Mechanism and Application of Chitosan and Its Derivatives in Promoting Permeation in Transdermal Drug Delivery Systems: A Review
Abstract
:1. Introduction
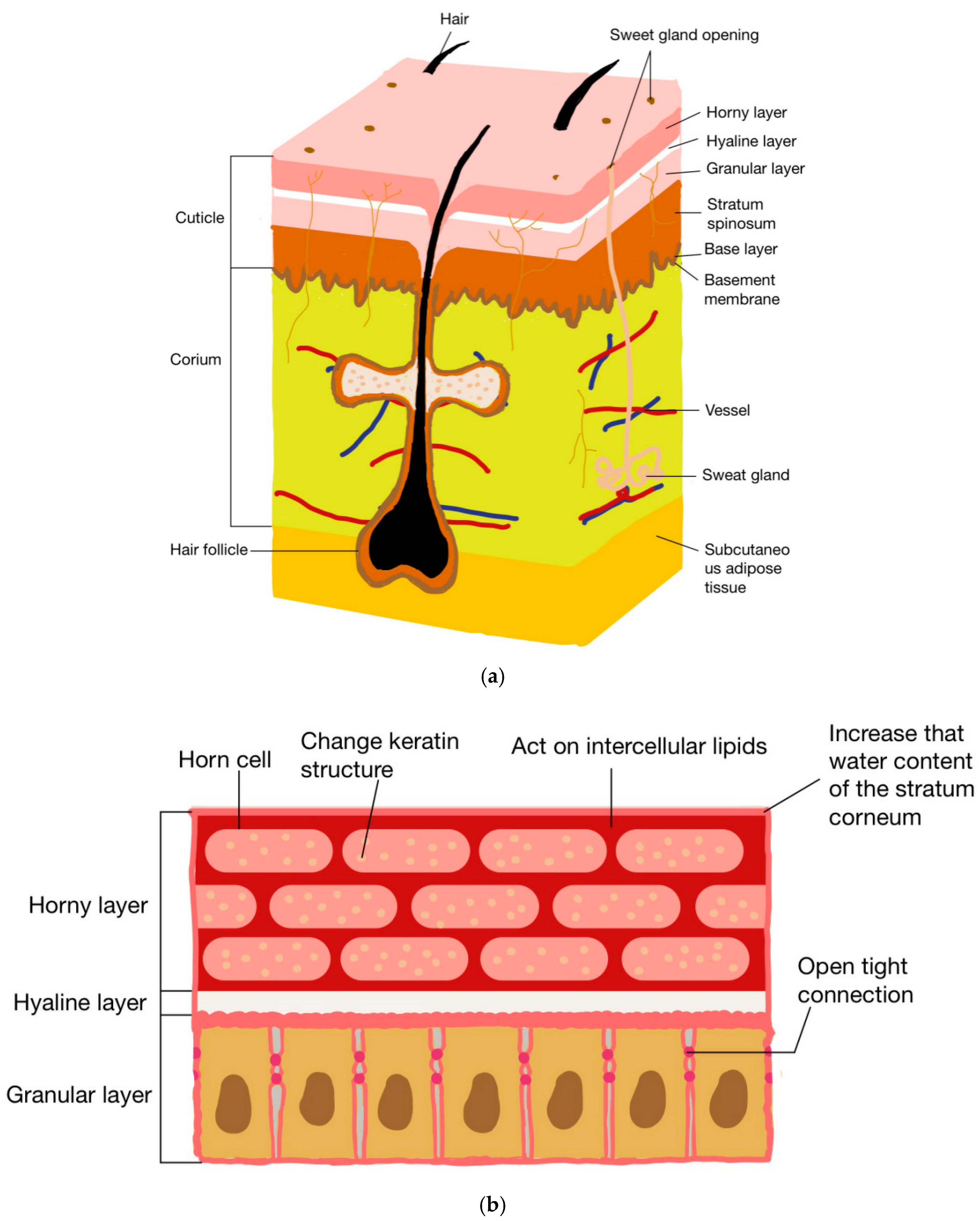
2. The Mechanism of Chitosan and Its Derivatives in Promoting Drug Penetration
2.1. Changing the Structure of Stratum Corneum Protein
2.2. Tight Junction Acting in Granular Layer
2.3. Interaction with Intercellular Lipids
2.4. Increasing the Stratum Corneum Water Content
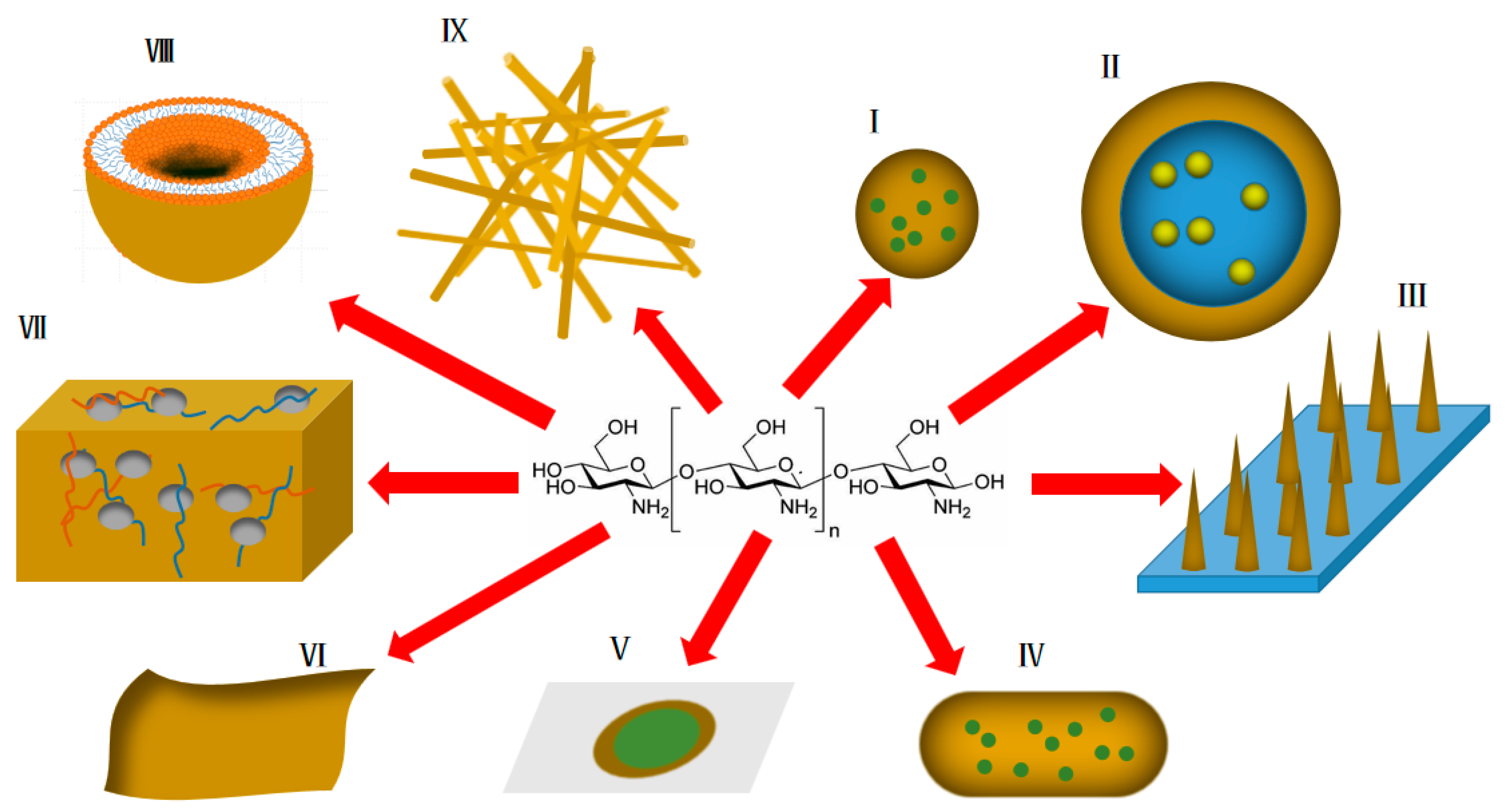
3. Application of Chitosan and Derivatives in Transdermal Drug Delivery Systems
3.1. Nanoparticles
3.2. Emulsions
3.3. Transdermal Microneedles
3.4. Nanocapsules
3.5. Transdermal Patches
3.6. Transdermal Membranes
3.7. Hydrogels
3.8. Liposomes
3.9. Nanometer Bracket
4. Status and Prospects
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wong, R.; Geyer, S.; Weninger, W.; Guimberteau, J.C.; Wong, J.K. The dynamic anatomy and patterning of skin. Exp. Dermatol. 2016, 25, 92–98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chambers, E.S.; Vukmanovic-Stejic, M. Skin barrier immunity and ageing. Immunology 2020, 160, 116–125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bukowski, K.; Kciuk, M.; Kontek, R. Mechanisms of Multidrug Resistance in Cancer Chemotherapy. Int. J. Mol. Sci. 2020, 21, 3233. [Google Scholar] [CrossRef] [PubMed]
- Tinoush, B.; Shirdel, I.; Wink, M. Phytochemicals: Potential Lead Molecules for MDR Reversal. Front. Pharmacol. 2020, 11, 832. [Google Scholar] [CrossRef] [PubMed]
- Berkey, C.; Kanno, D.; Mehling, A.; Koch, J.P.; Eisfeld, W.; Dierker, M.; Bhattacharya, S.; Dauskardt, R.H. Emollient structure and chemical functionality effects on the biomechanical function of human stratum corneum. Int. J. Cosmet. Sci. 2020, 42, 605–614. [Google Scholar] [CrossRef] [PubMed]
- Murthy, S.N. Approaches for Delivery of Drugs Topically. AAPS PharmSciTech 2019, 21, 30. [Google Scholar] [CrossRef] [PubMed]
- Barbieri, J.S.; Wanat, K.; Seykora, J. Skin: Basic Structure and Function. In Pathobiology of Human Disease; Academic Press: San Diego, CA, USA, 2014; pp. 1134–1144. [Google Scholar] [CrossRef]
- Paskeviciute, M.; Petrikaite, V. Overcoming transporter-mediated multidrug resistance in cancer: Failures and achievements of the last decades. Drug Deliv. Transl. Res. 2019, 9, 379–393. [Google Scholar] [CrossRef]
- Baveloni, F.G.; Riccio, B.V.F.; Di Filippo, L.D.; Fernandes, M.A.; Meneguin, A.B.; Chorilli, M. Nanotechnology-based Drug Delivery Systems as Potential for Skin Application: A Review. Curr. Med. Chem. 2021, 28, 3216–3248. [Google Scholar] [CrossRef]
- Akram, M.W.; Jamshaid, H.; Rehman, F.U.; Zaeem, M.; Khan, J.Z.; Zeb, A. Transfersomes: A Revolutionary Nanosystem for Efficient Transdermal Drug Delivery. AAPS PharmSciTech 2021, 23, 7. [Google Scholar] [CrossRef]
- N’Da, D.D. Prodrug strategies for enhancing the percutaneous absorption of drugs. Molecules 2014, 19, 20780–20807. [Google Scholar] [CrossRef] [Green Version]
- Singh Malik, D.; Mital, N.; Kaur, G. Topical drug delivery systems: A patent review. Expert. Opin. Ther. Pat. 2016, 26, 213–228. [Google Scholar] [CrossRef] [PubMed]
- Sabbagh, F.; Kim, B.S. Recent advances in polymeric transdermal drug delivery systems. J. Control. Release 2022, 341, 132–146. [Google Scholar] [CrossRef] [PubMed]
- Jeong, W.Y.; Kwon, M.; Choi, H.E.; Kim, K.S. Recent advances in transdermal drug delivery systems: A review. Biomater. Res. 2021, 25, 24. [Google Scholar] [CrossRef] [PubMed]
- Lodi, G.; Sannino, M.; Caterino, P.; Cannarozzo, G.; Bennardo, L.; Nistico, S.P. Fractional CO2 laser-assisted topical rifamycin drug delivery in the treatment of pediatric cutaneous leishmaniasis. Pediatr. Dermatol. 2021, 38, 717–720. [Google Scholar] [CrossRef]
- Ball, A.M.; Smith, K.M. Optimizing transdermal drug therapy. Am. J. Health Syst. Pharm. 2008, 65, 1337–1346. [Google Scholar] [CrossRef]
- Ghume, V.K.; Golhar, A.R.; Merekar, A.N.; Dokhe, M.D.; Parjane, S.K. Transdermal Drug Delivery System: A Review. Am. J. PharmTech Res. 2020, 10, 34–47. [Google Scholar] [CrossRef]
- Benson, H.A.E.; Grice, J.E.; Mohammed, Y.; Namjoshi, S.; Roberts, M.S. Topical and Transdermal Drug Delivery: From Simple Potions to Smart Technologies. Curr. Drug Deliv. 2019, 16, 444–460. [Google Scholar] [CrossRef]
- Waghule, T.; Singhvi, G.; Dubey, S.K.; Pandey, M.M.; Gupta, G.; Singh, M.; Dua, K. Microneedles: A smart approach and increasing potential for transdermal drug delivery system. Biomed. Pharmacother. 2019, 109, 1249–1258. [Google Scholar] [CrossRef]
- Al Hanbali, O.A.; Khan, H.M.S.; Sarfraz, M.; Arafat, M.; Ijaz, S.; Hameed, A. Transdermal patches: Design and current approaches to painless drug delivery. Acta Pharm. 2019, 69, 197–215. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, O.A.; El-Say, K.M.; Aljaeid, B.M.; Badr-Eldin, S.M.; Ahmed, T.A. Optimized vinpocetine-loaded vitamin E D-alpha-tocopherol polyethylene glycol 1000 succinate-alpha lipoic acid micelles as a potential transdermal drug delivery system: In vitro and ex vivo studies. Int. J. Nanomed. 2019, 14, 33–43. [Google Scholar] [CrossRef] [Green Version]
- Hirakawa, Y.; Ueda, H.; Miyano, T.; Kamiya, N.; Goto, M. New insight into transdermal drug delivery with supersaturated formulation based on co-amorphous system. Int. J. Pharm. 2019, 569, 118582. [Google Scholar] [CrossRef] [PubMed]
- Demir, B.; Rosselle, L.; Voronova, A.; Pagneux, Q.; Quenon, A.; Gmyr, V.; Jary, D.; Hennuyer, N.; Staels, B.; Hubert, T.; et al. Innovative transdermal delivery of insulin using gelatin methacrylate-based microneedle patches in mice and mini-pigs. Nanoscale Horiz. 2022, 7, 174–184. [Google Scholar] [CrossRef] [PubMed]
- Badhe, R.V.; Adkine, D.; Godse, A. Development of Polylactic Acid and Bovine Serum Albumin-layered-coated Chitosan Microneedles Using Novel Bees Wax Mould. Turk. J. Pharm. Sci. 2021, 18, 367–375. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Choi, S.O.; Seo, S.; Choy, Y.B.; Prausnitz, M.R. A microneedle roller for transdermal drug delivery. Eur. J. Pharm. Biopharm. 2010, 76, 282–289. [Google Scholar] [CrossRef]
- Sanchez-Salvador, J.L.; Balea, A.; Monte, M.C.; Negro, C.; Blanco, A. Chitosan grafted/cross-linked with biodegradable polymers: A review. Int. J. Biol. Macromol. 2021, 178, 325–343. [Google Scholar] [CrossRef]
- Ardean, C.; Davidescu, C.M.; Nemes, N.S.; Negrea, A.; Ciopec, M.; Duteanu, N.; Negrea, P.; Duda-Seiman, D.; Musta, V. Factors Influencing the Antibacterial Activity of Chitosan and Chitosan Modified by Functionalization. Int. J. Mol. Sci. 2021, 22, 7449. [Google Scholar] [CrossRef]
- Kriplani, P.; Guarve, K. Chitosan—A Potential Polymer to Prepare Anticancer Drug Delivery Systems: Patent Review. Recent Pat. Anticancer Drug Discov. 2021, 16, 249–257. [Google Scholar] [CrossRef]
- Patti, A.M.; Katsiki, N.; Nikolic, D.; Al-Rasadi, K.; Rizzo, M. Nutraceuticals in lipid-lowering treatment: A narrative review on the role of chitosan. Angiology 2015, 66, 416–421. [Google Scholar] [CrossRef]
- Li, X.; Xing, R.; Xu, C.; Liu, S.; Qin, Y.; Li, K.; Yu, H.; Li, P. Immunostimulatory effect of chitosan and quaternary chitosan: A review of potential vaccine adjuvants. Carbohydr. Polym. 2021, 264, 118050. [Google Scholar] [CrossRef]
- Ivanova, D.G.; Yaneva, Z.L. Antioxidant Properties and Redox-Modulating Activity of Chitosan and Its Derivatives: Biomaterials with Application in Cancer Therapy. Biores. Open Access 2020, 9, 64–72. [Google Scholar] [CrossRef] [Green Version]
- de Oliveira Pedro, R.; Ribeiro Pereira, A.; Oliveira, O.N.; Barbeitas Miranda, P. Interaction of chitosan derivatives with cell membrane models in a biologically relevant medium. Colloids Surf. B Biointerfaces 2020, 192, 111048. [Google Scholar] [CrossRef] [PubMed]
- Piosik, E.; Ziegler-Borowska, M.; Chelminiak-Dudkiewicz, D.; Martynski, T. Effect of Aminated Chitosan-Coated Fe3O4 Nanoparticles with Applicational Potential in Nanomedicine on DPPG, DSPC, and POPC Langmuir Monolayers as Cell Membrane Models. Int. J. Mol. Sci. 2021, 22, 2467. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.C.; Ling, M.H.; Lai, K.Y.; Pramudityo, E. Chitosan microneedle patches for sustained transdermal delivery of macromolecules. Biomacromolecules 2012, 13, 4022–4031. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Mishra, D.K.; Yoon, S.; Chauhan, A.K.; Koh, J. Synthesis of 2,5-furandicarboxylic acid-enriched-chitosan for anti-inflammatory and metal ion uptake. Int. J. Biol. Macromol. 2021, 179, 500–506. [Google Scholar] [CrossRef]
- Shariatinia, Z. Carboxymethyl chitosan: Properties and biomedical applications. Int. J. Biol. Macromol. 2018, 120, 1406–1419. [Google Scholar] [CrossRef]
- Taubner, T.; Marounek, M.; Synytsya, A. Preparation and characterization of hydrophobic and hydrophilic amidated derivatives of carboxymethyl chitosan and carboxymethyl beta-glucan. Int. J. Biol. Macromol. 2020, 163, 1433–1443. [Google Scholar] [CrossRef]
- Abdelwahab, H.E.; Hassan, S.Y.; Mostafa, M.A.; El Sadek, M.M. Synthesis and Characterization of Glutamic-Chitosan Hydrogel for Copper and Nickel Removal from Wastewater. Molecules 2016, 21, 684. [Google Scholar] [CrossRef] [Green Version]
- Zambito, Y.; Di Colo, G. Thiolated quaternary ammonium-chitosan conjugates for enhanced precorneal retention, transcorneal permeation and intraocular absorption of dexamethasone. Eur. J. Pharm. Biopharm. 2010, 75, 194–199. [Google Scholar] [CrossRef]
- Cardile, V.; Frasca, G.; Rizza, L.; Bonina, F.; Puglia, C.; Barge, A.; Chiambretti, N.; Cravotto, G. Improved adhesion to mucosal cells of water-soluble chitosan tetraalkylammonium salts. Int. J. Pharm. 2008, 362, 88–92. [Google Scholar] [CrossRef]
- Bombaldi de Souza, R.F.; Bombaldi de Souza, F.C.; Thorpe, A.; Mantovani, D.; Popat, K.C.; Moraes, A.M. Phosphorylation of chitosan to improve osteoinduction of chitosan/xanthan-based scaffolds for periosteal tissue engineering. Int. J. Biol. Macromol. 2020, 143, 619–632. [Google Scholar] [CrossRef]
- Amaral, I.F.; Granja, P.L.; Barbosa, M.A. Chemical modification of chitosan by phosphorylation: An XPS, FT-IR and SEM study. J. Biomater. Sci. Polym. Ed. 2005, 16, 1575–1593. [Google Scholar] [CrossRef] [PubMed]
- Villaseñor Rodríguez, I. Publicaciones españolas para la fundamentación de un marco teórico sobre los estudios de usuarios de información. Investig. Bibl. Arch. Bibl. Inf. 2014, 28, 223–257. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Jiang, Y.; Zhu, J.; Huang, J.; Zhang, H. Inhibition of bacterial adhesion and biofilm formation of sulfonated chitosan against Pseudomonas aeruginosa. Carbohydr. Polym. 2019, 206, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Liu, Y.; Yang, L.; Zhou, F. Synthesis of sulfonated chitosan and its antibiofilm formation activity against E. coli and S. aureus. Int. J. Biol. Macromol. 2019, 129, 980–988. [Google Scholar] [CrossRef]
- Kasaai, M.R.; Arul, J.; Charlet, G. Fragmentation of chitosan by ultrasonic irradiation. Ultrason. Sonochem. 2008, 15, 1001–1008. [Google Scholar] [CrossRef]
- Li, K.; Xing, R.; Liu, S.; Qin, Y.; Meng, X.; Li, P. Microwave-assisted degradation of chitosan for a possible use in inhibiting crop pathogenic fungi. Int. J. Biol. Macromol. 2012, 51, 767–773. [Google Scholar] [CrossRef]
- Hien, N.Q.; Phu, D.V.; Duy, N.N.; Lan, N.T.K. Degradation of chitosan in solution by gamma irradiation in the presence of hydrogen peroxide. Carbohydr. Polym. 2012, 87, 935–938. [Google Scholar] [CrossRef]
- Sikorski, D.; Gzyra-Jagiela, K.; Draczynski, Z. The Kinetics of Chitosan Degradation in Organic Acid Solutions. Mar. Drugs 2021, 19, 236. [Google Scholar] [CrossRef]
- Ma, Z.; Wang, W.; Wu, Y.; He, Y.; Wu, T. Oxidative degradation of chitosan to the low molecular water-soluble chitosan over peroxotungstate as chemical scissors. PLoS ONE 2014, 9, e100743. [Google Scholar] [CrossRef]
- Pierre, G.; Salah, R.; Gardarin, C.; Traikia, M.; Petit, E.; Delort, A.M.; Mameri, N.; Moulti-Mati, F.; Michaud, P. Enzymatic degradation and bioactivity evaluation of C-6 oxidized chitosan. Int. J. Biol. Macromol. 2013, 60, 383–392. [Google Scholar] [CrossRef]
- Pan, A.D.; Zeng, H.Y.; Foua, G.B.; Alain, C.; Li, Y.Q. Enzymolysis of chitosan by papain and its kinetics. Carbohydr. Polym. 2016, 135, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Laffleur, F.; Hintzen, F.; Rahmat, D.; Shahnaz, G.; Millotti, G.; Bernkop-Schnurch, A. Enzymatic degradation of thiolated chitosan. Drug Dev. Ind. Pharm. 2013, 39, 1531–1539. [Google Scholar] [CrossRef] [PubMed]
- Roman, D.L.; Ostafe, V.; Isvoran, A. Deeper inside the specificity of lysozyme when degrading chitosan. A structural bioinformatics study. J. Mol. Graph. Model. 2020, 100, 107676. [Google Scholar] [CrossRef] [PubMed]
- Islam, N.; Wang, H.; Maqbool, F.; Ferro, V. In Vitro Enzymatic Digestibility of Glutaraldehyde-Crosslinked Chitosan Nanoparticles in Lysozyme Solution and Their Applicability in Pulmonary Drug Delivery. Molecules 2019, 24, 1271. [Google Scholar] [CrossRef] [Green Version]
- Yomota, C.; Komuro, T.; Kimura, T. Studies on the degradation of chitosan films by lysozyme and release of loaded chemicals. Yakugazu-Zasshi 1990, 110, 442–448. [Google Scholar] [CrossRef] [Green Version]
- He, W.; Guo, X.; Xiao, L.; Feng, M. Study on the mechanisms of chitosan and its derivatives used as transdermal penetration enhancers. Int. J. Pharm. 2009, 382, 234–243. [Google Scholar] [CrossRef]
- Khan, T.A.; Azad, A.K.; Fuloria, S.; Nawaz, A.; Subramaniyan, V.; Akhlaq, M.; Safdar, M.; Sathasivam, K.V.; Sekar, M.; Porwal, O.; et al. Chitosan-Coated 5-Fluorouracil Incorporated Emulsions as Transdermal Drug Delivery Matrices. Polymers 2021, 13, 3345. [Google Scholar] [CrossRef]
- Yeh, T.H.; Hsu, L.W.; Tseng, M.T.; Lee, P.L.; Sonjae, K.; Ho, Y.C.; Sung, H.W. Mechanism and consequence of chitosan-mediated reversible epithelial tight junction opening. Biomaterials 2011, 32, 6164–6173. [Google Scholar] [CrossRef]
- Turner, J.R.; Buschmann, M.M.; Romero-Calvo, I.; Sailer, A.; Shen, L. The role of molecular remodeling in differential regulation of tight junction permeability. Semin. Cell Dev. Biol. 2014, 36, 204–212. [Google Scholar] [CrossRef] [Green Version]
- Zhou, X.; Liu, D.; Liu, H.; Yang, Q.; Yao, K.; Wang, X.; Wang, L.; Yang, X. Effect of low molecular weight chitosans on drug permeation through mouse skin: 1. Transdermal delivery of baicalin. J. Pharm. Sci. 2010, 99, 2991–2998. [Google Scholar] [CrossRef]
- Satheeshababu, B.K.; Shivakumar, K.L. Synthesis of conjugated chitosan and its effect on drug permeation from transdermal patches. Indian J. Pharm. Sci. 2013, 75, 162–170. [Google Scholar] [PubMed]
- Riezk, A.; Van Bocxlaer, K.; Yardley, V.; Murdan, S.; Croft, S.L. Activity of Amphotericin B-Loaded Chitosan Nanoparticles against Experimental Cutaneous Leishmaniasis. Molecules 2020, 25, 4002. [Google Scholar] [CrossRef] [PubMed]
- Fereig, S.A.; El-Zaafarany, G.M.; Arafa, M.G.; Abdel-Mottaleb, M.M.A. Tacrolimus-loaded chitosan nanoparticles for enhanced skin deposition and management of plaque psoriasis. Carbohydr. Polym. 2021, 268, 118238. [Google Scholar] [CrossRef]
- Al-Kassas, R.; Wen, J.; Cheng, A.E.; Kim, A.M.; Liu, S.S.M.; Yu, J. Transdermal delivery of propranolol hydrochloride through chitosan nanoparticles dispersed in mucoadhesive gel. Carbohydr. Polym. 2016, 153, 176–186. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, A.I.; Costa Lima, S.A.; Reis, S. Development of methotrexate loaded fucoidan/chitosan nanoparticles with anti-inflammatory potential and enhanced skin permeation. Int. J. Biol. Macromol. 2019, 124, 1115–1122. [Google Scholar] [CrossRef] [PubMed]
- Luesakul, U.; Puthong, S.; Sansanaphongpricha, K.; Muangsin, N. Quaternized chitosan-coated nanoemulsions: A novel platform for improving the stability, anti-inflammatory, anti-cancer and transdermal properties of Plai extract. Carbohydr. Polym. 2020, 230, 115625. [Google Scholar] [CrossRef]
- Kumari, B.; Kesavan, K. Effect of chitosan coating on microemulsion for effective dermal clotrimazole delivery. Pharm. Dev. Technol. 2017, 22, 617–626. [Google Scholar] [CrossRef]
- Sharkawy, A.; Casimiro, F.M.; Barreiro, M.F.; Rodrigues, A.E. Enhancing trans-resveratrol topical delivery and photostability through entrapment in chitosan/gum Arabic Pickering emulsions. Int. J. Biol. Macromol. 2020, 147, 150–159. [Google Scholar] [CrossRef]
- Williams, A.C.; Barry, B.W. Penetration enhancers. Adv. Drug Deliv. Rev. 2012, 64, 128–137. [Google Scholar] [CrossRef]
- Haq, M.I.; Smith, E.; John, D.N.; Kalavala, M.; Edwards, C.; Anstey, A.; Morrissey, A.; Birchall, J.C. Clinical administration of microneedles: Skin puncture, pain and sensation. Biomed. Microdevices 2009, 11, 35–47. [Google Scholar] [CrossRef]
- Gupta, J.; Gill, H.S.; Andrews, S.N.; Prausnitz, M.R. Kinetics of skin resealing after insertion of microneedles in human subjects. J. Control. Release 2011, 154, 148–155. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, Z.; Khan, M.I.; Siddique, M.I.; Sarwar, H.S.; Shahnaz, G.; Hussain, S.Z.; Bukhari, N.I.; Hussain, I.; Sohail, M.F. Fabrication and Characterization of Thiolated Chitosan Microneedle Patch for Transdermal Delivery of Tacrolimus. AAPS PharmSciTech 2020, 21, 68. [Google Scholar] [CrossRef]
- Chen, M.C.; Huang, S.F.; Lai, K.Y.; Ling, M.H. Fully embeddable chitosan microneedles as a sustained release depot for intradermal vaccination. Biomaterials 2013, 34, 3077–3086. [Google Scholar] [CrossRef] [PubMed]
- Chi, J.; Zhang, X.; Chen, C.; Shao, C.; Zhao, Y.; Wang, Y. Antibacterial and angiogenic chitosan microneedle array patch for promoting wound healing. Bioact. Mater. 2020, 5, 253–259. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Figueroa, M.J.; Narvaez-Araya, D.; Armijo-Escalona, N.; Carrasco-Flores, E.A.; Gonzalez-Aramundiz, J.V. Design of Chitosan Nanocapsules with Compritol 888 ATO(R) for Imiquimod Transdermal Administration. Evaluation of Their Skin Absorption by Raman Microscopy. Pharm. Res. 2020, 37, 195. [Google Scholar] [CrossRef]
- Frank, L.A.; Chaves, P.S.; D’Amore, C.M.; Contri, R.V.; Frank, A.G.; Beck, R.C.; Pohlmann, A.R.; Buffon, A.; Guterres, S.S. The use of chitosan as cationic coating or gel vehicle for polymeric nanocapsules: Increasing penetration and adhesion of imiquimod in vaginal tissue. Eur. J. Pharm. Biopharm. 2017, 114, 202–212. [Google Scholar] [CrossRef] [PubMed]
- Venkataprasanna, K.S.; Prakash, J.; Vignesh, S.; Bharath, G.; Venkatesan, M.; Banat, F.; Sahabudeen, S.; Ramachandran, S.; Devanand Venkatasubbu, G. Fabrication of Chitosan/PVA/GO/CuO patch for potential wound healing application. Int. J. Biol. Macromol. 2020, 143, 744–762. [Google Scholar] [CrossRef] [PubMed]
- Ghalayani Esfahani, A.; Altomare, L.; Varoni, E.M.; Bertoldi, S.; Fare, S.; De Nardo, L. Electrophoretic bottom up design of chitosan patches for topical drug delivery. J. Mater. Sci. Mater. Med. 2019, 30, 40. [Google Scholar] [CrossRef]
- Niranjan, R.; Kaushik, M.; Prakash, J.; Venkataprasanna, K.S.; Arpana, C.; Balashanmugam, P.; Venkatasubbu, G.D. Enhanced wound healing by PVA/Chitosan/Curcumin patches: In vitro and in vivo study. Colloids Surf. B Biointerfaces 2019, 182, 110339. [Google Scholar] [CrossRef]
- Malviya, R.; Tyagi, A.; Fuloria, S.; Subramaniyan, V.; Sathasivam, K.; Sundram, S.; Karupiah, S.; Chakravarthi, S.; Meenakshi, D.U.; Gupta, N.; et al. Fabrication and Characterization of Chitosan-Tamarind Seed Polysaccharide Composite Film for Transdermal Delivery of Protein/Peptide. Polymers 2021, 13, 1531. [Google Scholar] [CrossRef]
- Trombino, S.; Curcio, F.; Poerio, T.; Pellegrino, M.; Russo, R.; Cassano, R. Chitosan Membranes Filled with Cyclosporine A as Possible Devices for Local Administration of Drugs in the Treatment of Breast Cancer. Molecules 2021, 26, 1889. [Google Scholar] [CrossRef] [PubMed]
- Hemmingsen, L.M.; Julin, K.; Ahsan, L.; Basnet, P.; Johannessen, M.; Skalko-Basnet, N. Chitosomes-In-Chitosan Hydrogel for Acute Skin Injuries: Prevention and Infection Control. Mar. Drugs 2021, 19, 269. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, S.; Yu, W. Functionalized Graphene Oxide-Reinforced Chitosan Hydrogel as Biomimetic Dressing for Wound Healing. Macromol. BioSci. 2021, 21, e2000432. [Google Scholar] [CrossRef] [PubMed]
- Yan, T.; Kong, S.; Ouyang, Q.; Li, C.; Hou, T.; Chen, Y.; Li, S. Chitosan-Gentamicin Conjugate Hydrogel Promoting Skin Scald Repair. Mar. Drugs 2020, 18, 233. [Google Scholar] [CrossRef] [PubMed]
- Seong, J.S.; Yun, M.E.; Park, S.N. Surfactant-stable and pH-sensitive liposomes coated with N-succinyl-chitosan and chitooligosaccharide for delivery of quercetin. Carbohydr. Polym. 2018, 181, 659–667. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.H.; Lim, S.J.; Lee, M.K. Chitosan-coated liposomes to stabilize and enhance transdermal delivery of indocyanine green for photodynamic therapy of melanoma. Carbohydr. Polym. 2019, 224, 115143. [Google Scholar] [CrossRef] [PubMed]
- Jeon, S.; Yoo, C.Y.; Park, S.N. Improved stability and skin permeability of sodium hyaluronate-chitosan multilayered liposomes by Layer-by-Layer electrostatic deposition for quercetin delivery. Colloids Surf. B Biointerfaces 2015, 129, 7–14. [Google Scholar] [CrossRef]
- Li, L.; Zhang, Y.; Han, S.; Qu, Z.; Zhao, J.; Chen, Y.; Chen, Z.; Duan, J.; Pan, Y.; Tang, X. Penetration enhancement of lidocaine hydrochlorid by a novel chitosan coated elastic liposome for transdermal drug delivery. J. Biomed. Nanotechnol. 2011, 7, 704–713. [Google Scholar] [CrossRef]
- Shekh, M.I.; Amirian, J.; Stadler, F.J.; Du, B.; Zhu, Y. Oxidized chitosan modified electrospun scaffolds for controllable release of acyclovir. Int. J. Biol. Macromol. 2020, 151, 787–796. [Google Scholar] [CrossRef]

| Formula | Active Ingredient | Patent Approval Progress | Evaluate | Patent Application Number |
|---|---|---|---|---|
| Nanoparticle | Insulin | Under trial | The administration system is stable | 11/908,599 |
| Albumin | Lose effectiveness | It is suitable for transdermal administration of macromolecular protein drugs, and it is nontoxic, stable, and controllable | 201310289929.6 | |
| Emulsion | None | Valid patent | Good emulsification performance and long-term stability | 201810245672.7 |
| Aspirin | Valid patent | Good drug dispersibility | 201610598660.3 | |
| Transdermal microneedle | Chitosan | Under trial | The microneedle has an antibacterial effect, with a remarkable effect of growth inhibition of Escherichia coli and Staphylococcus aureus, and it can prevent infection possibly caused in the use proces of the microneedle | 201911381855.2 |
| Stem-cell exosome | Under trial | Solves the problem of transdermal absorption of exosome repair liquid | 202011148113.8 | |
| Nanocapsule | Fat-soluble drugs such as paclitaxel | Under trial | It has excellent skin permeability and improved bioavailability as compared to oral administration | 201980085253.4 |
| Transdermal patch | One or more of citalopram, lorazepam, alprazolam, and olanzapine | Valid patent | Stable and continuous administration; multiple drugs can be delivered simultaneously | 202011191501.4 |
| Wound healing drug | Valid patent | Good air permeability | 202121305386.9 | |
| Transdermal membrane | Chitosan | Valid patent | Chitosan is easy to release | 202110391721.X |
| Hydrogel | Hyaluronic acid, chitosan | Under trial | The chitosan forms a glue rapidly after azide reaction, and no photoinitiator is needed | 202111060377.2 |
| Salidroside | Under trial | No irritation and allergy, good absorption, and high safety | 202111271252.4 | |
| Liposome | Quercetin | Valid patent | The liposome is multilayered, the drug is gradually released, and the skin permeability is high | KR1020150080545 |
| Nano-scaffold | Ethyl orthosilicate, triethyl phosphate and calcium nitrate | Valid patent | The chitosan nano-stent has the mechanical properties of high tensile stress, high Young’s modulus, low elongation at break, and high water absorption; it can promote wound vascularization and shorten wound heal time, and it is a skin substitute with good performance | 202010654165.6 |
| Vascular endothelial growth factor (VEGF), follistatin-like 1 (FST-1), antibacterial agents (superparamagnetic iron oxide nanoparticles) | Under trial | The patch simulates the physical and mechanical properties of skin, the therapeutic biomolecules are uniformly distributed, and no additional adhesive is needed, so the patch can quickly repair skin wounds and is also effective for long-term inflammation | 17/055,786 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, J.; Wang, Y.; Lu, R. Mechanism and Application of Chitosan and Its Derivatives in Promoting Permeation in Transdermal Drug Delivery Systems: A Review. Pharmaceuticals 2022, 15, 459. https://doi.org/10.3390/ph15040459
Ma J, Wang Y, Lu R. Mechanism and Application of Chitosan and Its Derivatives in Promoting Permeation in Transdermal Drug Delivery Systems: A Review. Pharmaceuticals. 2022; 15(4):459. https://doi.org/10.3390/ph15040459
Chicago/Turabian StyleMa, Jinqian, Yuchen Wang, and Rong Lu. 2022. "Mechanism and Application of Chitosan and Its Derivatives in Promoting Permeation in Transdermal Drug Delivery Systems: A Review" Pharmaceuticals 15, no. 4: 459. https://doi.org/10.3390/ph15040459
APA StyleMa, J., Wang, Y., & Lu, R. (2022). Mechanism and Application of Chitosan and Its Derivatives in Promoting Permeation in Transdermal Drug Delivery Systems: A Review. Pharmaceuticals, 15(4), 459. https://doi.org/10.3390/ph15040459





