversal of rocuronium and bleeding tendency effect after septoplasty. QJM 2020, 113, i15–i16. [Google Scholar] [CrossRef]
- Gan, T.J.; Belani, K.G.; Bergese, S.; Chung, F.; Diemunsch, P.; Habib, A.S.; Jin, Z.; Kovac, A.L.; Meyer, T.A.; Urman, R.D.; et al. Fourth consensus guidelines for the management of postoperative nausea and vomiting. Anesth. Analg. 2020, 131, 411–448. [Google Scholar] [CrossRef]
- Sparr, H.J.; Beaufort, T.M.; Fuchs-Buder, T. Newer neuromuscular blocking agents: How do they compare with established agents? Drugs 2001, 61, 919–942. [Google Scholar] [CrossRef]
- Wierda, J.M.; Schuringa, M.; van den Broek, L. Cardiovascular effects of an intubating dose of rocuronium 0.6 mg kg-1 in anaesthetized patients, paralysed with vecuronium. Br. J. Anaesth. 1997, 78, 586–587. [Google Scholar] [CrossRef]
- Czarnetzki, C.; Albrecht, E.; Masouye, P.; Baeriswyl, M.; Poncet, A.; Robin, M.; Kern, C.; Tramer, M.R. Rapid Sequence Induction with a Standard Intubation Dose of Rocuronium After Magnesium Pretreatment Compared With Succinylcholine: A Randomized Clinical Trial. Anesth. Analg. 2021, 133, 1540–1549. [Google Scholar] [CrossRef]
- Oh, T.K.; Oh, A.Y.; Ryu, J.H.; Koo, B.W.; Song, I.A.; Nam, S.W.; Jee, H.J. Retrospective analysis of 30-day unplanned readmission after major abdominal surgery with reversal by sugammadex or neostigmine. Br. J. Anaesth. 2019, 122, 370–378. [Google Scholar] [CrossRef] [Green Version]
- Song, S.W.; Yoo, K.Y.; Ro, Y.S.; Pyeon, T.; Bae, H.B.; Kim, J. Sugammadex is associated with shorter hospital length of stay after open lobectomy for lung cancer: A retrospective observational study. J. Cardiothorac. Surg. 2021, 16, 45. [Google Scholar] [CrossRef]
- Futier, E.; Constantin, J.M.; Paugam-Burtz, C.; Pascal, J.; Eurin, M.; Neuschwander, A.; Marret, E.; Beaussier, M.; Gutton, C.; Lefrant, J.Y.; et al. A trial of intraoperative low-tidal-volume ventilation in abdominal surgery. N. Engl. J. Med. 2013, 369, 428–437. [Google Scholar] [CrossRef] [Green Version]
- Karalapillai, D.; Weinberg, L.; Peyton, P.; Ellard, L.; Hu, R.; Pearce, B.; Tan, C.O.; Story, D.; O’Donnell, M.; Hamilton, P.; et al. Effect of intraoperative low tidal volume vs conventional tidal volume on postoperative pulmonary complications in patients undergoing major surgery: A randomized clinical trial. JAMA 2020, 324, 848–858. [Google Scholar] [CrossRef]
- Meijer, F.; Honing, M.; Roor, T.; Toet, S.; Calis, P.; Olofsen, E.; Martini, C.; van Velzen, M.; Aarts, L.; Niesters, M.; et al. Reduced postoperative pain using Nociception Level-guided fentanyl dosing during sevoflurane anaesthesia: A randomised controlled trial. Br. J. Anaesth. 2020, 125, 1070–1078. [Google Scholar] [CrossRef]
- Hemmerling, T.M.; Desrosiers, A.M. Interference of electromagnetic operating systems in otorhinolaryngology surgery with bispectral index monitoring. Anesth. Analg. 2003, 96, 1698–1699. [Google Scholar] [CrossRef]
- Vandenbroucke, J.P.; von Elm, E.; Altman, D.G.; Gotzsche, P.C.; Mulrow, C.D.; Pocock, S.J.; Poole, C.; Schlesselman, J.J.; Egger, M.; STROBE Initiative. Strengthening the reporting of observational studies in epidemiology (STROBE): Explanation and elaboration. PLoS Med. 2007, 4, e297. [Google Scholar] [CrossRef] [Green Version]
- Back, I.N. Palliative Medicine Handbook, 3rd ed.; BPM Books: Cardiff, UK, 2001. [Google Scholar]
| Variables (Unit) | N(%)/Median (IQR) | Cisatracurium /Neostigmine n = 119 | Rocuronium /Sugammadex n = 119 | p-Value |
|---|---|---|---|---|
| Sex | ||||
| Female | 67 (28.2%) | 35 (29.4%) | 32 (26.9%) | 0.665 |
| Male | 171 (71.8%) | 84 (70.6%) | 87 (73.1%) | |
| Age | 43.0 (31.0–56.0) | 43.0 (29.0–57.0) | 43.0 (33.0–54.0) | 0.786 |
| Body weight (kg) | 75.0 (65.0–86.0) | 75.0 (67.0–87.0) | 75.0 (64.0–86.0) | 0.649 |
| ASA classification | ||||
| Ⅰ | 20 (8.4%) | 13 (10.9%) | 7 (5.9%) | 0.374 |
| Ⅱ | 181 (76.1%) | 88 (73.9%) | 93 (78.2%) | |
| Ⅲ | 37 (15.5%) | 18 (15.1%) | 19 (16.0%) | |
| Apfel score | ||||
| 0 | 27 (16.7%) | 11 (13.9%) | 16 (19.3%) | 0.822 |
| 1 | 55 (34.0%) | 27 (34.2%) | 28 (33.7%) | |
| 2 | 53 (32.7%) | 28 (35.4%) | 25 (30.1%) | |
| 3 | 24 (14.8%) | 11 (13.9%) | 13 (15.7%) | |
| 4 | 3 (1.9%) | 2 (2.5%) | 1 (1.2%) | |
| Hypertension | ||||
| No | 195 (81.9%) | 98 (82.4%) | 97 (81.5%) | 0.866 |
| Yes | 43 (18.1%) | 21 (17.6%) | 22 (18.5%) | |
| DM | (-) | (-) | (-) | |
| No | 218 (90.3%) | 106 (89.1%) | 109 (91.6%) | 0.510 |
| Yes | 23 (9.7%) | 13 (10.9%) | 10 (8.4%) | |
| CVA | ||||
| No | 237 (99.6%) | 118 (99.2%) | 119 (100.0%) | 1.000 |
| Yes | 1 (0.4%) | 1 (0.8%) | 0 (0.0%) | |
| Surgical indication for otorhinolaryngologic surgery | ||||
| Multiple sinusectomy | 38 (16.0%) | 17 (14.3%) | 21 (17.6%) | 0.830 |
| Pansinusectomy | 20 (8.4%) | 12 (10.1%) | 8 (6.7%) | |
| Septomeatal plasty | 34 (14.3%) | 18 (15.1%) | 16 (13.4%) | |
| Uvulopalatopharyngoplasty | 118 (49.6%) | 59 (49.6%) | 59 (49.6%) | |
| Oral tumor or oropharynx excision | 28 (11.8%) | 13 (10.9%) | 15 (12.6%) | |
| Variables (Unit) | N(%)/Median (IQR) | Cisatracurium /Neostigmine n = 119 | Rocuronium /Sugammadex n = 119 | p-Value |
|---|---|---|---|---|
| Intraoperative | ||||
| Duration of anesthesia (h) | 3.33 (2.35–6.17) | 2.82 (2.08–4.33) | 4.63 (2.92–6.75) | <0.001 |
| Baseline MAP | 97.2 (88.7–106.2) | 98.7 (89.7–107.5) | 95.8 (87.8–104.7) | 0.354 |
| Percentage of † MAP > 120% (%) | 1.4 (0–6.4) | 1.9 (0–6.5) | 1.4 (0–5.9) | 0.325 |
| Fluid mL/kg/h | 2.13 (1.63–2.67) | 2.22 (1.66–2.89) | 2.08 (1.50–2.56) | 0.064 |
| Sevoflurane consumption (mL/h) | 0.20 (0.18–0.21) | 0.21 (0.19–0.24) | 0.18 (0.16–0.19) | 0.009 |
| †† Intraoperative MME (mg/kg/h) | 0.073 (0.056–0.102) | 0.076 (0.057–0.116) | 0.067 (0.056–0.092) | 0.035 |
| Labetalol (mg) | 0 (0–2.5) | 0 (0–2.5) | 0 (0–5.0) | 0.002 |
| Nicardipine (mg) | 0 (0–1.0) | 0 (0–0.5) | 0 (0–1.0) | 0.245 |
| Postoperative | ||||
| Postoperative parecoxib (Dynastat®) | 36 (15%) | 21 (18%) | 15 (13%) | 0.278 |
| †† Postoperative MME (mg/kg/h) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0.054 |
| VAS at ward | 2 (2–3) | 2 (2–3) | 2 (1–3) | 0.086 |
| PONV at ward | 1 (0.4%) | 1 (0.8%) | 0 (0%) | 1.0 |
| Blood loss (mL) | 0 (0–100) | 0 (0–50) | 0 (0–100) | 0.507 |
| Extubation time (min) | 5 (5–10) | 5 (5–10) | 5 (5–10) | 0.176 |
| LOS (day) | 4 (2–6) | 4 (3–6) | 4 (2–6) | 0.553 |
| Percentage of delayed extubation (%) | 3 (1.3%) | 3 (2.5%) | 0 (0%) | 0.111 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, E.-B.; Hung, C.-T.; Luo, S.-D.; Wu, S.-C.; Lee, T.-Y.; Chin, J.-C.; Tsai, P.-N.; Yang, J.C.-S. Analgesic and Anesthetic Efficacy of Rocuronium/Sugammadex in Otorhinolaryngologic Surgery: A Propensity Score-Matched Analysis. Pharmaceuticals 2022, 15, 894. https://doi.org/10.3390/ph15070894
Wu E-B, Hung C-T, Luo S-D, Wu S-C, Lee T-Y, Chin J-C, Tsai P-N, Yang JC-S. Analgesic and Anesthetic Efficacy of Rocuronium/Sugammadex in Otorhinolaryngologic Surgery: A Propensity Score-Matched Analysis. Pharmaceuticals. 2022; 15(7):894. https://doi.org/10.3390/ph15070894
Chicago/Turabian StyleWu, En-Bo, Chao-Ting Hung, Sheng-Dean Luo, Shao-Chun Wu, Tsung-Yang Lee, Jo-Chi Chin, Peng-Neng Tsai, and Johnson Chia-Shen Yang. 2022. "Analgesic and Anesthetic Efficacy of Rocuronium/Sugammadex in Otorhinolaryngologic Surgery: A Propensity Score-Matched Analysis" Pharmaceuticals 15, no. 7: 894. https://doi.org/10.3390/ph15070894
APA StyleWu, E.-B., Hung, C.-T., Luo, S.-D., Wu, S.-C., Lee, T.-Y., Chin, J.-C., Tsai, P.-N., & Yang, J. C.-S. (2022). Analgesic and Anesthetic Efficacy of Rocuronium/Sugammadex in Otorhinolaryngologic Surgery: A Propensity Score-Matched Analysis. Pharmaceuticals, 15(7), 894. https://doi.org/10.3390/ph15070894










