Photodynamic Anti-Bacteria by Carbon Dots and Their Nano-Composites
Abstract
:1. Introduction
2. Synthesis Techniques of Carbon Dots Employed for Antimicrobials
3. Carbon Dots in Antimicrobial Photodynamic Therapy
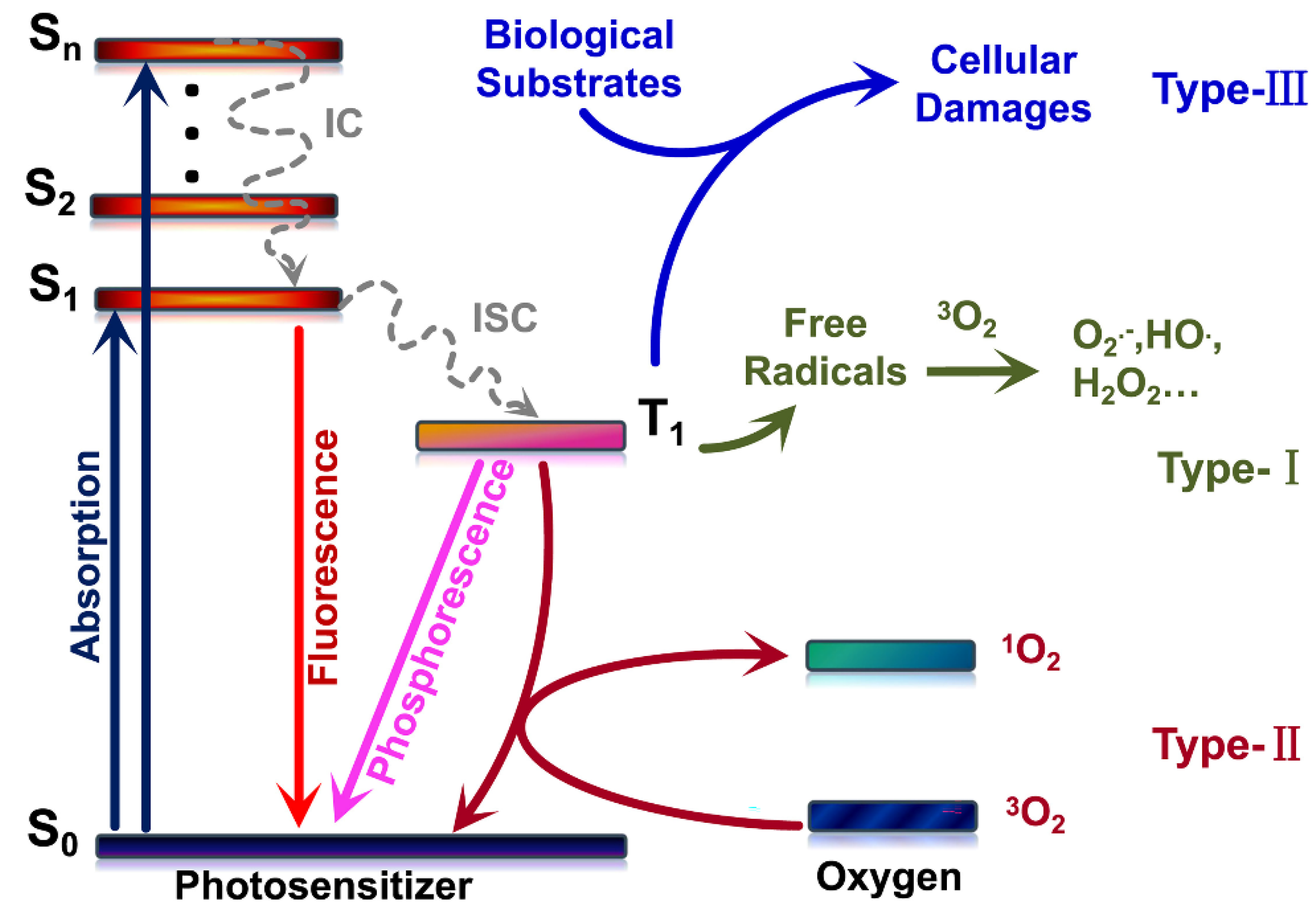
3.1. Photosensitization Mechanisms
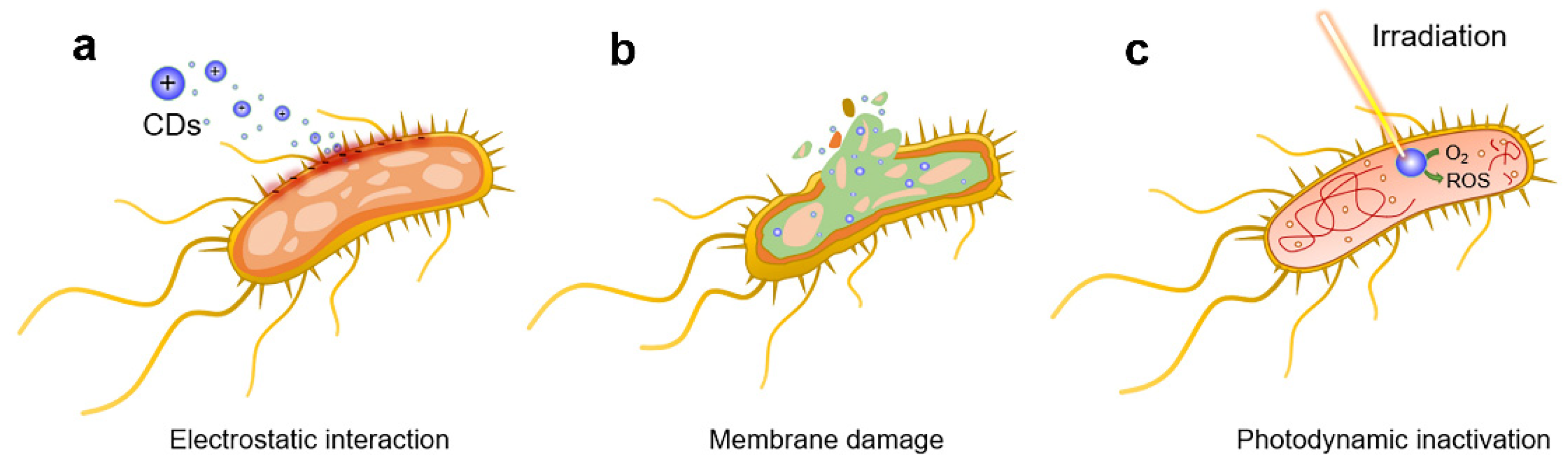
3.2. Photodynamic Anti-Bacteria by Carbon Dots
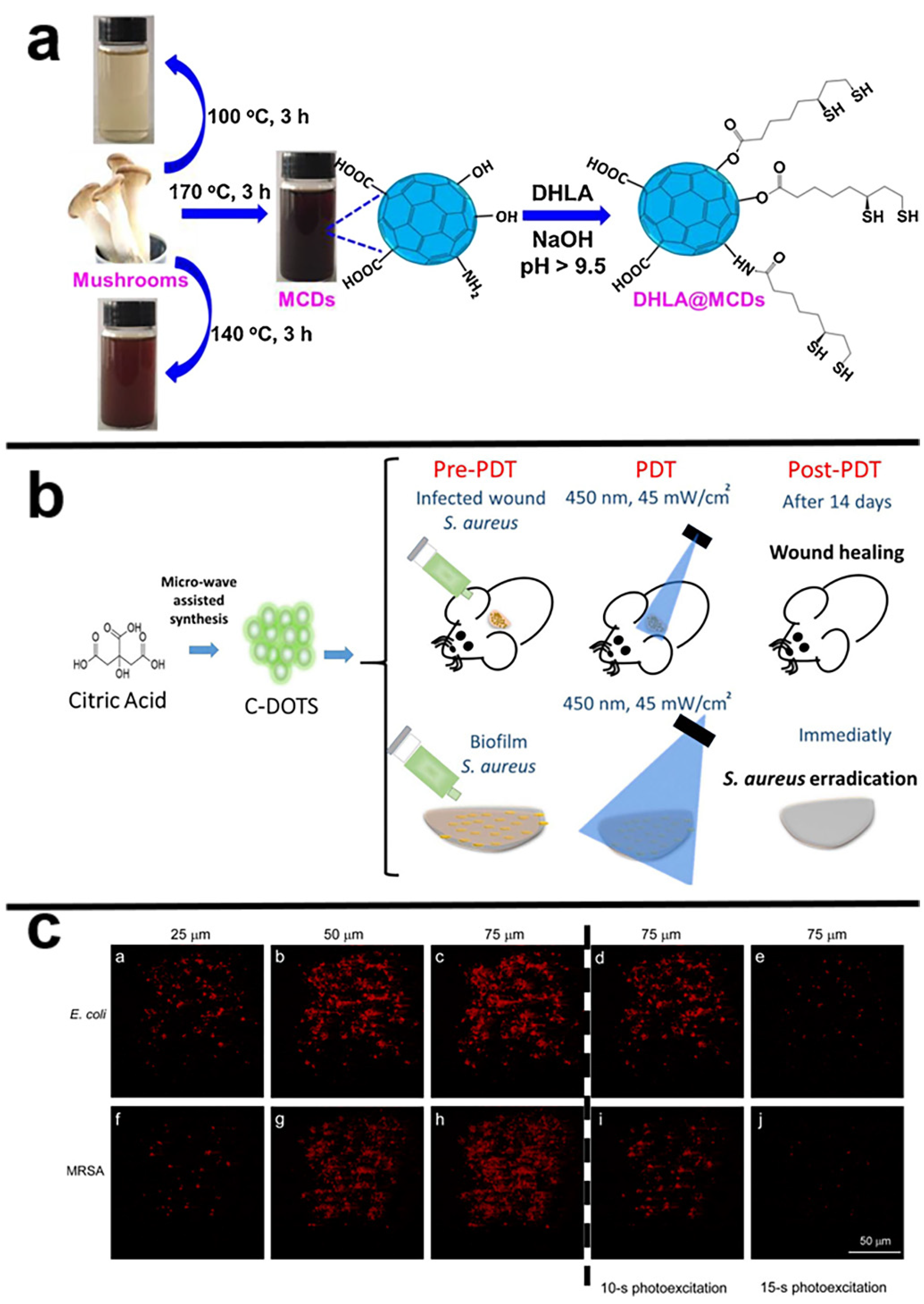
4. Carbon Dots-Based Nanocomposites in PACT
4.1. Antibiotic-Modified Carbon Dots
4.2. Carbon Dots as Nanocarriers for Photosensitizers
4.3. Carbon Dots/Metal Oxide Nanocomposites
4.4. Other Hybrid Carbon Dots
5. Toxicology and Safety Profile of Carbon Dots
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mu, H.; Tang, J.; Liu, Q.; Sun, C.; Wang, T.; Duan, J. Potent antibacterial nanoparticles against biofilm and intracellular bacteria. Sci. Rep. 2016, 6, 18877. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dong, X.; Al Awak, M.; Tomlinson, N.; Tang, Y.; Sun, Y.P.; Yang, L. Antibacterial effects of carbon dots in combination with other antimicrobial reagents. PLoS ONE 2017, 12, e0185324. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Varghese, M.; Balachandran, M. Antibacterial efficiency of carbon dots against Gram-positive and Gram-negative bacteria: A review. J. Environ. Chem. Eng. 2021, 9, 106821. [Google Scholar] [CrossRef]
- Durao, P.; Balbontin, R.; Gordo, I. Evolutionary mechanisms shaping the maintenance of antibiotic resistance. Trends Microbiol. 2018, 26, 677–691. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Liu, X.; Xiao, Y.; Chen, F.; Xiao, F. A multifunctional nanoplatform based on mesoporous silica nanoparticles for imaging-guided chemo/photodynamic synergetic therapy. RSC Adv. 2017, 7, 31133–31141. [Google Scholar] [CrossRef] [Green Version]
- Russell, S.P.; Neary, C.; Elwahab, S.A.; Powell, J.; O’Connell, N.; Power, L.; Tormey, S.; Merrigan, B.A.; Lowery, A.J. Breast infections–Microbiology and treatment in an era of antibiotic resistance. Surgeon 2020, 18, 1–7. [Google Scholar] [CrossRef]
- Sattarahmady, N.; Rezaie-Yazdi, M.; Tondro, G.H.; Akbari, N. Bactericidal laser ablation of carbon dots: An in vitro study on wild-type and antibiotic-resistant staphylococcus aureus. J. Photochem. Photobiol. B-Biol. 2017, 166, 323–332. [Google Scholar] [CrossRef]
- Pidot, S.J.; Gao, W.; Buultjens, A.H.; Monk, I.R.; Guerillot, R.; Carter, G.P.; Lee, J.Y.H.; Lam, M.M.C.; Grayson, M.L.; Ballard, S.A.; et al. Increasing tolerance of hospital enterococcus faecium to handwash alcohols. Sci. Transl. Med. 2018, 10, eaar6115. [Google Scholar] [CrossRef] [Green Version]
- Dong, X.; Liang, W.; Meziani, M.; Sun, Y.P.; Yang, L. Carbon dots as potent antimicrobial agents. Theranostics 2020, 10, 671–686. [Google Scholar] [CrossRef]
- Ghirardello, M.; Ramos-Soriano, J.; Galan, M.C. Carbon dots as an emergent class of antimicrobial agents. Nanomaterials 2021, 11, 1877. [Google Scholar] [CrossRef]
- Al Awak, M.M.; Wang, P.; Wang, S.; Tang, Y.; Sun, Y.P.; Yang, L. Correlation of carbon dots’ light-activated antimicrobial activities and fluorescence quantum yield. RSC Adv. 2017, 7, 30177–30184. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Peng, C.; Shi, C.; Jiang, M.; Chau, J.H.; Liu, Z.; Bai, H.; Kwok, R.T.; Lam, J.W.; Shi, Y. Mitochondria-specific aggregation-induced emission luminogens for selective photodynamic killing of fungi and efficacious treatment of keratitis. ACS Nano 2021, 15, 12129–12139. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Ren, B.; Tong, Y.; Dai, H.; Zhang, L. Synergistic combinations of antifungals and anti-virulence agents to fight against Candida albicans. Virulence 2015, 6, 362–371. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dong, X.; Ge, L.; Abu Rabe, D.I.; Mohammed, O.O.; Wang, P.; Tang, Y.; Kathariou, S.; Yang, L.; Sun, Y.P. Photoexcited state properties and antibacterial activities of carbon dots relevant to mechanistic features and implications. Carbon 2020, 170, 137–145. [Google Scholar] [CrossRef]
- Makabenta, J.M.V.; Nabawy, A.; Li, C.H.; Schmidt-Malan, S.; Patel, R.; Rotello, V.M. Nanomaterial-based therapeutics for antibiotic-resistant bacterial infections. Nat. Rev. Microbiol. 2021, 19, 23–36. [Google Scholar] [CrossRef]
- Yang, J.; Gao, G.; Zhang, X.; Ma, Y.H.; Jia, H.R.; Jiang, Y.W.; Wang, Z.; Wu, F.G. Ultrasmall and photostable nanotheranostic agents based on carbon quantum dots passivated with polyamine-containing organosilane molecules. Nanoscale 2017, 9, 15441–15452. [Google Scholar] [CrossRef]
- Hua, X.W.; Bao, Y.W.; Wu, F.G. Fluorescent carbon quantum dots with intrinsic nucleolus-targeting capability for nucleolus imaging and enhanced cytosolic and nuclear drug delivery. ACS Appl. Mater. Interfaces 2018, 10, 16924. [Google Scholar] [CrossRef]
- Jiang, L.; Ding, H.; Xu, M.; Hu, X.; Li, S.; Zhang, M.; Zhang, Q.; Wang, Q.; Lu, S.; Tian, Y.; et al. UV-Vis-NIR full-range responsive carbon dots with large multiphoton absorption cross sections and deep-red fluorescence at nucleoli and in vivo. Small 2020, 16, 2000680. [Google Scholar] [CrossRef]
- Sun, Y.; Qin, H.; Geng, X.; Yang, R.; Qu, L.; Kani, A.N.; Li, Z. Rational design of far-red to near-infrared emitting carbon dots for ultrafast lysosomal polarity imaging. ACS Appl. Mater. Interfaces 2020, 12, 31738–31744. [Google Scholar] [CrossRef]
- Kaminari, A.; Nikoli, E.; Athanasopoulos, A.; Sakellis, E.; Sideratou, Z.; Tsiourvas, D. Engineering mitochondriotropic carbon dots for targeting cancer cells. Pharmaceuticals 2021, 14, 932. [Google Scholar] [CrossRef]
- Wang, L.; Chang, Y.; Feng, Y.; Li, X.; Cheng, Y.; Jian, H.; Ma, X.; Zheng, R.; Wu, X.; Xu, K.; et al. Nitric oxide stimulated programmable drug release of nanosystem for multidrug resistance cancer therapy. Nano Lett. 2019, 19, 6800–6811. [Google Scholar] [CrossRef] [PubMed]
- Dou, Q.; Fang, X.; Jiang, S.; Chee, P.L.; Lee, T.C.; Loh, X.J. Multi-functional fluorescent carbon dots with antibacterial and gene delivery properties. RSC Adv. 2015, 5, 46817–46822. [Google Scholar] [CrossRef]
- Tuerhong, M.; Xu, Y.; Yin, X.B. Review on carbon dots and their applications. Chin. J. Anal. Chem. 2017, 45, 139–149. [Google Scholar] [CrossRef]
- Shen, C.; Dong, C.; Cheng, L.; Shi, X.; Bi, H. Fluorescent carbon dots from Shewanella oneidensis MR-1 for Hg2+ and tetracycline detection and selective fluorescence imaging of Gram-positive bacteria. J. Environ. Chem. Eng. 2022, 10, 107020. [Google Scholar] [CrossRef]
- Dong, X.; Bond, A.E.; Pan, N.Y.; Coleman, M.; Tang, Y.; Sun, Y.P.; Yang, L. Synergistic photoactivated antimicrobial effects of carbon dots combined with dye photosensitizers. Int. J. Nanomed. 2018, 13, 8025–8035. [Google Scholar] [CrossRef] [Green Version]
- Luo, P.G.; Yang, F.; Yang, S.T.; Sonkar, S.K.; Yang, L.; Broglie, J.J.; Liu, Y.; Sun, Y.P. Carbon-based quantum dots for fluorescence imaging of cells and tissues. RSC Adv. 2014, 4, 10791–10807. [Google Scholar] [CrossRef]
- Luo, Q.; Ding, H.; Hu, X.; Xu, J.; Sadat, A.; Xu, M.; Primo, F.L.; Tedesco, A.C.; Zhang, H.; Bi, H. Sn4+ complexation with sulfonated-carbon dots in pursuit of enhanced fluorescence and singlet oxygen quantum yield. Dalton Trans. 2020, 49, 6950–6956. [Google Scholar] [CrossRef]
- Wang, X.; Feng, Y.; Dong, P.; Huang, J. A mini review on carbon quantum dots: Preparation, properties, and electrocatalytic application. Front. Chem. 2019, 7, 671. [Google Scholar] [CrossRef]
- Zheng, X.; Ananthanarayanan, A.; Luo, K.Q.; Chen, P. Glowing graphene quantum dots and carbon dots: Properties, syntheses, and biological applications. Small 2015, 11, 1620–1636. [Google Scholar] [CrossRef]
- De, B.; Karak, N. Recent progress in carbon dot-metal based nanohybrids for photochemical and electrochemical applications. J. Mater. Chem. A 2017, 5, 1826–1859. [Google Scholar] [CrossRef]
- Wu, Y.; Li, C.; van der Mei, H.C.; Busscher, H.J.; Ren, Y. Carbon quantum dots derived from different carbon sources for antibacterial applications. Antibiotics 2021, 10, 623. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Wang, X.; Li, Y.; Wang, Z.; Yang, F.; Yang, X. Microwave synthesis of fluorescent carbon nanoparticles with electrochemiluminescence properties. Chem. Commun. 2009, 34, 5118–5120. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, H.Q.; Shimizu, Y.; Pyatenko, A.; Kawaguchi, K.; Koshizaki, N. Preparation of carbon quantum dots with tunable photoluminescence by rapid laser passivation in ordinary organic solvents. Chem. Commun. 2011, 47, 932–934. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Ming, H.; Liu, Y.; Yu, H.; He, X.; Huang, H.; Pan, K.; Kang, Z.; Lee, S.T. Fluorescent carbon nanoparticles: Electrochemical synthesis and their pH sensitive photoluminescence properties. New J. Chem. 2011, 35, 2666–2670. [Google Scholar] [CrossRef]
- Dong, Y.; Pang, H.; Yang, H.; Guo, C.; Shao, J.; Chi, Y.; Li, C.; Yu, T. Carbon-based dots co-doped with nitrogen and sulfur for high quantum yield and excitation-independent emission. Angew. Chem.-Int. Ed. 2013, 52, 7800–7804. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Li, C.; Ren, Y.; Sun, X.; Pan, W.; Li, Y.; Wang, J.; Wang, W. Carbon dots: Surface engineering and applications. J. Mater. Chem. B 2016, 4, 5772–5788. [Google Scholar] [CrossRef]
- Li, B.; Zhao, S.; Huang, L.; Wang, Q.; Xiao, J.; Lan, M. Recent advances and prospects of carbon dots in phototherapy. Chem. Eng. J. 2021, 408, 127245. [Google Scholar] [CrossRef]
- Xiao, D.; Qi, H.X.; Teng, Y.; Pierre, D.; Kutoka, P.T.; Liu, D. Advances and challenges of fluorescent nanomaterials for synthesis and biomedical applications. Nanoscale Res. Lett. 2021, 16, 167. [Google Scholar] [CrossRef]
- Wang, B.; Song, H.; Qu, X.; Chang, J.; Yang, B.; Lu, S. Carbon dots as a new class of nanomedicines: Opportunities and challenges. Coord. Chem. Rev. 2021, 442, 214010. [Google Scholar] [CrossRef]
- Anwar, S.; Ding, H.; Xu, M.; Hu, X.; Li, Z.; Wang, J.; Liu, L.; Jiang, L.; Wang, D.; Dong, C.; et al. Recent advances in synthesis, optical properties, and biomedical applications of carbon dots. ACS Appl. Bio Mater. 2019, 2, 2317–2338. [Google Scholar] [CrossRef]
- Zuo, P.; Lu, X.; Sun, Z.; Guo, Y.; He, H. A review on syntheses, properties, characterization and bioanalytical applications of fluorescent carbon dots. Microchim. Acta 2016, 183, 519–542. [Google Scholar] [CrossRef]
- Miao, S.; Liang, K.; Zhu, J.; Yang, B.; Zhao, D.; Kong, B. Hetero-atom-doped carbon dots: Doping strategies, properties and applications. Nano Today 2020, 33, 100879. [Google Scholar] [CrossRef]
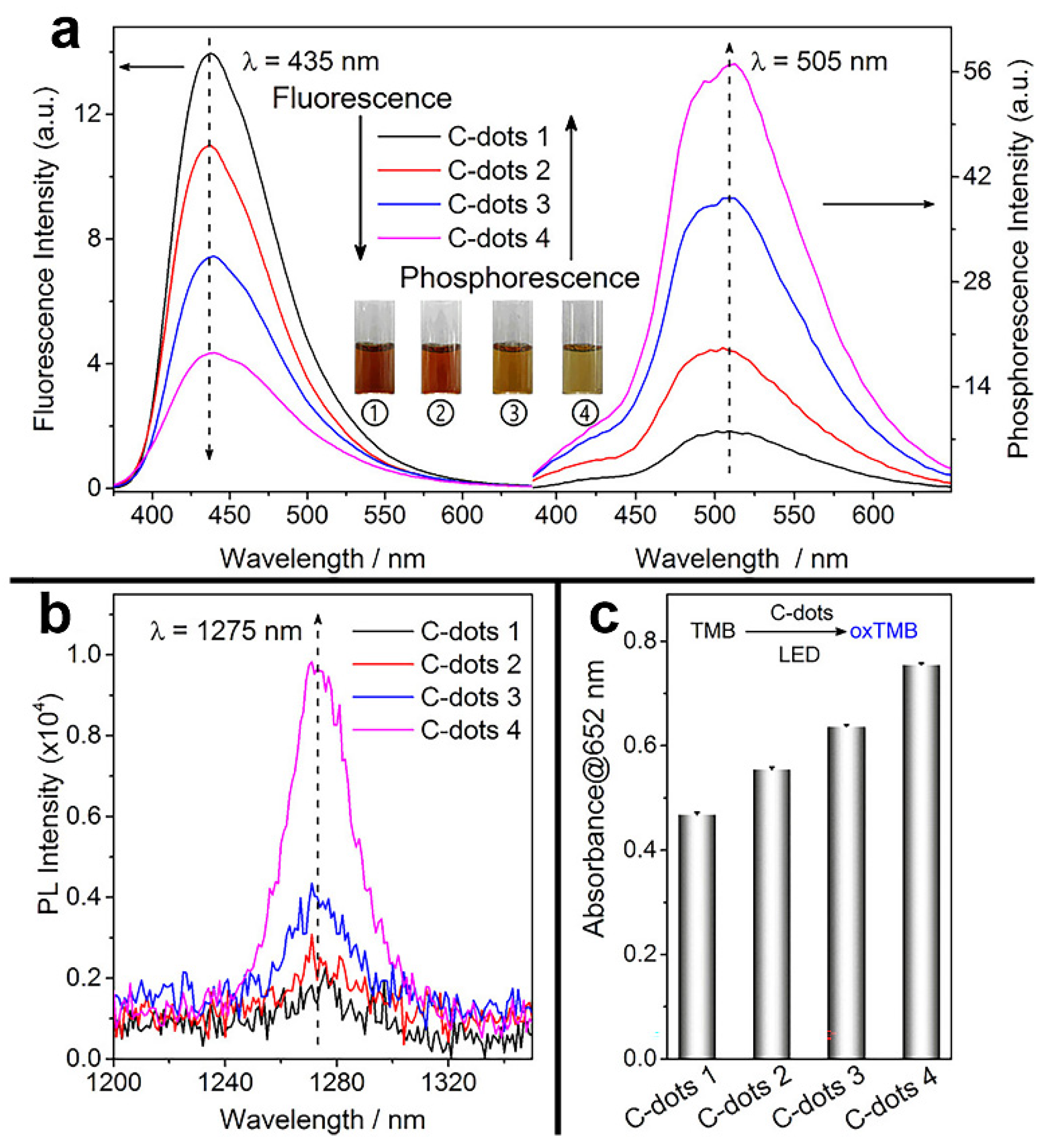
- Zhang, J.; Lu, X.; Tang, D.; Wu, S.; Hou, X.; Liu, J.; Wu, P. Phosphorescent carbon dots for highly efficient oxygen photosensitization and as photo-oxidative nanozymes. ACS Appl. Mater. Interfaces 2018, 10, 40808–40814. [Google Scholar] [CrossRef] [PubMed]
- Markovic, Z.M.; Labudova, M.; Danko, M.; Matijasevic, D.; Micusik, M.; Nadazdy, V.; Kovacova, M.; Kleinova, A.; Spitalsky, Z.; Pavlovic, V.; et al. Highly efficient antioxidant F- and Cl-doped carbon quantum dots for bioimaging. ACS Sustain. Chem. Eng. 2020, 8, 16327–16338. [Google Scholar] [CrossRef]
- Noh, S.-H.; Na, W.; Jang, J.-T.; Lee, J.-H.; Lee, E.J.; Moon, S.H.; Lim, Y.; Shin, J.-S.; Cheon, J. Nanoscale magnetism control via surface and exchange anisotropy for optimized ferrimagnetic hysteresis. Nano Lett. 2012, 12, 3716–3721. [Google Scholar] [CrossRef]
- Feng, Z.; Adolfsson, K.H.; Xu, Y.; Fang, H.; Hakkarainen, M.; Wu, M. Carbon dot/polymer nanocomposites: From green synthesis to energy, environmental and biomedical applications. Sustain. Mater. Technol. 2021, 29, e00304. [Google Scholar] [CrossRef]
- Saravanan, A.; Maruthapandi, M.; Das, P.; Ganguly, S.; Margel, S.; Luong, J.H.T.; Gedanken, A. Applications of N-doped carbon dots as antimicrobial agents, antibiotic carriers, and selective fluorescent probes for nitro explosives. ACS Appl. Bio Mater. 2020, 3, 8023–8031. [Google Scholar] [CrossRef]
- Bing, W.; Sun, H.; Yan, Z.; Ren, J.; Qu, X. Programmed bacteria death induced by carbon dots with different surface charge. Small 2016, 12, 4713–4718. [Google Scholar] [CrossRef]
- Vatansever, F.; de Melo, W.; Avci, P.; Vecchio, D.; Sadasivam, M.; Gupta, A.; Chandran, R.; Karimi, M.; Parizotto, N.A.; Yin, R.; et al. Antimicrobial strategies centered around reactive oxygen species-bactericidal antibiotics, photodynamic therapy, and beyond. Fems Microbiol. Rev. 2013, 37, 955–989. [Google Scholar] [CrossRef] [Green Version]
- Lacey, J.A.; Phillips, D. The photosensitisation of Escherichia coli using disulphonated aluminium phthalocyanine. J. Photochem. Photobio. A Chem. 2001, 142, 145–150. [Google Scholar] [CrossRef]
- Reddi, E.; Ceccon, M.; Valduga, G.; Jori, G.; Bommer, J.C.; Elisei, F.; Latterini, L.; Mazzucato, U. Photophysical properties and antibacterial activity of meso-substituted cationic porphyrins. Photochem. Photobio. 2002, 75, 462–470. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, X.; Ma, Y.H.; Gao, G.; Chen, X.; Jia, H.R.; Li, Y.H.; Chen, Z.; Wu, F.G. Carbon dot-based platform for simultaneous bacterial distinguishment and antibacterial applications. ACS Appl. Mater. Interfaces 2016, 8, 32170–32181. [Google Scholar] [CrossRef] [PubMed]
- Xu, N.; Du, J.; Yao, Q.; Ge, H.; Li, H.; Xu, F.; Gao, F.; Xian, L.; Fan, J.; Peng, X. Precise photodynamic therapy: Penetrating the nuclear envelope with photosensitive carbon dots. Carbon 2020, 159, 74–82. [Google Scholar] [CrossRef]
- Chilakamarthi, U.; Giribabu, L. Photodynamic therapy: Past, present and future. Chem. Rev. 2017, 17, 775–802. [Google Scholar] [CrossRef]
- He, H.; Zheng, X.; Liu, S.; Zheng, M.; Xie, Z.; Wang, Y.; Yu, M.; Shuai, X. Diketopyrrolopyrrole-based carbon dots for photodynamic therapy. Nanoscale 2018, 10, 10991–10998. [Google Scholar] [CrossRef]
- Xie, J.; Wang, Y.; Choi, W.; Jangili, P.; Ge, Y.; Xu, Y.; Kang, J.; Liu, L.; Zhang, B.; Xie, Z.; et al. Overcoming barriers in photodynamic therapy harnessing nano-formulation strategies. Chem. Soc. Rev. 2021, 50, 9152–9201. [Google Scholar] [CrossRef]
- Bayona, A.M.D.; Mroz, P.; Thunshelle, C.; Hamblin, M.R. Design features for optimization of tetrapyrrole macrocycles as antimicrobial and anticancer photosensitizers. Chem. Biol. Drug Des. 2017, 89, 192–206. [Google Scholar] [CrossRef] [Green Version]
- Horne, T.K.; Cronje, M.J. Mechanistics and photo-energetics of macrocycles and photodynamic therapy: An overview of aspects to consider for research. Chem. Biol. Drug Des. 2017, 89, 221–242. [Google Scholar] [CrossRef] [Green Version]
- Yao, Q.; Fan, J.; Long, S.; Zhao, X.; Li, H.; Du, J.; Shao, K.; Peng, X. The concept and examples of type-III photosensitizers for cancer photodynamic therapy. Chem 2022, 8, 197–209. [Google Scholar] [CrossRef]
- Nguyen, V.N.; Qi, S.; Kim, S.; Kwon, N.; Kim, G.; Yim, Y.; Park, S.; Yoon, J. An emerging molecular design approach to heavy-atom-free photosensitizers for enhanced photodynamic therapy under hypoxia. J. Am. Chem. Soc. 2019, 141, 16243–16248. [Google Scholar] [CrossRef]
- Shi, Z.; Zhang, K.; Zada, S.; Zhang, C.; Meng, X.; Yang, Z.; Dong, H. Upconversion nanoparticle-induced multimode photodynamic therapy based on a metal-organic framework/titanium dioxide nanocomposite. ACS Appl. Mater. Interfaces 2020, 12, 12600–12608. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Zhao, H.; Yao, L.; Li, Y.; Qi, B.; Wang, J.; Yang, X. Simple and multifunctional natural self-assembled sterols with anticancer activity-mediated supramolecular photosensitizers for enhanced antitumor photodynamic therapy. ACS Appl. Mater. Interfaces 2019, 11, 29498–29511. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Xu, M.; Wang, S.; Abbas, K.; Huang, X.; Zhang, R.; Tedesco, A.C.; Bi, H. F,N-doped carbon dots as efficient Type I photosensitizers for photodynamic therapy. Dalton Trans. 2022, 51, 2296–2303. [Google Scholar] [CrossRef] [PubMed]
- Foote, C.S. Definition of type I and type II photosensitized oxidation. Photochem. Photobio. 1991, 54, 659. [Google Scholar] [CrossRef] [PubMed]
- Wainwright, M. Special issue on photoantimicrobials. J. Photochem. Photobiol. B-Biol. 2015, 150, 1. [Google Scholar] [CrossRef]
- Planas, O.; Bresoli-Obach, R.; Nos, J.; Gallavardin, T.; Ruiz-Gonzalez, R.; Agut, M.; Nonell, S. Synthesis, Photophysical characterization, and photoinduced antibacterial activity of methylene blue-loaded amino- and mannose-targeted mesoporous silica nanoparticles. Molecules 2015, 20, 6284–6298. [Google Scholar] [CrossRef] [Green Version]
- DeRosa, M.C.; Crutchley, R.J. Photosensitized singlet oxygen and its applications. Coord. Chem. Rev. 2002, 233, 351–371. [Google Scholar] [CrossRef]
- Baptista, M.S.; Cadet, J.; Di Mascio, P.; Ghogare, A.A.; Greer, A.; Hamblin, M.R.; Lorente, C.; Nunez, S.C.; Ribeiro, M.S.; Thomas, A.H.; et al. Type I and Type II photosensitized oxidation reactions: Guidelines and mechanistic pathways. Photochem. Photobiol. 2017, 93, 912–919. [Google Scholar] [CrossRef] [Green Version]
- Wainwright, M.; Maisch, T.; Nonell, S.; Plaetzer, K.; Almeida, A.; Tegos, G.P.; Hamblin, M.R. Photoantimicrobials-are we afraid of the light? Lancet Infect. Dis. 2017, 17, E49–E55. [Google Scholar] [CrossRef]
- Alves, E.; Faustino, M.A.F.; Neves, M.; Cunha, A.; Tome, J.; Almeida, A. An insight on bacterial cellular targets of photodynamic inactivation. Future Med. Chem. 2014, 6, 141–164. [Google Scholar] [CrossRef]
- Wang, J.; Xu, M.; Wang, D.; Li, Z.; Primo, F.L.; Tedesco, A.C.; Bi, H. Copper-doped carbon dots for optical bioimaging and photodynamic therapy. Inorg. Chem. 2019, 58, 13394–13402. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.; Goel, S.; Ni, D.; Ellison, P.A.; Siamof, C.M.; Jiang, D.; Cheng, L.; Kang, L.; Yu, F.; Liu, Z.; et al. Reassembly of Zr-89-labeled cancer cell membranes into multicompartment membrane-derived liposomes for PET-trackable tumor-targeted theranostics. Adv. Mater. 2018, 30, 1704934. [Google Scholar] [CrossRef] [PubMed]
- Abrahamse, H.; Hamblin, M.R. New photosensitizers for photodynamic therapy. Biochem. J. 2016, 473, 347–364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ge, J.; Lan, M.; Zhou, B.; Liu, W.; Guo, L.; Wang, H.; Jia, Q.; Niu, G.; Huang, X.; Zhou, H.; et al. A graphene quantum dot photodynamic therapy agent with high singlet oxygen generation. Nat. Commun. 2014, 5, 4596. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Wang, D.; Xu, M.; Wang, J.; Hu, X.; Anwar, S.; Tedesco, A.C.; Morais, P.C.; Bi, H. Fluorine-containing graphene quantum dots with a high singlet oxygen generation applied for photodynamic therapy. J. Mat. Chem. B 2020, 8, 2598–2606. [Google Scholar] [CrossRef]
- Ristic, B.Z.; Milenkovic, M.M.; Dakic, I.R.; Todorovic-Markovic, B.M.; Milosavljevic, M.S.; Budimir, M.D.; Paunovic, V.G.; Dramicanin, M.D.; Markovic, Z.M.; Trajkovic, V.S. Photodynamic antibacterial effect of graphene quantum dots. Biomaterials 2014, 35, 4428–4435. [Google Scholar] [CrossRef]
- Stankovic, N.K.; Bodik, M.; Siffalovic, P.; Kotlar, M.; Micusik, M.; Spitalsky, Z.; Danko, M.; Milivojevic, D.D.; Kleinova, A.; Kubat, P.; et al. Antibacterial and antibiofouling properties of light triggered fluorescent hydrophobic carbon quantum dots langmuir-blodgett thin films. ACS Sustain. Chem. Eng. 2018, 6, 4154. [Google Scholar] [CrossRef]
- Venkateswarlu, S.; Viswanath, B.; Reddy, A.S.; Yoon, M. Fungus-derived photoluminescent carbon nanodots for ultrasensitive detection of Hg2+ ions and photoinduced bactericidal activity. Sens. Actuator B. Chem. 2018, 258, 172–183. [Google Scholar] [CrossRef]
- Dong, X.; Al Awak, M.; Wang, P.; Sun, Y.P.; Yang, L. Carbon dot incorporated multi-walled carbon nanotube coated filters for bacterial removal and inactivation. RSC Adv. 2018, 8, 8292–8301. [Google Scholar] [CrossRef]
- Romero, M.P.; Alves, F.; Stringasci, M.D.; Buzza, H.H.; Ciol, H.; Inada, N.M.; Bagnato, V.S. One-pot microwave-assisted synthesis of carbon dots and in vivo and in vitro antimicrobial photodynamic applications. Front. Microbiol. 2021, 12, 662149. [Google Scholar] [CrossRef]
- Cai, Y.; Wei, Z.; Song, C.H.; Tang, C.; Han, W.; Dong, X. Optical nano-agents in the second near-infrared window for biomedical applications. Chem. Soc. Rev. 2019, 48, 22–37. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Wang, G. NIR light-responsive nanocarriers for controlled release. J. Photochem. Photobiol. C-Photochem. Rev. 2021, 47, 100420. [Google Scholar] [CrossRef]
- Kuo, W.S.; Chang, C.Y.; Chen, H.H.; Hsu, C.L.L.; Wang, J.Y.; Kao, H.F.; Chou, L.C.S.; Chen, Y.C.; Chen, S.J.; Chang, W.T.; et al. Two-photon photoexcited photodynamic therapy and contrast agent with antimicrobial graphene quantum dots. ACS Appl. Mater. Interfaces 2016, 8, 30467–30474. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zheng, X.; Zhang, X.; Liu, S.; Pei, Q.; Zheng, M.; Xie, Z. Porphyrin-based carbon dots for photodynamic therapy of hepatoma. Adv. Healthc. Mater. 2017, 6, 1600924. [Google Scholar] [CrossRef]
- Jia, Q.; Ge, J.; Liu, W.; Zheng, X.; Chen, S.; Wen, Y.; Zhang, H.; Wang, P. A magnetofluorescent carbon dot assembly as an acidic H2O2-driven oxygenerator to regulate tumor hypoxia for simultaneous bimodal imaging and enhanced photodynamic therapy. Adv. Mater. 2018, 30, 1706090. [Google Scholar] [CrossRef]
- Wen, Y.; Jia, Q.; Nan, F.; Zheng, X.; Liu, W.; Wu, J.; Ren, H.; Ge, J.; Wang, P. Pheophytin derived near-infrared-light responsive carbon dot assembly as a new phototheranotic agent for bioimaging and photodynamic therapy. Chem.-Asian J. 2019, 14, 2162–2168. [Google Scholar] [CrossRef]
- Yue, J.; Li, L.; Jiang, C.; Mei, Q.; Dong, W.F.; Yan, R. Riboflavin-based carbon dots with high singlet oxygen generation for photodynamic therapy. J. Mater. Chem. B 2021, 9, 7972–7978. [Google Scholar] [CrossRef]
- Su, R.; Yan, H.; Jiang, X.; Zhang, Y.; Li, P.; Su, W. Orange-red to NIR emissive carbon dots for antimicrobial, bioimaging and bacteria diagnosis. J. Mater. Chem. B 2022, 10, 1250–1264. [Google Scholar] [CrossRef]
- Kuo, W.S.; Chen, H.H.; Chen, S.Y.; Chang, C.Y.; Chen, P.C.; Hou, Y.I.; Shao, Y.T.; Kao, H.F.; Hsu, C.L.L.; Chen, Y.C.; et al. Graphene quantum dots with nitrogen-doped content dependence for highly efficient dual-modality photodynamic antimicrobial therapy and bioimaging. Biomaterials 2017, 120, 185–194. [Google Scholar] [CrossRef]
- Knoblauch, R.; Geddes, C.D. Carbon Nanodots in Photodynamic Antimicrobial Therapy: A Review. Materials 2020, 13, 4004. [Google Scholar] [CrossRef]
- Knoblauch, R.; Harvey, A.; Ra, E.; Greenberg, K.M.; Lau, J.; Hawkins, E.; Geddes, C.D. Antimicrobial carbon nanodots: Photodynamic inactivation and dark antimicrobial effects on bacteria by brominated carbon nanodots. Nanoscale 2021, 13, 85–99. [Google Scholar] [CrossRef] [PubMed]
- Turro, N.J. Modern Molecular Photochemistry; University Science Books: Herndon, VA, USA, 1991. [Google Scholar]
- Abu Rabe, D.I.; Al Awak, M.M.; Yang, F.; Okonjo, P.A.; Dong, X.; Teisl, L.R.; Wang, P.; Tang, Y.; Pan, N.; Sun, Y.P.; et al. The dominant role of surface functionalization in carbon dots’ photo-activated antibacterial activity. Int. J. Nanomed. 2019, 14, 2655–2665. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Samphire, J.; Takebayashi, Y.; Hill, S.A.; Hill, N.; Heesom, K.J.; Lewis, P.A.; Alibhai, D.; Bragginton, E.C.; Dorh, J.; Dorh, N. Green fluorescent carbon dots as targeting probes for LED-dependent bacterial killing. Nano Select 2021, 3, 662–672. [Google Scholar] [CrossRef]
- Pan, C.L.; Chen, M.H.; Tung, F.I.; Liu, T.Y. A nanovehicle developed for treating deep-seated bacteria using low-dose X-ray. Acta Biomater. 2017, 47, 159–169. [Google Scholar] [CrossRef] [PubMed]
- Jijie, R.; Barras, A.; Bouckaert, J.; Dumitrascu, N.; Szunerits, S.; Boukherroub, R. Enhanced antibacterial activity of carbon dots functionalized with ampicillin combined with visible light triggered photodynamic effects. Colloid Surf. B-Biointerf. 2018, 170, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Sidhu, J.S.; Mayank; Pandiyan, T.; Kaur, N.; Singh, N. The Photochemical degradation of bacterial cell wall using penicillin-based carbon dots: Weapons against multi-drug resistant (MDR) Strains. Chem. Sel. 2017, 2, 9277–9283. [Google Scholar] [CrossRef]
- Thakur, M.; Pandey, S.; Mewada, A.; Patil, V.; Khade, M.; Goshi, E.; Sharon, M. Antibiotic conjugated fluorescent carbon dots as a theranostic agent for controlled drug release, bioimaging, and enhanced antimicrobial activity. J. Drug Deliv. 2014, 2014, 282193. [Google Scholar] [CrossRef]
- Hou, P.; Yang, T.; Liu, H.; Li, Y.; Huang, C. An active structure preservation method for developing functional graphitic carbon dots as an effective antibacterial agent and a sensitive pH and Al (III) nanosensor. Nanoscale 2017, 9, 17334–17341. [Google Scholar] [CrossRef]
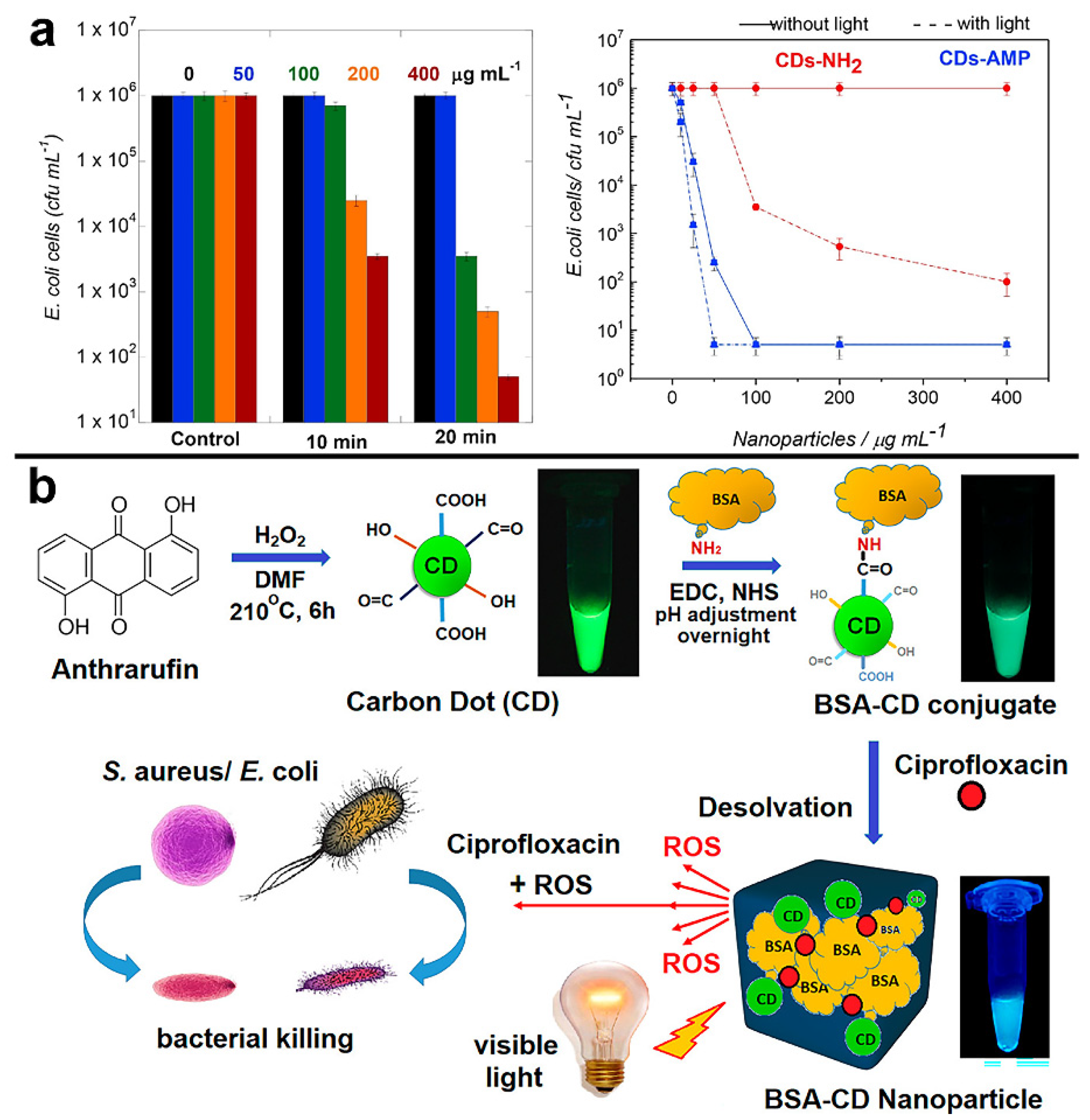
- Mandal, S.; Prasad, S.R.; Mandal, D.; Das, P. Bovine serum albumin amplified reactive oxygen species generation from anthrarufin-derived carbon dot and concomitant nanoassembly for combination antibiotic-photodynamic therapy application. ACS Appl. Mater. Interfaces 2019, 11, 33273–33284. [Google Scholar] [CrossRef]
- Cao, Y.; Dong, H.; Yang, Z.; Zhong, X.; Chen, Y.; Dai, W.; Zhang, X. Aptamer-conjugated graphene quantum dots/porphyrin derivative theranostic agent for intracellular cancer-related microRNA detection and fluorescence-guided photothermal/photodynamic synergetic therapy. ACS Appl. Mater. Interfaces 2017, 9, 159–166. [Google Scholar] [CrossRef]
- Kholikov, K.; Ilhom, S.; Sajjad, M.; Smith, M.E.; Monroe, J.D.; San, O.; Er, A.O. Improved singlet oxygen generation and antimicrobial activity of sulphur-doped graphene quantum dots coupled with methylene blue for photodynamic therapy applications. Photodiagn. Photodyn. Therp. 2018, 24, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Mushtaq, S.; Yasin, T.; Saleem, M.; Dai, T.; Yameen, M.A. Potentiation of antimicrobial photodynamic therapy by curcumin-loaded graphene quantum dots. Photochem. Photobio. 2022, 98, 202–210. [Google Scholar] [CrossRef] [PubMed]
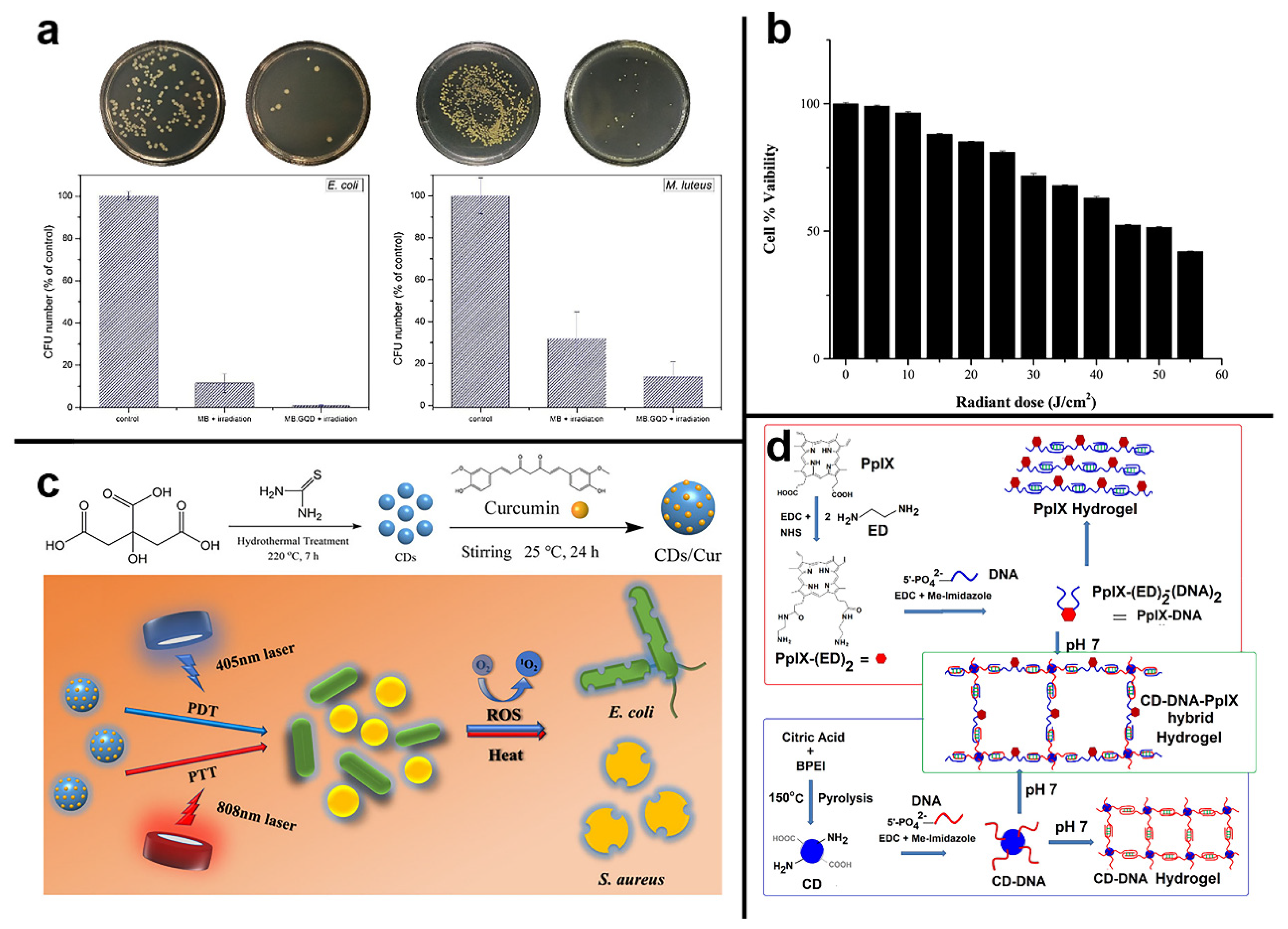
- Yan, H.; Zhang, B.; Zhang, Y.; Su, R.; Li, P.; Su, W. Fluorescent carbon dot-curcumin nanocomposites for remarkable antibacterial activity with synergistic photodynamic and photothermal abilities. ACS Appl. Bio. Mater. 2021, 4, 6703–6718. [Google Scholar] [CrossRef] [PubMed]
- Kumari, S.; Prasad, S.R.; Mandal, D.; Das, P. Carbon dot-DNA-protoporphyrin hybrid hydrogel for sustained photoinduced antimicrobial activity. J. Colloid Interface Sci. 2019, 553, 228–238. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Rojas-Andrade, M.D.; Chata, G.; Peng, Y.; Roseman, G.; Lu, J.E.; Millhauser, G.L.; Saltikov, C.; Chen, S. Photo-enhanced antibacterial activity of ZnO/graphene quantum dot nanocomposites. Nanoscale 2018, 10, 158–166. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, X.; Wang, X.; Mu, L.; Yuan, M.; Liu, B.; Shi, H. Carbon dots-decorated Na2W4O13 composite with WO3 for highly efficient photocatalytic antibacterial activity. J. Hazard. Mater. 2018, 359, 1–8. [Google Scholar] [CrossRef]
- Yan, Y.; Kuang, W.; Shi, L.; Ye, X.; Yang, Y.; Xie, X.; Shi, Q.; Tan, S. Carbon quantum dot-decorated TiO2 for fast and sustainable antibacterial properties under visible-light. J. Alloy. Compd. 2019, 777, 234–243. [Google Scholar] [CrossRef]
- De, B.; Gupta, K.; Mandal, M.; Karak, N. Biocide immobilized OMMT-carbon dot reduced Cu2O nanohybrid/hyperbranched epoxy nanocomposites: Mechanical, thermal, antimicrobial and optical properties. Mater. Sci. Eng. C-Mater. Biol. Appl. 2015, 56, 74–83. [Google Scholar] [CrossRef]
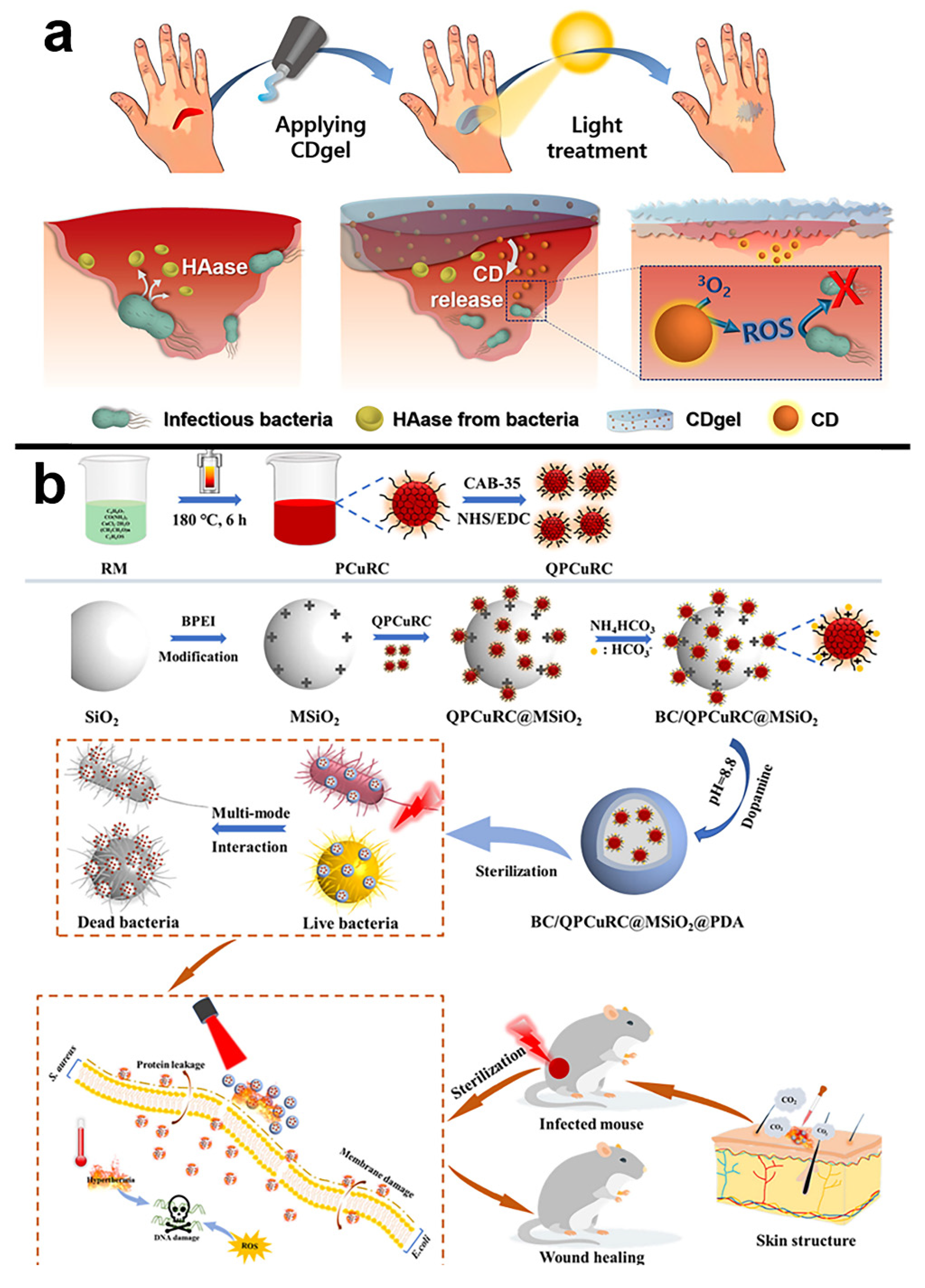
- Lee, C.H.; Song, S.Y.; Chung, Y.J.; Choi, E.K.; Jang, J.; Lee, D.H.; Kim, H.D.; Kim, D.-U.; Park, C.B. Light-stimulated carbon dot hydrogel: Targeting and clearing infectious bacteria in vivo. ACS Appl. Bio Mater. 2022, 5, 761–770. [Google Scholar] [CrossRef]
- Chu, X.; Liu, Y.; Zhang, P.; Li, K.; Feng, W.; Sun, B.; Zhou, N.; Shen, J. Silica-supported near-infrared carbon dots and bicarbonate nanoplatform for triple synergistic sterilization and wound healing promotion therapy. J. Colloid Interface Sci. 2022, 608, 1308–1322. [Google Scholar] [CrossRef]
- Wang, J.; Qiu, J. A review of carbon dots in biological applications. J. Mater. Sci. 2016, 51, 4728–4738. [Google Scholar] [CrossRef]
- Tao, H.; Yang, K.; Ma, Z.; Wan, J.; Zhang, Y.; Kang, Z.; Liu, Z. In vivo NIR fluorescence imaging, biodistribution, and toxicology of photoluminescent carbon dots produced from carbon nanotubes and graphite. Small 2012, 8, 281–290. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Ohulchanskyy, T.Y.; Liu, R.; Koynov, K.; Wu, D.; Best, A.; Kumar, R.; Bonoiu, A.; Prasad, P.N. Photoluminescent carbon dots as biocompatible nanoprobes for targeting cancer cells in vitro. J. Phys. Chem. C 2010, 114, 12062–12068. [Google Scholar] [CrossRef]
- Wang, S.; Cole, I.S.; Li, Q. The toxicity of graphene quantum dots. RSC Adv. 2016, 6, 89867–89878. [Google Scholar] [CrossRef]
- Li, Y.J.; Harroun, S.G.; Su, Y.C.; Huang, C.F.; Unnikrishnan, B.; Lin, H.J.; Lin, C.H.; Huang, C.C. Synthesis of self-assembled spermidine-carbon quantum dots effective against multidrug-resistant bacteria. Adv. Healthcare Mater. 2016, 5, 2545–2554. [Google Scholar] [CrossRef] [PubMed]
- Jian, H.J.; Wu, R.S.; Lin, T.Y.; Li, Y.J.; Lin, H.J.; Harroun, S.G.; Lai, J.Y.; Huang, C.C. Super-cationic carbon quantum dots synthesized from spermidine as an eye drop formulation for topical treatment of bacterial keratitis. ACS Nano 2017, 11, 6703–6716. [Google Scholar] [CrossRef]
| CDs Label a | The Precursor of CDs | Excitation Wavelength | Emission Wavelength | QY | Light Wavelength | Light Power | ROS Sensitization Yields | Microorganism | Reduction of Bacteria | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|
| GQD | graphite rods | 328 nm | 494 nm | -- | blue light (470 nm) | 1 W | -- | S. aureus and E. coli | 80% E. coli and 90–95% S. aureu were eliminated after 15 min * | [76] |
| MCDs | edible mushroom | 360 nm | 456 nm | 25% | visible LED light | 2.70 mW cm−2 | -- | E. coli | >90% elimination of E. coli in 12 h | [78] |
| CDs | citric acid | 370 nm | 450 nm | -- | blue light (450 nm) | 40 J cm−2 | -- | S. aureus | total elimination of S. aureus suspension was achieved (CDs: 6.90 mg/mL) and total elimination of the biofilm cultures was achieved (CDs: 13.80 mg/mL) | [80] |
| GQD | graphite | 480/740 nm | 618–647 nm | 18.50% | 800 nm | 2.64 mW | QY = 0.51 (1O2) | E. coli and MRSA | all E. coli and MRSA to be dead after the 15 s laser photoexcitation | [83] |
| Cur-NRCDs | curcumin, neutral red and citric acid | 540 nm | 635 nm | -- | xenon light (400–450 nm) | -- | -- | S. aureus and E. coli | after 10 min of xenon irradiation, 10 mM and 15 mM of Cur-NRCDs can kill 100% of S. aureus and E. coli, respectively | [88] |
| N-GQD (5.1%) | graphite | 365 nm | 624 nm | 25.90% | 670 nm laser | 0.10 W cm−2 | QY = 0.64 (1O2) | E. coli | 100% was eliminated by N-GQDs (5.1%) after a 3-min exposure | [89] |
| CDs | citric acid and ethylenediamine | 350 nm * | 450 nm | 20% | LED light (365 nm) | 3 V/3 W. | QY = 0.82 (1O2) | E. coli and Salmonella | bacteria growth inhibition efficiencies of 92% and 86% were obtained for E. coli and Salmonella in the presence of 5 μM CDs with light in 1 h, respectively | [43] |
| BrCDs | natural gas, HBr | 302 nm | >355 nm | -- | Ultraviolet lamp (365 nm) | 3 mW | -- | Listeria monocytogenes, S. aureus and E. coli. | with 10 min of UV exposure the growth of each bacterium is further decreased, achieving minimal to no colony formation visible for each | [91] |
| EDA-CDs/EPA-CDs | carbon nano-powders | -- | -- | 20% | 400–800 nm light bulb | 36 W, 12 V | -- | Bacillus subtilis | 1 h of EDA-CDs and EPA-CDs treatment resulted in a reduction of approximately 5.80 log and 0.84 log, respectively | [93] |
| FCDs | glucosamine hydrochloride and m-phenylenediamine | -- | -- | -- | blue-LED strip lights (460 nm) | 24 W, 12 V | -- | Klebsiella pneumoniae, Pseudomonas aeruginosa, E. coli and S. aureus | complete killing of each bacterium was reproducibly observed after treatment with 200 µg/mL FCDs with 4 h of irradiation, and significant killing (>95%) could be observed after only 90 min LED irradiation | [94] |
| Antibiotic-Modified CDs | ||||||||||
| CDs-AMP | citric acid and ethylenediamine | 350 nm | 450 nm | 19% | visible light | 0.30 W | -- | E. coli | >4 log10 inhibition of E. coli by CDs-AMP after 20 min of irradiation * | [96] |
| BSA-CDs NP | 1,5-dihydroxyanthraquinone | 395 nm | 525 nm | 75% (CDs) | Tungsten bulb (300–900 nm) | 100 W | -- | S. aureus and E. coli | 99.97% and 99.53% elimination of E. coli and S. aureus in 1h | [100] |
| CDs as nanocarriers for photosensitizers | ||||||||||
| CDs/MB or CDs/TB | carbon nanopowders | 400 nm | -- | 12% (CDs) | white light bulb | 36 W | -- | E. coli | 5 μg/mL CDs combined with 1 μg/mL MB completely inhibited bacteria growth, resulting in 6.20 log viable cell number reduction | [25] |
| GQDs | sulfur and nickel (II) oxide powder and benzene | 310 nm | 420 nm | -- | 660 nm red light | 12 W | -- | E. coli and Micrococcus luteus | 106 CFU/mL E. coli and Micrococcus luteus can be eradicated entirely in 10 min with MB-GQD irradiation | [102] |
| cur-GQDs | coal and curcumin | 407 nm | 525–550 nm * | -- | 405 nm LEDs | 30 J cm−2. | -- | Pseudomonas aeruginosa, MRSA, E. coli and Candida albicans. | for S. aureus Pseudomonas aeruginosa, MRSA, E. coli and Candida albicans, cur-GQDs caused 5.68 log10, 5.02 log10, 5.44 log10, 2.26 log10 and 3.82 log10 CFU reduction, respectively | [103] |
| CDs/Cur | citric acid and thiourea | 420 nm | 550–575 nm* | -- | 405 + 808 nm light | 808 nm (500 mW cm−2), 405 nm (200 mW cm−2) | -- | E. coli and S. aureus | death rate of E. coli and S. aureus increased to 100% for 1 μM and 0.1 nM CD/Cur, respectively | [104] |
| CD-DNA-PpIX hybrid hydrogel | citric acid and Branched Polyethylenimine | 350 nm | 625–650 nm * | -- | UV lamp (302 nm) | -- | -- | S. aureus | UV irradiation for 2.50 min followed by incubation for 24 h affected > 4.50 log (>99.99%) reduction of S. aureus cells | [105] |
| CDs/metal oxide nanocomposites | ||||||||||
| ZnO/GQDs | citric acid | 365 nm | 460 nm | -- | UV light (365nm) | 100 W, 1000–1500 lumen | -- | E. coli | 100% was eliminated by ZnO/GQDs after 5 min of UV exposure | [106] |
| Other hybrid CDs | ||||||||||
| CDgel | ammonium citrate and polyethylenimine | 390 nm | 400–500 nm | -- | white light irradiation | 5 mW cm−2 | -- | S. aureus and E. coli | CDgel under light giving approximately 99% and 97% mortality for S. aureus and E. coli, respectively | [110] |
| BC/ QPCuRC@MSiO2@PDA | Citric, urea and CuCl2·2H2O | 360 nm | 722 nm | -- | 808 nm | 2 W cm−2 | -- | S. aureus and E. coli | antibacterial rate up to 99.60% and 99.99% to E. coli and S. aureus, respectively | [111] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, X.; Abbas, K.; Yang, Y.; Li, Z.; Tedesco, A.C.; Bi, H. Photodynamic Anti-Bacteria by Carbon Dots and Their Nano-Composites. Pharmaceuticals 2022, 15, 487. https://doi.org/10.3390/ph15040487
Wu X, Abbas K, Yang Y, Li Z, Tedesco AC, Bi H. Photodynamic Anti-Bacteria by Carbon Dots and Their Nano-Composites. Pharmaceuticals. 2022; 15(4):487. https://doi.org/10.3390/ph15040487
Chicago/Turabian StyleWu, Xiaoyan, Khurram Abbas, Yuxiang Yang, Zijian Li, Antonio Claudio Tedesco, and Hong Bi. 2022. "Photodynamic Anti-Bacteria by Carbon Dots and Their Nano-Composites" Pharmaceuticals 15, no. 4: 487. https://doi.org/10.3390/ph15040487
APA StyleWu, X., Abbas, K., Yang, Y., Li, Z., Tedesco, A. C., & Bi, H. (2022). Photodynamic Anti-Bacteria by Carbon Dots and Their Nano-Composites. Pharmaceuticals, 15(4), 487. https://doi.org/10.3390/ph15040487






