Hematological and Extra-Hematological Autoimmune Complications after Checkpoint Inhibitors
Abstract
:1. Introduction
2. Hematological Autoimmune Complications
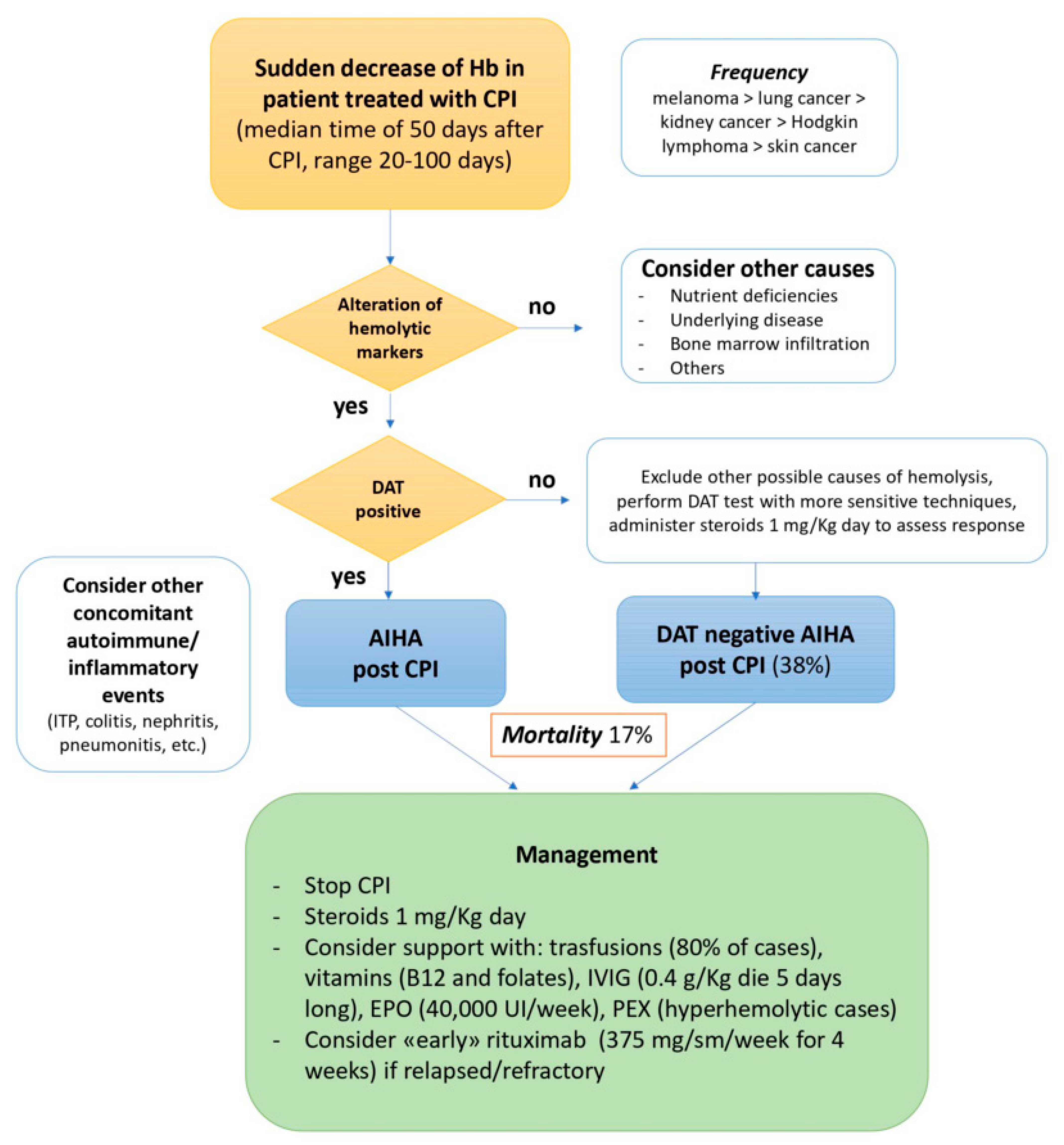
2.1. Autoimmune Hemolytic Anemia
2.2. Other Hematological Toxicities
| References | Type of Study | Patients | Frequency | Main Findings | CPI Interruption |
|---|---|---|---|---|---|
| Delanoy N et al. (2019) [17] | Observational study | 745 | 3.7% | The most-frequent hematologic IRAEs after anti-PD-1 or anti-PD-L1 were AIHA, ITP or neutropenia (26%), followed by pancytopenia or aplastic anemia (14%). The median time of onset was 10 weeks; most events were grade 4 and resolved after immunosuppressive therapy. | 80% of the cases 20% rechallenge |
| Michot JM et al. (2019) [19] | Review | 63 | 3.6% | An incidence of 0.7% for grades 3 to 4 IRAEs, mostly immune cytopenias (17 to 29%), aplastic anemia (19%) and HLH (11%). The median time of onset was of 10 weeks. Resolution varied from 25% for aplastic anemia to 80% for ITP and AIHA, and 14% died. The risk of recurrence after CPI rechallenge was around 50%. | Not reported |
| Davis E.J. et al. (2019) [20] | Observational study | 164 | 1% (among all reported adverse events) | AIHA was the most common, mostly associated with melanoma and lung cancer; 23% had an extra-hematological IRAEs; mortality was 11% but increased to 23% in the case of HLH. | Not reported |
| Zaremba A. et al. (2021) [18] | Observational study | 6961 | 0.14% | 10 patients experienced grade 4 neutropenia (60% possibly due to metamizole), with median time of onset of 6.4 weeks; 40% required systemic steroids, and neutropenia responded to G-CSF. No recurrence was reported after CPI rechallenge. | 70% |
| Kramer R et al. (2021) [16] | Observational study | 7626 | 0.6% | Mostly autoimmune cytopenias (28–34%), rarely HLH (4%), aplastic anemia (2%), coagulation dysfunction (2%) and acquired hemophilia A (2%). The median time of onset was 25 weeks. 60% required hospitalization, and 80% had complete resolution. AIHA and ITP tended to persist. | 60% |
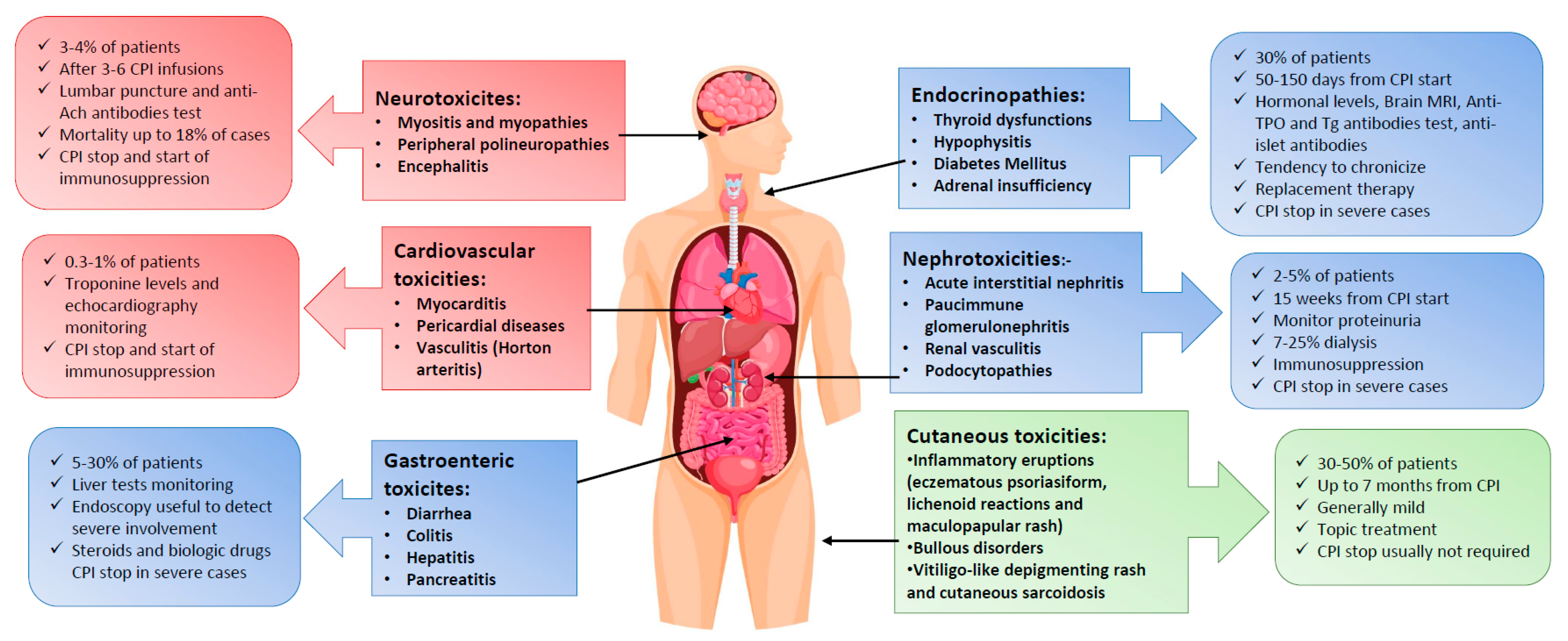
3. Extra-Hematological Toxicities after Checkpoint-Inhibitors
3.1. Immune-Related Endocrinopathies
| References | Type of Study | Patients | Frequency | Main Findings | CPI Interruption |
|---|---|---|---|---|---|
| Faje AT et al. (2014) [24] | Observational study | 154 | 11% | Immune hypophysitis in melanoma patients after Ipilimumab in a dose-dependent manner. Brain MRI may detect pituitary enlargement in symptomatic patients. Hormone deficiencies may persist. | Not reported |
| Morganstein D. et al. (2017) [27] | Observational study | 191 | 23% with anti-CTLA4, 39% with anti-PD-1 50% if in combination | Thyroid IRAEs occurred after a median of 30–60 days, more frequently in males. A hyperthyroidic phase followed by hypothyroidism is mainly observed. Altered TSH before treatment may be a predictor. | Not reported |
| Osorio J. et al. (2017) [29] | Observational study | 51 | 21% | Lung cancer patients treated with pembrolizumab with anti-thyroid antibodies were at higher risk of thyroid IRAEs. A biphasic pattern (hyperthyroidism followed by hypothyroidism) was described and replacement therapy was needed. | 0% |
| Garon-Czmil J. et al. (2019) [26] | Observational study | 249 | 37% among endocrine ir-AEs | Hypophysitis was more frequent with Ipilimumab, after 80 to 160 days; brain MRI may show pituitary enlargement. Nearly all patients required hydrocortisone supplementation (90%) and 20% thyroid hormones. | 1 patient |
| Faje, A. et al. (2019) [25] | Observational study | 22 | 0.5% anti-PD1 13.6% anti-CTLA4 | Hypophysitis developed after 77 to 500 days. Symptoms were more subtle after anti-PD1 (fatigue, loss of appetite and myalgias/arthralgias) versus anti-CTLA4. Brain MRI was not informative. | 5 patients |
| Presotto E.M. et al. (2020) [28] | Observational study | 179 | 30.2% | Thyroid alterations occurred in 29.6%. Pre-existing thyroid dysfunction was a risk factor. IRAE occurred within 2 months and 75.5% of cases required replacement therapy. | Not reported |
| Kotwal A. et al. (2020) [30] | Observational study | 91 | 25% | TPO antibodies were detected only in 22% of patients with thyroid IRAEs. Higher TPO titer may be related to more severe thyroid dysfunction. Longer time from thyrotoxicosis to hypothyroidism was described as compared to other thyroid disorders. | 0% |
| Quandt, Z. et al. (2020) [31] | Review | 53 | 0-2-1.4% | Diabetes mellitus was most frequent with anti-PD1/PD-L1, after 7–17 weeks (shorter in patients with anti-islet antibodies). Steroids worsened insulin resistance. | Not reported |
| Rubino R. et al. (2021) [22] | Observational study | 251 | 27.89% | Thyroid IRAEs were the most frequent and may be predicted by pre-existing endocrinopathy. Female were more affected and required replacement in 45%. A correlation between IRAEs and a better outcome (PFS and OS) was reported. | 25% |
| Muniz et al. (2021) [32] | Observational study | 34 | Not reported | Diabetes mellitus developed after a median of 2.4 months and was more frequent with anti-PD1/PDL1. 62% of patients had an acute onset with ketoacidosis with a mortality of 5%, and some became chronic. All patients were treated with insulin therapy and in 12% with immunosuppressive therapy. | 56% |
3.2. Cutaneous Adverse Events
| References | Type of Study | Patients | Frequency | Main Findings | CPI Interruption |
|---|---|---|---|---|---|
| Naidoo J et al. (2016) [38] | Case series | 3 | Not reported | Bullous Pemphigoides (BP) on anti–PD1/PDL1 inhibitors may occur after several months and may be accompanied or preceded by pruritus. Discontinuation of CPI may not determine resolution of BP. | 100% |
| Hwang SJ et al. (2016) [33] | Observational study | 82 | 49% | Cutaneous IRAEs included lichenoid reaction (17%), eczema (17%) and vitiligo (15%) in melanoma patients with anti-PD1/PD-L1. | Not reported |
| Siegel J et al. (2018) [36] | Observational study | 853 | 1% | BP occurred after anti-PD1/PDL1 CPIs and had mucosal involvement in 30%; may be determined by autoantibodies against hemidesmosome protein BP180. Steroids were recommended if > 30% of body surface was involved. | 1% |
| Lee YJ et al. (2019) [37] | Observational study | 211 | 16,4% | Pruritus was reported as the main manifestation, followed by eczema and maculopapular rash, after a median onset of 50 days. Longer PFS may occur in such patients. | 0% |
| Chan L. et al. (2020) [40] | Observational study | 82 | 40% | Cutaneous IRAEs occurred after a median of 6 months. Longer PFS may occur in patients experiencing cutaneous IRAEs | Not reported |
3.3. Gastroenteric Side Effects
| References | Type of Study | Patients | Frequency | Main Findings | CPI Interruption |
|---|---|---|---|---|---|
| Abu-Sbeih H et al. (2018) [43] | Retrospective study | 182 | 43% grade 3/4 diarrhea 32.4% grade 3/4 colitis | Grade 3 colitis affected mostly left colon; at endoscopy one third showed ulcerative pattern. 77.5% patients required immunosuppressant treatment. All patients reached clinical remission and 30% histological remission. The recurrence of colitis occurred in 28% of subjects. | 66% |
| Geukes Foppen et al. (2018) [42] | Systematic review and meta-analysis | 92 | 56% with anti-CTLA4 22% with anti-PD1 | In 44% of cases, diarrhea was grade 3 and 30% had ulcers at endoscopy; half of patients was refractory to steroids and required Infliximab. The presence of ulcers and pancolitis (≥3 affected colon segments) predicted refractoriness to steroids. | Not reported |
| Cheung et al. (2020) [43] | Retrospective study | 134 | 10% | Higher risk of colitis with combination therapy (anti-PD1/PD-L1 and anti-CTLA4 inhibitors). No predictors; 23% of patients were rescued with Infliximab due to erosions; earlier administration does not seem beneficial. | Not reported |
| Bellaguarda et al. (2020) [41] | Systematic review | Not reported | 30.2–35.4% after anti-CTLA4 12.1–13.7% after anti-PD1 | The median onset time of gastroenteric toxicities was 4 weeks with anti-CTLA4 and 2–4 months with anti-PD1/PD-L1. Supportive therapies, CPI discontinuation, systemic steroids (effective in 85% of patients) and biological drugs (Infliximab and vedolizumab) were used. | Grade 3 temporarily discontinuation Grade 4 permanently discontinuation |
| Riveiro-Barciela et al. (2020) [46] | Retrospective study | 414 | 6.8% | Severe hepatitis resulted in acute liver failure in 7.7% of cases. Mostly related to anti-PD1/PD-L1 agents, after a median of 12 weeks. All were treated with steroids, and 35.7% required a second line. No recurrence after CPI rechallenge. | 100% |
3.4. Neuromuscular Complications
| References | Type of Study | Patients | Frequency | Main Findings | CPI Interruption |
|---|---|---|---|---|---|
| Möhn, N et al. (2019) [50] | Systematic review | 81 | 3.8% with anti-CTLA4 6.1% with anti-PD1 | Myasthenia and Guillain–Barrè syndromes (GBS) were the most common, followed by peripheral polyneuropathies. Complete response occurred in 37.2% of cases. | Not reported |
| Galmiche S et al. (2019) [51] | Case series | 5 | Not reported | Encephalitis manifested with headaches, confusion, ataxia, anisocoria and/or dysarthria and meningeal symptoms, with negative CSF and brain MRI findings. The median time of onset was 42 days and required early discontinuation of CPI and prompt immunosuppression. Mortality rate reached 18%. | 100% |
| Liewluck T. et al. (2018) [49] | Observational study | 654 | 0.76% | Pembrolizumab-related myopathies mostly affected oculobulbar muscles. AChR antibodies were detected in 50%. Overall, non-necrotizing myopathy responded well to immunosuppressive therapies. Evaluation of myocardium involvement is recommended. | 100% |
| Moreira A. et al. (2019) [48] | Observational study | 38 | Not reported | Myositis occurred at median of 19 weeks after CPI start, often with oculomotor symptoms and usually preceded by other IRAEs. Myocarditis was present in 32% of cases with increased CPK in 43% of patients. 50% responded to steroids and 2 patients died. | 50% permanently stopped 25% interrupted |
| Johansen A. et al. (2019) [47] | Systematic review | 85 | Not reported | Myastenia Gravis (27%), neuropathy (23%, mostly Guillain–Barrè syndrome) and myopathy (34%) were the most frequent. The median time of onset was of 3.6 cycles of anti-PD1/PD-L1 inhibitors. Ach-R antibodies were detected in 50% of patients. 79% responded to steroids. | Not reported |
3.5. Nephrotoxicity
| References | Type of Study | Patients | Frequency | Main Findings | Stop CPI |
|---|---|---|---|---|---|
| Shirali A. et al. (2016) [58] | Case series | 6 | Not reported | Consider concomitant therapies that may cause idiosyncratic AKI (PPI and NSAID). | 100% |
| Gallan A.J. et al. (2019) [56] | Case series | 4 | Not reported | ANCA antibodies were always negative and all responded to steroids. | 25% |
| Mamlouk O. et al. (2019) [57] | Observational study | 16 | 0.07% | Glomerulopathies were associated acute tubulointerstitial nephritis (ATIN) without glomerulonephritis and nine cases of ATIN with glomerulopathies. CPI were discontinued and steroids given. For AKI > grade 2 or proteinuria >1 gram/day, kidney biopsy should be performed. | 93% |
| Kitchlu A. et al. (2020) [55] | Systematic review | 45 | Not reported | Most frequent manifestations were pauci-immune GN and renal vasculitis (27%), followed by podocytopathies (minimal change disease MCD; 20%) and C3 GN (11%). | 88% |
| Cortazar F.B. (2020) [53] | Observational study | 138 | Not reported | AKI occurred at a median time of 14 weeks, grade 3 in about 57% and requiring renal replacement therapy in 9% with persistent renal damage in 15%. At rechallenge with CPI, recurrence rate was of 23%. Risk factors include use of PPI, lower eGFR at baseline and concomitant anti-PD1 and anti-CTLA4 therapy. Renal biopsy should be always performed. | 3% at diagnosis |
| Gupta S. et al. (2021) [54] | Observational study | 429 | Not reported | AKI occurred mostly after 16 weeks from CPI. Lower baseline eGFR, PPI use and prior or concomitant extrarenal IRAEs were associated. In 60% of cases there were concomitant kidney toxic drugs. 5% of patients required other immunosuppressive therapy and 7% received renal replacement. | 10% |
3.6. Cardiovascular Toxicities
| References | Type of Study | Patients | Frequency | Main Findings |
|---|---|---|---|---|
| Mahmood S.S. et al. (2018) [61] | Observational study | 35 | 1.14% for myocarditis 0.52% for MACE | Cardiovascular IRAEs were more common with combination therapy (anti-PD1 + anti-CTLA4 inhibitors), with median onset of 34 days. Higher level of troponin was detected at admission in nearly all patients. Treatment with high doses of steroids was associated with reduced incidence of major cardiologic events. |
| Salem JE et al. (2018) [60] | Observational study | 31,321 evaluated records | Not reported | Higher incidence of myocarditis, pericardial diseases, supraventricular arrhythmias and vasculitis was described after CPI versus the general population. The median time to onset was of about 30 days. Epidosed were mainly severe (>80%), with a mortality of 50% for myocarditis. |
| Hu J. et al. (2019) [59] | Systematic Review | Not reported | 0.27–1.14% of myositis No data for pericarditis | Most frequent cardiovascular IRAEs were myocarditis, pericardial diseases and vasculitis. Patients receiving CPI had 11-fold increase of myocarditis compared with the general populations. |
| Awadalla M et al. (2020) [62] | Observational study | 101 | Not reported | Global longitudinal strain (GLS) at echocardiography did not predict overall cardiac IRAEs but identified patients at a higher risk of MACE. |
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hodi, F.S.; O’Day, S.J.; McDermott, D.F.; Weber, R.W.; Sosman, J.A.; Haanen, J.B.; Gonzalez, R.; Robert, C.; Schadendorf, D.; Hassel, J.C.; et al. Improved Survival with Ipilimumab in Patients with Metastatic Melanoma. N. Engl. J. Med. 2010, 363, 711–723. [Google Scholar] [CrossRef] [PubMed]
- Larkin, J.; Chiarion-Sileni, V.; Gonzalez, R.; Grob, J.-J.; Cowey, C.L.; Lao, C.D.; Schadendorf, D.; Dummer, R.; Smylie, M.; Rutkowski, P.; et al. Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. N. Engl. J. Med. 2015, 373, 23–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borghaei, H.; Paz-Ares, L.; Horn, L.; Spigel, D.R.; Steins, M.; Ready, N.E.; Chow, L.Q.; Vokes, E.E.; Felip, E.; Holgado, E.; et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2015, 373, 1627–1639. [Google Scholar] [CrossRef] [PubMed]
- Mok, T.S.K.; Wu, Y.-L.; Kudaba, I.; Kowalski, D.M.; Cho, B.C.; Turna, H.Z.; Castro, G., Jr.; Srimuninnimit, V.; Laktionov, K.K.; Bondarenko, I.; et al. Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): A randomised, open-label, controlled, phase 3 trial. Lancet 2019, 393, 1819–1830. [Google Scholar] [CrossRef]
- Motzer, R.J.; Escudier, B.; McDermott, D.F.; George, S.; Hammers, H.J.; Srinivas, S.; Tykodi, S.S.; Sosman, J.A.; Procopio, G.; Plimack, E.R.; et al. Nivolumab versus Everolimus in Advanced Renal-Cell Carcinoma. N. Engl. J. Med. 2015, 373, 1803–1813. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Zinzani, P.L.; Fanale, M.A.; Armand, P.; Johnson, N.A.; Brice, P.; Radford, J.; Ribrag, V.; Molin, D.; Vassilakopoulos, T.P.; et al. Phase II Study of the Efficacy and Safety of Pembrolizumab for Relapsed/Refractory Classic Hodgkin Lymphoma. J. Clin. Oncol. 2017, 35, 2125–2132. [Google Scholar] [CrossRef]
- Younes, A.; Santoro, A.; Shipp, M.; Zinzani, P.L.; Timmerman, J.M.; Ansell, S.; Armand, P.; Fanale, M.; Ratanatharathorn, V.; Kuruvilla, J.; et al. Nivolumab for classical Hodgkin’s lymphoma after failure of both autologous stem-cell transplantation and brentuximab vedotin: A multicentre, multicohort, single-arm phase 2 trial. Lancet Oncol. 2016, 17, 1283–1294. [Google Scholar] [CrossRef] [Green Version]
- Postow, M.A.; Sidlow, R.; Hellmann, M.D. Immune-Related Adverse Events Associated with Immune Checkpoint Blockade. N. Engl. J. Med. 2018, 378, 158–168. [Google Scholar] [CrossRef]
- Petrelli, F.; Ardito, R.; Borgonovo, K.; Lonati, V.; Cabiddu, M.; Ghilardi, M.; Barni, S. Haematological toxicities with immunotherapy in patients with cancer: A systematic review and meta-analysis. Eur. J. Cancer 2018, 103, 7–16. [Google Scholar] [CrossRef]
- Jäger, U.; Barcellini, W.; Broome, C.M.; Gertz, M.A.; Hill, A.; Hill, Q.A.; Jilma, B.; Kuter, D.J.; Michel, M.; Montillo, M.; et al. Diagnosis and treatment of autoimmune hemolytic anemia in adults: Recommendations from the First International Consensus Meeting. Blood Rev. 2019, 41, 100648. [Google Scholar] [CrossRef]
- Barcellini, W.; Fattizzo, B. The Changing Landscape of Autoimmune Hemolytic Anemia. Front. Immunol. 2020, 11, 946. [Google Scholar] [CrossRef] [PubMed]
- Leaf, R.K.; Ferreri, C.; Rangachari, D.; Mier, J.; Witteles, W.; Ansstas, G.; Anagnostou, T.; Zubiri, L.; Piotrowska, Z.; Oo, T.H.; et al. Clinical and laboratory features of autoimmune hemolytic anemia associated with immune checkpoint inhibitors. Am. J. Hematol. 2019, 94, 563–574. [Google Scholar] [CrossRef] [PubMed]
- Barcellini, W.; Giannotta, J.A.; Fattizzo, B. Autoimmune Complications in Hematologic Neoplasms. Cancers 2021, 13, 1532. [Google Scholar] [CrossRef] [PubMed]
- Palla, A.R.; Kennedy, D.; Mosharraf, H.; Doll, D. Autoimmune Hemolytic Anemia as a Complication of Nivolumab Therapy. Case Rep. Oncol. 2016, 9, 691–697. [Google Scholar] [CrossRef]
- Fattizzo, B.; Michel, M.; Zaninoni, A.; Giannotta, J.; Guillet, S.; Frederiksen, H.; Vos, J.M.; Mauro, F.R.; Jilma, B.; Patriarca, A.; et al. Efficacy of recombinant erythropoietin in autoimmune haemolytic anaemia: A multicentre international study. Haematologica 2020, 106, 622–625. [Google Scholar] [CrossRef]
- Grewal, U.S.; Thotamgari, S.R.; Shah, P.R.; Uppal, J.K.; Gaddam, S.J. Re: Hematological immune related adverse events after treatment with immune checkpoint inhibitors. Eur. J. Cancer 2021, 147, 170–181. [Google Scholar] [CrossRef]
- Delanoy, N.; Michot, J.-M.; Comont, T.; Kramkimel, N.; Lazarovici, J.; Dupont, R.; Champiat, S.; Chahine, C.; Robert, C.; Herbaux, C.; et al. Haematological immune-related adverse events induced by anti-PD-1 or anti-PD-L1 immunotherapy: A descriptive observational study. Lancet Haematol. 2019, 6, e48–e57. [Google Scholar] [CrossRef]
- Zaremba, A.; Kramer, R.; De Temple, V.; Bertram, S.; Salzmann, M.; Gesierich, A.; Reinhardt, L.; Baroudjian, B.; Sachse, M.M.; Mechtersheimer, G.; et al. Grade 4 Neutropenia Secondary to Immune Checkpoint Inhibition—A Descriptive Observational Retrospective Multicenter Analysis. Front. Oncol. 2021, 11, 765608. [Google Scholar] [CrossRef]
- Michot, J.; Lazarovici, J.; Tieu, A.; Champiat, S.; Voisin, A.; Ebbo, M.; Godeau, B.; Michel, M.; Ribrag, V.; Lambotte, O. Haematological immune-related adverse events with immune checkpoint inhibitors, how to manage? Eur. J. Cancer 2019, 122, 72–90. [Google Scholar] [CrossRef]
- Davis, E.J.; Salem, J.-E.; Young, A.; Green, J.R.; Ferrell, P.B.; Ancell, K.K.; Lebrun-Vignes, B.; Moslehi, J.J.; Johnson, D.B. Hematologic Complications of Immune Checkpoint Inhibitors. Oncologist 2019, 24, 584–588. [Google Scholar] [CrossRef] [Green Version]
- Kalmuk, J.; Puchalla, J.; Feng, G.; Giri, A.; Kaczmar, J. Pembrolizumab-induced Hemophagocytic Lymphohistiocytosis: An immunotherapeutic challenge. Cancers Head Neck 2020, 5, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rubino, R.; Marini, A.; Roviello, G.; Presotto, E.M.; Desideri, I.; Ciardetti, I.; Brugia, M.; Pimpinelli, N.; Antonuzzo, L.; Mini, E.; et al. Endocrine-related adverse events in a large series of cancer patients treated with anti-PD1 therapy. Endocrine 2021, 74, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Barroso-Sousa, R.; Barry, W.T.; Garrido-Castro, A.C.; Hodi, F.S.; Min, L.; Krop, I.E.; Tolaney, S.M. Incidence of endocrine dysfunction following the use of different immune checkpoint inhibitor regimens a systematic review and meta-analysis. JAMA Oncol. Am. Med. Assoc. 2018, 4, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Faje, A.T.; Sullivan, R.; Lawrence, D.; Tritos, N.A.; Fadden, R.; Klibanski, A.; Nachtigall, L. Ipilimumab-Induced Hypophysitis: A Detailed Longitudinal Analysis in a Large Cohort of Patients With Metastatic Melanoma. J. Clin. Endocrinol. Metab. 2014, 99, 4078–4085. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Faje, A.; Reynolds, K.; Zubiri, L.; Lawrence, D.; Cohen, J.V.; Sullivan, R.J.; Nachtigall, L.; Tritos, N. Hypophysitis secondary to nivolumab and pembrolizumab is a clinical entity distinct from ipilimumab-associated hypophysitis. Eur. J. Endocrinol. 2019, 181, 211–219. [Google Scholar] [CrossRef] [PubMed]
- Garon-Czmil, J.; Petitpain, N.; Rouby, F.; Sassier, M.; Babai, S.; Yéléhé-Okouma, M.; Weryha, G.; Klein, M.; Gillet, P. Immune check point inhibitors-induced hypophysitis: A retrospective analysis of the French Pharmacovigilance database. Sci. Rep. 2019, 9, 19419. [Google Scholar] [CrossRef]
- Morganstein, D.; Lai, Z.; Spain, L.; Diem, S.; Levine, D.; Mace, C.; Gore, M.; Larkin, J. Thyroid abnormalities following the use of cytotoxic T-lymphocyte antigen-4 and programmed death receptor protein-1 inhibitors in the treatment of melanoma. Clin. Endocrinol. 2017, 86, 614–620. [Google Scholar] [CrossRef]
- Presotto, E.M.; Rastrelli, G.; Desideri, I.; Scotti, V.; Gunnella, S.; Pimpinelli, N.; Vaccher, E.; Bearz, A.; Di Costanzo, F.; Bruggia, M.; et al. Endocrine toxicity in cancer patients treated with nivolumab or pembrolizumab: Results of a large multicentre study. J. Endocrinol. Investig. 2019, 43, 337–345. [Google Scholar] [CrossRef]
- Osorio, J.C.; Ni, A.; Chaft, J.E.; Pollina, R.; Kasler, M.K.; Stephens, D.; Rodriguez, C.; Cambridge, L.; Rizvi, H.; Wolchok, J.D.; et al. Antibody-mediated thyroid dysfunction during T-cell checkpoint blockade in patients with non-small-cell lung cancer. Ann. Oncol. 2017, 28, 583–589. [Google Scholar] [CrossRef]
- Kotwal, A.; Kottschade, L.; Ryder, M. PD-L1 Inhibitor-Induced Thyroiditis Is Associated with Better Overall Survival in Cancer Patients. Thyroid 2020, 30, 177–184. [Google Scholar] [CrossRef]
- Quandt, Z.; Young, A.; Anderson, M. Immune checkpoint inhibitor diabetes mellitus: A novel form of autoimmune diabetes. Clin. Exp. Immunol. 2020, 200, 131–140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muniz, T.P.; Araujo, D.V.; Savage, K.J.; Cheng, T.; Saha, M.; Song, X.; Gill, S.; Monzon, J.G.; Grenier, D.; Genta, S.; et al. CANDIED: A Pan-Canadian Cohort of Immune Checkpoint Inhibitor-Induced Insulin-Dependent Diabetes Mellitus. Cancers 2021, 14, 89. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.J.E.; Carlos, G.; Wakade, D.; Byth, K.; Kong, B.Y.; Chou, S.; Carlino, M.S.; Kefford, R.; Fernandez-Penas, P. Cutaneous adverse events (AEs) of anti-programmed cell death (PD)-1 therapy in patients with metastatic melanoma: A single-institution cohort. J. Am. Acad. Dermatol. 2016, 74, 455–461.e1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geisler, A.N.; Phillips, G.S.; Barrios, D.M.; Wu, J.; Leung, D.Y.M.; Moy, A.P.; Kern, J.A.; Lacouture, M.E. Immune checkpoint inhibitor–related dermatologic adverse events. J. Am. Acad. Dermatol. 2020, 83, 1255–1268. [Google Scholar] [CrossRef]
- Coleman, E.; Ko, C.; Dai, F.; Tomayko, M.M.; Kluger, H.; Leventhal, J.S. Inflammatory eruptions associated with immune checkpoint inhibitor therapy: A single-institution retrospective analysis with stratification of reactions by toxicity and implications for management. J. Am. Acad. Dermatol. 2019, 80, 990–997. [Google Scholar] [CrossRef] [PubMed]
- Siegel, J.; Totonchy, M.; Damsky, W.; Berk-Krauss, J.; Castiglione, F.; Sznol, M.; Petrylak, D.P.; Fischbach, N.; Goldberg, S.B.; Decker, R.H.; et al. Bullous disorders associated with anti–PD-1 and anti–PD-L1 therapy: A retrospective analysis evaluating the clinical and histopathologic features, frequency, and impact on cancer therapy. J. Am. Acad. Dermatol. 2018, 79, 1081–1088. [Google Scholar] [CrossRef]
- Lee, Y.J.; Kim, H.T.; Won, C.H.; Chang, S.E.; Lee, M.W.; Choi, J.H.; Lee, W.J. Characterization and Prognostic Significance of Cutaneous Adverse Events to Anti-Programmed Cell Death-1 Therapy. J. Korean Med. Sci. 2019, 34, e186. [Google Scholar] [CrossRef]
- Naidoo, J.; Schindler, K.; Querfeld, C.; Busam, K.; Cunningham, J.; Page, D.B.; Postow, M.A.; Weinstein, A.; Lucas, A.S.; Ciccolini, K.T.; et al. Autoimmune Bullous Skin Disorders with Immune Checkpoint Inhibitors Targeting PD-1 and PD-L1. Cancer Immunol. Res. 2016, 4, 383–389. [Google Scholar] [CrossRef] [Green Version]
- Matsuya, T.; Nakamura, Y.; Matsushita, S.; Tanaka, R.; Teramoto, Y.; Asami, Y.; Uehara, J.; Aoki, M.; Yamamura, K.; Nakamura, Y.; et al. Vitiligo expansion and extent correlate with durable response in anti-programmed death 1 antibody treatment for advanced melanoma: A multi-institutional retrospective study. J. Dermatol. 2020, 47, 629–635. [Google Scholar] [CrossRef]
- Chan, L.; Hwang, S.J.; Byth, K.; Kyaw, M.; Carlino, M.S.; Chou, S.; Fernandez-Penas, P. Survival and prognosis of individuals receiving programmed cell death 1 inhibitor with and without immunologic cutaneous adverse events. J. Am. Acad. Dermatol. 2020, 82, 311–316. [Google Scholar] [CrossRef]
- Bellaguarda, E.; Hanauer, S. Checkpoint Inhibitor–Induced Colitis. Am. J. Gastroenterol. 2020, 115, 202–210. [Google Scholar] [CrossRef] [PubMed]
- Foppen MH, G.; Rozeman, E.A.; Van Wilpe, S.; Postma, C.; Snaebjornsson, P.; Van Thienen, J.V.; van Leerdam, M.E.; van den Heuvel, M.; Blank, C.U.; van Dieren, J.; et al. Immune checkpoint inhibition-related colitis: Symptoms, endoscopic features, histology and response to management. ESMO Open. BMJ Publ. Group 2018, 3, e000278. [Google Scholar]
- Abu-Sbeih, H.; Ali, F.S.; Luo, W.; Qiao, W.; Raju, G.S.; Wang, Y. Importance of endoscopic and histological evaluation in the management of immune checkpoint inhibitor-induced colitis. J. Immunother. Cancer 2018, 6, 95. [Google Scholar] [CrossRef] [PubMed]
- Abu-Sbeih, H.; Ali, F.S.; Wang, X.; Mallepally, N.; Chen, E.; Altan, M.; Bresalier, R.S.; Charabaty, A.; Dadu, R.; Jazaeri, A.; et al. Early introduction of selective immunosuppressive therapy associated with favorable clinical outcomes in patients with immune checkpoint inhibitor–induced colitis. J. Immunother. Cancer 2019, 7, 93. [Google Scholar] [CrossRef]
- Cheung, V.T.F.; Gupta, T.; Olsson-Brown, A.; Subramanian, S.; Sasson, S.C.; Heseltine, J.; Fryer, E.; Collantes, E.; Sacco, J.J.; Pirmohamed, M.; et al. Immune checkpoint inhibitor-related colitis assessment and prognosis: Can IBD scoring point the way? Br. J. Cancer 2020, 123, 207–215. [Google Scholar] [CrossRef]
- Riveiro-Barciela, M.; Barreira-Díaz, A.; Vidal-González, J.; Muñoz-Couselo, E.; Martínez-Valle, F.; Viladomiu, L.; Mínguez, B.; Ortiz-Velez, C.; Castells, L.; Esteban, R.; et al. Immune-related hepatitis related to checkpoint inhibitors: Clinical and prognostic factors. Liver Int. 2020, 40, 1906–1916. [Google Scholar] [CrossRef]
- Johansen, A.; Christensen, S.J.; Scheie, D.; Højgaard, J.L.; Kondziella, D. Neuromuscular adverse events associated with anti-PD-1 monoclonal antibodies. Neurology 2019, 92, 663–674. [Google Scholar] [CrossRef]
- Moreira, A.; Loquai, C.; Pföhler, C.; Kähler, K.C.; Knauss, S.; Heppt, M.V.; Gutzmer, R.; Dimitriou, F.; Meier, F.; Mitzel-Rink, H.; et al. Myositis and neuromuscular side-effects induced by immune checkpoint inhibitors. Eur. J. Cancer 2019, 106, 12–23. [Google Scholar] [CrossRef]
- Liewluck, T.; Kao, J.C.; Mauermann, M.L. PD-1 Inhibitor-associated Myopathies: Emerging Immune-mediated Myopathies. J. Immunother. 2018, 41, 208–211. [Google Scholar] [CrossRef]
- Möhn, N.; Beutel, G.; Gutzmer, R.; Ivanyi, P.; Satzger, I.; Skripuletz, T. Neurological Immune Related Adverse Events Associated with Nivolumab, Ipilimumab, and Pembrolizumab Therapy—Review of the Literature and Future Outlook. J. Clin. Med. 2019, 8, 1777. [Google Scholar] [CrossRef] [Green Version]
- Morrone, A.; Scarabello, A.; Sperduti, I.; Cota, C.; Donà, M.; Orsini, D.; Cristaudo, A. Donovanosis in migrants: A clinical case series in an Italian dermatological hospital. J. Eur. Acad. Dermatol. Venereol. 2019, 33, e438–e440. [Google Scholar] [CrossRef] [PubMed]
- Zimmer, L.; Goldinger, S.M.; Hassel, J.C.; Meier, F.; Tietze, J.K.; Forschner, A.; Weishaupt, C.; Leverkus, M.; Wahl, R.; Dietrich, U.; et al. Neurological, respiratory, musculoskeletal, cardiac and ocular side-effects of anti-PD-1 therapy. Eur. J. Cancer 2016, 60, 210–225. [Google Scholar] [CrossRef] [PubMed]
- Cortazar, F.B.; Kibbelaar, Z.A.; Glezerman, I.G.; Abudayyeh, A.; Mamlouk, O.; Motwani, S.S.; Murakami, N.; Herrmann, S.M.; Manohar, S.; Shirali, A.C.; et al. Clinical Features and Outcomes of Immune Checkpoint Inhibitor–Associated AKI: A Multicenter Study. J. Am. Soc. Nephrol. 2020, 31, 435–446. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Short, S.A.P.; Sise, M.E.; Prosek, J.M.; Madhavan, S.M.; Soler, M.J.; Ostermann, M.; Herrmann, S.M.; Abudayyeh, A.; Anand, S.; et al. Acute kidney injury in patients treated with immune checkpoint inhibitors. J. Immunother. Cancer 2021, 9, e003467. [Google Scholar] [CrossRef]
- Kitchlu, A.; Jhaveri, K.D.; Wadhwani, S.; Deshpande, P.; Harel, Z.; Kishibe, T.; Henriksen, K.; Wanchoo, R. A Systematic Review of Immune Checkpoint Inhibitor–Associated Glomerular Disease. Kidney Int. Rep. 2021, 6, 66–77. [Google Scholar] [CrossRef]
- Gallan, A.J.; Alexander, E.; Reid, P.; Kutuby, F.; Chang, A.; Henriksen, K.J. Renal Vasculitis and Pauci-immune Glomerulonephritis Associated With Immune Checkpoint Inhibitors. Am. J. Kidney Dis. 2019, 74, 853–856. [Google Scholar] [CrossRef]
- Mamlouk, O.; Selamet, U.; Machado, S.; Abdelrahim, M.; Glass, W.F.; Tchakarov, A.; Gaber, L.; Lahoti, A.; Workeneh, B.; Chen, S.; et al. Nephrotoxicity of immune checkpoint inhibitors beyond tubulointerstitial nephritis: Single-center experience. J. Immunother. Cancer 2019, 7, 2. [Google Scholar] [CrossRef]
- Shirali, A.C.; Perazella, M.A.; Gettinger, S. Association of Acute Interstitial Nephritis With Programmed Cell Death 1 Inhibitor Therapy in Lung Cancer Patients. Am. J. Kidney Dis. 2016, 68, 287–291. [Google Scholar] [CrossRef]
- Hu, J.-R.; Florido, R.; Lipson, E.J.; Naidoo, J.; Ardehali, R.; Tocchetti, C.G.; Lyon, A.R.; Padera, R.F.; Johnson, D.B.; Moslehi, J. Cardiovascular toxicities associated with immune checkpoint inhibitors. Cardiovasc. Res. 2019, 115, 854–868. [Google Scholar] [CrossRef] [Green Version]
- Salem, J.-E.; Manouchehri, A.; Moey, M.; Lebrun-Vignes, B.; Bastarache, L.; Pariente, A.; Gobert, A.; Spano, J.-P.; Balko, J.M.; Bonaca, M.P.; et al. Cardiovascular toxicities associated with immune checkpoint inhibitors: An observational, retrospective, pharmacovigilance study. Lancet Oncol. 2018, 19, 1579–1589. [Google Scholar] [CrossRef]
- Mahmood, S.S.; Fradley, M.G.; Cohen, J.V.; Nohria, A.; Reynolds, K.L.; Heinzerling, L.M.; Sullivan, R.J.; Damrongwatanasuk, R.; Chen, C.L.; Gupta, D.; et al. Myocarditis in Patients Treated With Immune Checkpoint Inhibitors. J. Am. Coll. Cardiol. 2018, 71, 1755–1764. [Google Scholar] [CrossRef] [PubMed]
- Awadalla, M.; Mahmood, S.S.; Groarke, J.D.; Hassan, M.Z.; Nohria, A.; Rokicki, A.; Murphy, S.P.; Mercaldo, N.D.; Zhang, L.; Zlotoff, D.A.; et al. Global Longitudinal Strain and Cardiac Events in Patients With Immune Checkpoint Inhibitor-Related Myocarditis. J. Am. Coll. Cardiol. 2020, 75, 467–478. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fattizzo, B.; Rampi, N.; Barcellini, W. Hematological and Extra-Hematological Autoimmune Complications after Checkpoint Inhibitors. Pharmaceuticals 2022, 15, 557. https://doi.org/10.3390/ph15050557
Fattizzo B, Rampi N, Barcellini W. Hematological and Extra-Hematological Autoimmune Complications after Checkpoint Inhibitors. Pharmaceuticals. 2022; 15(5):557. https://doi.org/10.3390/ph15050557
Chicago/Turabian StyleFattizzo, Bruno, Nicolò Rampi, and Wilma Barcellini. 2022. "Hematological and Extra-Hematological Autoimmune Complications after Checkpoint Inhibitors" Pharmaceuticals 15, no. 5: 557. https://doi.org/10.3390/ph15050557
APA StyleFattizzo, B., Rampi, N., & Barcellini, W. (2022). Hematological and Extra-Hematological Autoimmune Complications after Checkpoint Inhibitors. Pharmaceuticals, 15(5), 557. https://doi.org/10.3390/ph15050557






