A Clinical Perspective of the Multifaceted Mechanism of Metformin in Diabetes, Infections, Cognitive Dysfunction, and Cancer
Abstract
1. Introdaction
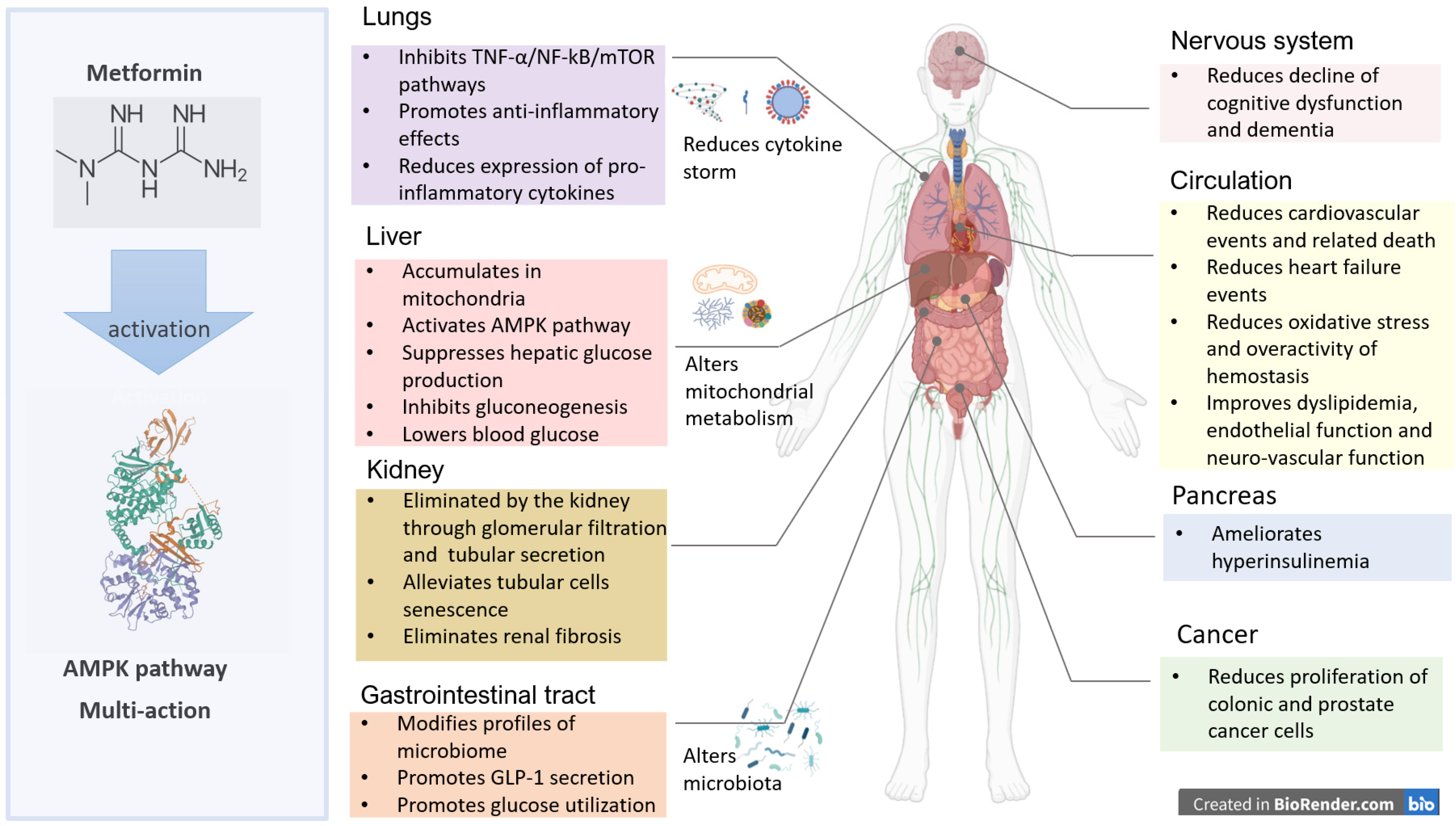
2. Metformin Pharmacology and Mechanisms of Action
2.1. Metformin Pharmacology
2.2. Inhibition of Mitochondrial Metabolism and Endogenous Glucose Production
2.3. Metformin and Hepatic Gluconeogenesis
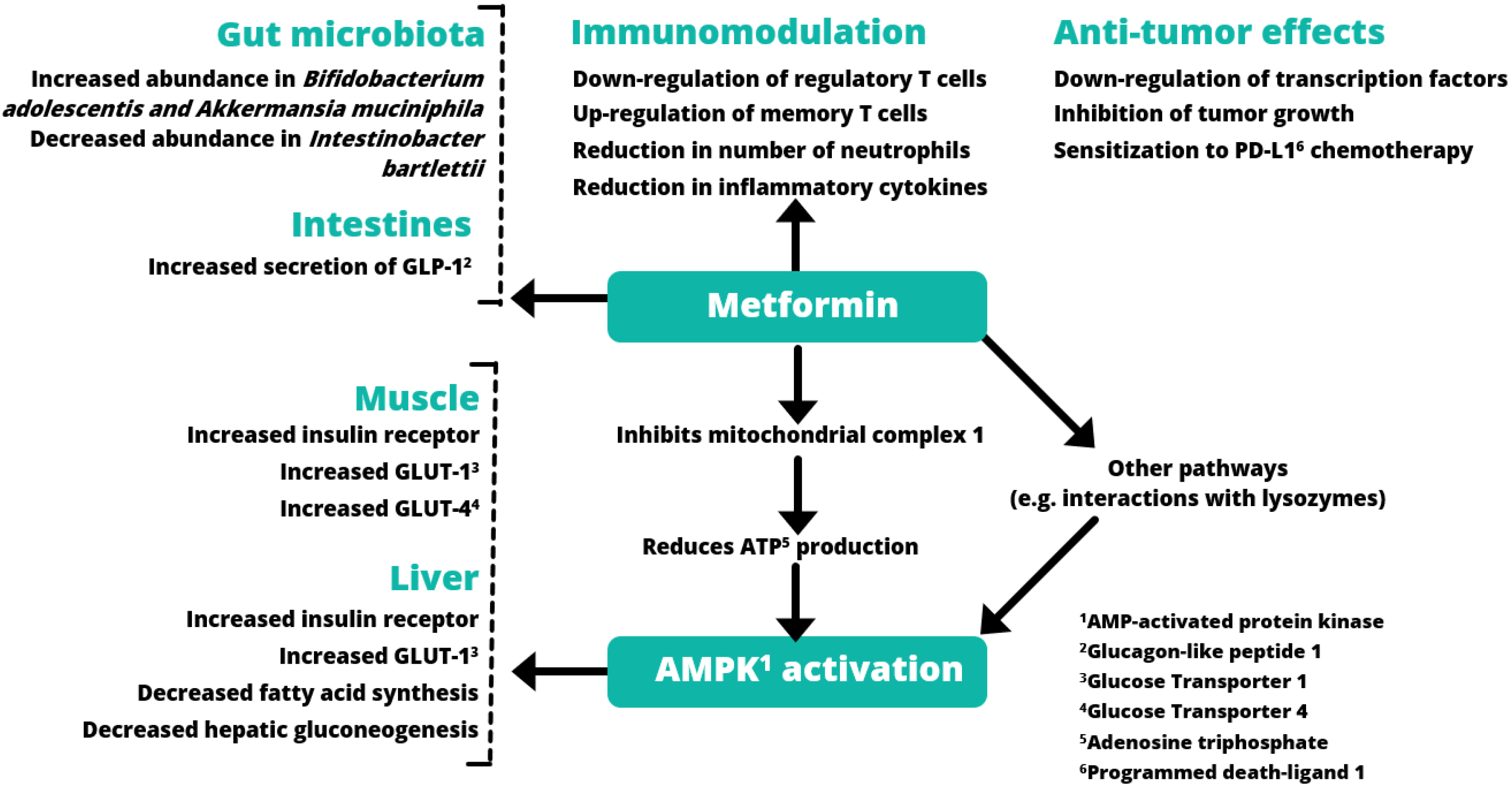
2.4. Modulation of Gut Microbiota and Inflammation
3. Clinical Evidence for Pleiotropic Effects of Metformin
3.1. Putative Mechanisms of Metformin on Cardiovascular Systems
| Author/ Year | Study Design | Region | No. of Participants | No. of Cases | Follow-Up (Years) | Comparations and Outcomes | Main Conclusion |
|---|---|---|---|---|---|---|---|
| Raee, 2017 [46] | Cohort | Iran | 717 | 446 | 3.0 | Glyburide versus metformin All-cause mortality: HR = 0.27, 95% CI: 0.10–0.73 Cardiovascular mortality: HR = 0.12, 95% CI: 0.20–0.66 | Compared with metformin, glyburide was associated with increased all-cause and cardiovascular mortality in patients with diabetes. |
| Scheller, 2014 [47] | Retrospective cohort | Denmark | 84,756 | 83,528 | 5.0 | Sitagliptin versus metformin All-cause mortality: HR = 1.25, 95% CI: 0.92–1.71 Incidence of CVD: HR = 1.22, 95% CI: 0.96–1.61 | Compared with metformin monotherapy, sitagliptin monotherapy was not associated with increased risk of all-cause mortality or CVD. |
| Roumie, 2012 [48] | Retrospective cohort | USA | 253,690 | 155,025 | 5.5 | Sulfonylurea versus metformin CVD (acute myocardial infarction and stroke) or death: HR = 1.21, 95% CI: 1.13–1.30 | Compared with metformin, use of sulfonylureas was associated with an increased hazard of CVD events or death. |
| Roumie, 2017 [49] | Retrospective cohort | USA | 131,972 | 65,986 | 0.9–1.1 | Sulfonylurea versus metformin Heart failure and cardiovascular death: HR = 1.32, 95% CI: 1.21–1.43 | Compared with metformin, sulfonylurea had a higher risk of heart failure and cardiovascular death. |
| Johnson, 2002 [50] | Cross-sectional | Canada | 4183 | 1150 | 5.1 | Metformin versus sulfonylurea All-cause mortality: OR = 0.60, 95% CI: 0.49–0.74 cardiovascular–related mortality: OR = 0.53, 95% CI: 0.41–0.68 | Metformin therapy, alone or in combination with sulfonylurea, was associated with reduced all-cause and cardiovascular mortality. |
| Ekstrom, 2012 [51] | Register-based cohort | Sweden | 32,152 | 14,696 | 3.9 | Other-GLDs versus metformin Incidence CVD: HR = 1.02, 95% CI: 0.93–1.12 All–cause mortality: HR = 1.13, 95% CI: 1.01–1.27 | Metformin showed lower risk than insulin for CVD and all-cause mortality and slightly lowered risk for all-cause mortality compared with other GLDs. |
| Pantalone, 2012 [52] | Retrospective cohort | USA | 23,915 | 12,774 | 2.2 | Glipizide, glyburide, glimepiride versus metformin All-cause mortality:
| Glipizide, glyburide and glimepiride were associated with an increased risk of overall mortality versus metformin. |
| Charytan, 2019 [53] | Clinical trails | USA | 4038 | 591 | 4.0 | Metformin versus non-metformin All-cause mortality: HR = 0.49, 95% CI: 0.36–0.69 Cardiovascular death: HR = 0.49, 95% CI: 0.32–0.74 Cardiovascular composite: HR = 0.67, 95% CI: 0.51–0.88 Kidney disease composite: HR = 0.77, 95% CI: 0.61–0.98 ESKD (end stage kidney disease): HR = 1.01,95% CI: 0.65–1.55 | Metformin might be safer for use in CKD than previously considered with reduced risk of death and cardiovascular events in individuals with stage 3 CKD. |
| Cheng, 2014 [54] | Retrospective cohort | Taiwan | 14,856 | 10,857 | 4.0 | Metformin versus non-metformin Incidence stroke: HR = 0.38, 95% CI: 0.35–0.42 | Compared with non-metformin use, metformin use was associated with lower risk of stroke especially in high-risk patients |
| Mogensen, 2015 [55] | Retrospective cohort | Danish | 28,236 | 16,910 | 13.0 | Sulfonylureas + metformin versus metformin/metformin + insulin All-cause mortality: RR = 1.81, 95% CI: 1.63–2.01 cardiovascular death: RR = 1.35, 95% CI: 1.14–1.60 Composite endpoint (myocardial infarction, stroke and cardiovascular death): RR = 1.25, 95% CI: 1.09–1.42 | In combination with insulin, the use of sulfonylureas was associated with increased mortality compared with metformin. |
| Evans, 2006 [56] | Retrospective cohort | UK | 5617 | 2286 | 8.0 | Sulfonylurea versus metformin All-cause mortality: HR = 1.43, 95% CI: 1.15–1.77 Cardiovascular mortality: HR = 1.70, 95% CI: 1.18–2.45 | Patients treated with sulfonylureas only, or combinations of sulfonylureas and metformin, were at higher risk of adverse cardiovascular outcomes than those treated with metformin alone. |
| Sillars, 2010 [57] | Retrospective cohort | Australia | 1271 | 390 | 10.4 | Metformin–sulphonylurea versus diet and metformin monotherapy All-cause mortality: HR = 0.82, 95% CI: 0.58–1.23 Cardiovascular mortality: HR = 0.82, 95% CI: 0.53–1.27 | Combination metformin–sulphonylurea appeared to be as safe as other blood glucose-lowering therapies used in type 2 diabetes. |
| Morgan, 2014 [58] | Retrospective cohort | UK | 80,999 | 68,139 | 2.9–3.1 | Sulfonylurea versus metformin All-cause mortality: HR = 1.27, 95% CI: 1.02–1.58 MACE (adverse cardiovascular events): HR = 0.81, 95% CI: 0.57–1.15 | All-cause mortality was increased in patients prescribed with sulphonylureas compared with metformin monotherapy. |
| Breunig, 2014 [59] | Retrospective cohort | USA | 6271 | 5548 | 1.6 | Rosiglitazone, pioglitazone versus metformin Incidence of heart failure: Rosiglitazone: HR = 1.57, 95% CI: 1.15–2.15 | Compared with metformin, there appeared to be higher risk of heart failure in patients started on rosiglitazone but not pioglitazone |
| Fung, 2015 [60] | Retrospective cohort | Hong Kong | 11,293 | 7493 | 5.0 | Metformin versus non–metformin All-cause mortality: HR = 0.73, 95% CI: 0.58–0.90 Incidence CVD: HR = 0.72, 95% CI: 0.60–0.87 Incidence of coronary heart disease: HR = 0.67, 95% CI: 0.52–0.86 Incidence of stroke: HR = 0.75, 95% CI: 0.57–0.98 Incidence of CKD (eGFR < 30): HR = 1.08, 95% CI: 0.84–1.38 | Patients who were started on metformin monotherapy showed improvement in many of the clinical parameters and a reduction in all-cause mortality and CVD events than lifestyle modifications alone |
3.1.1. Metformin and Endothelial Dysfunction, Inflammation and Oxidative Stress
3.1.2. Metformin on Blood Flow and Haemostasis
3.1.3. Metformin and Kidney Disease
| Author/ Year | Study Design | Sample Size | Comparation | Duration/ Dose | Outcomes, Hazard Ratio (95% CI) | Main Conclusion |
|---|---|---|---|---|---|---|
| Whitlock, 2020 [78] | Retrospective Cohort (2006–2017) FU: 1.4 vs. 1.1 years | 21,996 (metformin: 19,990) | metformin vs. sulfonylurea among patients with T2D (age > 18 years) | NA | All-cause mortality: Overall: 0.48 (0.40–0.58) eGFR ≥90: 0.38 (0.27–0.53) eGFR 60–89: 0.42 (0.31–0.56) eGFR 45–59: 0.92 (0.53–1.61) eGFR 30–44: 0.85 (0.46–1.57) eGFR <30: 1.51 (0.58–3.95) CVD: Overall: 0.64 (0.41–1.00) eGFR ≥90: 0.78 (0.52–1.2) eGFR 60–89: 0.86 (0.45–1.64) eGFR 45–59: 0.62 (0.3–1.29) eGFR 30–44: 0.85 (0.46–1.57) eGFR <30: 0.56 (0.18–1.69) | Metformin use was associated with lower risk for all-cause mortality, cardiovascular events, and major hypoglycemic episodes when compared with sulfonylureas. CKD was a significant effect modifier for all-cause mortality, but not for cardiovascular events or major hypoglycemic episodes. |
| Kwon, 2020 [79] | Retrospective Cohort (2001–2016) FU: 7.3 years | 10,426 | metformin vs. non-metformin among patients with type 2 diabetes kidney disease | Duration and dose | All-cause mortality: Overall: 0.48 (0.40–0.58) eGFR ≥45: 0.38 (0.27–0.53) eGFR 45–30: 0.42 (0.31–0.56) eGFR <30: 0.55 (0.37–0.81) ESKD: Overall: 0.67 (0.58–0.77) eGFR ≥45: 0.62 (0.51–0.76) eGFR 45–30: 0.73 (0.54–0.99) eGFR <30: 0.87 (0.67–1.12) | Metformin usage in advanced CKD patients, especially those with CKD 3b, was associated with reduced risk of all-cause mortality and incident ESKD. Metformin did not increase the risk of lactic acidosis. |
| Charytan, 2019 [53] | Retrospective analysis in trials | 4038 (591) | metformin vs. non-metformin among patients with diabetes and chronic kidney disease | NA | All-cause mortality: Overall: 0.49 (0.36–0.69) CKD S1–3: 0.61 (0.44–0.82) CKD S4–5: 0.83 (0.54–1.27) CV-death: Overall: 0.49 (0.32–0.74) CKD S1–3: 0.59 (0.38–0.9) CKD S4–5: 0.80 (0.46–1.39) ESKD: Overall: 1.01 (0.65–1.55) CKD S1–3: 0.70 (0.53–0.92) CKD S4–5: 0.95 (0.7–1.29) | Metformin might be safer for use in CKD than previously considered with reduced risk of death and cardiovascular events in individuals with stage 3 CKD. |
| Bergmark, 2019 [80] | Retrospective analysis in trials (2010–2013) FU: 2.1 years | 12,156 (8971) | metformin vs. non-metformin among patients with diabetes and high CV risk | NA | All-cause mortality: 0.75 (0.59–0.95) CV-death: 0.68 (0.51–0.91) MI: 1.08 (0.83–1.41) Stroke: 1.07 (0.77–1.48) Hear failure: 1.23 (0.94–1.6) | Metformin use was associated with reduced risk of all-cause mortality, including after adjustment for clinical variables and biomarkers, but not lower rates of the composite end point of cardiovascular death, myocardial infarction, or ischemic stroke. |
| Roumie, 2019 [81] | Retrospective Cohort (2001–2016) FU: 1.1 year | 174,882 metformin and sulfonylureas users | metformin vs. sulfonylureas | NA | MACE: Overall: 0.80 (0.75–0.86) | Among patients with diabetes and reduced kidney function persisting with monotherapy, treatment with metformin, compared with a sulfonylurea, was associated with a lower risk of MACE. |
| Hung, 2015 [82] | Retrospective Cohort (2000–2009) FU: 2.1 years | 3252 (metformin 813) | metformin vs. non-metformin among patients with type 2 diabetes and stage 5 chronic kidney disease | Daily dose | All-cause mortality: 1.35 (1.2–1.51) | Use of metformin in people with type 2 diabetes and a serum creatinine concentration greater than 530 μmol/L was associated with an increased risk of all-cause mortality compared with non-users. Metformin use should not be encouraged in this patient group. |
| Ekstrom, 2012 [51] | Retrospective analysis in Swedish register (2004–2007) FU: 3.9 years | 51,675 patients with type 2 diabetes | Metformin monotherapy vs. other GLDs | NA | All-cause mortality: Overall: 1.13 (1.01–1.27) Fatal/non-fatal CVD: Overall: 1.02 (0.93–1.12) | Metformin showed lower risk vs. insulin for CVD and all-cause mortality, and lower risk for all-cause mortality vs. other GLDs |
3.2. Metformin and Infection
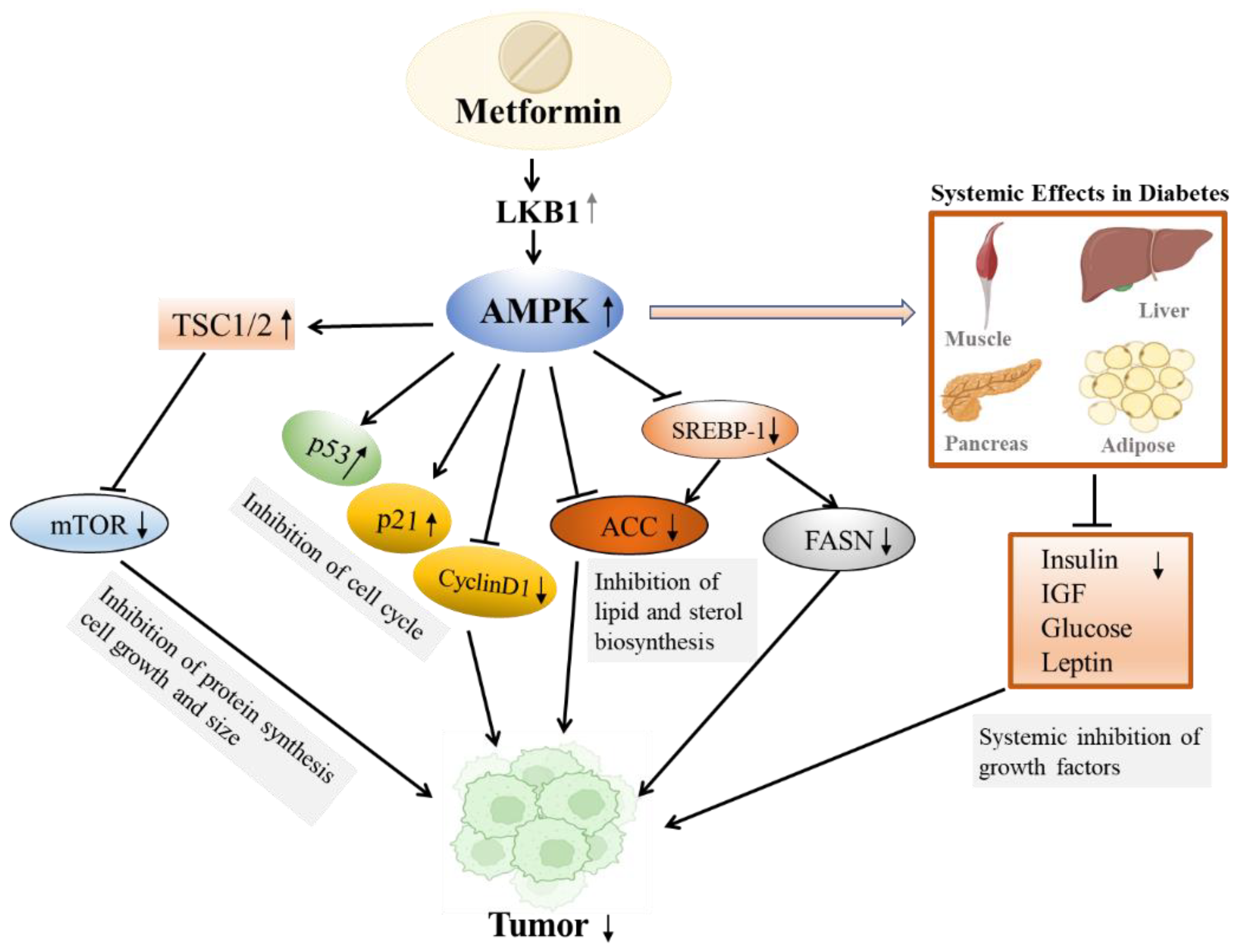
3.3. Metformin and Cancer
3.4. Metformin and NAFLD
3.5. Metformin and Cognitive Function
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sun, H.; Saeedi, P.; Karuranga, S.; Pinkepank, M.; Ogurtsova, K.; Duncan, B.B.; Stein, C.; Basit, A.; Chan, J.C.N.; Mbanya, J.C.; et al. IDF diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res. Clin. Pract. 2021, 183, 109119. [Google Scholar] [CrossRef] [PubMed]
- Gerich, J.E. Physiology of glucose homeostasis. Diabetes Obes. Metab. 2000, 2, 345–350. [Google Scholar] [CrossRef] [PubMed]
- Schrijvers, B.F.; De Vriese, A.S.; Flyvbjerg, A. From hyperglycemia to diabetic kidney disease: The role of metabolic, hemodynamic, intracellular factors and growth factors/cytokines. Endocr. Rev. 2004, 25, 971–1010. [Google Scholar] [CrossRef] [PubMed]
- Tahrani, A.A.; Bailey, C.J.; Del Prato, S.; Barnett, A.H. Management of type 2 diabetes: New and future developments in treatment. Lancet 2011, 378, 182–197. [Google Scholar] [CrossRef]
- American Diabetes Association. 9. Pharmacologic Approaches to Glycemic Treatment: Standards of Medical Care in Diabetes-2021. Diabetes Care 2021, 44 (Suppl. S1), S111–S124. [Google Scholar] [CrossRef] [PubMed]
- Marshall, S.M. 60 years of metformin use: A glance at the past and a look to the future. Diabetologia 2017, 60, 1561–1565. [Google Scholar] [CrossRef] [PubMed]
- Bailey, C.J. Metformin: Historical overview. Diabetologia 2017, 60, 1566–1576. [Google Scholar] [CrossRef] [PubMed]
- Cetin, M.; Sahin, S. Microparticulate and nanoparticulate drug delivery systems for metformin hydrochloride. Drug Deliv. 2016, 23, 2796–2805. [Google Scholar] [CrossRef]
- Gormsen, L.C.; Sundelin, E.I.; Jensen, J.B.; Vendelbo, M.H.; Jakobsen, S.; Munk, O.L.; Hougaard Christensen, M.M.; Brøsen, K.; Frøkiær, J.; Jessen, N. In Vivo Imaging of Human 11C-Metformin in Peripheral Organs: Dosimetry, Biodistribution, and Kinetic Analyses. J. Nucl. Med. 2016, 57, 1920–1926. [Google Scholar] [CrossRef]
- Bailey, C.J.; Wilcock, C.; Scarpello, J.H. Metformin and the intestine. Diabetologia 2008, 51, 1552–1553. [Google Scholar] [CrossRef]
- Chen, Y.; Shan, X.; Luo, C.; He, Z. Emerging nanoparticulate drug delivery systems of metformin. J. Pharm. Investig. 2020, 50, 219–230. [Google Scholar] [CrossRef]
- LaMoia, T.E.; Shulman, G.I. Cellular and Molecular Mechanisms of Metformin Action. Endocr. Rev. 2020, 42, 77–96. [Google Scholar] [CrossRef] [PubMed]
- Rena, G.; Hardie, D.G.; Pearson, E.R. The mechanisms of action of metformin. Diabetologia 2017, 60, 1577–1585. [Google Scholar] [CrossRef] [PubMed]
- Madiraju, A.K.; Erion, D.M.; Rahimi, Y.; Zhang, X.-M.; Braddock, D.T.; Albright, R.A.; Prigaro, B.J.; Wood, J.L.; Bhanot, S.; Macdonald, M.J.; et al. Metformin suppresses gluconeogenesis by inhibiting mitochondrial glycerophosphate dehydrogenase. Nature 2014, 510, 542–546. [Google Scholar] [CrossRef] [PubMed]
- Pernicova, I.; Korbonits, M. Metformin—mode of action and clinical implications for diabetes and cancer. Nat. Rev. Endocrinol. 2014, 10, 143–156. [Google Scholar] [CrossRef]
- Li, M.; Li, X.; Zhang, H.; Lu, Y. Molecular Mechanisms of Metformin for Diabetes and Cancer Treatment. Front. Physiol. 2018, 9, 1039. [Google Scholar] [CrossRef]
- Song, A.; Zhang, C.; Meng, X. Mechanism and application of metformin in kidney diseases: An update. Biomed. Pharmacother. 2021, 138, 111454. [Google Scholar] [CrossRef]
- Jaune, E.; Rocchi, S. Metformin: Focus on Melanoma. Front. Endocrinol. 2018, 9, 472. [Google Scholar] [CrossRef]
- Ma, R.; Yi, B.; Riker, A.I.; Xi, Y. Metformin and cancer immunity. Acta Pharmacol. Sin. 2020, 41, 1403–1409. [Google Scholar] [CrossRef]
- Giaccari, A.; Solini, A.; Frontoni, S.; Del Prato, S. Metformin Benefits: Another Example for Alternative Energy Substrate Mechanism? Diabetes Care 2021, 44, 647–654. [Google Scholar] [CrossRef]
- Salpeter, S.R.; Greyber, E.; Pasternak, G.A.; Salpeter, E.E. Risk of Fatal and Nonfatal Lactic Acidosis With Metformin Use in Type 2 Diabetes Mellitus. Arch. Intern. Med. 2003, 163, 2594. [Google Scholar] [CrossRef] [PubMed]
- Lipska, K.J.; Yao, X.; Herrin, J.; McCoy, R.G.; Ross, J.S.; Steinman, M.A.; Inzucchi, S.E.; Gill, T.M.; Krumholz, H.M.; Shah, N.D. Trends in Drug Utilization, Glycemic Control, and Rates of Severe Hypoglycemia, 2006–2013. Diabetes Care 2017, 40, 468–475. [Google Scholar] [CrossRef] [PubMed]
- Yang, A.; Wu, H.; Lau, E.S.; Ma, R.C.; Kong, A.P.; So, W.Y.; Luk, A.O.; Chan, J.C.; Chow, E. Trends in Glucose-Lowering Drug Use, Glycemic Control, and Severe Hypoglycemia in Adults With Diabetes in Hong Kong, 2002–2016. Diabetes Care 2020, 43, 2967–2974. [Google Scholar] [CrossRef] [PubMed]
- Harris, S.T.; Patorno, E.; Zhuo, M.; Kim, S.C.; Paik, J.M. Prescribing Trends of Antidiabetes Medications in Patients With Type 2 Diabetes and Diabetic Kidney Disease, a Cohort Study. Diabetes Care 2021, 44, 2293–2301. [Google Scholar] [CrossRef]
- Ala, M.; Ala, M. Metformin for Cardiovascular Protection, Inflammatory Bowel Disease, Osteoporosis, Periodontitis, Polycystic Ovarian Syndrome, Neurodegeneration, Cancer, Inflammation and Senescence: What Is Next? ACS Pharmacol. Transl. Sci. 2021, 4, 1747–1770. [Google Scholar] [CrossRef]
- Foretz, M.; Hébrard, S.; Leclerc, J.; Zarrinpashneh, E.; Soty, M.; Mithieux, G.; Sakamoto, K.; Andreelli, F.; Viollet, B. Metformin inhibits hepatic gluconeogenesis in mice independently of the LKB1/AMPK pathway via a decrease in hepatic energy state. J. Clin. Investig. 2010, 120, 2355–2369. [Google Scholar] [CrossRef]
- Lv, Z.; Guo, Y. Metformin and its benefits for various diseases. Front. Endocrinol. 2020, 11, 191. [Google Scholar] [CrossRef]
- Gurung, M.; Li, Z.; You, H.; Rodrigues, R.; Jump, D.B.; Morgun, A.; Shulzhenko, N. Role of gut microbiota in type 2 diabetes pathophysiology. EBioMedicine 2020, 51, 102590. [Google Scholar] [CrossRef]
- Wu, H.; Esteve, E.; Tremaroli, V.; Khan, M.T.; Caesar, R.; Mannerås-Holm, L.; Ståhlman, M.; Olsson, L.M.; Serino, M.; Planas-Fèlix, M. Metformin alters the gut microbiome of individuals with treatment-naive type 2 diabetes, contributing to the therapeutic effects of the drug. Nat. Med. 2017, 23, 850–858. [Google Scholar] [CrossRef]
- Vallianou, N.G.; Stratigou, T.; Tsagarakis, S. Metformin and gut microbiota: Their interactions and their impact on diabetes. Hormones 2019, 18, 141–144. [Google Scholar] [CrossRef]
- De La Cuesta-Zuluaga, J.; Mueller, N.T.; Corrales-Agudelo, V.; Velásquez-Mejía, E.P.; Carmona, J.A.; Abad, J.M.; Escobar, J.S. Metformin is associated with higher relative abundance of mucin-degrading Akkermansia muciniphila and several short-chain fatty acid–producing microbiota in the gut. Diabetes Care 2017, 40, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Turroni, F.; Milani, C.; Duranti, S.; Mancabelli, L.; Mangifesta, M.; Viappiani, A.; Lugli, G.A.; Ferrario, C.; Gioiosa, L.; Ferrarini, A. Deciphering bifidobacterial-mediated metabolic interactions and their impact on gut microbiota by a multi-omics approach. ISME J. 2016, 10, 1656–1668. [Google Scholar] [CrossRef] [PubMed]
- Jeon, B.S.; Choi, O.; Um, Y.; Sang, B.-I. Production of medium-chain carboxylic acids by Megasphaera sp. MH with supplemental electron acceptors. Biotechnol. Biofuels 2016, 9, 129. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Xie, C.; Wang, G.; Wu, Y.; Wu, Q.; Wang, X.; Liu, J.; Deng, Y.; Xia, J.; Chen, B. Gut microbiota and intestinal FXR mediate the clinical benefits of metformin. Nat. Med. 2018, 24, 1919–1929. [Google Scholar] [CrossRef] [PubMed]
- Cabreiro, F.; Au, C.; Leung, K.Y.; Vergara-Irigaray, N.; Cochemé, H.M.; Noori, T.; Weinkove, D.; Schuster, E.; Greene, N.D.; Gems, D. Metformin retards aging in C. elegans by altering microbial folate and methionine metabolism. Cell 2013, 153, 228–239. [Google Scholar] [CrossRef]
- Hsu, S.-K.; Cheng, K.-C.; Mgbeahuruike, M.O.; Lin, Y.-H.; Wu, C.-Y.; Wang, H.-M.D.; Yen, C.-H.; Chiu, C.-C.; Sheu, S.-J. New Insight into the Effects of Metformin on Diabetic Retinopathy, Aging and Cancer: Nonapoptotic Cell Death, Immunosuppression, and Effects beyond the AMPK Pathway. Int. J. Mol. Sci. 2021, 22, 9453. [Google Scholar] [CrossRef]
- Kristófi, R.; Eriksson, J.W. Metformin as an anti-inflammatory agent: A short review. J. Endocrinol. 2021, 251, R11–R22. [Google Scholar] [CrossRef]
- Holman, R.R.; Paul, S.K.; Bethel, M.A.; Matthews, D.R.; Neil, H.A. 10-year follow-up of intensive glucose control in type 2 diabetes. N. Engl. J. Med. 2008, 359, 1577–1589. [Google Scholar] [CrossRef]
- Aroda, V.R.; Knowler, W.C.; Crandall, J.P.; Perreault, L.; Edelstein, S.L.; Jeffries, S.L.; Molitch, M.E.; Pi-Sunyer, X.; Darwin, C.; Heckman-Stoddard, B.M.; et al. Metformin for diabetes prevention: Insights gained from the Diabetes Prevention Program/Diabetes Prevention Program Outcomes Study. Diabetologia 2017, 60, 1601–1611. [Google Scholar] [CrossRef]
- Griffin, S.J.; Leaver, J.K.; Irving, G.J. Impact of metformin on cardiovascular disease: A meta-analysis of randomised trials among people with type 2 diabetes. Diabetologia 2017, 60, 1620–1629. [Google Scholar] [CrossRef]
- Zhang, K.; Yang, W.; Dai, H.; Deng, Z. Cardiovascular risk following metformin treatment in patients with type 2 diabetes mellitus: Results from meta-analysis. Diabetes Res. Clin. Pract. 2020, 160, 108001. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Xie, H.; Liu, Y.; Gao, P.; Yang, X.; Shen, Z. Effect of metformin on all-cause and cardiovascular mortality in patients with coronary artery diseases: A systematic review and an updated meta-analysis. Cardiovasc. Diabetol. 2019, 18, 96. [Google Scholar] [CrossRef] [PubMed]
- Campbell, J.M.; Bellman, S.M.; Stephenson, M.D.; Lisy, K. Metformin reduces all-cause mortality and diseases of ageing independent of its effect on diabetes control: A systematic review and meta-analysis. Ageing Res. Rev. 2017, 40, 31–44. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Celotto, S.; Pizzol, D.; Gasevic, D.; Ji, M.M.; Barnini, T.; Solmi, M.; Stubbs, B.; Smith, L.; López Sánchez, G.F.; et al. Metformin and health outcomes: An umbrella review of systematic reviews with meta-analyses. Eur. J. Clin. Investig. 2021, 51, e13536. [Google Scholar] [CrossRef]
- Monami, M.; Candido, R.; Pintaudi, B.; Targher, G.; Mannucci, E. Effect of metformin on all-cause mortality and major adverse cardiovascular events: An updated meta-analysis of randomized controlled trials. Nutr. Metab Cardiovasc. Dis. 2021, 31, 699–704. [Google Scholar] [CrossRef]
- Raee, M.R.; Nargesi, A.A.; Heidari, B.; Mansournia, M.A.; Larry, M.; Rabizadeh, S.; Zarifkar, M.; Esteghamati, A.; Nakhjavani, M. All-Cause and Cardiovascular Mortality following Treatment with Metformin or Glyburide in Patients with Type 2 Diabetes Mellitus. Arch. Iran. Med. 2017, 20, 141–146. [Google Scholar]
- Scheller, N.M.; Mogensen, U.M.; Andersson, C.; Vaag, A.; Torp-Pedersen, C. All-cause mortality and cardiovascular effects associated with the DPP-IV inhibitor sitagliptin compared with metformin, a retrospective cohort study on the Danish population. Diabetes Obes. Metab. 2014, 16, 231–236. [Google Scholar] [CrossRef]
- Roumie, C.L.; Hung, A.M.; Greevy, R.A.; Grijalva, C.G.; Liu, X.; Murff, H.J.; Elasy, T.A.; Griffin, M.R. Comparative effectiveness of sulfonylurea and metformin monotherapy on cardiovascular events in type 2 diabetes mellitus: A cohort study. Ann. Intern. Med. 2012, 157, 601–610. [Google Scholar] [CrossRef]
- Roumie, C.L.; Min, J.Y.; D’Agostino McGowan, L.; Presley, C.; Grijalva, C.G.; Hackstadt, A.J.; Hung, A.M.; Greevy, R.A.; Elasy, T.; Griffin, M.R. Comparative Safety of Sulfonylurea and Metformin Monotherapy on the Risk of Heart Failure: A Cohort Study. J. Am. Heart Assoc. 2017, 6, e005379. [Google Scholar] [CrossRef]
- Johnson, J.A.; Majumdar, S.R.; Simpson, S.H.; Toth, E.L. Decreased mortality associated with the use of metformin compared with sulfonylurea monotherapy in type 2 diabetes. Diabetes Care 2002, 25, 2244–2248. [Google Scholar] [CrossRef]
- Ekström, N.; Schiöler, L.; Svensson, A.M.; Eeg-Olofsson, K.; Jonasson, J.M.; Zethelius, B.; Cederholm, J.; Eliasson, B.; Gudbjörnsdottir, S. Effectiveness and safety of metformin in 51,675 patients with type 2 diabetes and different levels of renal function: A cohort study from the Swedish National Diabetes Register. BMJ Open 2012, 2, e001076. [Google Scholar] [CrossRef] [PubMed]
- Pantalone, K.M.; Kattan, M.W.; Yu, C.; Wells, B.J.; Arrigain, S.; Jain, A.; Atreja, A.; Zimmerman, R.S. Increase in overall mortality risk in patients with type 2 diabetes receiving glipizide, glyburide or glimepiride monotherapy versus metformin: A retrospective analysis. Diabetes Obes. Metab. 2012, 14, 803–809. [Google Scholar] [CrossRef] [PubMed]
- Charytan, D.M.; Solomon, S.D.; Ivanovich, P.; Remuzzi, G.; Cooper, M.E.; McGill, J.B.; Parving, H.H.; Parfrey, P.; Singh, A.K.; Burdmann, E.A.; et al. Metformin use and cardiovascular events in patients with type 2 diabetes and chronic kidney disease. Diabetes Obes. Metab. 2019, 21, 1199–1208. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.Y.; Leu, H.B.; Chen, T.J.; Chen, C.L.; Kuo, C.H.; Lee, S.D.; Kao, C.L. Metformin-inclusive therapy reduces the risk of stroke in patients with diabetes: A 4-year follow-up study. J. Stroke Cerebrovasc. Dis. 2014, 23, e99–e105. [Google Scholar] [CrossRef] [PubMed]
- Mogensen, U.M.; Andersson, C.; Fosbøl, E.L.; Schramm, T.K.; Vaag, A.; Scheller, N.M.; Torp-Pedersen, C.; Gislason, G.; Køber, L. Sulfonylurea in combination with insulin is associated with increased mortality compared with a combination of insulin and metformin in a retrospective Danish nationwide study. Diabetologia 2015, 58, 50–58. [Google Scholar] [CrossRef][Green Version]
- Evans, J.M.; Ogston, S.A.; Emslie-Smith, A.; Morris, A.D. Risk of mortality and adverse cardiovascular outcomes in type 2 diabetes: A comparison of patients treated with sulfonylureas and metformin. Diabetologia 2006, 49, 930–936. [Google Scholar] [CrossRef]
- Sillars, B.; Davis, W.A.; Hirsch, I.B.; Davis, T.M. Sulphonylurea-metformin combination therapy, cardiovascular disease and all-cause mortality: The Fremantle Diabetes Study. Diabetes Obes. Metab. 2010, 12, 757–765. [Google Scholar] [CrossRef]
- Morgan, C.L.; Mukherjee, J.; Jenkins-Jones, S.; Holden, S.E.; Currie, C.J. Association between first-line monotherapy with sulphonylurea versus metformin and risk of all-cause mortality and cardiovascular events: A retrospective, observational study. Diabetes Obes. Metab. 2014, 16, 957–962. [Google Scholar] [CrossRef]
- Breunig, I.M.; Shaya, F.T.; McPherson, M.L.; Snitker, S. Development of heart failure in Medicaid patients with type 2 diabetes treated with pioglitazone, rosiglitazone, or metformin. J. Manag. Care Spec. Pharm. 2014, 20, 895–903. [Google Scholar] [CrossRef]
- Fung, C.S.; Wan, E.Y.; Wong, C.K.; Jiao, F.; Chan, A.K. Effect of metformin monotherapy on cardiovascular diseases and mortality: A retrospective cohort study on Chinese type 2 diabetes mellitus patients. Cardiovasc. Diabetol. 2015, 14, 137. [Google Scholar] [CrossRef]
- Mather, K.J.; Verma, S.; Anderson, T.J. Improved endothelial function with metformin in type 2 diabetes mellitus. J. Am. Coll. Cardiol. 2001, 37, 1344–1350. [Google Scholar] [CrossRef]
- de Jager, J.; Kooy, A.; Schalkwijk, C.; van der Kolk, J.; Lehert, P.; Bets, D.; Wulffelé, M.G.; Donker, A.J.; Stehouwer, C.D. Long-term effects of metformin on endothelial function in type 2 diabetes: A randomized controlled trial. J. Intern. Med. 2014, 275, 59–70. [Google Scholar] [CrossRef] [PubMed]
- Triggle, C.R.; Ding, H. Metformin is not just an antihyperglycaemic drug but also has protective effects on the vascular endothelium. Acta Physiol. 2017, 219, 138–151. [Google Scholar] [CrossRef] [PubMed]
- Davis, B.J.; Xie, Z.; Viollet, B.; Zou, M.H. Activation of the AMP-activated kinase by antidiabetes drug metformin stimulates nitric oxide synthesis in vivo by promoting the association of heat shock protein 90 and endothelial nitric oxide synthase. Diabetes 2006, 55, 496–505. [Google Scholar] [CrossRef]
- Yu, J.W.; Deng, Y.P.; Han, X.; Ren, G.F.; Cai, J.; Jiang, G.J. Metformin improves the angiogenic functions of endothelial progenitor cells via activating AMPK/eNOS pathway in diabetic mice. Cardiovasc. Diabetol. 2016, 15, 88. [Google Scholar] [CrossRef]
- An, H.; Wei, R.; Ke, J.; Yang, J.; Liu, Y.; Wang, X.; Wang, G.; Hong, T. Metformin attenuates fluctuating glucose-induced endothelial dysfunction through enhancing GTPCH1-mediated eNOS recoupling and inhibiting NADPH oxidase. J. Diabetes Complicat. 2016, 30, 1017–1024. [Google Scholar] [CrossRef]
- Nafisa, A.; Gray, S.G.; Cao, Y.; Wang, T.; Xu, S.; Wattoo, F.H.; Barras, M.; Cohen, N.; Kamato, D.; Little, P.J. Endothelial function and dysfunction: Impact of metformin. Pharmacol. Ther. 2018, 192, 150–162. [Google Scholar] [CrossRef]
- Zheng, Z.; Chen, H.; Li, J.; Li, T.; Zheng, B.; Zheng, Y.; Jin, H.; He, Y.; Gu, Q.; Xu, X. Sirtuin 1-mediated cellular metabolic memory of high glucose via the LKB1/AMPK/ROS pathway and therapeutic effects of metformin. Diabetes 2012, 61, 217–228. [Google Scholar] [CrossRef]
- Marfella, R.; Acampora, R.; Verrazzo, G.; Ziccardi, P.; De Rosa, N.; Giunta, R.; Giugliano, D. Metformin improves hemodynamic and rheological responses to L-arginine in NIDDM patients. Diabetes Care 1996, 19, 934–939. [Google Scholar] [CrossRef]
- Grant, P.J. Beneficial effects of metformin on haemostasis and vascular function in man. Diabetes Metab. 2003, 29 Pt 2, 6S44–6S52. [Google Scholar] [CrossRef]
- Grant, P.J.; Stickland, M.H.; Booth, N.A.; Prentice, C.R. Metformin causes a reduction in basal and post-venous occlusion plasminogen activator inhibitor-1 in type 2 diabetic patients. Diabet. Med. 1991, 8, 361–365. [Google Scholar] [CrossRef] [PubMed]
- Xin, G.; Wei, Z.; Ji, C.; Zheng, H.; Gu, J.; Ma, L.; Huang, W.; Morris-Natschke, S.L.; Yeh, J.L.; Zhang, R.; et al. Metformin Uniquely Prevents Thrombosis by Inhibiting Platelet Activation and mtDNA Release. Sci. Rep. 2016, 6, 36222. [Google Scholar] [CrossRef] [PubMed]
- Cabrera, C.S.; Lee, A.S.; Olsson, M.; Schnecke, V.; Westman, K.; Lind, M.; Greasley, P.J.; Skrtic, S. Impact of CKD Progression on Cardiovascular Disease Risk in a Contemporary UK Cohort of Individuals With Diabetes. Kidney Int. Rep. 2020, 5, 1651–1660. [Google Scholar] [CrossRef]
- Petrie, J.R.; Rossing, P.R.; Campbell, I.W. Metformin and cardiorenal outcomes in diabetes: A reappraisal. Diabetes Obes. Metab. 2020, 22, 904–915. [Google Scholar] [CrossRef] [PubMed]
- Gansevoort, R.T.; Correa-Rotter, R.; Hemmelgarn, B.R.; Jafar, T.H.; Heerspink, H.J.; Mann, J.F.; Matsushita, K.; Wen, C.P. Chronic kidney disease and cardiovascular risk: Epidemiology, mechanisms, and prevention. Lancet 2013, 382, 339–352. [Google Scholar] [CrossRef]
- Phung, O.J.; Scholle, J.M.; Talwar, M.; Coleman, C.I. Effect of noninsulin antidiabetic drugs added to metformin therapy on glycemic control, weight gain, and hypoglycemia in type 2 diabetes. JAMA 2010, 303, 1410–1418. [Google Scholar] [CrossRef]
- Neven, E.; Vervaet, B.; Brand, K.; Gottwald-Hostalek, U.; Opdebeeck, B.; De Maré, A.; Verhulst, A.; Lalau, J.D.; Kamel, S.; De Broe, M.E.; et al. Metformin prevents the development of severe chronic kidney disease and its associated mineral and bone disorder. Kidney Int. 2018, 94, 102–113. [Google Scholar] [CrossRef]
- Whitlock, R.H.; Hougen, I.; Komenda, P.; Rigatto, C.; Clemens, K.K.; Tangri, N. A Safety Comparison of Metformin vs Sulfonylurea Initiation in Patients With Type 2 Diabetes and Chronic Kidney Disease: A Retrospective Cohort Study. Mayo Clin. Proc. 2020, 95, 90–100. [Google Scholar] [CrossRef]
- Kwon, S.; Kim, Y.C.; Park, J.Y.; Lee, J.; An, J.N.; Kim, C.T.; Oh, S.; Park, S.; Kim, D.K.; Oh, Y.K.; et al. The Long-term Effects of Metformin on Patients with Type 2 Diabetic Kidney Disease. Diabetes Care 2020, 43, 948–955. [Google Scholar] [CrossRef]
- Bergmark, B.A.; Bhatt, D.L.; McGuire, D.K.; Cahn, A.; Mosenzon, O.; Steg, P.G.; Im, K.; Kanevsky, E.; Gurmu, Y.; Raz, I.; et al. Metformin Use and Clinical Outcomes among Patients with Diabetes Mellitus with or Without Heart Failure or Kidney Dysfunction: Observations from the SAVOR-TIMI 53 Trial. Circulation 2019, 140, 1004–1014. [Google Scholar] [CrossRef]
- Roumie, C.L.; Chipman, J.; Min, J.Y.; Hackstadt, A.J.; Hung, A.M.; Greevy, R.A., Jr.; Grijalva, C.G.; Elasy, T.; Griffin, M.R. Association of Treatment With Metformin vs Sulfonylurea With Major Adverse Cardiovascular Events Among Patients With Diabetes and Reduced Kidney Function. JAMA 2019, 322, 1167–1177. [Google Scholar] [CrossRef] [PubMed]
- Hung, S.C.; Chang, Y.K.; Liu, J.S.; Kuo, K.L.; Chen, Y.H.; Hsu, C.C.; Tarng, D.C. Metformin use and mortality in patients with advanced chronic kidney disease: National, retrospective, observational, cohort study. Lancet Diabetes Endocrinol. 2015, 3, 605–614. [Google Scholar] [CrossRef]
- Navaneethan, S.D.; Zoungas, S.; Caramori, M.L.; Chan, J.C.N.; Heerspink, H.J.L.; Hurst, C.; Liew, A.; Michos, E.D.; Olowu, W.A.; Sadusky, T.; et al. Diabetes Management in Chronic Kidney Disease: Synopsis of the 2020 KDIGO Clinical Practice Guideline. Ann. Intern. Med. 2020, 174, 385–394. [Google Scholar] [CrossRef] [PubMed]
- Buse, J.B.; Wexler, D.J.; Tsapas, A.; Rossing, P.; Mingrone, G.; Mathieu, C.; D’Alessio, D.A.; Davies, M.J. 2019 Update to: Management of Hyperglycemia in Type 2 Diabetes, 2018. A Consensus Report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2020, 43, 487–493. [Google Scholar] [CrossRef]
- Kim, J.; Shon, E.; Kim, C.S.; Kim, J.S. Renal podocyte injury in a rat model of type 2 diabetes is prevented by metformin. Exp. Diabetes Res. 2012, 2012, 210821. [Google Scholar] [CrossRef]
- Kim, D.I.; Park, M.J.; Heo, Y.R.; Park, S.H. Metformin ameliorates lipotoxicity-induced mesangial cell apoptosis partly via upregulation of glucagon like peptide-1 receptor (GLP-1R). Arch. Biochem. Biophys. 2015, 584, 90–97. [Google Scholar] [CrossRef]
- Yu, S.; Zhao, H.; Yang, W.; Amat, R.; Peng, J.; Li, Y.; Deng, K.; Mao, X.; Jiao, Y. The alcohol extract of Coreopsis tinctoria Nutt ameliorates diabetes and diabetic nephropathy in db/db mice through miR-192/miR-200b and PTEN/AKT and ZEB2/ECM pathways. BioMed Res. Int. 2019, 2019, 5280514. [Google Scholar] [CrossRef]
- Zhao, Y.; Sun, M. Metformin rescues Parkin protein expression and mitophagy in high glucose-challenged human renal epithelial cells by inhibiting NF-κB via PP2A activation. Life Sci. 2020, 246, 117382. [Google Scholar] [CrossRef]
- Chang, M.Y.; Ma, T.L.; Hung, C.C.; Tian, Y.C.; Chen, Y.C.; Yang, C.W.; Cheng, Y.C. Metformin Inhibits Cyst Formation in a Zebrafish Model of Polycystin-2 Deficiency. Sci. Rep. 2017, 7, 7161. [Google Scholar] [CrossRef]
- Sharma, S.; Ray, A.; Sadasivam, B. Metformin in COVID-19: A possible role beyond diabetes. Diabetes Res. Clin. Pract. 2020, 164, 108183. [Google Scholar] [CrossRef]
- Dalan, R. Metformin, neutrophils and COVID-19 infection. Diabetes Res. Clin. Pract. 2020, 164, 108230. [Google Scholar] [CrossRef] [PubMed]
- Samuel, S.M.; Varghese, E.; Büsselberg, D. Therapeutic Potential of Metformin in COVID-19: Reasoning for Its Protective Role. Trends Microbiol. 2021, 29, 894–907. [Google Scholar] [CrossRef] [PubMed]
- Bramante, C.T.; Ingraham, N.E.; Murray, T.A.; Marmor, S.; Hovertsen, S.; Gronski, J.; McNeil, C.; Feng, R.; Guzman, G.; Abdelwahab, N.; et al. Metformin and risk of mortality in patients hospitalised with COVID-19: A retrospective cohort analysis. Lancet Healthy Longev. 2021, 2, e34–e41. [Google Scholar] [CrossRef]
- Poly, T.N.; Islam, M.M.; Li, Y.J.; Lin, M.C.; Hsu, M.H.; Wang, Y.C. Metformin Use Is Associated with Decreased Mortality in COVID-19 Patients with Diabetes: Evidence from Retrospective Studies and Biological Mechanism. J. Clin. Med. 2021, 10, 3507. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Yang, D.; Cheng, B.; Chen, J.; Peng, A.; Yang, C.; Liu, C.; Xiong, M.; Deng, A.; Zhang, Y.; et al. Clinical Characteristics and Outcomes of Patients With Diabetes and COVID-19 in Association With Glucose-Lowering Medication. Diabetes Care 2020, 43, 1399–1407. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Liu, Y.-M.; Li, H.; Zhang, X.; Lei, F.; Qin, J.-J.; Chen, Z.; Deng, K.-Q.; Lin, L.; Chen, M.-M. Metformin Use Is Associated with Increased Incidence of Acidosis but not Mortality in Individuals with COVID-19 and Pre-existing Type 2 Diabetes. Cell Metab. 2020, 32, 537–547. [Google Scholar] [CrossRef] [PubMed]
- De Jager, C.P.; Wever, P.C.; Gemen, E.F.; Kusters, R.; van Gageldonk-Lafeber, A.B.; van der Poll, T.; Laheij, R.J. The neutrophil-lymphocyte count ratio in patients with community-acquired pneumonia. PLoS ONE 2012, 7, e46561. [Google Scholar] [CrossRef] [PubMed]
- Cameron, A.R.; Morrison, V.L.; Levin, D.; Mohan, M.; Forteath, C.; Beall, C.; McNeilly, A.D.; Balfour, D.J.; Savinko, T.; Wong, A.K. Anti-inflammatory effects of metformin irrespective of diabetes status. Circ. Res. 2016, 119, 652–665. [Google Scholar] [CrossRef]
- Mor, A.; Petersen, I.; Sørensen, H.T.; Thomsen, R.W. Metformin and other glucose-lowering drug initiation and rates of community-based antibiotic use and hospital-treated infections in patients with type 2 diabetes: A Danish nationwide population-based cohort study. BMJ Open 2016, 6, e011523. [Google Scholar] [CrossRef]
- Mortensen, E.; Anzueto, A. Association of metformin and mortality for patients with diabetes who are hospitalized with pneumonia. Eur. Respir. J. 2018, 52, PA2639. [Google Scholar]
- Yang, A.; Shi, M.; Wu, H.; Lau, E.S.H.; Ma, R.C.W.; Kong, A.P.S.; So, W.Y.; Luk, A.O.Y.; Chan, J.C.N.; Chow, E. Long-term metformin use and risk of pneumonia and related death in type 2 diabetes: A registry-based cohort study. Diabetologia 2021, 64, 1760–1765. [Google Scholar] [CrossRef] [PubMed]
- Pernicova, I.; Kelly, S.; Ajodha, S.; Sahdev, A.; Bestwick, J.P.; Gabrovska, P.; Akanle, O.; Ajjan, R.; Kola, B.; Stadler, M.; et al. Metformin to reduce metabolic complications and inflammation in patients on systemic glucocorticoid therapy: A randomised, double-blind, placebo-controlled, proof-of-concept, phase 2 trial. Lancet Diabetes Endocrinol. 2020, 8, 278–291. [Google Scholar] [CrossRef]
- Chen, X.; Walther, F.J.; Sengers, R.M.; Laghmani, E.H.; Salam, A.; Folkerts, G.; Pera, T.; Wagenaar, G.T. Metformin attenuates hyperoxia-induced lung injury in neonatal rats by reducing the inflammatory response. Am. J. Physiol. Lung Cell Mol. Physiol. 2015, 309, L262–L270. [Google Scholar] [CrossRef] [PubMed]
- Menegazzo, L.; Scattolini, V.; Cappellari, R.; Bonora, B.M.; Albiero, M.; Bortolozzi, M.; Romanato, F.; Ceolotto, G.; de Kreutzeberg, S.V.; Avogaro, A. The antidiabetic drug metformin blunts NETosis in vitro and reduces circulating NETosis biomarkers in vivo. Acta Diabetol. 2018, 55, 593–601. [Google Scholar] [CrossRef]
- Yu, X.; Li, L.; Xia, L.; Feng, X.; Chen, F.; Cao, S.; Wei, X. Impact of metformin on the risk and treatment outcomes of tuberculosis in diabetics: A systematic review. BMC Infect. Dis. 2019, 19, 859. [Google Scholar] [CrossRef] [PubMed]
- Singhal, A.; Jie, L.; Kumar, P.; Hong, G.S.; Leow, M.K.-S.; Paleja, B.; Tsenova, L.; Kurepina, N.; Chen, J.; Zolezzi, F. Metformin as adjunct antituberculosis therapy. Sci. Transl. Med. 2014, 6, 263ra159. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Carlos, A.; Valdez-Miramontes, C.; Marin-Luevano, P.; González-Curiel, I.; Enciso-Moreno, J.A.; Rivas-Santiago, B. Metformin promotes Mycobacterium tuberculosis killing and increases the production of human β-defensins in lung epithelial cells and macrophages. Microbes. Infect. 2020, 22, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Lee, H.M.; Chan, J.C. Drug-subphenotype interactions for cancer in type 2 diabetes mellitus. Nat. Rev. Endocrinol. 2015, 11, 372–379. [Google Scholar] [CrossRef]
- Mao, D.; Lau, E.S.H.; Wu, H.; Yang, A.; Shi, M.; Fan, B.; Tam, C.; Chow, E.; Kong, A.P.S.; Ma, R.C.W.; et al. Risk associations of long-term HbA1c variability and obesity on cancer events and cancer-specific death in 15,286 patients with diabetes—A prospective cohort study. Lancet Reg. Health—West. Pac. 2022, 18, 100315. [Google Scholar] [CrossRef]
- Mao, D.; Lau, E.S.H.; Wu, H.; Yang, A.; Shi, M.; Fan, B.; Tam, C.; Chow, E.; Kong, A.P.S.; Ma, R.C.W.; et al. Risk associations of glycemic burden and obesity with liver cancer—A 10-year analysis of 16,410 patients with type 2 diabetes. Hepatol. Commun. 2022. [Google Scholar] [CrossRef]
- Vancura, A.; Bu, P.; Bhagwat, M.; Zeng, J.; Vancurova, I. Metformin as an Anticancer Agent. Trends Pharmacol. Sci. 2018, 39, 867–878. [Google Scholar] [CrossRef] [PubMed]
- Heckman-Stoddard, B.M.; Decensi, A.; Sahasrabuddhe, V.V.; Ford, L.G. Repurposing metformin for the prevention of cancer and cancer recurrence. Diabetologia 2017, 60, 1639–1647. [Google Scholar] [CrossRef] [PubMed]
- Ben Sahra, I.; Regazzetti, C.; Robert, G.; Laurent, K.; Le Marchand-Brustel, Y.; Auberger, P.; Tanti, J.F.; Giorgetti-Peraldi, S.; Bost, F. Metformin, independent of AMPK, induces mTOR inhibition and cell-cycle arrest through REDD1. Cancer Res. 2011, 71, 4366–4372. [Google Scholar] [CrossRef] [PubMed]
- Rattan, R.; Giri, S.; Hartmann, L.C.; Shridhar, V. Metformin attenuates ovarian cancer cell growth in an AMP-kinase dispensable manner. J. Cell Mol. Med. 2011, 15, 166–178. [Google Scholar] [CrossRef]
- Rattan, R.; Ali Fehmi, R.; Munkarah, A. Metformin: An emerging new therapeutic option for targeting cancer stem cells and metastasis. J. Oncol. 2012, 2012, 928127. [Google Scholar] [CrossRef]
- He, H.; Ke, R.; Lin, H.; Ying, Y.; Liu, D.; Luo, Z. Metformin, an old drug, brings a new era to cancer therapy. Cancer J. 2015, 21, 70–74. [Google Scholar] [CrossRef]
- Xiong, Y.; Song, W.; Shen, L.; Wang, Y.; Zhang, J.; Hu, M.; Liu, Y.; Li, J.; Musetti, S.; Liu, R.; et al. Oral Metformin and Polymetformin Reprogram Immunosuppressive Microenvironment and Boost Immune Checkpoint Inhibitor Therapy in Colorectal Cancer. Adv. Ther. 2020, 3, 2000168. [Google Scholar] [CrossRef]
- Scharping, N.E.; Menk, A.V.; Whetstone, R.D.; Zeng, X.; Delgoffe, G.M. Efficacy of PD-1 Blockade Is Potentiated by Metformin-Induced Reduction of Tumor Hypoxia. Cancer Immunol. Res. 2017, 5, 9–16. [Google Scholar] [CrossRef]
- Bai, M.; Yang, L.; Liao, H.; Liang, X.; Xie, B.; Xiong, J.; Tao, X.; Chen, X.; Cheng, Y.; Chen, X.; et al. Metformin sensitizes endometrial cancer cells to chemotherapy through IDH1-induced Nrf2 expression via an epigenetic mechanism. Oncogene 2018, 37, 5666–5681. [Google Scholar] [CrossRef]
- Mallik, R.; Chowdhury, T.A. Metformin in cancer. Diabetes Res. Clin. Pract. 2018, 143, 409–419. [Google Scholar] [CrossRef]
- Lee, M.-S.; Hsu, C.-C.; Wahlqvist, M.L.; Tsai, H.-N.; Chang, Y.-H.; Huang, Y.-C. Type 2 diabetes increases and metformin reduces total, colorectal, liver and pancreatic cancer incidences in Taiwanese: A representative population prospective cohort study of 800,000 individuals. BMC Cancer 2011, 11, 20. [Google Scholar] [CrossRef]
- Currie, C.J.; Poole, C.D.; Gale, E.A.M. The influence of glucose-lowering therapies on cancer risk in type 2 diabetes. Diabetologia 2009, 52, 1766–1777. [Google Scholar] [CrossRef]
- Bodmer, M.; Meier, C.; Krahenbuhl, S.; Jick, S.S.; Meier, C.R. Long-Term Metformin Use Is Associated With Decreased Risk of Breast Cancer. Diabetes Care 2010, 33, 1304–1308. [Google Scholar] [CrossRef]
- Libby, G.; Donnelly, L.A.; Donnan, P.T.; Alessi, D.R.; Morris, A.D.; Evans, J.M.M. New Users of Metformin Are at Low Risk of Incident Cancer: A cohort study among people with type 2 diabetes. Diabetes Care 2009, 32, 1620–1625. [Google Scholar] [CrossRef]
- Yang, X.; So, W.Y.; Ma, R.C.W.; Kong, A.P.S.; Lee, H.M.; Yu, L.W.L.; Chow, C.C.; Ozaki, R.; Ko, G.T.C.; Chan, J.C.N. Low HDL Cholesterol, Metformin Use, and Cancer Risk in Type 2 Diabetes: The Hong Kong Diabetes Registry. Diabetes Care 2011, 34, 375–380. [Google Scholar] [CrossRef]
- Viberti, G.; Kahn, S.E.; Greene, D.A.; Herman, W.H.; Zinman, B.; Holman, R.R.; Haffner, S.M.; Levy, D.; Lachin, J.M.; Berry, R.A.; et al. A diabetes outcome progression trial (ADOPT): An international multicenter study of the comparative efficacy of rosiglitazone, glyburide, and metformin in recently diagnosed type 2 diabetes. Diabetes Care 2002, 25, 1737–1743. [Google Scholar] [CrossRef]
- Home, P.D.; Kahn, S.E.; Jones, N.P.; Noronha, D.; Beck-Nielsen, H.; Viberti, G. Experience of malignancies with oral glucose-lowering drugs in the randomised controlled ADOPT (A Diabetes Outcome Progression Trial) and RECORD (Rosiglitazone Evaluated for Cardiovascular Outcomes and Regulation of Glycaemia in Diabetes) clinical trials. Diabetologia 2010, 53, 1838–1845. [Google Scholar] [CrossRef]
- Suissa, S.; Azoulay, L. Metformin and the risk of cancer: Time-related biases in observational studies. Diabetes Care 2012, 35, 2665–2673. [Google Scholar] [CrossRef]
- Decensi, A.; Puntoni, M.; Goodwin, P.; Cazzaniga, M.; Gennari, A.; Bonanni, B.; Gandini, S. Metformin and Cancer Risk in Diabetic Patients: A Systematic Review and Meta-analysis. Cancer Prev. Res. 2010, 3, 1451–1461. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Kim, J.H.; Jang, H.J.; Lee, J. The addition of metformin to systemic anticancer therapy in advanced or metastatic cancers: A meta-analysis of randomized controlled trials. Int. J. Med. Sci. 2020, 17, 2551–2560. [Google Scholar] [CrossRef] [PubMed]
- Higurashi, T.; Hosono, K.; Takahashi, H.; Komiya, Y.; Umezawa, S.; Sakai, E.; Uchiyama, T.; Taniguchi, L.; Hata, Y.; Uchiyama, S.; et al. Metformin for chemoprevention of metachronous colorectal adenoma or polyps in post-polypectomy patients without diabetes: A multicentre double-blind, placebo-controlled, randomised phase 3 trial. Lancet Oncol. 2016, 17, 475–483. [Google Scholar] [CrossRef]
- Pimentel, I.; Lohmann, A.E.; Ennis, M.; Dowling, R.J.O.; Cescon, D.; Elser, C.; Potvin, K.R.; Haq, R.; Hamm, C.; Chang, M.C.; et al. A phase II randomized clinical trial of the effect of metformin versus placebo on progression-free survival in women with metastatic breast cancer receiving standard chemotherapy. Breast 2019, 48, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Farkhondeh, T.; Amirabadizadeh, A.; Aramjoo, H.; Llorens, S.; Roshanravan, B.; Saeedi, F.; Talebi, M.; Shakibaei, M.; Samarghandian, S. Impact of Metformin on Cancer Biomarkers in Non-Diabetic Cancer Patients: A Systematic Review and Meta-Analysis of Clinical Trials. Curr. Oncol. 2021, 28, 1412–1423. [Google Scholar] [CrossRef] [PubMed]
- Goodwin, P.J.; Dowling, R.J.O.; Ennis, M.; Chen, B.E.; Parulekar, W.R.; Shepherd, L.E.; Burnell, M.J.; Vander Meer, R.; Molckovsky, A.; Gurjal, A.; et al. Effect of metformin versus placebo on metabolic factors in the MA.32 randomized breast cancer trial. NPJ Breast Cancer 2021, 7, 74. [Google Scholar] [CrossRef] [PubMed]
- Pimentel, I.; Chen, B.E.; Lohmann, A.E.; Ennis, M.; Ligibel, J.; Shepherd, L.; Hershman, D.L.; Whelan, T.; Stambolic, V.; Mayer, I.; et al. The Effect of Metformin vs Placebo on Sex Hormones in Canadian Cancer Trials Group MA.32. JNCI J. Natl. Cancer Inst. 2021, 113, 192–198. [Google Scholar] [CrossRef]
- Liu, F.; Wang, C.; Zhang, L.; Xu, Y.; Jang, L.; Gu, Y.; Cao, X.; Zhao, X.; Ye, J.; Li, Q. Metformin prevents hepatic steatosis by regulating the expression of adipose differentiation-related protein. Int. J. Mol. Med. 2014, 33, 51–58. [Google Scholar] [CrossRef]
- Lin, H.Z.; Yang, S.Q.; Chuckaree, C.; Kuhajda, F.; Ronnet, G.; Diehl, A.M. Metformin reverses fatty liver disease in obese, leptin-deficient mice. Nat. Med. 2000, 6, 998–1003. [Google Scholar] [CrossRef]
- Kita, Y.; Takamura, T.; Misu, H.; Ota, T.; Kurita, S.; Takeshita, Y.; Uno, M.; Matsuzawa-Nagata, N.; Kato, K.-I.; Ando, H.; et al. Metformin Prevents and Reverses Inflammation in a Non-Diabetic Mouse Model of Nonalcoholic Steatohepatitis. PLoS ONE 2012, 7, e43056. [Google Scholar] [CrossRef]
- Marchesini, G.; Brizi, M.; Bianchi, G.; Tomassetti, S.; Zoli, M.; Melchionda, N. Metformin in non-alcoholic steatohepatitis. Lancet 2001, 358, 893–894. [Google Scholar] [CrossRef]
- Loomba, R.; Lutchman, G.; Kleiner, D.E.; Ricks, M.; Feld, J.J.; Borg, B.B.; Modi, A.; Nagabhyru, P.; Sumner, A.E.; Liang, T.J.; et al. Clinical trial: Pilot study of metformin for the treatment of non-alcoholic steatohepatitis. Aliment. Pharmacol. Ther. 2009, 29, 172–182. [Google Scholar] [CrossRef]
- Uygun, A.; Kadayifci, A.; Isik, A.T.; Ozgurtas, T.; Deveci, S.; Tuzun, A.; Yesilova, Z.; Gulsen, M.; Dagalp, K. Metformin in the treatment of patients with non-alcoholic steatohepatitis. Aliment. Pharmacol. Ther. 2004, 19, 537–544. [Google Scholar] [CrossRef] [PubMed]
- Bugianesi, E.; Gentilcore, E.; Manini, R.; Natale, S.; Vanni, E.; Villanova, N.; David, E.; Rizzetto, M.; Marchesini, G. A randomized controlled trial of metformin versus vitamin E or prescriptive diet in nonalcoholic fatty liver disease. Am. J. Gastroenterol. 2005, 100, 1082–1090. [Google Scholar] [CrossRef] [PubMed]
- Omer, Z.; Cetinkalp, S.; Akyildiz, M.; Yilmaz, F.; Batur, Y.; Yilmaz, C.; Akarca, U. Efficacy of insulin-sensitizing agents in nonalcoholic fatty liver disease. Eur. J. Gastroenterol. Hepatol. 2010, 22, 18–23. [Google Scholar] [CrossRef] [PubMed]
- Haukeland, J.W.; Konopski, Z.; Eggesbø, H.B.; Von Volkmann, H.L.; Raschpichler, G.; Bjøro, K.; Haaland, T.; Løberg, E.M.; Birkeland, K. Metformin in patients with non-alcoholic fatty liver disease: A randomized, controlled trial. Scand. J. Gastroenterol. 2009, 44, 853–860. [Google Scholar] [CrossRef] [PubMed]
- Lavine, J.E.; Schwimmer, J.B.; Van Natta, M.L.; Molleston, J.P.; Murray, K.F.; Rosenthal, P.; Abrams, S.H.; Scheimann, A.O.; Sanyal, A.J.; Chalasani, N.; et al. Effect of vitamin E or metformin for treatment of nonalcoholic fatty liver disease in children and adolescents: The TONIC randomized controlled trial. JAMA 2011, 305, 1659–1668. [Google Scholar] [CrossRef] [PubMed]
- Krakoff, J.; Clark, J.M.; Crandall, J.P.; Wilson, C.; Molitch, M.E.; Brancati, F.L.; Edelstein, S.L.; Knowler, W.C. Effects of metformin and weight loss on serum alanine aminotransferase activity in the diabetes prevention program. Obesity 2010, 18, 1762–1767. [Google Scholar] [CrossRef]
- Li, Y.; Liu, L.; Wang, B.; Wang, J.; Chen, D. Metformin in non-alcoholic fatty liver disease: A systematic review and meta-analysis. Biomed. Rep. 2013, 1, 57–64. [Google Scholar] [CrossRef]
- Ou, Z.; Kong, X.; Sun, X.; He, X.; Zhang, L.; Gong, Z.; Huang, J.; Xu, B.; Long, D.; Li, J.; et al. Metformin treatment prevents amyloid plaque deposition and memory impairment in APP/PS1 mice. Brain Behav. Immun. 2018, 69, 351–363. [Google Scholar] [CrossRef]
- Hardie, D.G. AMP-Activated Protein Kinase: Maintaining Energy Homeostasis at the Cellular and Whole-Body Levels. Annu. Rev. Nutr. 2014, 34, 31–55. [Google Scholar] [CrossRef]
- Jin, Q.; Cheng, J.; Liu, Y.; Wu, J.; Wang, X.; Wei, S.; Zhou, X.; Qin, Z.; Jia, J.; Zhen, X. Improvement of functional recovery by chronic metformin treatment is associated with enhanced alternative activation of microglia/macrophages and increased angiogenesis and neurogenesis following experimental stroke. Brain Behav. Immun. 2014, 40, 131–142. [Google Scholar] [CrossRef]
- Hohnholt, M.C.; Blumrich, E.-M.; Waagepetersen, H.S.; Dringen, R. The antidiabetic drug metformin decreases mitochondrial respiration and tricarboxylic acid cycle activity in cultured primary rat astrocytes. J. Neurosci. Res. 2017, 95, 2307–2320. [Google Scholar] [CrossRef] [PubMed]
- Mostafa, D.K.; Ismail, C.A.; Ghareeb, D.A. Differential metformin dose-dependent effects on cognition in rats: Role of Akt. Psychopharmacology 2016, 233, 2513–2524. [Google Scholar] [CrossRef] [PubMed]
- McNeilly, A.D.; Williamson, R.; Balfour, D.J.K.; Stewart, C.A.; Sutherland, C. A high-fat-diet-induced cognitive deficit in rats that is not prevented by improving insulin sensitivity with metformin. Diabetologia 2012, 55, 3061–3070. [Google Scholar] [CrossRef]
- Oliveira, W.H.; Nunes, A.K.; França, M.E.R.; Santos, L.A.; Lós, D.B.; Rocha, S.W.; Barbosa, K.P.; Rodrigues, G.B.; Peixoto, C.A. Effects of metformin on inflammation and short-term memory in streptozotocin-induced diabetic mice. Brain Res. 2016, 1644, 149–160. [Google Scholar] [CrossRef] [PubMed]
- Ghadernezhad, N.; Khalaj, L.; Pazoki-Toroudi, H.; Mirmasoumi, M.; Ashabi, G. Metformin pretreatment enhanced learning and memory in cerebral forebrain ischaemia: The role of the AMPK/BDNF/P70SK signalling pathway. Pharm. Biol. 2016, 54, 2211–2219. [Google Scholar] [CrossRef]
- Chaudhari, K.; Reynolds, C.D.; Yang, S.-H. Metformin and cognition from the perspectives of sex, age, and disease. GeroScience 2020, 42, 97–116. [Google Scholar] [CrossRef]
- Moore, E.; Mander, A.; Ames, D.; Carne, R.; Sanders, K.; Watters, D. Cognitive impairment and vitamin B12: A review. Int. Psychogeriatr. 2012, 24, 541–556. [Google Scholar] [CrossRef]
- Moore, E.M.; Mander, A.G.; Ames, D.; Kotowicz, M.A.; Carne, R.P.; Brodaty, H.; Woodward, M.; Boundy, K.; Ellis, K.A.; Bush, A.I.; et al. Increased Risk of Cognitive Impairment in Patients With Diabetes Is Associated With Metformin. Diabetes Care 2013, 36, 2981–2987. [Google Scholar] [CrossRef]
- Porter, K.M.; Ward, M.; Hughes, C.F.; O’Kane, M.; Hoey, L.; McCann, A.; Molloy, A.M.; Cunningham, C.; Casey, M.C.; Tracey, F.; et al. Hyperglycemia and Metformin Use Are Associated With B Vitamin Deficiency and Cognitive Dysfunction in Older Adults. J. Clin. Endocrinol. Metab. 2019, 104, 4837–4847. [Google Scholar] [CrossRef]
- Ng, T.P.; Feng, L.; Yap, K.B.; Lee, T.S.; Tan, C.H.; Winblad, B. Long-term metformin usage and cognitive function among older adults with diabetes. J. Alzheimer’s Dis. 2014, 41, 61–68. [Google Scholar] [CrossRef]
- Samaras, K.; Makkar, S.; Crawford, J.D.; Kochan, N.A.; Wen, W.; Draper, B.; Trollor, J.N.; Brodaty, H.; Sachdev, P.S. Metformin Use Is Associated With Slowed Cognitive Decline and Reduced Incident Dementia in Older Adults With Type 2 Diabetes: The Sydney Memory and Ageing Study. Diabetes Care 2020, 43, 2691–2701. [Google Scholar] [CrossRef] [PubMed]
- Koenig, A.M.; Mechanic-Hamilton, D.; Xie, S.X.; Combs, M.F.; Cappola, A.R.; Xie, L.; Detre, J.A.; Wolk, D.A.; Arnold, S.E. Effects of the Insulin Sensitizer Metformin in Alzheimer Disease. Alzheimer Dis. Assoc. Disord. 2017, 31, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.; Mi, J.; Jiang, Q.M.; Xu, J.M.; Tang, Y.Y.; Tian, G.; Wang, B. Metformin may produce antidepressant effects through improvement of cognitive function among depressed patients with diabetes mellitus. Clin. Exp. Pharmacol. Physiol. 2014, 41, 650–656. [Google Scholar] [CrossRef] [PubMed]
- Luchsinger, J.A.; Ma, Y.; Christophi, C.A.; Florez, H.; Golden, S.H.; Hazuda, H.; Crandall, J.; Venditti, E.; Watson, K.; Jeffries, S.; et al. Metformin, Lifestyle Intervention, and Cognition in the Diabetes Prevention Program Outcomes Study. Diabetes Care 2017, 40, 958–965. [Google Scholar] [CrossRef]
- Campbell, J.M.; Stephenson, M.D.; de Courten, B.; Chapman, I.; Bellman, S.M.; Aromataris, E. Metformin Use Associated with Reduced Risk of Dementia in Patients with Diabetes: A Systematic Review and Meta-Analysis. J. Alzheimer’s Dis. 2018, 65, 1225–1236. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chow, E.; Yang, A.; Chung, C.H.L.; Chan, J.C.N. A Clinical Perspective of the Multifaceted Mechanism of Metformin in Diabetes, Infections, Cognitive Dysfunction, and Cancer. Pharmaceuticals 2022, 15, 442. https://doi.org/10.3390/ph15040442
Chow E, Yang A, Chung CHL, Chan JCN. A Clinical Perspective of the Multifaceted Mechanism of Metformin in Diabetes, Infections, Cognitive Dysfunction, and Cancer. Pharmaceuticals. 2022; 15(4):442. https://doi.org/10.3390/ph15040442
Chicago/Turabian StyleChow, Elaine, Aimin Yang, Colin H. L. Chung, and Juliana C. N. Chan. 2022. "A Clinical Perspective of the Multifaceted Mechanism of Metformin in Diabetes, Infections, Cognitive Dysfunction, and Cancer" Pharmaceuticals 15, no. 4: 442. https://doi.org/10.3390/ph15040442
APA StyleChow, E., Yang, A., Chung, C. H. L., & Chan, J. C. N. (2022). A Clinical Perspective of the Multifaceted Mechanism of Metformin in Diabetes, Infections, Cognitive Dysfunction, and Cancer. Pharmaceuticals, 15(4), 442. https://doi.org/10.3390/ph15040442







