Pharmacovigilance Signals of the Opioid Epidemic over 10 Years: Data Mining Methods in the Analysis of Pharmacovigilance Datasets Collecting Adverse Drug Reactions (ADRs) Reported to EudraVigilance (EV) and the FDA Adverse Event Reporting System (FAERS)
Abstract
:1. Introduction
1.1. The Opioid Epidemic
1.2. Post-Marketing Studies
2. Results
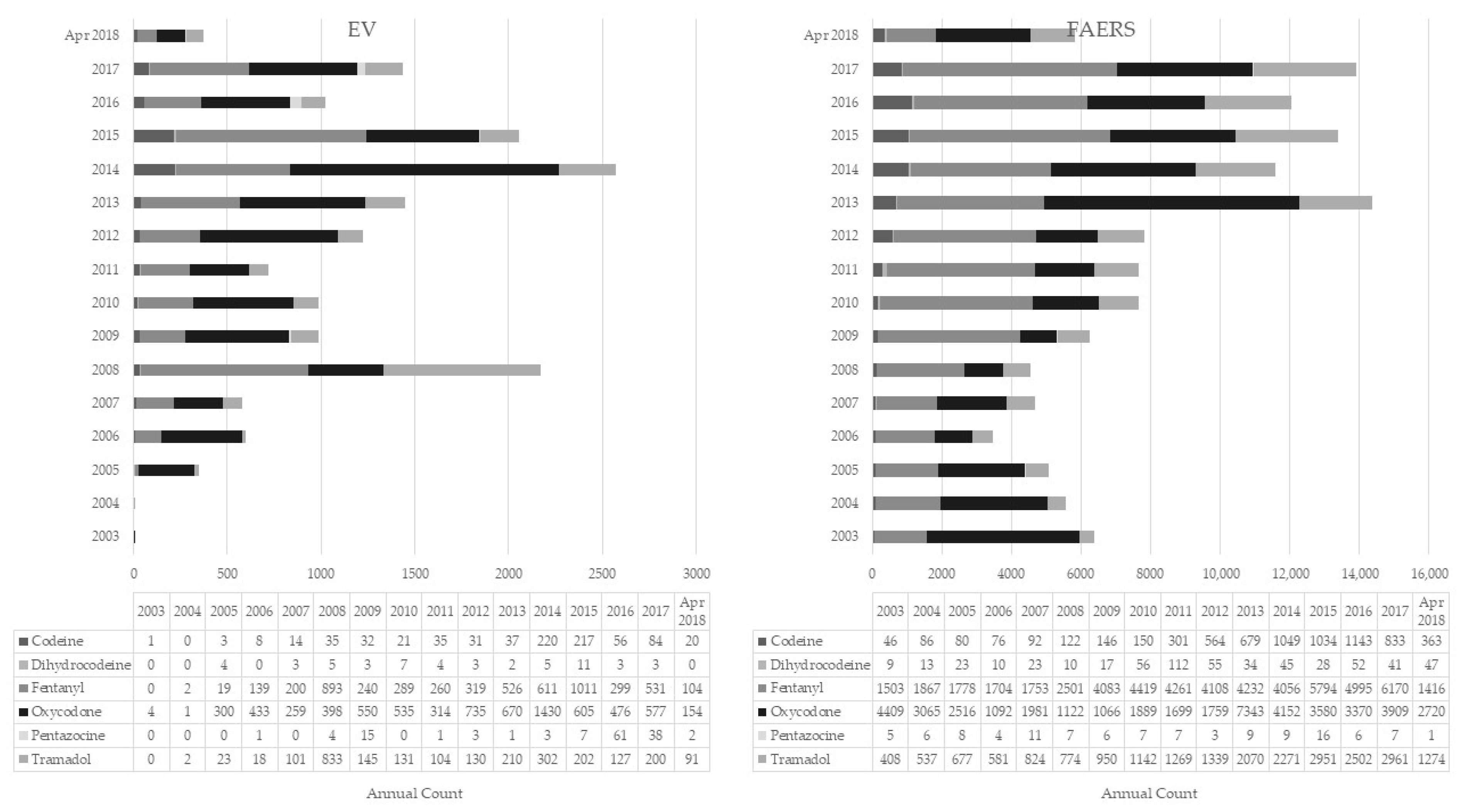
2.1. EMA versus FAERS Datasets
2.2. Pharmacovigilance Signals
2.3. New Psychoactive Substances (NPS)
3. Discussion
3.1. Opioid Differences
3.1.1. Epidemiology
3.1.2. Pharmacology
3.1.3. Abuse and Diversion Issues
3.1.4. Concomitant Drugs Used
3.1.5. Fatalities
3.2. NPS
3.3. Limitations
4. Materials and Methods
4.1. Data Sources
4.2. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chiappini, S.; Schifano, F. What about “Pharming”? Issues Regarding the Misuse of Prescription and over-the-Counter Drugs. Brain Sci. 2020, 10, 736. [Google Scholar] [CrossRef] [PubMed]
- Chiappini, S.; Guirguis, A.; Corkery, J.M.; Schifano, F. Misuse of Prescription and Over-the-Counter Drugs to Obtain Illicit Highs: How Pharmacists Can Prevent Abuse. Pharm. J. 2020, 305, 1–31. [Google Scholar]
- Schifano, F.; Chiappini, S.; Corkery, J.M.; Guirguis, A. Abuse of Prescription Drugs in the Context of Novel Psychoactive Substances (NPS): A Systematic Review. Brain Sci. 2018, 8, 73. [Google Scholar] [CrossRef] [Green Version]
- Huang, B.; Dawson, D.A.; Stinson, F.S.; Hasin, D.S.; Ruan, W.J.; Saha, T.D.; Smith, S.M.; Goldstein, R.B.; Grant, B.F. Prevalence, Correlates, and Comorbidity of Nonmedical Prescription Drug Use and Drug Use Disorders in the United States: Results of the National Epidemiologic Survey on Alcohol and Related Conditions. J. Clin. Psych. 2006, 67, 1062–1073. [Google Scholar] [CrossRef] [PubMed]
- United Nations Office on Drugs and Crime (UNODC, 2021). World Drug Report 2021—Drug Market Trends: Cannabis and Opioids. Vienna: United Nations publication, Sales No. E.21.XI.8. Available online: https://www.unodc.org/res/wdr2021/field/WDR21_Booklet_4.pdf%0Ahttps://www.unodc.org/res/wdr2021/field/WDR21_Booklet_3.pdf (accessed on 6 March 2022).
- Pathan, H.; Williams, J. Basic opioid pharmacology: An update. Br. J. Pain 2012, 6, 11–16. [Google Scholar] [CrossRef] [Green Version]
- Schifano, F.; Chiappini, S.; Corkery, J.M.; Guirguis, A. Assessing the 2004–2018 Fentanyl Misusing Issues Reported to an International Range of Adverse Reporting Systems. Front. Pharmacol. 2019, 10, 46. [Google Scholar] [CrossRef]
- Schifano, F.; Chiappini, S.; Corkery, J.M.; Scherbaum, N.; Guirguis, A. The E-Psychonaut Drugs’ Psychopharmacology. Curr. Opin. Pharmacol. 2021, 7, 165–174. [Google Scholar] [CrossRef]
- Lyden, J.; Binswanger, I.A. The United States opioid epidemic. Semin. Perinatol. 2019, 43, 123–131. [Google Scholar] [CrossRef]
- Kolodny, A. Viewpoint: How FDA Failures Contributed to the Opioid Crisis. AMA J. Ethics 2020, 22, 743–750. [Google Scholar]
- European Monitoring Centre for Drugs and Drug Addiction (EMCDDA, 2021). European Monitoring Centre for Drugs and Drug Addiction (2021) European Drug Report 2021: Trends and Developments. Available online: https://www.emcdda.europa.eu/system/files/publications/13838/TDAT21001ENN.pdf (accessed on 6 March 2022).
- Pichini, S.; Zaami, S.; Pacifici, R.; Tagliabracci, A.; Busardò, F.P. Editorial: The Challenge Posed by New Synthetic Opioids: Pharmacology and Toxicology. Front. Pharmacol. 2019, 10, 563. [Google Scholar] [CrossRef]
- United Nations Office on Drugs and Crime (UNODC, 2021). World Drug Report 2021-COVID-19 and Drugs: Impact Outlook. Available online: https://www.unodc.org/res/wdr2021/field/WDR21_Booklet_5.pdf (accessed on 6 March 2022).
- Singh, G.K.; Kim, I.E.; Girmay, M.; Perry, C.; Daus, G.P.; Vedamuthu, I.P.; De Los Reyes, A.A.; Ramey, C.T.; Martin, E.K.; Allender, M. Opioid Epidemic in the United States: Empirical Trends, and A Literature Review of Social Determinants and Epidemiological, Pain Management, and Treatment Patterns. Int. J. Matern. Child Health AIDS 2019, 8, 89–100. [Google Scholar] [CrossRef] [PubMed]
- Seyler, T.; Giraudon, I.; Noor, A.; Mounteney, J.; Griffiths, P. Is Europe Facing an Opioid Epidemic: What Does European Monitoring Data Tell Us? Eur. J. Pain 2021, 25, 1072–1080. [Google Scholar] [CrossRef] [PubMed]
- Häuser, W.; Buchser, E.; Finn, D.P.; Dom, G.; Fors, E.; Heiskanen, T.; Jarlbaek, L.; Knaggs, R.D.; Kosek, E.; Krcevski-Škvarč, N.; et al. Is Europe also facing an opioid crisis?—A survey of European Pain Federation chapters. Eur. J. Pain 2021, 25, 1760–1769. [Google Scholar] [CrossRef] [PubMed]
- van Amsterdam, J.; van den Brink, W. The Misuse of Prescription Opioids: A Threat for Europe? Curr. Drug Abuse Rev. 2015, 8, 3–14. [Google Scholar] [CrossRef]
- van Amsterdam, J.; Pierce, M.; van den Brink, W. Is Europe Facing an Emerging Opioid Crisis Comparable to the U.S.? Ther. Drug Monit. 2021, 43, 42–51. [Google Scholar] [CrossRef]
- Pierce, M.; van Amsterdam, J.; Kalkman, G.A.; Schellekens, A.; van den Brink, W. Is Europe facing an opioid crisis like the United States? An analysis of opioid use and related adverse effects in 19 European countries between 2010 and 2018. Eur. Psychiatry 2021, 64, e47. [Google Scholar] [CrossRef]
- Office for National Statistics. Deaths Related to Drug Poisoning in England and Wales: 2020. 2021. Available online: https://www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/deaths/bulletins/deathsrelatedtodrugpoisoninginenglandandwales/2020 (accessed on 6 March 2022).
- Dasgupta, N.; Bailey, E.J.; Cicero, T.; Inciardi, J.; Parrino, M.; Rosenblum, A.; Dart, R.C. Post-marketing surveillance of methadone and buprenorphine in the United States. Pain Med. 2010, 11, 1078–1091. [Google Scholar] [CrossRef] [Green Version]
- Veronin, M.A.; Schumaker, R.P.; Dixit, R.R.; Elath, H. Opioids and frequency counts in the US Food and Drug Administration Adverse Event Reporting System (FAERS) database: A quantitative view of the epidemic. Drug Healthc Patient Saf. 2019, 11, 65–70. [Google Scholar] [CrossRef] [Green Version]
- Blazes, C.K.; Morrow, J.D. Reconsidering the Usefulness of Adding Naloxone to Buprenorphine. Front. Psychiatry 2020, 11, 549272. [Google Scholar] [CrossRef]
- Cicero, T.J.; Ellis, M.S. Abuse-Deterrent Formulations and the Prescription Opioid Abuse Epidemic in the United States: Lessons Learned from OxyContin. JAMA Psychiatry 2015, 72, 424–430. [Google Scholar] [CrossRef] [Green Version]
- Moorman-Li, R.; Motycka, C.A.; Inge, L.D.; Congdon, J.M.; Hobson, S.; Pokropski, B. A review of abuse-deterrent opioids for chronic nonmalignant pain. P T 2012, 37, 412–418. [Google Scholar] [PubMed]
- Peacock, A.; Larance, B.; Bruno, R.; Pearson, S.A.; Buckley, N.A.; Farrell, M.; Degenhardt, L. Post-marketing studies of pharmaceutical opioid abuse-deterrent formulations: A framework for research design and reporting. Addiction 2019, 114, 389–399. [Google Scholar] [CrossRef] [PubMed]
- United Nations Office on Drugs and Crime (UNODC, 2021). SMART Update—Regional Diversity and the Impact of Scheduling on NPS Trends. Volume 25. Available online: www.unodc.org/tox (accessed on 6 March 2022).
- Goldman, S.A. Limitations and strengths of spontaneous reports data. Clin. Ther. 1998, 20, C40–C44. [Google Scholar] [CrossRef]
- Trifirò, G.; Crisafulli, S. A New Era of Pharmacovigilance: Future Challenges and Opportunities. Front. Drug Saf. Regul. 2022, 2, 2020–2023. [Google Scholar] [CrossRef]
- Schepis, T.S.; McCabe, V.V.; Boyd, C.J.; McCabe, S.E. The epidemiology of prescription fentanyl misuse in the United States. Addict. Behav. 2019, 96, 89–93. [Google Scholar] [CrossRef]
- Casati, A.; Sedefov, R.; Pfeiffer-Gerschel, T. Misuse of Medicines in the European Union: A Systematic Review of the Literature. Eur. Addict. Res. 2012, 18, 228–245. [Google Scholar] [CrossRef]
- Serdarevic, M.; Striley, C.W.; Gurka, K.K.; Leeman, R.F.; Cottler, L.B. Sex differences in prescription opioid use patterns assessed through a community engagement program in Florida. Drug Alcohol Depend. 2019, 204, 107568. [Google Scholar] [CrossRef]
- Silver, E.R.; Hur, C. Gender differences in prescription opioid use and misuse: Implications for men’s health and the opioid epidemic. Prev. Med. 2020, 131, 105946. [Google Scholar] [CrossRef]
- Han, B.; Compton, W.M.; Blanco, C.; Colpe, L.J. Prevalence, Treatment, And Unmet Treatment Needs of US Adults with Mental Health and Substance Use Disorders. Health Aff. 2017, 36, 1739–1747. [Google Scholar] [CrossRef]
- Trescot, A.M.; Datta, S.; Lee, M.; Hansen, H. Opioid Pharmacology. Pain Physician 2008, 11, S133–S153. [Google Scholar] [CrossRef]
- Cicero, T.J.; Ellis, M.S.; Paradis, A.; Ortbal, Z. Determinants of fentanyl and other potent µ opioid agonist misuse in opioid-dependent individuals. Pharmacoepidemiol. Drug Saf. 2010, 19, 1057–1063. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuczyńska, K.; Grzonkowski, P.; Kacprzak, Ł.; Zawilska, J.B. Abuse of fentanyl: An emerging problem to face. Forensic Sci Int. 2018, 289, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Schoedel, K.A.; McMorn, S.; Chakraborty, B.; Potts, S.L.; Zerbe, K.; Sellers, E.M. Positive and negative subjective effects of extended-release oxymorphone versus controlled-release oxycodone in recreational opioid users. J. Opioid Manag. 2011, 7, 179–192. [Google Scholar] [CrossRef] [PubMed]
- Zacny, J.P.; Lichtor, S.A. Within-subject comparison of the psychopharmacological profiles of oral oxycodone and oral morphine in non-drug-abusing volunteers. Psychopharmacology 2008, 196, 105–116. [Google Scholar] [CrossRef] [Green Version]
- Wightman, R.; Perrone, J.; Portelli, I.; Nelson, L. Likeability and abuse liability of commonly prescribed opioids. J. Med. Toxicol. 2012, 8, 335–340. [Google Scholar] [CrossRef]
- Remillard, D.; Kaye, A.D.; McAnally, H. Oxycodone’s Unparalleled Addictive Potential: Is it Time for a Moratorium? Curr. Pain Headache Rep. 2019, 23, 15. [Google Scholar] [CrossRef]
- Cicero, T.J.; Ellis, M.S.; Surratt, H.L.; Kurtz, S.P. Factors influencing the selection of hydrocodone and oxycodone as primary opioids in substance abusers seeking treatment in the United States. Pain 2013, 154, 2639–2648. [Google Scholar] [CrossRef]
- Morton, T.; Kostenbader, K.; Montgomery, J.; Devarakonda, K.; Barrett, T.; Webster, L. Comparison of subjective effects of extended-release versus immediate-release oxycodone/acetaminophen tablets in healthy nondependent recreational users of prescription opioids: A randomized trial. Postgrad. Med. 2014, 126, 20–32. [Google Scholar] [CrossRef]
- Kopecky, E.A.; Fleming, A.B.; Levy-Cooperman, N.; O’Connor, M.; Sellers, E.M. Oral Human Abuse Potential of Oxycodone DETERx® (Xtampza® ER). J. Clin. Pharmacol. 2017, 57, 500–512. [Google Scholar] [CrossRef]
- Balhara, Y.P.S.; Parmar, A.; Sarkar, S. Use of Tramadol for Management of Opioid Use Disorders: Rationale and Recommendations. J. Neurosci. Rural Pract. 2018, 9, 397–403. [Google Scholar] [CrossRef] [Green Version]
- Shah, K.; Stout, B.; Caskey, H. Tramadol for the Management of Opioid Withdrawal: A Systematic Review of Randomized Clinical Trials. Cureus 2020, 12, e9128. [Google Scholar] [CrossRef] [PubMed]
- Rajabizadeh, G.; Kheradmand, A.; Nasirian, M. Psychosis Following Tramadol Withdrawal. Addict. Health 2009, 1, 58–61. [Google Scholar] [PubMed]
- Abou Taam, M.; de Boissieu, P.; Abou Taam, R.; Breton, A.; Trenque, T. Drug-Induced Hallucination: A Case/Non Case Study in the French Pharmacovigilance Database. Eur. J. Psychiatry 2015, 29, 21–31. [Google Scholar] [CrossRef] [Green Version]
- Jean, Y.K.; Gitlin, M.C.; Reynolds, J.; Candiotti, K.A. Tramadol-associated hallucinations: A systematic review and narrative synthesis of their pathophysiology, diagnosis, and treatment. Can. J. Anaesth. 2020, 67, 360–368. [Google Scholar] [CrossRef]
- Adverse Drug Reactions Advisory Committee (ADRAC). Tramadol—Four Years’ Experience. Aust. Adv. Drug React. Bull. 2003, 22, 2–3. [Google Scholar]
- Faller, R.W.; Toller Erausquin, J.; McCoy, T.P. Misuse of Prescription and Illicit Drugs in Middle Adulthood in the Context of the Opioid Epidemic. Subst. Use Misuse 2021, 56, 333–337. [Google Scholar] [CrossRef]
- Mccance-Katz, E.F.; Webcast Slides for the 2019 National Survey on Drug Use and Health, no. September: 1–63. 2020. Available online: https://www.samhsa.gov/data/sites/default/files/reports/rpt29392/Assistant-Secretary-nsduh2019_presentation/Assistant-Secretary-nsduh2019_presentation.pdf (accessed on 6 March 2022).
- Compton, W.M.; Valentino, R.J.; DuPont, R.L. Polysubstance Use in the U.S. Opioid Crisis. Mol. Psych. 2021, 26, 41–50. [Google Scholar] [CrossRef]
- European Monitoring Centre for Drugs and Drug Addiction (EMCDDA, 2019). Drug-Related Deaths and Mortality in Europe’. Publications Office of the European Union, no. July: 28. Available online: https://dataunodc.un.org/Drugs/Mortality/Europe (accessed on 6 March 2022).
- Fischer, B.; Jürgen, R. Deaths Related to the Use of Prescription Opioids. CMAJ 2009, 181, 881–882. [Google Scholar] [CrossRef] [Green Version]
- Elzey, M.J.; Barden, S.M.; Edwards, E.S. Patient Characteristics and Outcomes in Unintentional, Non-fatal Prescription Opioid Overdoses: A Systematic Review. Pain Physician 2016, 19, 215–228. [Google Scholar] [CrossRef]
- Floyd, C.N.; Warren, J.B. Opioids out of Control. Br. J. Clin. Pharmacol. 2018, 84, 813–815. [Google Scholar] [CrossRef]
- Wolff, C.; Dowd, W.N.; Ali, M.M.; McClellan, C.; Meinhofer, A.; Glos, L.; Mutter, R.; Rosenberg, M.; Schick, A. The impact of the abuse-deterrent reformulation of extended-release OxyContin on prescription pain reliever misuse and heroin initiation. Addict. Behav. 2020, 105, 106268. [Google Scholar] [CrossRef] [PubMed]
- Knight, K.R.; Kushel, M.; Chang, J.S.; Zamora, K.; Ceasar, R.; Hurstak, E.; Miaskowski, C. Opioid pharmacovigilance: A clinical-social history of the changes in opioid prescribing for patients with co-occurring chronic non-cancer pain and substance use. Soc. Sci. Med. 2017, 186, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Postigo, R.; Brosch, S.; Slattery, J.; van Haren, A.; Dogné, J.M.; Kurz, X.; Candore, G.; Domergue, F.; Arlett, P. EudraVigilance Medicines Safety Database: Publicly Accessible Data for Research and Public Health Protection. Drug Saf. 2018, 41, 665–675. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- World Health Organization (WHO). Reporting and Learning Systems for Medication Errors: The Role of Pharmacovigilance Centres. 2014. Available online: https://apps.who.int/iris/handle/10665/137036 (accessed on 6 March 2022).
- Alomar, M.; Tawfiq, A.M.; Hassan, N.; Palaian, S. Post Marketing Surveillance of Suspected Adverse Drug Reactions through Spontaneous Reporting: Current Status, Challenges and the Future. Ther. Adv Drug Saf. 2020, 11, 2042098620938595. [Google Scholar] [CrossRef]
- Evans, S.J.; Waller, P.C.; Davis, S. Use of proportional reporting ratios (PRRs) for signal generation from spontaneous adverse drug reaction reports. Pharmacoepidemiol. Drug Saf. 2001, 10, 483–486. [Google Scholar] [CrossRef]
- Mann, R.D.; Andrews, E.B. Pharmacovigilance. In Pharmacovigilance, 2nd ed.; John Wiley & Sons: West Sussex, UK, 2007. [Google Scholar]
- Schifano, F.; Napoletano, F.; Chiappini, S.; Guirguis, A.; Corkery, J.M.; Bonaccorso, S.; Ricciardi, A.; Scherbaum, N.; Vento, A. New/Emerging Psychoactive Substances and Associated Psychopathological Consequences. Psychol. Med. 2021, 51, 30–42. [Google Scholar] [CrossRef] [Green Version]
- Schifano, F.; Chiappini, S.; Miuli, A.; Corkery, J.M.; Scherbaum, N.; Napoletano, F.; Arillotta, D.; Zangani, C.; Catalani, V.; Vento, A.; et al. New Psychoactive Substances (NPS) and Serotonin Syndrome Onset: A Systematic Review. Exp. Neurol. 2021, 339, 113638. [Google Scholar] [CrossRef]
- Schifano, F.; Corkery, J.; Ghodse, A.H. Suspected and confirmed fatalities associated with mephedrone (4-methylmethcathinone, “meow meow”) in the United Kingdom. J. Clin. Psychopharmacol. 2012, 32, 710–714. [Google Scholar] [CrossRef]
- Schifano, F.; Napoletano, F.; Arillotta, D.; Zangani, C.; Gilgar, L.; Guirguis, A.; Corkery, J.M.; Vento, A. The clinical challenges of synthetic cathinones. Br. J. Clin. Pharmacol. 2020, 86, 410–419. [Google Scholar] [CrossRef]
- Corkery, J.M.; Streete, P.; Claridge, H.; Goodair, C.; Papanti, D.; Orsolini, L.; Schifano, F.; Sikka, K.; Körber, S.; Hendricks, A. Characteristics of deaths associated with kratom use. J. Psychopharmacol. 2019, 33, 1102–1123. [Google Scholar] [CrossRef]
- Schifano, F.; Chiappini, S. Is There Such a Thing as a “lope” dope? Analysis of Loperamide-Related European Medicines Agency (EMA) Pharmacovigilance Database Reports’. PLoS ONE 2018, 13, e0204443. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grond, S.; Sablotzki, A. Clinical Pharmacology of Tramadol. Clin. Pharmacokinet. 2004, 43, 879–923. [Google Scholar] [CrossRef] [PubMed]
- Orsolini, L.; Corkery, J.M.; Chiappini, S.; Guirguis, A.; Vento, A.; De Berardis, D.; Papanti, D.; Schifano, F. New/Designer Benzodiazepines: An Analysis of the Literature and Psychonauts Trip Reports. Curr. Neuropharmacol. 2020, 18, 809–837. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency (2017). Module VI—Collection, Management and Submission of Reports of Suspected Adverse Reactions to Medicinal Products (Rev 2). Guideline on Good Pharmacovigilance Practices (GVP) Revision 2 (July): 144. Available online: http://www.ema.europa.eu/docs/en_GB/document_library/Regulatory_and_procedural_guideline/2017/08/WC500232767.pdf (accessed on 6 March 2022).
- U.S. Food & Drug Administration (FDA, 2021). FDA Adverse Event Reporting System (FAERS) Public Dashboard. U.S. Food & Drug Administration. 2021. Available online: https://www.fda.gov/drugs/questions-and-answers-fdas-adverse-event-reporting-system-faers/fda-adverse-event-reporting-system-faers-public-dashboard (accessed on 6 March 2022).
- Ahmed, I.; Poncet, A. PhViD: An R Package for PharmacoVigilance Signal Detection. R Package Version 1.0.6., no. December. Available online: https://cran.r-project.org/web/packages/PhViD/PhViD.pdf (accessed on 6 March 2022).
- Subeesh, V.; Maheswari, E.; Saraswathy, G.R.; Swaroop, A.M.; Minnikanti, S.S. A Comparative Study of Data Mining Algorithms Used for Signal Detection in FDA AERS Database. J. Young Pharm. 2018, 10, 444–449. [Google Scholar] [CrossRef] [Green Version]
- Poluzzi, E.; Raschi, E.; Piccinni, C.; De Ponti, F. Data Mining Techniques in Pharmacovigilance: Analysis of the Publicly Accessible FDA Adverse Event Reporting System (AERS). In Data Mining Applications in Engineering and Medicine; IntechOpen: London, UK, 2012. [Google Scholar]
- Suling, M.; Pigeot, I. Signal detection and monitoring based on longitudinal healthcare data. Pharmaceutics 2012, 4, 607–640. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, I.; Thiessard, F.; Miremont-Salam, G.; Haramburu, F.; Kreft-Jais, C.; Bgaud, B.; Tubert-Bitter, P. Early Detection of Pharmacovigilance Signals with Automated Methods Based on False Discovery Rates: A Comparative Study. Drug Saf. 2012, 35, 495–506. [Google Scholar] [CrossRef]
- Campbell, G.; Lintzeris, N.; Gisev, N.; Larance, B.; Pearson, S.; Degenhardt, L. Regulatory and other responses to the pharmaceutical opioid problem. Med. J. Aust. 2019, 210, 6–8.e1. [Google Scholar] [CrossRef]
- Fermont, I. Pharmacovigilance Strategy: Opportunities for Cross-National Learning. Isr. J. Health Pol. Res. 2019, 8, 1–5. [Google Scholar] [CrossRef]
- Throckmorton, D.C.; Gottlieb, S.; Woodstock, J. The FDA and the Next Wave of Drug Abuse—Proactive Pharmacovigilance. N. Engl. J. Med. 2018, 379, 205–207. [Google Scholar] [CrossRef]
- Medawar, C.; Herxheimer, A. A Comparison of Adverse Drug Reaction Reports from Professionals and Users, Relating to Risk of Dependence and Suicidal Behaviour with Paroxetine. Intern J. Risk Saf. Med. 2003, 6, 5–19. [Google Scholar]
- ICH. MedDRA ® TERM SELECTION: POINTS TO CONSIDER. ICH-Endorsed Guide for MedDRA Users. London Release 4. (Version 17.1): 1–49. 2014. Available online: https://www.meddra.org/how-to-use/support-documentation/english (accessed on 6 March 2022).
- Vickers-Smith, R.; Sun, J.; Charnigo, R.J.; Lofwall, M.R.; Walsh, S.L.; Havens, J.R. Gabapentin drug misuse signals: A pharmacovigilance assessment using the FDA adverse event reporting system. Drug Alcohol Depend. 2020, 206, 107709. [Google Scholar] [CrossRef] [PubMed]
- van Puijenbroek, E.P.; Bate, A.; Leufkens, H.G.; Lindquist, M.; Orre, R.; Egberts, A.C. A comparison of measures of disproportionality for signal detection in spontaneous reporting systems for adverse drug reactions. Pharmacoepidemiol. Drug Saf. 2002, 11, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, I.; Dalmasso, C.; Haramburu, F.; Thiessard, F.; Broët, P.; Tubert-Bitter, P. False Discovery Rate Estimation for Frequentist Pharmacovigilance Signal Detection Methods. Biometrics 2010, 66, 301–309. [Google Scholar] [CrossRef] [PubMed]
| ADR Report Characteristics | Codeine | Dihydrocodeine | Fentanyl | Oxycodone | Pentazocine | Tramadol | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| EMA | FAERS | EMA | FAERS | EMA | FAERS | EMA | FAERS | EMA | FAERS | EMA | FAERS | |
| Individual cases | 814 | 6764 | 53 | 575 | 5443 | 54,640 | 7441 | 45,672 | 136 | 112 | 2619 | 22,530 |
| Mean age in years (SD) | 38.3 (13.6) | 50.7 (19.6) | 37.9 (12.7) | 43.4 (22.2) | 43.3 (16.0) | 53.2 (19.2) | 38.0 (13.6) | 45.6 (18.2) | 46.3 (16.5) | 51.4 (21.1) | 42.7 (15.7) | 52.8 (20.4) |
| M (%) | 73.8% (540) 26.2% (192) | 32.2% (1983) | 36.2% (17) | 48.2% (244) | 53.0% (2459) | 40.5% (19,354) | 61.4% (3929) | 54.2% (22,504) | 20.7% (28) | 51.9% (54) | 48.9% (1142) | 38.7% (7890) |
| F (%) | 67.8% (4167) | 63.8% (30) | 51.8% (262) | 47.0% (2178) | 59.5% (28,382) | 38.6% (2468) | 45.8% (19,036) | 79.3% (107) | 48.1% (50) | 51.1% (1195) | 61.3% (12,479) | |
| Most common | Drug abuse (1.9%) | Pain (7.2%) | Pain (20.0%) | Pain (12.3%) | Pain (25.0%) | Pain (31.0%) | Drug abuse (15.3%) | Pain (30.5%) | Pain (24.4%) | Pain (17.3%) | Pain (18.9%) | Pain (21.6%) |
| indications recorded for the index opioid when reported (%) | Pain (1.6%) | Rheumatoid arthritis (4.9%) | Procedural pain (10.0%) | Back Pain (5.9%) | Intentional product misuse (7.3%) | Back pain (9.1%) | Pain (13.8%) | Back Pain (5.8%) | Drug abuse (7.7%) | Analgesic therapy (14.3%) | Back pain (7.8%) | Back pain (6.8%) |
| Cough (1.4%) | Cough (2.6%) | Drug dependence (6.7%) | Rheumatoid arthritis (5.4%) | Back pain (4.7%) | Cancer pain (6.2%) | Back pain (4.7%) | Drug abuse (4.0%) | Migraine (3.8%) | Drug abuse (8.2%) | Headache (2.7%) | Depression (6.1%) | |
| ROA (%) | Oral (26.9%) | Oral (32.2%) | Oral (63.0%) | Oral (40.1%) | Transdermal (44.9%) | Transdermal (75.0%) | Oral (56.0%) | Oral (76.1%) | Intravenous (70.0%) | Intramuscular (32.7%) | Oral (86.5%) | Oral (63.9%) |
| Parenteral (9.0%) | Parenteral (2.3%) | Parenteral (0%) | Transplacental (16.5%) | Oral (22.6%) | Intravenous (6.0%) | Intravenous (3.2%) | Intravenous (1.3%) | Intramuscular (19.2%) | Intravenous (32.7%) | Intravenous (0.8%) | Intravenous (2.1%) | |
| Nasal/inhalation (1.8%) | Transplacental (1.3%) | Nasal/inhalation (0%) | Intrauterine (0.6%) | Intravenous (4.6%) | Oral (3.6%) | Nasal/inhalation (2.5%) | Nasal/inhalation (1.0%) | Oral (2.5%) | Oral (7.3%) | Parenteral (0.3%) | Transplacental (1.0%) | |
| Intravenous (0.6%) | Intravenous (0.6%) | Intravenous (0%) | Parenteral (3.7%) | Intrathecal (1.4%) | Parenteral (0.4%) | Transplacental (0.5%) | Parenteral (2.5%) | Parenteral (7.3%) | Oropharyngeal (0.5%) | |||
| Rectal (0.2%) | Nasal/inhalation (0.4%) | Rectal (0%) | Nasal/inhalation (3.1%) | Topical (1.1%) | Rectal (0%) | Parenteral (0.3%) | Subcutaneous (5.5%) | Intramuscular (0.3%) | ||||
| Fatal outcome (%) | 69.50% | 29.70% | 24.50% | 32.70% | 46.80% | 21.00% | 31.30% | 36.90% | 1.50% | 13.40% | 21.70% | 22.40% |
| Most important concomitant prescription psychotropic drugs recorded | ||||||||||||
| Antidepressants (%) | 20.90% | 23.40% | 9.40% | 47.10% | 14.30% | 11.10% | 13.70% | 13.20% | 1.50% | 9.80% | 17.60% | 26.60% |
| Antipsychotics (%) | 5.20% | 6.60% | 9.40% | 21.40% | 2.70% | 2.90% | 3.30% | 4.10% | 1.50% | 7.10% | 3.20% | 6.60% |
| Benzodiazepines (%) | 31.20% | 19.60% | 24.50% | 35.10% | 18.20% | 13.60% | 23.00% | 18.80% | 5.10% | 27.70% | 15.40% | 18.20% |
| Gabapentinoids (%) | 2.20% | 9.40% | 1.90% | 20.30% | 5.00% | 5.60% | 3.20% | 6.20% | 0.70% | 1.80% | 4.30% | 12.30% |
| Mood Stabilizers (%) | 2.00% | 5.20% | 0% | 12.30% | 2.20% | 2.20% | 1.60% | 2.50% | 0.70% | 1.80% | 2.40% | 5.40% |
| OTCs (%): | ||||||||||||
| Anticholinergics (%) | 1.40% | 2.50% | 3.40% | 1.60% | 0.70% | 2.20% | 0.40% | 1.20% | 0% | 9.80% | 0.90% | 2.70% |
| Antihistamines (%) | 19.70% | 12.10% | 9.40% | 0% | 6.00% | 3.70% | 8.70% | 5.30% | 5.10% | 33.90% | 5.60% | 9.00% |
| Dextromethorphan (%) | 12.50% | 3.00% | 0% | 0.30% | 0.70% | 0.20% | 1.50% | 0.60% | 0% | 0% | 1.50% | 0.40% |
| Loperamide (%) | 0% | 0.80% | 0% | 0.30% | 0.10% | 0.10% | 0.10% | 0.20% | 0% | 0.90% | 0.20% | 0.50% |
| Paracetamol/Acetaminophen (%) | 14.30% | 17.50% | 3.80% | 25.10% | 3.00% | 2.70% | 5.50% | 5.70% | 2.20% | 8.90% | 5.80% | 14.00% |
| Pseudoephedrine and Pseudoephedrine-Containing Products (%) | 0.40% | 0.90% | 0% | 0% | 0.10% | 0% | 0.30% | 0.20% | 0% | 0% | 0.10% | 0.20% |
| Other Opioids (%) | 67.60% | 39.70% | 20.80% | 37.40% | 21.50% | 43.00% | 31.00% | 22.80% | 5.90% | 14.30% | 16.60% | 16.70% |
| Z-Drugs (%) | 4.20% | 4.10% | 3.80% | 2.40% | 2.70% | 2.10% | 2.50% | 2.90% | 0.70% | 5.40% | 2.60% | 5.60% |
| Most important concomitant recreational drugs recorded | ||||||||||||
| Alcohol (%) | 8.10% | 3.60% | 11.30% | 8.70% | 3.10% | 0.90% | 8.70% | 4.20% | 2.20% | 0.90% | 3.60% | 2.60% |
| Amphetamines and Methamphetamines (%) | 4.50% | 2.80% | 3.40% | 1.90% | 1.70% | 0.40% | 3.80% | 1.70% | 0% | 0% | 1.50% | 0.90% |
| Cannabis and Cannabinoids (%) | 2.70% | 1.00% | 0% | 0.50% | 1.10% | 0.30% | 4.70% | 1.80% | 0.70% | 0.90% | 1.50% | 0.50% |
| Cocaine (%) | 19.30% | 4.40% | 1.90% | 0.70% | 3.50% | 0.80% | 8.80% | 3.20% | 0% | 0% | 2.60% | 0.90% |
| Hallucinogens (%) | 2.00% | 0.60% | 0% | 0.70% | 0.10% | 0.10% | 0.90% | 0.40% | 0% | 0% | 0.70% | 0.20% |
| Heroin (%) | 0% | 9.10% | 0% | 4.00% | 0% | 1.00% | 0% | 1.80% | 0% | 0.90% | 0% | 0.40% |
| Ketamine (%) | 0.40% | 0.10% | 0% | 0.30% | 0.20% | 0.30% | 0.20% | 0.10% | 0% | 0% | 0% | 0.20% |
| NPS (%) | 0% | 0.10% | 0% | 0% | 0% | 0.00% | 0% | 0.00% | 0% | 0% | 0.20% | 0.10% |
| Preferred Term (PT) | Codeine | Dihydrocodeine | Fentanyl | Oxycodone | Pentazocine | Tramadol |
|---|---|---|---|---|---|---|
| PRR (FDR) | PRR (FDR) | PRR (FDR) | PRR (FDR) | PRR (FDR) | PRR (FDR) | |
| Misuse-/Abuse-Related Terms | ||||||
| Drug Abuse | ||||||
| EV | 1.94 (<0.01) | 0.90 (0.44) | 0.93 (0.71) | 0.91 (0.70) | 2.23 (<0.01) | 1.01 (0.02) |
| FAERS | 1.96 (<0.01) | 0.32 (0.41) | 0.40 (0.43) | 2.48 (<0.01) | 1.17 (0.05) | 0.62 (0.43) |
| Drug Abuser | ||||||
| EV | NA | NA | 0.31 (0.68) | 2.52 (<0.01) | NA | 0.65 (0.49) |
| FAERS | 0.17 (0.42) | NA | 0.13 (0.43) | 10.17 (<0.01) | NA | 0.29 (0.43) |
| Drug Diversion | ||||||
| EV | 0.88 (0.26) | NA | 2.30 (<0.01) | 0.72 (0.68) | NA | 0.18 (0.71) |
| FAERS | NA | 2.12 (<0.01) * | 1.70 (<0.01) | 1.17 (<0.01) | NA | 0.25 (0.42) |
| Drug Use Disorder | ||||||
| EV | NA | NA | NA | NA | NA | NA |
| FAERS | NA | NA | 1.25 (0.07) | NA | NA | 2.81 (<0.01) * |
| Intentional Product Misuse | ||||||
| EV | 2.23 (<0.01) | 1.35 (<0.01) * | 2.20 (<0.01) | 0.33 (0.70) | 0.34 (0.68) | 1.24 (<0.01) |
| FAERS | 1.25 (<0.01) | 1.18 (0.03) * | 1.09 (<0.01) | 1.07 (<0.01) | NA | 0.72 (0.42) |
| Substance Abuse | ||||||
| EV | 1.11 (<0.01) * | NA | 0.09 (0.70) | 8.84 (<0.01) | NA | 0.14 (0.70) |
| FAERS | 0.91 (0.23) | 0.70 (0.25) | 0.03 (0.43) | 17.61 (<0.01) | NA | 0.13 (0.43) |
| Substance Use | ||||||
| EV | NA | NA | NA | NA | NA | NA |
| FAERS | NA | NA | 0.53 (0.31) | NA | NA | 3.51 (<0.01) |
| Dependence-Related Terms | ||||||
| Dependence | ||||||
| EV | 0.92 (0.27) | NA | 1.13 (<0.01) | 0.17 (0.70) | NA | 5.38 (<0.01) |
| FAERS | 0.98 (0.14) | NA | 0.92 (0.23) | 0.64 (0.39) | NA | 1.88 (<0.01) |
| Drug Dependence | ||||||
| EV | 0.78 (0.69) | 1.24 (<0.01) * | 0.21 (0.70) | 2.75 (<0.01) | 0.70 (0.52) | 0.99 (0.22) |
| FAERS | 0.24 (0.43) | 0.30 (0.40) | 0.09 (0.43) | 11.53 (<0.01) | 1.56 (<0.01) * | 0.31 (0.43) |
| Substance Dependence | ||||||
| EV | NA | NA | 0.13 (0.70) | 13.19 (<0.01) | NA | NA |
| FAERS | NA | NA | 0.04 (0.42) | 53.88 (<0.01) | NA | NA |
| Withdrawal-Related Terms | ||||||
| Drug Withdrawal Syndrome | ||||||
| EV | 0.22 (0.70) | 0.81 (0.39) | 0.66 (0.70) | 1.92 (<0.01) | 0.57 (0.55) | 0.65 (0.70) |
| FAERS | 0.19 (0.42) | NA | 0.68 (0.43) | 2.82 (<0.01) | NA | 0.32 (0.43) |
| Overdose and Off-Label-Use Terms | ||||||
| Intentional Overdose | ||||||
| EV | 1.68 (<0.01) * | NA | 0.47 (0.71) | 0.53 (0.71) | NA | 4.00 (<0.01) |
| FAERS | 2.03 (<0.01) | 2.39 (<0.01) | 0.14 (0.43) | 1.48 (<0.01) | NA | 2.49 (<0.01) |
| Off-Label Use | ||||||
| EV | 0.88 (0.24) | NA | 4.67 (<0.01) | 0.28 (0.70) | NA | 0.37 (0.71) |
| FAERS | 0.59 (0.40) | 1.70 (<0.01) | 2.74 (<0.01) | 0.44 (0.43) | NA | 0.57 (0.42) |
| Overdose | ||||||
| EV | 0.93 (0.32) | 1.78 (<0.01) * | 1.02 (0.02) | 0.77 (0.70) | NA | 1.55 (<0.01) |
| FAERS | 0.96 (0.23) | 1.53 (<0.01) | 0.51 (0.43) | 2.24 (<0.01) | NA | 0.72 (0.42) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chiappini, S.; Vickers-Smith, R.; Guirguis, A.; Corkery, J.M.; Martinotti, G.; Harris, D.R.; Schifano, F. Pharmacovigilance Signals of the Opioid Epidemic over 10 Years: Data Mining Methods in the Analysis of Pharmacovigilance Datasets Collecting Adverse Drug Reactions (ADRs) Reported to EudraVigilance (EV) and the FDA Adverse Event Reporting System (FAERS). Pharmaceuticals 2022, 15, 675. https://doi.org/10.3390/ph15060675
Chiappini S, Vickers-Smith R, Guirguis A, Corkery JM, Martinotti G, Harris DR, Schifano F. Pharmacovigilance Signals of the Opioid Epidemic over 10 Years: Data Mining Methods in the Analysis of Pharmacovigilance Datasets Collecting Adverse Drug Reactions (ADRs) Reported to EudraVigilance (EV) and the FDA Adverse Event Reporting System (FAERS). Pharmaceuticals. 2022; 15(6):675. https://doi.org/10.3390/ph15060675
Chicago/Turabian StyleChiappini, Stefania, Rachel Vickers-Smith, Amira Guirguis, John M. Corkery, Giovanni Martinotti, Daniel R. Harris, and Fabrizio Schifano. 2022. "Pharmacovigilance Signals of the Opioid Epidemic over 10 Years: Data Mining Methods in the Analysis of Pharmacovigilance Datasets Collecting Adverse Drug Reactions (ADRs) Reported to EudraVigilance (EV) and the FDA Adverse Event Reporting System (FAERS)" Pharmaceuticals 15, no. 6: 675. https://doi.org/10.3390/ph15060675
APA StyleChiappini, S., Vickers-Smith, R., Guirguis, A., Corkery, J. M., Martinotti, G., Harris, D. R., & Schifano, F. (2022). Pharmacovigilance Signals of the Opioid Epidemic over 10 Years: Data Mining Methods in the Analysis of Pharmacovigilance Datasets Collecting Adverse Drug Reactions (ADRs) Reported to EudraVigilance (EV) and the FDA Adverse Event Reporting System (FAERS). Pharmaceuticals, 15(6), 675. https://doi.org/10.3390/ph15060675










