Druggable Targets and Compounds with Both Antinociceptive and Antipruritic Effects
Abstract
1. Introduction
2. Overlapping Nature of Pain and Itch
2.1. Overlapping of Algogens and Pruritogens
2.2. Central and Peripheral Sensitization in Pathological Itch and Pain Conditions
2.2.1. Peripheral Sensitization
2.2.2. Central Sensitization
3. Therapeutic Targets for Pain and Itch
3.1. TRPV1 (Transient Receptor Potential Vanilloid 1)
3.2. TRPV3 (Transient Receptor Potential Vanilloid 3)
3.3. TRPV4 (Transient Receptor Potential Vanilloid 4)
3.4. TRPA1 (Transient Receptor Potential Cation Channel Subfamily A Member 1)
3.5. TRPM8 (Transient Receptor Potential Cation Channel Subfamily M Member 8)
3.6. TRPC3 (Transient Receptor Potential Channel Subfamily C Member 3)
3.7. Kappa and Miu Opioid Receptors
3.8. Histamine Receptors
3.8.1. Histamine H1 Receptor (H1R)
3.8.2. Histamine H4 Receptor (H4R)
3.9. Cannabinoid Receptors
3.10. Oncostatin M (OSM)
3.11. JAK-STAT (Janus Kinase/Signal Transducer and Activator of Transcription) Pathway
3.12. Nerve Growth Factor (NGF)
3.13. Protease-Activated Receptor 2 (PAR2)
3.14. Other Agents with Analgesic and Antipruritic Effects
3.14.1. Botulinum Toxin
3.14.2. Local Anesthetics
3.14.3. Gapapentin/Pregabalin
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Honma, M.; Kanai, Y.; Murotani, K.; Nomura, T.; Ito, K.; Imafuku, S. Itching and skin pain in real-life patients with plaque psoriasis: Baseline analysis of the ProLOGUE study. J. Dermatol. Sci. 2022, 105, 189–191. [Google Scholar] [CrossRef] [PubMed]
- Newton, L.; DeLozier, A.M.; Griffiths, P.C.; Hill, J.N.; Hudgens, S.; Symonds, T.; Gable, J.C.; Paik, J.; Wyrwich, K.W.; Eichenfield, L.F. Exploring content and psychometric validity of newly developed assessment tools for itch and skin pain in atopic dermatitis. J. Patient-Rep. Outcomes 2019, 3, 42. [Google Scholar] [CrossRef] [PubMed]
- Oaklander, A.L.; Bowsher, D.; Galer, B.; Haanpää, M.; Jensen, M.P. Herpes zoster itch: Preliminary epidemiologic data. J. Pain 2003, 4, 338–343. [Google Scholar] [CrossRef]
- Maarouf, M.; Kromenacker, B.; Capozza, K.cL.; Kempton, D.; Hendricks, A.; Tran, K.; Shi, V.Y. Pain and itch are dual burdens in atopic dermatitis. Dermatitis 2018, 29, 278–281. [Google Scholar] [CrossRef] [PubMed]
- Dawn, A.; Papoiu, A.; Chan, Y.; Rapp, S.; Rassette, N.; Yosipovitch, G. Itch characteristics in atopic dermatitis: Results of a web-based questionnaire. Br. J. Dermatol. 2009, 160, 642–644. [Google Scholar] [CrossRef] [PubMed]
- Van Wijck, A.J.; Aerssens, Y.R. Pain, itch, quality of life, and costs after herpes zoster. Pain Pract. 2017, 17, 738–746. [Google Scholar] [CrossRef] [PubMed]
- Sauver, J.L.S.; Warner, D.O.; Yawn, B.P.; Jacobson, D.J.; McGree, M.E.; Pankratz, J.J.; Melton, L.J., III; Roger, V.L.; Ebbert, J.O.; Rocca, W.A. Why patients visit their doctors: Assessing the most prevalent conditions in a defined American population. Mayo Clin. Proc. 2013, 88, 56–67. [Google Scholar] [CrossRef]
- Vos, T.; Allen, C.; Arora, M.; Barber, R.M.; Bhutta, Z.A.; Brown, A.; Carter, A.; Casey, D.C.; Charlson, F.J.; Chen, A.Z. Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990–2015: A systematic analysis for the Global Burden of Disease Study 2015. Lancet 2016, 388, 1545–1602. [Google Scholar] [CrossRef]
- Dalgard, F.; Svensson, Å.; Holm, J.Ø.; Sundby, J. Self-reported skin morbidity among adults: Associations with quality of life and general health in a Norwegian survey. J. Investig. Dermatol. Symp. Proc. 2004, 9, 120–125. [Google Scholar] [CrossRef]
- Boston, A.; Sharpe, L. The role of threat-expectancy in acute pain: Effects on attentional bias, coping strategy effectiveness and response to pain. Pain 2005, 119, 168–175. [Google Scholar] [CrossRef]
- Roelofs, J.; Peters, M.L.; Vlaeyen, J.W. Selective attention for pain-related information in healthy individuals: The role of pain and fear. Eur. J. Pain 2002, 6, 331–339. [Google Scholar] [CrossRef]
- Fortune, D.G.; Richards, H.L.; Corrin, A.; Taylor, R.J.; Griffiths, C.E.; Main, C.J. Attentional bias for psoriasis-specific and psychosocial threat in patients with psoriasis. J. Behav. Med. 2003, 26, 211–224. [Google Scholar] [CrossRef] [PubMed]
- Van Laarhoven, A.I.; Kraaimaat, F.W.; Wilder-Smith, O.; Evers, A.W. Role of attentional focus on bodily sensations in sensitivity to itch and pain. Acta Derm. Venereol. 2010, 90, 46–51. [Google Scholar] [CrossRef] [PubMed]
- Seminowicz, D.A.; Davis, K.D. Pain enhances functional connectivity of a brain network evoked by performance of a cognitive task. J. Neurophysiol. 2007, 97, 3651–3659. [Google Scholar] [CrossRef] [PubMed]
- McGowan, N.; Sharpe, L.; Refshauge, K.; Nicholas, M. The effect of attentional re-training and threat expectancy in response to acute pain. Pain 2009, 142, 101–107. [Google Scholar] [CrossRef]
- Sikand, P.; Shimada, S.G.; Green, B.G.; LaMotte, R.H. Similar itch and nociceptive sensations evoked by punctate cutaneous application of capsaicin, histamine and cowhage. Pain 2009, 144, 66–75. [Google Scholar] [CrossRef]
- Whitty, C.W.M. Substances producing pain and itch. J. Neurol. Neurosurg. Psychiatry 1964, 27, 483. [Google Scholar] [CrossRef][Green Version]
- Davidson, S.; Giesler, G.J. The multiple pathways for itch and their interactions with pain. Trends Neurosci. 2010, 33, 550–558. [Google Scholar] [CrossRef]
- Simpson, J. Pain and the Neurosurgeon a Forty-Year Experience. J. Neurol. Neurosurg. Psychiatry 1970, 33, 129. [Google Scholar] [CrossRef][Green Version]
- Clark, K. Use of cordotomy in the relief of intractable pain. Arch. Surg. 1961, 82, 440–442. [Google Scholar] [CrossRef]
- Vedantam, A.; Bruera, E.; Hess, K.R.; Dougherty, P.M.; Viswanathan, A. Somatotopy and organization of spinothalamic tracts in the human cervical spinal cord. Neurosurgery 2019, 84, E311–E317. [Google Scholar] [CrossRef] [PubMed]
- Hyndman, O.R.; Wolkin, J. Anterior chordotomy: Further observations on physiologic results and optimum manner of performance. Arch. Neurol. Psychiatry 1943, 50, 129–148. [Google Scholar] [CrossRef]
- Han, L.; Ma, C.; Liu, Q.; Weng, H.-J.; Cui, Y.; Tang, Z.; Kim, Y.; Nie, H.; Qu, L.; Patel, K.N. A subpopulation of nociceptors specifically linked to itch. Nat. Neurosci. 2013, 16, 174–182. [Google Scholar] [CrossRef] [PubMed]
- Jinks, S.L.; Carstens, E. Responses of superficial dorsal horn neurons to intradermal serotonin and other irritants: Comparison with scratching behavior. J. Neurophysiol. 2002, 87, 1280–1289. [Google Scholar] [CrossRef]
- Johanek, L.M.; Meyer, R.A.; Friedman, R.M.; Greenquist, K.W.; Shim, B.; Borzan, J.; Hartke, T.; LaMotte, R.H.; Ringkamp, M. A role for polymodal C-fiber afferents in nonhistaminergic itch. J. Neurosci. 2008, 28, 7659–7669. [Google Scholar] [CrossRef]
- LaMotte, R.H.; Dong, X.; Ringkamp, M. Sensory neurons and circuits mediating itch. Nat. Rev. Neurosci. 2014, 15, 19–31. [Google Scholar] [CrossRef]
- Ma, Q. Labeled lines meet and talk: Population coding of somatic sensations. J. Clin. Investig. 2010, 120, 3773–3778. [Google Scholar] [CrossRef]
- Patel, K.N.; Dong, X. An itch to be scratched. Neuron 2010, 68, 334–339. [Google Scholar] [CrossRef]
- Liu, T.; Ji, R.-R. New insights into the mechanisms of itch: Are pain and itch controlled by distinct mechanisms? Pflügers Arch. Eur. J. Physiol. 2013, 465, 1671–1685. [Google Scholar] [CrossRef]
- Wang, H.; Papoiu, A.; Coghill, R.; Patel, T.; Wang, N.; Yosipovitch, G. Ethnic differences in pain, itch and thermal detection in response to topical capsaicin: African Americans display a notably limited hyperalgesia and neurogenic inflammation. Br. J. Dermatol. 2010, 162, 1023–1029. [Google Scholar] [CrossRef]
- Moser, H.R.; Giesler, G.J., Jr. Characterization of pruriceptive trigeminothalamic tract neurons in rats. J. Neurophysiol. 2014, 111, 1574–1589. [Google Scholar] [CrossRef] [PubMed]
- Klein, A.; Carstens, M.I.; Carstens, E. Facial injections of pruritogens or algogens elicit distinct behavior responses in rats and excite overlapping populations of primary sensory and trigeminal subnucleus caudalis neurons. J. Neurophysiol. 2011, 106, 1078–1088. [Google Scholar] [CrossRef] [PubMed]
- McNeil, B.; Dong, X. Peripheral mechanisms of itch. Neurosci. Bull. 2012, 28, 100–110. [Google Scholar] [CrossRef] [PubMed]
- Yosipovitch, G.; Carstens, E.; McGlone, F. Chronic itch and chronic pain: Analogous mechanisms. Pain 2007, 131, 4–7. [Google Scholar] [CrossRef]
- Van Laarhoven, A.; Kraaimaat, F.; Wilder-Smith, O.; Van de Kerkhof, P.; Cats, H.; Van Riel, P.; Evers, A. Generalized and symptom-specific sensitization of chronic itch and pain. J. Eur. Acad. Dermatol. Venereol. 2007, 21, 1187–1192. [Google Scholar] [CrossRef]
- Davidson, R.J.; Jackson, D.C.; Kalin, N.H. Emotion, plasticity, context, and regulation: Perspectives from affective neuroscience. Psychol. Bull. 2000, 126, 890. [Google Scholar] [CrossRef]
- Brosschot, J.F. Cognitive-emotional sensitization and somatic health complaints. Scand. J. Psychol. 2002, 43, 113–121. [Google Scholar] [CrossRef]
- Houtveen, J.H.; Rietveld, S.; de Geus, E.J. Exaggerated perception of normal physiological responses to stress and hypercapnia in young women with numerous functional somatic symptoms. J. Psychosom. Res. 2003, 55, 481–490. [Google Scholar] [CrossRef][Green Version]
- Rietvelt, S.; Houtveen, J.H. Acquired sensitivity to relevant physiological activity in patients with chronic health problems. Behav. Res. Ther. 2004, 42, 137–153. [Google Scholar] [CrossRef]
- Van den Bergh, O.; Winters, W.; Devriese, S.; Van Diest, I. Learning subjective health complaints. Scand. J. Psychol. 2002, 43, 147–152. [Google Scholar] [CrossRef]
- Pisoni, R.L.; Wikström, B.; Elder, S.J.; Akizawa, T.; Asano, Y.; Keen, M.L.; Saran, R.; Mendelssohn, D.C.; Young, E.W.; Port, F.K. Pruritus in haemodialysis patients: International results from the Dialysis Outcomes and Practice Patterns Study (DOPPS). Nephrol. Dial. Transplant. 2006, 21, 3495–3505. [Google Scholar] [CrossRef] [PubMed]
- Giesecke, T.; Williams, D.A.; Harris, R.E.; Cupps, T.R.; Tian, X.; Tian, T.X.; Gracely, R.H.; Clauw, D.J. Subgrouping of fibromyalgia patients on the basis of pressure-pain thresholds and psychological factors. Arthritis Rheum. 2003, 48, 2916–2922. [Google Scholar] [CrossRef] [PubMed]
- Giesecke, T.; Gracely, R.H.; Grant, M.A.; Nachemson, A.; Petzke, F.; Williams, D.A.; Clauw, D.J. Evidence of augmented central pain processing in idiopathic chronic low back pain. Arthritis Rheum. 2004, 50, 613–623. [Google Scholar] [CrossRef] [PubMed]
- Ikoma, A.; Fartasch, M.; Heyer, G.; Miyachi, Y.; Handwerker, H.; Schmelz, M. Painful stimuli evoke itch in patients with chronic pruritus: Central sensitization for itch. Neurology 2004, 62, 212–217. [Google Scholar] [CrossRef] [PubMed]
- Thieme, K.; Rose, U.; Pinkpank, T.; Spies, C.; Turk, D.C.; Flor, H. Psychophysiological responses in patients with fibromyalgia syndrome. J. Psychosom. Res. 2006, 61, 671–679. [Google Scholar] [CrossRef]
- Staud, R.; Rodriguez, M.E. Mechanisms of disease: Pain in fibromyalgia syndrome. Nat. Clin. Pract. Rheumatol. 2006, 2, 90–98. [Google Scholar] [CrossRef]
- Kidd, B.; Urban, L. Mechanisms of inflammatory pain. Br. J. Anaesth. 2001, 87, 3–11. [Google Scholar] [CrossRef]
- Schmelz, M.; Schmidt, R.; Weidner, C.; Hilliges, M.; Torebjork, H.; Handwerker, H.O. Chemical response pattern of different classes of C-nociceptors to pruritogens and algogens. J. Neurophysiol. 2003, 89, 2441–2448. [Google Scholar] [CrossRef]
- Baral, P.; Mills, K.; Pinho-Ribeiro, F.A.; Chiu, I.M. Pain and itch: Beneficial or harmful to antimicrobial defense? Cell Host Microbe 2016, 19, 755–759. [Google Scholar] [CrossRef]
- Toyoda, M.; Nakamura, M.; Makino, T.; Hino, T.; Kagoura, M.; Morohashi, M. Nerve growth factor and substance P are useful plasma markers of disease activity in atopic dermatitis. Br. J. Dermatol. 2002, 147, 71–79. [Google Scholar] [CrossRef]
- Kinkelin, I.; Mötzing, S.; Koltzenburg, M.; Bröcker, E.-B. Increase in NGF content and nerve fiber sprouting in human allergic contact eczema. Cell Tissue Res. 2000, 302, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Johansson, O.; Liang, Y.; Emtestam, L. Increased nerve growth factor-and tyrosine kinase A-like immunoreactivities in prurigo nodularis skin–an exploration of the cause of neurohyperplasia. Arch. Dermatol. Res. 2002, 293, 614–619. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.-C.; Yang, J.-H.; Chang, S.-E.; Choi, J.-H. Pruritus and nerve growth factor in psoriasis. Korean J. Dermatol. 2005, 43, 769–773. [Google Scholar]
- Bohm-Starke, N.; Hilliges, M.; Falconer, C.; Rylander, E. Increased intraepithelial innervation in women with vulvar vestibulitis syndrome. Gynecol. Obstet. Investig. 1998, 46, 256–260. [Google Scholar] [CrossRef] [PubMed]
- Halvorson, K.G.; Kubota, K.; Sevcik, M.A.; Lindsay, T.H.; Sotillo, J.E.; Ghilardi, J.R.; Rosol, T.J.; Boustany, L.; Shelton, D.L.; Mantyh, P.W. A blocking antibody to nerve growth factor attenuates skeletal pain induced by prostate tumor cells growing in bone. Cancer Res. 2005, 65, 9426–9435. [Google Scholar] [CrossRef]
- Schmelz, M.; Mantyh, P.; Malfait, A.-M.; Farrar, J.; Yaksh, T.; Tive, L.; Viktrup, L. Nerve growth factor antibody for the treatment of osteoarthritis pain and chronic low-back pain: Mechanism of action in the context of efficacy and safety. Pain 2019, 160, 2210–2220. [Google Scholar] [CrossRef] [PubMed]
- Atanassoff, P.G.; Brull, S.J.; Zhang, J.; Greenquist, K.; Silverman, D.G.; Lamotte, R.H. Enhancement of experimental pruritus and mechanically evoked dysesthesiae with local anesthesia. Somatosens. Mot. Res. 1999, 16, 291–298. [Google Scholar] [CrossRef]
- LaMotte, R.H. Subpopulations of “nocifensor neurons” contributing to pain and allodynia, itch and alloknesis. APS J. 1992, 1, 115–126. [Google Scholar] [CrossRef]
- Yosipovitch, G.; Rosen, J.D.; Hashimoto, T. Itch: From mechanism to (novel) therapeutic approaches. J. Allergy Clin. Immunol. 2018, 142, 1375–1390. [Google Scholar] [CrossRef]
- Simone, D.A.; Alreja, M.; Lamotte, R.H. Psychophysical studies of the itch sensation and itchy skin (“alloknesis”) produced by intracutaneous injection of histamine. Somatosens. Mot. Res. 1991, 8, 271–279. [Google Scholar] [CrossRef]
- Torebjörk, H.; Schmelz, M.; Handwerker, H. Functional Properties of Human Cutaneous Nociceptors and Their Role in Pain and Hyperalgesia. In Neurobiology of Nociceptors; Oxford University Press: Oxford, UK, 1996; pp. 349–369. [Google Scholar]
- LaMotte, R.H.; Shain, C.N.; Simone, D.A.; Tsai, E. Neurogenic hyperalgesia: Psychophysical studies of underlying mechanisms. J. Neurophysiol. 1991, 66, 190–211. [Google Scholar] [CrossRef] [PubMed]
- Koltzenburg, M. Neural mechanisms of cutaneous nociceptive pain. Clin. J. Pain 2000, 16, S131–S138. [Google Scholar] [CrossRef] [PubMed]
- Torebjörk, H.; Lundberg, L.; LaMotte, R. Central changes in processing of mechanoreceptive input in capsaicin-induced secondary hyperalgesia in humans. J. Physiol. 1992, 448, 765–780. [Google Scholar] [CrossRef] [PubMed]
- Magerl, W.; Fuchs, P.N.; Meyer, R.A.; Treede, R.-D. Roles of capsaicin-insensitive nociceptors in cutaneous pain and secondary hyperalgesia. Brain 2001, 124, 1754–1764. [Google Scholar] [CrossRef]
- Ziegler, E.; Magerl, W.; Meyer, R.; Treede, R.-D. Secondary hyperalgesia to punctate mechanical stimuli: Central sensitization to A-fibre nociceptor input. Brain 1999, 122, 2245–2257. [Google Scholar] [CrossRef]
- Fuchs, P.N.; Campbell, J.N.; Meyer, R.A. Secondary hyperalgesia persists in capsaicin desensitized skin. Pain 2000, 84, 141–149. [Google Scholar] [CrossRef]
- Baron, R.; Schwarz, K.; Kleinert, A.; Schattschneider, J.; Wasner, G. Histamine-induced itch converts into pain in neuropathic hyperalgesia. Neuroreport 2001, 12, 3475–3478. [Google Scholar] [CrossRef]
- Birklein, F.; Claus, D.; Riedl, B.; Neundörfer, B.; Handwerker, H.O. Effects of cutaneous histamine application in patients with sympathetic reflex dystrophy. Muscle Nerve 1997, 20, 1389–1395. [Google Scholar] [CrossRef]
- Kwatra, S.G.; Stander, S.; Bernhard, J.D.; Weisshaar, E.; Yosipovitch, G. Brachioradial pruritus: A trigger for generalization of itch. J. Am. Acad. Dermatol. 2013, 68, 870–873. [Google Scholar] [CrossRef]
- Hosogi, M.; Schmelz, M.; Miyachi, Y.; Ikoma, A. Bradykinin is a potent pruritogen in atopic dermatitis: A switch from pain to itch. Pain 2006, 126, 16–23. [Google Scholar] [CrossRef]
- Ishiuji, Y.; Coghill, R.; Patel, T.; Dawn, A.; Fountain, J.; Oshiro, Y.; Yosipovitch, G. Repetitive scratching and noxious heat do not inhibit histamine-induced itch in atopic dermatitis. Br. J. Dermatol. 2008, 158, 78–83. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, H.-J.; Schouenborg, J. Differential inhibitory effect on human nociceptive skin senses induced by local stimulation of thin cutaneous fibers. Pain 1999, 80, 103–112. [Google Scholar] [CrossRef]
- Oaklander, A.L.; Cohen, S.P.; Raju, S.V. Intractable postherpetic itch and cutaneous deafferentation after facial shingles. Pain 2002, 96, 9–12. [Google Scholar] [CrossRef]
- Ishikawa, R.; Iseki, M.; Koga, R.; Yamaguchi, K.; Inada, E. Investigation of neuropathic pain component by the stage of a disease of the herpes zoster associated pain patients received pain clinic treatments. Pain Res. 2016, 31, 156–165. [Google Scholar] [CrossRef][Green Version]
- Bueller, H.A. Gabapentin treatment for brachioradial pruritus. J. Eur. Acad. Derm. Venereol. 1999, 13, 227–230. [Google Scholar] [CrossRef]
- Caterina, M.J.; Schumacher, M.A.; Tominaga, M.; Rosen, T.A.; Levine, J.D.; Julius, D. The capsaicin receptor: A heat-activated ion channel in the pain pathway. Nature 1997, 389, 816–824. [Google Scholar] [CrossRef]
- Tominaga, M.; Caterina, M.J.; Malmberg, A.B.; Rosen, T.A.; Gilbert, H.; Skinner, K.; Raumann, B.E.; Basbaum, A.I.; Julius, D. The cloned capsaicin receptor integrates multiple pain-producing stimuli. Neuron 1998, 21, 531–543. [Google Scholar] [CrossRef]
- Caterina, M.J.; Leffler, A.; Malmberg, A.B.; Martin, W.; Trafton, J.; Petersen-Zeitz, K.; Koltzenburg, M.; Basbaum, A.; Julius, D. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science 2000, 288, 306–313. [Google Scholar] [CrossRef]
- Wooten, M.; Weng, H.-J.; Hartke, T.V.; Borzan, J.; Klein, A.H.; Turnquist, B.; Dong, X.; Meyer, R.A.; Ringkamp, M. Three functionally distinct classes of C-fibre nociceptors in primates. Nat. Commun. 2014, 5, 4122. [Google Scholar] [CrossRef]
- Imamachi, N.; Park, G.H.; Lee, H.; Anderson, D.J.; Simon, M.I.; Basbaum, A.I.; Han, S.-K. TRPV1-expressing primary afferents generate behavioral responses to pruritogens via multiple mechanisms. Proc. Natl. Acad. Sci. USA 2009, 106, 11330–11335. [Google Scholar] [CrossRef]
- Lim, K.-M.; Park, Y.-H. Development of PAC-14028, a novel transient receptor potential vanilloid type 1 (TRPV1) channel antagonist as a new drug for refractory skin diseases. Arch. Pharmacal Res. 2012, 35, 393–396. [Google Scholar] [CrossRef] [PubMed]
- Yun, J.-W.; Seo, J.A.; Jang, W.-H.; Koh, H.J.; Bae, I.-H.; Park, Y.-H.; Lim, K.-M. Antipruritic effects of TRPV1 antagonist in murine atopic dermatitis and itching models. J. Investig. Dermatol. 2011, 131, 1576–1579. [Google Scholar] [CrossRef] [PubMed]
- Park, C.W.; Kim, B.J.; Lee, Y.W.; Won, C.; Park, C.O.; Chung, B.Y.; Lee, D.H.; Jung, K.; Nam, H.-J.; Choi, G. Asivatrep, a TRPV1 antagonist, for the topical treatment of atopic dermatitis: Phase 3, randomized, vehicle-controlled study (CAPTAIN-AD). J. Allergy Clin. Immunol. 2022, 149, 1340–1347.e1344. [Google Scholar] [CrossRef] [PubMed]
- Nikolaeva-Koleva, M.; Butron, L.; González-Rodríguez, S.; Devesa, I.; Valente, P.; Serafini, M.; Genazzani, A.A.; Pirali, T.; Ballester, G.F.; Fernández-Carvajal, A. A capsaicinoid-based soft drug, AG1529, for attenuating TRPV1-mediated histaminergic and inflammatory sensory neuron excitability. Sci. Rep. 2021, 11, 246. [Google Scholar] [CrossRef]
- Backonja, M.M.; Malan, T.P.; Vanhove, G.F.; Tobias, J.K. NGX-4010, a high-concentration capsaicin patch, for the treatment of postherpetic neuralgia: A randomized, double-blind, controlled study with an open-label extension. Pain Med. 2010, 11, 600–608. [Google Scholar] [CrossRef]
- Papoiu, A.D.; Yosipovitch, G. Topical capsaicin. The fire of a ‘hot’medicine is reignited. Expert Opin. Pharmacother. 2010, 11, 1359–1371. [Google Scholar] [CrossRef]
- Andersen, H.H.; Arendt-Nielsen, L.; Elberling, J. Topical capsaicin 8% for the treatment of neuropathic itch conditions. Clin. Exp. Dermatol. 2017, 42, 596–598. [Google Scholar] [CrossRef]
- Fazio, S.B.; Yosipovitch, G. Pruritus: Therapies for Localized Pruritus; Dellavalle, R.P., Callen, J., Eds.; Uptodate: Waltham, MA, USA, 2022. [Google Scholar]
- Smith, G.; Gunthorpe, M.; Kelsell, R.; Hayes, P.; Reilly, P.; Facer, P.; Wright, J.; Jerman, J.; Walhin, J.-P.; Ooi, L. TRPV3 is a temperature-sensitive vanilloid receptor-like protein. Nature 2002, 418, 186–190. [Google Scholar] [CrossRef]
- Moqrich, A.; Hwang, S.W.; Earley, T.J.; Petrus, M.J.; Murray, A.N.; Spencer, K.S.; Andahazy, M.; Story, G.M.; Patapoutian, A. Impaired thermosensation in mice lacking TRPV3, a heat and camphor sensor in the skin. Science 2005, 307, 1468–1472. [Google Scholar] [CrossRef]
- Yoshioka, T.; Imura, K.; Asakawa, M.; Suzuki, M.; Oshima, I.; Hirasawa, T.; Sakata, T.; Horikawa, T.; Arimura, A. Impact of the Gly573Ser substitution in TRPV3 on the development of allergic and pruritic dermatitis in mice. J. Investig. Dermatol. 2009, 129, 714–722. [Google Scholar] [CrossRef]
- Han, Y.; Luo, A.; Kamau, P.M.; Takomthong, P.; Hu, J.; Boonyarat, C.; Luo, L.; Lai, R. A plant-derived TRPV3 inhibitor suppresses pain and itch. Br. J. Pharmacol. 2021, 178, 1669–1683. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Wang, J.; Wei, X.; Hu, J.; Ping, C.; Gao, Y.; Xie, C.; Wang, P.; Cao, P.; Cao, Z. Therapeutic inhibition of keratinocyte TRPV3 sensory channel by local anesthetic dyclonine. eLife 2021, 10, e68128. [Google Scholar] [CrossRef] [PubMed]
- Shelmire, B.; Gastineau, F.; Shields, T.L. Evaluation of a new topical anesthetic, dyclonine hydrochloride. AMA Arch. Dermatol. 1955, 71, 728–730. [Google Scholar] [CrossRef] [PubMed]
- Broad, L.M.; Mogg, A.J.; Eberle, E.; Tolley, M.; Li, D.L.; Knopp, K.L. TRPV3 in drug development. Pharmaceuticals 2016, 9, 55. [Google Scholar] [CrossRef]
- Grubisha, O.; Mogg, A.J.; Sorge, J.L.; Ball, L.J.; Sanger, H.; Ruble, C.L.; Folly, E.A.; Ursu, D.; Broad, L.M. Pharmacological profiling of the TRPV3 channel in recombinant and native assays. Br. J. Pharmacol. 2014, 171, 2631–2644. [Google Scholar] [CrossRef]
- Liedtke, W.; Choe, Y.; Martí-Renom, M.A.; Bell, A.M.; Denis, C.S.; Hudspeth, A.; Friedman, J.M.; Heller, S. Vanilloid receptor–related osmotically activated channel (VR-OAC), a candidate vertebrate osmoreceptor. Cell 2000, 103, 525–535. [Google Scholar] [CrossRef]
- Alessandri-Haber, N.; Joseph, E.; Dina, O.A.; Liedtke, W.; Levine, J.D. TRPV4 mediates pain-related behavior induced by mild hypertonic stimuli in the presence of inflammatory mediator. Pain 2005, 118, 70–79. [Google Scholar] [CrossRef]
- Chen, Y.; Kanju, P.; Fang, Q.; Lee, S.H.; Parekh, P.K.; Lee, W.; Moore, C.; Brenner, D.; Gereau, R.W., IV; Wang, F. TRPV4 is necessary for trigeminal irritant pain and functions as a cellular formalin receptor. Pain 2014, 155, 2662–2672. [Google Scholar] [CrossRef]
- Akiyama, T.; Ivanov, M.; Nagamine, M.; Davoodi, A.; Carstens, M.I.; Ikoma, A.; Cevikbas, F.; Kempkes, C.; Buddenkotte, J.; Steinhoff, M. Involvement of TRPV4 in serotonin-evoked scratching. J. Investig. Dermatol. 2016, 136, 154–160. [Google Scholar] [CrossRef]
- Kim, S.; Barry, D.M.; Liu, X.-Y.; Yin, S.; Munanairi, A.; Meng, Q.-T.; Cheng, W.; Mo, P.; Wan, L.; Liu, S.-B. Facilitation of TRPV4 by TRPV1 is required for itch transmission in some sensory neuron populations. Sci. Signal. 2016, 9, ra71. [Google Scholar] [CrossRef]
- Lawhorn, B.G.; Brnardic, E.J.; Behm, D.J. TRPV4 antagonists: A patent review (2015–2020). Expert Opin. Ther. Pat. 2021, 31, 773–784. [Google Scholar] [CrossRef] [PubMed]
- Story, G.M.; Peier, A.M.; Reeve, A.J.; Eid, S.R.; Mosbacher, J.; Hricik, T.R.; Earley, T.J.; Hergarden, A.C.; Andersson, D.A.; Hwang, S.W. ANKTM1, a TRP-like channel expressed in nociceptive neurons, is activated by cold temperatures. Cell 2003, 112, 819–829. [Google Scholar] [CrossRef]
- Liu, Q.; Feng, L.; Han, X.; Zhang, W.; Zhang, H.; Xu, L. The TRPA1 Channel Mediates Mechanical Allodynia and Thermal Hyperalgesia in a Rat Bone Cancer Pain Model. Front. Pain Res. 2021, 2, 7. [Google Scholar] [CrossRef]
- Bandell, M.; Story, G.M.; Hwang, S.W.; Viswanath, V.; Eid, S.R.; Petrus, M.J.; Earley, T.J.; Patapoutian, A. Noxious cold ion channel TRPA1 is activated by pungent compounds and bradykinin. Neuron 2004, 41, 849–857. [Google Scholar] [CrossRef]
- Kwan, K.Y.; Glazer, J.M.; Corey, D.P.; Rice, F.L.; Stucky, C.L. TRPA1 modulates mechanotransduction in cutaneous sensory neurons. J. Neurosci. 2009, 29, 4808–4819. [Google Scholar] [CrossRef]
- Wilson, S.R.; Gerhold, K.A.; Bifolck-Fisher, A.; Liu, Q.; Patel, K.N.; Dong, X.; Bautista, D.M. TRPA1 is required for histamine-independent, Mas-related G protein–coupled receptor–mediated itch. Nat. Neurosci. 2011, 14, 595–602. [Google Scholar] [CrossRef]
- Kremeyer, B.; Lopera, F.; Cox, J.J.; Momin, A.; Rugiero, F.; Marsh, S.; Woods, C.G.; Jones, N.G.; Paterson, K.J.; Fricker, F.R. A gain-of-function mutation in TRPA1 causes familial episodic pain syndrome. Neuron 2010, 66, 671–680. [Google Scholar] [CrossRef]
- Rosengaard, C.; Andersen, H.H.; Arendt-Nielsen, L.; Gazerani, P. A human surrogate model of itch utilizing the TRPA1 agonist trans-cinnamaldehyde. Acta Derm. 2015, 95, 798–803. [Google Scholar]
- Chen, H.; Terrett, J.A. Transient receptor potential ankyrin 1 (TRPA1) antagonists: A patent review (2015–2019). Expert Opin. Ther. Pat. 2020, 30, 643–657. [Google Scholar] [CrossRef]
- Weng, H.-J.; Patel, K.N.; Jeske, N.A.; Bierbower, S.M.; Zou, W.; Tiwari, V.; Zheng, Q.; Tang, Z.; Mo, G.C.; Wang, Y. Tmem100 is a regulator of TRPA1-TRPV1 complex and contributes to persistent pain. Neuron 2015, 85, 833–846. [Google Scholar] [CrossRef]
- Dong, X.; Weng, H.-J. Tmem100 Peptides and Variants Thereof and Their Use in Treating or Preventing Diseases or Conditions. US11066455B2, 20 July 2021. [Google Scholar]
- Dhaka, A.; Murray, A.N.; Mathur, J.; Earley, T.J.; Petrus, M.J.; Patapoutian, A. TRPM8 is required for cold sensation in mice. Neuron 2007, 54, 371–378. [Google Scholar] [CrossRef] [PubMed]
- McKemy, D.D.; Neuhausser, W.M.; Julius, D. Identification of a cold receptor reveals a general role for TRP channels in thermosensation. Nature 2002, 416, 52–58. [Google Scholar] [CrossRef] [PubMed]
- González-Muñiz, R.; Bonache, M.A.; Martín-Escura, C.; Gómez-Monterrey, I. Recent progress in TRPM8 modulation: An update. Int. J. Mol. Sci. 2019, 20, 2618. [Google Scholar] [CrossRef] [PubMed]
- Davies, S.J.; Harding, L.M.; Baranowski, A.P. A novel treatment of postherpetic neuralgia using peppermint oil. Clin. J. Pain 2002, 18, 200–202. [Google Scholar] [CrossRef]
- Pergolizzi, J., Jr.; Taylor, R., Jr.; LeQuang, J.A.; Raffa, R.; Group, N.R. The role and mechanism of action of menthol in topical analgesic products. J. Clin. Pharm. Ther. 2018, 43, 313–319. [Google Scholar] [CrossRef]
- Caceres, A.I.; Liu, B.; Jabba, S.V.; Achanta, S.; Morris, J.B.; Jordt, S.E. Transient receptor potential cation channel subfamily M member 8 channels mediate the anti-inflammatory effects of eucalyptol. Br. J. Pharmacol. 2017, 174, 867–879. [Google Scholar] [CrossRef]
- Knowlton, W.M.; Palkar, R.; Lippoldt, E.K.; McCoy, D.D.; Baluch, F.; Chen, J.; McKemy, D.D. A sensory-labeled line for cold: TRPM8-expressing sensory neurons define the cellular basis for cold, cold pain, and cooling-mediated analgesia. J. Neurosci. 2013, 33, 2837–2848. [Google Scholar] [CrossRef]
- Andersen, H.H.; Gazerani, P.; Arendt-Nielsen, L. High-concentration L-menthol exhibits counter-irritancy to neurogenic inflammation, thermal and mechanical hyperalgesia caused by trans-cinnamaldehyde. J. Pain 2016, 17, 919–929. [Google Scholar] [CrossRef]
- Colvin, L.A.; Johnson, P.R.; Mitchell, R.; Fleetwood-Walker, S.M.; Fallon, M. From bench to bedside: A case of rapid reversal of bortezomib-induced neuropathic pain by the TRPM8 activator, menthol. J. Clin. Oncol. 2008, 26, 4519–4520. [Google Scholar] [CrossRef]
- Palkar, R.; Ongun, S.; Catich, E.; Li, N.; Borad, N.; Sarkisian, A.; McKemy, D.D. Cooling relief of acute and chronic itch requires TRPM8 channels and neurons. J. Investig. Dermatol. 2018, 138, 1391–1399. [Google Scholar] [CrossRef]
- Wei, E.T. Di-Isopropyl-Phosphinoyl-Alkanes (Dapa) Compounds as Topical Agents for the Treatment of Sensory Discomfort. US20210401857A1, 30 December 2021. [Google Scholar]
- Liu, B.; Fan, L.; Balakrishna, S.; Sui, A.; Morris, J.B.; Jordt, S.-E. TRPM8 is the principal mediator of menthol-induced analgesia of acute and inflammatory pain. Pain 2013, 154, 2169–2177. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, Y.; Narang, C.; Limjunyawong, N.; Jamaldeen, H.; Yu, S.; Patiram, S.; Nie, H.; Caterina, M.J.; Dong, X. Sensory Neuron Expressed TRPC3 Mediates Acute and Chronic Itch. 2021. Available online: https://assets.researchsquare.com/files/rs-1021582/v1/e34a7342-53a2-4e30-9468-4fa4759b6152.pdf?c=1648558243 (accessed on 4 June 2022).
- Ko, M.-C. Neuraxial Opioid-Induced Itch and Its Pharmacological Antagonism. In Pharmacology of Itch; Springer: Berlin/Heidelberg, Germany, 2015; pp. 315–335. [Google Scholar]
- Ko, M.H.; Song, M.; Edwards, T.; Lee, H.; Naughton, N. The role of central μ opioid receptors in opioid-induced itch in primates. J. Pharmacol. Exp. Ther. 2004, 310, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Bigliardi, P.L.; Stammer, H.; Jost, G.; Rufli, T.; Büchner, S.; Bigliardi-Qi, M. Treatment of pruritus with topically applied opiate receptor antagonist. J. Am. Acad. Dermatol. 2007, 56, 979–988. [Google Scholar] [CrossRef]
- Fishbane, S.; Jamal, A.; Munera, C.; Wen, W.; Menzaghi, F. A phase 3 trial of difelikefalin in hemodialysis patients with pruritus. N. Engl. J. Med. 2020, 382, 222–232. [Google Scholar] [CrossRef]
- Menzaghi, F.; Spencer, R.; Abrouk, N.; Lewis, M.; Chalmers, D. (422) CR845, a peripheral kappa opioid, provides better pain relief with less nausea and vomiting than placebo in patients after bunionectomy. J. Pain 2015, 16, S81. [Google Scholar] [CrossRef]
- Wang, X.; Gou, X.; Yu, X.; Bai, D.; Tan, B.; Cao, P.; Qian, M.; Zheng, X.; Wang, H.; Tang, P. Antinociceptive and antipruritic effects of HSK21542, a peripherally-restricted kappa opioid receptor agonist, in animal models of pain and itch. Front. Pharmacol. 2021, 12, 773204. [Google Scholar] [CrossRef] [PubMed]
- Brust, T.F.; Morgenweck, J.; Kim, S.A.; Rose, J.H.; Locke, J.L.; Schmid, C.L.; Zhou, L.; Stahl, E.L.; Cameron, M.D.; Scarry, S.M. Biased agonists of the kappa opioid receptor suppress pain and itch without causing sedation or dysphoria. Sci. Signal. 2016, 9, ra117. [Google Scholar] [CrossRef]
- Lee, B.; Elston, D.M. The uses of naltrexone in dermatologic conditions. J. Am. Acad. Dermatol. 2019, 80, 1746–1752. [Google Scholar] [CrossRef]
- Lee, J.; Shin, J.U.; Noh, S.; Park, C.O.; Lee, K.H. Clinical efficacy and safety of naltrexone combination therapy in older patients with severe pruritus. Ann. Dermatol. 2016, 28, 159–163. [Google Scholar] [CrossRef][Green Version]
- Levine, J.; Gordon, N.; Jones, R.; Fields, H. The narcotic antagonist naloxone enhances clinical pain. Nature 1978, 272, 826–827. [Google Scholar] [CrossRef]
- Chen, K.Y.; Chen, L.; Mao, J. Buprenorphine–naloxone therapy in pain management. Anesthesiology 2014, 120, 1262–1274. [Google Scholar] [CrossRef] [PubMed]
- Dawn, A.G.; Yosipovitch, G. Butorphanol for treatment of intractable pruritus. J. Am. Acad. Dermatol. 2006, 54, 527–531. [Google Scholar] [CrossRef] [PubMed]
- Golpanian, R.S.; Yosipovitch, G.; Levy, C. Use of butorphanol as treatment for cholestatic itch. Dig. Dis. Sci. 2021, 66, 1693–1699. [Google Scholar] [CrossRef] [PubMed]
- Mathur, V.S.; Kumar, J.; Crawford, P.W.; Hait, H.; Sciascia, T.; Investigators, T.S. A multicenter, randomized, double-blind, placebo-controlled trial of nalbuphine ER tablets for uremic pruritus. Am. J. Nephrol. 2017, 46, 450–458. [Google Scholar] [CrossRef]
- Jannuzzi, R.G. Nalbuphine for treatment of opioid-induced pruritus. Clin. J. Pain 2016, 32, 87–93. [Google Scholar] [CrossRef]
- Pereira, M.P.; Ständer, S. Novel drugs for the treatment of chronic pruritus. Expert Opin. Investig. Drugs 2018, 27, 981–988. [Google Scholar] [CrossRef]
- Thangam, E.B.; Jemima, E.A.; Singh, H.; Baig, M.S.; Khan, M.; Mathias, C.B.; Church, M.K.; Saluja, R. The role of histamine and histamine receptors in mast cell-mediated allergy and inflammation: The hunt for new therapeutic targets. Front. Immunol. 2018, 9, 1873. [Google Scholar] [CrossRef]
- Lewis, T. The blood vessels of the human skin. Br. Med. J. 1926, 2, 61. [Google Scholar] [CrossRef]
- Simone, D.A.; Ngeow, J.Y.; Whitehouse, J.; Becerra-Cabal, L.; Putterman, G.J.; Lamotte, R.H. The magnitude and duration of itch produced by intracutaneous injections of histamine. Somatosens. Res. 1987, 5, 81–92. [Google Scholar] [CrossRef]
- Obara, I.; Telezhkin, V.; Alrashdi, I.; Chazot, P.L. Histamine, histamine receptors, and neuropathic pain relief. Br. J. Pharmacol. 2020, 177, 580–599. [Google Scholar] [CrossRef]
- Santiago-Palma, J.; Fischberg, D.; Kornick, C.; Khjainova, N.; Gonzales, G. Diphenhydramine as an analgesic adjuvant in refractory cancer pain. J. Pain Symptom Manag. 2001, 22, 699–703. [Google Scholar] [CrossRef]
- Dunford, P.J.; Williams, K.N.; Desai, P.J.; Karlsson, L.; McQueen, D.; Thurmond, R.L. Histamine H4 receptor antagonists are superior to traditional antihistamines in the attenuation of experimental pruritus. J. Allergy Clin. Immunol. 2007, 119, 176–183. [Google Scholar] [CrossRef] [PubMed]
- Roßbach, K.; Wendorff, S.; Sander, K.; Stark, H.; Gutzmer, R.; Werfel, T.; Kietzmann, M.; Bäumer, W. Histamine H4 receptor antagonism reduces hapten-induced scratching behaviour but not inflammation. Exp. Dermatol. 2009, 18, 57–63. [Google Scholar] [CrossRef]
- Coruzzi, G.; Adami, M.; Guaita, E.; de Esch, I.J.; Leurs, R. Antiinflammatory and antinociceptive effects of the selective histamine H4-receptor antagonists JNJ7777120 and VUF6002 in a rat model of carrageenan-induced acute inflammation. Eur. J. Pharmacol. 2007, 563, 240–244. [Google Scholar] [CrossRef] [PubMed]
- Popiolek-Barczyk, K.; Łażewska, D.; Latacz, G.; Olejarz, A.; Makuch, W.; Stark, H.; Kieć-Kononowicz, K.; Mika, J. Antinociceptive effects of novel histamine H3 and H4 receptor antagonists and their influence on morphine analgesia of neuropathic pain in the mouse. Br. J. Pharmacol. 2018, 175, 2897–2910. [Google Scholar] [CrossRef] [PubMed]
- Schlosburg, J.E.; O’Neal, S.T.; Conrad, D.H.; Lichtman, A.H. CB1 receptors mediate rimonabant-induced pruritic responses in mice: Investigation of locus of action. Psychopharmacology 2011, 216, 323–331. [Google Scholar] [CrossRef]
- Clayton, N.; Marshall, F.; Bountra, C.; O’shaughnessy, C. CB1 and CB2 cannabinoid receptors are implicated in inflammatory pain. Pain 2002, 96, 253–260. [Google Scholar] [CrossRef]
- Bilir, K.; Anli, G.; Ozkan, E.; Gunduz, O.; Ulugol, A. Involvement of spinal cannabinoid receptors in the antipruritic effects of WIN 55,212-2, a cannabinoid receptor agonist. Clin. Exp. Dermatol. 2018, 43, 553–558. [Google Scholar] [CrossRef]
- Latek, D.; Kolinski, M.; Ghoshdastider, U.; Debinski, A.; Bombolewski, R.; Plazinska, A.; Jozwiak, K.; Filipek, S. Modeling of ligand binding to G protein coupled receptors: Cannabinoid CB1, CB2 and adrenergic β2AR. J. Mol. Model. 2011, 17, 2353–2366. [Google Scholar] [CrossRef]
- Avila, C.; Massick, S.; Kaffenberger, B.H.; Kwatra, S.G.; Bechtel, M. Cannabinoids for the treatment of chronic pruritus: A review. J. Am. Acad. Dermatol. 2020, 82, 1205–1212. [Google Scholar] [CrossRef]
- Ständer, S.; Schmelz, M.; Metze, D.; Luger, T.; Rukwied, R. Distribution of cannabinoid receptor 1 (CB1) and 2 (CB2) on sensory nerve fibers and adnexal structures in human skin. J. Dermatol. Sci. 2005, 38, 177–188. [Google Scholar] [CrossRef]
- Moreira, F.A.; Grieb, M.; Lutz, B. Central side-effects of therapies based on CB1 cannabinoid receptor agonists and antagonists: Focus on anxiety and depression. Best Pract. Res. Clin. Endocrinol. Metab. 2009, 23, 133–144. [Google Scholar] [CrossRef]
- Neff, G.W.; O’Brien, C.B.; Reddy, K.R.; Bergasa, N.V.; Regev, A.; Molina, E.; Amaro, R.; Rodriguez, M.J.; Chase, V.; Jeffers, L. Preliminary observation with dronabinol in patients with intractable pruritus secondary to cholestatic liver disease. Am. J. Gastroenterol. 2002, 97, 2117–2119. [Google Scholar] [CrossRef]
- Narang, S.; Gibson, D.; Wasan, A.D.; Ross, E.L.; Michna, E.; Nedeljkovic, S.S.; Jamison, R.N. Efficacy of dronabinol as an adjuvant treatment for chronic pain patients on opioid therapy. J. Pain 2008, 9, 254–264. [Google Scholar] [CrossRef]
- Dvorak, M.; Watkinson, A.; McGlone, F.; Rukwied, R. Histamine induced responses are attenuated by a cannabinoid receptor agonist in human skin. Inflamm. Res. 2003, 52, 238–245. [Google Scholar] [CrossRef]
- Yuan, C.; Wang, X.-M.; Guichard, A.; Tan, Y.-M.; Qian, C.-Y.; Yang, L.-J.; Humbert, P. N-palmitoylethanolamine and N-acetylethanolamine are effective in asteatotic eczema: Results of a randomized, double-blind, controlled study in 60 patients. Clin. Interv. Aging 2014, 9, 1163. [Google Scholar] [CrossRef]
- Szepietowski, J.C.; Szepietowski, T.; Reich, A. Efficacy and tolerance of the cream containing structured physiological lipids with endocannabinoids in the treatment of uremic pruritus: A preliminary study. Acta Dermatovenerol. Croat. 2005, 13, 97–103. [Google Scholar]
- Tamura, S.; Morikawa, Y.; Miyajima, A.; Senba, E. Expression of oncostatin M receptor β in a specific subset of nociceptive sensory neurons. Eur. J. Neurosci. 2003, 17, 2287–2298. [Google Scholar] [CrossRef]
- Morikawa, Y.; Tamura, S.; Minehata, K.-I.; Donovan, P.J.; Miyajima, A.; Senba, E. Essential function of oncostatin m in nociceptive neurons of dorsal root ganglia. J. Neurosci. 2004, 24, 1941–1947. [Google Scholar] [CrossRef]
- Garza Carbajal, A.; Ebersberger, A.; Thiel, A.; Ferrari, L.; Acuna, J.; Brosig, S.; Isensee, J.; Moeller, K.; Siobal, M.; Rose-John, S. Oncostatin M induces hyperalgesic priming and amplifies signaling of cAMP to ERK by RapGEF2 and PKA. J. Neurochem. 2021, 157, 1821–1837. [Google Scholar] [CrossRef]
- Hashimoto, T.; Nattkemper, L.A.; Kim, H.S.; Kursewicz, C.D.; Fowler, E.; Shah, S.M.; Nanda, S.; Fayne, R.A.; Paolini, J.F.; Romanelli, P. Itch intensity in prurigo nodularis is closely related to dermal interleukin-31, oncostatin M, IL-31 receptor alpha and oncostatin M receptor beta. Exp. Dermatol. 2021, 30, 804–810. [Google Scholar] [CrossRef]
- Tseng, P.-Y.; Hoon, M.A. Oncostatin M can sensitize sensory neurons in inflammatory pruritus. Sci. Transl. Med. 2021, 13, eabe3037. [Google Scholar] [CrossRef]
- Fu, X.-Y.; Kessler, D.S.; Veals, S.A.; Levy, D.E.; Darnell, J. ISGF3, the transcriptional activator induced by interferon alpha, consists of multiple interacting polypeptide chains. Proc. Natl. Acad. Sci. USA 1990, 87, 8555–8559. [Google Scholar] [CrossRef]
- Wilks, A.F. Two putative protein-tyrosine kinases identified by application of the polymerase chain reaction. Proc. Natl. Acad. Sci. USA 1989, 86, 1603–1607. [Google Scholar] [CrossRef]
- Hu, X.; Fu, M.; Zhao, X.; Wang, W. The JAK/STAT signaling pathway: From bench to clinic. Signal Transduct. Target. Ther. 2021, 6, 402. [Google Scholar] [CrossRef]
- Howell, M.D.; Kuo, F.I.; Smith, P.A. Targeting the Janus kinase family in autoimmune skin diseases. Front. Immunol. 2019, 10, 2342. [Google Scholar] [CrossRef]
- Shiratori-Hayashi, M.; Yamaguchi, C.; Eguchi, K.; Shiraishi, Y.; Kohno, K.; Mikoshiba, K.; Inoue, K.; Nishida, M.; Tsuda, M. Astrocytic STAT3 activation and chronic itch require IP3R1/TRPC-dependent Ca2+ signals in mice. J. Allergy Clin. Immunol. 2021, 147, 1341–1353. [Google Scholar] [CrossRef]
- Oetjen, L.K.; Mack, M.R.; Feng, J.; Whelan, T.M.; Niu, H.; Guo, C.J.; Chen, S.; Trier, A.M.; Xu, A.Z.; Tripathi, S.V. Sensory neurons co-opt classical immune signaling pathways to mediate chronic itch. Cell 2017, 171, 217–228.e213. [Google Scholar] [CrossRef]
- Kim, B.S. The translational revolution of itch. Neuron 2022, in press. [Google Scholar] [CrossRef]
- Tsuda, M.; Kohro, Y.; Yano, T.; Tsujikawa, T.; Kitano, J.; Tozaki-Saitoh, H.; Koyanagi, S.; Ohdo, S.; Ji, R.-R.; Salter, M.W. JAK-STAT3 pathway regulates spinal astrocyte proliferation and neuropathic pain maintenance in rats. Brain 2011, 134, 1127–1139. [Google Scholar] [CrossRef]
- Dominguez, E.; Rivat, C.; Pommier, B.; Mauborgne, A.; Pohl, M. JAK/STAT3 pathway is activated in spinal cord microglia after peripheral nerve injury and contributes to neuropathic pain development in rat. J. Neurochem. 2008, 107, 50–60. [Google Scholar] [CrossRef]
- Crispino, N.; Ciccia, F. JAK/STAT pathway and nociceptive cytokine signalling in rheumatoid arthritis and psoriatic arthritis. Clin. Exp. Rheumatol. 2021, 39, 668–675. [Google Scholar] [CrossRef]
- Salaffi, F.; Carotti, M.; Farah, S.; Ceccarelli, L.; Giovagnoni, A.; Di Carlo, M. Early response to JAK inhibitors on central sensitization and pain catastrophizing in patients with active rheumatoid arthritis. Inflammopharmacology 2022, 30, 1119–1128. [Google Scholar] [CrossRef]
- Reichardt, L.F. Neurotrophin-regulated signalling pathways. Philos. Trans. R. Soc. B Biol. Sci. 2006, 361, 1545–1564. [Google Scholar] [CrossRef]
- Sofroniew, M.V.; Howe, C.L.; Mobley, W.C. Nerve growth factor signaling, neuroprotection, and neural repair. Annu. Rev. Neurosci. 2001, 24, 1217–1281. [Google Scholar] [CrossRef]
- Aloe, L.; Rocco, M.L.; Balzamino, B.O.; Micera, A. Nerve growth factor: A focus on neuroscience and therapy. Curr. Neuropharmacol. 2015, 13, 294–303. [Google Scholar] [CrossRef]
- Aloe, L.; Rocco, M.L.; Bianchi, P.; Manni, L. Nerve growth factor: From the early discoveries to the potential clinical use. J. Transl. Med. 2012, 10, 239. [Google Scholar] [CrossRef]
- Micera, A.; Puxeddu, I.; Aloe, L.; Levi-Schaffer, F. New insights on the involvement of Nerve Growth Factor in allergic inflammation and fibrosis. Cytokine Growth Factor Rev. 2003, 14, 369–374. [Google Scholar] [CrossRef]
- Lambiase, A.; Micera, A.; Sgrulletta, R.; Bonini, S.; Bonini, S. Nerve growth factor and the immune system: Old and new concepts in the cross-talk between immune and resident cells during pathophysiological conditions. Curr. Opin. Allergy Clin. Immunol. 2004, 4, 425–430. [Google Scholar] [CrossRef]
- Yamaguchi, J.; Aihara, M.; Kobayashi, Y.; Kambara, T.; Ikezawa, Z. Quantitative analysis of nerve growth factor (NGF) in the atopic dermatitis and psoriasis horny layer and effect of treatment on NGF in atopic dermatitis. J. Dermatol. Sci. 2009, 53, 48–54. [Google Scholar] [CrossRef]
- Webb, M.P.; Helander, E.M.; Menard, B.L.; Urman, R.D.; Kaye, A.D. Tanezumab: A selective humanized mAb for chronic lower back pain. Ther. Clin. Risk Manag. 2018, 14, 361. [Google Scholar] [CrossRef]
- Dakin, P.; Kivitz, A.J.; Gimbel, J.S.; Skrepnik, N.; DiMartino, S.J.; Emeremni, C.A.; Gao, H.; Stahl, N.; Weinreich, D.M.; Yancopoulos, G.D. Efficacy and safety of fasinumab in patients with chronic low back pain: A phase II/III randomised clinical trial. Ann. Rheum. Dis. 2021, 80, 509–517. [Google Scholar] [CrossRef]
- Déry, O.; Corvera, C.U.; Steinhoff, M.; Bunnett, N.W. Proteinase-activated receptors: Novel mechanisms of signaling by serine proteases. Am. J. Physiol. Cell Physiol. 1998, 274, C1429–C1452. [Google Scholar] [CrossRef]
- Steinhoff, M.; Vergnolle, N.; Young, S.; Tognetto, M.; Amadesi, S.; Ennes, H.; Trevisani, M.; Hollenberg, M.; Wallace, J.; Caughey, G. Agonists of proteinase-activated receptor 2 induce inflammation by a neurogenic mechanism. Nat. Med. 2000, 6, 151–158. [Google Scholar] [CrossRef]
- Rothmeier, A.S.; Ruf, W. Protease-activated receptor 2 signaling in inflammation. Proc. Semin. Immunopathol. 2012, 34, 133–149. [Google Scholar] [CrossRef]
- Shpacovitch, V.; Feld, M.; Bunnett, N.; Steinhoff, M. Protease-activated receptors: Novel PARtners in innate immunity. Trends Immunol. 2007, 28, 541–550. [Google Scholar] [CrossRef]
- Liu, Q.; Weng, H.-J.; Patel, K.N.; Tang, Z.; Bai, H.; Steinhoff, M.; Dong, X. The distinct roles of two GPCRs, MrgprC11 and PAR2, in itch and hyperalgesia. Sci. Signal. 2011, 4, ra45. [Google Scholar] [CrossRef]
- Zhao, J.; Munanairi, A.; Liu, X.-Y.; Zhang, J.; Hu, L.; Hu, M.; Bu, D.; Liu, L.; Xie, Z.; Kim, B.S. PAR2 mediates itch via TRPV3 signaling in keratinocytes. J. Investig. Dermatol. 2020, 140, 1524–1532. [Google Scholar] [CrossRef]
- Yau, M.-K.; Liu, L.; Fairlie, D.P. Toward drugs for protease-activated receptor 2 (PAR2). J. Med. Chem. 2013, 56, 7477–7497. [Google Scholar] [CrossRef]
- Fiorucci, S.; Distrutti, E. Role of PAR2 in pain and inflammation. Trends Pharmacol. Sci. 2002, 23, 153–155. [Google Scholar] [CrossRef]
- Vergnolle, N.; Bunnett, N.; Sharkey, K.; Brussee, V.; Compton, S.; Grady, E.; Cirino, G.; Gerard, N.; Basbaum, A.; Andrade-Gordon, P. Proteinase-activated receptor-2 and hyperalgesia: A novel pain pathway. Nat. Med. 2001, 7, 821–826. [Google Scholar] [CrossRef]
- Lam, D.; Schmidt, B. Serine proteases and protease-activated receptor 2-dependent allodynia: A novel cancer pain pathway. PAIN 2010, 149, 263–272. [Google Scholar] [CrossRef]
- Buhl, T.; Ikoma, A.; Kempkes, C.; Cevikbas, F.; Sulk, M.; Buddenkotte, J.; Akiyama, T.; Crumrine, D.; Camerer, E.; Carstens, E. Protease-activated receptor-2 regulates neuro-epidermal communication in atopic dermatitis. Front. Immunol. 2020, 11, 1740. [Google Scholar] [CrossRef]
- Lam, D.K.; Dang, D.; Zhang, J.; Dolan, J.C.; Schmidt, B.L. Novel animal models of acute and chronic cancer pain: A pivotal role for PAR2. J. Neurosci. 2012, 32, 14178–14183. [Google Scholar] [CrossRef]
- McIntosh, K.A.; Cunningham, M.R.; Bushell, T.; Plevin, R. The development of proteinase-activated receptor-2 modulators and the challenges involved. Biochem. Soc. Trans. 2020, 48, 2525–2537. [Google Scholar] [CrossRef]
- Wei, H.; Wei, Y.; Tian, F.; Niu, T.; Yi, G. Blocking proteinase-activated receptor 2 alleviated neuropathic pain evoked by spinal cord injury. Physiol. Res. 2016, 65, 145. [Google Scholar] [CrossRef]
- Andoh, T.; Takayama, Y.; Yamakoshi, T.; Lee, J.-B.; Sano, A.; Shimizu, T.; Kuraishi, Y. Involvement of serine protease and proteinase-activated receptor 2 in dermatophyte-associated itch in mice. J. Pharmacol. Exp. Ther. 2012, 343, 91–96. [Google Scholar] [CrossRef]
- Egeo, G.; Fofi, L.; Barbanti, P. Botulinum neurotoxin for the treatment of neuropathic pain. Front. Neurol. 2020, 11, 716. [Google Scholar] [CrossRef]
- Hary, V.; Schitter, S.; Martinez, V. Efficacy and safety of botulinum A toxin for the treatment of chronic peripheral neuropathic pain: A systematic review of randomized controlled trials and meta-analysis. Eur. J. Pain 2022, 26, 980–990. [Google Scholar] [CrossRef]
- Portugal, D.M.; Ferreira, E.F.; Camões-Barbosa, A. Botulinum toxin type A therapy for bilateral focal neuropathic pruritus in multiple sclerosis: A case report. Int. J. Rehabil. Res. 2021, 44, 382–383. [Google Scholar] [CrossRef]
- Maari, C.; Marchessault, P.; Bissonnette, R. Treatment of notalgia paresthetica with botulinum toxin A: A double-blind randomized controlled trial. J. Am. Acad. Dermatol. 2014, 70, 1139–1141. [Google Scholar] [CrossRef]
- Shaarawy, E.; Hegazy, R.A.; Abdel Hay, R.M. Intralesional botulinum toxin type A equally effective and better tolerated than intralesional steroid in the treatment of keloids: A randomized controlled trial. J. Cosmet. Dermatol. 2015, 14, 161–166. [Google Scholar] [CrossRef]
- Klager, S.; Kumar, M.G. Treatment of pruritus with botulinum toxin in a pediatric patient with Fox-Fordyce disease. Pediatric Dermatol. 2021, 38, 950–951. [Google Scholar] [CrossRef]
- Meixiong, J.; Dong, X.; Weng, H.-J. Neuropathic itch. Cells 2020, 9, 2263. [Google Scholar] [CrossRef]
- Boozalis, E.; Sheu, M.; Selph, J.; Kwatra, S.G. Botulinum toxin type A for the treatment of localized recalcitrant chronic pruritus. J. Am. Acad. Dermatol. 2018, 78, 192–194. [Google Scholar] [CrossRef]
- Villamil, A.G.; Bandi, J.C.; Galdame, O.A.; Gerona, S.; Gadano, A.C. Efficacy of lidocaine in the treatment of pruritus in patients with chronic cholestatic liver diseases. Am. J. Med. 2005, 118, 1160–1163. [Google Scholar] [CrossRef]
- Lee, H.G.; Grossman, S.K.; Valdes-Rodriguez, R.; Berenato, F.; Korbutov, J.; Chan, Y.-H.; Lavery, M.J.; Yosipovitch, G. Topical ketamine-amitriptyline-lidocaine for chronic pruritus: A retrospective study assessing efficacy and tolerability. J. Am. Acad. Dermatol. 2017, 76, 760–761. [Google Scholar] [CrossRef]
- Kopecky, E.A.; Jacobson, S.; Hubley, P.; Palozzi, L.; Clarke, H.M.; Koren, G. Safety and pharmacokinetics of EMLA in the treatment of postburn pruritus in pediatric patients: A pilot study. J. Burn Care Rehabil. 2001, 22, 235–242. [Google Scholar] [CrossRef]
- Young, T.A.; Patel, T.S.; Camacho, F.; Clark, A.; Freedman, B.I.; Kaur, M.; Fountain, J.; Williams, L.L.; Yosipovitch, G.; Fleischer, A.B., Jr. A pramoxine-based anti-itch lotion is more effective than a control lotion for the treatment of uremic pruritus in adult hemodialysis patients. J. Dermatol. Treat. 2009, 20, 76–81. [Google Scholar] [CrossRef]
- Sutton, K.; Martin, D.; Pinnock, R.; Lee, K.; Scott, R. Gabapentin inhibits high-threshold calcium channel currents in cultured rat dorsal root ganglion neurones. Br. J. Pharmacol. 2002, 135, 257–265. [Google Scholar] [CrossRef]
- Matsuda, K.M.; Sharma, D.; Schonfeld, A.R.; Kwatra, S.G. Gabapentin and pregabalin for the treatment of chronic pruritus. J. Am. Acad. Dermajtol. 2016, 75, 619–625.e616. [Google Scholar] [CrossRef]
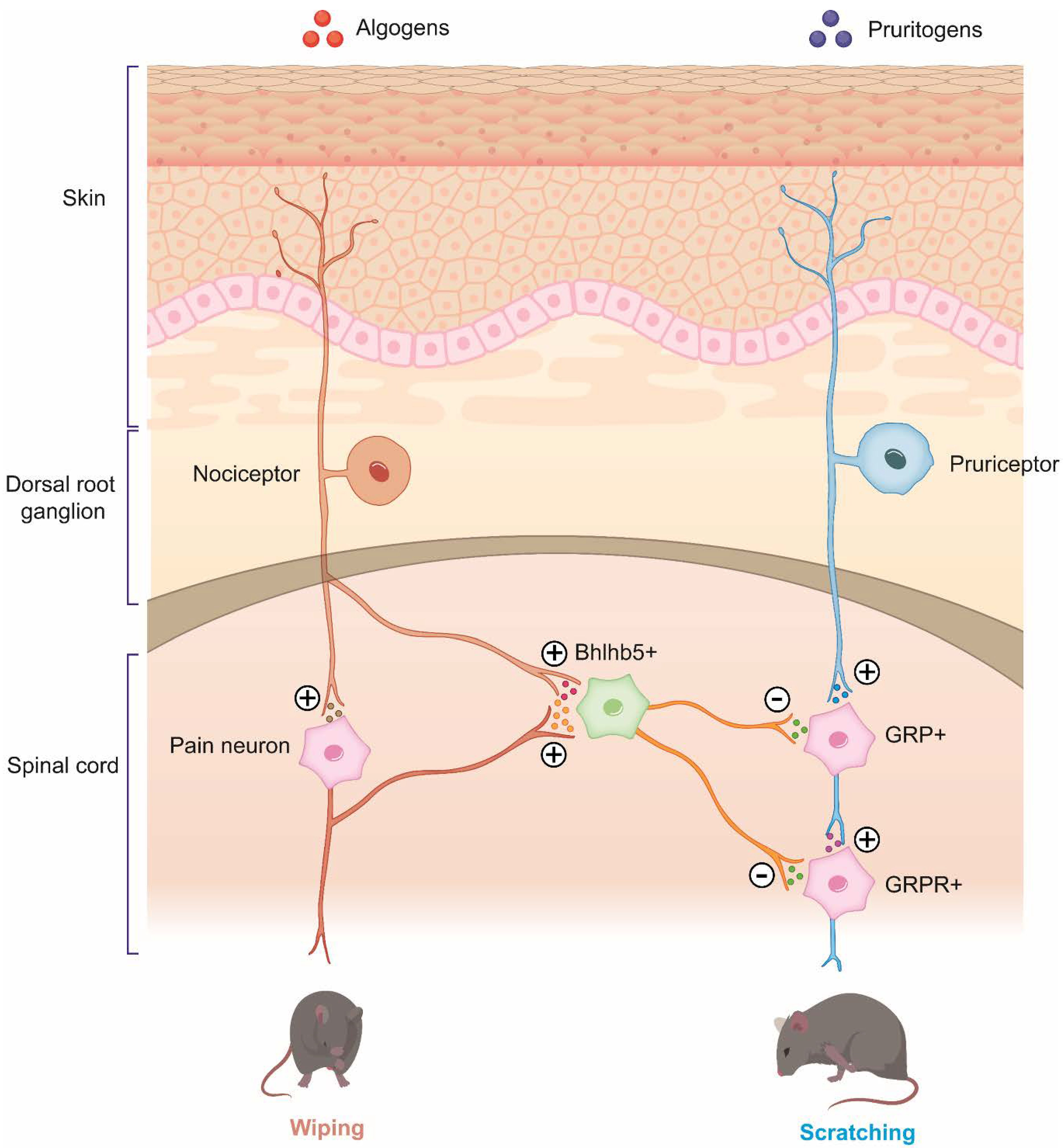
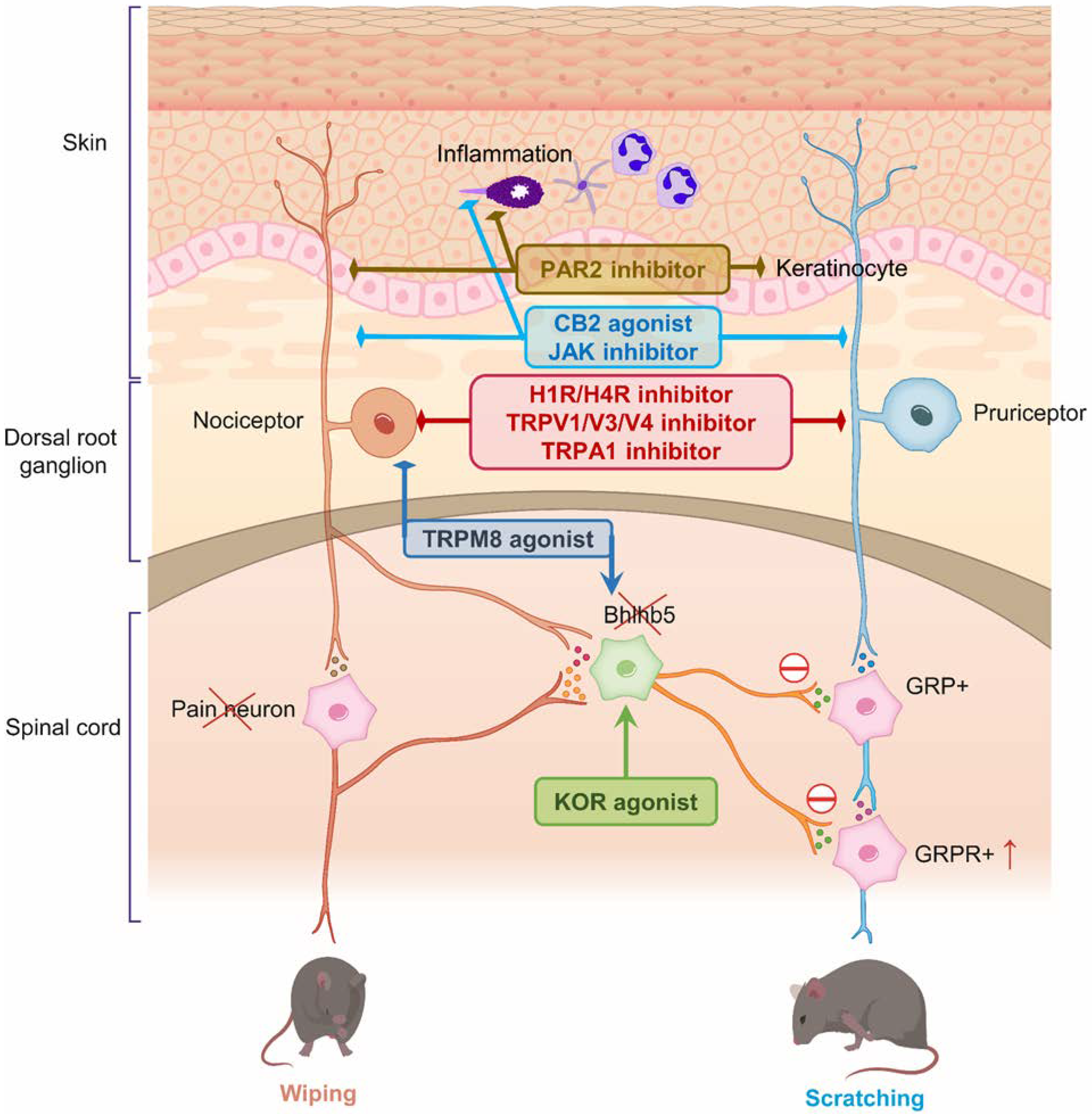
| Target | Effects on Itch and Pain | Therapeutic Compounds |
|---|---|---|
| TRPV1 | ↑ pain; ↑ itch | Asivatrep, AG1529, NGX-4010 |
| TRPV3 | ↑ pain; ↑ itch | Citrusinine-II, dyclonine, FTP-THQ |
| TRPV4 | ↑ pain; ↑ itch | isopropyl cyclohexane |
| TRPA1 | ↑ pain; ↑ itch | |
| TRPM8 | ↑↓ pain; ↑ itch | Di-isopropyl-phosphinoyl-alkanes, WS-12 |
| TRPC3 | ↑ pain; ↑ itch | |
| KOR | ↓ pain; ↓ itch | Difelikefalin, HSK21542, triazole 1.1, butorphanol, nalbuphine |
| MOR | ↓ pain; ↑ itch | Buprenorphine–naloxone, nalbuphine |
| H1R | ↑ pain; ↑ itch | Chlorpheniramine, fexofenadine, promethazine, diphenhydramine, orphenadrine, mepyramine, pyrilamine |
| H4R | ↓ pain(central); ↑ pain(peripheral); ↑ itch | JNJ7777120 |
| CB1 | ↓ pain; ↓ itch | Dronabinol |
| CB2 | ↓ pain; ↓ itch | Dronabinol |
| Oncostatin M | ↑ pain; ↑ itch | |
| JAK-STAT signaling | ↑ pain; ↑ itch | Baricitinib, upadacitinib |
| NGF | ↑ pain; ↑ itch | |
| PAR2 | ↑ pain; ↑ itch | FSLLRY-NH2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Weng, H.-J.; Pham, Q.T.T.; Chang, C.-W.; Tsai, T.-F. Druggable Targets and Compounds with Both Antinociceptive and Antipruritic Effects. Pharmaceuticals 2022, 15, 892. https://doi.org/10.3390/ph15070892
Weng H-J, Pham QTT, Chang C-W, Tsai T-F. Druggable Targets and Compounds with Both Antinociceptive and Antipruritic Effects. Pharmaceuticals. 2022; 15(7):892. https://doi.org/10.3390/ph15070892
Chicago/Turabian StyleWeng, Hao-Jui, Quoc Thao Trang Pham, Chia-Wei Chang, and Tsen-Fang Tsai. 2022. "Druggable Targets and Compounds with Both Antinociceptive and Antipruritic Effects" Pharmaceuticals 15, no. 7: 892. https://doi.org/10.3390/ph15070892
APA StyleWeng, H.-J., Pham, Q. T. T., Chang, C.-W., & Tsai, T.-F. (2022). Druggable Targets and Compounds with Both Antinociceptive and Antipruritic Effects. Pharmaceuticals, 15(7), 892. https://doi.org/10.3390/ph15070892








