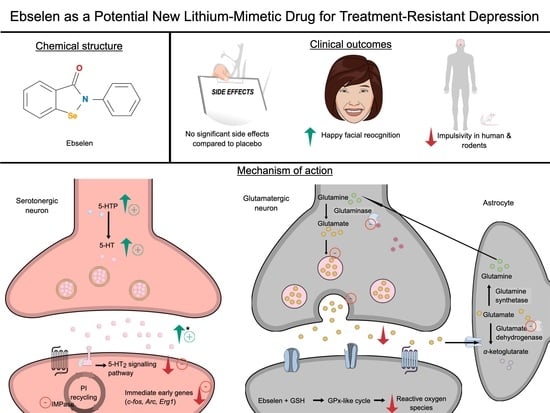
The Potential Use of Ebselen in Treatment-Resistant Depression
Abstract
:- Introduction p. 2
- The Development of Ebselen as a Pharmaceutical p. 3
- Clinical Pharmacokinetics of Ebselen p. 4
- Toxicity and Side Effects of Ebselen p. 4
- Psychopharmacology of Ebselen in Animal Studies p. 5
- 5.1
- Ebselen as an IMPase Inhibitor p. 5
- 5.2
- Effects of Ebselen on Animal Models of Mood Disorders p. 6
- 5.3
- Effects of Ebselen on Serotonin Neurotransmission p. 6
- 5.4
- Effects of Ebselen on Animal Models of Impulsivity p. 7
- 5.5
- Effects of Ebselen on Oxidative Stress/Neuroprotection p. 7
- Psychoparmacology of Ebselen in Human Studies p. 8
- 6.1
- Effects on Neuropsychological Tasks p. 8
- 6.2
- Effects of Ebselen on the Sleep Polysomnogram p. 9
- 6.3
- Effects of Ebselen on Brain Neurochemistry p. 9
- 6.4
- Effects of Ebselen in Bipolar Disorder p. 10
- 6.5
- Effects of Ebselen in TRD p. 10
- Conclusion p. 17
1. Introduction
2. The Development of Ebselen as a Pharmaceutical
3. Clinical Pharmacokinetics of Ebselen
4. Toxicity and Side Effects of Ebselen
5. Psychopharmacology of Ebselen in Animal Studies
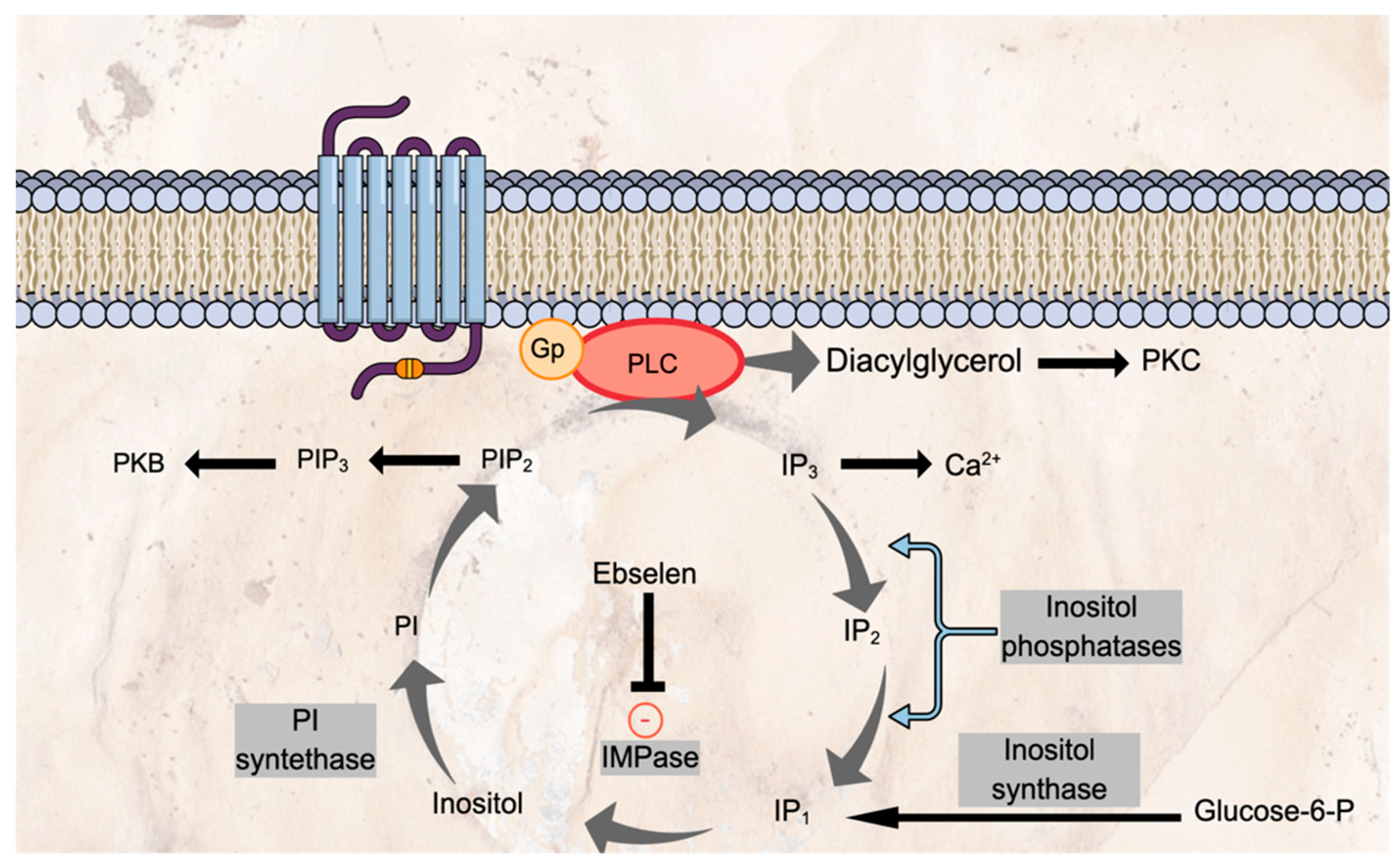
5.1. Ebselen as an IMPase Inhibitor
5.2. Effects of Ebselen on Animal Models of Mood Disorder
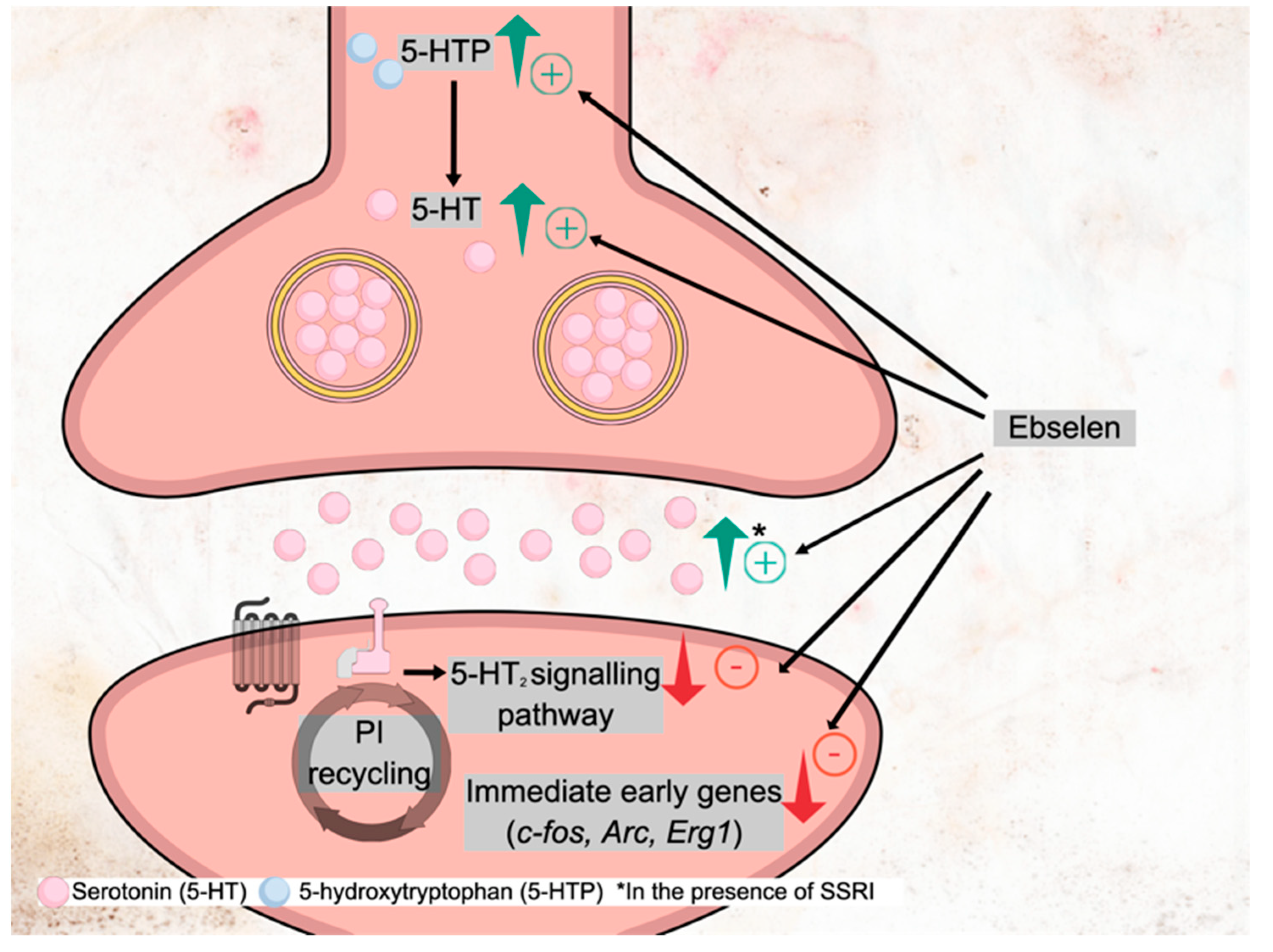
5.3. Effects of Ebselen on Serotonin Neurotransmission
5.4. Effects of Ebselen on Animal Models of Impulsivity
5.5. Effects of Ebselen on Oxidative Status/Neuroprotection
6. Psychopharmacology of Ebselen in Humans
6.1. Effects on Neuropsychological Tasks
6.2. Effects of Ebselen on the Sleep Polysomnogram
6.3. Effects of Ebselen on Brain Neurochemistry
6.4. Effect of Ebselen in Bipolar Disorder
6.5. Ebselen in TRD
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. Depression and Other Common Mental Disorders: Global Health Estimates; World Health Organization: Geneva, Switzerland, 2017. [Google Scholar]
- GBD 2016 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1789–1858. [Google Scholar] [CrossRef] [Green Version]
- GBD 2016 Disease and Injury Incidence and Prevalence Collaborators. Global prevalence and burden of depressive and anxiety disorders in 204 countries and territories in 2020 due to the COVID-19 pandemic. Lancet 2021, 398, 1700–1712. [Google Scholar] [CrossRef]
- Voineskos, D.; Daskalakis, Z.J.; Blumberger, D.M. Management of Treatment-Resistant Depression: Challenges and Strategies. Neuropsychiatr. Dis. Treat. 2020, 16, 221–234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rush, A.J.; Trivedi, M.H.; Wisniewski, S.R.; Nierenberg, A.A.; Stewart, J.W.; Warden, D.; Niederehe, G.; Thase, M.E.; Lavori, P.W.; Lebowitz, B.D.; et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: A STAR*D report. Am. J. Psychiatry 2006, 163, 1905–1917. [Google Scholar] [CrossRef]
- Cowen, P.J. Backing into the future: Pharmacological approaches to the management of resistant depression. Psychol. Med. 2017, 47, 2569–2577. [Google Scholar] [CrossRef] [Green Version]
- Kessler, D.; Burns, A.; Tallon, D.; Lewis, G.; MacNeill, S.; Round, J.; Hollingworth, W.; Chew-Graham, C.; Anderson, I.; Campbell, J.; et al. Combining mirtazapine with SSRIs or SNRIs for treatment-resistant depression: The MIR RCT. Health Technol. Assess. 2018, 22, 1–136. [Google Scholar] [CrossRef]
- Spielmans, G.I.; Berman, M.I.; Linardatos, E.; Rosenlicht, N.Z.; Perry, A.; Tsai, A.C. Adjunctive Atypical Antipsychotic Treatment for Major Depressive Disorder: A Meta-Analysis of Depression, Quality of Life, and Safety Outcomes. Focus 2016, 14, 244–265. [Google Scholar] [CrossRef] [Green Version]
- Nelson, J.C.; Baumann, P.; Delucchi, K.; Joffe, R.; Katona, C. A systematic review and meta-analysis of lithium augmentation of tricyclic and second generation antidepressants in major depression. J. Affect. Disord. 2014, 168, 269–275. [Google Scholar] [CrossRef]
- Vázquez, G.H.; Bahji, A.; Undurraga, J.; Tondo, L.; Baldessarini, R.J. Efficacy and Tolerability of Combination Treatments for Major Depression: Antidepressants plus Second-Generation Antipsychotics vs. Esketamine vs. Lithium. J. Psychopharmacol. 2021, 35, 890–900. [Google Scholar] [CrossRef]
- Cipriani, A.; Hawton, K.; Stockton, S.; Geddes, J.R. Lithium in the prevention of suicide in mood disorders: Updated systematic review and meta-analysis. BMJ 2013, 346, f3646. [Google Scholar] [CrossRef] [Green Version]
- McKnight, R.F.; Adida, M.; Budge, K.; Stockton, S.; Goodwin, G.M.; Geddes, J.R. Lithium toxicity profile: A systematic review and meta-analysis. Lancet 2012, 379, 721–728. [Google Scholar] [CrossRef]
- Nierenberg, A.A.; Fava, M.; Trivedi, M.H.; Wisniewski, S.R.; Thase, M.E.; McGrath, P.J.; Alpert, J.E.; Warden, D.; Luther, J.F.; Niederehe, G.; et al. A comparison of lithium and T(3) augmentation following two failed medication treatments for depression: A STAR*D report. Am. J. Psychiatry 2006, 163, 1519–1530. [Google Scholar] [CrossRef] [PubMed]
- Berridge, M.J.; Downes, C.P.; Hanley, M.R. Neural and developmental actions of lithium: A unifying hypothesis. Cell 1989, 59, 411–419. [Google Scholar] [CrossRef]
- Agam, G.; Bersudsky, Y.; Berry, G.T.; Moechars, D.; Lavi-Avnon, Y.; Belmaker, R.H. Knockout mice in understanding the mechanism of action of lithium. Biochem. Soc. Trans. 2009, 37, 1121–1125. [Google Scholar] [CrossRef] [Green Version]
- Parnham, M.J.; Sies, H. The early research and development of ebselen. Biochem. Pharmacol. 2013, 86, 1248–1253. [Google Scholar] [CrossRef]
- Engman, L.; Hallberg, A. Expedient synthesis of ebselen and related compounds. J. Org. Chem. 1989, 54, 2964–2966. [Google Scholar] [CrossRef]
- Santi, C.; Scimmi, C.; Sancineto, L. Ebselen and Analogues: Pharmacological Properties and Synthetic Strategies for Their Preparation. Molecules 2021, 26, 4230. [Google Scholar] [CrossRef]
- Shaaban, S.; Negm, A.; Sobh, M.A.; Wessjohann, L.A. Organoselenocyanates and symmetrical diselenides redox modulators: Design, synthesis and biological evaluation. Eur. J. Med. Chem. 2015, 97, 190–201. [Google Scholar] [CrossRef]
- Wendel, A.; Fausel, M.; Safayhi, H.; Tiegs, G.; Otter, R. A novel biologically active seleno-organic compound--II. Activity of PZ 51 in relation to glutathione peroxidase. Biochem. Pharmacol. 1984, 33, 3241–3245. [Google Scholar] [CrossRef]
- Sies, H. Ebselen, a selenoorganic compound as glutathione peroxidase mimic. Free Radic. Biol. Med. 1993, 14, 313–323. [Google Scholar] [CrossRef]
- Dawson, D.A.; Masayasu, H.; Graham, D.I.; Macrae, I.M. The neuroprotective efficacy of ebselen (a glutathione peroxidase mimic) on brain damage induced by transient focal cerebral ischaemia in the rat. Neurosci. Lett. 1995, 185, 65–69. [Google Scholar] [CrossRef]
- Johshita, H.; Sasaki, T.; Matsui, T.; Hanamura, T.; Masayasu, H.; Asano, T.; Takakura, K. Effects of ebselen (PZ51) on ischaemic brain oedema after focal ischaemia in cats. Acta Neurochir. Suppl. 1990, 51, 239–241. [Google Scholar] [CrossRef] [PubMed]
- Saito, I.; Asano, T.; Sano, K.; Takakura, K.; Abe, H.; Yoshimoto, T.; Kikuchi, H.; Ohta, T.; Ishibashi, S. Neuroprotective effect of an antioxidant, ebselen, in patients with delayed neurological deficits after aneurysmal subarachnoid hemorrhage. Neurosurgery 1998, 42, 269–277. [Google Scholar] [CrossRef] [Green Version]
- Yamaguchi, T.; Sano, K.; Takakura, K.; Saito, I.; Shinohara, Y.; Asano, T.; Yasuhara, H. Ebselen in acute ischemic stroke: A placebo-controlled, double-blind clinical trial. Ebselen Study Group. Stroke 1998, 29, 12–17. [Google Scholar] [CrossRef] [PubMed]
- Sharpley, A.L.; Williams, C.; Holder, A.A.; Godlewska, B.R.; Singh, N.; Shanyinde, M.; MacDonald, O.; Cowen, P.J. A phase 2a randomised, double-blind, placebo-controlled, parallel-group, add-on clinical trial of ebselen (SPI-1005) as a novel treatment for mania or hypomania. Psychopharmacology 2020, 237, 3773–3782. [Google Scholar] [CrossRef] [PubMed]
- Antoniadou, I.; Kouskou, M.; Arsiwala, T.; Singh, N.; Vasudevan, S.R.; Fowler, T.; Cadirci, E.; Churchill, G.C.; Sharp, T. Ebselen has lithium-like effects on central 5-HT(2A) receptor function. Br. J. Pharmacol. 2018, 175, 2599–2610. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martini, F.; Rosa, S.G.; Klann, I.P.; Fulco, B.C.W.; Carvalho, F.B.; Rahmeier, F.L.; Fernandes, M.C.; Nogueira, C.W. A multifunctional compound ebselen reverses memory impairment, apoptosis and oxidative stress in a mouse model of sporadic Alzheimer’s disease. J. Psychiatr. Res. 2019, 109, 107–117. [Google Scholar] [CrossRef]
- Xie, Y.; Tan, Y.; Zheng, Y.; Du, X.; Liu, Q. Ebselen ameliorates β-amyloid pathology, tau pathology, and cognitive impairment in triple-transgenic Alzheimer’s disease mice. J. Biol. Inorg. Chem. 2017, 22, 851–865. [Google Scholar] [CrossRef]
- Cheng, B.; Zhong, J.P.; Wu, F.X.; Li, G.L.; Ruan, Q.X.; Luo, G.; Jiang, H. Ebselen protects rat hearts against myocardial ischemia-reperfusion injury. Exp. Ther. Med. 2019, 17, 1412–1419. [Google Scholar] [CrossRef] [Green Version]
- Sies, H.; Parnham, M.J. Potential therapeutic use of ebselen for COVID-19 and other respiratory viral infections. Free Radic. Biol. Med. 2020, 156, 107–112. [Google Scholar] [CrossRef]
- Kil, J.; Lobarinas, E.; Spankovich, C.; Griffiths, S.K.; Antonelli, P.J.; Lynch, E.D.; Le Prell, C.G. Safety and efficacy of ebselen for the prevention of noise-induced hearing loss: A randomised, double-blind, placebo-controlled, phase 2 trial. Lancet 2017, 390, 969–979. [Google Scholar] [CrossRef]
- Lynch, E.; Kil, J. Development of ebselen, a glutathione peroxidase mimic, for the prevention and treatment of noise-induced hearing loss. In Proceedings of Seminars in Hearing; Thieme Medical Publishers: New York, NY, USA, 2009; pp. 47–55. [Google Scholar]
- Kil, J.; Harruff, E.E.; Longenecker, R.J. Development of ebselen for the treatment of sensorineural hearing loss and tinnitus. Hear. Res. 2022, 413, 108209. [Google Scholar] [CrossRef] [PubMed]
- Kil, J.; Huang, M.; Nguyen, S.; Chandrasekhar, S.; Lambert, P. SPI-1005 A Novel Investigational Drug For The Treatment of Meniere’s Disease. In Proceedings of 2018 ARO Abstract; Association for Research in Otolaryngology: Brenrwood, TN, USA, 2018; p. 519. [Google Scholar]
- Menéndez, C.A.; Byléhn, F.; Perez-Lemus, G.R.; Alvarado, W.; de Pablo, J.J. Molecular characterization of ebselen binding activity to SARS-CoV-2 main protease. Sci. Adv. 2020, 6, eabd0345. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.Y.; Chen, C.; Su, J.; Li, J.Q.; Jiang, Z.; Gao, H.; Chigan, J.Z.; Ding, H.H.; Zhai, L.; Yang, K.W. Ebsulfur and Ebselen as highly potent scaffolds for the development of potential SARS-CoV-2 antivirals. Bioorg. Chem. 2021, 112, 104889. [Google Scholar] [CrossRef]
- Masaki, C.; Sharpley, A.L.; Godlewska, B.R.; Berrington, A.; Hashimoto, T.; Singh, N.; Vasudevan, S.R.; Emir, U.E.; Churchill, G.C.; Cowen, P.J. Effects of the potential lithium-mimetic, ebselen, on brain neurochemistry: A magnetic resonance spectroscopy study at 7 tesla. Psychopharmacology 2016, 233, 1097–1104. [Google Scholar] [CrossRef] [Green Version]
- Singh, N.; Sharpley, A.L.; Emir, U.E.; Masaki, C.; Herzallah, M.M.; Gluck, M.A.; Sharp, T.; Harmer, C.J.; Vasudevan, S.R.; Cowen, P.J.; et al. Effect of the Putative Lithium Mimetic Ebselen on Brain Myo-Inositol, Sleep, and Emotional Processing in Humans. Neuropsychopharmacology 2016, 41, 1768–1778. [Google Scholar] [CrossRef]
- Caeran Bueno, D.; Meinerz, D.F.; Allebrandt, J.; Waczuk, E.P.; dos Santos, D.B.; Mariano, D.O.; Rocha, J.B. Cytotoxicity and genotoxicity evaluation of organochalcogens in human leucocytes: A comparative study between ebselen, diphenyl diselenide, and diphenyl ditelluride. BioMed Res. Int. 2013, 2013, 537279. [Google Scholar] [CrossRef]
- Bueno, D.; Meinerz, D.; Waczuk, E.; de Souza, D.; Batista Rocha, J. Toxicity of organochalcogens in human leukocytes is associated, but not directly related with reactive species production, apoptosis and changes in antioxidant gene expression. Free Radic. Res. 2018, 52, 1158–1169. [Google Scholar] [CrossRef]
- Meotti, F.C.; Borges, V.C.; Zeni, G.; Rocha, J.B.; Nogueira, C.W. Potential renal and hepatic toxicity of diphenyl diselenide, diphenyl ditelluride and Ebselen for rats and mice. Toxicol. Lett. 2003, 143, 9–16. [Google Scholar] [CrossRef]
- Farina, M.; Soares, F.A.; Zeni, G.; Souza, D.O.; Rocha, J.B. Additive pro-oxidative effects of methylmercury and ebselen in liver from suckling rat pups. Toxicol. Lett. 2004, 146, 227–235. [Google Scholar] [CrossRef]
- Puntel, R.L.; Roos, D.H.; Folmer, V.; Nogueira, C.W.; Galina, A.; Aschner, M.; Rocha, J.B. Mitochondrial dysfunction induced by different organochalchogens is mediated by thiol oxidation and is not dependent of the classical mitochondrial permeability transition pore opening. Toxicol. Sci. 2010, 117, 133–143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Puntel, R.L.; Roos, D.H.; Seeger, R.L.; Rocha, J.B. Mitochondrial electron transfer chain complexes inhibition by different organochalcogens. Toxicol. Vitr. 2013, 27, 59–70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, N.; Halliday, A.C.; Thomas, J.M.; Kuznetsova, O.V.; Baldwin, R.; Woon, E.C.Y.; Aley, P.K.; Antoniadou, I.; Sharp, T.; Vasudevan, S.R.; et al. A safe lithium mimetic for bipolar disorder. Nat. Commun. 2013, 4, 1332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fenn, G.D.; Waller-Evans, H.; Atack, J.R.; Bax, B.D. Crystallization and structure of ebselen bound to Cys141 of human inositol monophosphatase. Acta Crystallogr. Sect. F Struct. Biol. Commun. 2020, 76, 469–476. [Google Scholar] [CrossRef]
- Harwood, A.J. Lithium and bipolar mood disorder: The inositol-depletion hypothesis revisited. Mol. Psychiatry 2005, 10, 117–126. [Google Scholar] [CrossRef]
- Posser, T.; Kaster, M.P.; Baraúna, S.C.; Rocha, J.B.; Rodrigues, A.L.; Leal, R.B. Antidepressant-like effect of the organoselenium compound ebselen in mice: Evidence for the involvement of the monoaminergic system. Eur. J. Pharmacol. 2009, 602, 85–91. [Google Scholar] [CrossRef]
- Lerer, B.; Globus, M.; Brik, E.; Hamburger, R.; Belmaker, R.H. Effect of treatment and withdrawal from chronic lithium in rats on stimulant-induced responses. Neuropsychobiology 1984, 11, 28–32. [Google Scholar] [CrossRef]
- Jacobs, D.; Silverstone, T. Dextroamphetamine-induced arousal in human subjects as a model for mania. Psychol. Med. 1986, 16, 323–329. [Google Scholar] [CrossRef]
- Cipriani, A.; Barbui, C.; Salanti, G.; Rendell, J.; Brown, R.; Stockton, S.; Purgato, M.; Spineli, L.M.; Goodwin, G.M.; Geddes, J.R. Comparative efficacy and acceptability of antimanic drugs in acute mania: A multiple-treatments meta-analysis. Lancet 2011, 378, 1306–1315. [Google Scholar] [CrossRef]
- Lan, A.; Einat, H. Questioning the predictive validity of the amphetamine-induced hyperactivity model for screening mood stabilizing drugs. Behav. Brain Res. 2019, 362, 109–113. [Google Scholar] [CrossRef]
- Cowen, P.J.; Browning, M. What has serotonin to do with depression? World Psychiatry 2015, 14, 158–160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blier, P.; De Montigny, C. Short-term lithium administration enhances serotonergic neurotransmission: Electrophysiological evidence in the rat CNS. Eur. J. Pharmacol. 1985, 113, 69–77. [Google Scholar] [CrossRef]
- Cowen, P.J.; McCance, S.L.; Cohen, P.R.; Julier, D.L. Lithium increases 5-HT-mediated neuroendocrine responses in tricyclic resistant depression. Psychopharmacology 1989, 99, 230–232. [Google Scholar] [CrossRef] [PubMed]
- Sharp, T.; Bramwell, S.R.; Lambert, P.; Grahame-Smith, D.G. Effect of short- and long-term administration of lithium on the release of endogenous 5-HT in the hippocampus of the rat in vivo and in vitro. Neuropharmacology 1991, 30, 977–984. [Google Scholar] [CrossRef]
- Wegener, G.; Bandpey, Z.; Heiberg, I.L.; Mørk, A.; Rosenberg, R. Increased extracellular serotonin level in rat hippocampus induced by chronic citalopram is augmented by subchronic lithium: Neurochemical and behavioural studies in the rat. Psychopharmacology 2003, 166, 188–194. [Google Scholar] [CrossRef]
- Goodwin, G.M.; DeSouza, R.J.; Wood, A.J.; Green, A.R. Lithium decreases 5-HT1A and 5-HT2 receptor and alpha 2-adrenoceptor mediated function in mice. Psychopharmacology 1986, 90, 482–487. [Google Scholar] [CrossRef]
- Boothman, L.J.; Mitchell, S.N.; Sharp, T. Investigation of the SSRI augmentation properties of 5-HT(2) receptor antagonists using in vivo microdialysis. Neuropharmacology 2006, 50, 726–732. [Google Scholar] [CrossRef]
- Antoniadou, I.; Buchmueller, D.; Walker, P.; Singh, N.; Vasudevan, S.; Churchill, G.; Sharp, T. Effect of ebselen, a putative lithium mimetic, on central 5-HT2C receptor function in the mouse. Proc. Br. Pharmacol. Soc. 2015, 1, 215P. [Google Scholar]
- Sotty, F.; Folgering, J.H.; Brennum, L.T.; Hogg, S.; Mørk, A.; Hertel, P.; Cremers, T.I. Relevance of dorsal raphe nucleus firing in serotonin 5-HT(2C) receptor blockade-induced augmentation of SSRIs effects. Neuropharmacology 2009, 57, 18–24. [Google Scholar] [CrossRef]
- Di Nicola, M.; De Crescenzo, F.; D’Alò, G.L.; Remondi, C.; Panaccione, I.; Moccia, L.; Molinaro, M.; Dattoli, L.; Lauriola, A.; Martinelli, S.; et al. Pharmacological and Psychosocial Treatment of Adults With Gambling Disorder: A Meta-Review. J. Addict. Med. 2020, 14, e15–e23. [Google Scholar] [CrossRef]
- Fink, L.H.; Anastasio, N.C.; Fox, R.G.; Rice, K.C.; Moeller, F.G.; Cunningham, K.A. Individual Differences in Impulsive Action Reflect Variation in the Cortical Serotonin 5-HT2A Receptor System. Neuropsychopharmacology 2015, 40, 1957–1968. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barkus, C.; Ferland, J.N.; Adams, W.K.; Churchill, G.C.; Cowen, P.J.; Bannerman, D.M.; Rogers, R.D.; Winstanley, C.A.; Sharp, T. The putative lithium-mimetic ebselen reduces impulsivity in rodent models. J. Psychopharmacol. 2018, 32, 1018–1026. [Google Scholar] [CrossRef] [PubMed]
- Azad, G.K.; Tomar, R.S. Ebselen, a promising antioxidant drug: Mechanisms of action and targets of biological pathways. Mol. Biol. Rep. 2014, 41, 4865–4879. [Google Scholar] [CrossRef] [PubMed]
- Mishra, B.; Priyadarsini, K.I.; Mohan, H.; Mugesh, G. Horseradish peroxidase inhibition and antioxidant activity of ebselen and related organoselenium compounds. Bioorg. Med. Chem. Lett. 2006, 16, 5334–5338. [Google Scholar] [CrossRef] [PubMed]
- Noguchi, N.; Yoshida, Y.; Kaneda, H.; Yamamoto, Y.; Niki, E. Action of ebselen as an antioxidant against lipid peroxidation. Biochem. Pharmacol. 1992, 44, 39–44. [Google Scholar] [CrossRef]
- Klann, I.P.; Martini, F.; Rosa, S.G.; Nogueira, C.W. Ebselen reversed peripheral oxidative stress induced by a mouse model of sporadic Alzheimer’s disease. Mol. Biol. Rep. 2020, 47, 2205–2215. [Google Scholar] [CrossRef]
- Cabungcal, J.H.; Counotte, D.S.; Lewis, E.; Tejeda, H.A.; Piantadosi, P.; Pollock, C.; Calhoon, G.G.; Sullivan, E.; Presgraves, E.; Kil, J.; et al. Juvenile antioxidant treatment prevents adult deficits in a developmental model of schizophrenia. Neuron 2014, 83, 1073–1084. [Google Scholar] [CrossRef] [Green Version]
- Heffetz-Giterman, L.; Lander, S.S.; Cohen, R.; Gross, A.; Gaisler-Salomon, I. T220. The glutaminase inhibitor ebselen prevents amphetamine sensitization in mice. Schizophr. Bull. 2018, 44, S202. [Google Scholar] [CrossRef] [Green Version]
- Kade, I.J.; Balogun, B.D.; Rocha, J.B. In vitro glutathione peroxidase mimicry of ebselen is linked to its oxidation of critical thiols on key cerebral suphydryl proteins-A novel component of its GPx-mimic antioxidant mechanism emerging from its thiol-modulated toxicology and pharmacology. Chem. Biol. Interact. 2013, 206, 27–36. [Google Scholar] [CrossRef] [Green Version]
- Nogueira, C.W.; Rocha, J.B. Toxicology and pharmacology of selenium: Emphasis on synthetic organoselenium compounds. Arch. Toxicol. 2011, 85, 1313–1359. [Google Scholar] [CrossRef]
- Harmer, C.J.; Duman, R.S.; Cowen, P.J. How do antidepressants work? New perspectives for refining future treatment approaches. Lancet Psychiatry 2017, 4, 409–418. [Google Scholar] [CrossRef] [Green Version]
- Godlewska, B.R.; Harmer, C.J. Cognitive neuropsychological theory of antidepressant action: A modern-day approach to depression and its treatment. Psychopharmacology 2021, 238, 1265–1278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Masaki, C.; Sharpley, A.L.; Cooper, C.M.; Godlewska, B.R.; Singh, N.; Vasudevan, S.R.; Harmer, C.J.; Churchill, G.C.; Sharp, T.; Rogers, R.D.; et al. Effects of the potential lithium-mimetic, ebselen, on impulsivity and emotional processing. Psychopharmacology 2016, 233, 2655–2661. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ohmura, Y.; Tsutsui-Kimura, I.; Kumamoto, H.; Minami, M.; Izumi, T.; Yamaguchi, T.; Yoshida, T.; Yoshioka, M. Lithium, but not valproic acid or carbamazepine, suppresses impulsive-like action in rats. Psychopharmacology 2012, 219, 421–432. [Google Scholar] [CrossRef]
- Sheard, M.H.; Marini, J.L.; Bridges, C.I.; Wagner, E. The effect of lithium on impulsive aggressive behavior in man. Am. J. Psychiatry 1976, 133, 1409–1413. [Google Scholar] [CrossRef]
- Murphy, F.C.; Rubinsztein, J.S.; Michael, A.; Rogers, R.D.; Robbins, T.W.; Paykel, E.S.; Sahakian, B.J. Decision-making cognition in mania and depression. Psychol. Med. 2001, 31, 679–693. [Google Scholar] [CrossRef]
- Sharpley, A.L.; Cowen, P.J. Effect of pharmacologic treatments on the sleep of depressed patients. Biol. Psychiatry 1995, 37, 85–98. [Google Scholar] [CrossRef]
- Friston, K.J.; Sharpley, A.L.; Solomon, R.A.; Cowen, P.J. Lithium increases slow wave sleep: Possible mediation by brain 5-HT2 receptors? Psychopharmacology 1989, 98, 139–140. [Google Scholar] [CrossRef]
- Kadriu, B.; Musazzi, L.; Henter, I.D.; Graves, M.; Popoli, M.; Zarate, C.A., Jr. Glutamatergic Neurotransmission: Pathway to Developing Novel Rapid-Acting Antidepressant Treatments. Int. J. Neuropsychopharmacol. 2019, 22, 119–135. [Google Scholar] [CrossRef] [Green Version]
- Thomas, A.G.; Rojas, C.; Tanega, C.; Shen, M.; Simeonov, A.; Boxer, M.B.; Auld, D.S.; Ferraris, D.V.; Tsukamoto, T.; Slusher, B.S. Kinetic characterization of ebselen, chelerythrine and apomorphine as glutaminase inhibitors. Biochem. Biophys. Res. Commun. 2013, 438, 243–248. [Google Scholar] [CrossRef] [Green Version]
- Yüksel, C.; Öngür, D. Magnetic resonance spectroscopy studies of glutamate-related abnormalities in mood disorders. Biol. Psychiatry 2010, 68, 785–794. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Servaes, S.; Kara, F.; Glorie, D.; Stroobants, S.; Van Der Linden, A.; Staelens, S. In Vivo Preclinical Molecular Imaging of Repeated Exposure to an N-methyl-d-aspartate Antagonist and a Glutaminase Inhibitor as Potential Glutamatergic Modulators. J. Pharmacol. Exp. Ther. 2019, 368, 382–390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mota, F.; Sementa, T.; Taddei, C.; Moses, N.; Bordoloi, J.; Hader, S.; Eykyn, T.; Cash, D.; Turkheimer, F.; Veronese, M.; et al. Investigating the effects of ebselen, a potential new lithium mimetic, on glutamate transmission. Synapse 2020, 74, e22151. [Google Scholar] [CrossRef] [PubMed]
- Nogueira, C.W.; Rotta, L.N.; Zeni, G.; Souza, D.O.; Rocha, J.B. Exposure to ebselen changes glutamate uptake and release by rat brain synaptosomes. Neurochem. Res. 2002, 27, 283–288. [Google Scholar] [CrossRef]
- Porciúncula, L.O.; Rocha, J.B.; Boeck, C.R.; Vendite, D.; Souza, D.O. Ebselen prevents excitotoxicity provoked by glutamate in rat cerebellar granule neurons. Neurosci. Lett. 2001, 299, 217–220. [Google Scholar] [CrossRef]
- Gigante, A.D.; Bond, D.J.; Lafer, B.; Lam, R.W.; Young, L.T.; Yatham, L.N. Brain glutamate levels measured by magnetic resonance spectroscopy in patients with bipolar disorder: A meta-analysis. Bipolar Disord. 2012, 14, 478–487. [Google Scholar] [CrossRef]
- Pawlas, N.; Małecki, A. Effects of ebselen on glutathione level in neurons exposed to arachidonic acid and 4-hydroxynonenal during simulated ischemia in vitro. Pharmacol. Rep. 2007, 59, 708–714. [Google Scholar]
- Satoh, T.; Yoshioka, Y. Contribution of reduced and oxidized glutathione to signals detected by magnetic resonance spectroscopy as indicators of local brain redox state. Neurosci. Res. 2006, 55, 34–39. [Google Scholar] [CrossRef]
- Berk, M.; Ng, F.; Dean, O.; Dodd, S.; Bush, A.I. Glutathione: A novel treatment target in psychiatry. Trends Pharmacol. Sci. 2008, 29, 346–351. [Google Scholar] [CrossRef]
- Bahji, A.; Ermacora, D.; Stephenson, C.; Hawken, E.R.; Vazquez, G. Comparative efficacy and tolerability of pharmacological treatments for the treatment of acute bipolar depression: A systematic review and network meta-analysis. J. Affect. Disord. 2020, 269, 154–184. [Google Scholar] [CrossRef]
- Godlewska, B.R. Cognitive neuropsychological theory: Reconciliation of psychological and biological approaches for depression. Pharmacol. Ther. 2019, 197, 38–51. [Google Scholar] [CrossRef] [PubMed]
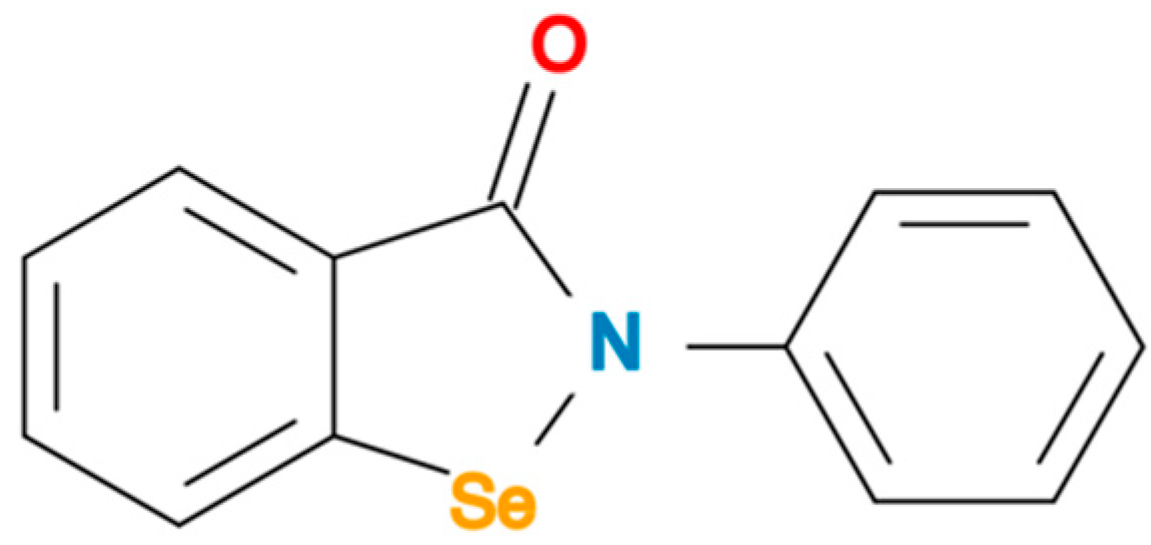
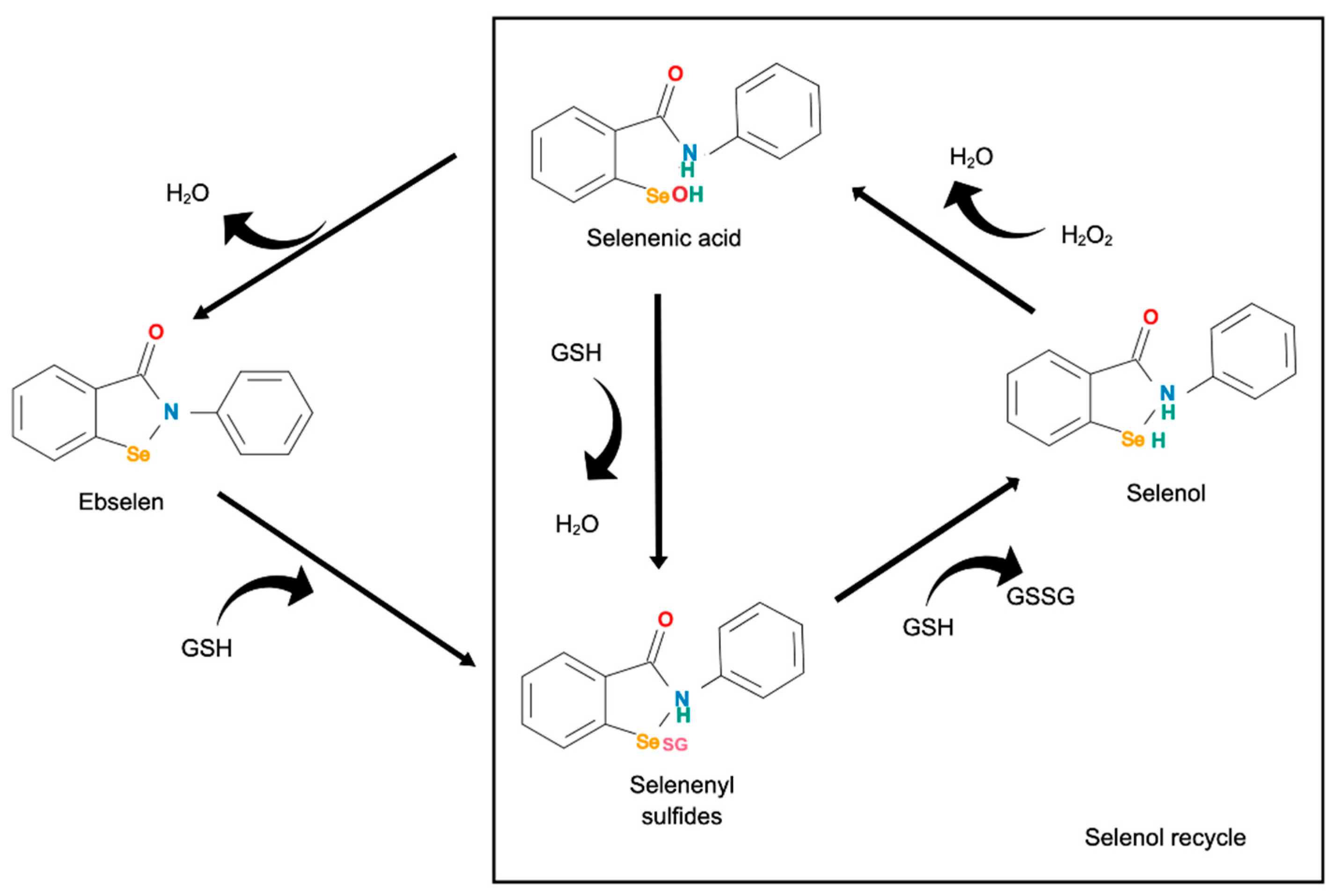
| Ref | Focus on | Methods—Main Aspects | Important Findings | Remarks |
|---|---|---|---|---|
| Pre-Clinical Studies | ||||
| Ischemic Models—Neuroprotection | ||||
| Dawson et al. 1995 [22] | The neuroprotective effect of ebselen in the model of transient focal ischaemia in rats | Temporary occlusion of the middle cerebral artery (MCA) with vasoconstrictor endothelin-1. Pre-treatment with ebselen (10 or 30 mg/kg p.o.) or vehicle, 40 min pre-MCA occlusion (n = 15 in each group). | Dose-dependent reduction in the volume of ischaemic damage 4-h post-endothelin-1 application (non-significant 35% at 10 mg/kg and significant 48–53% at 30 mg/kg compared with the vehicle control). No alterations in blood pressure, body temperature or arterial blood gases, i.e., the neuroprotective effect of ebselen was not attributable to alterations in physiological variables. | Suggested neuroprotective mechanism: decrease in oxidative stress. Ebselen may be an effective neuroprotective agent against acute focal ischaemic-reperfusion injury. |
| Johshita et al. 1990 [23] | The neuroprotective effect of ebselen in the model of ischaemic cortical oedema in cats | Temporary occlusion of the MCA: prolonged ischaemia and recirculation. Local cerebral blood flow (lCBF) measured by the hydrogen clearance in the MCA territory. | Ebselen significantly ameliorated post-ischaemic hypoperfusion following recirculation. No significant effect on normal and ischaemic lCBF. | Proposed main mechanism: anti-inflammatory action. |
| Cheng et al. 2019 [30] | The effect of ebselen on myocardial ischaemia-reperfusion (I/R) injury in rats | Temporary occlusion (30 min) of the left anterior descending coronary artery, followed by 2 h of reperfusion. Pre-treatment with ebselen (20 mg/kg) intragastrically 24 h prior to the I/R-inducing surgery and throughout the experimental period. | Ebselen: -reduced I/R-induced myocardial infarct size -prevented I/R-induced decreases in ejection fraction and fractional shortening -attenuated I/R-induced heart injury and apoptosis (histological and ultrastructural changes, reduction of serum CK, CK-MB and LDH activity, decreased cell apoptosis) -ameliorated oxidative stress | Proposed main mechanism: suppression of cardiomyocyte apoptosis and promotion of antioxidant activity. |
| Studies Relevant To Mental Health and Central Effects of Ebselen | ||||
| Singh et al. 2013 [46] | Ebselen as a lithium mimetic; mechanisms of action | Animal models Ex vivo assessments | Ebselen: -is pharmacologically active in the brain (ex vivo methods based on IMPase activity in brain homogenate) -alters the function of the CNS (a decrease in 5-HT2 agonist-induced head twitches in a dose-dependent manner, with decreased expression of Arc mRNA (a marker of neural activity) in the PFC and ACC) - exhibits lithium-like effects on behavior (reduced amphetamine-induced hyperactivity, dependent on the dose of amphetamine and ebselen, baseline activity unaffected) -inhibits IMPase in irreversible and covalent manner - acts through inositol depletion (intracerebroventricular injection of inositol reversed the behavioral effects of ebselen; intraperitoneal injection of ebselen decreased brain inositol) | Ebselen suggested as a lithium mimetic acting via inhibition of IMPase. |
| Antoniadou et al. 2018 [27] | The effect of ebselen on 5-HT2A receptor function in mice | Behavioural and molecular models of 5-HT2A receptor function: -behavioural responses (head-twitches, ear scratches) -molecular responses (levels of mRNA for cortical immediate early gene, IEG: Arc, c-fos, Egr2) to 5-HT2A receptor agonist DOI -augmentation of SSRI action, similar to lithium and 5-HT2A antagonists Ebselen: acute (1, 5 or 10 mg/kg i.p.) and repeated (10 mg/kg i.p., bd for seven days) administration prior to assessment of 5-HT2A receptor function; co-administration of ebselen with the SSRI citalopram in microdialysis experiments | Ebselen: -inhibited behavioural and IEG responses to DOI; -increased extracellular 5-HT; -increased regional brain 5-HT synthesis. | Suggested mechanism of action: IMPase inhibition. The study also tested the effects of lithium, IMPase inhibitor L-690330, GSK-3 inhibitor AR-A 014418 |
| Martini et al. 2019 [28] | The effect of ebselen on memory impairment, hippocampal oxidative stress, apoptosis and cell proliferation in a mouse model of sporadic Alzheimer Disease (AD). | Metabolic model of sporadic AD induced by intracerebroventricular (icv) injection of streptozotocin (STZ). Ebselen (1−10 mg/kg i.p.) administered with icv STZ (3 mg/kg, 1 μL/min). Behavioural tests of memory (object recognition test, object location test, Y- maze test, spontaneous locomotion test) Ex-vivo analyses of glycemia, parameters of oxidative stress and markers of cell proliferation (BrdU) | Ebselen: -reversed memory impairment -reversed hippocampal oxidative stress -had an anti-apoptotic effect. No effect against decreased cell proliferation induced by icv STZ. | The study also tested the effects of donepezil |
| Xie et al. 2017 [29] | The effect of ebselen on cognitive dysfunction and neuropathology in a mouse model of AD, AD model cell, and primary culture. | Mice expressing mutations of human genes relevant to AD. Ebselen 3 μg/mL for six months between two and eight months of age Behavioral tests of spatial learning and memory (Morris test, place navigation test, probe trial) Ex-vivo biochemical analyses | Ebselen: -improved spatial learning and memory -reduced oxidative stress in both AD model cells and mouse brains -decreased tau pathology -reduced levels of Aβ in, especially the most toxic soluble oligomers - reversed synaptic deficits | Suggested as a potential novel therapeutic approach for the prevention of AD |
| Cabungcal et al. 2014 [70] | The effect of ebselen on behavioral deficits caused by oxidative stress in a developmental rodent model of schizophrenia. | Model: rats with neonatal ventral hippocampal lesion (NVHL), yielding adolescent and adult animals with PFC-dependent electrophysiological, neurochemical, and behavioral anomalies, reflecting changes in schizophrenia. Ebselen (10 mg/kg) i.p. five days per week from postnatal day 35 to 60 (PPI testing). N-acetylcysteine (NAC). Prepulse inhibition of the acoustic startle response (PPI), a measure of sensorimotor gating. Biochemical analyses for NAC only. | Ebselen and NAC reversed behavioural deficits in the model. NAC prevented oxidative stress, the reduction of prefrontal parvalbumin interneuron activity and electrophysiological deficits (not tested for ebselen). | Adolescent treatment with NAC or ebselen sufficient to prevent PPI deficits. Redox modulation suggested as a potential target for early intervention in schizophrenia. |
| Posser at al. 2009 [49] | Antidepressant effect of ebselen, and its mechanisms, in a rodent model of depression. | Mouse model of depression: the forced swimming test (FST), tail suspension test (TST) Ebselen s.c. 3−10 mg/kg Specific mechanisms tested via pretreatment with appropriate compounds: (1) Serotonergic mechanisms: -inhibitor of serotonin synthesis, p-chlorophenylalanine (PCPA) -serotonin 5HT1A receptor antagonist NAN-190 -serotonin 5-HT2A/2C receptor antagonist ketanserin (2) Noradrenergic mechanisms: -alpha1-adrenoceptor antagonist prazosin -alpha2-adrenoceptor antagonist yohimbine (3) Dopaminergic mechanisms: -dopamine D1 receptor antagonist SCH23390 -dopamine D2 receptor antagonist sulpiride | Ebselen: - ↓ immobility time in the FST (i.e antidepressant-like effect of ebselen) at 10−20 mg/kg but not 3 or 30 mg/kg, with no effect in the open field test (i.e., effect in FST not attributable to a psychostimulant effect) - ↓ immobility time present with pre-treatment with serotonergic agents but not with noradrenergic and dopaminergic agents -no effect in TST | Ebselen produced an antidepressant-like effect. This effect was likely related to noradrenergic and dopaminergic, but not serotonergic, action. |
| Barkus et al. 2018 [65] | The effect of ebselen on 5-HT2A receptor function in rat models of impulsive behavior. | Ebselen in doses decreasing 5-HT2A receptor function (DOI-induced wet dog shakes) Two models of impulsivity: -five-choice serial reaction time task (5-CSRTT) -rodent gambling task (rGT). The main outcome measures: -premature responses (5-CSRTT and rGT), model of motor impulsivity -choice behaviour (rGT), model of choice impulsivity | The 5-CSRTT: Ebselen decreased premature responding both in the absence and presence of cocaine; The 5-HT2A receptor antagonist MDL 100,907 reduced premature responding only in the absence of cocaine The rGT: Ebselen reduced premature responding, with no effect on choice behaviour. | Ebselen preferentially reduced motor impulsivity over choice impulsivity, with inhibition of 5-HT2A receptor function as a contributing mechanism. Suggested as a potential compound in the management of disorders with poor impulse control. |
| Antiviral Activity | ||||
| Menéndez et al. 2020 [36] | The potential of ebselen against severe respiratory syndrome coronavirus 2 (SARS-CoV-2). | Atomistic molecular simulations. | Two highly probable interaction sites between SARS-CoV-2 Mpro and ebselen: within the catalytic region and in the previously unknown binding sites between the II and III domains, essential for Mpro dimerization. | Ebselen deemed a potential drug against SARS-CoV-2. |
| Sun et al. 2021 [37] | The potential and mechanism of action of ebselen (and ebsulfur) as an anti-SARS-CoV-2 agent. | Enzymatic kinetics and fluorescent labeling, molecular docking. | The half-maximal inhibitory concentration (IC50) Ebselen: 0.074 μM) and Ebsulfur: 0.11 μM). The action mechanism: covalent and irreversibly bind to Mpro, an SS bond with the Cys145 at the enzymatic active site. | Ebsulfur and Ebselen potent scaffolds for the development of covalent inhibitors of Mpro against COVID-19. |
| Human Studies | ||||
| Pharmacokinetics | ||||
| Lynch and Kil [33] | Pharmacokinetics of ebselen | An FDA approved, placebo-controlled, Phase 1 trial of ebselen in 32 healthy participants who received single doses of ebselen varying between 200 mg and 1600 mg. | Maximum blood concentrations of ebselen: between 1.5 and 2.25 h The half- life increased with dose, around 6.5 h with 200 mg and 16.7 h with 1600 mg. Maximum plasma concentration and area under the curve (i.e., exposure to ebselen) increased with dose but not proportionately. | |
| Studies Relevant To Mental Health and Central Effects of Ebselen | ||||
| Masaki et al. 2016 [38] | The effect of ebselen on brain biochemistry | Double-blind, random-order, crossover study in 20 healthy volunteers tested on two occasions receiving either ebselen (3600 mg over 24 h) or placebo. Neurometabolites in the ACC and OCC were measured using 7 Tesla H1-MRS. | Ebselen: ↓ concentrations of inositol, glutathione, glutamine, glutamate and Glx in the ACC but not the OCC. . | Ebselen suggested to inhibit both IMPase and glutaminase in the human brain. Adverse events comparable between groups and mild. |
| Singh et al. 2016 [39] | The effect of ebselen on brain biochemistry, sleep and reward processing | Treatment: 1800 mg ebselen or placebo over two days Sleep and inositol study: 16 healthy volunteers, a double-blind, random-order, crossover design. Emotional processing study: 40 healthy volunteers, a double-blind, random-order, parallel-group design. Emotional processing assessment: -Auditory Verbal Learning Task -Emotional Testing Battery (ETB) Reward processing assessment: Reward and Punishment Learning Task Sleep assessment: polysomnograms recorded at home Brain biochemistry: H1-MRS at 3 Tesla, voxels in the ACC and OCC | Ebselen: ↓ inositol levels in the ACC (no effect in the OCC) ↓ slow-wave sleep episodes ↓ total correct reward choices made, reward reinforcement, latency of response to the acoustic stimuli in the startle test ↑ punishment reinforcement, recognition of disgust and happiness | Ebselen affected the phosphoinositide cycle and had CNS effects on surrogate markers that may be relevant to the treatment of bipolar disorder. Adverse events comparable between groups and mild. |
| Masaki et al. 2016 [76] | The effect of ebselen on emotional processing and risk-taking behaviour. | Double-blind, randomised, cross-over study in 20 healthy participants who were tested on two occasions receiving either ebselen (3600 mg over 24 h) or identical placebo. The Cambridge Gambling Task (CGT) and facial emotion recognition task (FERT) 3 h after the final dose of ebselen/placebo | The CGT: Ebselen reduced delay aversion. The FERT: Ebselen increased the recognition of positive vs. negative facial expressions. | Ebselen can decrease impulsivity and produce a positive bias in emotional processing. Adverse events comparable between groups and mild. |
| Sharpley et al. 2020 [26] | The efficacy of adjunctive ebselen in mania. | Randomised, double-blind, placebo-controlled, parallel-group trial. Patients with mania or hypomania received ebselen (600 mg bd) (n = 33) or placebo (n = 35) for three weeks, added to their usual psychotropic medication. Primary outcome: the Young Mania Rating Scale (YMRS) Secondary outcomes: the Altman Self-Rating Mania (ASRM) Scale and Clinical Global Impression-Severity Scale (CGI-S) | Ebselen was numerically, but not statistically, superior to the placebo in lowering scores on the YMRS and ASRM. CGI-S scores were significantly lower at week three in ebselen-treated participants. Differences were magnified by exclusion of patients taking concomitant valproate treatment. | Adverse events comparable between groups and mild. |
| Other Clinical Studies | ||||
| Yamaguchi et al. 1998 [25] | The effect of ebselen on the outcome of acute ischaemic stroke | A multicenter, placebo-controlled, double-blind clinical trial. Patients with acute ischaemic stroke in whom treatment was started within 48 h of stroke onset received 150mg bd ebselen p.o. (n = 151) or placebo (n = 149) for two weeks, with treatment started immediately after admission. | A significantly better outcome on the Glasgow Outcome Scale, the modified Mathew Scale and modified Barthel Index scores after ebselen treatment at 1 month but not at three months. Significant improvement in patients who started ebselen within 24 h but not after 24 h of stroke onset. | Early treatment with ebselen improved the outcome of acute ischaemic stroke. Ebselen suggested as a promising neuroprotective agent. |
| Saito et al. 1998 [24] | The effect of ebselen on the outcome of aneurysmal subarachnoid hemorrhages | A multicenter placebo-controlled double-blind clinical trial. Patients with aneurysmal subarachnoid hemorrhages of Hunt and Kosnik Grades II through IV in whom treatment was started within 96 h of the ictus received 150 mg bd ebselen p.o. (n = 145) or placebo (n = 141) for two weeks, with treatment started immediately after admission. | A significantly better outcome the Glasgow Outcome Scale after ebselen treatment, with a corresponding decrease in the incidence and extent of low-density areas on postoperative computed tomographic scans. Unaltered incidence of clinically diagnosed delayed ischemic neurological deficits. | Ebselen reduced brain damage in patients with delayed neurological deficits after subarachnoid hemorrhage. Ebselen suggested as a promising neuroprotective agent. |
| Kil et al. 2017 [32] | Effect of ebselen in noise-induced hearing loss in young adults | Single-centre, randomised, double-blind, placebo-controlled phase 2 trial in healthy adults aged 18−31 years. Intervention: ebselen 200 mg (n = 22), 400 mg (n = 20), or 600 mg (n = 21), or placebo (n = 20) p.o. bd for four days. Calibrated sound challenge: 4 h of pre-recorded music delivered by insert earphones. | Significant reduction (68%) in mean temporary threshold shift (TTS) at 4 kHz measured 15 min after the calibrated sound challenge by pure tone audiometry with 400 mg ebselen compared with placebo. Non-significant TTS reduction with ebselen 200 mg and 600 mg. | Ebselen well tolerated across all doses. Support for a role of GPx1 activity in acute noise-induced hearing loss. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramli, F.F.; Cowen, P.J.; Godlewska, B.R. The Potential Use of Ebselen in Treatment-Resistant Depression. Pharmaceuticals 2022, 15, 485. https://doi.org/10.3390/ph15040485
Ramli FF, Cowen PJ, Godlewska BR. The Potential Use of Ebselen in Treatment-Resistant Depression. Pharmaceuticals. 2022; 15(4):485. https://doi.org/10.3390/ph15040485
Chicago/Turabian StyleRamli, Fitri Fareez, Philip J. Cowen, and Beata R. Godlewska. 2022. "The Potential Use of Ebselen in Treatment-Resistant Depression" Pharmaceuticals 15, no. 4: 485. https://doi.org/10.3390/ph15040485
APA StyleRamli, F. F., Cowen, P. J., & Godlewska, B. R. (2022). The Potential Use of Ebselen in Treatment-Resistant Depression. Pharmaceuticals, 15(4), 485. https://doi.org/10.3390/ph15040485