A New Pyrroloquinoline-Derivative-Based Fluorescent Probe for the Selective Detection and Cell Imaging of Lysine
Abstract
:1. Introduction
2. Results and Discussion
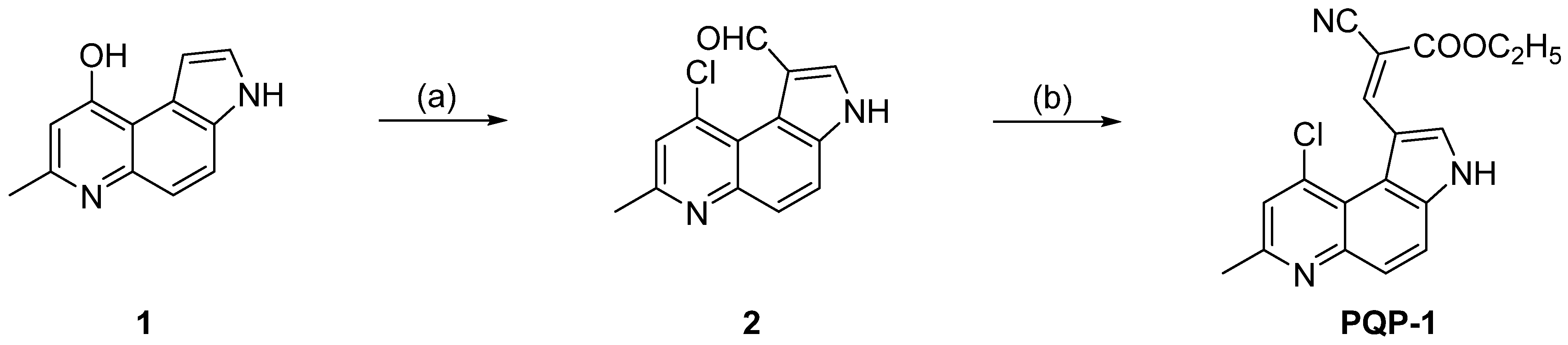
2.1. Synthesis of the Probe PQP-1
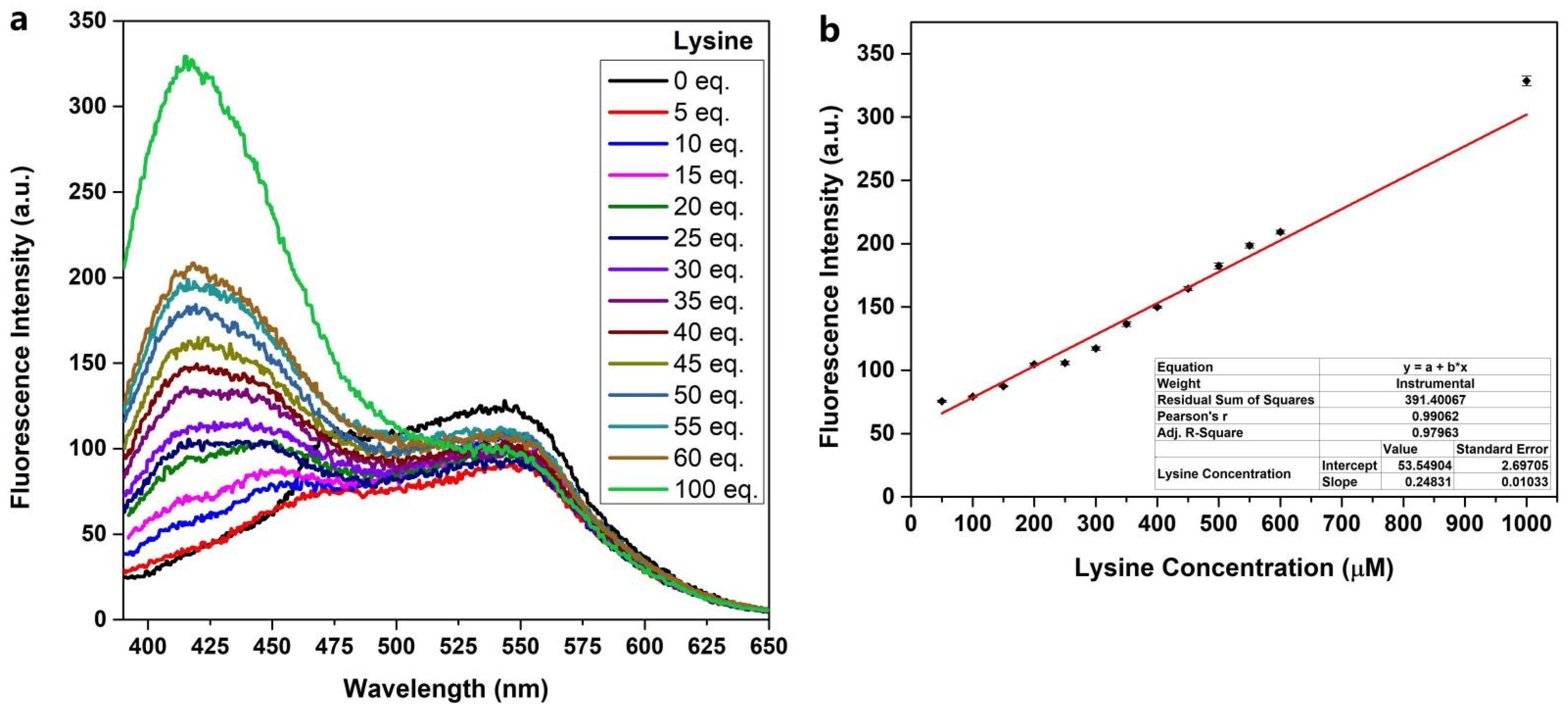
2.2. Fluorescent Response of Probe PQP-1 to Lysine
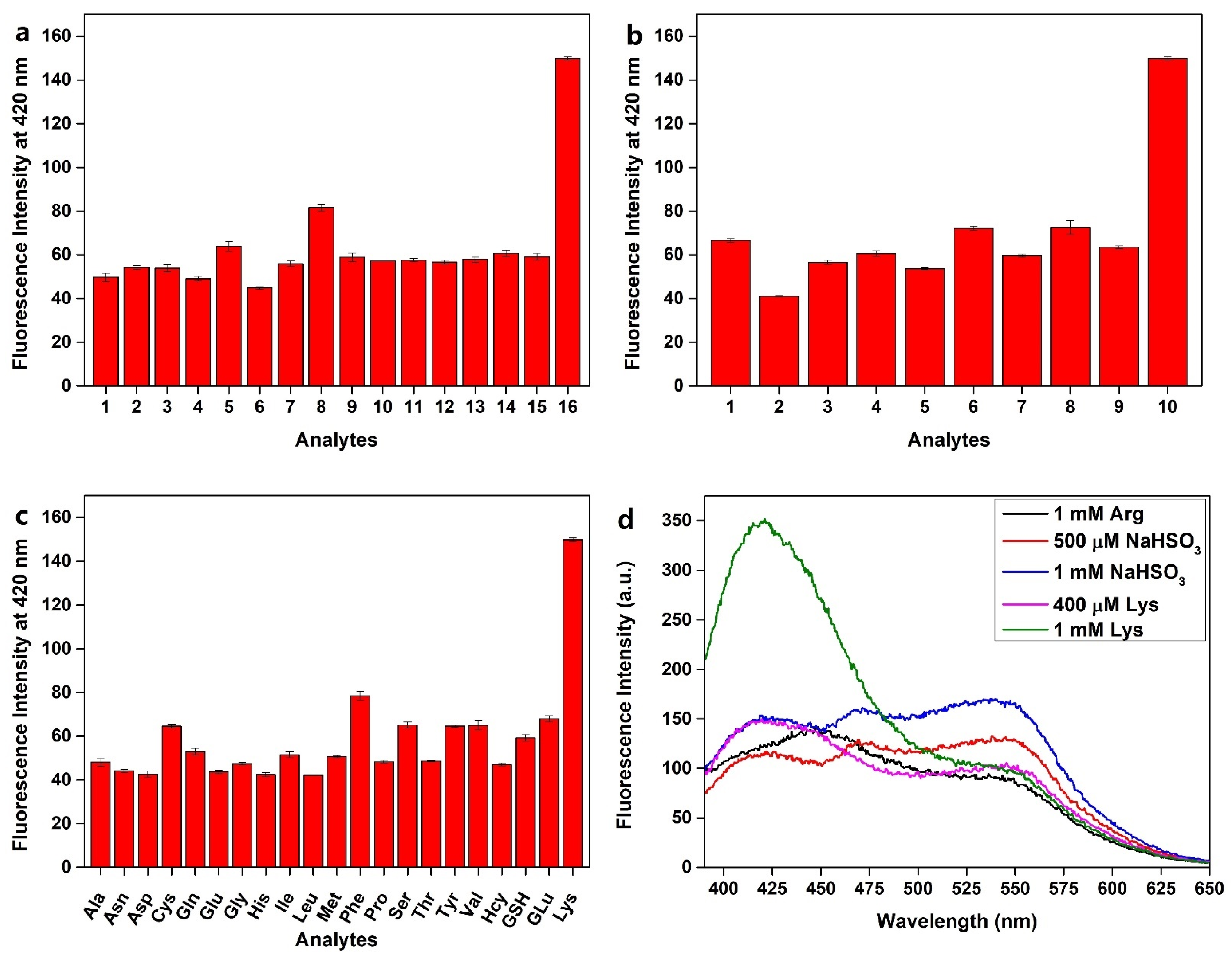
2.3. Selective Detection for Lysine
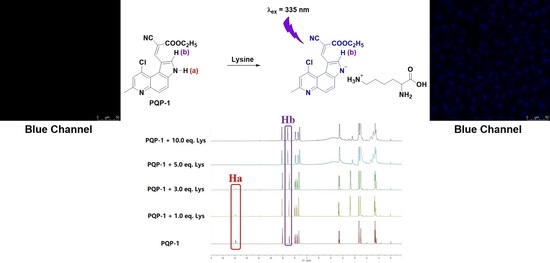
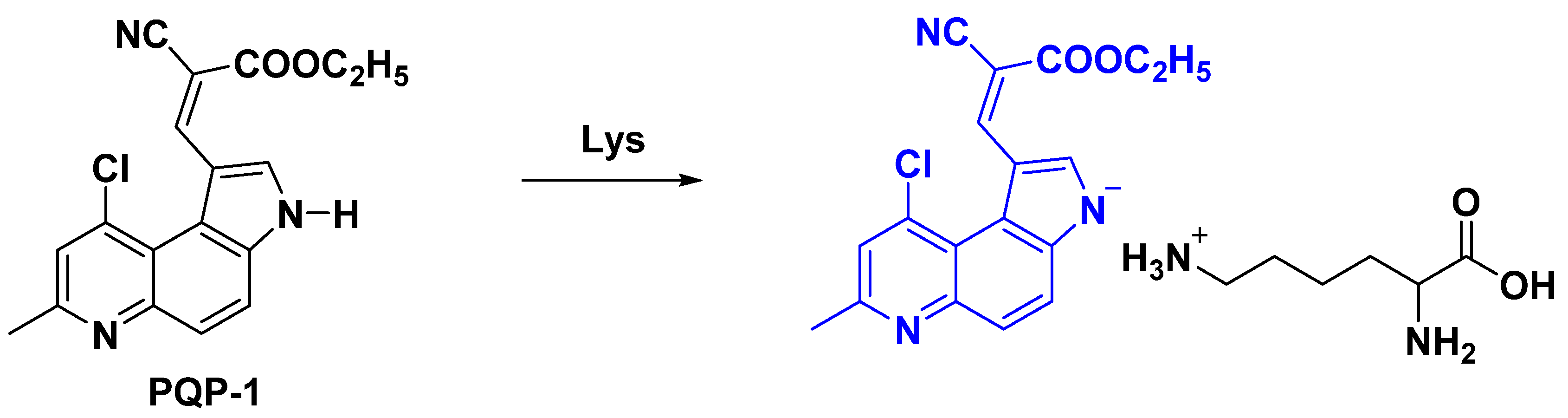
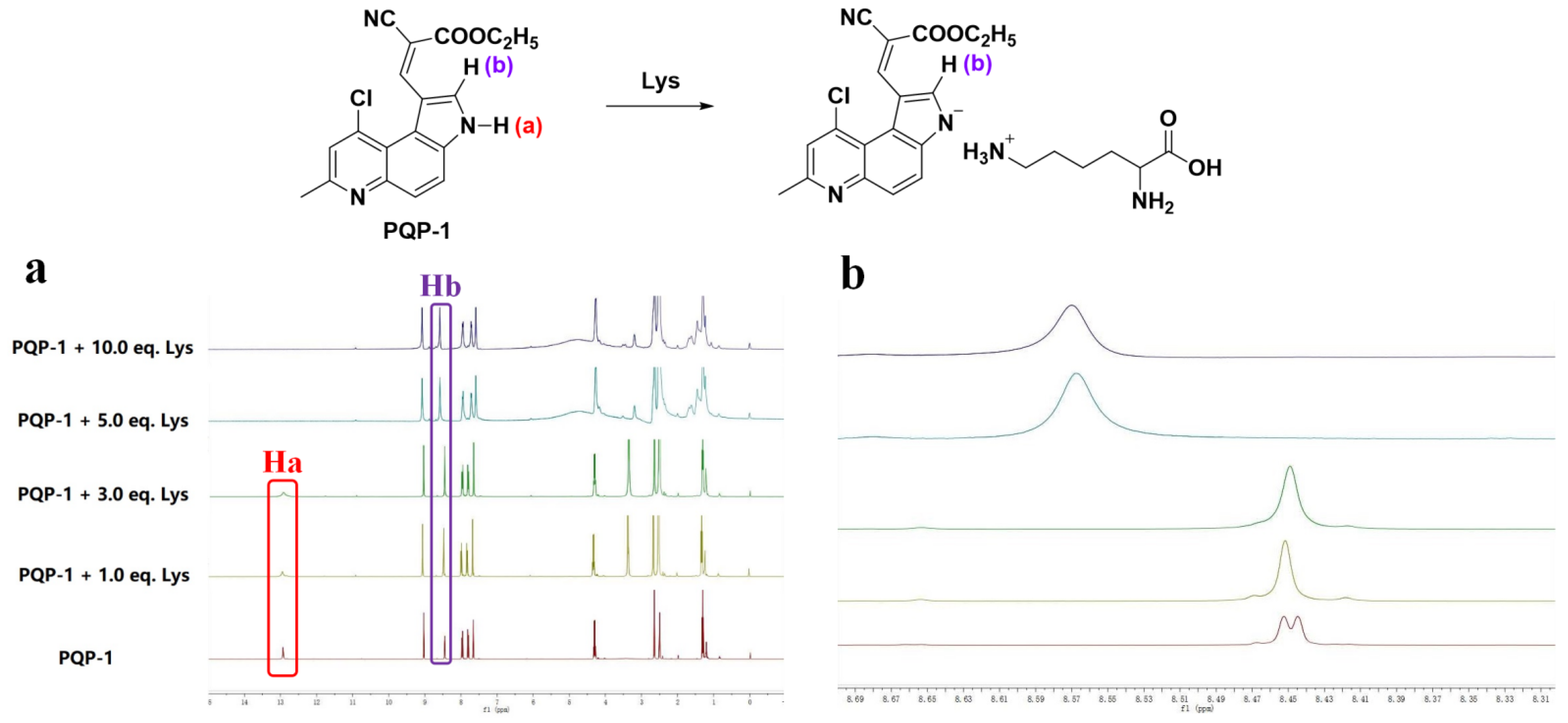
2.4. Proposed Response Mechanism
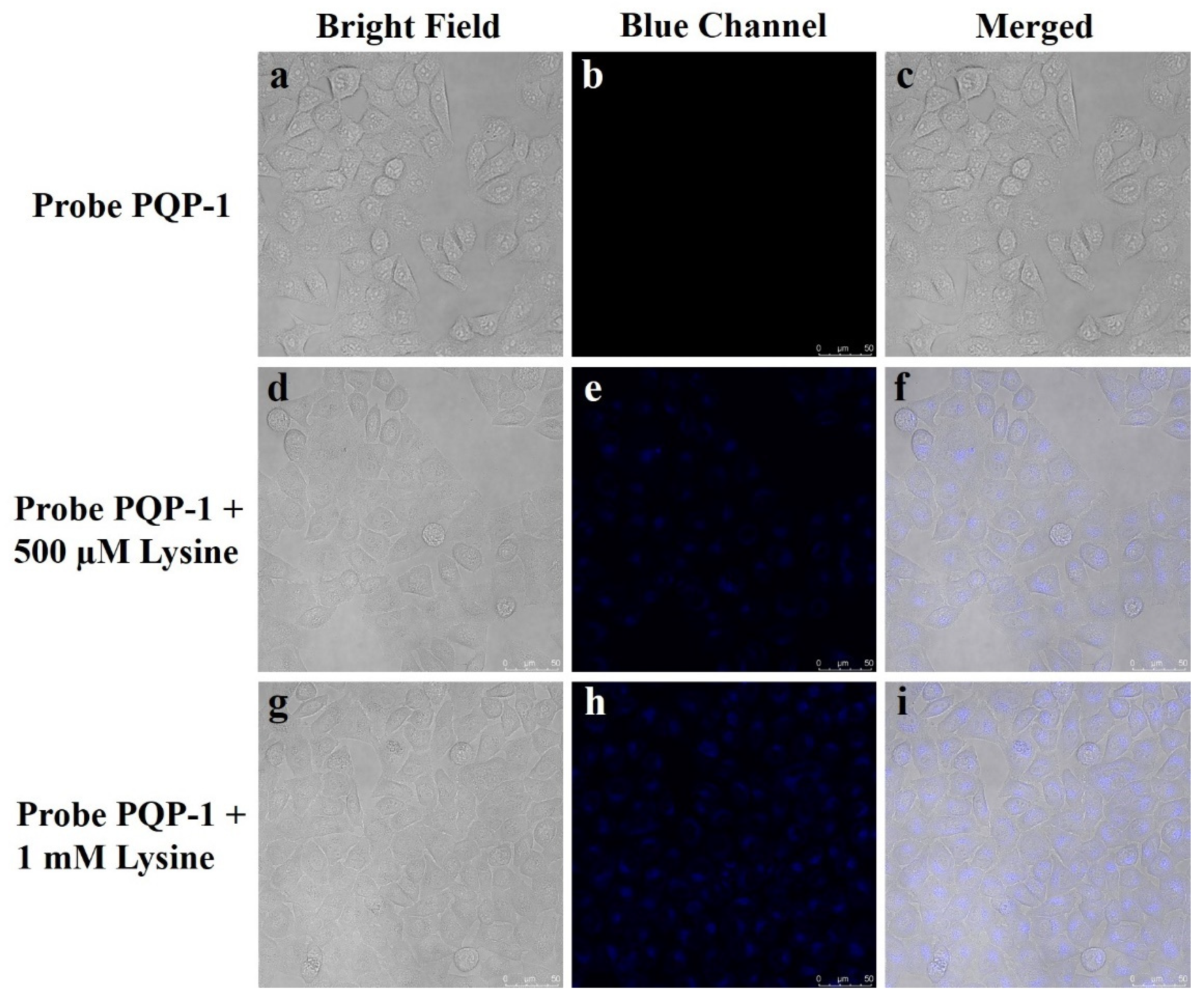
2.5. Imaging Study
2.6. Detection of Lysine Concentrations in Natural Mineral Water for Drinking
3. Materials and Methods
3.1. Materials and Apparatus
3.2. Preparation of the Probe PQP-1
3.3. Testing Conditions
3.4. Calculation of the Fluorescence Quantum Yield
Au = 0.0207, Fu = 27.036, n = 1.3330;
Quantum yield: Φu = 0.05.
3.5. Calculation of the Detection Limit
3.6. Imaging Study
3.7. Water Sample Preparation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Galili, G.; Amir, R. Fortifying plants with the essential amino acids lysine and methionine to improve nutritional quality. Palnt Biotechnol. J. 2013, 11, 211–222. [Google Scholar] [CrossRef]
- Lin, X.; Li, S.; Zou, Y.; Zhao, F.Q.; Liu, J.; Liu, H. Lysine stimulates protein synthesis by promoting the expression of ATB (0,+) and activating the mTOR pathway in bovine mammary epithelial cells. J. Nutr. 2018, 148, 1426–1433. [Google Scholar] [CrossRef]
- Austin, S.A.; Clemens, M.J. Stimulation of protein synthesis by lysine analogues in lysine-deprived Ehrlich ascites tumour cells. Biochim. Biophys. Acta Mol. Cell Res. 1984, 804, 16–22. [Google Scholar] [CrossRef]
- Zhao, Y.; Han, Y.; Sun, Y.; Wei, Z.; Chen, J.; Niu, X.; An, Q.; Zhang, L.; Qi, R.; Gao, X. Comprehensive succinylome profiling reveals the pivotal role of lysine succinylation in energy metabolism and quorum sensing of staphylococcus epidermidis. Front. Microbiol. 2021, 11, 632367. [Google Scholar] [CrossRef] [PubMed]
- De Marchi, U.; Galindo, A.N.; Thevenet, J.; Hermant, A.; Bermont, F.; Lassueur, S.; Domingo, J.S.; Kussmann, M.; Dayon, L.; Wiederkehr, A. Mitochondrial lysine deacetylation promotes energy metabolism and calcium signaling in insulin-secreting cells. FASEB J. 2019, 33, 4660–4674. [Google Scholar] [CrossRef] [PubMed]
- Rushton, D.H. Nutritional factors and hair loss. Clin. Exp. Dermatol. 2002, 27, 396–404. [Google Scholar] [CrossRef] [PubMed]
- Civitelli, R.; Villareal, D.T.; Agnusdei, D.; Nardi, P.; Gennari, C. Dietary L-lysine and calcium metabolism in humans. Nutrition 1992, 8, 400–405. [Google Scholar] [PubMed]
- Wang, H.; Elsaadawy, S.A.; Wu, Z.; Bu, D.P. Maternal supply of ruminally-protected lysine and methionine during close-up period enhances immunity and growth rate of neonatal calves. Front. Vet. Sci. 2021, 8, 780731. [Google Scholar] [CrossRef]
- Smriga, M.; Kameishi, M.; Uneyama, H.; Torii, K. Dietary L-lysine deficiency increases stress-induced anxiety and fecal excretion in rats. J. Nutr. 2002, 132, 3744–3746. [Google Scholar] [CrossRef] [Green Version]
- Unni, U.S.; Raj, T.; Sambashivaiah, S.; Kuriyan, R.; Uthappa, S.; Vaz, M.; Regan, M.M.; Kurpad, A.V. The effect of a controlled 8-week metabolic ward based lysine supplementation on muscle function, insulin sensitivity and leucine kinetics young men. Clin. Nutr. 2012, 31, 903–910. [Google Scholar] [CrossRef]
- Zhou, Y.; Yang, Z.; Xu, M. Colorimetric detection of lysine using gold nanoparticles aggregation. Anal. Methods 2012, 4, 2711–2714. [Google Scholar] [CrossRef]
- Kugimiya, A.; Takamitsu, E. Spectrophotometric detection of histidine and lysine using combined enzymatic reactions. Mater. Sci. Eng. C 2013, 33, 4867–4870. [Google Scholar] [CrossRef]
- Rawat, K.A.; Kailasa, S.K. Visual detection of arginine, histidine and lysine using quercetin-functionalized gold nanoparticles. Microchim. Acta 2014, 181, 1917–1929. [Google Scholar] [CrossRef]
- Zeußel, L.; Mai, P.; Sharma, S.; Schober, A.; Ren, S.; Singh, S. Colorimetric method for instant of lysine and arginine using novel meldrum’s acid-furfural conjugate. Chemistryselect 2021, 6, 6834–6840. [Google Scholar] [CrossRef]
- García-Villar, N.; Saurina, J.; Hernández-Cassou, S. Liquid chromatographic determination of lysine by potentionmetric detection with a biosensor. Anal. Lett. 2002, 35, 1313–1325. [Google Scholar] [CrossRef]
- Zakrzewski, R.; Ciesielski, W.; Kaźmierczak, D. Detection of proline, arginine, and lysine using iodine-azide reaction in TLC and HPTLC. J. Sep. Sci. 2003, 26, 1063–1066. [Google Scholar] [CrossRef]
- Arendowski, A.; Ruman, T. Lysine detection and quantification by laser desorption/ionization mass spectrometry on gold nanoparticle-enhanced target. Anal. Methods 2018, 10, 5398–5405. [Google Scholar] [CrossRef]
- Liu, Y.; Huangfu, M.; Wu, P.; Jiang, M.; Zhao, X.; Liang, L.; Xie, L.; Bai, J.; Wang, J. Post-imparting Brønsted acidity into an amino functionalized MOF as a bifunctional luminescent turn-ON sensor for the detection of aluminum ions and lysine. Dalton Trans. 2019, 48, 13834–13840. [Google Scholar] [CrossRef]
- Sahin, O.G.; Gulce, H.; Gulce, A. Polyvinylferrocenium based platinum electrodeposited amperometric biosensors for lysine detection. J. Electroanal. Chem. 2013, 690, 1–7. [Google Scholar] [CrossRef]
- Chauhan, N.; Narang, J.; Sunny; Pundir, C.S. Immobilization of lysine oxidase on a gold-platinum nanoparticles modified Au electrode for detection of lysine. Enzyme Microb. Technol. 2013, 52, 265–271. [Google Scholar] [CrossRef]
- Yu, H.; Xu, L.; You, T. Indirect electrochemiluminescence detection of lysine and histidine separated by capillary electrophoresis based on charge displacement. Luminescence 2013, 28, 217–221. [Google Scholar] [CrossRef] [PubMed]
- Heli, H.; Sattarahmady, N.; Hajjizadeh, M. Electrocatalytic oxidation and electrochemical detection of guanine, L-arginine and L-lysine at a copper nanoparticles-modified electrode. Anal. Methods 2014, 6, 6981–6989. [Google Scholar] [CrossRef]
- Cheng, J.; Zhong, S.; Wan, W.; Chen, X.; Chen, A.; Cheng, Y. Novel graphene/In2O3 nanocubes preparation and selective electrochemical detection for L-lysine of Camellia nitidissima Chi. Materials 2020, 13, 1999. [Google Scholar] [CrossRef] [PubMed]
- Butko, A.V.; Butko, V.Y.; Lebedev, S.P.; Lebedev, A.A.; Davydov, V.Y.; Eliseyev, I.A.; Kumzerov, Y.A. Detection of lysine molecular ions in solution gated field effect transistors based on unmodified graphene. J. Appl. Phys. 2020, 128, 215302. [Google Scholar] [CrossRef]
- Ma, H.; Qi, C.; Cao, H.; Zhang, Z.; Yang, Z.; Zhang, B.; Chen, C.; Lei, Z.Q. Water-soluble fluorescent probes for selective recognition of lysine and its application in an object carry-and-release system. Chem. Asian J. 2016, 11, 58–63. [Google Scholar] [CrossRef]
- Liu, G.; Feng, D.Q.; Hua, D.; Liu, T.; Qi, G.; Wang, W. Fluorescence enhancement of terminal amine assembled on gold nanoclusters and its application to ratiometric lysine detection. Langmuir 2017, 33, 14643–14648. [Google Scholar] [CrossRef]
- Song, W.; Duan, W.; Liu, Y.; Ye, Z.; Chen, Y.; Chen, H.; Qi, S.; Wu, J.; Liu, D.; Xiao, L.; et al. Ratiometric detection of intracellular lysine and pH with one-pot synthesized dual emissive carbon dots. Anal. Chem. 2017, 89, 13626–13633. [Google Scholar] [CrossRef]
- Jiang, X.D.; Yue, S.; Jia, L.; Li, S.; Li, C.; Li, Q.; Xiao, L. NIR fluorescent azaBODPIY-based probe for the specific detection of L-lysine. Chemistryselect 2018, 3, 7581–7585. [Google Scholar] [CrossRef]
- Zhang, Z.; Wei, T.; Chen, Y.; Chen, T.; Chi, B.; Wang, F.; Chen, X. A polydiacetylenes-based colorimetric and fluorescent probe for L-arginine and L-lysine and its application for logic gate. Sens. Actuators B 2018, 255, 2211–2217. [Google Scholar] [CrossRef]
- Du, G.; Pu, L. Micelle-encapsulated fluorescent probe: Chemoselective and enantioselective recognition of lysine in aqueous solution. Org. Lett. 2019, 21, 4777–4781. [Google Scholar] [CrossRef]
- Mi, G.; Yang, M.; Wang, C.; Zhang, B.; Hu, X.; Hao, H.; Fan, J. A simple “turn off-on” ratio fluorescent probe for sensitive detection of dopamine and lysine/arginine. Spectrochim. Acta Part A 2021, 253, 119555. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Won, J.; Lee, J.Y.; Yoon, J. Studies leading to the development of a highly selective colorimetric and fluorescent chemosensor for lysine. Chem. Commun. 2011, 47, 1997–1999. [Google Scholar] [CrossRef] [PubMed]
- Lohar, S.; Safin, D.A.; Sengupta, A.; Chattopadhyay, A.; Matalobos, J.S.; Babashkina, M.G.; Robeyns, K.; Mitoraj, M.P.; Kubisiak, P.; Garcia, Y.; et al. Ratiometric sensing of lysine through the formation of the pyrene excimer: Experimental and computational studies. Chem. Commun. 2015, 51, 8536–8539. [Google Scholar] [CrossRef] [PubMed]
- Adhikari, S.; Ghosh, A.; Mandal, S.; Guria, S.; Banerjee, P.P.; Chatterjee, A.; Das, D. Colorimetric and fluorescence probe for the detection of nano-molar lysine in aqueous medium. Org. Biomol. Chem. 2016, 14, 10688–10694. [Google Scholar] [CrossRef]
- Yang, L.; Xie, Y.; Chen, Q.; Zhang, J.; Li, L.; Sun, H. Colorimetric and fluorescent dual-signal chemosensor for lysine and arginine and its application to detect amines in solid-phase peptide synthesis. ACS Appl. Bio Mater. 2021, 4, 6558–6564. [Google Scholar] [CrossRef]
- Zhu, Z.; Wang, Y.; Ding, H.; Fan, C.; Tu, Y.; Liu, G.; Pu, S. A novel full symmetric diarylethene-based ratiometric fluorescent sensor for lysine and the application for a logic circuit. Luminescence 2021, 36, 691–697. [Google Scholar] [CrossRef]
- Hou, J.-T.; Li, K.; Liu, B.-Y.; Liao, Y.-X.; Yu, X.-Q. The first ratiometric probe for lysine in water. Tetrahedron 2013, 69, 2118–2123. [Google Scholar] [CrossRef]
- Qian, X.; Gong, W.; Wang, F.; Lin, Y.; Ning, G. A pyrylium-based colorimetric and fluorimetric chemosensor for the selective detection of lysine in aqueous environment and real sample. Tetrahedron Lett. 2015, 56, 2764–2767. [Google Scholar] [CrossRef]
- Bhosale, R.S.; Shitre, G.V.; Kumar, R.; Biradar, D.O.; Bhosale, S.V.; Narayan, R.; Bhosale, S.V. A 8-hydroxypyrene-1,3,6-trisulfonic acid trisodium salt (HPTS) based colorimetric and green turn-on fluorescent sensor for the detection of arginine and lysine in aqueous solution. Sens. Actuators B 2017, 241, 1270–1275. [Google Scholar] [CrossRef]
- Zhao, H.; Li, Y.; Cao, Y.; Gong, G.; Zhou, Y.; Gao, X.X.; Pu, L.; Zhao, G. Spectroscopic studies of a BINAM-based sensor: Highly selective fluorescent recognition of lysine in water solution through a nucleophilic substitution reaction. Tetrahedron Lett. 2019, 60, 1238–1242. [Google Scholar] [CrossRef]
- Hao, J.; Wang, M.; Wang, S.; Huang, Y.; Cao, D. Dissolution-enhanced emission of 1,3,6,8-Tetrakis(p-benzoic acid)pyrene for detecting arginine and lysine amino acids. Dyes Pigm. 2020, 175, 108131. [Google Scholar] [CrossRef]
- Gong, Y.; Du, C.; Wang, X.; Guo, H.; Yang, F. First stable (Z)-configuration of cyanostilbene derivative: An effective “turn-on” fluorescent sensor for lysine in aqueous media. Microchem. J. 2021, 162, 105866. [Google Scholar] [CrossRef]
- Wang, T.; Pang, Q.; Tong, Z.; Xiang, H.; Xiao, N. A hydrazone-based spectroscopic off-on probe for sensing of basic arginine and lysine. Spectrochim. Acta Part A 2021, 258, 119824. [Google Scholar] [CrossRef]
- Ferlin, M.; Gatto, B.; Chiarelotto, G.; Palumbo, M. Pyrrolo-quinoline derivatives as potential antineoplastic drugs. Bioorg. Med. Chem. 2000, 8, 1415–1422. [Google Scholar]
- Ferlin, M.; Gatto, B.; Chiarelotto, G.; Palumbo, M. Novel pyrrolo [3, 2-f] quinolines: Synthesis and antiproliferative activity. Bioorg. Med. Chem. 2001, 9, 1843–1848. [Google Scholar] [CrossRef]
| Entry | Added Concentrations (μM) | Detected Concentrations (μM) | Recovery (%) |
|---|---|---|---|
| 1 | 80 | 80.37 ± 1.43 | 100.46 |
| 2 | 220 | 224.25 ± 2.86 | 101.93 |
| 3 | 490 | 484.95 ± 0.45 | 98.97 |
| 4 | 750 | 724.84 ± 3.04 | 96.65 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, B.; Zhou, J.; Huang, X.; Chen, Z.; Tian, S.; Shi, Y. A New Pyrroloquinoline-Derivative-Based Fluorescent Probe for the Selective Detection and Cell Imaging of Lysine. Pharmaceuticals 2022, 15, 474. https://doi.org/10.3390/ph15040474
Yang B, Zhou J, Huang X, Chen Z, Tian S, Shi Y. A New Pyrroloquinoline-Derivative-Based Fluorescent Probe for the Selective Detection and Cell Imaging of Lysine. Pharmaceuticals. 2022; 15(4):474. https://doi.org/10.3390/ph15040474
Chicago/Turabian StyleYang, Bing, Jiahua Zhou, Xu Huang, Zhongping Chen, Shu Tian, and Yujun Shi. 2022. "A New Pyrroloquinoline-Derivative-Based Fluorescent Probe for the Selective Detection and Cell Imaging of Lysine" Pharmaceuticals 15, no. 4: 474. https://doi.org/10.3390/ph15040474
APA StyleYang, B., Zhou, J., Huang, X., Chen, Z., Tian, S., & Shi, Y. (2022). A New Pyrroloquinoline-Derivative-Based Fluorescent Probe for the Selective Detection and Cell Imaging of Lysine. Pharmaceuticals, 15(4), 474. https://doi.org/10.3390/ph15040474