New Membrane Active Antibacterial and Antiviral Amphiphiles Derived from Heterocyclic Backbone of Pyridinium-4-Aldoxime
Abstract
:1. Introduction
2. Results and Discussion
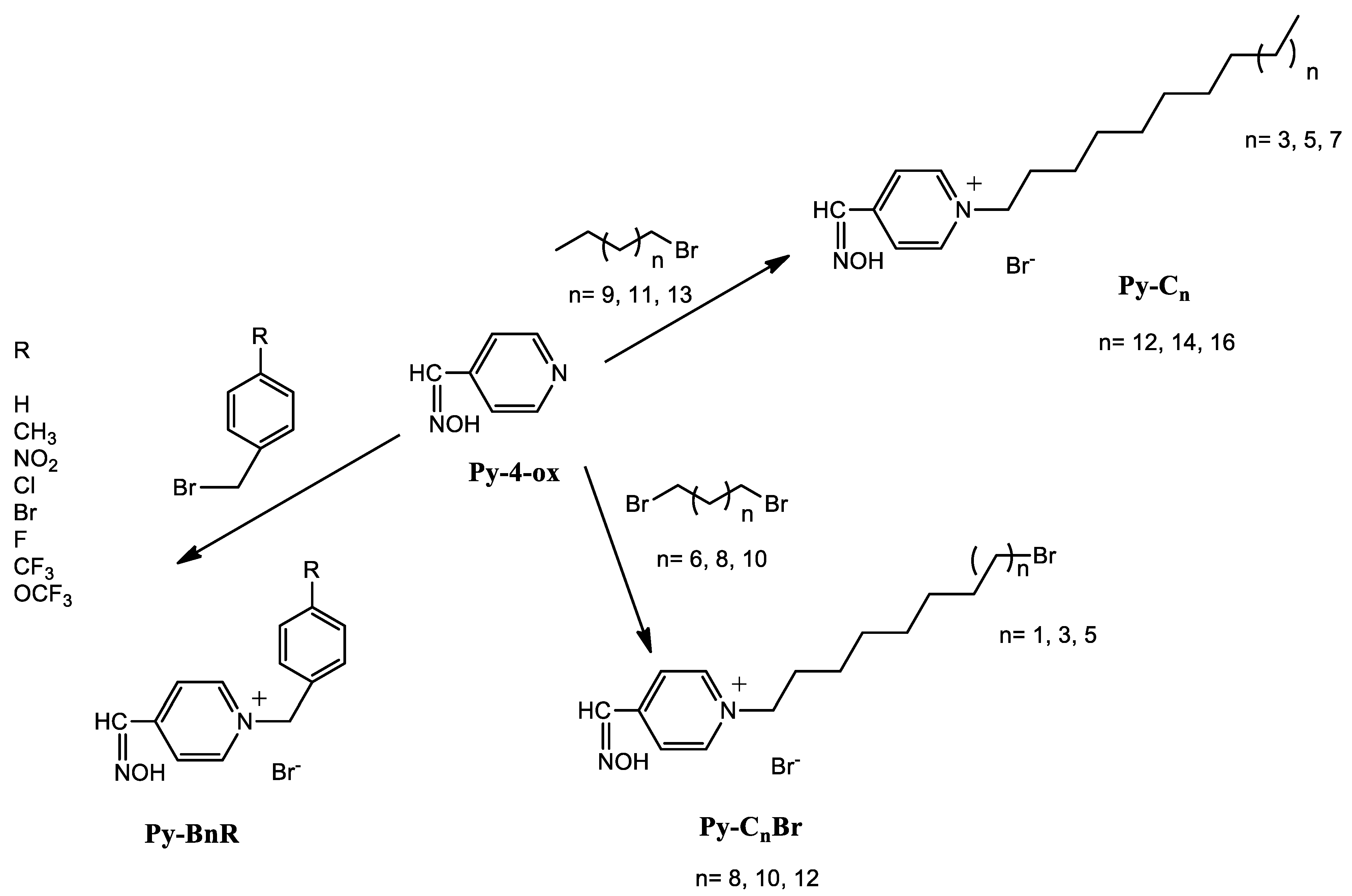
2.1. Chemistry
2.2. In Vitro Evaluation of Biological Activity
2.2.1. Antibacterial Activity
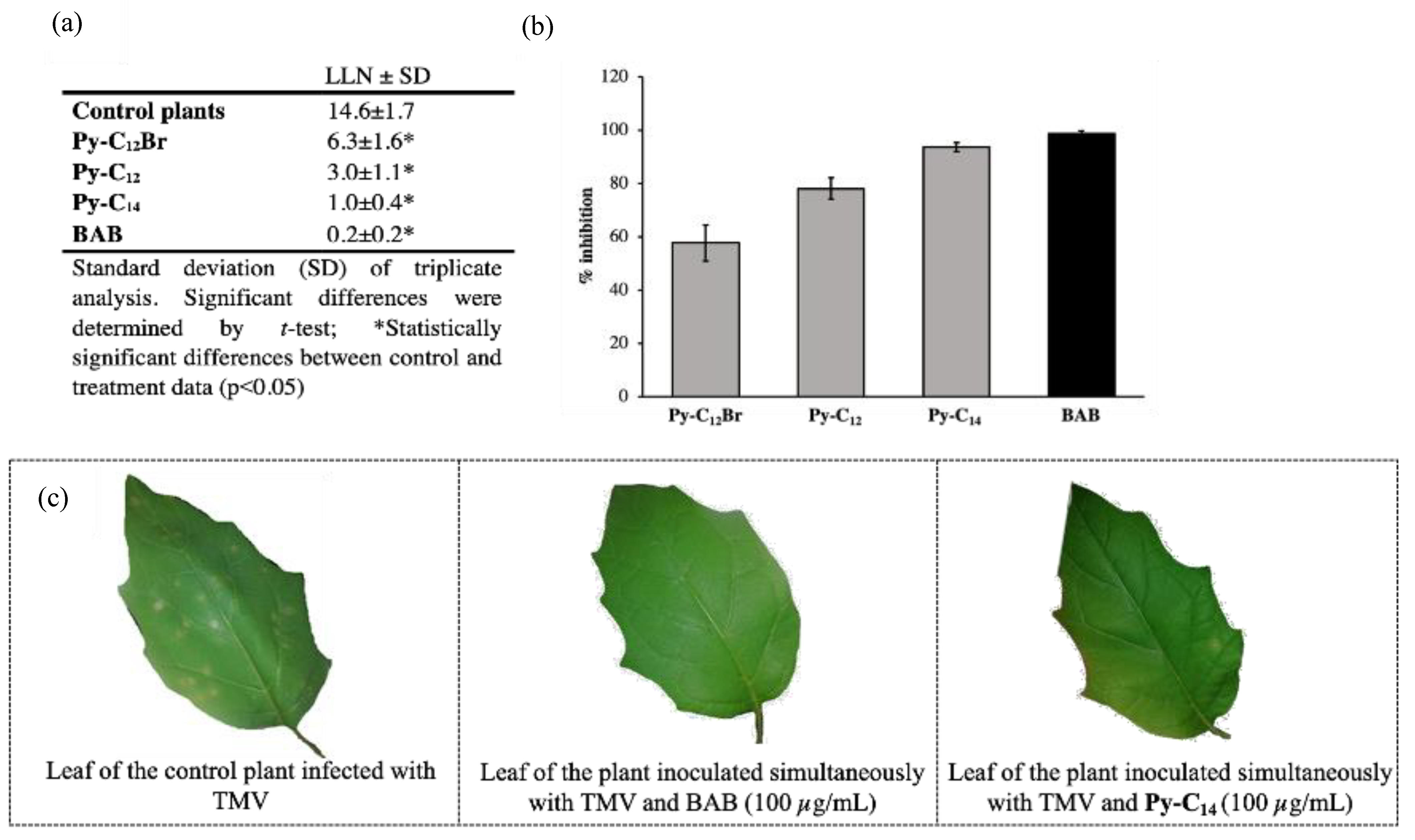
2.2.2. Antiviral Activity
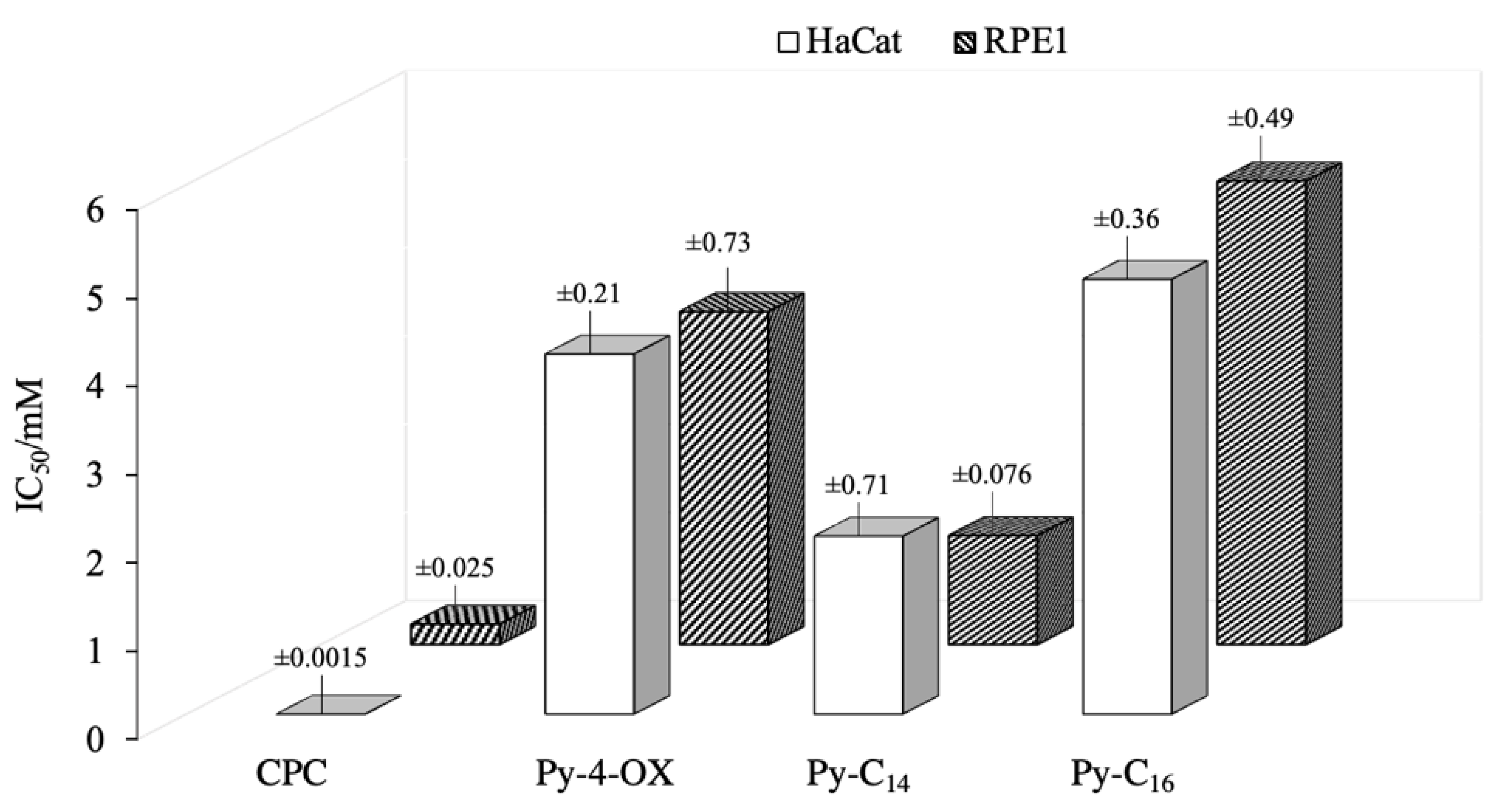
2.2.3. Cytotoxic Activity
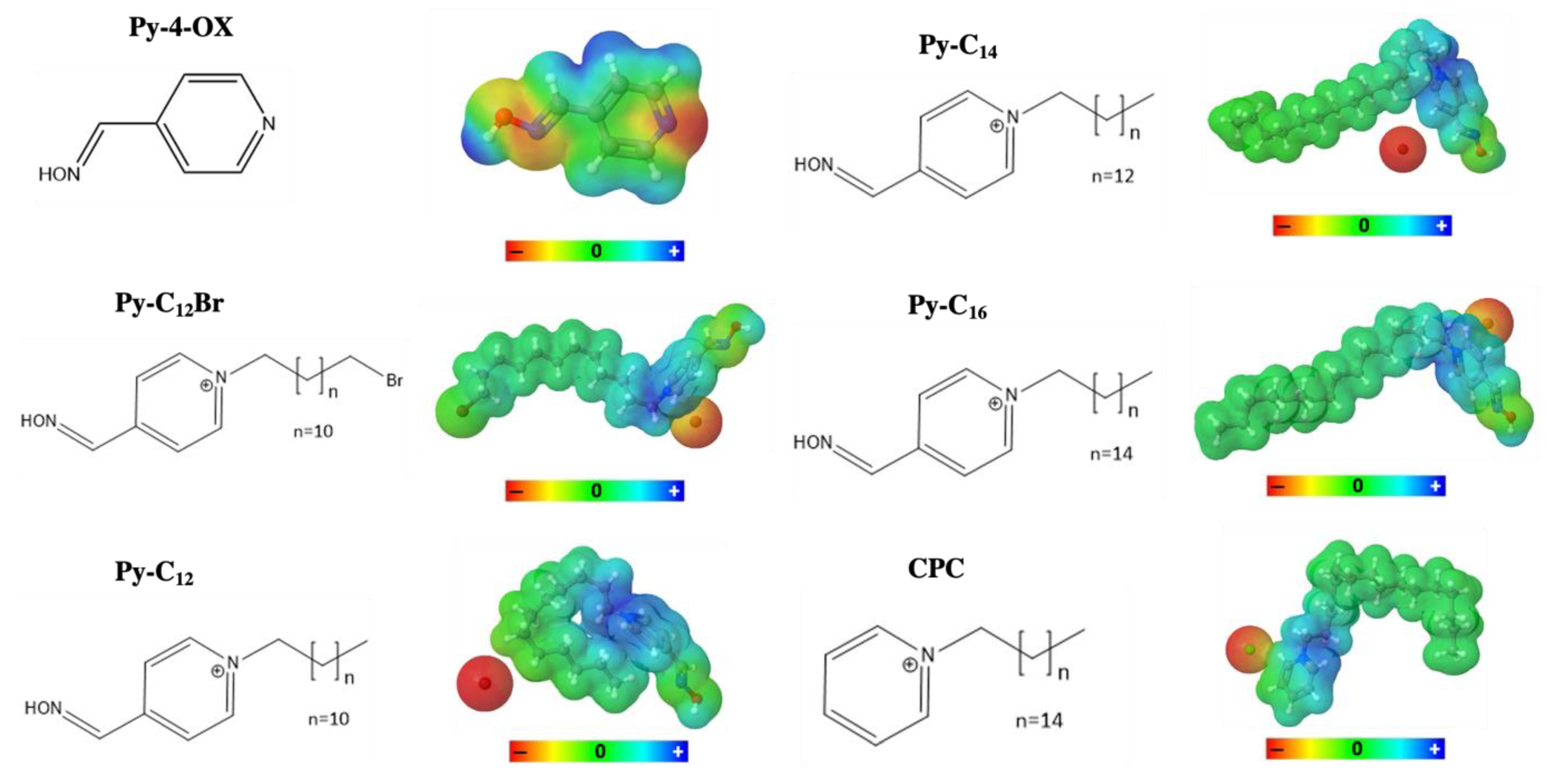
2.3. Hydrophobicity and Electron Density Distribution of Synthesized QAS
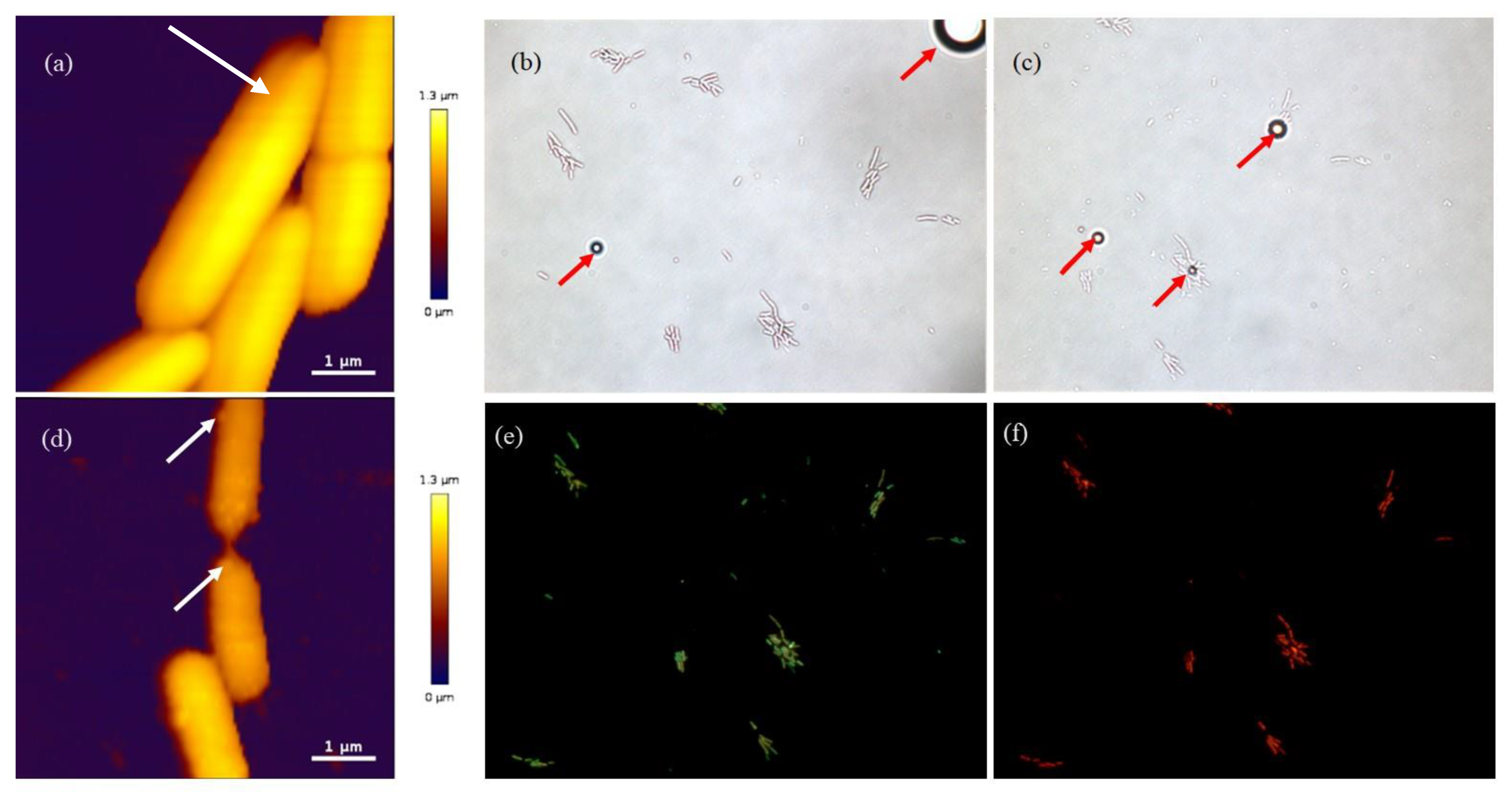
2.4. Atomic Force Microscopy (AFM)
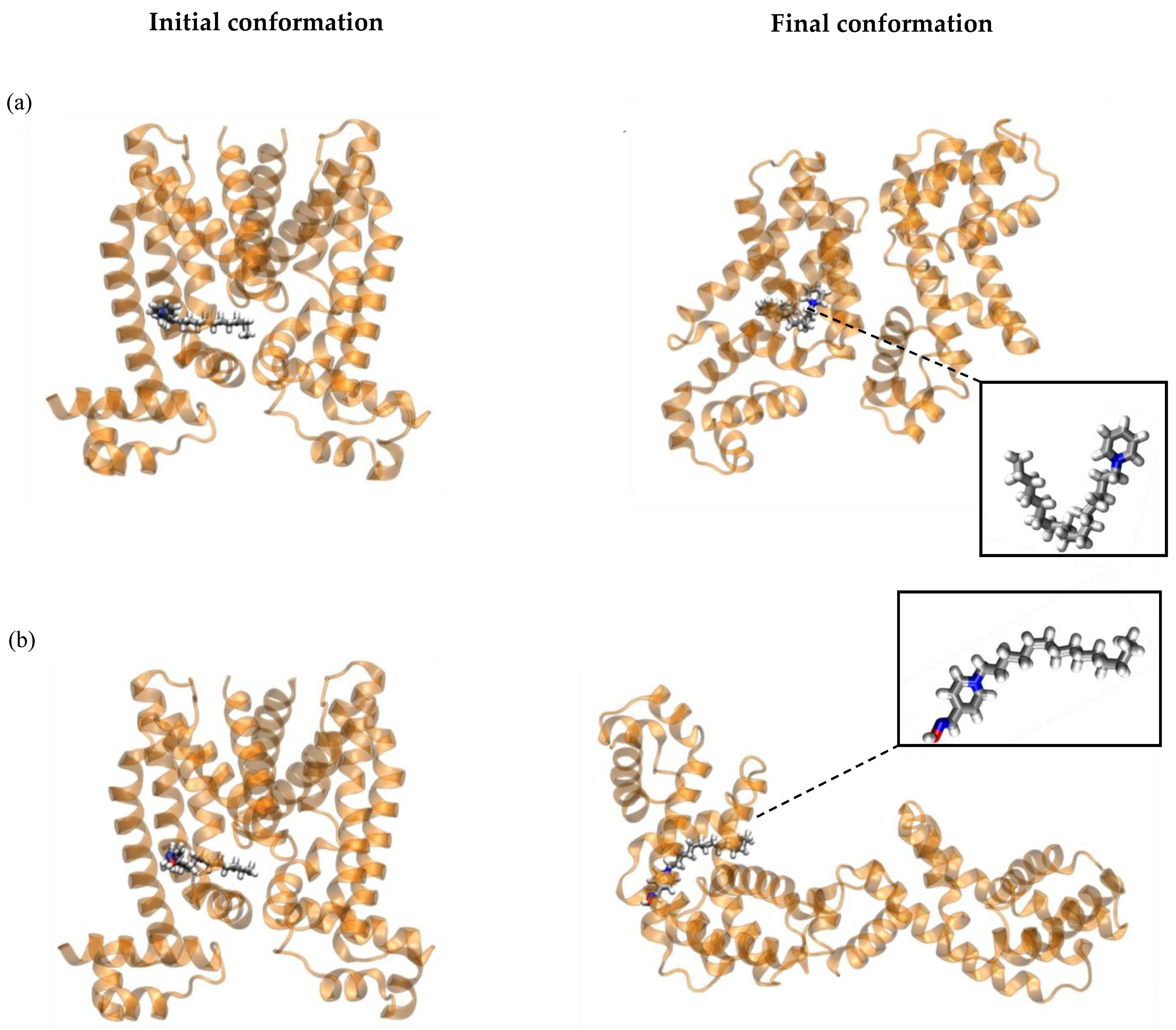
2.5. The Effect of Py-C14 Binding to the Transcriptional Factor QacR Dimer
3. Material and Methods
3.1. Synthesis
3.2. In Vitro Evaluation of Biological Activity
3.2.1. Bacterial Strains
3.2.2. The Standard Curves for Bacterial Colony Forming Units per Milliliter (CFU/mL) versus A600
3.2.3. Broth Microdilution Assays
3.2.4. Virus and Plant Hosts
3.2.5. Antiphytoviral Activity Assay
3.2.6. Cytotoxicity
3.3. SwissADME Calculations and Visualization of Electron Density Distribution
3.4. Atomic Force Microscopy
3.5. Docking Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Loftsson, T.; Thorsteinsson, T.; Hilmarsson, H.; Hjálmarsdóttir, M.A.; Kristinsson, K.G.; Másson, M. Soft Antimicrobial Agents: Synthesis and Activity of Labile Environmentally Friendly Long Chain Quaternary Ammonium Compounds. J. Med. Chem. 2003, 46, 4173–4181. [Google Scholar] [CrossRef]
- Minbiole, K.P.C.; Jennings, M.C.; Ator, L.E.; Black, J.W.; Grenier, M.C.; LaDow, J.E.; Caran, K.L.; Seifert, K.; Wuest, W.M. From antimicrobial activity to mechanism of resistance: The multifaceted role of simple quaternary ammonium compounds in bacterial eradication. Tetrahedron 2016, 72, 3559–3566. [Google Scholar] [CrossRef] [Green Version]
- Jennings, M.C.; Minbiole, K.P.C.; Wuest, W.M. Quaternary ammonium compounds: An antimicrobial mainstay and platform for innovation to address bacterial resistance. ACS Infect. Dis. 2016, 1, 288–303. [Google Scholar] [CrossRef] [PubMed]
- Alkhalifa, S.; Jennings, M.C.; Granata, D.; Klein, M.; Wuest, W.M.; Minbiole, K.P.C.; Carnevale, V. Analysis of the Destabilization of Bacterial Membranes by Quaternary Ammonium Compounds: A Combined Experimental and Computational Study. ChemBioChem 2020, 21, 1510–1516. [Google Scholar] [CrossRef]
- Tischer, M.; Pradel, G.; Ohlsen, K.; Holzgrabe, U. Quaternary ammonium salts and their antimicrobial potential: Targets or nonspecific interactions? ChemMedChem 2012, 7, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Xiling, G.; Yin, C.; Ling, W.; Xiaosong, W.; Jingjing, F.; Fang, L.; Xiaoyan, Z.; Yiyue, G.; Ying, C.; Lunbiao, C.; et al. In vitro inactivation of SARS-CoV-2 by commonly used disinfection products and methods. Sci. Rep. 2021, 11, 2418. [Google Scholar] [CrossRef] [PubMed]
- Buffet-Bataillon, S.; Tattevin, P.; Bonnaure-Mallet, M.; Jolivet-Gougeon, A. Emergence of resistance to antibacterial agents: The role of quaternary ammonium compounds—A critical review. Int. J. Antimicrob. Agents 2012, 39, 381–389. [Google Scholar] [CrossRef]
- Jennings, M.C.; Buttaro, B.A.; Minbiole, K.P.C.; Wuest, W.M. Bioorganic Investigation of Multicationic Antimicrobials to Combat QAC-Resistant Staphylococcus aureus. ACS Infect. Dis. 2016, 1, 304–309. [Google Scholar] [CrossRef]
- Schumacher, M.A.; Miller, M.C.; Grkovic, S.; Brown, M.H.; Skurray, R.A.; Brennan, R.G. Structural basis for cooperative DNA binding by two dimers of the multidrug-binding protein QacR. EMBO J. 2002, 21, 1210–1218. [Google Scholar] [CrossRef]
- Forman, M.E.; Fletcher, M.H.; Jennings, M.C.; Duggan, S.M.; Minbiole, K.P.C.; Wuest, W.M. Structure-Resistance Relationships: Interrogating Antiseptic Resistance in Bacteria with Multicationic Quaternary Ammonium Dyes. ChemMedChem 2016, 11, 958–962. [Google Scholar] [CrossRef]
- Jennings, M.C.; Forman, M.E.; Duggan, S.M.; Minbiole, K.P.C.; Wuest, W.M. Efflux Pumps Might Not Be the Major Drivers of QAC Resistance in Methicillin-Resistant Staphylococcus aureus. ChemBioChem 2017, 18, 1573–1577. [Google Scholar] [CrossRef] [PubMed]
- Al-Khalifa, S.E.; Jennings, M.C.; Wuest, W.M.; Minbiole, K.P.C. The Development of Next-Generation Pyridinium-Based multiQAC Antiseptics. ChemMedChem 2017, 12, 280–283. [Google Scholar] [CrossRef] [PubMed]
- Ator, L.E.; Jennings, M.C.; McGettigan, A.R.; Paul, J.J.; Wuest, W.M.; Minbiole, K.P.C. Beyond paraquats: Dialkyl 3,3′- and 3,4′-bipyridinium amphiphiles as antibacterial agents. Bioorganic Med. Chem. Lett. 2014, 24, 3706–3709. [Google Scholar] [CrossRef]
- Garrison, M.A.; Mahoney, A.R.; Wuest, W.M. Tricepyridinium-inspired QACs yield potent antimicrobials and provide insight into QAC resistance. ChemMedChem 2021, 16, 463–466. [Google Scholar] [CrossRef] [PubMed]
- Haldar, J.; Kondaiah, P.; Bhattacharya, S. Synthesis and Antibacterial Properties of Novel Hydrolyzable Cationic Amphiphiles. Incorporation of Multiple Head Groups Leads to Impressive Antibacterial Activity. J. Med. Chem. 2005, 48, 3823–3831. [Google Scholar] [CrossRef]
- Grenier, M.C.; Davis, R.W.; Wilson-Henjum, K.L.; Ladow, J.E.; Black, J.W.; Caran, K.L.; Seifert, K.; Minbiole, K.P.C. The antibacterial activity of 4,4′-bipyridinium amphiphiles with conventional, bicephalic and gemini architectures. Bioorganic Med. Chem. Lett. 2012, 22, 4055–4058. [Google Scholar] [CrossRef]
- Rodríguez-Morales, S.; Compadre, R.L.; Castillo, R.; Breen, P.J.; Compadre, C.M. 3D-QSAR, synthesis, and antimicrobial activity of 1-alkylpyridinium compounds as potential agents to improve food safety. Eur. J. Med. Chem. 2005, 40, 840–849. [Google Scholar] [CrossRef]
- Marek, J.; Malinak, D.; Dolezal, R.; Soukup, O.; Pasdiorova, M.; Dolezal, M.; Kuca, K. Synthesis and disinfection effect of the pyridine-4-aldoxime based salts. Molecules 2015, 20, 3681–3696. [Google Scholar] [CrossRef]
- Abele, E.; Abele, R.; Lukevics, E. Pyridine Oximes: Synthesis, Reactions, and Biological Activity. Chem. Heterocycl. Compd. 2003, 39, 825–865. [Google Scholar] [CrossRef]
- Gašo-Sokač, D.; Katalinić, M.; Kovarik, Z.; Bušić, V.; Kovač, S. Synthesis and evaluation of novel analogues of vitamin B6 as reactivators of tabun and paraoxon inhibited acetylcholinesterase. Chem.-Biol. Interact. 2010, 187, 234–237. [Google Scholar] [CrossRef]
- Čalić, M.; Vrdoljak, A.L.; Radić, B.; Jelić, D.; Jun, D.; Kuča, K.; Kovarik, Z. In vitro and in vivo evaluation of pyridinium oximes: Mode of interaction with acetylcholinesterase, effect on tabun- and soman-poisoned mice and their cytotoxicity. Toxicology 2006, 219, 85–96. [Google Scholar] [CrossRef] [PubMed]
- Odžak, R.; Čalić, M.; Hrenar, T.; Primožič, I.; Kovarik, Z. Evaluation of monoquaternary pyridinium oximes potency to reactivate tabun-inhibited human acetylcholinesterase. Toxicology 2007, 233, 85–96. [Google Scholar] [CrossRef] [PubMed]
- Foretić, B.; Damjanović, V.; Vianello, R.; Picek, I. Novel insights into the thioesterolytic activity of N-substituted pyridinium-4-oximes. Molecules 2020, 25, 2385. [Google Scholar] [CrossRef] [PubMed]
- Crnčević, D.; Odžak, R. Synthesis of quaternary ammonium salts based on quinuclidin-3-ol and pyridine-4-aldoxime with alkyl chains. ST-OPEN 2020, 1, 1–8. [Google Scholar] [CrossRef]
- Bazina, L.; Maravić, A.; Krce, L.; Soldo, B.; Odžak, R.; Popović, V.B.; Aviani, I.; Primožič, I.; Šprung, M. Discovery of novel quaternary ammonium compounds based on quinuclidine-3-ol as new potential antimicrobial candidates. Eur. J. Med. Chem. 2019, 163, 626–635. [Google Scholar] [CrossRef]
- Crnčević, D.; Krce, L.; Mastelić, L.; Maravić, A.; Soldo, B.; Aviani, I.; Primožič, I.; Odžak, R.; Šprung, M. The mode of antibacterial action of quaternary N-benzylimidazole salts against emerging opportunistic pathogens. Bioorganic Chem. 2021, 112, 104938. [Google Scholar] [CrossRef]
- Zhang, N.; Ma, S. Recent development of membrane-active molecules as antibacterial agents. Eur. J. Med. Chem. 2019, 184, 111743. [Google Scholar] [CrossRef]
- Kontos, R.C.; Schallenhammer, S.A.; Bentley, B.S.; Morrison, K.R.; Feliciano, J.A.; Tasca, J.A.; Kaplan, A.R.; Bezpalko, M.W.; Kassel, W.S.; Wuest, W.M.; et al. An Investigation into Rigidity–Activity Relationships in BisQAC Amphiphilic Antiseptics. ChemMedChem 2019, 14, 83–87. [Google Scholar] [CrossRef]
- Mereghetti, L.; Quentin, R.; der Mee, N.M.-V.; Audurier, A. Low sensitivity of Listeria monocytogenes to quaternary ammonium compounds. Appl. Environ. Microbiol. 2000, 66, 5083–5086. [Google Scholar] [CrossRef] [Green Version]
- Kwaśniewska, D.; Chen, Y.L.; Wieczorek, D. Biological activity of quaternary ammonium salts and their derivatives. Pathogens 2020, 9, 459. [Google Scholar] [CrossRef]
- Sunde, M.; Langsrud, S.; Yazdankhah, S.P.; Hegstad, K.; Lunestad, B.T.; Scheie, A.A. Does the wide use of Quaternary Ammonium Compounds enhance the selection and spread of antimicrobial resistance and thus threaten our health? Microb. Drug Resist. 2010, 16, 91–104. [Google Scholar] [CrossRef]
- Ogilvie, B.H.; Solis-Leal, A.; Lopez, J.B.; Poole, B.D.; Robison, R.A.; Berges, B.K. Alcohol-free hand sanitizer and other quaternary ammonium disinfectants quickly and effectively inactivate SARS-CoV-2. J. Hosp. Infect. 2021, 108, 142–145. [Google Scholar] [CrossRef]
- Rusak, G.; Kraja, M.; Krsnik-Rasol, M.; Gutzeit, H.O. Quercetin influences response in Nicotiana megalosiphon infected by satellite-associated cucumber mosaic virus. J. Plant Dis. Prot. 2007, 114, 145–150. [Google Scholar] [CrossRef]
- Othman, B. Antiphytoviral Activity of the Plectranthus Tenuiflorus on Some Important Viruses. 2016. Available online: https://www.researchgate.net/publication/228906668 (accessed on 2 February 2022).
- Krcatović, E.; Rusak, G.; Bezić, N.; Krajačić, M. Inhibition of tobacco mosaic virus infection by quercetin and vitexin. Acta Virol. 2008, 52, 119–124. [Google Scholar] [PubMed]
- Vuko, E.; Dunkić, V.; Maravić, A.; Ruščić, M.; Nazlić, M.; Radan, M.; Ljubenkov, I.; Soldo, B.; Fredotović, Ž. Not only a weed plant—biological activities of essential oil and hydrosol of Dittrichia viscosa (L.) greuter. Plants 2021, 10, 1837. [Google Scholar] [CrossRef]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stocks, S.M. Mechanism and use of the commercially available viability stain, BacLight. Cytom. Part A 2004, 61, 189–195. [Google Scholar] [CrossRef]
- Clsi, M07-A9: Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically; Approved Standard—Ninth Edition, n.d. Available online: www.clsi.org (accessed on 11 November 2021).
- Vuko, E.; Dunkić, V.; Ruščić, M.; Nazlić, M.; Mandić, N.; Soldo, B.; Šprung, M.; Fredotović, Ž. Chemical composition and new biological activities of essential oil and hydrosol of Hypericum perforatum l. Ssp. veronense (schrank) h. lindb. Plants 2021, 10, 1014. [Google Scholar] [CrossRef]
- Leatherbarrow, R.J. GraFit Version 7; Erithacus Software Ltd.: Horley, UK, 2009. [Google Scholar]
- PerkinElmer, Chemdraw, (RRID:SCR_016768), (n.d.). Available online: https://perkinelmerinformatics.com/ (accessed on 6 October 2021).
- Jmol Development Team, Jmol. 2016. Available online: http://jmol.sourceforge.net (accessed on 6 October 2021).
- Krce, L.; Šprung, M.; Maravić, A.; Umek, P.; Salamon, K.; Krstulović, N.; Aviani, I. Bacteria exposed to silver nanoparticles synthesized by laser ablation in water: Modelling E. coli growth and inactivation. Materials 2020, 13, 653. [Google Scholar] [CrossRef] [Green Version]
- Maier, J.A.; Martinez, C.; Kasavajhala, K.; Wickstrom, L.; Hauser, K.E.; Simmerling, C. ff14SB: Improving the Accuracy of Protein Side Chain and Backbone Parameters from ff99SB. J. Chem. Theory Comput. 2015, 11, 3696–3713. [Google Scholar] [CrossRef] [Green Version]
- Joung, S.; Cheatham, T.E., III. Determination of alkali and halide monovalent ion parameters for use in explicitly solvated biomolecular simulations. J. Phys. Chem. B 2008, 112, 9020–9041. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Wolf, R.M.; Caldwell, J.W.; Kollman, P.A.; Case, D.A. Development and testing of a general AMBER force field. J. Comput. Chem. 2004, 25, 1157–1174. [Google Scholar] [CrossRef] [PubMed]
- Cieplak, P.; Cornell, W.D.; Bayly, C.I.; Kollman, P.A. Application of the multimolecule and multiconformational RESP methodology to biopolymers: Charge derivation for DNA, RNA, and proteins. J. Comput. Chem. 1995, 16, 1357–1377. [Google Scholar] [CrossRef]
- Schrödinger, LLC. Schrödinger Release 2022-1: Maestro; Schrödinger, LLC: New York, NY, USA, 2021. [Google Scholar]
- Darden, T.; York, D.; Pedersen, L. Particle mesh Ewald: An N·log(N) method for Ewald sums in large systems. J. Chem. Phys. 1993, 98, 10089–10092. [Google Scholar] [CrossRef] [Green Version]
- Abraham, M.J.; Murtola, T.; Schulz, R.; Páll, S.; Smith, J.C.; Hess, B.; Lindahl, E. GROMACS: High performance molecular simulations through multi-level parallelism from laptops to supercomputers. SoftwareX 2015, 1, 19–25. [Google Scholar] [CrossRef] [Green Version]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD—Visual Molecular Dynamics. J. Mol. Graph. 1996, 14, 33–38. [Google Scholar] [CrossRef]

| Bacterial Strain | Strain Origin | MIC (mg mL−1/mM) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Py-4-ox | Py-Bn | Py-BnCH3 | Py-BnNO2 | Py-BnCl | Py-BnF | Py-BnBr | Py-BnCF3 | Py-BnOCF3 | ||
| Mr = 122.1 | Mr = 293.2 | Mr = 307.2 | Mr = 338.2 | Mr = 327.6 | Mr = 311.2 | Mr = 372.1 | Mr = 361.2 | Mr = 377.2 | ||
| Gram-positive bacteria | ||||||||||
| Staphylococcus aureus | ATCC25923 | >5/>40.9 | >5/>17.05 | 2.5/8.14 | 5/14.78 | 1.25/3.82 | 5/16.07 | 1.25/3.36 | 0.63/1.74 | 0.31/0.82 |
| Staphylococcus aureus | Clinical/MRSA | >5/>40.9 | >5/>17.05 | 5/16.28 | >5/>14.78 | 2.5/7.63 | >5/>16.07 | 1.25/3.36 | 1.25/3.46 | 1.25/3.31 |
| Bacillus cereus | ATCC14579 | >5/>40.9 | >5/>17.05 | 5/16.28 | >5/>14.78 | 5/15.26 | >5/>16.07 | 2.5/6.72 | 1.25/3.46 | 2.5/6.63 |
| Enterococcus faecalis | ATCC29212 | 5/40.9 | >5/>17.05 | 2.5/8.14 | >5/>14.78 | 5/15.26 | >5/>16.07 | 2.5/6.72 | 2.5/6.92 | 2.5/6.63 |
| Listeria monocytogenes | ATCC7644 | >5/>40.9 | >5/>17.05 | 1.25/4.07 | >5/>14.78 | 2.5/7.63 | >5/>16.07 | 2.5/6.72 | 2.5/6.92 | 2.5/6.63 |
| Gram-negative bacteria | ||||||||||
| Escherichia coli | ATCC25922 | >5/>40.9 | 1.25/4.26 | 5/16.28 | 2.5/7.39 | 0.31/0.95 | 0.63/2.02 | 0.16/0.43 | 2.5/6.92 | 0.31/0.82 |
| Salmonella enterica | Food isolate | 5/40.9 | >5/>17.05 | 0.63/2.05 | 5/14.78 | 2.5/7.63 | >5/>16.07 | 0.63/1.69 | 1.25/3.46 | 2.5/6.63 |
| Pseudomonas aeruginosa | ATCC27853 | >5/>40.9 | >5/>17.05 | >5/>16.28 | >5/>14.78 | 5/15.26 | >5/>16.07 | 5/13.44 | 5/13.84 | 2.5/6.63 |
| Bacterial Strain | Strain Origin | MIC (mg mL−1/mM) | MIC (μg mL−1/μM) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Py-C8Br | Py-C10Br | Py-C12Br | Py-C12 | Py-C14 | Py-C16 | CPC | Cefotaxime | ||
| Mr = 394.2 | Mr = 422.2 | Mr = 450.3 | Mr = 371.4 | Mr = 399.4 | Mr = 427.5 | Mr = 339.9 | Mr = 477.5 | ||
| Gram-positive bacteria | |||||||||
| Staphylococcus aureus | ATCC25923 | 5/12.68 | 0.63/1.49 | 0.16/0.36 | 0.08/0.22 | 0.08/0.20 | 0.04/0.09 | 1.4/4.1 | 3.7/10 |
| Staphylococcus aureus | Clinical/MRSA | 2.5/6.34 | 0.31/0.73 | 0.31/0.69 | 0.31/0.83 | 0.16/0.40 | 0.16/0.37 | 2.7/10 | >119.4/>250 |
| Bacillus cereus | ATCC14579 | 2.5/6.34 | 0.63/1.49 | 0.63/1.40 | 1.25/3.37 | 1.25/3.13 | 0.16/0.37 | 5.3/20 | 3.7/10 |
| Enterococcus faecalis | ATCC29212 | 2.5/6.34 | 5/11.84 | 1.25/2.78 | 0.63/1.70 | 0.31/0.78 | 2.5/5.85 | 2.7/10 | 29.8/60 |
| Listeria monocytogenes | ATCC7644 | 1.25/3.17 | 5/11.84 | 1.25/2.78 | 0.63/1.70 | 0.31/0.78 | 1.25/2.92 | 2.7/10 | 3.7/10 |
| Gram-negative bacteria | |||||||||
| Escherichia coli | ATCC25922 | 1.25/3.17 | 0.63/1.49 | 0.63/1.40 | 0.63/1.70 | 0.63/1.58 | 0.16/0.37 | 5.3/20 | 0.2/0.4 |
| Salmonella enterica | Food isolate | >5/>12.68 | 5/11.84 | 2.5/5.55 | 0.63/1.70 | 2.5/6.26 | 5/11.70 | 21.2/60 | 0.1/0.2 |
| Pseudomonas aeruginosa | ATCC27853 | >5/>12.68 | >5/>11.84 | 2.5/5.55 | 2.5/6.73 | 2.5/6.26 | 2.5/5.85 | 850/250 | 59.7/130 |
| Compound | cLogP | TPSA/Ų | |
|---|---|---|---|
| Py-4-ox | 0.73 | 45.48 | |
| Aryl substituent | Py-Bn | 0.95 | 36.47 |
| Py-BnCH3 | 0.69 | 36.47 | |
| Py-BnNO2 | -0.62 | 82.29 | |
| Py-BnCl | 0.86 | 36.47 | |
| Py-BnF | 0.63 | 36.47 | |
| Py-BnBr | 0.93 | 36.47 | |
| Py-BnCF3 | 1.50 | 36.47 | |
| Py-BnOCF3 | 0.90 | 45.70 | |
| Alkyl substituent | Py-C8Br | 1.76 | 36.47 |
| Py-C10Br | 2.40 | 36.47 | |
| Py-C12Br | 3.12 | 36.47 | |
| Py-C12 | 1.51 | 36.47 | |
| Py-C14 | 2.07 | 36.47 | |
| Py-C16 | 2.82 | 36.47 | |
| CPC | 3.24 | 3.88 | |
| BAB | 3.55 | 0.00 |
| Amino Acid | Overall Hydrogen-Bond (HB) Count (%) |
|---|---|
| Glu67 | 50 |
| Gln63 | 23 |
| Leu94 | 12 |
| Tyr91 | 9 |
| Thr87 | 5 |
| Leu80 | 1 |
| Compound | SMILES |
|---|---|
| Py-4-ox | ON=CC1=CC=NC=C1 |
| Py-Bn | ON=CC1=CC=[N+](CC2=CC=CC=C2)C=C1.[Br-] |
| Py-BnCH3 | CC(C=C1)=CC=C1C[N+]2=CC=C(C=NO)C=C2.[Br-] |
| Py-BnNO2 | ON=CC1=CC=[N+](CC2=CC=C([N+]([O-])=O)C=C2)C=C1.[Br-] |
| Py-BnCl | ClC(C=C1)=CC=C1C[N+]2=CC=C(C=NO)C=C2.[Br-] |
| Py-BnF | FC(C=C1)=CC=C1C[N+]2=CC=C(C=NO)C=C2.[Br-] |
| Py-BnBr | BrC(C=C1)=CC=C1C[N+]2=CC=C(C=NO)C=C2.[Br-] |
| Py-BnCF3 | ON=CC1=CC=[N+](CC2=CC=C(C(F)(F)F)C=C2)C=C1.[Br-] |
| Py-BnOCF3 | ON=CC1=CC=[N+](CC2=CC=C(OC(F)(F)F)C=C2)C=C1.[Br-] |
| Py-C8Br | ON=CC1=CC=[N+](CCCCCCCCBr)C=C1.[Br-] |
| Py-C10Br | ON=CC1=CC=[N+](CCCCCCCCCCBr)C=C1.[Br-] |
| Py-C12Br | ON=CC1=CC=[N+](CCCCCCCCCCCCBr)C=C1.[Br-] |
| Py-C12 | ON=CC1=CC=[N+](CCCCCCCCCCCC)C=C1.[Br-] |
| Py-C14 | ON=CC1=CC=[N+](CCCCCCCCCCCCCC)C=C1.[Br-] |
| Py-C16 | ON=CC1=CC=[N+](CCCCCCCCCCCCCCCC)C=C1.[Br-] |
| CPC | CCCCCCCCCCCCCCCC[N+]1=CC=CC=C1.[Cl-] |
| BAB | C[N+](CC1=CC=CC=C1)(C)CCCCCCCCCCCC.[Br-] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Crnčević, D.; Krce, L.; Cvitković, M.; Brkljača, Z.; Sabljić, A.; Vuko, E.; Primožič, I.; Odžak, R.; Šprung, M. New Membrane Active Antibacterial and Antiviral Amphiphiles Derived from Heterocyclic Backbone of Pyridinium-4-Aldoxime. Pharmaceuticals 2022, 15, 775. https://doi.org/10.3390/ph15070775
Crnčević D, Krce L, Cvitković M, Brkljača Z, Sabljić A, Vuko E, Primožič I, Odžak R, Šprung M. New Membrane Active Antibacterial and Antiviral Amphiphiles Derived from Heterocyclic Backbone of Pyridinium-4-Aldoxime. Pharmaceuticals. 2022; 15(7):775. https://doi.org/10.3390/ph15070775
Chicago/Turabian StyleCrnčević, Doris, Lucija Krce, Mislav Cvitković, Zlatko Brkljača, Antonio Sabljić, Elma Vuko, Ines Primožič, Renata Odžak, and Matilda Šprung. 2022. "New Membrane Active Antibacterial and Antiviral Amphiphiles Derived from Heterocyclic Backbone of Pyridinium-4-Aldoxime" Pharmaceuticals 15, no. 7: 775. https://doi.org/10.3390/ph15070775
APA StyleCrnčević, D., Krce, L., Cvitković, M., Brkljača, Z., Sabljić, A., Vuko, E., Primožič, I., Odžak, R., & Šprung, M. (2022). New Membrane Active Antibacterial and Antiviral Amphiphiles Derived from Heterocyclic Backbone of Pyridinium-4-Aldoxime. Pharmaceuticals, 15(7), 775. https://doi.org/10.3390/ph15070775







