Unveiling the Multitarget Anti-Alzheimer Drug Discovery Landscape: A Bibliometric Analysis
Abstract
1. Introduction
- (i)
- There is a clear feeling that the number of publications on multitarget anti-AD agents has steadily increased over the past one or two decades, especially since the publication in 2008 of a seminal review article by Cavalli, Bolognesi, Melchiorre, and coworkers [27], but the actual magnitude and evolution of the number of publications on this topic over the years has not been assessed so far.
- (ii)
- A second, quite general notion is that most multitarget anti-AD compounds are designed to hit AChE and another target of interest [28]. Indeed, a large number of multitarget compounds feature pharmacophoric moieties of the approved AChE inhibitors [29,30,31,32], even though publications on multitarget anti-AD agents based on other pharmacophores, especially naturally occurring scaffolds such as β-carbolines [33], naphthoquinones and anthraquinones [34], or chalcones [35], just to name a few of those recently reviewed, are continuously appearing. Apart from AChE, other targets and pathogenic mechanisms that, intuitively, seem to have been commonly addressed when designing multitarget anti-AD compounds are glutamante NMDA receptors [36,37], β-amyloid and tau aggregation [38,39], glycogen synthase kinase 3β (GSK-3β) [40], biometal dyshomeostasis [41], monoamine oxidases (MAOs) [42], or oxidative stress [43].
- (iii)
- It is usually assumed that the linked-pharmacophore strategy is the most popular design strategy, likely because AChE is thought to be the most commonly pursued target in multitarget anti-AD drug design and it is one of those proteins with binding sites buried at the bottom of a deep cavity.
2. Results and Discussion
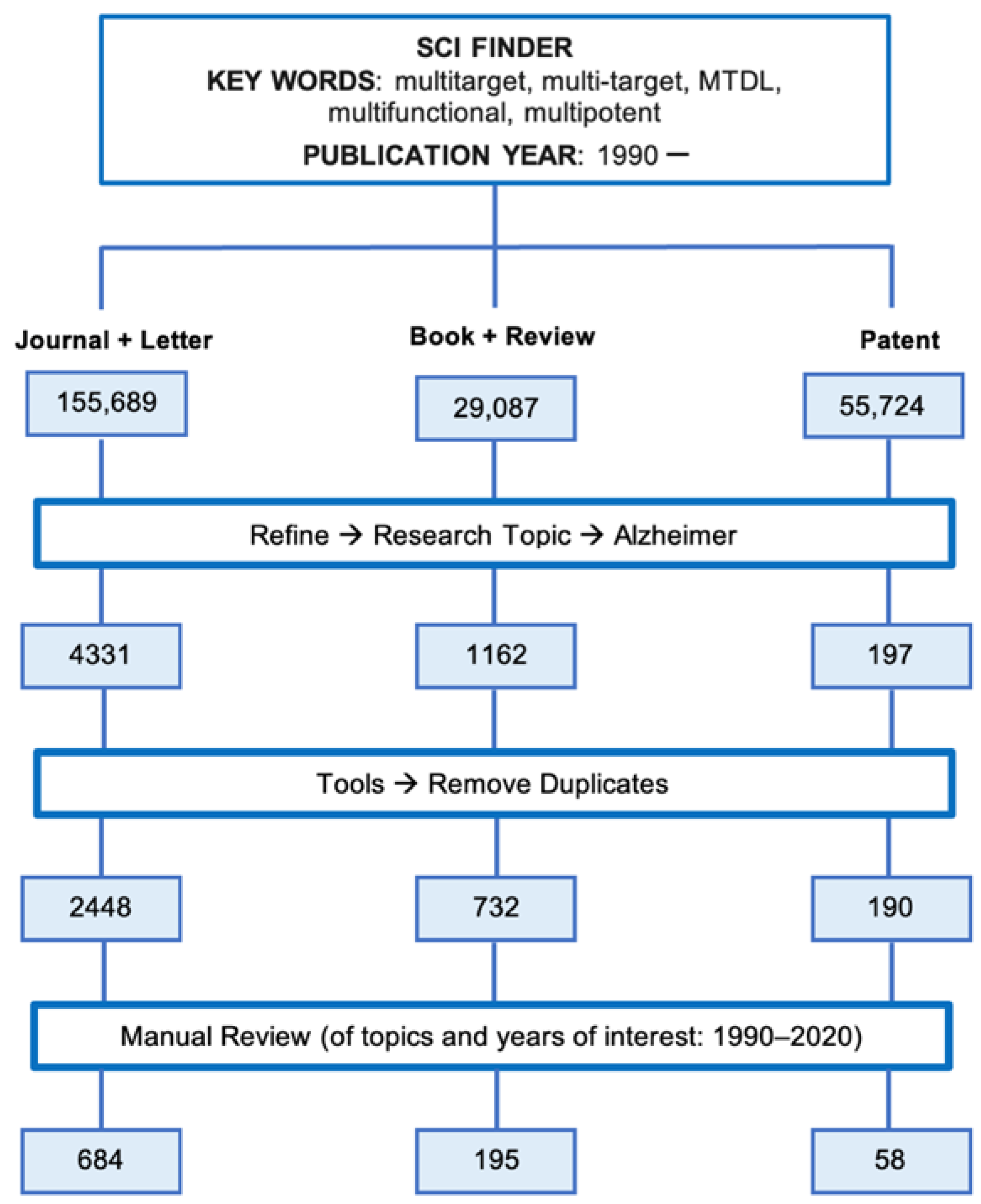
2.1. Bibliographic Search
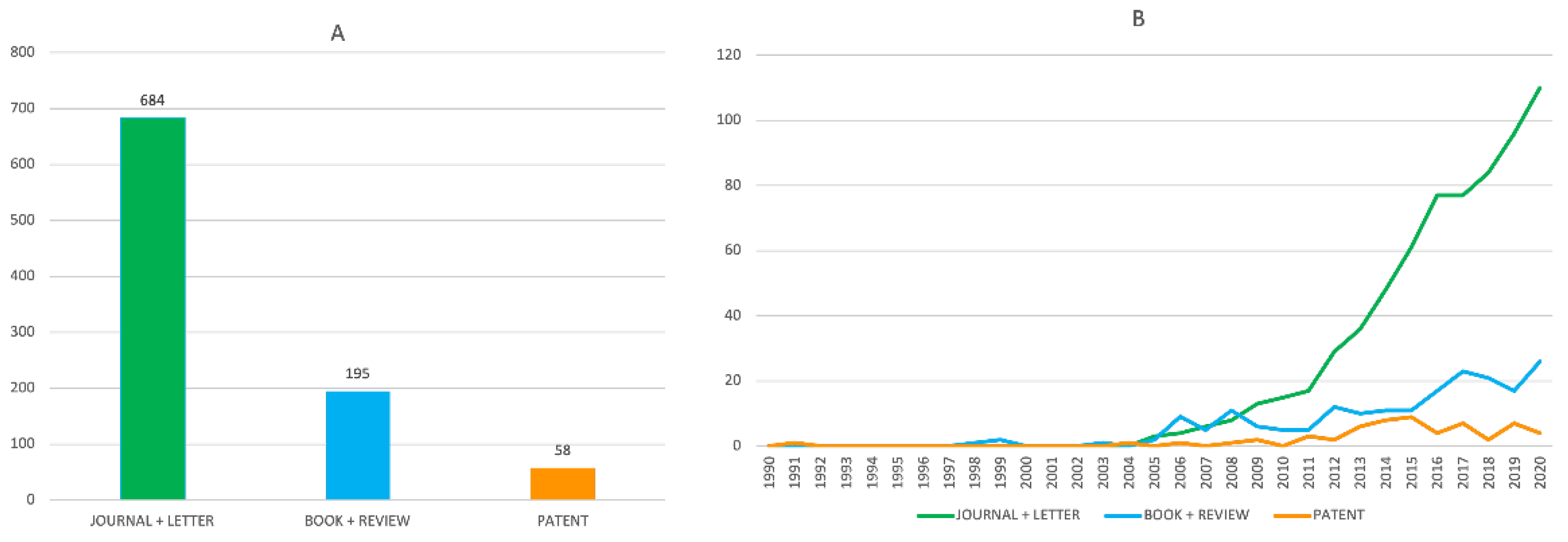
2.2. Evolution of the Number of Publications on Multitarget Anti-Alzheimer Compounds in the Period 1990–2020
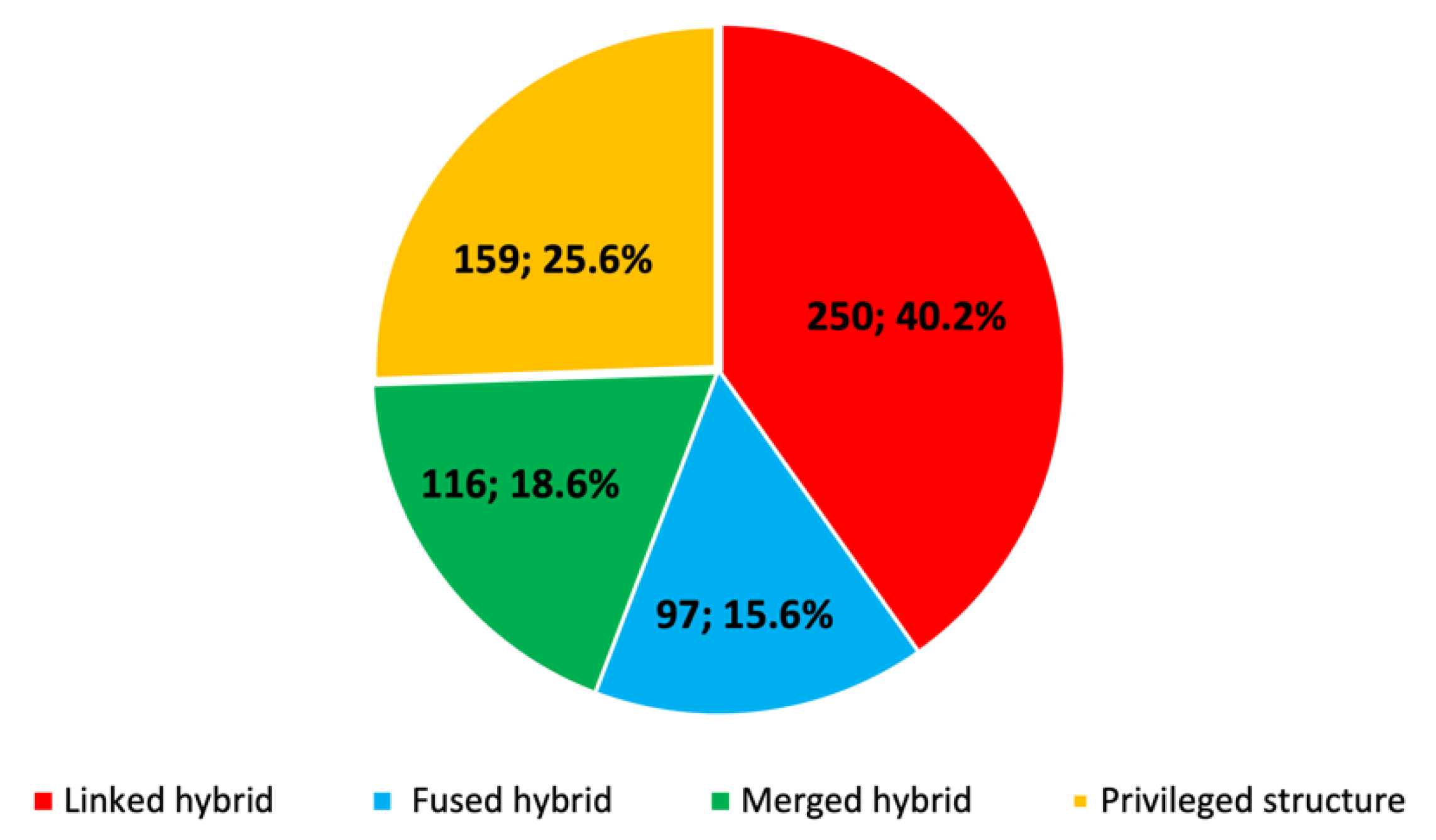
2.3. Design Strategies of Multitarget Anti-Alzheimer Compounds in the Period 1990–2020
2.4. Pharmacological Profiling of Multitarget Anti-Alzheimer Compounds in the Period 1990–2020
2.5. Geographical Origin of the Works on Multitarget Anti-Alzheimer Compounds in the Period 1990–2020
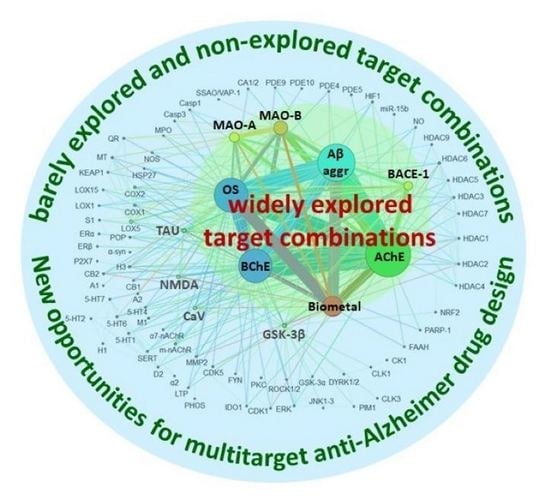
2.6. Biological Targets and Target Combinations Hit by Multitarget Anti-Alzheimer Compounds in the Period 1990–2020
3. Materials and Methods
3.1. Bibliographic Search
3.2. Creation of the Gephi Graph
4. Conclusions
- It was not until one decade ago (around 2011) that the number of publications on this topic experienced an explosive growth. This upward growth remained at the end of the studied period.
- It is very likely that, because of their ease of design and synthesis and the appropriateness to hit targets with binding sites that extend along deep gorges, commonly pursued in multitarget anti-AD compounds such as AChE, linked hybrids are more common than the smaller-sized fused or merged hybrids and privileged structures, even though the latter should be preferable in terms of better physicochemical and pharmacokinetic properties.
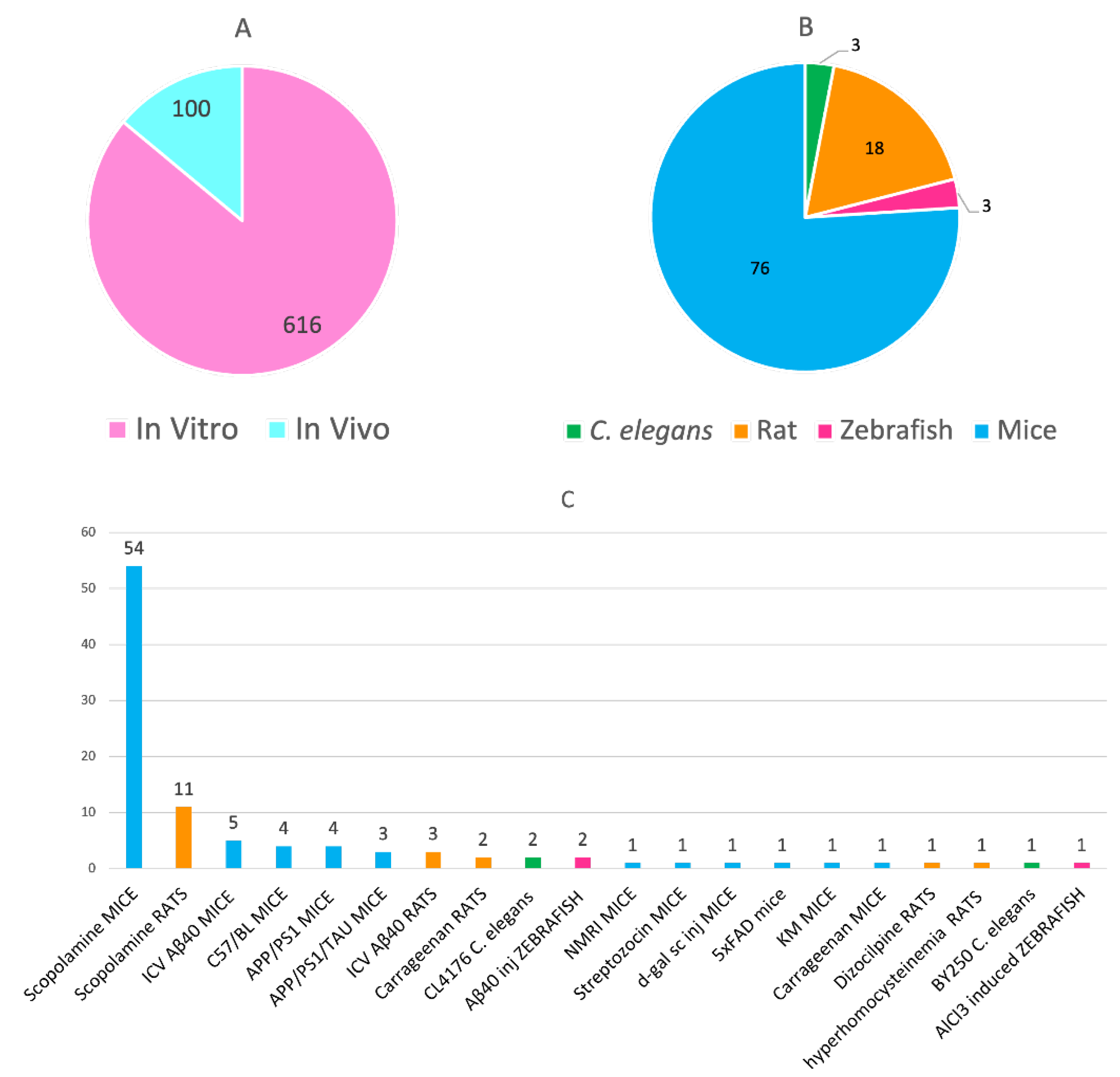
- In addition to the mandatory in vitro evaluation towards the multiple targets, a significant number of in vivo studies have been performed to assess the efficacy of multitarget anti-AD compounds, especially from 2017 (72 in vivo studies out of a total of 100 in the whole period), indicating a clear willingness to advance these candidates to preclinical and clinical testing. By far, the model of amnesia induced by scopolamine in wild-type mice and, to a minor extent, in rats, has been the most widely used animal model, which enables the evaluation of behavioral effects but not the impact on the underlying disease mechanisms. When affordable, other models which better recapitulate the different pathologies of AD, e.g., suitable transgenic animals, should be used to more consistently assess the in vivo efficacy of the multitarget compounds.
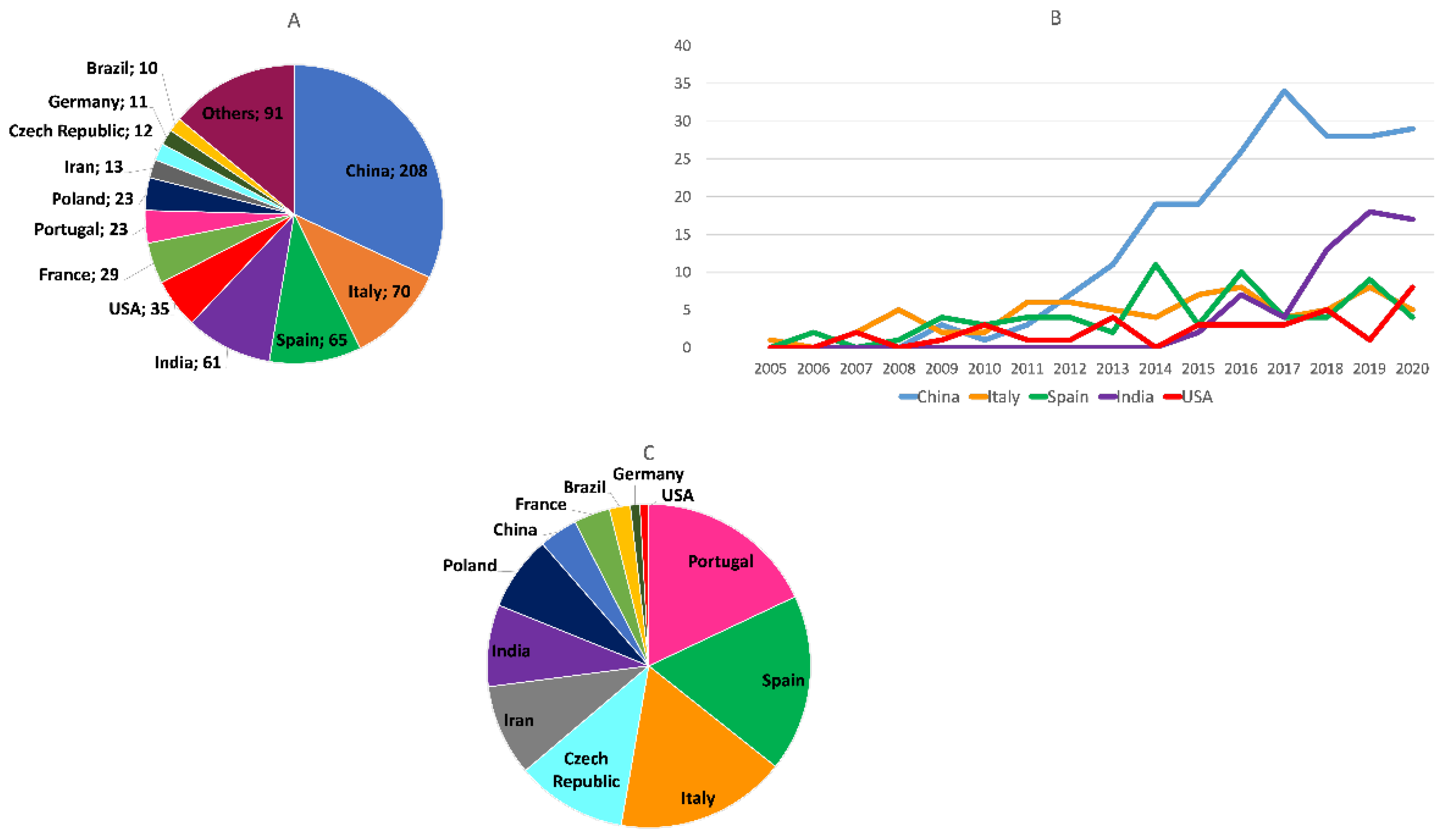
- Within the overall studied period, China has been the major contributor to the publications on this topic. Italy, Spain, India, the USA, France, Portugal, Poland, Iran, and the Czech Republic complete the list of the top ten most contributing countries. At the end of the period, China and India were clearly the countries with a greatest productivity. When the number of publications was normalized by the number of researchers of each country, we found that Portugal, Spain, and Italy are the countries in whose scientific communities multitarget anti-AD agents have attracted more interest, followed by the Czech Republic, Iran, India, Poland, China, France, Brazil, Germany, and the USA.
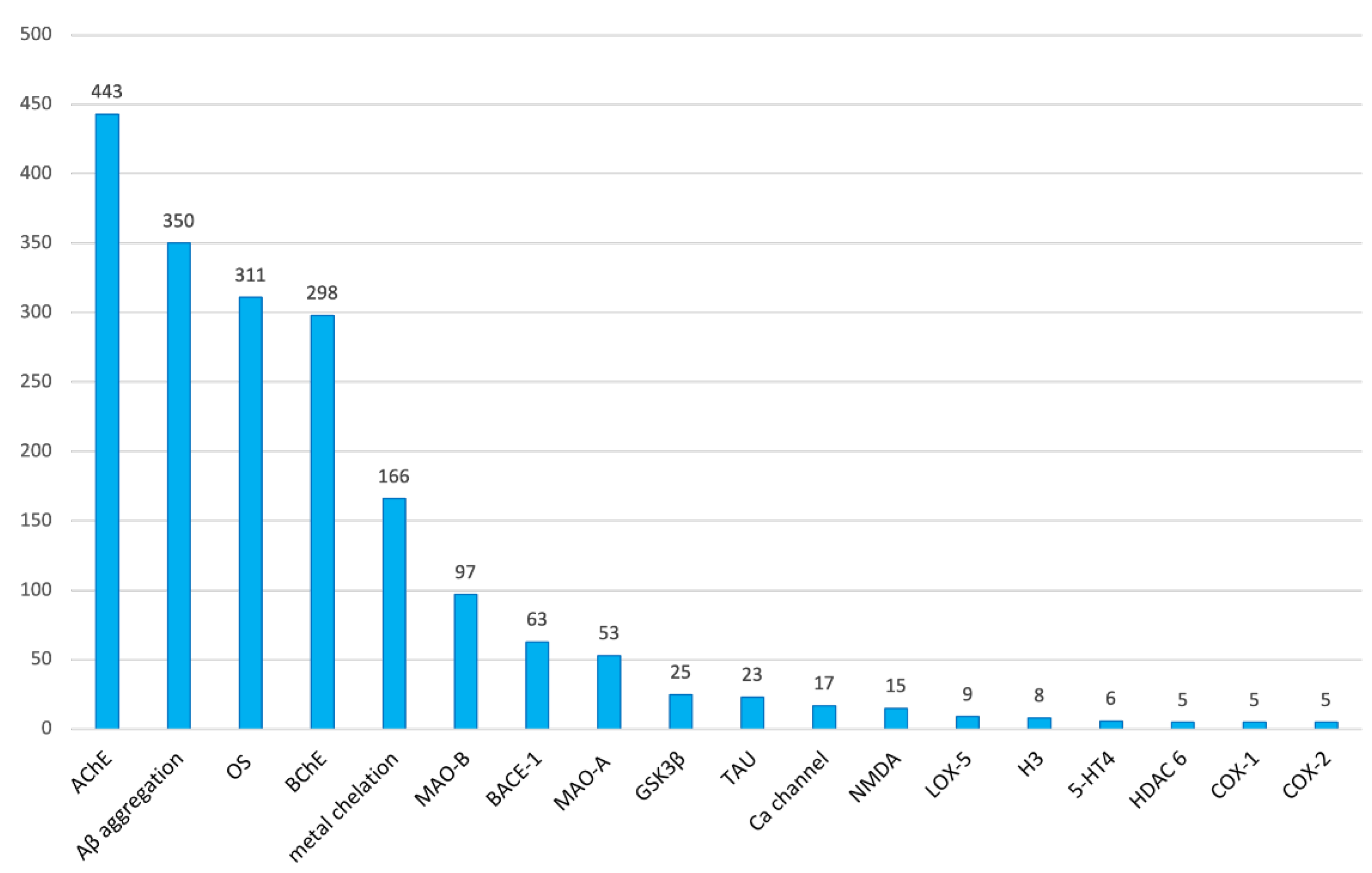
- AChE is clearly the most commonly pursued biological target in multitarget anti-AD compounds, appearing in up to 71.2% of all the research articles on this topic. In the list of the top ten most common targets, we also found Aβ aggregation (56.3%), oxidative stress (50.0%), BChE (47.9%), metal chelation (26.7%), MAO-B (15.6%), BACE-1 (10.1%), MAO-A (8.5%), GSK-3β (4.0%), and tau aggregation (3.7%). Eighty other different targets, including different subtypes and isoforms of some receptors and enzymes, were also pursued in the multitarget compounds developed in the studied period, but most of them only very sporadically.
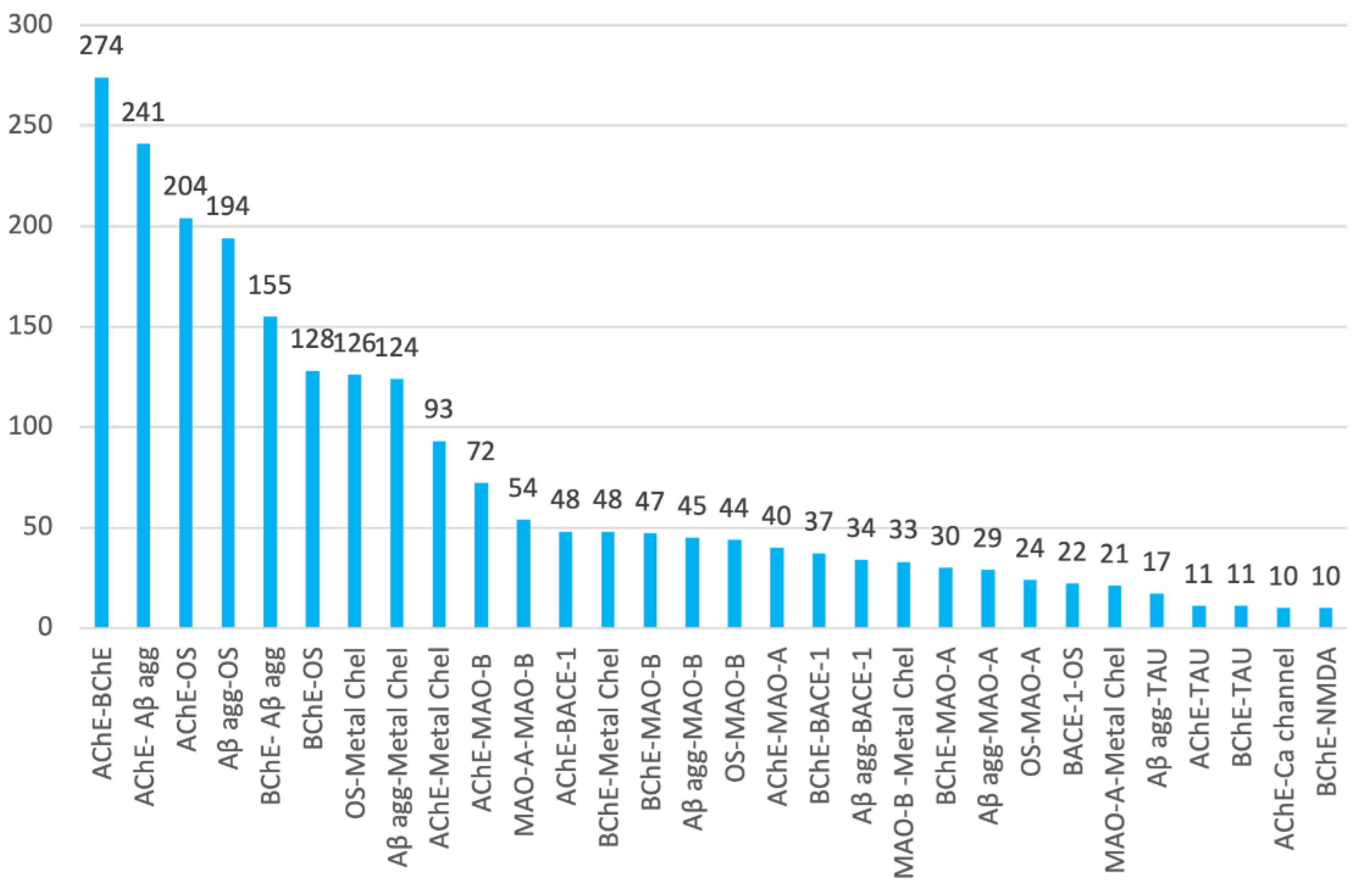
- Paralleling the frequency at which individual biological targets have been considered in multitarget anti-AD drug design, the binary combinations of the most common targets have been the most widely pursued. In the list of the top ten binary target combinations, we found AChE–BChE (in 44.1% of research articles), AChE–Aβ aggregation (38.7%), AChE–oxidative stress (32.8%), Aβ aggregation–oxidative stress (31.2%), BChE–Aβ aggregation (24.9%), BChE–oxidative stress (20.6%), oxidative stress–metal chelation (20.3%), Aβ aggregation–metal chelation (19.9%), AChE–metal chelation (15.0%), and AChE–MAO-B (11.6%).
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alzheimer’s Disease International. World Alzheimer Report 2019: Attitudes to Dementia; Alzheimer’s Disease International: London, UK, 2019. [Google Scholar]
- World Health Organization. The Top 10 Causes of Death. Available online: https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death (accessed on 5 January 2022).
- Joe, E.; Ringman, J.M. Cognitive Symptoms of Alzheimer’s Disease: Clinical Management and Prevention. BMJ 2019, 367, l6217. [Google Scholar] [CrossRef] [PubMed]
- Syed, Y.Y. Sodium Oligomannate: First Approval. Drugs 2020, 80, 441–444. [Google Scholar] [CrossRef] [PubMed]
- ClinicalTrials.gov. A Study of Sodium Oligomannate (GV-971) in Participants with Mild to Moderate Alzheimer’s Disease (GREEN MEMORY). Available online: https://clinicaltrials.gov/ct2/show/NCT04520412 (accessed on 5 January 2022).
- EU Clinical Trials Register. Available online: https://www.clinicaltrialsregister.eu/ctr-search/trial/2020-001755-41/CZ (accessed on 5 January 2022).
- Mullard, A. 2020 FDA Drug Approvals. Nat. Rev. Drug Discov. 2021, 20, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Mahase, E. Aducanumab: European Agency Rejects Alzheimer’s Drug over Efficacy and Safety Concerns. BMJ 2021, 375, n3127. [Google Scholar] [CrossRef]
- Cummings, J.; Lee, G.; Ritter, A.; Sabbagh, M.; Zhong, K. Alzheimer’s Disease Drug Development Pipeline: 2020. Alzheimer’s Dement. 2020, 6, e12050. [Google Scholar] [CrossRef]
- Liu, P.-P.; Xie, Y.; Meng, X.-Y.; Kang, J.-S. History and Progress of Hypotheses and Clinical Trials for Alzheimer’s Disease. Signal Transduct. Target Ther. 2019, 4, 29. [Google Scholar] [CrossRef]
- Cummings, J.; Lee, G.; Zhong, K.; Fonseca, J.; Taghva, K. Alzheimer’s Disease Drug Development Pipeline: 2021. Alzheimer’s Dement. 2021, 7, e12179. [Google Scholar] [CrossRef]
- Benek, O.; Korabecny, J.; Soukup, O. A Perspective on Multi-Target Drugs for Alzheimer’s Disease. Trends Pharmacol. Sci. 2020, 41, 434–445. [Google Scholar] [CrossRef]
- Du, X.; Wang, X.; Geng, M. Alzheimer’s Disease Hypothesis and Related Therapies. Transl. Neurodegener. 2018, 7, 2. [Google Scholar] [CrossRef]
- Gadhave, K.; Kumar, D.; Uversky, V.N.; Giri, R. A Multitude of Signaling Pathways Associated with Alzheimer’s Disease and Their Roles in AD Pathogenesis and Therapy. Med. Res. Rev. 2021, 41, 2689–2745. [Google Scholar] [CrossRef]
- Hopkins, A.L. Network Pharmacology: The next Paradigm in Drug Discovery. Nat. Chem. Biol. 2008, 4, 682–690. [Google Scholar] [CrossRef]
- Viayna, E.; Sola, I.; Di Pietro, O.; Muñoz-Torrero, D. Human Disease and Drug Pharmacology, Complex as Real Life. Curr. Med. Chem. 2013, 20, 1623–1634. [Google Scholar] [CrossRef][Green Version]
- Albertini, C.; Salerno, A.; Sena Murteira Pinheiro, P.; Bolognesi, M.L. From Combinations to Multitarget-directed Ligands: A Continuum in Alzheimer’s Disease Polypharmacology. Med. Res. Rev. 2021, 41, 2606–2633. [Google Scholar] [CrossRef]
- Yang, T.; Sui, X.; Yu, B.; Shen, Y.; Cong, H. Recent Advances in the Rational Drug Design Based on Multi-Target Ligands. Curr. Med. Chem. 2020, 27, 4720–4740. [Google Scholar] [CrossRef]
- Matos, M.J. Multitarget Therapeutic Approaches for Alzheimer’s and Parkinson’s Diseases: An Opportunity or an Illusion? Future Med. Chem. 2021, 13, 1301–1309. [Google Scholar] [CrossRef]
- Alarcón-Espósito, J.; Mallea, M.; Rodríguez-Lavado, J. From Hybrids to New Scaffolds: The Latest Medicinal Chemistry Goals in Multi-Target Directed Ligands for Alzheimer’s Disease. Curr. Neuropharmacol. 2021, 19, 832–867. [Google Scholar] [CrossRef]
- Pirolla, N.F.F.; Batista, V.S.; Dias Viegas, F.P.; Gontijo, V.S.; McCarthy, C.R.; Viegas, C.; Nascimento-Júnior, N.M. Alzheimer’s Disease: Related Targets, Synthesis of Available Drugs, Bioactive Compounds Under Development and Promising Results Obtained from Multi-Target Approaches. Curr. Drug Targets 2021, 22, 505–538. [Google Scholar] [CrossRef]
- Proschak, E.; Stark, H.; Merk, D. Polypharmacology by Design: A Medicinal Chemist’s Perspective on Multitargeting Compounds. J. Med. Chem. 2019, 62, 420–444. [Google Scholar] [CrossRef]
- Zhou, J.; Jiang, X.; He, S.; Jiang, H.; Feng, F.; Liu, W.; Qu, W.; Sun, H. Rational Design of Multitarget-Directed Ligands: Strategies and Emerging Paradigms. J. Med. Chem. 2019, 62, 8881–8914. [Google Scholar] [CrossRef]
- Li, X.; Li, X.; Liu, F.; Li, S.; Shi, D. Rational Multitargeted Drug Design Strategy from the Perspective of a Medicinal Chemist. J. Med. Chem. 2021, 64, 10581–10605. [Google Scholar] [CrossRef]
- Morphy, R.; Rankovic, Z. Designed Multiple Ligands. An Emerging Drug Discovery Paradigm. J. Med. Chem. 2005, 48, 6523–6543. [Google Scholar] [CrossRef] [PubMed]
- Morphy, R.; Rankovic, Z. The Physicochemical Challenges of Designing Multiple Ligands. J. Med. Chem. 2006, 49, 4961–4970. [Google Scholar] [CrossRef] [PubMed]
- Cavalli, A.; Bolognesi, M.L.; Minarini, A.; Rosini, M.; Tumiatti, V.; Recanatini, M.; Melchiorre, C. Multi-Target-Directed Ligands to Combat Neurodegenerative Diseases. J. Med. Chem. 2008, 51, 347–372. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Xu, S.; Zhu, Z.; Xu, J. Multi-Target Design Strategies for the Improved Treatment of Alzheimer’s Disease. Eur. J. Med. Chem. 2019, 176, 228–247. [Google Scholar] [CrossRef]
- Bautista-Aguilera, Ó.M.; Ismaili, L.; Iriepa, I.; Diez-Iriepa, D.; Chabchoub, F.; Marco-Contelles, J.; Pérez, M. Tacrines as Therapeutic Agents for Alzheimer’s Disease. V. Recent Developments. Chem. Rec. 2021, 21, 162–174. [Google Scholar] [CrossRef]
- Eckroat, T.J.; Manross, D.L.; Cowan, S.C. Merged Tacrine-Based, Multitarget-Directed Acetylcholinesterase Inhibitors 2015–Present: Synthesis and Biological Activity. Int. J. Mol. Sci. 2020, 21, 5965. [Google Scholar] [CrossRef]
- Mak, S.; Li, W.; Fu, H.; Luo, J.; Cui, W.; Hu, S.; Pang, Y.; Carlier, P.R.; Tsim, K.W.; Pi, R.; et al. Promising Tacrine/Huperzine A-based Dimeric Acetylcholinesterase Inhibitors for Neurodegenerative Disorders: From Relieving Symptoms to Modifying Diseases through Multitarget. J. Neurochem. 2021, 158, 1381–1393. [Google Scholar] [CrossRef]
- Iraji, A.; Khoshneviszadeh, M.; Firuzi, O.; Khoshneviszadeh, M.; Edraki, N. Novel Small Molecule Therapeutic Agents for Alzheimer Disease: Focusing on BACE1 and Multi-Target Directed Ligands. Bioorg. Chem. 2020, 97, 103649. [Google Scholar] [CrossRef]
- Beato, A.; Gori, A.; Boucherle, B.; Peuchmaur, M.; Haudecoeur, R. β-Carboline as a Privileged Scaffold for Multitarget Strategies in Alzheimer’s Disease Therapy. J. Med. Chem. 2021, 64, 1392–1422. [Google Scholar] [CrossRef]
- Campora, M.; Francesconi, V.; Schenone, S.; Tasso, B.; Tonelli, M. Journey on Naphthoquinone and Anthraquinone Derivatives: New Insights in Alzheimer’s Disease. Pharmaceuticals 2021, 14, 33. [Google Scholar] [CrossRef]
- Antoniolli, G.; Almeida, W.P.; Frias, C.C.; de Oliveira, T.B. Chalcones Acting as Inhibitors of Cholinesterases, β-Secretase and β- Amyloid Aggregation and Other Targets for Alzheimer’s Disease: A Critical Review. Curr. Med. Chem. 2021, 28, 4259–4282. [Google Scholar] [CrossRef]
- Uddin, M.S.; Al Mamun, A.; Kabir, M.T.; Ashraf, G.M.; Bin-Jumah, M.N.; Abdel-Daim, M.M. Multi-Target Drug Candidates for Multifactorial Alzheimer’s Disease: AChE and NMDAR as Molecular Targets. Mol. Neurobiol. 2021, 58, 281–303. [Google Scholar] [CrossRef]
- Marotta, G.; Basagni, F.; Rosini, M.; Minarini, A. Memantine Derivatives as Multitarget Agents in Alzheimer’s Disease. Molecules 2020, 25, 4005. [Google Scholar] [CrossRef]
- Du, Z.; Li, M.; Ren, J.; Qu, X. Current Strategies for Modulating Aβ Aggregation with Multifunctional Agents. Acc. Chem. Res. 2021, 54, 2172–2184. [Google Scholar] [CrossRef]
- Malafaia, D.; Albuquerque, H.M.T.; Silva, A.M.S. Amyloid-β and Tau Aggregation Dual-Inhibitors: A Synthetic and Structure-Activity Relationship Focused Review. Eur. J. Med. Chem. 2021, 214, 113209. [Google Scholar] [CrossRef]
- De Simone, A.; Tumiatti, V.; Andrisano, V.; Milelli, A. Glycogen Synthase Kinase 3β: A New Gold Rush in Anti-Alzheimer’s Disease Multitarget Drug Discovery? J. Med. Chem. 2021, 64, 26–41. [Google Scholar] [CrossRef]
- Chaves, S.; Várnagy, K.; Santos, M.A. Recent Multi-Target Approaches on the Development of Anti-Alzheimer’s Agents Integrating Metal Chelation Activity. Curr. Med. Chem. 2021, 28, 7247–7277. [Google Scholar] [CrossRef]
- Zhang, C.; Lv, Y.; Bai, R.; Xie, Y. Structural Exploration of Multifunctional Monoamine Oxidase B Inhibitors as Potential Drug Candidates against Alzheimer’s Disease. Bioorg. Chem. 2021, 114, 105070. [Google Scholar] [CrossRef]
- Bacci, A.; Runfola, M.; Sestito, S.; Rapposelli, S. Beyond Antioxidant Effects: Nature-Based Templates Unveil New Strategies for Neurodegenerative Diseases. Antioxidants 2021, 10, 367. [Google Scholar] [CrossRef]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and Computational Approaches to Estimate Solubility and Permeability in Drug Discovery and Development Settings. Adv. Drug Deliv. Rev. 1997, 23, 3–25. [Google Scholar] [CrossRef]
- Pajouhesh, H.; Lenz, G.R. Medicinal Chemical Properties of Successful Central Nervous System Drugs. Neurotherapeutics 2005, 2, 541–553. [Google Scholar] [CrossRef] [PubMed]
- Wager, T.T.; Hou, X.; Verhoest, P.R.; Villalobos, A. Moving beyond Rules: The Development of a Central Nervous System Multiparameter Optimization (CNS MPO) Approach to Enable Alignment of Druglike Properties. ACS Chem. Neurosci. 2010, 1, 435–449. [Google Scholar] [CrossRef] [PubMed]
- Viayna, E.; Coquelle, N.; Cieslikiewicz-Bouet, M.; Cisternas, P.; Oliva, C.A.; Sánchez-López, E.; Ettcheto, M.; Bartolini, M.; De Simone, A.; Ricchini, M.; et al. Discovery of a Potent Dual Inhibitor of Acetylcholinesterase and Butyrylcholinesterase with Antioxidant Activity That Alleviates Alzheimer-like Pathology in Old APP/PS1 Mice. J. Med. Chem. 2021, 64, 812–839. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Torrero, D. Multitarget Anti-Alzheimer Hybrid Compounds: Do They Work In Vivo? In Design of Hybrid Molecules for Drug Development; Decker, M., Ed.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 167–192. ISBN 9780081010112. [Google Scholar]
- UNESCO Institute for Statistics. Data as of September 2021. Available online: https://data.worldbank.org/indicator/SP.POP.SCIE.RD.P6?end=2018&most_recent_value_desc=true&start=1996&view=chart (accessed on 5 January 2022).
- Gephi. The Open Graph Viz Platform. Available online: https://gephi.org/ (accessed on 10 January 2022).
- Zagórska, A.; Jaromin, A. Perspectives for New and More Efficient Multifunctional Ligands for Alzheimer′s Disease Therapy. Molecules 2020, 25, 3337. [Google Scholar] [CrossRef]
- Martins, M.; Silva, R.; Pinto, M.M.M.; Sousa, E. Marine Natural Products, Multitarget Therapy and Repurposed Agents in Alzheimer’s Disease. Pharmaceuticals 2020, 13, 242. [Google Scholar] [CrossRef]
- Maramai, S.; Benchekroun, M.; Gabr, M.T.; Yahiaoui, S. Multitarget Therapeutic Strategies for Alzheimer’s Disease: Review on Emerging Target Combinations. BioMed Res. Int. 2020, 2020, 5120230. [Google Scholar] [CrossRef]
- Rodrigues, D.A.; Pinheiro, P.D.S.M.; Sagrillo, F.S.; Bolognesi, M.L.; Fraga, C.A.M. Histone Deacetylases as Targets for the Treatment of Neurodegenerative Disorders: Challenges and Future Opportunities. Med. Res. Rev. 2020, 40, 2177–2211. [Google Scholar] [CrossRef]
- Demuro, S.; Di Martino, R.M.C.; Ortega, J.A.; Cavalli, A. GSK-3β, FYN, and DYRK1A: Master Regulators in Neurodegenerative Pathways. Int. J. Mol. Sci. 2021, 22, 9098. [Google Scholar] [CrossRef]
- Martín-Cámara, O.; Cores, Á.; López-Alvarado, P.; Menéndez, J.C. Emerging Targets in Drug Discovery against Neurodegenerative Diseases: Control of Synapsis Disfunction by the RhoA/ROCK Pathway. Eur. J. Med. Chem. 2021, 225, 113742. [Google Scholar] [CrossRef]
- Millan, M.J.; Dekeyne, A.; Gobert, A.; Brocco, M.; Mannoury la Cour, C.; Ortuno, J.-C.; Watson, D.; Fone, K.C.F. Dual-Acting Agents for Improving Cognition and Real-World Function in Alzheimer’s Disease: Focus on 5-HT6 and D3 Receptors as Hubs. Neuropharmacology 2020, 177, 108099. [Google Scholar] [CrossRef]
- Kandimalla, R.; Reddy, P.H. Therapeutics of Neurotransmitters in Alzheimer’s disease. J. Alzheimer’s Dis. 2017, 57, 1049–1069. [Google Scholar] [CrossRef]
- Aso, E.; Ferrer, I. CB2 Cannabinoid Receptor as Potential Target against Alzheimer’s Disease. Front. Neurosci. 2016, 10, 243. [Google Scholar] [CrossRef]
- Wu, J.; Bie, B.; Yang, H.; Xu, J.J.; Brown, D.L.; Naguib, M. Activation of the CB2 Receptor System Reverses Amyloid-Induced Memory Deficiency. Neurobiol. Aging 2013, 34, 791–804. [Google Scholar] [CrossRef]
- Brimson, J.M.; Brimson, S.; Chomchoei, C.; Tencomnao, T. Using Sigma-Ligands as Part of a Multi-Receptor Approach to Target Diseases of the Brain. Expert Opin. Ther. Targets 2020, 24, 1009–1028. [Google Scholar] [CrossRef]
- Shevtsova, E.F.; Maltsev, A.V.; Vinogradova, D.V.; Shevtsov, P.N.; Bachurin, S.O. Mitochondria as a Promising Target for Developing Novel Agents for Treating Alzheimer’s Disease. Med. Res. Rev. 2021, 41, 803–827. [Google Scholar] [CrossRef]
- Biber, K.; Bhattacharya, A.; Campbell, B.M.; Piro, J.R.; Rohe, M.; Staal, R.G.W.; Talanian, R.V.; Möller, T. Microglial Drug Targets in AD: Opportunities and Challenges in Drug Discovery and Development. Front. Pharmacol. 2019, 10, 840. [Google Scholar] [CrossRef]
- Schmidt, J.; Rotter, M.; Weiser, T.; Wittmann, S.; Weizel, L.; Kaiser, A.; Heering, J.; Goebel, T.; Angioni, C.; Wurglics, M.; et al. A Dual Modulator of Farnesoid X Receptor and Soluble Epoxide Hydrolase to Counter Nonalcoholic Steatohepatitis. J. Med. Chem. 2017, 60, 7703–7724. [Google Scholar] [CrossRef]
- Hiesinger, K.; Wagner, K.M.; Hammock, B.D.; Proschak, E.; Hwang, S.H. Development of Multitarget Agents Possessing Soluble Epoxide Hydrolase Inhibitory Activity. Prostaglandins Other Lipid Mediat. 2019, 140, 31–39. [Google Scholar] [CrossRef]
- Das Mahapatra, A.; Choubey, R.; Datta, B. Small Molecule Soluble Epoxide Hydrolase Inhibitors in Multitarget and Combination Therapies for Inflammation and Cancer. Molecules 2020, 25, 5488. [Google Scholar] [CrossRef]
- Griñán-Ferré, C.; Codony, S.; Pujol, E.; Yang, J.; Leiva, R.; Escolano, C.; Puigoriol-Illamola, D.; Companys-Alemany, J.; Corpas, R.; Sanfeliu, C.; et al. Pharmacological Inhibition of Soluble Epoxide Hydrolase as a New Therapy for Alzheimer’s Disease. Neurotherapeutics 2020, 17, 1825–1835. [Google Scholar] [CrossRef]
- Ghosh, A.; Comerota, M.M.; Wan, D.; Chen, F.; Propson, N.E.; Hwang, S.H.; Hammock, B.D.; Zheng, H. An Epoxide Hydrolase Inhibitor Reduces Neuroinflammation in a Mouse Model of Alzheimer’s Disease. Sci. Transl. Med. 2020, 12, eabb1206. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Wang, M.; Zhu, M.; Xiong, W.; Qin, X.; Zhu, X. 14,15-Epoxyeicosatrienoic Acid Alleviates Pathology in a Mouse Model of Alzheimer’s Disease. J. Neurosci. 2020, 40, 8188–8203. [Google Scholar] [CrossRef] [PubMed]
- Borkowski, K.; Pedersen, T.L.; Seyfried, N.T.; Lah, J.J.; Levey, A.I.; Hales, C.M.; Dammer, E.B.; Blach, C.; Louie, G.; Kaddurah-Daouk, R.; et al. Association of Plasma and CSF Cytochrome P450, Soluble Epoxide Hydrolase, and Ethanolamide Metabolism with Alzheimer’s Disease. Alzheimer’s Res. Ther. 2021, 13, 149. [Google Scholar] [CrossRef] [PubMed]
- Ginsberg, S.D.; Neubert, T.A.; Sharma, S.; Digwal, C.S.; Yan, P.; Timbus, C.; Wang, T.; Chiosis, G. Disease-specific Interactome Alterations via Epichaperomics: The Case for Alzheimer’s Disease. FEBS J. 2021, 289, 2047–2066. [Google Scholar] [CrossRef]
- Haenig, C.; Atias, N.; Taylor, A.K.; Mazza, A.; Schaefer, M.H.; Russ, J.; Riechers, S.-P.; Jain, S.; Coughlin, M.; Fontaine, J.-F.; et al. Interactome Mapping Provides a Network of Neurodegenerative Disease Proteins and Uncovers Widespread Protein Aggregation in Affected Brains. Cell Rep. 2020, 32, 108050. [Google Scholar] [CrossRef]
- Drummond, E.; Pires, G.; MacMurray, C.; Askenazi, M.; Nayak, S.; Bourdon, M.; Safar, J.; Ueberheide, B.; Wisniewski, T. Phosphorylated Tau Interactome in the Human Alzheimer’s Disease Brain. Brain 2020, 143, 2803–2817. [Google Scholar] [CrossRef]
- Drummond, E.; Wisniewski, T. Using Proteomics to Understand Alzheimer’s Disease Pathogenesis. In Alzheimer’s Disease; Wisniewski, T., Ed.; Codon Publications: Singapore, 2019; pp. 37–51. ISBN 9780646809687. [Google Scholar]
- Soler-López, M.; Zanzoni, A.; Lluís, R.; Stelzl, U.; Aloy, P. Interactome Mapping Suggests New Mechanistic Details Underlying Alzheimer’s Disease. Genome Res. 2011, 21, 364–376. [Google Scholar] [CrossRef]
- Alam, J.; Blackburn, K.; Patrick, D. Neflamapimod: Clinical Phase 2b-Ready Oral Small Molecule Inhibitor of p38α to Reverse Synaptic Dysfunction in Early Alzheimer’s Disease. J. Prev. Alzheimer’s Dis. 2017, 4, 273–278. [Google Scholar] [CrossRef]
- Codony, S.; Pont, C.; Griñán-Ferré, C.; Di Pede-Mattatelli, A.; Calvó-Tusell, C.; Feixas, F.; Osuna, S.; Jarné-Ferrer, J.; Naldi, M.; Bartolini, M.; et al. Discovery and In Vivo Proof of Concept of a Highly Potent Dual Inhibitor of Soluble Epoxide Hydrolase and Acetylcholinesterase for the Treatment of Alzheimer’s Disease. J. Med. Chem. 2022, 65, 4909–4925. [Google Scholar] [CrossRef]
- Villapol, S. Roles of Peroxisome Proliferator-Activated Receptor Gamma on Brain and Peripheral Inflammation. Cell Mol. Neurobiol. 2018, 38, 121–132. [Google Scholar] [CrossRef]
- Jankiewicz, W.K.; Barnett, S.D.; Stavniichuk, A.; Hwang, S.H.; Hammock, B.D.; Belayet, J.B.; Khan, A.H.; Imig, J.D. Dual SEH/COX-2 Inhibition Using PTUPB—A Promising Approach to Antiangiogenesis-Induced Nephrotoxicity. Front. Pharmacol. 2021, 12, 744776. [Google Scholar] [CrossRef]
- Moser, D.; Wisniewska, J.M.; Hahn, S.; Achenbach, J.; Buscató, E.; Klingler, F.-M.; Hofmann, B.; Steinhilber, D.; Proschak, E. Dual-Target Virtual Screening by Pharmacophore Elucidation and Molecular Shape Filtering. ACS Med. Chem. Lett. 2012, 3, 155–158. [Google Scholar] [CrossRef]
- Hye Khan, M.A.; Kolb, L.; Skibba, M.; Hartmann, M.; Blöcher, R.; Proschak, E.; Imig, J.D. A Novel Dual PPAR-γ Agonist/SEH Inhibitor Treats Diabetic Complications in a Rat Model of Type 2 Diabetes. Diabetologia 2018, 61, 2235–2246. [Google Scholar] [CrossRef]
- Kovacs, G.G. Tauopathies. In Handbook of Clinical Neurology; Elsevier: Amsterdam, The Netherlands, 2018; Volume 145, pp. 355–368. ISBN 9780128023952. [Google Scholar]
- Yadikar, H.; Torres, I.; Aiello, G.; Kurup, M.; Yang, Z.; Lin, F.; Kobeissy, F.; Yost, R.; Wang, K.K. Screening of Tau Protein Kinase Inhibitors in a Tauopathy-Relevant Cell-Based Model of Tau Hyperphosphorylation and Oligomerization. PLoS ONE 2020, 15, e0224952. [Google Scholar] [CrossRef]
- Prins, N.D.; Harrison, J.E.; Chu, H.-M.; Blackburn, K.; Alam, J.J.; Scheltens, P. A Phase 2 Double-Blind Placebo-Controlled 24-Week Treatment Clinical Study of the P38 Alpha Kinase Inhibitor Neflamapimod in Mild Alzheimer’s Disease. Alzheimer’s Res. Ther. 2021, 13, 106. [Google Scholar] [CrossRef]
- Schnöder, L.; Hao, W.; Qin, Y.; Liu, S.; Tomic, I.; Liu, X.; Fassbender, K.; Liu, Y. Deficiency of Neuronal P38α MAPK Attenuates Amyloid Pathology in Alzheimer Disease Mouse and Cell Models through Facilitating Lysosomal Degradation of BACE1. J. Biol. Chem. 2016, 291, 2067–2079. [Google Scholar] [CrossRef]
- Martin, L.; Latypova, X.; Wilson, C.M.; Magnaudeix, A.; Perrin, M.-L.; Yardin, C.; Terro, F. Tau Protein Kinases: Involvement in Alzheimer’s Disease. Ageing Res. Rev. 2013, 12, 289–309. [Google Scholar] [CrossRef]


| Abbreviation | Target | Abbreviation | Target |
|---|---|---|---|
| A1/A2 | adenosine A1/A2 receptor | JNK1-3 | c-Jun N-terminal kinases 1-3 |
| AChE | acetylcholinesterase | KEAP1 | Kelch-like ECH-associated protein 1 |
| Aβ aggr | β-amyloid aggregation | LOX1/5/15 | Lipoxygenase 1/5/15 |
| α2 | α2 adrenergic receptor | LTP | long-term potentiation |
| α7-nAChR | α7 nicotinic receptor | M1 | muscarinic M1 receptor |
| α-syn | α-synuclein aggregation | MAO-A/MAO-B | monoamine oxidase A/B |
| BACE-1 | β-secretase | miR-15b | microRNA 15b |
| BChE | butyrylcholinesterase | MMP2 | matrix metalloproteinase-2 |
| Biometal | biometals chelation | m-nAChR | muscle-type nicotinic receptor |
| CA1/CA2 | carbonic anhydrase 1/2 | MPO | myeloperoxidase |
| Casp1/Casp3 | caspase 1/3 | MT | microtubule |
| CaV | voltage-gated calcium channel | NMDA | N-methyl-D-aspartate receptor |
| CB1/CB2 | cannabinoid receptor 1/2 | NO | nitric oxide release |
| CDK1/CDK5 | cyclin-dependent kinase 1/5 | NOS | nitric oxide synthase |
| CK1 | casein kinase 1 | NRF2 | nuclear factor-erythroid 2 p45-related factor 2 |
| CLK1/3 | cdc2-like kinase 1/3 | OS | oxidative stress |
| COX1/COX2 | cyclooxygenase 1/2 | P2X7 | purinergic P2X7 receptors |
| D2 | dopamine D2 receptor | PARP-1 | poly(ADP-ribose) polymerase 1 |
| DYRK1/2 | dual-specificity tyrosine phosphory-lation-regulated kinase 1/2 | PDE4/5/9/10 | phosphodiesterases 4/5/9/10 |
| ERK | extracellular signal-regulated kinase | PHOS | serine/threonine phosphatases |
| ERα/ERβ | estrogen receptor α/β | PIM1 | Pim1 kinase |
| FAAH | fatty acid amide hydrolase | PKC | protein kinase C |
| FYN | Fyn kinase | POP | prolyl oligopeptidase |
| GSK-3α/3β | glycogen synthase kinase 3α/3β | QR | quinone reductase |
| H1/H3 | histamine H1/H3 receptor | ROCK1/2 | Rho-associated coiled-coil kinase 1/2 |
| 5-HT1/2/4/6/7 | serotonin receptor 1/2/4/6/7 | S1 | sigma-1 receptor |
| HDAC1-7,9 | histone deacetylase 1-7,9 | SERT | serotonin reuptake transporter |
| HIF1 | hypoxia-inducible factor 1 | SSAO/VAP-1 | semicarbazide-sensitive amine oxidase/vascular adhesion protein-1 |
| HSP27 | heat shock protein 27 | TAU | tau aggregation |
| IDO1 | indoleamine 2,3-dioxygenase 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sampietro, A.; Pérez-Areales, F.J.; Martínez, P.; Arce, E.M.; Galdeano, C.; Muñoz-Torrero, D. Unveiling the Multitarget Anti-Alzheimer Drug Discovery Landscape: A Bibliometric Analysis. Pharmaceuticals 2022, 15, 545. https://doi.org/10.3390/ph15050545
Sampietro A, Pérez-Areales FJ, Martínez P, Arce EM, Galdeano C, Muñoz-Torrero D. Unveiling the Multitarget Anti-Alzheimer Drug Discovery Landscape: A Bibliometric Analysis. Pharmaceuticals. 2022; 15(5):545. https://doi.org/10.3390/ph15050545
Chicago/Turabian StyleSampietro, Anna, F. Javier Pérez-Areales, Paula Martínez, Elsa M. Arce, Carles Galdeano, and Diego Muñoz-Torrero. 2022. "Unveiling the Multitarget Anti-Alzheimer Drug Discovery Landscape: A Bibliometric Analysis" Pharmaceuticals 15, no. 5: 545. https://doi.org/10.3390/ph15050545
APA StyleSampietro, A., Pérez-Areales, F. J., Martínez, P., Arce, E. M., Galdeano, C., & Muñoz-Torrero, D. (2022). Unveiling the Multitarget Anti-Alzheimer Drug Discovery Landscape: A Bibliometric Analysis. Pharmaceuticals, 15(5), 545. https://doi.org/10.3390/ph15050545