An Albumin-Binding PSMA Ligand with Higher Tumor Accumulation for PET Imaging of Prostate Cancer
Abstract
:1. Introduction
2. Results
2.1. Radiosynthesis and Quality Control
2.2. In Vitro Stability
2.3. Partition Coefficient
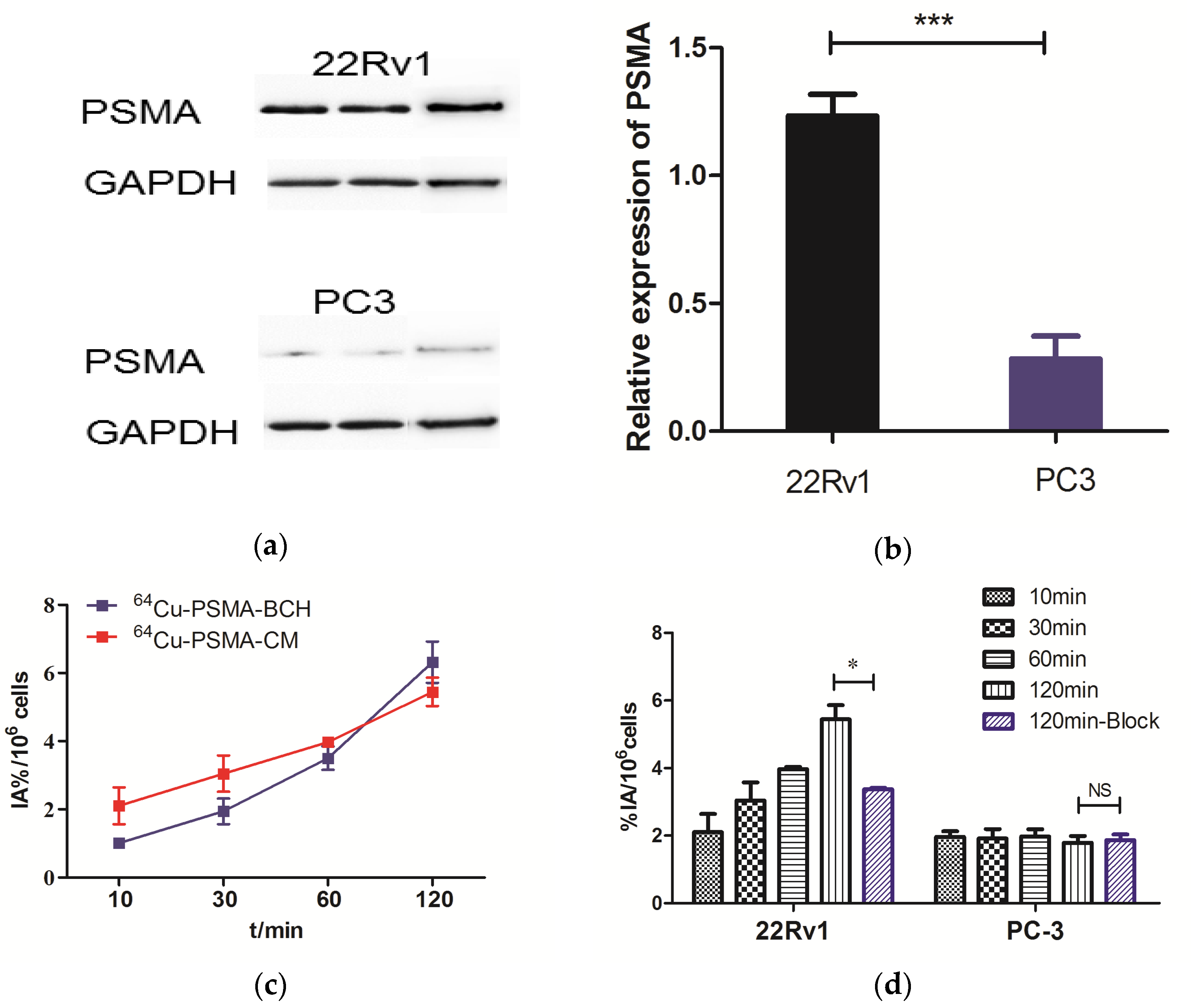
2.4. Western Blotting and In Vitro Cell Experiments
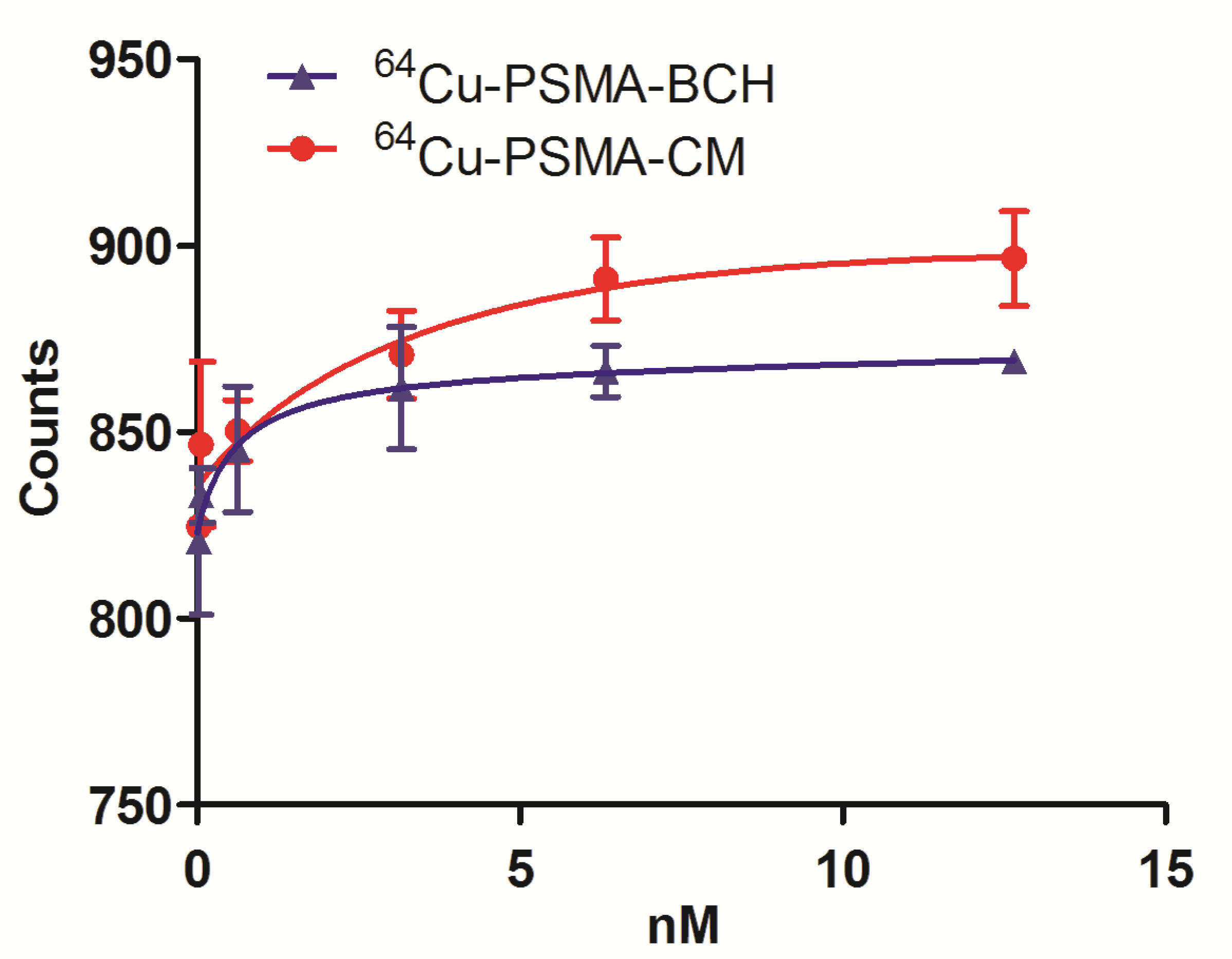
2.5. Binding Affinity Assay
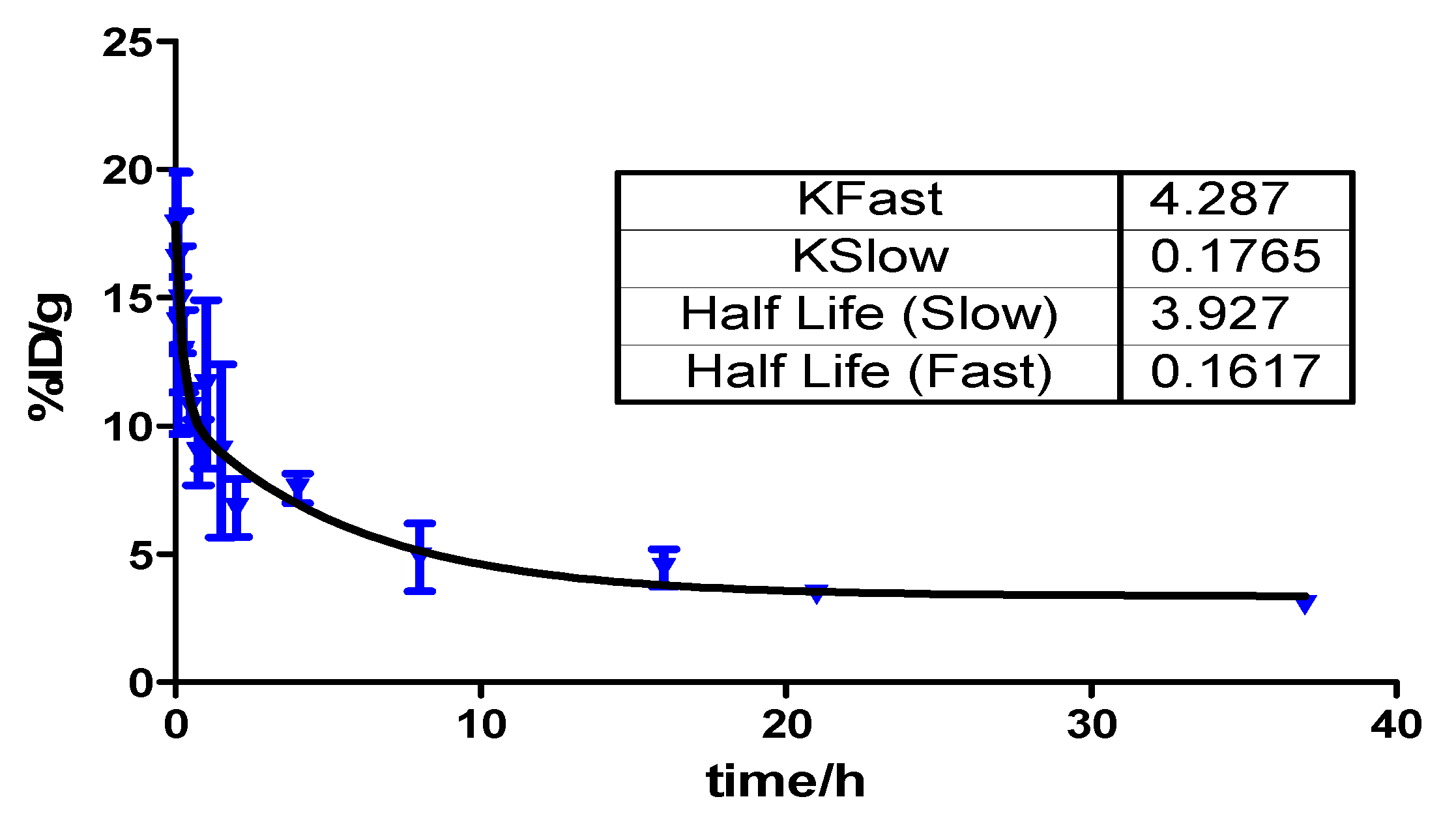
2.6. Pharmacokinetics
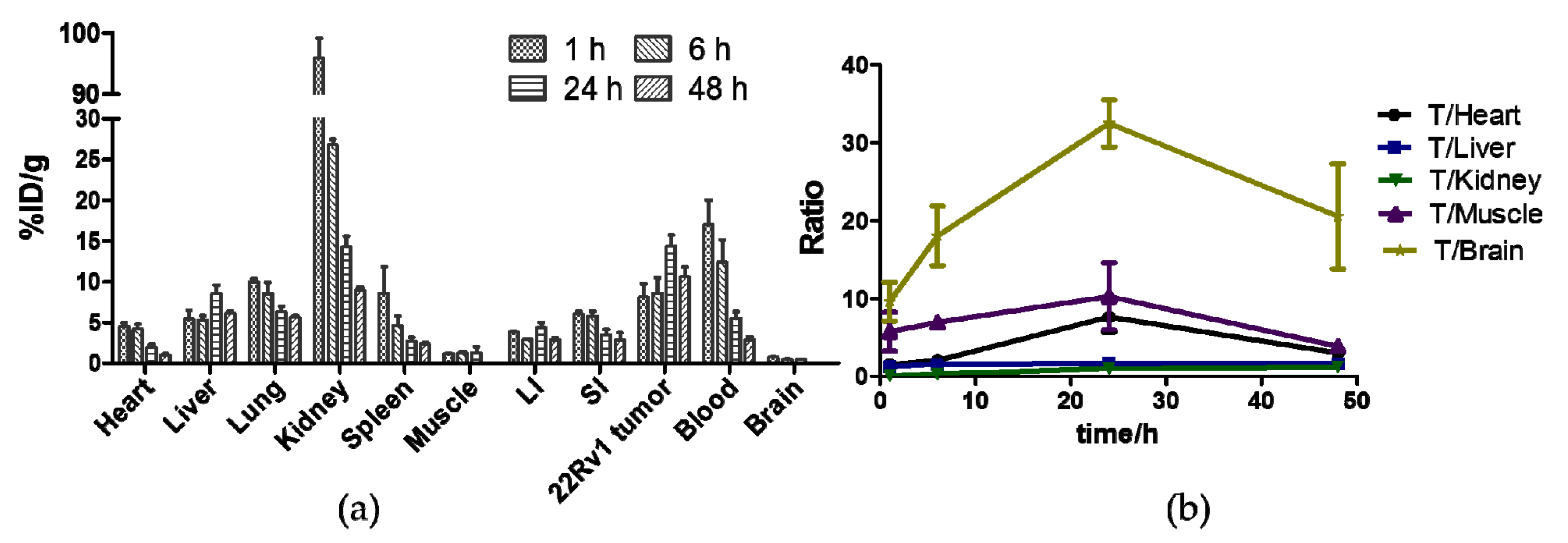
2.7. Biodistribution Studies
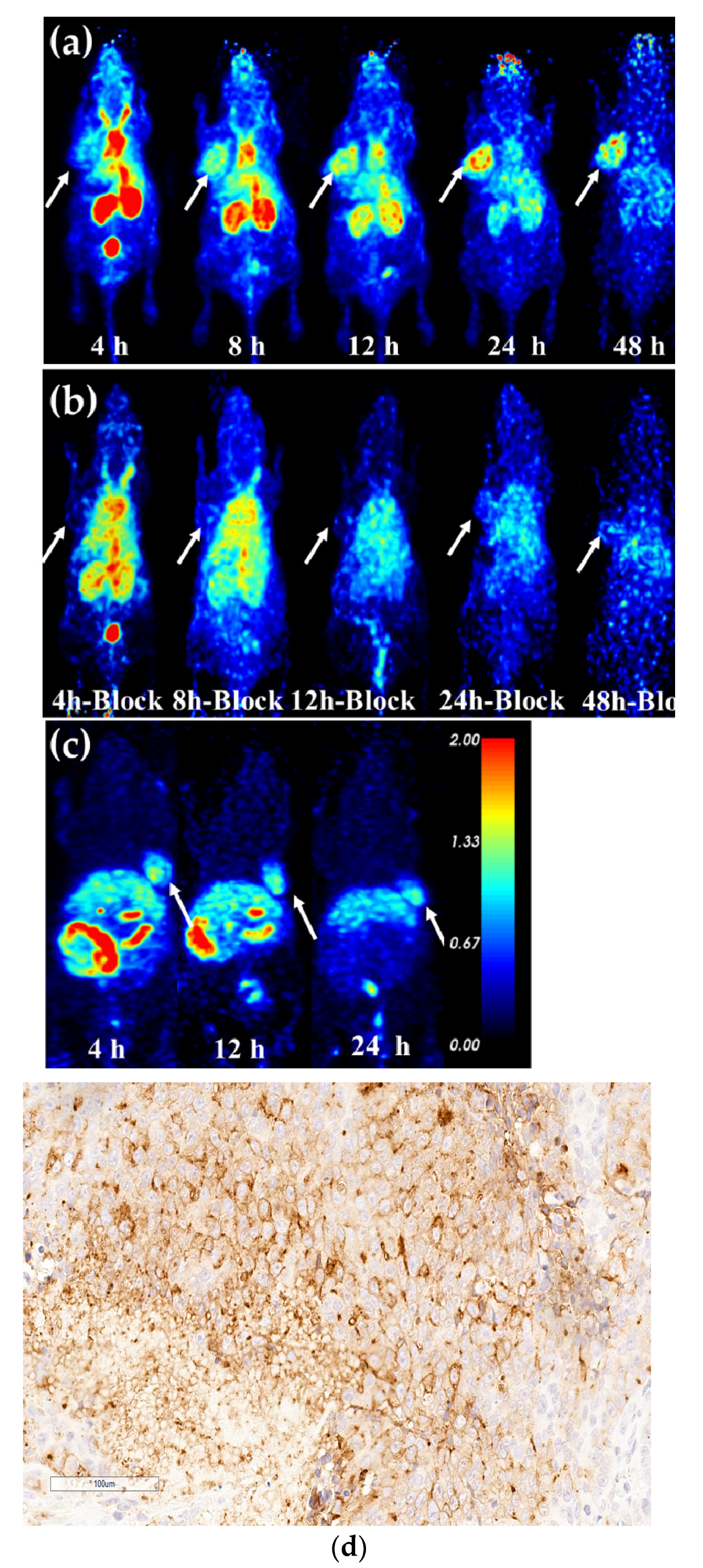
2.8. Micro-PET Imaging
2.9. Estimation of Radiation Dosimetry
3. Discussion
4. Materials and Methods
4.1. General Materials
4.2. Radiosynthesis and Quality Control of 64Cu-PSMA-CM
4.3. In Vitro Stability
4.4. Partition Coefficient
4.5. Cell Culture and Animal Models
4.6. Western Blotting and Cell Uptake
4.7. Binding Affinity Assay
4.8. Pharmacokinetics in Blood
4.9. Biodistribution
4.10. Micro-PET Imaging and Immunohistochemical Staining
4.11. Estimation of Radiation Dosimetry in Human Organs
4.12. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Okarvi, S.M. Recent developments of prostate-specific membrane antigen (PSMA)-specific radiopharmaceuticals for precise imaging and therapy of prostate cancer: An overview. Clin. Transl. Imaging 2019, 7, 189–208. [Google Scholar] [CrossRef]
- Hofman, M.S.; Emmett, L.; Sandhu, S.; Iravani, A.; Joshua, A.M.; Goh, J.C.; Pattison, D.A.; Tan, T.H.; Kirkwood, I.D.; Ng, S.; et al. [177Lu]Lu-PSMA-617 versus cabazitaxel in patients with metastatic castration-resistant prostate cancer (TheraP): A randomised, open-label, phase 2 trial. Lancet 2021, 397, 797–804. [Google Scholar] [CrossRef]
- Zacho, H.D.; Ravn, S.; Afshar-Oromieh, A.; Fledelius, J.; Ejlersen, J.A.; Petersen, L.J. Added value of (68)Ga-PSMA PET/CT for the detection of bone metastases in patients with newly diagnosed prostate cancer and a previous (99m)Tc bone scintigraphy. EJNMMI Res. 2020, 10, 31. [Google Scholar] [CrossRef] [PubMed]
- Werner, P.; Neumann, C.; Eiber, M.; Wester, H.J.; Schottelius, M. [(99cm)Tc]Tc-PSMA-I&S-SPECT/CT: Experience in prostate cancer imaging in an outpatient center. EJNMMI Res. 2020, 10, 45. [Google Scholar] [CrossRef]
- Schmidkonz, C.; Götz, T.I.; Atzinger, A.; Ritt, P.; Prante, O.; Kuwert, T.; Bäuerle, T.; Goebell, P.; Cordes, M. 99mTc-MIP-1404 SPECT/CT for Assessment of Whole-Body Tumor Burden and Treatment Response in Patients with Biochemical Recurrence of Prostate Cancer. Clin. Nucl. Med. 2020, 45, e349–e357. [Google Scholar] [CrossRef]
- Piron, S.; Verhoeven, J.; Descamps, B.; Kersemans, K.; De Man, K.; Van Laeken, N.; Pieters, L.; Vral, A.; Vanhove, C.; De Vos, F. Intra-individual dynamic comparison of (18)F-PSMA-11 and (68)Ga-PSMA-11 in LNCaP xenograft bearing mice. Sci. Rep. 2020, 10, 21068. [Google Scholar] [CrossRef]
- Liu, C.; Liu, T.; Zhang, Z.; Zhang, N.; Du, P.; Yang, Y.; Liu, Y.; Yu, W.; Li, N.; Gorin, M.A.; et al. PSMA PET/CT and standard plus PET/CT-Ultrasound fusion targeted prostate biopsy can diagnose clinically significant prostate cancer in men with previous negative biopsies. J. Nucl. Med. 2020, 61, 1314–1319. [Google Scholar] [CrossRef]
- Lawal, I.O.; Mokoala, K.M.G.; Mahapane, J.; Kleyhans, J.; Meckel, M.; Vorster, M.; Ebenhan, T.; Rösch, F.; Sathekge, M.M. A prospective intra-individual comparison of [68Ga]Ga-PSMA-11 PET/CT, [68Ga]Ga-NODAGAZOL PET/CT, and [99mTc]Tc-MDP bone scintigraphy for radionuclide imaging of prostate cancer skeletal metastases. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 134–142. [Google Scholar] [CrossRef]
- Hofman, M.S.; Lawrentschuk, N.; Francis, R.J.; Tang, C.; Vela, I.; Thomas, P.; Rutherford, N.; Martin, J.M.; Frydenberg, M.; Shakher, R.; et al. Prostate-specific membrane antigen PET-CT in patients with high-risk prostate cancer before curative-intent surgery or radiotherapy (proPSMA): A prospective, randomised, multicentre study. Lancet 2020, 395, 1208–1216. [Google Scholar] [CrossRef]
- Liu, T.; Liu, C.; Xu, X.; Liu, F.; Guo, X.; Li, N.; Wang, X.; Yang, J.; Yang, X.; Zhu, H.; et al. Preclinical Evaluation and Pilot Clinical Study of Al18F-PSMA-BCH for Prostate Cancer PET Imaging. J. Nucl. Med. 2019, 60, 1284–1292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giesel, F.L.; Hadaschik, B.; Cardinale, J.; Radtke, J.; Vinsensia, M.; Lehnert, W.; Kesch, C.; Tolstov, Y.; Singer, S.; Grabe, N. F-18 labelled PSMA-1007: Biodistribution, radiation dosimetry and histopathological validation of tumor lesions in prostate cancer patients. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 678–688. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hofman, M.S.; Violet, J.; Hicks, R.J.; Ferdinandus, J.; Thang, S.P.; Akhurst, T.; Iravani, A.; Kong, G.; Ravi Kumar, A.; Murphy, D.G.; et al. [(177)Lu]-PSMA-617 radionuclide treatment in patients with metastatic castration-resistant prostate cancer (LuPSMA trial): A single-centre, single-arm, phase 2 study. Lancet Oncol. 2018, 19, 825–833. [Google Scholar] [CrossRef]
- Kurth, J.; Krause, B.J.; Schwarzenbock, S.M.; Stegger, L.; Schafers, M.; Rahbar, K. External radiation exposure, excretion, and effective half-life in (177)Lu-PSMA-targeted therapies. EJNMMI Res. 2018, 8, 32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kelly, J.M.; Amor-Coarasa, A.; Nikolopoulou, A.; Wustemann, T.; Barelli, P.; Kim, D.; Williams, C., Jr.; Zheng, X.; Bi, C.; Hu, B.; et al. Dual-Target Binding Ligands with Modulated Pharmacokinetics for Endoradiotherapy of Prostate Cancer. J. Nucl. Med. 2017, 58, 1442–1449. [Google Scholar] [CrossRef] [Green Version]
- Choy, C.J.; Ling, X.; Geruntho, J.J.; Beyer, S.K.; Latoche, J.D.; Langton-Webster, B.; Anderson, C.J.; Berkman, C.E. (177)Lu-Labeled Phosphoramidate-Based PSMA Inhibitors: The Effect of an Albumin Binder on Biodistribution and Therapeutic Efficacy in Prostate Tumor-Bearing Mice. Theranostics 2017, 7, 1928–1939. [Google Scholar] [CrossRef]
- Liu, Z.; Chen, X. Simple bioconjugate chemistry serves great clinical advances: Albumin as a versatile platform for diagnosis and precision therapy. Chem. Soc. Rev. 2016, 45, 1432–1456. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jacobson, O.; Kiesewetter, D.O.; Chen, X. Albumin-Binding Evans Blue Derivatives for Diagnostic Imaging and Production of Long-Acting Therapeutics. Bioconjug. Chem. 2016, 27, 2239–2247. [Google Scholar] [CrossRef]
- Umbricht, C.A.; Benešová, M.; Schibli, R.; Müller, C. Preclinical Development of Novel PSMA-Targeting Radioligands: Modulation of Albumin-Binding Properties to Improve Prostate Cancer Therapy. Mol. Pharm. 2018, 15, 2297–2306. [Google Scholar] [CrossRef]
- Benesova, M.; Umbricht, C.A.; Schibli, R.; Muller, C. Albumin-Binding PSMA Ligands: Optimization of the Tissue Distribution Profile. Mol. Pharm. 2018, 15, 934–946. [Google Scholar] [CrossRef]
- Zang, J.; Fan, X.; Wang, H.; Liu, Q.; Wang, J.; Li, H.; Li, F.; Jacobson, O.; Niu, G.; Zhu, Z.; et al. First-in-human study of (177)Lu-EB-PSMA-617 in patients with metastatic castration-resistant prostate cancer. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 148–158. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Tian, R.; Niu, G.; Ma, Y.; Lang, L.; Szajek, L.P.; Kiesewetter, D.O.; Jacobson, O.; Chen, X. Single Low-Dose Injection of Evans Blue Modified PSMA-617 Radioligand Therapy Eliminates Prostate-Specific Membrane Antigen Positive Tumors. Bioconjug. Chem. 2018, 29, 3213–3221. [Google Scholar] [CrossRef] [PubMed]
- Gunnoo, S.B.; Madder, A. Chemical Protein Modification through Cysteine. ChemBioChem 2016, 17, 529–553. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Timms, M.; Ganio, K.; Forbes, G.; Bailey, S.; Steel, R. An immuno polymerase chain reaction screen for the detection of CJC-1295 and other growth-hormone-releasing hormone analogs in equine plasma. Drug Test. Anal. 2019, 11, 804–812. [Google Scholar] [CrossRef]
- Xie, D.; Yao, C.; Wang, L.; Min, W.; Xu, J.; Xiao, J.; Huang, M.; Chen, B.; Liu, B.; Li, X.; et al. An albumin-conjugated peptide exhibits potent anti-HIV activity and long in vivo half-life. Antimicrob. Agents Chemother. 2010, 54, 191–196. [Google Scholar] [CrossRef] [Green Version]
- Feng, J.; Zhao, C.; Wang, L.; Qu, L.; Zhu, H.; Yang, Z.; An, G.; Tian, H.; Shou, C. Development of a novel albumin-based and maleimidopropionic acid-conjugated peptide with prolonged half-life and increased in vivo anti-tumor efficacy. Theranostics 2018, 8, 2094–2106. [Google Scholar] [CrossRef]
- Liu, T.; Liu, C.; Ren, Y.; Guo, X.; Jiang, J.; Xie, Q.; Xia, L.; Wang, F.; Zhu, H.; Yang, Z. Development of an Albumin-Based PSMA Probe with Prolonged Half-Life. Front. Mol. Biosci. 2020, 7, 585024. [Google Scholar] [CrossRef]
- Carlos Dos Santos, J.; Beijer, B.; Bauder-Wust, U.; Schafer, M.; Leotta, K.; Eder, M.; Benesova, M.; Kleist, C.; Giesel, F.; Kratochwil, C.; et al. Development of Novel PSMA Ligands for Imaging and Therapy with Copper Isotopes. J. Nucl. Med. 2020, 61, 70–79. [Google Scholar] [CrossRef]
- Liu, T.; Liu, C.; Zhang, Z.; Zhang, N.; Guo, X.; Xia, L.; Jiang, J.; Xie, Q.; Yan, K.; Rowe, S.P.; et al. (64)Cu-PSMA-BCH: A new radiotracer for delayed PET imaging of prostate cancer. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 4508–4516. [Google Scholar] [CrossRef]
- Kelly, J.; Amor-Coarasa, A.; Ponnala, S.; Nikolopoulou, A.; Williams, C., Jr.; Schlyer, D.; Zhao, Y.; Kim, D.; Babich, J.W. Trifunctional PSMA-targeting constructs for prostate cancer with unprecedented localization to LNCaP tumors. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 1841–1851. [Google Scholar] [CrossRef]
- Kelly, J.M.; Amor-Coarasa, A.; Ponnala, S.; Nikolopoulou, A.; Williams, C., Jr.; DiMagno, S.G.; Babich, J.W. Albumin-Binding PSMA Ligands: Implications for Expanding the Therapeutic Window. J. Nucl. Med. 2019, 60, 656–663. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zang, J.; Liu, Q.; Sui, H.; Wang, R.; Jacobson, O.; Fan, X.; Zhu, Z.; Chen, X. (177)Lu-EB-PSMA Radioligand Therapy with Escalating Doses in Patients with Metastatic Castration-Resistant Prostate Cancer. J. Nucl. Med. 2020, 61, 1772–1778. [Google Scholar] [CrossRef] [PubMed]


| Target Organ | Absorbed Dose (mGy/MBq) |
|---|---|
| Adrenals | 3.94 × 10−2 |
| Brain | 2.84 × 10−2 |
| Esophagus | 2.95 × 10−2 |
| Eyes | 2.53 × 10−2 |
| Gallbladder Wall | 4.14 × 10−2 |
| Left colon | 3.16 × 10−2 |
| Small Intestine | 4.00 × 10−2 |
| Stomach Wall | 3.09 × 10−2 |
| Right colon | 3.33 × 10−2 |
| Rectum | 3.80 × 10−2 |
| Heart Wall | 3.25 × 10−2 |
| Kidneys | 9.97 × 10−2 |
| Liver | 1.07 × 10−1 |
| Lungs | 3.27 × 10−2 |
| Pancreas | 3.32 × 10−2 |
| Prostate | 3.08 × 10−2 |
| Salivary Glands | 2.80 × 10−2 |
| Red Marrow | 2.40 × 10−2 |
| Osteogenic Cells | 3.46 × 10−2 |
| Spleen | 1.74 × 10−2 |
| Testes | 2.73 × 10−2 |
| Thymus | 2.83 × 10−2 |
| Thyroid | 2.82 × 10−2 |
| Urinary Bladder Wall | 3.06 × 10−2 |
| Total Body | 3.09 × 10−2 |
| Effective Dose (mSv/MBq) | 2.76 × 10−2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, Y.; Liu, T.; Liu, C.; Guo, X.; Wang, F.; Zhu, H.; Yang, Z. An Albumin-Binding PSMA Ligand with Higher Tumor Accumulation for PET Imaging of Prostate Cancer. Pharmaceuticals 2022, 15, 513. https://doi.org/10.3390/ph15050513
Ren Y, Liu T, Liu C, Guo X, Wang F, Zhu H, Yang Z. An Albumin-Binding PSMA Ligand with Higher Tumor Accumulation for PET Imaging of Prostate Cancer. Pharmaceuticals. 2022; 15(5):513. https://doi.org/10.3390/ph15050513
Chicago/Turabian StyleRen, Ya’nan, Teli Liu, Chen Liu, Xiaoyi Guo, Feng Wang, Hua Zhu, and Zhi Yang. 2022. "An Albumin-Binding PSMA Ligand with Higher Tumor Accumulation for PET Imaging of Prostate Cancer" Pharmaceuticals 15, no. 5: 513. https://doi.org/10.3390/ph15050513
APA StyleRen, Y., Liu, T., Liu, C., Guo, X., Wang, F., Zhu, H., & Yang, Z. (2022). An Albumin-Binding PSMA Ligand with Higher Tumor Accumulation for PET Imaging of Prostate Cancer. Pharmaceuticals, 15(5), 513. https://doi.org/10.3390/ph15050513






