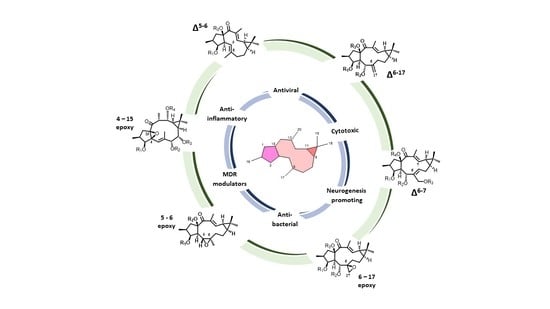
Pharmacological Potential of Lathyrane-Type Diterpenoids from Phytochemical Sources
Abstract
1. Introduction
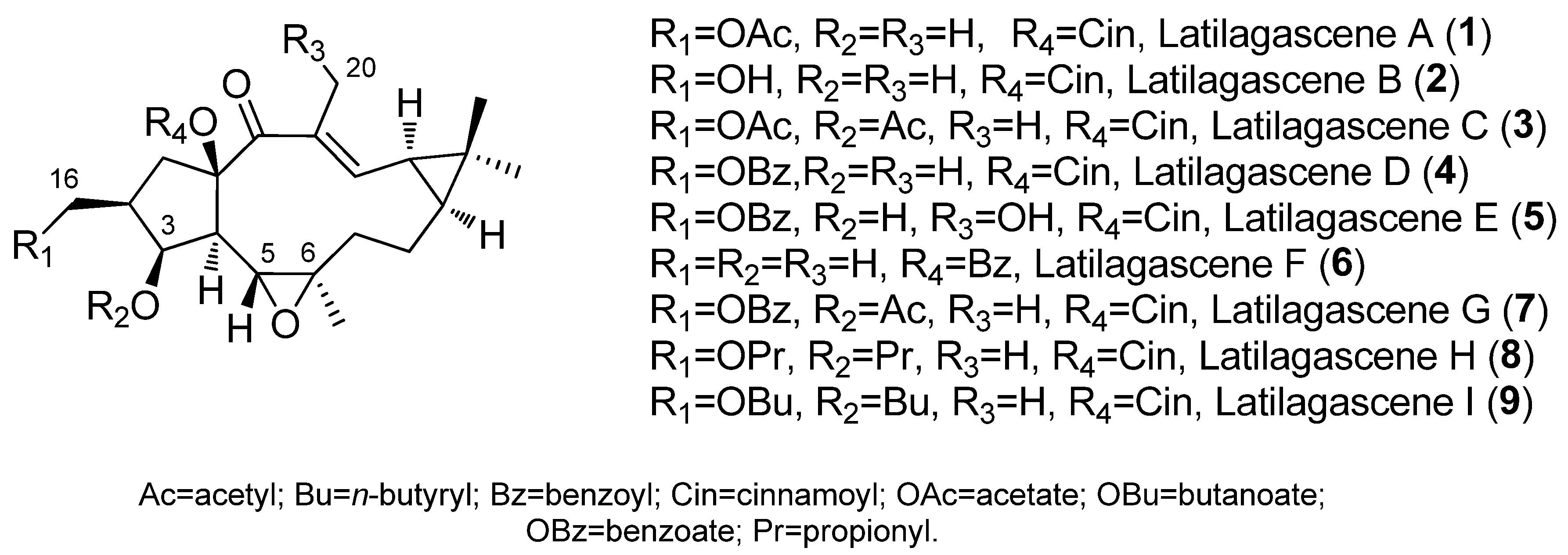
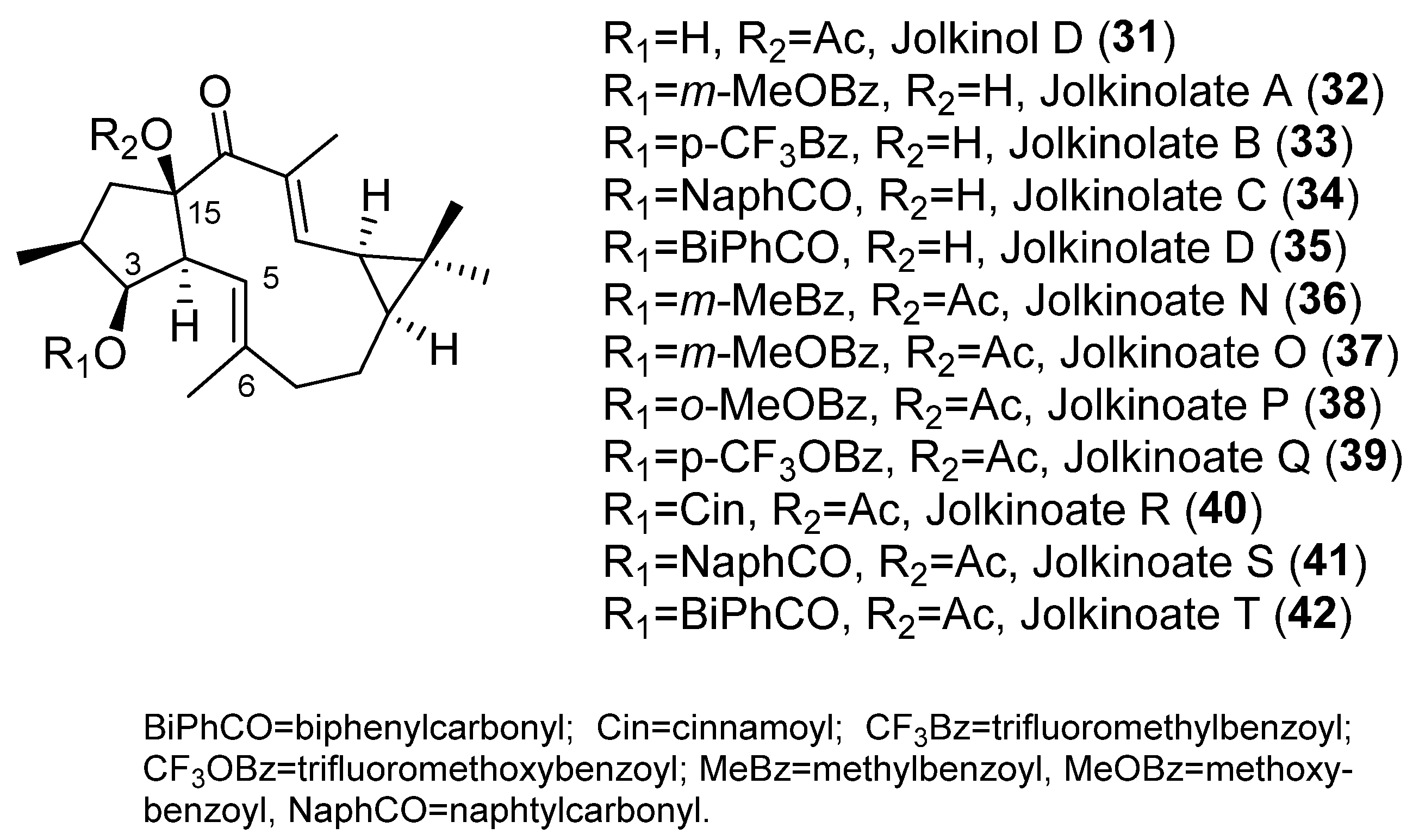
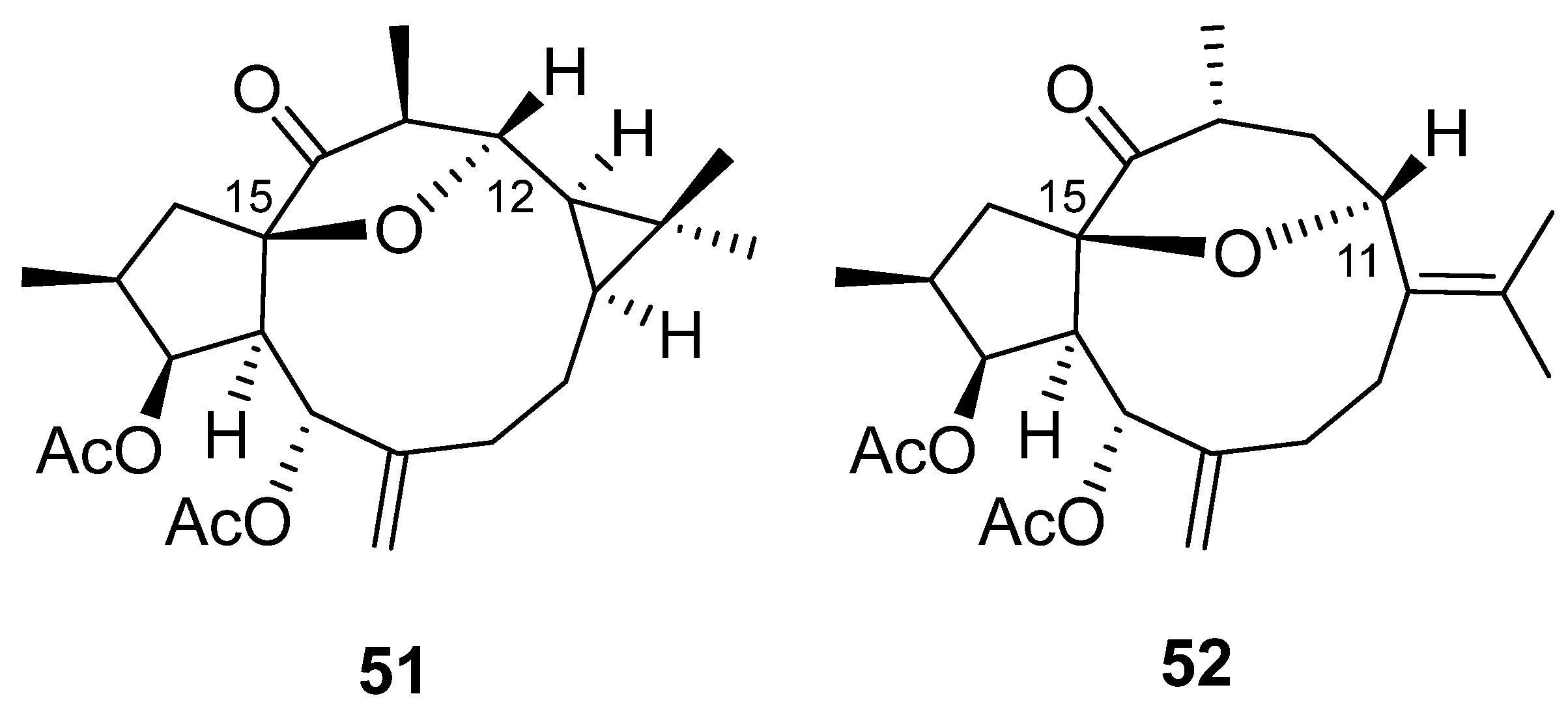
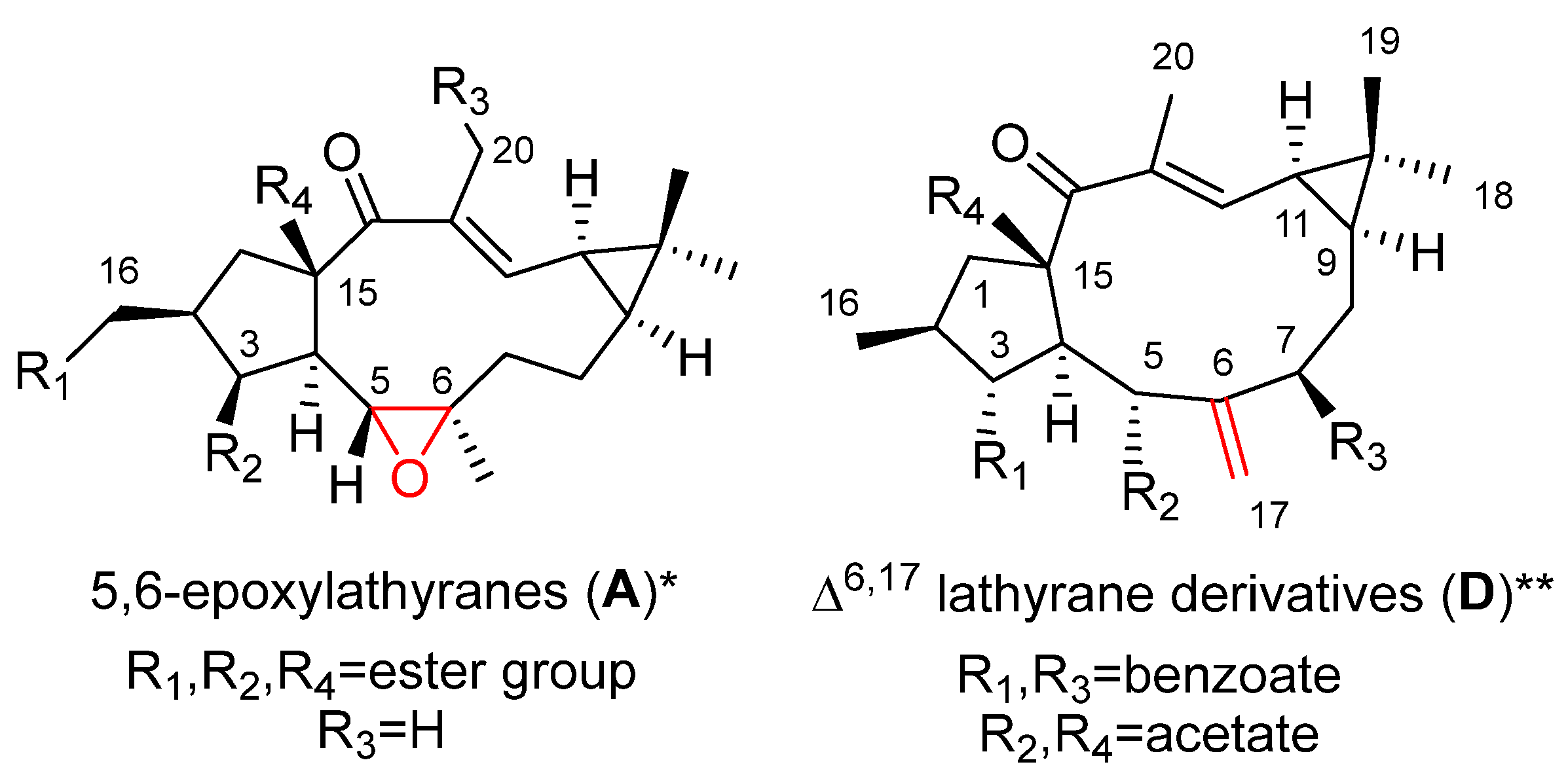
2. Bioactive Lathyranes
3. Biological Activities
3.1. Modulation of Multidrug Resistance (MDR)
Mode of Action
3.2. Cytotoxic
Mode of Action
3.3. Anti-Inflammatory Activity
Mode of Action
3.4. Antiviral and HIV-1 Reactivation Activities
3.5. Neurogenesis Promoting
3.6. Others
3.6.1. Anticholestasis
3.6.2. Antibacterial
3.6.3. Vascular-Relaxing Activity
3.6.4. Gastrointestinal Toxicity
3.6.5. Osteoclastogenesis Inhibition
3.6.6. Inhibition of 11β-HDS1
3.6.7. Induction of Lysosomal Biosynthesis
3.6.8. PGE2 Inhibition
4. Drug Delivery
5. Conclusions
6. Forward-Looking Outlook and Recommendations
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shi, Q.W.; Su, X.H.; Kiyota, H. Chemical and Pharmacological Research of the Plants in Genus Euphorbia. Chem. Rev. 2008, 108, 4295–4327. [Google Scholar] [CrossRef] [PubMed]
- Durán-Peña, M.J.; Botubol Ares, J.M.; Collado, I.G.; Hernández-Galán, R. Biologically Active Diterpenes Containing a Gem-Dimethylcyclopropane Subunit: An Intriguing Source of PKC Modulators. Nat. Prod. Rep. 2014, 31, 940–952. [Google Scholar] [CrossRef] [PubMed]
- Vasas, A.; Hohmann, J. Euphorbia Diterpenes: Isolation, Structure, Biological Activity, and Synthesis (2008–2012). Chem. Rev. 2014, 114, 8579–8612. [Google Scholar] [CrossRef] [PubMed]
- Domínguez-García, S.; Geribaldi-Doldán, N.; Gómez-Oliva, R.; Ruiz, F.A.; Carrascal, L.; Bolívar, J.; Verástegui, C.; Garcia-Alloza, M.; Macías-Sánchez, A.J.; Hernández-Galán, R.; et al. A Novel PKC Activating Molecule Promotes Neuroblast Differentiation and Delivery of Newborn Neurons in Brain Injuries. Cell Death Dis. 2020, 11, 262. [Google Scholar] [CrossRef] [PubMed]
- Murillo-Carretero, M.; Geribaldi-Doldán, N.; Flores-Giubi, E.; García-Bernal, F.; Navarro-Quiroz, E.A.; Carrasco, M.; Macías-Sánchez, A.J.; Herrero-Foncubierta, P.; Delgado-Ariza, A.; Verástegui, C.; et al. ELAC (3,12-Di-O-Acetyl-8-O-Tigloilingol), a Plant-Derived Lathyrane Diterpene, Induces Subventricular Zone Neural Progenitor Cell Proliferation through PKCβ Activation. Br. J. Pharmacol. 2017, 174, 2373–2392. [Google Scholar] [CrossRef] [PubMed]
- Evans, B.E.; Rittle, K.E.; Bock, M.G.; DiPardo, R.M.; Freidinger, R.M.; Whitter, W.L.; Lundell, G.F.; Veber, D.F.; Anderson, P.S.; Chang, R.S.L.; et al. Methods for Drug Discovery: Development of Potent, Selective, Orally Effective Cholecystokinin Antagonists. J. Med. Chem. 1988, 31, 2235–2246. [Google Scholar] [CrossRef]
- Reis, M.; Ferreira, R.J.; Santos, M.M.M.; Dos Santos, D.J.V.A.; Molnár, J.; Ferreira, M.J.U. Enhancing Macrocyclic Diterpenes as Multidrug-Resistance Reversers: Structure-Activity Studies on Jolkinol D Derivatives. J. Med. Chem. 2013, 56, 748–760. [Google Scholar] [CrossRef]
- Reis, M.A.; Ahmed, O.B.; Spengler, G.; Molnár, J.; Lage, H.; Ferreira, M.J.U. Exploring Jolkinol D Derivatives to Overcome Multidrug Resistance in Cancer. J. Nat. Prod. 2017, 80, 1411–1420. [Google Scholar] [CrossRef]
- Duarte, N.; Járdánházy, A.; Molnár, J.; Hilgeroth, A.; Ferreira, M.J.U. Synergistic Interaction between P-Glycoprotein Modulators and Epirubicine on Resistant Cancer Cells. Bioorganic Med. Chem. 2008, 16, 9323–9330. [Google Scholar] [CrossRef]
- Duarte, N.; Varga, A.; Cherepnev, G.; Radics, R.; Molnár, J.; Ferreira, M.J.U. Apoptosis Induction and Modulation of P-Glycoprotein Mediated Multidrug Resistance by New Macrocyclic Lathyrane-Type Diterpenoids. Bioorganic Med. Chem. 2007, 15, 546–554. [Google Scholar] [CrossRef]
- Jian, B.; Zhang, H.; Han, C.; Liu, J. Anti-Cancer Activities of Diterpenoids Derived from Euphorbia Fischeriana Steud. Molecules 2018, 23, 387. [Google Scholar] [CrossRef] [PubMed]
- Jian, B.; Zhang, H.; Liu, J. Structural Diversity and Biological Activities of Diterpenoids Derived from Euphorbia Fischeriana Steud. Molecules 2018, 23, 935. [Google Scholar] [CrossRef] [PubMed]
- Vasas, A.; Rédei, D.; Csupor, D.; Molnár, J.; Hohmann, J. Diterpenes from European Euphorbia Species Serving as Prototypes for Natural-Product-Based Drug Discovery. Eur. J. Org. Chem. 2012, 1, 5115–5130. [Google Scholar] [CrossRef]
- Ferreira, M.J.U.; Duarte, N.; Reis, M.; Madureira, A.M.; Molnár, J. Euphorbia and Momordica Metabolites for Overcoming Multidrug Resistance. Phytochem. Rev. 2014, 13, 915–935. [Google Scholar] [CrossRef]
- Wang, W.; Wu, Y.; Li, C.; Yang, Y.; Li, X.; Li, H.; Chen, L. Synthesis of New Lathyrane Diterpenoid Derivatives from Euphorbia Lathyris and Evaluation of Their Anti-Inflammatory Activities. Chem. Biodivers. 2020, 17, 531. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.Y.; Wu, Y.L.; Zhang, P.; Chen, Z.Z.; Li, H.; Chen, L.X. Anti-Inflammatory Lathyrane Diterpenoids from Euphorbia Lathyris. J. Nat. Prod. 2019, 82, 756–764. [Google Scholar] [CrossRef]
- Zuo, Q.; Mu, H.-Y.; Gong, Q.; Ding, X.; Wang, W.; Zhang, H.-Y.; Zhao, W.-M. Diterpenoids from the Seeds of Euphorbia Lathyris and Their Effects on Microglial Nitric Oxide Production. Fitoterapia 2021, 150, 104834. [Google Scholar] [CrossRef]
- Lee, J.W.; Jin, Q.; Jang, H.; Kim, J.G.; Lee, D.; Kim, Y.; Hong, J.T.; Lee, M.K.; Hwang, B.Y. Lathyrane-Type Diterpenoids from the Seeds of Euphorbia lathyris L. with Inhibitory Effects on NO Production in RAW 264.7 Cells. Chem. Biodivers. 2018, 15, 144. [Google Scholar] [CrossRef]
- Wang, Y.; Song, Z.; Guo, Y.; Xie, H.; Zhang, Z.; Sun, D.; Li, H.; Chen, L. Diterpenoids from the Seeds of Euphorbia Lathyris and Their Anti-Inflammatory Activity. Bioorganic Chem. 2021, 112, 104944. [Google Scholar] [CrossRef]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and Computational Approaches to Estimate Solubility and Permeability in Drug Discovery and Development Settings. Adv. Drug Deliv. Rev. 1997, 23, 3–25. [Google Scholar] [CrossRef]
- Vieira, C.; Duarte, N.; Reis, M.A.; Spengler, G.; Madureira, A.M.; Molnár, J.; Ferreira, M.J.U. Improving the MDR Reversal Activity of 6,17-Epoxylathyrane Diterpenes. Bioorganic Med. Chem. 2014, 22, 6392–6400. [Google Scholar] [CrossRef] [PubMed]
- Baptista, R.; Ferreira, R.J.; Dos Santos, D.J.; Fernandes, M.; Ferreira, M. Optimizing the Macrocyclic Diterpenic Core toward the Reversal of Multidrug Resistance in Cancer. Future Med. Chem. 2016, 8, 629–645. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, R.J.; Dos Santos, D.J.V.A.; Ferreira, M.J.U.; Guedes, R.C. Toward a Better Pharmacophore Description of P-Glycoprotein Modulators, Based on Macrocyclic Diterpenes from Euphorbia Species. J. Chem. Inf. Model. 2011, 51, 1315–1324. [Google Scholar] [CrossRef]
- Matos, A.M.; Reis, M.; Duarte, N.; Spengler, G.; Molnár, J.; Ferreira, M.J.U. Epoxylathyrol Derivatives: Modulation of ABCB1-Mediated Multidrug Resistance in Human Colon Adenocarcinoma and Mouse T-Lymphoma Cells. J. Nat. Prod. 2015, 78, 2215–2228. [Google Scholar] [CrossRef] [PubMed]
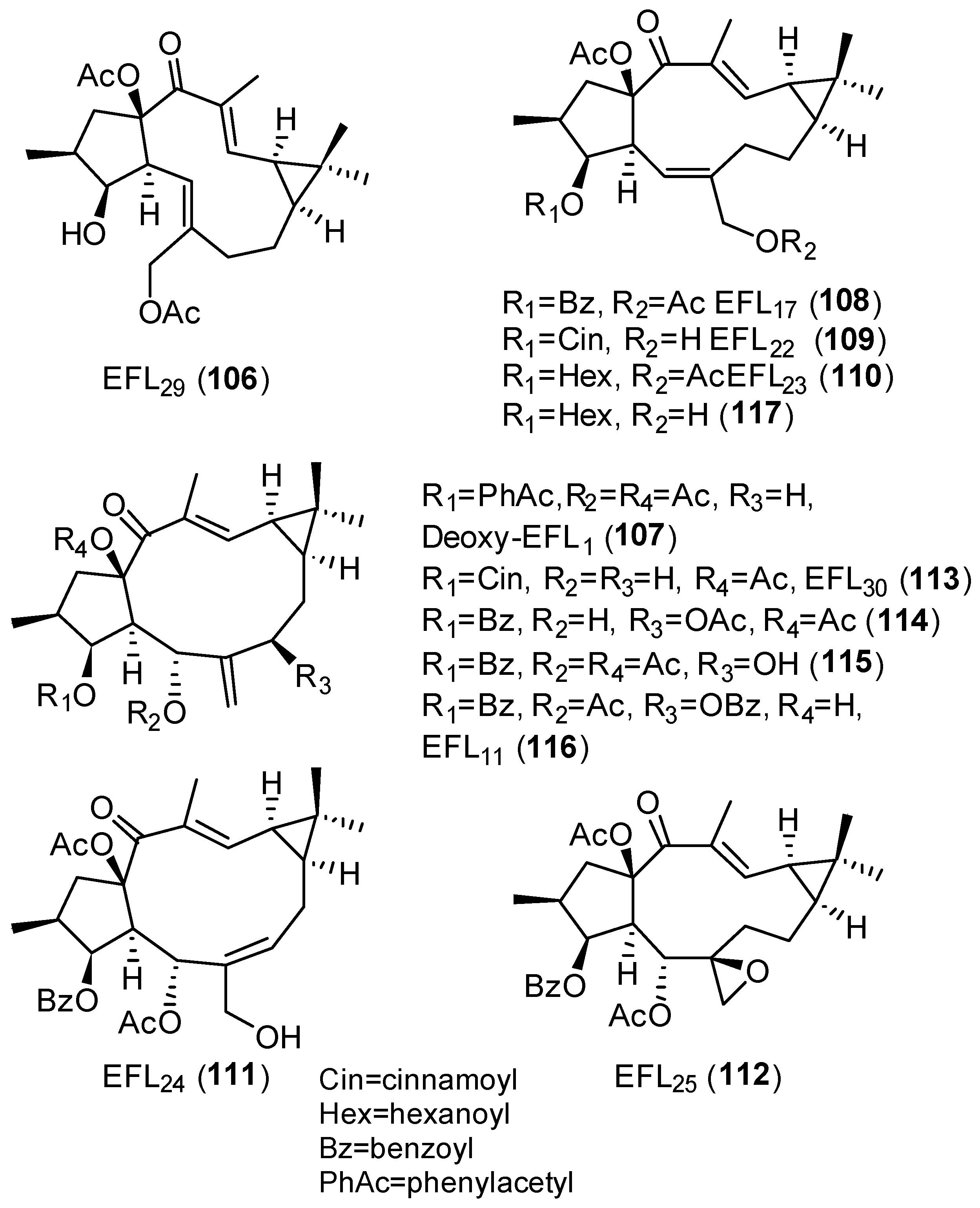
- Jiao, W.; Wan, Z.; Chen, S.; Lu, R.; Chen, X.; Fang, D.; Wang, J.; Pu, S.; Huang, X.; Gao, H.; et al. Lathyrol Diterpenes as Modulators of P-Glycoprotein Dependent Multidrug Resistance: Structure-Activity Relationship Studies on Euphorbia Factor L3 Derivatives. J. Med. Chem. 2015, 58, 3720–3738. [Google Scholar] [CrossRef]
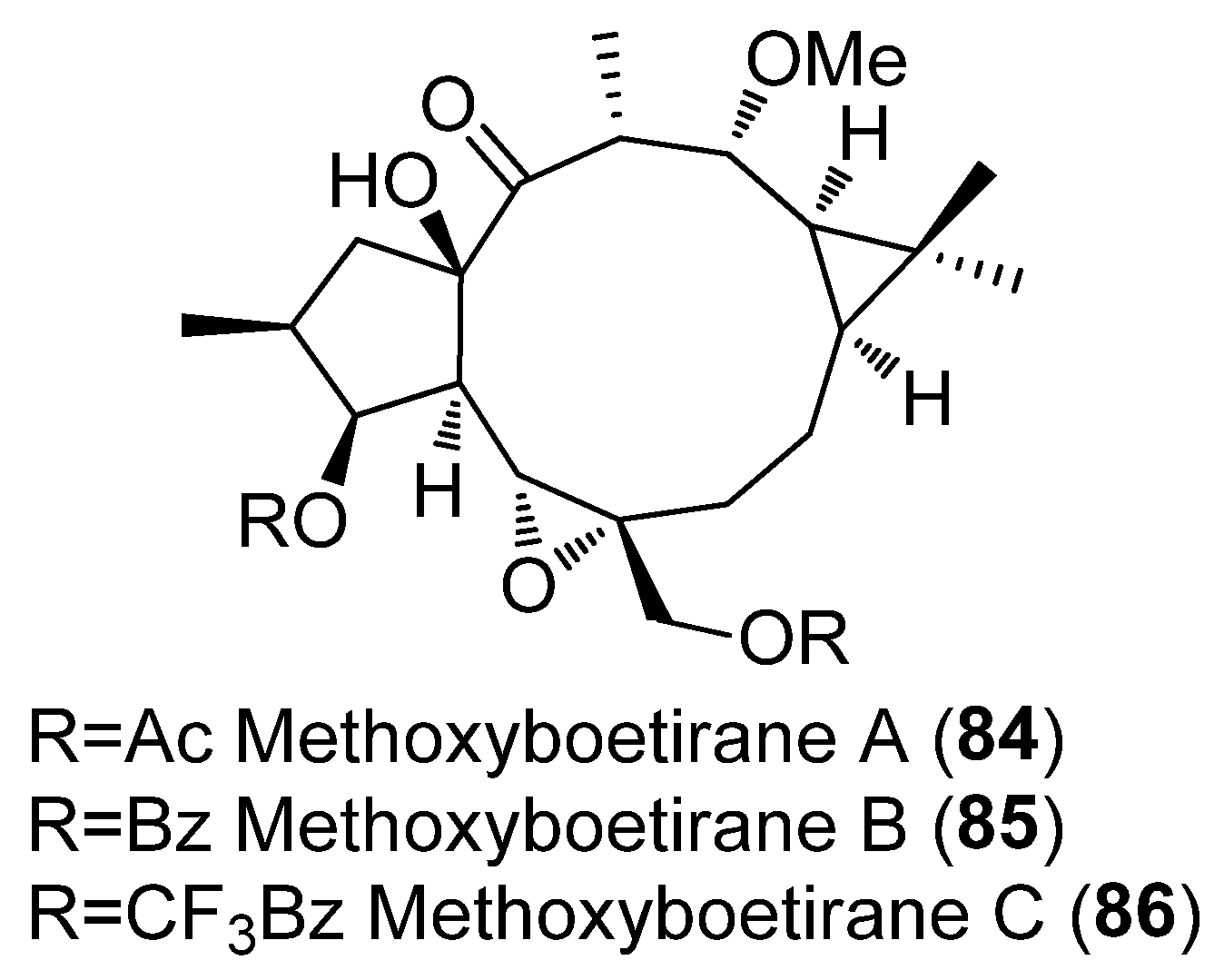
- Neto, S.; Duarte, N.; Pedro, C.; Spengler, G.; Molnár, J.; Ferreira, M.J.U. Effective MDR Reversers through Phytochemical Study of Euphorbia Boetica. Phytochem. Anal. 2019, 30, 498–511. [Google Scholar] [CrossRef]
- Mónico, A.; Nim, S.; Duarte, N.; Rawal, M.K.; Prasad, R.; Di Pietro, A.; Ferreira, M.J.U. Lathyrol and Epoxylathyrol Derivatives: Modulation of Cdr1p and Mdr1p Drug-Efflux Transporters of Candida Albicans in Saccharomyces Cerevisiae Model. Bioorganic Med. Chem. 2017, 25, 3278–3284. [Google Scholar] [CrossRef]
- Jiao, W.; Dong, W.; Li, Z.; Deng, M.; Lu, R. Lathyrane Diterpenes from Euphorbia Lathyris as Modulators of Multidrug Resistance and Their Crystal Structures. Bioorganic Med. Chem. 2009, 17, 4786–4792. [Google Scholar] [CrossRef]
- Yang, T.; Wang, S.; Li, H.; Zhao, Q.; Yan, S.; Dong, M.; Liu, D.; Chen, X.; Li, R. Lathyrane Diterpenes from Euphorbia Lathyris and the Potential Mechanism to Reverse the Multi-Drug Resistance in HepG2/ADR Cells. Biomed. Pharmacother. 2020, 121, 109663. [Google Scholar] [CrossRef]
- Gottesman, M.M.; Lavi, O.; Hall, M.D.; Gillet, J.-P. Toward a Better Understanding of the Complexity of Cancer Drug Resistance. Annu. Rev. Pharmacol. Toxicol. 2016, 56, 85–102. [Google Scholar] [CrossRef]
- Sarkadi, B.; Homolya, L.; Szakács, G.; Váradi, A. Human Multidrug Resistance ABCB and ABCG Transporters: Participation in a Chemoimmunity Defense System. Physiol. Rev. 2006, 86, 1179–1236. [Google Scholar] [CrossRef]
- Ozben, T. Mechanisms and Strategies to Overcome Multiple Drug Resistance in Cancer. FEBS Lett. 2006, 580, 2903–2909. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Cai, P.; Guo, S.; Shi, J.; Sun, H. Identification of a Lathyrane-Type Diterpenoid EM-E-11-4 as a Novel Paclitaxel Resistance Reversing Agent with Multiple Mechanisms of Action. Aging 2020, 12, 3713–3729. [Google Scholar] [CrossRef]
- Sève, P.; Dumontet, C. Is Class III β-Tubulin a Predictive Factor in Patients Receiving Tubulin-Binding Agents? Lancet Oncol. 2008, 9, 168–175. [Google Scholar] [CrossRef]
- Moktan, S.; Ryppa, C.; Kratz, F.; Raucher, D. A Thermally Responsive Biopolymer Conjugated to an Acid-Sensitive Derivative of Paclitaxel Stabilizes Microtubules, Arrests Cell Cycle, and Induces Apoptosis. Invest. New Drugs 2012, 30, 236–248. [Google Scholar] [CrossRef] [PubMed]
- Gan, P.P.; Pasquier, E.; Kavallaris, M. Class III β-Tubulin Mediates Sensitivity to Chemotherapeutic Drugs in Non–Small Cell Lung Cancer. Cancer Res. 2007, 67, 9356–9363. [Google Scholar] [CrossRef] [PubMed]
- Stepien, A.; Grzanka, A.; Grzanka, D.; Andrzej Szczepanski, M.; Helmin-Basa, A.; Gackowska, L. Taxol-Induced Polyploidy and Cell Death in CHO AA8 Cells. Acta Histochem. 2010, 112, 62–71. [Google Scholar] [CrossRef]
- Reis, M.A.; Matos, A.M.; Duarte, N.; Ahmed, O.B.; Ferreira, R.J.; Lage, H.; Ferreira, M.J.U. Epoxylathyrane Derivatives as MDR-Selective Compounds for Disabling Multidrug Resistance in Cancer. Front. Pharmacol. 2020, 11, 599. [Google Scholar] [CrossRef]
- Lage, H.; Duarte, N.; Coburger, C.; Hilgeroth, A.; Ferreira, M.J.U. Antitumor Activity of Terpenoids against Classical and Atypical Multidrug Resistant Cancer Cells. Phytomedicine 2010, 17, 441–448. [Google Scholar] [CrossRef]
- Pusztai, R.; Ferreira, M.J.U.; Duarte, N.; Engi, H.; Molnar, J. Macrocyclic Lathyrane Diterpenes as Antitumor Promoters. Anticancer Res. 2007, 27, 201–206. [Google Scholar]
- Li, J.; He, J.; Yang, C.; Yan, X.; Yin, Z. Cytotoxic Lathyrane Diterpenoids from the Roots of Euphorbia Fischeriana. Rec. Nat. Prod. 2020, 14, 286–291. [Google Scholar] [CrossRef]
- Kuang, X.; Li, W.; Kanno, Y.; Yamashita, N.; Kikkawa, S.; Azumaya, I.; Nemoto, K.; Asada, Y.; Koike, K. Euphorins A–H: Bioactive Diterpenoids from Euphorbia Fischeriana. J. Nat. Med. 2016, 70, 412–422. [Google Scholar] [CrossRef]
- Yuan, W.J.; Yang, G.P.; Zhang, J.H.; Zhang, Y.; Chen, D.Z.; Li, S.L.; Di, Y.T.; Hao, X.J. Three New Diterpenes with Cytotoxic Activity from the Roots of Euphorbia Ebracteolata Hayata. Phytochem. Lett. 2016, 18, 176–179. [Google Scholar] [CrossRef]
- Teng, Y.N.; Wang, Y.; Hsu, P.L.; Xin, G.; Zhang, Y.; Morris-Natschke, S.L.; Goto, M.; Lee, K.H. Mechanism of Action of Cytotoxic Compounds from the Seeds of Euphorbia Lathyris. Phytomedicine 2018, 41, 62–66. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.X.; Wang, Q.; Zhen, Y.Q.; Zhao, S.M.; Gao, F.; Zhou, X.L. Cytotoxic Lathyrane-Type Diterpenes from Seeds of Euphorbia Lathyris. Chem. Pharm. Bull. 2018, 66, 674–677. [Google Scholar] [CrossRef]
- Yuan, H.T.; Li, Q.F.; Tian, T.; Zhang, C.Y.; Huang, Z.Q.; Fan, C.X.; Mei, K.; Zhou, J.; Zhai, X.X.; Li, S.B.; et al. Lathyrane Diterpenoids from Jatropha Podagrica and Their Antitumor Activities in Human Osteosarcoma Cells. Nat. Prod. Res. 2020, 35, 5089–5095. [Google Scholar] [CrossRef]
- Ye, Y.; Liu, G.H.; Dawa, D.; Ding, L.S.; Cao, Z.X.; Zhou, Y. Cytotoxic Diterpenoids from the Roots of Euphorbia Stracheyi. Phytochem. Lett. 2020, 36, 183–187. [Google Scholar] [CrossRef]
- Ravikanth, V.; Lakshmi Niranjan Reddy, V.; Vijender Reddy, A.; Ravinder, K.; Prabhakar Rao, T.; Siva Ram, T.; Anand Kumar, K.; Prakesh Vamanarao, D.; Venkateswarlu, Y. Three New Ingol Diterpenes from Euphorbia Nivulia: Evaluation of Cytotoxic Activity. Chem. Pharm. Bull. 2003, 51, 431–434. [Google Scholar] [CrossRef]
- Kröger, N.; Achterrath, W.; Hegewisch-Becker, S.; Mross, K.; Zander, A.R. Current Options in Treatment of Anthracycline-Resistant Breast Cancer. Cancer Treat. Rev. 1999, 25, 279–291. [Google Scholar] [CrossRef]
- Penson, R.T.; Oliva, E.; Skates, S.J.; Glyptis, T.; Fuller, A.F.; Goodman, A.; Seiden, M.V. Expression of Multidrug Resistance-1 Protein Inversely Correlates with Paclitaxel Response and Survival in Ovarian Cancer Patients: A Study in Serial Samples. Gynecol. Oncol. 2004, 93, 98–106. [Google Scholar] [CrossRef]
- Pokharel, D.; Roseblade, A.; Oenarto, V.; Lu, J.; Bebawy, M. Proteins Regulating the Intercellular Transfer and Function of P-Glycoprotein in Multidrug-Resistant Cancer. Ecancermedicalscience 2017, 11, 768. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.Y.; Lin, M.T.; Yi, T.; Tang, Y.N.; Fan, L.L.; He, X.C.; Zhao, Z.Z.; Chen, H.B. Apoptosis Sensitization by Euphorbia Factor L1 in ABCB1-Mediated Multidrug Resistant K562/ADR Cells. Molecules 2013, 18, 12793–12808. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.Y.; Mi, Y.J.; Chen, S.P.; Wang, F.; Liang, Y.J.; Zheng, L.S.; Shi, C.J.; Tao, L.Y.; Chen, L.M.; Chen, H.B.; et al. Euphorbia Factor L1 Reverses ABCB1-Mediated Multidrug Resistance Involving Interaction with ABCB1 Independent of ABCB1 Downregualtion. J. Cell. Biochem. 2011, 112, 1076–1083. [Google Scholar] [CrossRef]
- Duarte, N.; Gyémánt, N.; Abreu, P.M.; Molnár, J.; Ferreira, M.J.U. New Macrocyclic Lathyrane Diterpenes, from Euphorbia Lagascae, as Inhibitors of Multidrug Resistance of Tumour Cells. Planta Med. 2006, 72, 162–168. [Google Scholar] [CrossRef]
- Zhang, J.Y.; Liang, Y.J.; Chen, H.B.; Zheng, L.S.; Mi, Y.J.; Wang, F.; Zhao, X.Q.; Wang, X.K.; Zhang, H.; Fu, L.W. Structure Identification of Euphorbia Factor L3 and Its Induction of Apoptosis through the Mitochondrial Pathway. Molecules 2011, 16, 3222–3231. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhu, S.; Cheng, X.; Lu, C.; Tao, W.; Zhang, Y.; William, B.C.; Cao, X.; Yi, S.; Liu, Y.; et al. Euphorbia Factor L2 Alleviates Lipopolysaccharide-Induced Acute Lung Injury and Inflammation in Mice through the Suppression of NF-ΚB Activation. Biochem. Pharmacol. 2018, 155, 444–454. [Google Scholar] [CrossRef] [PubMed]
- Lu, F.; Huayun, Z.; Weiwei, T.; Li, L.; Xin, S.; Ming, Z.; Dongdong, S. Euphorbia Factor L2 Inhibits TGF-β-Induced Cell Growth and Migration of Hepatocellular Carcinoma through AKT/STAT3. Phytomedicine 2019, 62, 152931. [Google Scholar] [CrossRef]
- Chang, S.; He, H.Q.; Kong, R.; Xie, Z.J.; Hu, J.P. Study on Molecular Recognition between Euphorbia Factor L713283 and β -Tubulin via Molecular Simulation Methods. J. Chem. 2015, 2015, 9238. [Google Scholar] [CrossRef]
- Dal Piaz, F.; Vera Saltos, M.B.; Franceschelli, S.; Forte, G.; Marzocco, S.; Tuccinardi, T.; Poli, G.; Nejad Ebrahimi, S.; Hamburger, M.; De Tommasi, N.; et al. Drug Affinity Responsive Target Stability (DARTS) Identifies Laurifolioside as a New Clathrin Heavy Chain Modulator. J. Nat. Prod. 2016, 79, 2681–2692. [Google Scholar] [CrossRef]
- Medzhitov, R. Origin and Physiological Roles of Inflammation. Nature 2008, 454, 428–435. [Google Scholar] [CrossRef]
- Atsumi, T.; Singh, R.; Sabharwal, L.; Bando, H.; Meng, J.; Arima, Y.; Yamada, M.; Harada, M.; Jiang, J.J.; Kamimura, D.; et al. Inflammation Amplifier, a New Paradigm in Cancer Biology. Cancer Res. 2014, 74, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Baummann, H.; Gauldie, J. The Acute Phase Response. Inmunol. Today 1994, 75, 74–80. [Google Scholar] [CrossRef]
- Edwards, A.D. The Pharmacology of Inhaled Nitric Oxide. Arch. Dis. Child. 1995, 72, F127–F130. [Google Scholar] [CrossRef] [PubMed]
- Mario Scuri, M.; Samsell, L.; Piedimonte, G. The Role of Neurotrophins in Inflammation and Allergy. Inflamm. Allergy-Drug Targets 2010, 9, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Xu, D.; Liu, P.; Liu, F.; Dai, L.; Yan, H.; Wen, F. Effects of Calcium Gluconate on Lipopolysaccharide-Induced Acute Lung Injury in Mice. Biochem. Biophys. Res. Commun. 2018, 503, 2931–2935. [Google Scholar] [CrossRef]
- Lee, J.W.; Lee, C.; Jin, Q.; Jang, H.; Lee, D.; Lee, H.-J.; Shin, J.W.; Han, S.B.; Hong, J.T.; Kim, Y.; et al. Diterpenoids from the Roots of Euphorbia Fischeriana with Inhibitory Effects on Nitric Oxide Production. J. Nat. Prod. 2016, 79, 126–131. [Google Scholar] [CrossRef]
- Kim, Y.M.; Ahn, J.; Chae, H.S.; Choi, Y.H.; Kim, J.; Chin, Y.W. Two New Lathyrane-Type Diterpenoid Glycosides with IL-6 Production Inhibitory Activity from the Roots of Euphorbia Kansui. Bioorganic Med. Chem. Lett. 2018, 28, 1207–1210. [Google Scholar] [CrossRef]
- An, L.; Liang, Y.; Yang, X.; Wang, H.; Zhang, J.; Tuerhong, M.; Li, D.; Wang, C.; Lee, D.; Xu, J.; et al. NO Inhibitory Diterpenoids as Potential Anti-Inflammatory Agents from Euphorbia Antiquorum. Bioorganic Chem. 2019, 92, 103237. [Google Scholar] [CrossRef]
- Huang, J.-D.; Zhang, C.; Xu, W.-J.; Lian, C.-L.; Liu, X.-M.; Wang, C.-F.; Liu, J.-Q. New Lathyrane Diterpenoids with Anti-Inflammatory Activity Isolated from the Roots of Jatropha curcas L. J. Ethnopharmacol. 2021, 268, 113673. [Google Scholar] [CrossRef]
- Daoubi, M.; Marquez, N.; Mazoir, N.; Benharref, A.; Hernández-Galán, R.; Muñoz, E.; Collado, I.G. Isolation of New Phenylacetylingol Derivatives That Reactivate HIV-1 Latency and a Novel Spirotriterpenoid from Euphorbia Officinarum Latex. Bioorganic Med. Chem. 2007, 15, 4577–4584. [Google Scholar] [CrossRef]
- Avila, L.; Perez, M.; Sanchez-Duffhues, G.; Hernández-Galán, R.; Muñoz, E.; Cabezas, F.; Quiñones, W.; Torres, F.; Echeverri, F. Effects of Diterpenes from Latex of Euphorbia Lactea and Euphorbia Laurifolia on Human Immunodeficiency Virus Type 1 Reactivation. Phytochemistry 2010, 71, 243–248. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Xu, W.; Zhu, C.; Lin, S.; Li, Y.; Xiong, L.; Wang, S.; Wang, L.; Yang, Y.; Guo, Y.; et al. Lathyrane Diterpenoids from the Roots of Euphorbia Micractina and Their Biological Activities. J. Nat. Prod. 2011, 74, 1221–1229. [Google Scholar] [CrossRef]
- Yan, S.L.; Li, Y.H.; Chen, X.Q.; Liu, D.; Chen, C.H.; Li, R.T. Diterpenes from the Stem Bark of Euphorbia Neriifolia and Their in Vitro Anti-HIV Activity. Phytochemistry 2018, 145, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Li, H.; Liu, D.; Zhao, Q.; Yang, T.; Li, R.; Chen, X. Diterpenoids from the Seeds of Euphorbia Lathyris and Their in Vitro Anti-HIV Activity. Chem. Nat. Compd. 2020, 56, 78–85. [Google Scholar] [CrossRef]
- Li, S.F.; Jiao, Y.Y.; Zhang, Z.Q.; Chao, J.B.; Jia, J.; Shi, X.L.; Zhang, L.W. Diterpenes from Buds of Wikstroemia Chamaedaphne Showing Anti-Hepatitis B Virus Activities. Phytochemistry 2018, 151, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.-S.; Luo, S.-H.; Li, Y.-L.; Li, C.-H.; Hua, J.; Liu, Y.; Jing, S.-X.; Wang, Y.; Yang, M.-J.; Li, S.-H. Antifeedant and Antiviral Diterpenoids from the Fresh Roots of Euphorbia Jolkinii. Nat. Prod. Bioprospect. 2014, 4, 91–100. [Google Scholar] [CrossRef][Green Version]
- Remy, S.; Olivon, F.; Desrat, S.; Blanchard, F.; Eparvier, V.; Leyssen, P.; Neyts, J.; Roussi, F.; Touboul, D.; Litaudon, M. Structurally Diverse Diterpenoids from Sandwithia Guyanensis. J. Nat. Prod. 2018, 81, 901–912. [Google Scholar] [CrossRef]
- Flores-Giubi, E.; Geribaldi-Doldán, N.; Murillo-Carretero, M.; Castro, C.; Durán-Patrón, R.; MacÍas-Sánchez, A.J.; Hernández-Galán, R. Lathyrane, Premyrsinane, and Related Diterpenes from Euphorbia Boetica: Effect on in Vitro Neural Progenitor Cell Proliferation. J. Nat. Prod. 2019, 82, 2517–2528. [Google Scholar] [CrossRef]
- Huang, D.; Wang, R.-M.; Li, W.; Zhao, Y.-Y.; Yuan, F.-Y.; Yan, X.-L.; Chen, Y.; Tang, G.-H.; Bi, H.-C.; Yin, S. Lathyrane Diterpenoids as Novel HPXR Agonists: Isolation, Structural Modification, and Structure–Activity Relationships. ACS Med. Chem. Lett. 2021, 12, 1159–1165. [Google Scholar] [CrossRef]
- Aiyelaagbe, O.O.; Adesogan, K.; Ekundayo, O.; Gloer, J.B. Antibacterial Diterpenoids from Jatropha Podagrica Hook. Phytochemistry 2007, 68, 2420–2425. [Google Scholar] [CrossRef]
- Chopra, R.N.; Nayar, S.L.; Chopra, I.C. Glossary of Indian Medicinal Plants; CSIR: New Delhi, India, 1956. [Google Scholar]
- Zhu, A.; Zhang, T.; Wang, Q. The Phytochemistry, Pharmacokinetics, Pharmacology and Toxicity of Euphorbia Semen. J. Ethnopharmacol. 2018, 227, 41–55. [Google Scholar] [CrossRef] [PubMed]
- Zhu, A.; Ji, Z.; Zhao, J.; Zhang, W.; Sun, Y.; Zhang, T.; Gao, S.; Li, G.; Wang, Q. Effect of Euphorbia Factor L1 on Intestinal Barrier Impairment and Defecation Dysfunction in Caenorhabditis Elegans. Phytomedicine 2019, 65, 3102. [Google Scholar] [CrossRef] [PubMed]
- Zhu, A.; Sun, Y.; Zhong, Q.; Yang, J.; Zhang, T.; Zhao, J.; Wang, Q. Effect of Euphorbia Factor L1 on Oxidative Stress, Apoptosis, and Autophagy in Human Gastric Epithelial Cells. Phytomedicine 2019, 64, 152929. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.E.; Lee, J.; Seo, D.H.; In Lee, H.; Ri Park, D.; Lee, G.R.; Jo, Y.J.; Kim, N.; Kwon, M.; Shon, H.; et al. Euphorbia Factor L1 Inhibits Osteoclastogenesis by Regulating Cellular Redox Status and Induces Fas-Mediated Apoptosis in Osteoclast. Free Radic. Biol. Med. 2017, 112, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Han, M.L.; Shen, Y.; Wang, G.C.; Leng, Y.; Zhang, H.; Yue, J.M. 11β-HSD1 Inhibitors from Walsura Cochinchinensis. J. Nat. Prod. 2013, 76, 1319–1327. [Google Scholar] [CrossRef] [PubMed]
- Qi, W.-Y.; Zhang, W.-Y.; Shen, Y.; Leng, Y.; Gao, K.; Yue, J.-M. Ingol-Type Diterpenes from Euphorbia Antiquorum with Mouse 11β-Hydroxysteroid Dehydrogenase Type 1 Inhibition Activity. J. Nat. Prod. 2014, 77, 1452–1458. [Google Scholar] [CrossRef]
- Zhao, N.D.; Ding, X.; Song, Y.; Yang, D.Q.; Yu, H.L.; Adelakun, T.A.; Qian, W.D.; Zhang, Y.; Di, Y.T.; Gao, F.; et al. Identification of Ingol and Rhamnofolane Diterpenoids from Euphorbia Resinifera and Their Abilities to Induce Lysosomal Biosynthesis. J. Nat. Prod. 2018, 81, 1209–1218. [Google Scholar] [CrossRef]
- Ravikanth, V.; Niranjan Reddy, V.L.; Prabhakar Rao, T.; Diwan, P.V.; Ramakrishna, S.; Venkateswarlu, Y. Macrocyclic Diterpenes from Euphorbia Nivulia. Phytochemistry 2002, 59, 331–335. [Google Scholar] [CrossRef]
- Maxmen, A. Busting the Billion-Dollar Myth: How to Slash the Cost of Drug Development. Nature 2016, 536, 389–390. [Google Scholar] [CrossRef]
- Hunter, A.C.; Elsom, J.; Wibroe, P.P.; Moghimi, S.M. Polymeric Particulate Technologies for Oral Drug Delivery and Targeting: A Pathophysiological Perspective. Maturitas 2012, 73, 5–18. [Google Scholar] [CrossRef]
- Vermunt, M.; Marchetti, S.; Beijnen, J. Pharmacokinetics and Toxicities of Oral Docetaxel Formulations Co-Administered with Ritonavir in Phase I Trials. Clin. Pharmacol. Adv. Appl. 2021, 13, 21–32. [Google Scholar] [CrossRef] [PubMed]
- Szebeni, J.; Alving, C.R.; Savay, S.; Barenholz, Y.; Priev, A.; Danino, D.; Talmon, Y. Formation of Complement-Activating Particles in Aqueous Solutions of Taxol: Possible Role in Hypersensitivity Reactions. Int. Immunopharmacol. 2001, 1, 721–735. [Google Scholar] [CrossRef]
- Hunter, A.C.; Moghimi, S.M. Therapeutic Synthetic Polymers: A Game of Russian Roulette? Drug Discov. Today 2002, 7, 998–1001. [Google Scholar] [CrossRef]
- Kessel, D.; Woodburn, K.; Decker, D.; Sykes, E. Fractionation of Cremophor EL Delineates Components Responsible for Plasma Lipoprotein Alterations and Multidrug Resistance Reversal. Oncol. Res. 1995, 7, 207–212. [Google Scholar] [PubMed]
- Woodburn, K.; Sykes, E.; Kessel, D. Interactions of Solutol HS 15 and Cremophore EL with Plasma Lipoproteins. Int. J. Biochem. Cell Biol. 1995, 27, 693–699. [Google Scholar] [CrossRef]
- Moghimi, S.M.; Andersen, A.J.; Ahmadvand, D.; Wibroe, P.P.; Andresen, T.L.; Hunter, A.C. Material Properties in Complement Activation. Adv. Drug Deliv. Rev. 2011, 63, 1000–1007. [Google Scholar] [CrossRef]
- Gabizon, A.; Shmeeda, H.; Tahover, E.; Kornev, G.; Patil, Y.; Amitay, Y.; Ohana, P.; Sapir, E.; Zalipsky, S. Development of Promitil®, a Lipidic Prodrug of Mitomycin c in PEGylated Liposomes: From Bench to Bedside. Adv. Drug Deliv. Rev. 2020, 154–155, 13–26. [Google Scholar] [CrossRef]
- Yu Helvig, S.; Woythe, L.; Pham, S.; Bor, G.; Andersen, H.; Moein Moghimi, S.; Yaghmur, A. A Structurally Diverse Library of Glycerol Monooleate/Oleic Acid Non-Lamellar Liquid Crystalline Nanodispersions Stabilized with Nonionic Methoxypoly(Ethylene Glycol) (MPEG)-Lipids Showing Variable Complement Activation Properties. J. Colloid Interface Sci. 2021, 582, 906–917. [Google Scholar] [CrossRef]
- Wu, L.P.; Ahmadvand, D.; Su, J.; Hall, A.; Tan, X.; Farhangrazi, Z.S.; Moghimi, S.M. Crossing the Blood-Brain-Barrier with Nanoligand Drug Carriers Self-Assembled from a Phage Display Peptide. Nat. Commun. 2019, 10, 12554. [Google Scholar] [CrossRef]
- Pilati, D.; Howard, K.A. Albumin-Based Drug Designs for Pharmacokinetic Modulation. Expert Opin. Drug Metab. Toxicol. 2020, 16, 783–795. [Google Scholar] [CrossRef]
- Gifford, G.; Vu, V.P.; Banda, N.K.; Holers, V.M.; Wang, G.; Groman, E.V.; Backos, D.; Scheinman, R.; Moghimi, S.M.; Simberg, D. Complement Therapeutics Meets Nanomedicine: Overcoming Human Complement Activation and Leukocyte Uptake of Nanomedicines with Soluble Domains of CD55. J. Control. Release 2019, 302, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Abrahao, A.; Meng, Y.; Llinas, M.; Huang, Y.; Hamani, C.; Mainprize, T.; Aubert, I.; Heyn, C.; Black, S.E.; Hynynen, K.; et al. First-in-Human Trial of Blood–Brain Barrier Opening in Amyotrophic Lateral Sclerosis Using MR-Guided Focused Ultrasound. Nat. Commun. 2019, 10, 12426. [Google Scholar] [CrossRef]
- Boyé, K.; Geraldo, L.H.; Furtado, J.; Pibouin-Fragner, L.; Poulet, M.; Kim, D.; Nelson, B.; Xu, Y.; Jacob, L.; Maissa, N.; et al. Endothelial Unc5B Controls Blood-Brain Barrier Integrity. Nat. Commun. 2022, 13, 28785. [Google Scholar] [CrossRef] [PubMed]
- Crowe, T.P.; Greenlee, M.H.W.; Kanthasamy, A.G.; Hsu, W.H. Mechanism of Intranasal Drug Delivery Directly to the Brain. Life Sci. 2018, 195, 44–52. [Google Scholar] [CrossRef]
- Xu, J.; Tao, J.; Wang, J. Design and Application in Delivery System of Intranasal Antidepressants. Front. Bioeng. Biotechnol. 2020, 8, 6882. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Hu, J.; Wang, B.; Sheng, L.; Liu, Z.; Yang, S.; Li, Y. Inhibitory effects of herbal constituents on P-glycoprotein in vitro and in vivo: Herb–drug interactions mediated via P-gp. Toxicol. Appl. Pharmacol. 2014, 275, 163–175. [Google Scholar] [CrossRef]
- Wang, Q.; Zhen, Y.Q.; Gao, F.; Huang, S.; Zhou, X.L. Five New Diterpenoids from the Seeds of Euphorbia lathyris. Chem. Biodivers. 2018, 15, 9–16. [Google Scholar] [CrossRef]
- Lu, J.; Li, G.; Huang, J.; Zhang, C.; Zhang, L.; Zhang, K.; Li, P.; Lin, R.; Wang, J. Lathyrane-type diterpenoids from the seeds of Euphorbia lathyris. Phytochemistry 2014, 104, 79–88. [Google Scholar] [CrossRef]
- Sousa, I.J.; Ferreira, M.J.U.; Molnár, J.; Fernandes, M.X. QSAR studies of macrocyclic diterpenes with P-glycoprotein inhibitory activity. Eur. J. Pharm. Sci. 2013, 48, 542–553. [Google Scholar] [CrossRef]
- Engi, H.; Vasas, A.; Rédei, D.; Molnár, J.; Hohmann, J. New MDR modulators and apoptosis inducers from Euphorbia species. Anticancer Res. 2007, 27, 3451–3458. [Google Scholar] [PubMed]
- Lin, M.; Tang, S.; Zhang, C.; Chen, H.; Huang, W.; Liu, Y.; Zhang, J. Euphorbia factor L2 induces apoptosis in A549 cells through the mitochondrial pathway. Acta Pharm. Sin. B 2017, 7, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Huang, J.; Lyu, H.; Guan, F.; Li, P.; Tian, M.; Xu, S.; Zhao, X.; Liu, F.; Paetz, C.; et al. Two lathyrane diterpenoid stereoisomers containing an unusual: Trans-gem -dimethylcyclopropane from the seeds of Euphorbia lathyris. RSC Adv. 2021, 11, 3183–3189. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Jin, D.Q.; Song, H.; Guo, Y.; He, Y. Lathyrane diterpenes from Euphorbia prolifera and their inhibitory activities on LPS-induced NO production. Fitoterapia 2012, 83, 1205–1209. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.Y.; Zhang, C.; Chen, H.B.; Fu, L.W.; Tao, Y.W.; Zheng, X.Q.; Cao, Z.M.; Zhong, Y.F.; Yu, L.H. Assignments of 1H and 13C NMR signals of Euphorbia factor L1 and investigation of its anticancer activity in vitro. J. Med. Plants Res. 2010, 4, 335–338. [Google Scholar] [CrossRef]
- Nabatchian, F.; Moradi, A.; Aghaei, M.; Ghanadian, M.; Jafari, S.M.; Tabesh, S. New 6(17)-epoxylathyrane diterpene: Aellinane from Euphorbia aellenii induces apoptosis via mitochondrial pathway in ovarian cancer cell line. Toxicol. Mech. Methods 2017, 27, 622–630. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Chen, Q.B.; Liu, Y.Q.; Xin, X.L.; Yili, A.; Aisa, H.A. Diterpenoid Constituents of Euphorbia Macrorrhiza. Phytochemistry 2016, 122, 246–253. [Google Scholar] [CrossRef]
- Appendino, G.; Porta, C.D.; Conseil, G.; Sterner, O.; Mercalli, E.; Dumontet, C.; Di Pietro, A. A New P-Glycoprotein Inhibitor from the Caper Spurge (Euphorbia Lathyris). J. Nat. Prod. 2003, 66, 140–142. [Google Scholar] [CrossRef]
- Valente, C.; Pedro, M.; Ascenso, J.R.; Abreu, P.M.; Nascimento, M.S.J.; Ferreira, M.J.U. Euphopubescenol and Euphopubescene, Two New Jatrophane Polyesters, and Lathyrane-Type Diterpenes from Euphorbia Pubescens. Planta Med. 2004, 70, 244–249. [Google Scholar] [CrossRef]
- Ricciotti, E.; FitzGerald, G.A. Prostaglandins and Inflammation. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 986–1000. [Google Scholar] [CrossRef]
- Zhang, J.-S.; Zhang, Y.; Li, S.; Ahmed, A.; Tang, G.-H.; Yin, S. Cytotoxic Macrocyclic Diterpenoids from Jatropha Multifida. Bioorg. Chem. 2018, 80, 511–518. [Google Scholar] [CrossRef] [PubMed]
- Chianese, G.; Fattorusso, E.; Aiyelaagbe, O.O.; Luciano, P.; Schröder, H.C.; Müller, W.E.G.; Taglialatela-Scafati, O. Spirocurcasone, a Diterpenoid with a Novel Carbon Skeleton from Jatropha curcas. Org. Lett. 2011, 13, 316–319. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.Q.; Yang, Y.F.; Xia, J.J.; Li, X.Y.; Li, Z.R.; Zhou, L.; Qiu, M.H. Cytotoxic Diterpenoids from Jatropha curcas cv. Nigroviensrugosus CY Yang Roots. Phytochemistry 2015, 117, 462–468. [Google Scholar] [CrossRef] [PubMed]
- Yin, Z.-Y.; Dai, Y.; Hua, P.; Sun, Z.-J.; Cheng, Y.-F.; Yuan, S.-H.; Chen, Z.-Y.; Gu, Q. Discovery of diverse diterpenoid scaffolds from Euphorbia antiquorum and their activity against RANKL-induced osteoclastogenesis. Bioorg. Chem. 2019, 92, 103292. [Google Scholar] [CrossRef]
- Baloch, I.B.; Baloch, M.K.; Saqib, Q.N.U. Cytotoxic macrocyclic diterpenoid esters from Euphorbia cornigera. Planta Med. 2006, 72, 830–834. [Google Scholar] [CrossRef]
- Marco, J.A.; Sanz-Cervera, J.F.; Yuste, A. Ingenane and lathyrane diterpenes from the latex of Euphorbia canariensis. Phytochemistry 1997, 45, 563–570. [Google Scholar] [CrossRef]
- Miranda, F.J.; Alabadi, J.A.; Orti, M.; Centeno, J.M.; Pión, M.; Yuste, A.; Sanz-Cervera, J.F.; Marco, J.A.; Alborch, E. Comparative Analysis of the Vascular Actions of Diterpenes Isolated from Euphorbia canariensis. J. Pharm. Pharmacol. 1998, 50, 237–241. [Google Scholar] [CrossRef] [PubMed]










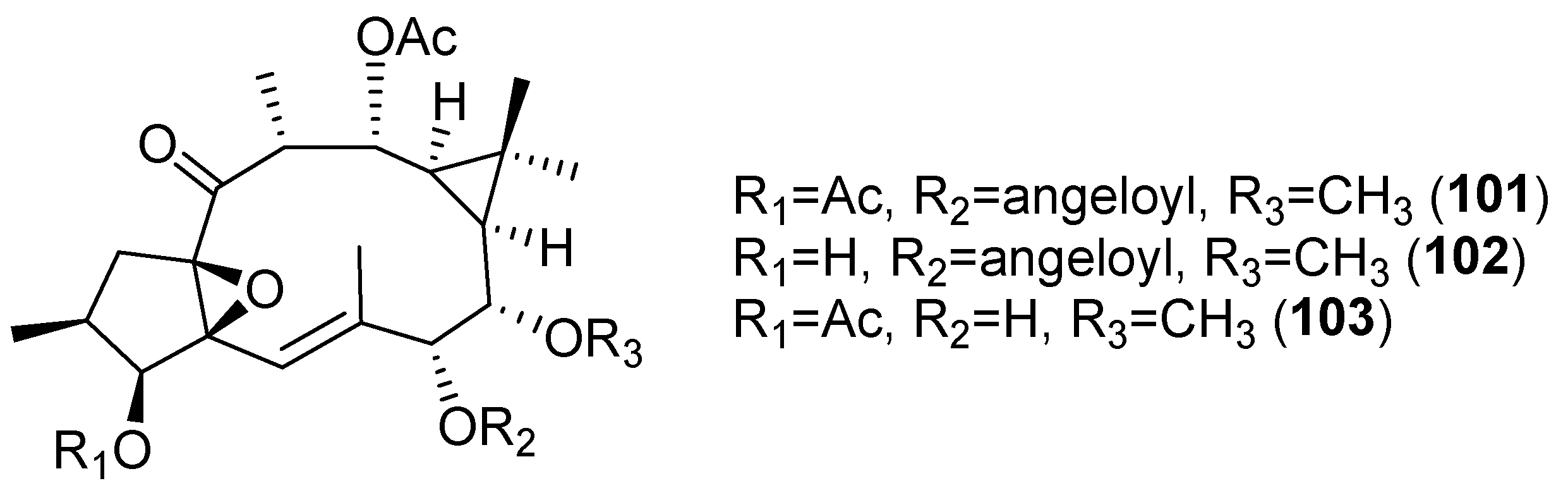



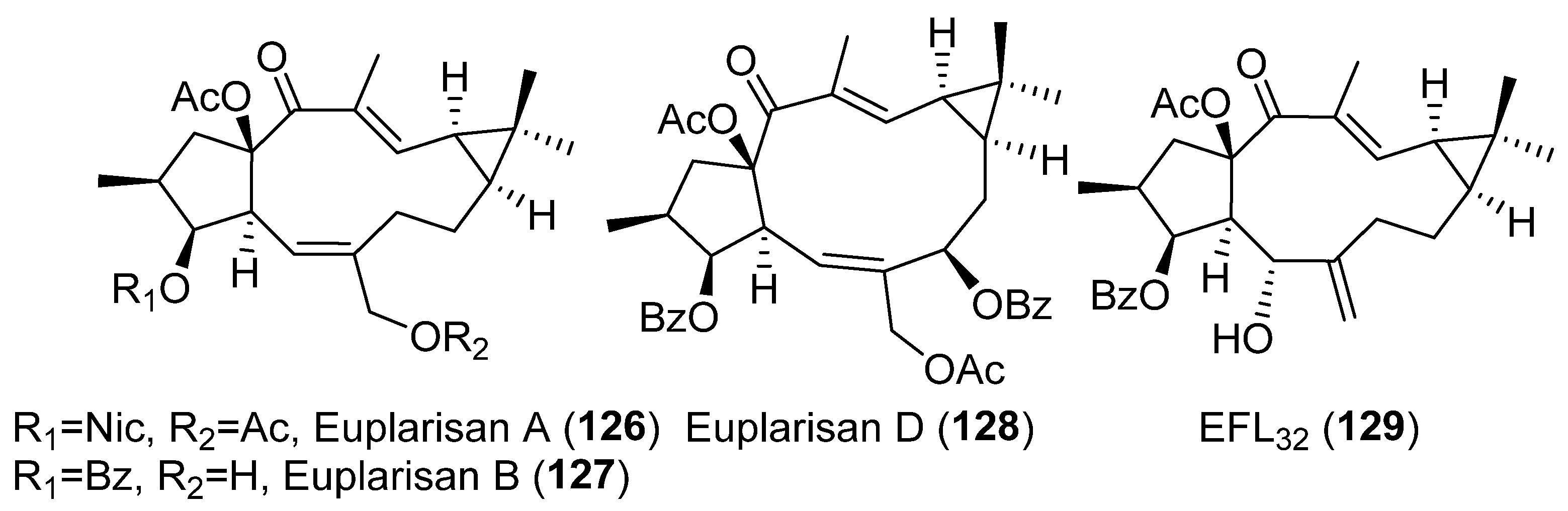
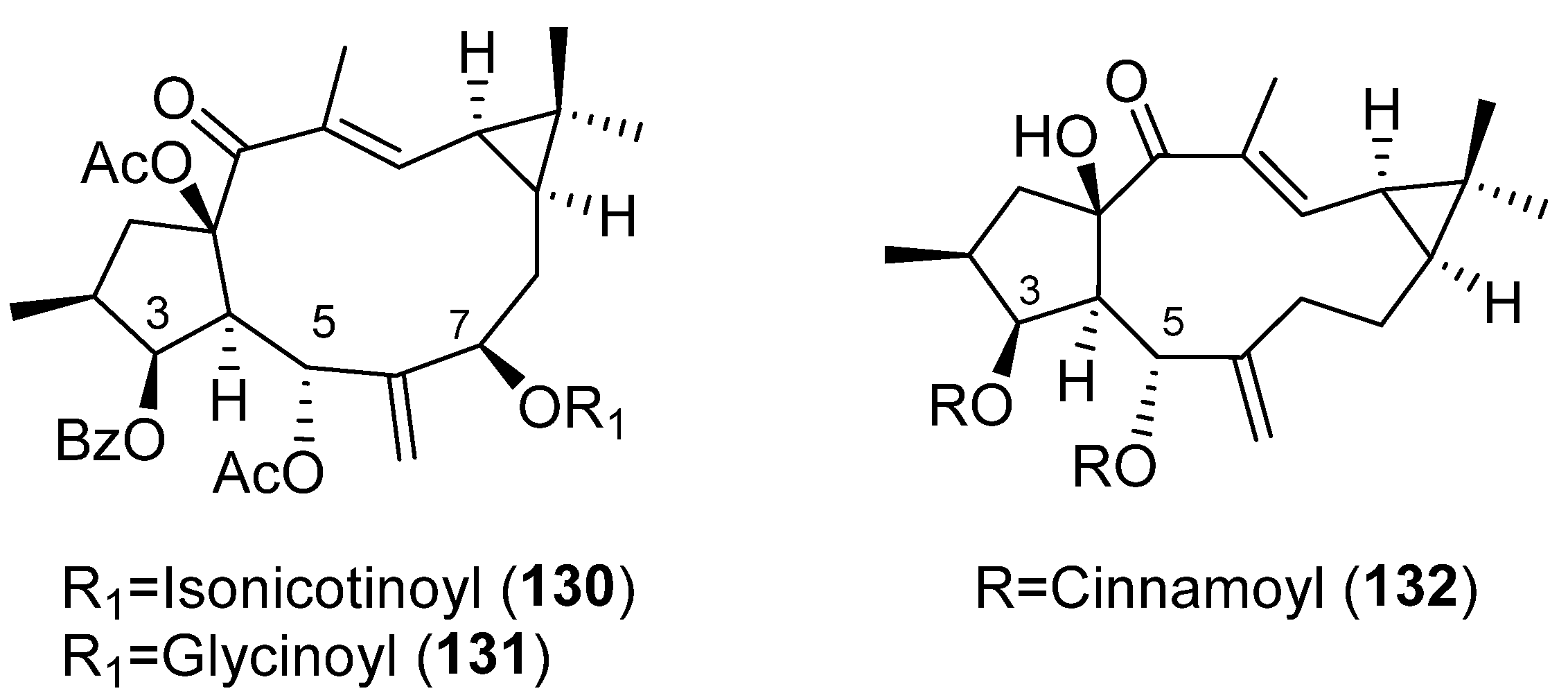
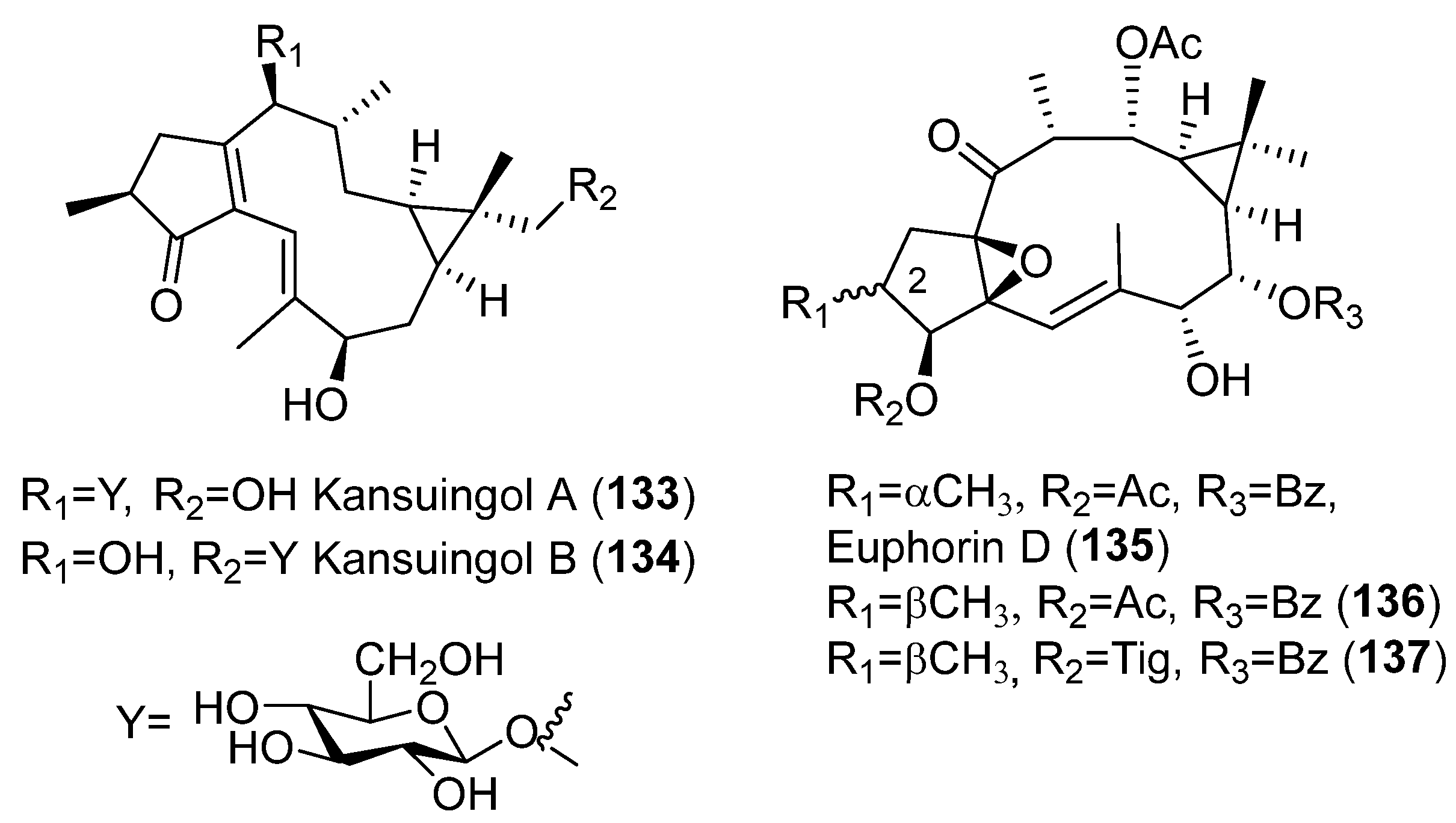
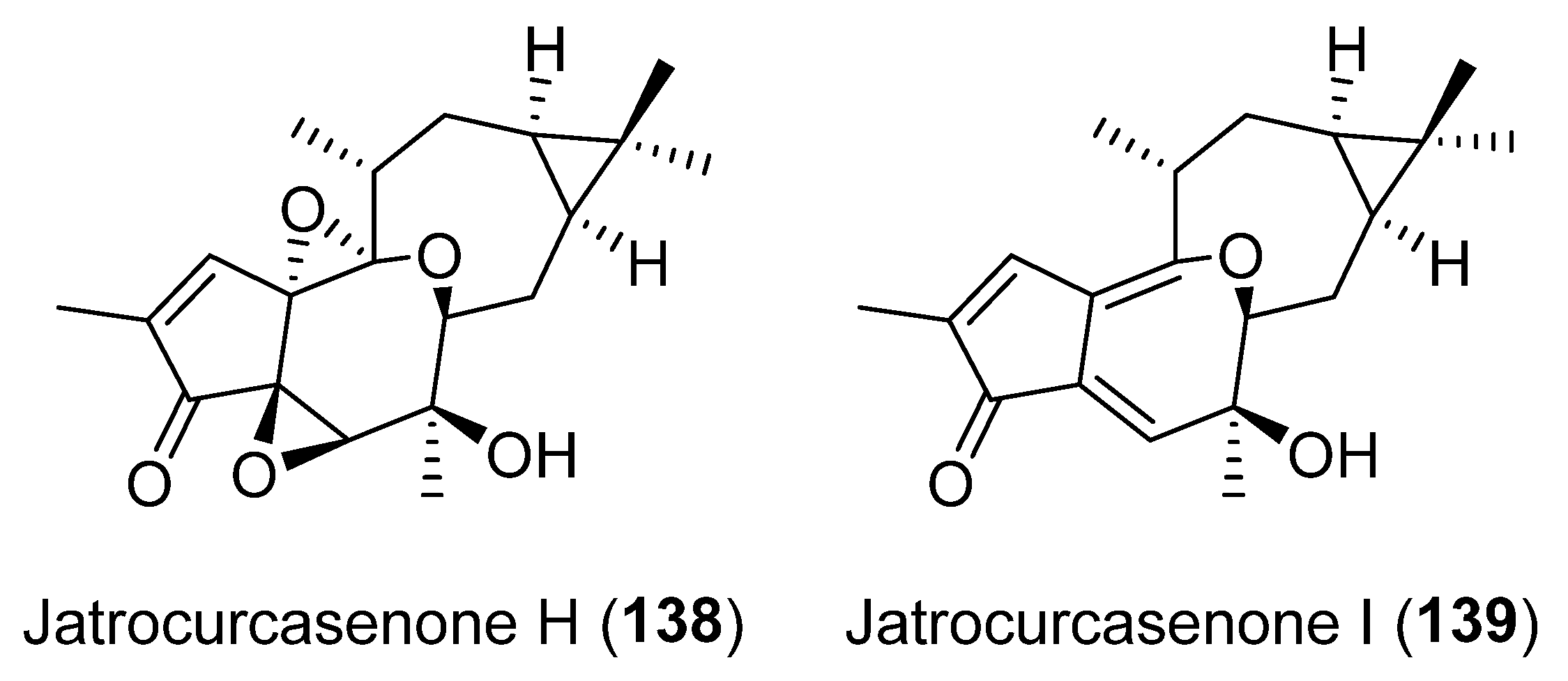
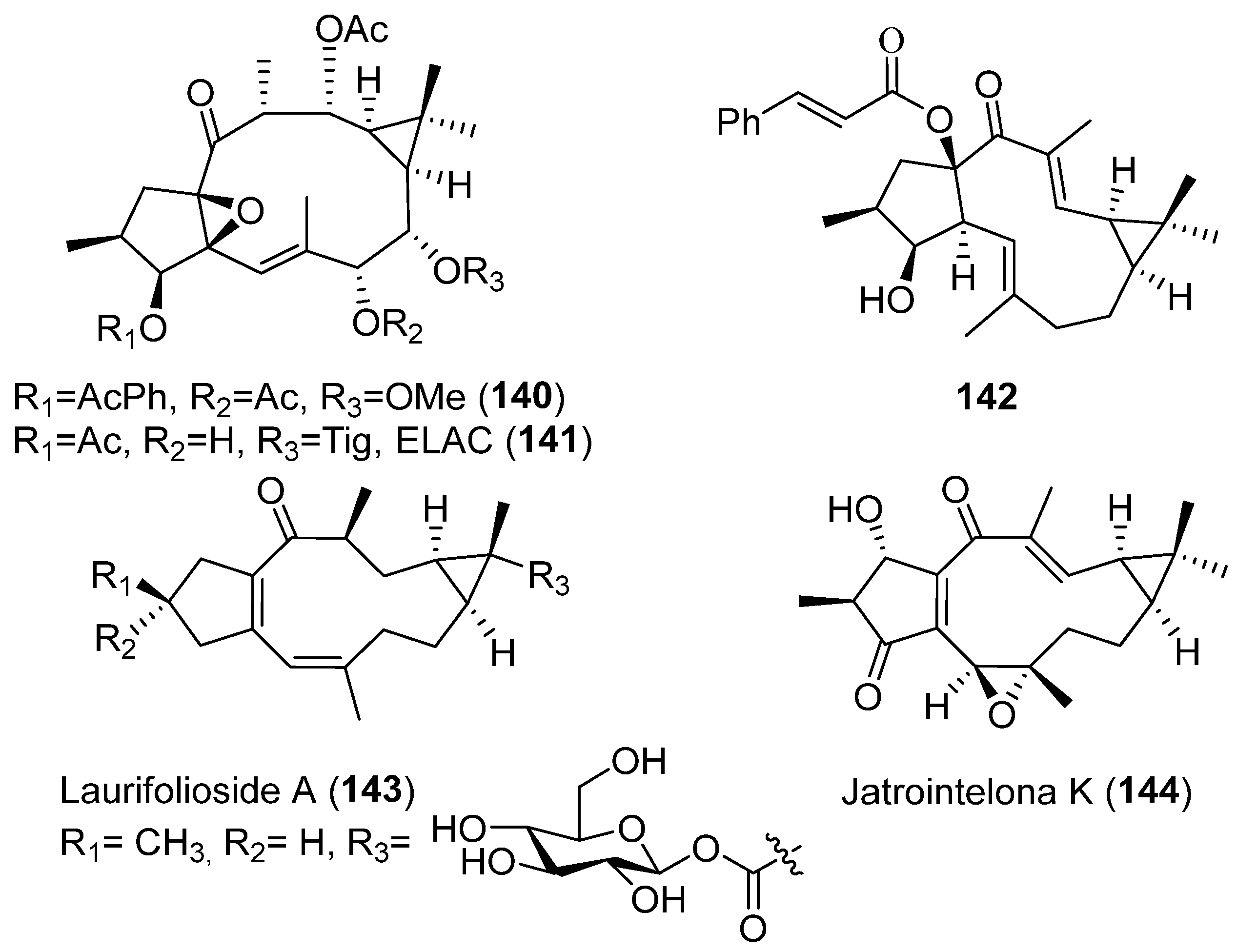
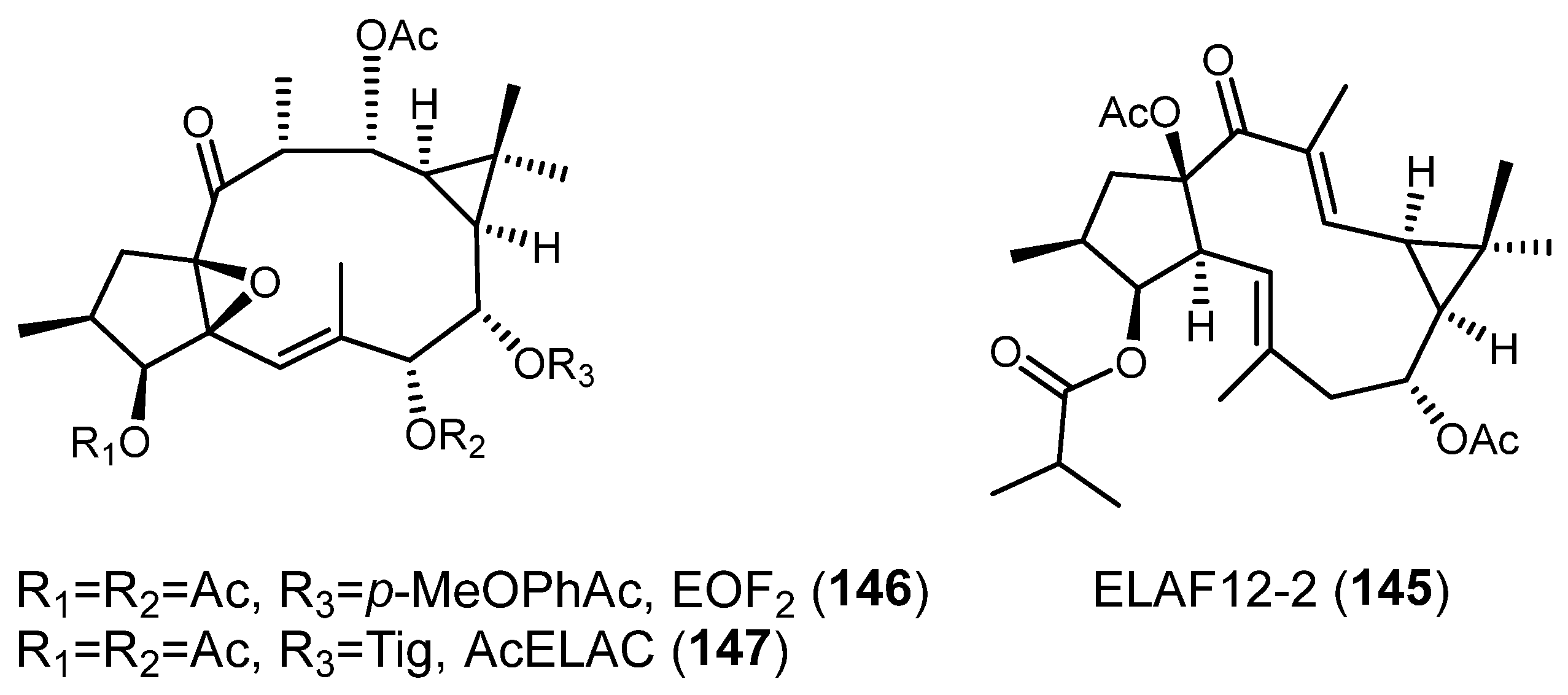
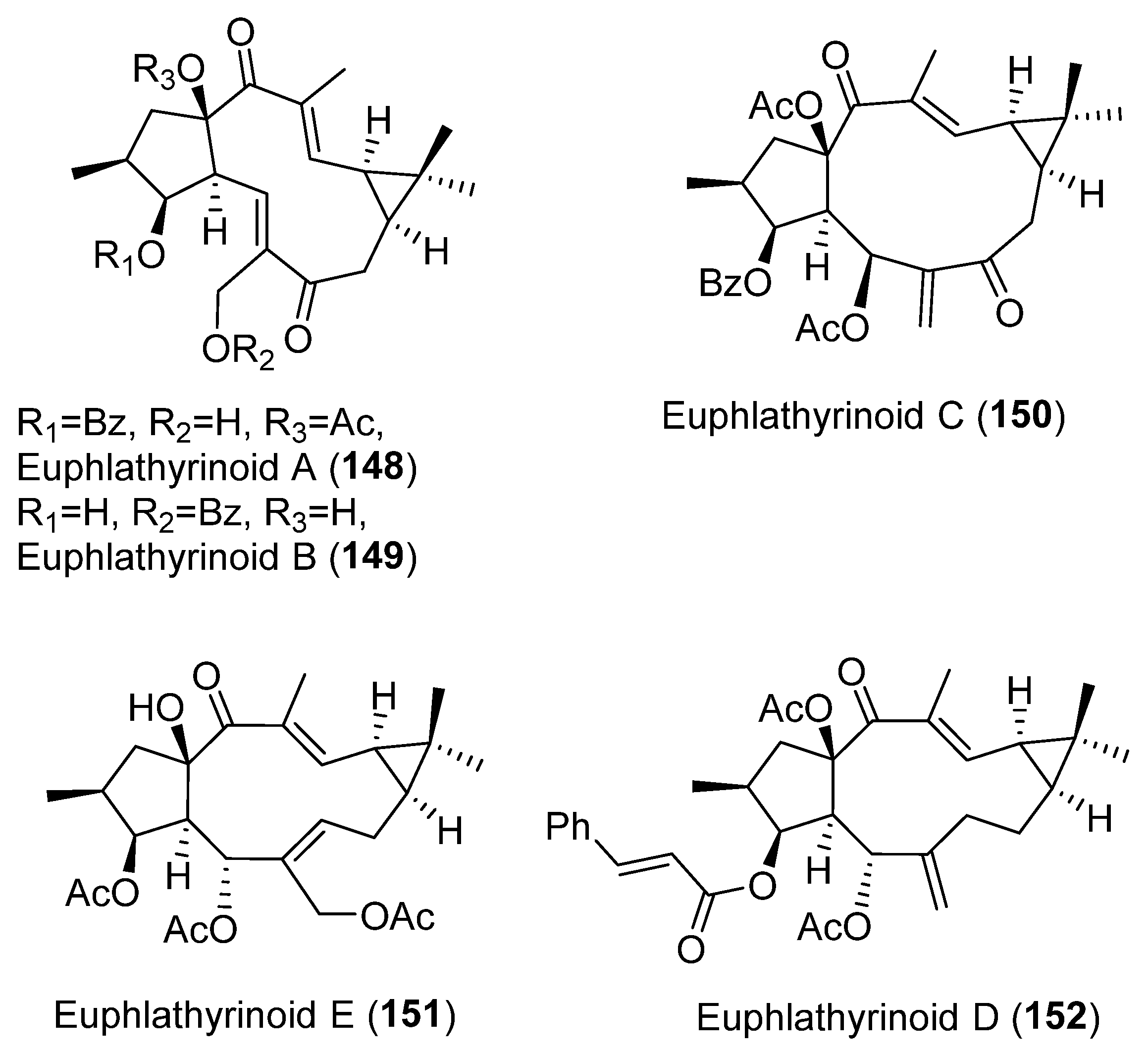


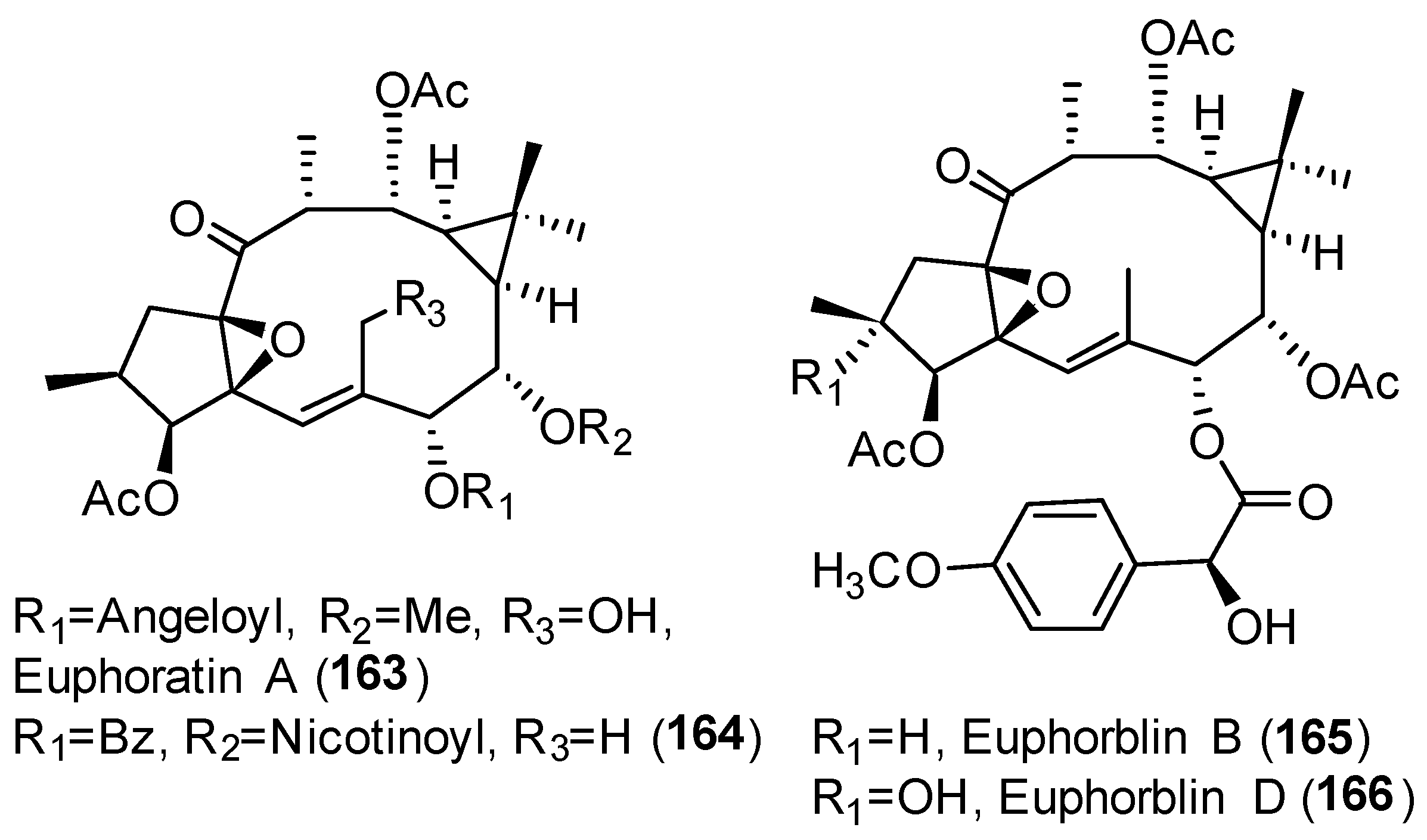
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vela, F.; Ezzanad, A.; Hunter, A.C.; Macías-Sánchez, A.J.; Hernández-Galán, R. Pharmacological Potential of Lathyrane-Type Diterpenoids from Phytochemical Sources. Pharmaceuticals 2022, 15, 780. https://doi.org/10.3390/ph15070780
Vela F, Ezzanad A, Hunter AC, Macías-Sánchez AJ, Hernández-Galán R. Pharmacological Potential of Lathyrane-Type Diterpenoids from Phytochemical Sources. Pharmaceuticals. 2022; 15(7):780. https://doi.org/10.3390/ph15070780
Chicago/Turabian StyleVela, Fátima, Abdellah Ezzanad, Alan Christy Hunter, Antonio José Macías-Sánchez, and Rosario Hernández-Galán. 2022. "Pharmacological Potential of Lathyrane-Type Diterpenoids from Phytochemical Sources" Pharmaceuticals 15, no. 7: 780. https://doi.org/10.3390/ph15070780
APA StyleVela, F., Ezzanad, A., Hunter, A. C., Macías-Sánchez, A. J., & Hernández-Galán, R. (2022). Pharmacological Potential of Lathyrane-Type Diterpenoids from Phytochemical Sources. Pharmaceuticals, 15(7), 780. https://doi.org/10.3390/ph15070780