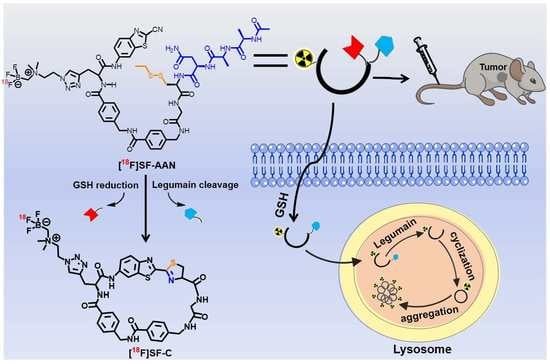
Development of a Promising 18F-Radiotracer for PET Imaging Legumain Activity In Vivo
Abstract
:1. Introduction
2. Results
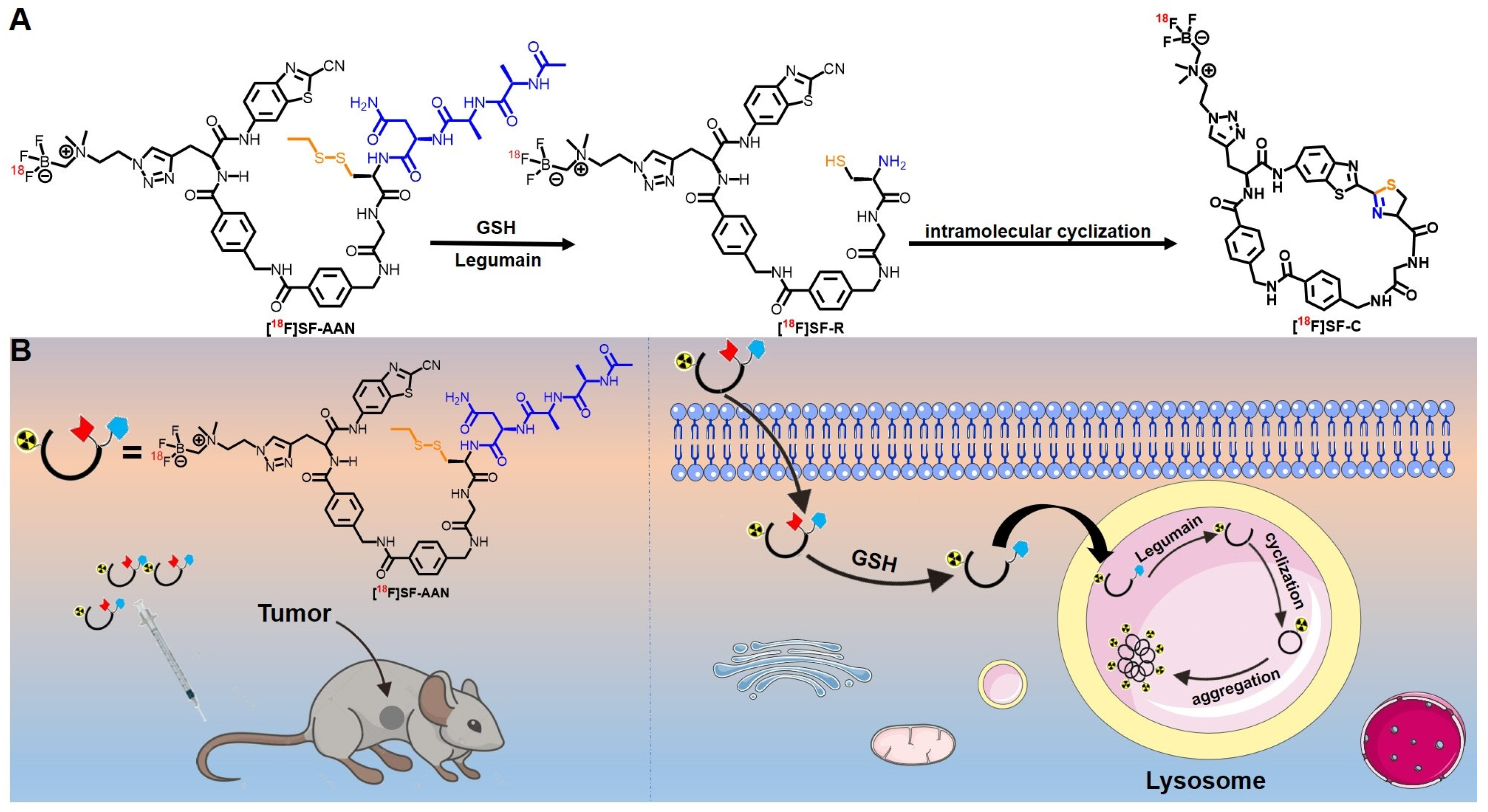
2.1. Chemical Synthesis and Characterization
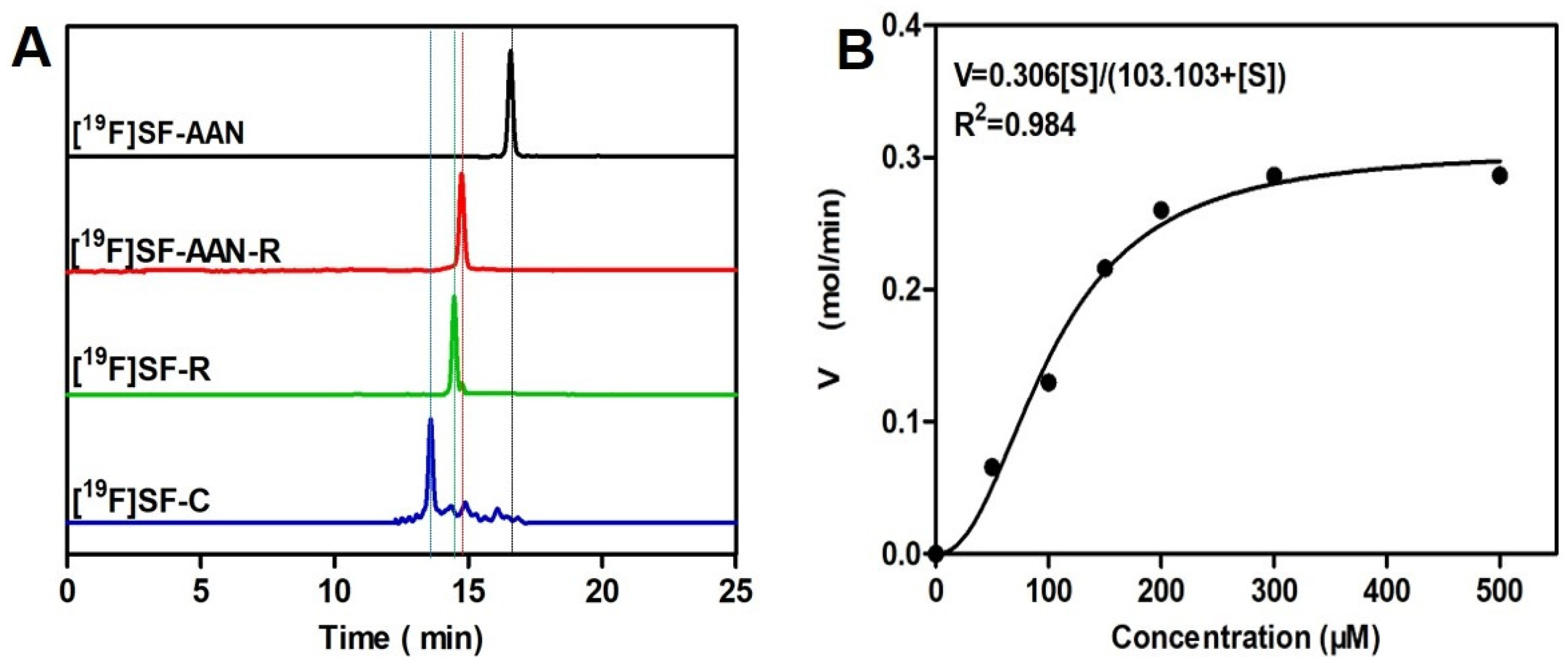
2.2. Radiolabeling and Stability Assay
2.3. Reduction and Legumain-Controlled Self-Assembly
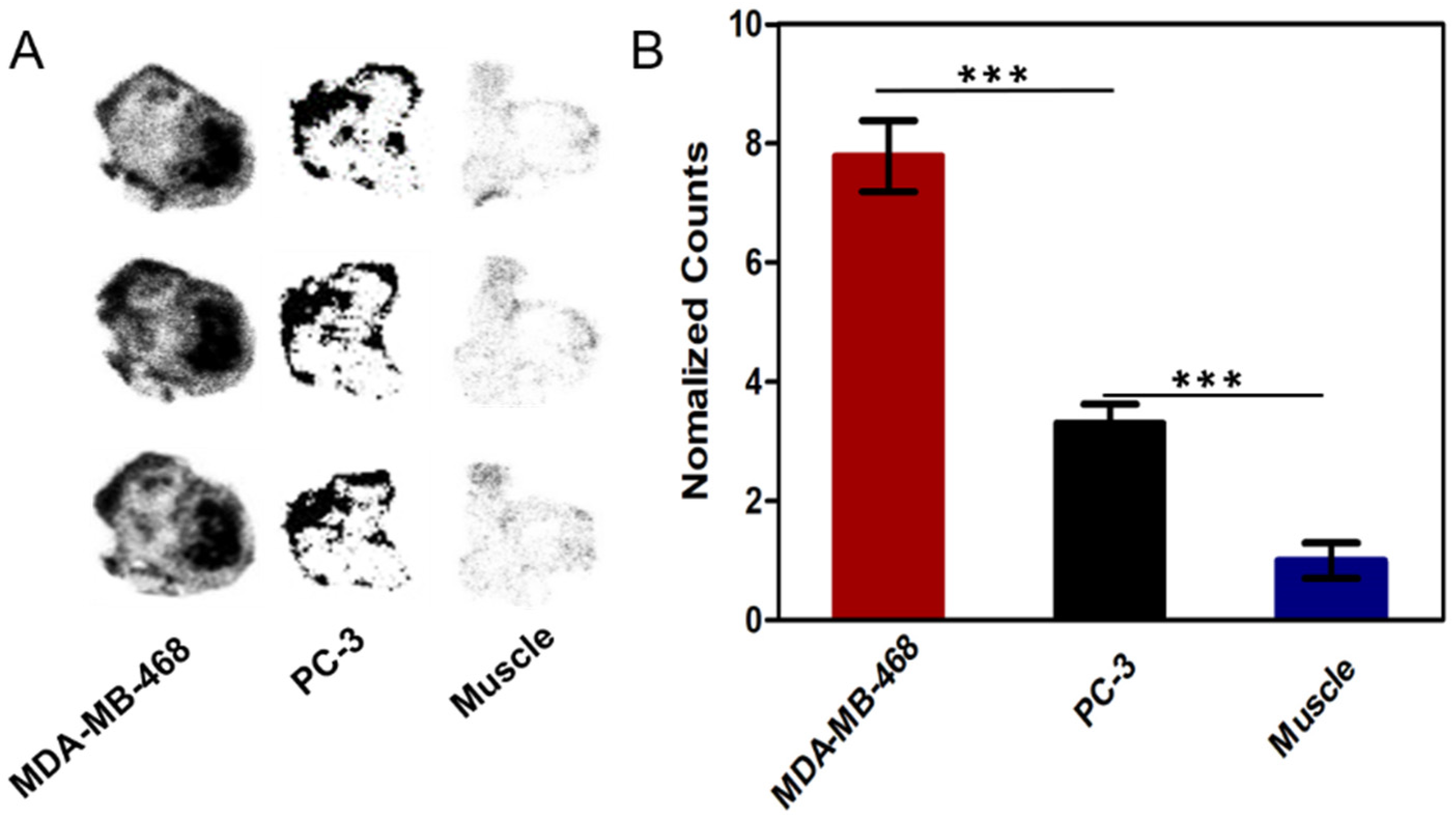
2.4. Cellular Uptake Assay
2.5. Intramolecular Condensation in Cancer Cells
2.6. Colocalization Assay
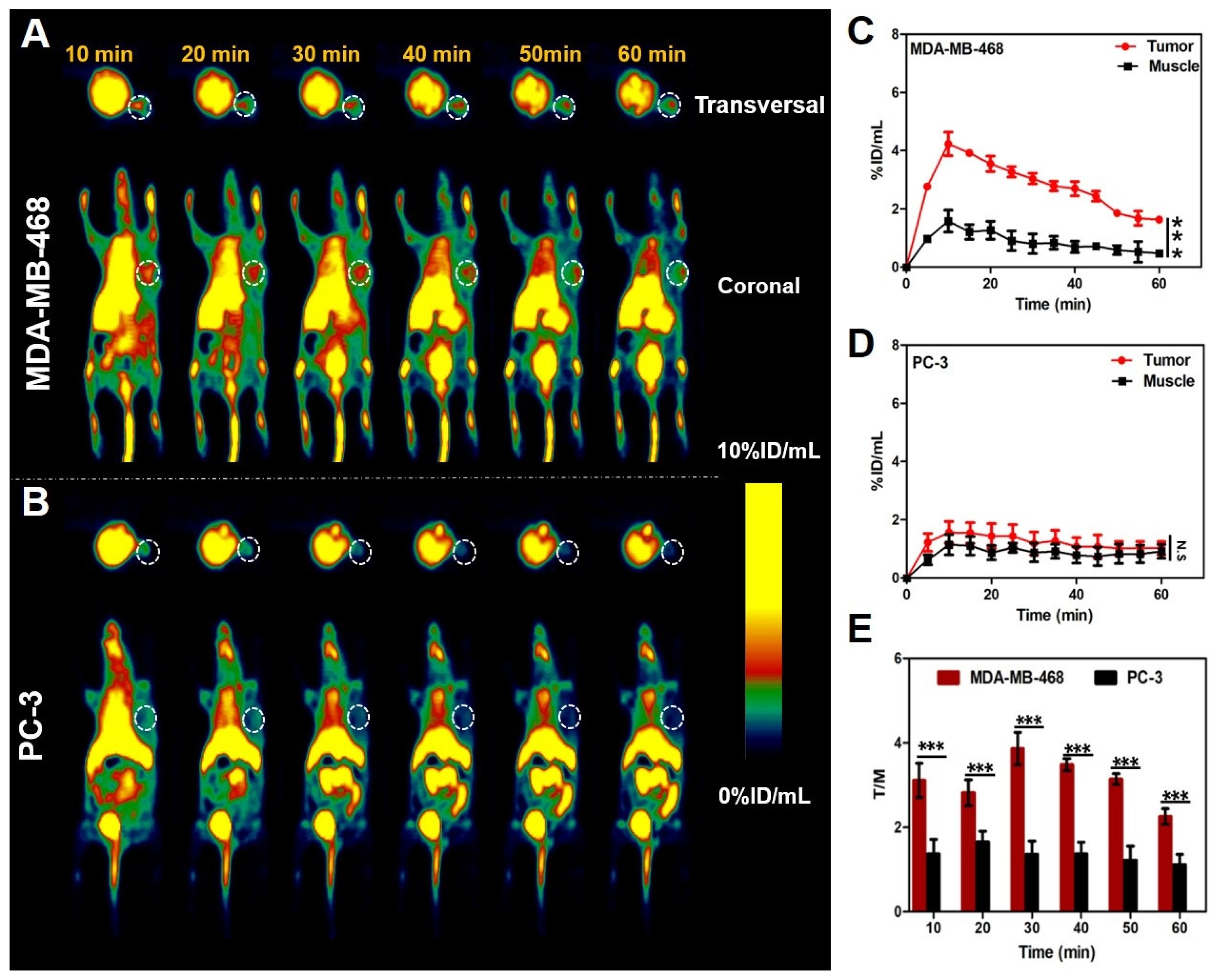
2.7. PET Imaging of Tumor-Bearing Mice
2.8. Biodistribution and Histopathological Analysis
3. Discussion
4. Materials and Methods
4.1. General Information
4.2. Synthesis of Nonradioactive Probe [19F]SF-AAN
4.3. Radiosynthesis of Tracer [18F]SF-AAN
4.4. Stability Assay
4.5. Measurement of Water Partition Coefficient
4.6. Reduction and Legumain-Controlled Self-Assembly
4.7. Cell Culture and Western Blot Analysis
4.8. In Vitro Biocompatibility Test
4.9. Cellular Uptake Assay
4.10. Intramolecular Condensation in Cancer Cells
4.11. Colocalization Assay
4.12. Pharmacokinetics Study
4.13. In Vivo PET Imaging
4.14. Ex Vivo Biodistribution and Histopathological Analysis
4.15. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Chen, W.; Lin, W.; Yuan, W.; Liu, H.; Xie, H.; Zhang, Q.; Zhang, P.; Ding, C. A turn on fluorescent assay for γ-glutamytransferase activity and its application in biological imaging. Talanta 2021, 239, 123126. [Google Scholar] [CrossRef] [PubMed]
- Singal, A.; Mittal, S.; Yerokun, O.; Ahn, C.; Marrero, J.; Yopp, A.; Parikh, N.; Scaglione, S. Hepatocellular Carcinoma Screening Associated with Early Tumor Detection and Improved Survival among Patients with Cirrhosis in the US. Am. J. Med. 2017, 130, 1099–1106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, H.; Gu, Z.; An, H.; Chen, C.; Chen, J.; Cui, R.; Chen, S.; Chen, W.; Chen, X.; Chen, X.; et al. Precise nanomedicine for intelligent therapy of cancer. Sci. China Chem. 2018, 61, 1503–1552. [Google Scholar] [CrossRef]
- Lin, Y.; Zhou, Q.; Li, J.; Shu, J.; Qiu, Z.; Lin, Y.; Tang, D. Magnetic Graphene Nanosheet-Based Microfluidic Device for Homogeneous Real-Time Electronic Monitoring of Pyrophosphatase Activity Using Enzymatic Hydrolysate-Induced Release of Copper Ion. Anal. Chem. 2016, 88, 1030–1038. [Google Scholar] [CrossRef]
- Edgington, L.; Verdoes, M.; Ortega, A.; Withana, N.; Lee, J.; Syed, S.; Bachmann, M.; Blum, G.; Bogyo, M. Functional Imaging of Legumain in Cancer Using a New Quenched Activity-Based Probe. J. Am. Chem. Soc. 2013, 135, 174–182. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.; Sun, C.; Huang, H.; Janda, K.; Edgington, T. Overexpression of Legumain in Tumors Is Significant for Invasion/Metastasis and a Candidate Enzymatic Target for Prodrug Therapy. Cancer Res. 2003, 63, 2957–2964. [Google Scholar]
- Mai, C.; Chung, F.; Leong, C.O. Targeting Legumain As a Novel Therapeutic Strategy in Cancers. Curr. Drug. Targets 2017, 18, 1259–1268. [Google Scholar] [CrossRef]
- Mathur, S.; Turnbull, A.; Akaev, I.; Stevens, C.; Agrawal, N.; Chopra, M.; Mincher, D. Design of a New Peptide Substrate Probe of the Putative Biomarker Legumain with Potential Application in Prostate Cancer Diagnosis Ex vivo. Int. J. Pept. Res. Ther. 2019, 26, 1965–1980. [Google Scholar] [CrossRef] [Green Version]
- Lin, Q.; Zhang, Y.; Fu, Z.; Hu, B.; Si, Z.; Zhao, Y.; Shi, H.; Cheng, D. Synthesis and evaluation of 18F labeled crizotinib derivative [18F]FPC as a novel PET probe for imaging c-MET-positive NSCLC tumor. Bioorg. Med. Chem. 2020, 28, 115577. [Google Scholar] [CrossRef]
- Zhao, Y.; Hai, Z.; Wang, H.; Su, L.; Liang, G. Legumain-Specific Near-Infrared Fluorescence “Turn On” for Tumor-Targeted Imaging. Anal. Chem. 2018, 90, 8732–8735. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamane, T.; Kozuka, M.; Yamamoto, Y.; Nakano, Y.; Nakagaki, T.; Ohkubo, I.; Ariga, H. Protease activity of legumain is inhibited by an increase of cystatin E/M in the DJ-1-knockout mouse spleen, cerebrum and heart. Biochem. Biophys. Rep. 2017, 9, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Xiong, M.; Gong, J.; Zhang, Y.; Bai, N.; Luo, Y.; Li, L.; Wei, Y.; Liu, Y.; Tan, X.; et al. Legumain protease-activated TAT-liposome cargo for targeting tumours and their microenvironment. Nat. Commun. 2014, 5, 4280. [Google Scholar] [CrossRef] [PubMed]
- Gawenda, J.; Traub, F.; Luck, H.; Kreipe, H.; Von Wasielewski, R. Legumain expression as a prognostic factor in breast cancer patients. Breast Cancer Res. Treat. 2007, 102, 1–6. [Google Scholar] [CrossRef]
- He, B.; Tan, T.; Wang, H.; Hu, H.; Wang, Z.; Wang, J.; Li, J.; Sun, K.; Zhang, Z.; Li, Y. Rational Design of Tumor Microenvironment-Activated Micelles for Programed Targeting of Breast Cancer Metastasis. Adv. Funct. Mater. 2018, 28, 1705622. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, S.; Wang, H.; Cui, Y.; Wang, Z.; Cheng, X.; Li, W.; Hou, J.; Ji, Y.; Liu, T. High Level of Legumain Was Correlated with Worse Prognosis and Peritoneal Metastasis in Gastric Cancer Patients. Front. Oncol. 2020, 10, 966. [Google Scholar] [CrossRef]
- Harbeck, N.; Gnant, M. Breast cancer. Lancet 2017, 389, 1134–1150. [Google Scholar] [CrossRef]
- Romeo, V.; Clauser, P.; Rasul, S.; Kapetas, P.; Gibbs, P.; Baltzer, P.; Hacker, M.; Woitek, R.; Helbich, T.; Pinker, K. AI-enhanced simultaneous multiparametric 18F-FDG PET/MRI for accurate breast cancer diagnosis. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 596–608. [Google Scholar] [CrossRef]
- Li, X.; Liu, Q.; Ye, S.; Wang, S.; Li, K.; Lv, G.; Peng, Y.; Qiu, L.; Lin, J. A protease-responsive fluorescent probe for sensitive imaging of legumain activity in living tumor cells. Chem. Biol. Drug. Des. 2019, 94, 1494–1503. [Google Scholar]
- Yuan, Y.; Ge, S.; Sun, H.; Dong, X.; Zhao, H.; An, L.; Zhang, J.; Wang, J.; Hu, B.; Liang, G. Intracellular Self-assembly and Disassembly of 19F Nanoparticles Confer Respective “Off” and “On” 19F NMR/MRI Signals for Legumain Activity Detection in Zebrafish. ACS Nano 2015, 9, 5117–5124. [Google Scholar] [CrossRef]
- Luo, M.; Li, Q.; Wang, D.; Ge, C.; Wang, J.; Nan, K.; Lin, S. Fabrication of chitosan based nanocomposite with legumain sensitive properties using charge driven self-assembly strategy. J. Mater. Sci. Mater. Med. 2018, 29, 142. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Xie, P.; Luo, M.; Li, Q.; Li, L.; Zhang, J.; Zheng, Q.; Chen, H.; Nan, K. Efficiency against multidrug resistance by co-delivery of doxorubicin and curcumin with a legumain-sensitive nanocarrier. Nano Res. 2018, 11, 3619–3635. [Google Scholar] [CrossRef]
- Lin, S.; Li, T.; Xie, P.; Li, Q.; Wang, B.; Wang, L.; Li, L.; Wang, Y.; Chen, H.; Nan, K. Targeted delivery of doxorubicin to tumour tissues by a novel legumain sensitive polygonal nanogel. Nanoscale 2016, 8, 18400–18411. [Google Scholar] [CrossRef] [PubMed]
- Qiu, L.; Li, X.; Lv, G.; Seimbille, Y.; Li, K.; Peng, Y.; Liu, Q.; Xie, M.; Lin, J. Radiofluorinated Smart Probes for Noninvasive PET Imaging of Legumain Activity in Living Subjects. Anal. Chem. 2020, 92, 11627–11634. [Google Scholar] [CrossRef] [PubMed]
- Ruan, S.; Hu, C.; Tang, X.; Cun, X.; Xiao, W.; Shi, K.; He, Q.; Gao, H. Increased Gold Nanoparticle Retention in Brain Tumors by in situ Enzyme-Induced aggregation. ACS Nano 2016, 10, 10086–10098. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Li, J.; Lin, S.; Lu, C.; Chiu, S.; Jeng, F.; Chang, C.; Yang, B.; Chang, M.; Ke, C.; et al. Effect of Cerenkov Radiation-Induced Photodynamic Therapy with 18F-FDG in an Intraperitoneal Xenograft Mouse model of ovarian cancer. Int. J. Mol. Sci. 2021, 22, 4934. [Google Scholar] [CrossRef]
- Stotz, S.; Bowden, G.; Cotton, J.; Pichler, B.; Maurer, A. Covalent 18F-Radiotracers for SNAPTag: A New Toolbox for Reporter Gene Imaging. Pharmaceuticals 2021, 14, 897. [Google Scholar] [CrossRef]
- Vandergaast, R.; Khongwichit, S.; Jiang, H.; DeGrado, T.; Peng, K.; Smith, D.; Russell, S.; Suksanpaisan, L. Enhanced noninvasive imaging of oncology models using the NIS reporter gene and bioluminescence imaging. Cancer Gene Ther. 2020, 27, 179–188. [Google Scholar] [CrossRef] [Green Version]
- Zhang, M.; Li, S.; Zhang, H.; Xu, H. Research progress of 18F labeled small molecule positron emission tomography (PET) imaging agents. Eur. J. Med. Chem. 2020, 205, 112629. [Google Scholar] [CrossRef]
- An, F.; Nurili, F.; Sayman, H.; Ozer, Z.; Cakiroglu, H.; Aras, O.; Ting, R. One-Step, Rapid, 18F-19F Isotopic Exchange Radiolabeling of Difluoro-dioxaborinins: Substituent Effect on Stability and In Vivo Applications. J. Med. Chem. 2020, 63, 12693–12706. [Google Scholar] [CrossRef]
- Ye, D.; Liang, G.; Ma, M.; Rao, J. Controlling Intracellular Macrocyclization for the Imaging of Protease Activity. Angew. Chem. Int. Ed. Engl. 2011, 50, 2275–2279. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Chen, M.; Cheng, Y.; Kowada, T.; Xie, J.; Zheng, X.; Rao, J. Exploring the Condensation Reaction between Aromatic Nitriles and Amino Thiols to Optimize in Situ Nanoparticle Formation for the Imaging of Proteases and Glycosidases in Cells. Angew. Chem. Int. Ed. Engl. 2020, 59, 3272–3279. [Google Scholar] [CrossRef] [PubMed]
- Qiang, L.; Li, H.; Dong, X.; Lv, M.; Lu, K. Effect of Alanine and Glycine on the Assembly of Surfactant-like Peptides with Tyrosine as Hydrophilic Head in Basic Solution. J. Chin. Chem. Soc. 2017, 64, 1220–1226. [Google Scholar] [CrossRef]
- Qiu, L.; Lin, J.; Lv, G.; Liu, Q.; Li, K.; Gao, D.; Lu, C. A Self-Assembly Molecular Probe Activated by Tumor Protease and the Preparative Method and Application. CN Patent 202110656191.7, 14 September 2021. [Google Scholar]
- Sadowsky, J.; Pillow, T.; Chen, J.; Fan, F.; He, C.; Wang, Y.; Yan, G.; Yao, H.; Xu, Z.; Martin, S.; et al. Development of Efficient Chemistry to Generate Site-Specific Disulfide-Linked Protein-and Peptide-Payload Conjugates: Application to THIOMAB Antibody-drug Conjugates. Bioconjug. Chem. 2017, 28, 2086–2098. [Google Scholar] [CrossRef]
- Liang, G.; Ren, H.; Rao, J. A biocompatible condensation reaction for controlled assembly of nanostructures in live cells. Nat. Chem. 2009, 2, 239. [Google Scholar] [CrossRef] [Green Version]
- Zhang, M.; Liang, G. Applications of CBT-Cys click reaction: Past, present, and future. Sci. China Chem. 2018, 61, 1088–1098. [Google Scholar] [CrossRef]
- Yuan, Y.; Liang, G. A biocompatible, highly efficient click reaction and its applications. Org. Biomol. Chem. 2014, 12, 865–871. [Google Scholar] [CrossRef]
- Yuan, Y.; Zhang, J.; Qi, X.; Li, S.; Liu, G.; Siddhanta, S.; Barman, I.; Song, X.; McMahon, M.; Bulte, J. Furin-mediated intracellular self-assembly of olsalazine nanoparticles for enhanced magnetic resonance imaging and tumour therapy. Nat. Mater. 2019, 18, 1376–1383. [Google Scholar] [CrossRef]
- Liu, Z.; Pourghiasian, M.; Radtke, M.; Lau, J.; Pan, J.; Dias, G.; Yapp, D.; Lin, K.; Benard, F.; Perrin, D. An organotrifluoroborate for broadly applicable one-step 18F-labeling. Angew. Chem. Int. Ed. Engl. 2014, 53, 11876–11880. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, C.; Wang, X.; Wang, Q.; Zhang, L.; Lin, J.; Qiu, L. Development of a Promising 18F-Radiotracer for PET Imaging Legumain Activity In Vivo. Pharmaceuticals 2022, 15, 543. https://doi.org/10.3390/ph15050543
Lu C, Wang X, Wang Q, Zhang L, Lin J, Qiu L. Development of a Promising 18F-Radiotracer for PET Imaging Legumain Activity In Vivo. Pharmaceuticals. 2022; 15(5):543. https://doi.org/10.3390/ph15050543
Chicago/Turabian StyleLu, Chunmei, Xiuting Wang, Qiqi Wang, Lixia Zhang, Jianguo Lin, and Ling Qiu. 2022. "Development of a Promising 18F-Radiotracer for PET Imaging Legumain Activity In Vivo" Pharmaceuticals 15, no. 5: 543. https://doi.org/10.3390/ph15050543
APA StyleLu, C., Wang, X., Wang, Q., Zhang, L., Lin, J., & Qiu, L. (2022). Development of a Promising 18F-Radiotracer for PET Imaging Legumain Activity In Vivo. Pharmaceuticals, 15(5), 543. https://doi.org/10.3390/ph15050543