Thymoquinone: Review of Its Potential in the Treatment of Neurological Diseases
Abstract
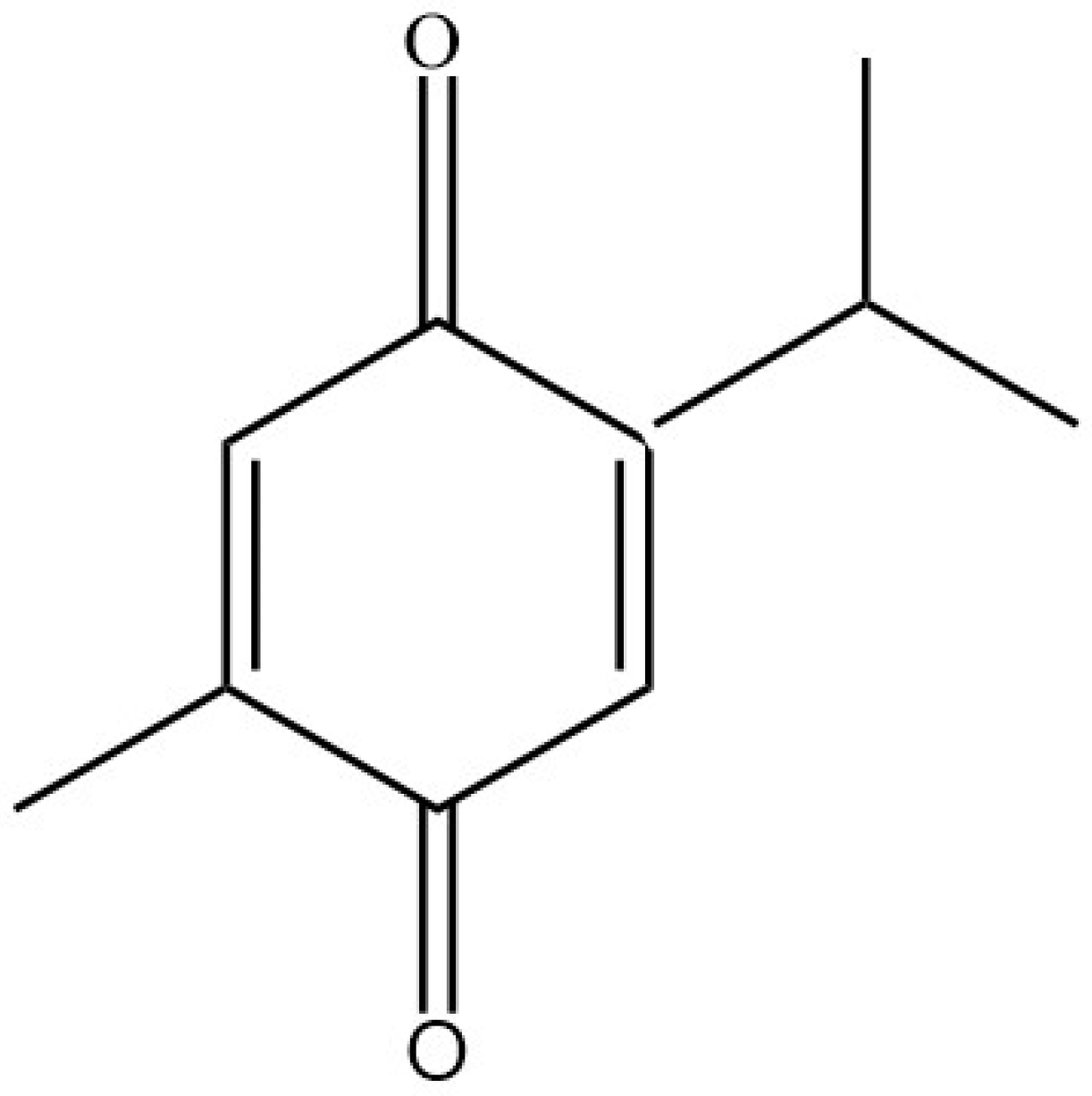
:1. Introduction
2. Effect of Thymoquinone on Neuroinflammation
3. Effect of Thymoquinone on Neurological Diseases
3.1. Alzheimer’s Disease
3.2. Parkinson’s Disease
3.3. Epilepsy
4. Effect of Thymoquinone on Learning and Memory
5. Safety and Adverse Effects
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ibrahim AbdEl Fattah, L.; Zickri, M.B.; Aal, L.A.; Heikal, O.; Osama, E. The Effect of Thymoquinone, α7 Receptor Agonist and α7 Receptor Allosteric Modulator on the Cerebral Cortex in Experimentally Induced Alzheimer’s Disease in Relation to MSCs Activation. IJSC Int. J. Stem Cells 2016, 9, 230–238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abulfadl, Y.; El-Maraghy, N.; Ahmed, A.E.; Nofal, S.; Abdel-Mottaleb, Y.; Badary, O. Thymoquinone alleviates the experimentally induced Alzheimer’s disease inflammation by modulation of TLRs signaling. Hum. Exp. Toxicol. 2018, 37, 1092–1104. [Google Scholar] [CrossRef] [PubMed]
- Ardah, M.T.; Merghani, M.M.; Haque, M.E. Thymoquinone prevents neurodegeneration against MPTP in vivo and modulates α-synuclein aggregation in vitro. Neurochem. Int. 2019, 128, 115–126. [Google Scholar] [CrossRef] [PubMed]
- Badary, O.A.; Taha, R.A.; Gamal El-Din, A.M.; Abdel-Wahab, M.H. Thymoquinone Is a Potent Superoxide Anion Scavenger. Drug Chem. Toxicol. 2003, 26, 87–98. [Google Scholar] [CrossRef]
- Malik, S.; Singh, A.; Negi, P.; Kapoor, V.K. Thymoquinone: A small molecule from nature with high therapeutic potential. Drug Discov. Today 2021, 26, 2716–2725. [Google Scholar] [CrossRef] [PubMed]
- Akhondian, J.; Kianifar, H.; Raoofziaee, M.; Moayedpour, A.; Toosi, M.B.; Khajedaluee, M. The effect of thymoquinone on intractable pediatric seizures (pilot study). Epilepsy Res. 2011, 93, 39–43. [Google Scholar] [CrossRef]
- Hosseinzadeh, H.; Parvardeh, S.; Asl, M.N.; Sadeghnia, H.R.; Ziaee, T. Effect of thymoquinone and Nigella sativa seeds oil on lipid peroxidation level during global cerebral ischemia-reperfusion injury in rat hippocampus. Phytomedicine 2007, 14, 621–627. [Google Scholar] [CrossRef]
- Jakaria Md Cho, D.-Y.; Ezazul Haque Md Karthivashan, G.; Kim, I.-S.; Ganesan, P.; Choi, D.-K. Neuropharmacological Potential and Delivery Prospects of Thymoquinone for Neurological Disorders. Oxidative Med. Cell. Longev. 2018, 2018, 1209801. [Google Scholar]
- Pal, R.R.; Rajpal, V.; Singh, P.; Saraf, S.A. Recent Findings on Thymoquinone and Its Applications as a Nanocarrier for the Treatment of Cancer and Rheumatoid Arthritis. Pharmaceutics 2021, 13, 775. [Google Scholar] [CrossRef]
- Kohandel, Z.; Farkhondeh, T.; Aschner, M.; Samarghandian, S. Anti-inflammatory effects of thymoquinone and its protective effects against several diseases. Biomed. Pharmacother. 2021, 138, 111492. [Google Scholar] [CrossRef]
- Sarkar, C.; Jamaddar, S.; Islam, T.; Mondal, M.; Islam, M.T.; Mubarak, M.S. Therapeutic perspectives of the black cumin component thymoquinone: A review. Food Funct. 2021, 12, 6167–6213. [Google Scholar] [CrossRef] [PubMed]
- Alkharfy, K.M.; Ahmad, A.; Khan, R.M.A.; Al-Asmari, M. High-Performance Liquid Chromatography of Thymoquinone in Rabbit Plasma and Its Application to Pharmacokinetics. J. Liq. Chromatogr. Relat. Technol. 2013, 36, 2242–2250. [Google Scholar] [CrossRef]
- Ahmad, A.; Khan, R.M.A.; Alkharfy, K.M.; Raish, M.; Al-Jenoobi, F.I.; Al-Mohizea, A.M. Effects of Thymoquinone on the Pharmacokinetics and Pharmacodynamics of Glibenclamide in a Rat Model. Nat. Prod. Commun. 2015, 10, 1395–1398. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alkharfy, K.M.; Ahmad, A.; Khan, R.M.A.; Al-Shagha, W.M. Pharmacokinetic plasma behaviors of intravenous and oral bioavailability of thymoquinone in a rabbit model. Eur. J. Drug Metab. Pharmacokinet. 2015, 40, 319–323. [Google Scholar] [CrossRef]
- Elmaci, I.; Altinoz, M.A. Thymoquinone: An edible redox-active quinone for the pharmacotherapy of neurodegenerative conditions and glial brain tumors. A short review. Biomed. Pharmacother. 2016, 83, 635–640. [Google Scholar] [CrossRef]
- Guler, E.M.; Sisman, B.H.; Kocyigit, A.; Hatiboglu, M.A. Investigation of cellular effects of thymoquinone on glioma cell. Toxicol. Rep. 2021, 8, 162–170. [Google Scholar] [CrossRef]
- Darakhshan, S.; Bidmeshki Pour, A.; Hosseinzadeh Colagar, A.; Sisakhtnezhad, S. Thymoquinone and its therapeutic potentials. Pharmacol. Res. 2015, 95–96, 138–158. [Google Scholar] [CrossRef]
- Landucci, E.; Mazzantini, C.; Buonvicino, D.; Pellegrini-Giampietro, D.E.; Bergonzi, M.C. Neuroprotective Effects of Thymoquinone by the Modulation of ER Stress and Apoptotic Pathway in In Vitro Model of Excitotoxicity. Molecules 2021, 26, 1592. [Google Scholar] [CrossRef]
- Xiao, X.-Y.; Zhu, Y.-X.; Bu, J.-Y.; Li, G.-W.; Zhou, J.-H.; Zhou, S.-P. Evaluation of Neuroprotective Effect of Thymoquinone Nanoformulation in the Rodent Cerebral Ischemia-Reperfusion Model. BioMed Res. Int. 2016, 2016, 2571060. [Google Scholar] [CrossRef] [Green Version]
- Al-Majed, A.A.; Al-Omar, F.A.; Nagi, M.N. Neuroprotective effects of thymoquinone against transient forebrain ischemia in the rat hippocampus. Eur. J. Pharmacol. 2006, 543, 40–47. [Google Scholar] [CrossRef]
- Türkerï, Ö.N.; Tanyelï, A.; Kurt, N.; Bakan, N.; Akdemïr, F.N.E.; Mokhtare, B. Biochemical and Histopathological Evaluation of the Protective Efficacy of Thymoquinone in Experimentally Ischemia Reperfusion Induced Rat Ovaries. New Trends Med. Sci. 2021, 2, 136–143. [Google Scholar]
- Sahak, M.K.A.; Mohamed, A.M.; Hashim, N.H.; Hasan Adli, D.S. Nigella sativa Oil Enhances the Spatial Working Memory Performance of Rats on a Radial Arm Maze. Evid. Based Complement. Altern. Med. 2013, 2013, 180598. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farkhondeh, T.; Samarghandian, S.; Hozeifi, S.; Azimi-Nezhad, M. Therapeutic effects of thymoquinone for the treatment of central nervous system tumors: A review. Biomed. Pharmacother. 2017, 96, 1440–1444. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Vaibhav, K.; Javed, H.; Khan, M.M.; Tabassum, R.; Ahmed, M.E.; Srivastava, P.; Khuwaja, G.; Islam, F.; Siddiqui, M.S.; et al. Attenuation of Aβ-induced neurotoxicity by thymoquinone via inhibition of mitochondrial dysfunction and oxidative stress. Mol. Cell. Biochem. 2012, 369, 55–65. [Google Scholar] [CrossRef]
- Das, S.S.; Kannan, R.; George, S.; Chakrapani, B.P.S.; Maliakel, B.; Ittiyavirah, S.; Krishnakumar, I.M. Thymoquinone-rich black cumin oil improves sleep quality, alleviates anxiety/stress on healthy subjects with sleep disturbances—A pilot polysomnography study. J. Herb. Med. 2022, 32, 100507. [Google Scholar] [CrossRef]
- Roohbakhsh, A.; Moshiri, M.; Salehi Kakhki, A.; Iranshahy, M.; Amin, F.; Etemad, L. Thymoquinone abrogates methamphetamine-induced striatal neurotoxicity and hyperlocomotor activity in mice. Res. Pharm. Sci. 2021, 16, 391–399. [Google Scholar] [CrossRef]
- Alhmied, F.; Alammar, A.; Alsultan, B.; Alshehri, M.; Pottoo, F.H. Molecular Mechanisms of Thymoquinone as Anticancer Agent. Comb. Chem. High Throughput Screen. 2021, 24, 1644–1653. [Google Scholar] [CrossRef] [PubMed]
- Bargi, R.; Hosseini, M.; Asgharzadeh, F.; Khazaei, M.; Shafei, M.N.; Beheshti, F. Protection against blood-brain barrier permeability as a possible mechanism for protective effects of thymoquinone against sickness behaviors induced by lipopolysaccharide in rats. Jundishapur J. Nat. Pharm. Prod. 2021, 16, e67765. [Google Scholar] [CrossRef]
- Alshyarba, M.; Otifi, H.; Al Fayi, M.; ADera, A.; Rajagopalan, P. Thymoquinone inhibits IL-7-induced tumor progression and metastatic invasion in prostate cancer cells by attenuating matrix metalloproteinase activity and Akt/NF-κB signaling. Biotechnol. Appl. Biochem. 2021, 68, 1403–1411. [Google Scholar] [CrossRef]
- Farkhondeh, T.; Samarghandian, S.; Shahri, A.M.P.; Samini, F. The Neuroprotective Effects of Thymoquinone: A Review. Dose-Response 2018, 16, 155932581876145. [Google Scholar] [CrossRef]
- Talebi, M.; Talebi, M.; Farkhondeh, T.; Samarghandian, S. Biological and therapeutic activities of thymoquinone: Focus on the Nrf2 signaling pathway. Phytother. Res. 2021, 35, 1739–1753. [Google Scholar] [CrossRef] [PubMed]
- Junaid Md Akter, Y.; Afrose, S.S.; Tania, M.; Khan, M.d.A. Biological Role of AKT and Regulation of AKT Signaling Pathway by Thymoquinone: Perspectives in Cancer Therapeutics. Mini Rev. Med. Chem. 2021, 21, 288–301. [Google Scholar] [CrossRef] [PubMed]
- Bargi, R.; Asgharzadeh, F.; Beheshti, F.; Hosseini, M.; Sadeghnia, H.R.; Khazaei, M. The effects of thymoquinone on hippocampal cytokine level, brain oxidative stress status and memory deficits induced by lipopolysaccharide in rats. Cytokine 2017, 96, 173–184. [Google Scholar] [CrossRef]
- Yusuf, M.; Khan, M.; Alrobaian, M.M.; Alghamdi, S.A.; Warsi, M.H.; Sultana, S.; Khan, R.A. Brain targeted Polysorbate-80 coated PLGA thymoquinone nanoparticles for the treatment of Alzheimer’s disease, with biomechanistic insights. J. Drug Deliv. Sci. Technol. 2021, 61, 102214. [Google Scholar] [CrossRef]
- Yu, T.-W.; Lane, H.-Y.; Lin, C.-H. Novel Therapeutic Approaches for Alzheimer’s Disease: An Updated Review. Int. J. Mol. Sci. 2021, 22, 8208. [Google Scholar] [CrossRef] [PubMed]
- Ismail, N.; Ismail, M.; Latiff, L.A.; Mazlan, M.; Mariod, A.A. Black Cumin Seed (Nigella Sativa Linn.) Oil and Its Fractions Protect Against Beta Amyloid Peptide-Induced Toxicity in Primary Cerebellar Granule Neurons: Neuroprotective Effect of N. Sativa Oil. J. Food Lipids 2008, 15, 519–533. [Google Scholar] [CrossRef]
- Nampoothiri, N.; Sundararajan, V.; Pallavi, D.; Venkatasubbu, G.D.; Mohideen, S.S. Thymoquinone as a potential therapeutic for Alzheimer’s disease in transgenic Drosophila melanogaster model. BIOCELL 2021, 45, 1251–1262. [Google Scholar] [CrossRef]
- Ozbolat, G.; Alizade, A. Investigation of the protective effect of thymoquinone of U87 cells induced by beta-amyloid. Bratisl. Lek. Listy 2021, 122, 748–752. [Google Scholar] [CrossRef]
- El-Marasy, S.A.; El-Shenawy, S.M.; El-Khatib, A.S.; El-Shabrawy, O.A.; Kenawy, S.A. Effect of Nigella sativa and wheat germ oils on scopolamine-induced memory impairment in rats. Bull. Fac. Pharm. Cairo Univ. 2012, 50, 81–88. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Drew, J.; Berney, W.; Lei, W. Neuroprotective Natural Products for Alzheimer’s Disease. Cells 2021, 10, 1309. [Google Scholar] [CrossRef]
- Samad, N.; Manzoor, N.; Muneer, Z.; Bhatti, S.A.; Imran, I. Reserpine-induced altered neuro-behavioral, biochemical and histopathological assessments prevent by enhanced antioxidant defence system of thymoquinone in mice. Metab. Brain Dis. 2021, 36, 2535–2552. [Google Scholar] [CrossRef] [PubMed]
- Cascella, M.; Bimonte, S.; Barbieri, A.; Del Vecchio, V.; Muzio, M.R.; Vitale, A.; Benincasa, G.; Ferriello, A.B.; Azzariti, A.; Arra, C.; et al. Dissecting the Potential Roles of Nigella sativa and Its Constituent Thymoquinone on the Prevention and on the Progression of Alzheimer’s Disease. Front. Aging Neurosci. 2018, 10, 16. [Google Scholar] [CrossRef] [PubMed]
- Ganguly, U.; Kaur, U.; Chakrabarti, S.S.; Sharma, P.; Agrawal, B.K.; Saso, L.; Chakrabarti, S. Oxidative Stress, Neuroinflammation, and NADPH Oxidase: Implications in the Pathogenesis and Treatment of Alzheimer’s Disease. Oxid. Med. Cell. Longev. 2021, 2021, 7086512. [Google Scholar] [CrossRef] [PubMed]
- Fouad, I.A.; Sharaf, N.M.; Abdelghany, R.M.; El Sayed, N.S.E.D. Neuromodulatory Effect of Thymoquinone in Attenuating Glutamate-Mediated Neurotoxicity Targeting the Amyloidogenic and Apoptotic Pathways. Front. Neurol. 2018, 9, 236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ali, M.d.Y.; Akter, Z.; Mei, Z.; Zheng, M.; Tania, M.; Khan, M.d.A. Thymoquinone in autoimmune diseases: Therapeutic potential and molecular mechanisms. Biomed. Pharmacother. 2021, 134, 111157. [Google Scholar] [CrossRef]
- Cobourne-Duval, M.K.; Taka, E.; Mendonca, P.; Soliman, K.F.A. Thymoquinone increases the expression of neuroprotective proteins while decreasing the expression of pro-inflammatory cytokines and the gene expression NFκB pathway signaling targets in LPS/IFNγ -activated BV-2 microglia cells. J. Neuroimmunol. 2018, 320, 87–97. [Google Scholar] [CrossRef]
- Alhebshi, A.H.; Gotoh, M.; Suzuki, I. Thymoquinone protects cultured rat primary neurons against amyloid β-induced neurotoxicity. Biochem. Biophys. Res. Commun. 2013, 433, 362–367. [Google Scholar] [CrossRef]
- Poorgholam, P.; Yaghmaei, P.; Hajebrahimi, Z. Thymoquinone recovers learning function in a rat model of Alzheimer’s disease. Avicenna J. Phytomed. 2018, 8, 188–197. [Google Scholar]
- Fiasal Zaher, M.; Abdelfattah Bendary, M.; Saeed Abd El-Aziz, G.; Shaker Ali, A. Potential Protective Role of Thymoquinone on Experimentally-induced Alzheimer Rats. JPRI J. Pharm. Res. Int. 2019, 31, 1–18. [Google Scholar] [CrossRef]
- Elibol, B.; Beker, M.; Terzioglu-Usak, S.; Dalli, T.; Kilic, U. Thymoquinone administration ameliorates Alzheimer’s disease-like phenotype by promoting cell survival in the hippocampus of amyloid beta1–42 infused rat model. Phytomedicine 2020, 79, 153324. [Google Scholar] [CrossRef]
- Song, J.-X.; Sze, S.C.-W.; Ng, T.-B.; Lee, C.K.-F.; Leung, G.P.H.; Shaw, P.-C.; Tong, Y.; Zhang, Y.B. Anti-Parkinsonian drug discovery from herbal medicines: What have we got from neurotoxic models? J. Ethnopharmacol. 2012, 139, 698–711. [Google Scholar] [CrossRef] [PubMed]
- Uddin, M.N.; Hoq, M.I.; Jahan, I.; Siddiqui, S.A.; Clinton, C.D.; Ibrahim, M.; Islam, M.S.; Jakaria, M. The Mechanistic Role of Thymoquinone in Parkinson’s Disease: Focus on Neuroprotection in Pre-Clinical Studies. Curr. Mol. Pharmacol. 2021, 14, 1083–1092. [Google Scholar] [CrossRef] [PubMed]
- Jahromy, M.H.; Jalili, M.; Mohajer, A.J.; Poor, F.K.; Dara, S.M. Effects of Nigella sativa Seed Extract on Perphenzine-Induced Muscle Rigidity in Male Mice. WJNS 2014, 04, 313–318. [Google Scholar] [CrossRef] [Green Version]
- Khalilullah, H. Anti-epileptic Action of Thymoquinone. In Molecular and Therapeutic Actions of Thymoquinone; Younus, H., Ed.; Springer: Singapore, 2018; pp. 75–80. Available online: http://link.springer.com/10.1007/978-981-10-8800-1_7 (accessed on 13 March 2022).
- Iranshahy, M.; Javadi, B.; Sahebkar, A. Protective effects of functional foods against Parkinson’s disease: A narrative review on pharmacology, phytochemistry, and molecular mechanisms. Phytother. Res. 2022. [Google Scholar] [CrossRef] [PubMed]
- Radad, K.; Moldzio, R.; Taha, M.; Rausch, W.-D. Thymoquinone protects dopaminergic neurons against MPP + and rotenone. Phytother. Res. 2009, 23, 696–700. [Google Scholar] [CrossRef] [PubMed]
- Butt, M.S.; Imran, M.; Imran, A.; Arshad, M.S.; Saeed, F.; Gondal, T.A.; Shariati, M.A.; Gilani, S.A.; Tufail, T.; Ahmad, I.; et al. Therapeutic perspective of thymoquinone: A mechanistic treatise. Food Sci. Nutr. 2021, 9, 1792–1809. [Google Scholar] [CrossRef] [PubMed]
- Al Mamun, A.; Matsuzaki, K.; Islam, R.; Hossain, S.; Hossain, M.E.; Katakura, M.; Arai, H.; Shido, O.; Hashimoto, M. Chronic Administration of Thymoquinone Enhances Adult Hippocampal Neurogenesis and Improves Memory in Rats Via Regulating the BDNF Signaling Pathway. Neurochem. Res. 2022, 47, 933–951. [Google Scholar] [CrossRef]
- Liang, J.; Lian, L.; Wang, X.; Li, L. Thymoquinone, extract from Nigella sativa seeds, protects human skin keratinocytes against UVA-irradiated oxidative stress, inflammation and mitochondrial dysfunction. Mol. Immunol. 2021, 135, 21–27. [Google Scholar] [CrossRef]
- Alrafiah, A. Thymoquinone Protects Neurons in the Cerebellum of Rats through Mitigating Oxidative Stress and Inflammation Following High-Fat Diet Supplementation. Biomolecules 2021, 11, 165. [Google Scholar] [CrossRef]
- Radad, K.S.; Al-Shraim, M.M.; Moustafa, M.F.; Rausch, W.-D. Neuroprotective role of thymoquinone against 1-methyl-4-phenylpyridinium-induced dopaminergic cell death in primary mesencephalic cell culture. Neurosciences 2015, 20, 10–16. [Google Scholar]
- Sedaghat, R.; Roghani, M.; Khalili, M. Neuroprotective effect of thymoquinone, the nigella sativa bioactive compound, in 6-hydroxydopamine-induced hemi-parkinsonian rat model. Iran. J. Pharm. Res. 2014, 13, 227–234. [Google Scholar] [PubMed]
- Ebrahimi, S.S.; Oryan, S.; Izadpanah, E.; Hassanzadeh, K. Thymoquinone exerts neuroprotective effect in animal model of Parkinson’s disease. Toxicol. Lett. 2017, 276, 108–114. [Google Scholar] [CrossRef] [PubMed]
- Sandhua, K.S.; Ranab, A.C. Evaluation of Anti Parkinson’s Activity of Nigella Sativa (Kalonji) Seeds in Chlorpromazine Induced Experimental Animal Model. Int. J. Pharm. Pharm. Sci. 2013, 5, 884–888. [Google Scholar]
- Malik, T.; Hasan, S.; Pervez, S.; Fatima, T.; Haleem, D.J. Nigella sativa Oil Reduces Extrapyramidal Symptoms (EPS)-Like Behavior in Haloperidol-Treated Rats. Neurochem. Res. 2016, 41, 3386–3398. [Google Scholar] [CrossRef] [PubMed]
- Beyazcicek, E.; Ankarali, S.; Beyazcicek, O.; Ankarali, H.; Demir, S.; Ozmerdivenli, R. Effects of thymoquinone, the major constituent of Nigella sativa seeds, on penicillin-induced epileptiform activity in rats. NSJ 2016, 21, 131–137. [Google Scholar] [CrossRef] [Green Version]
- Pottoo, F.H.; Salahuddin, M.; Khan, F.A.; Alomar, F.; Al Dhamen, M.A.; Alhashim, A.F.; Alqattan, H.H.; Gomaa, M.S.; Alomary, M.N. Thymoquinone Potentiates the Effect of Phenytoin against Electroshock-Induced Convulsions in Rats by Reducing the Hyperactivation of m-TOR Pathway and Neuroinflammation: Evidence from In Vivo, In Vitro and Computational Studies. Pharmaceuticals 2021, 14, 1132. [Google Scholar] [CrossRef]
- Ayaz, M.; Sadiq, A.; Junaid, M.; Ullah, F.; Subhan, F.; Ahmed, J. Neuroprotective and Anti-Aging Potentials of Essential Oils from Aromatic and Medicinal Plants. Front. Aging Neurosci. 2017, 9, 168. [Google Scholar] [CrossRef] [Green Version]
- Seghatoleslam, M.; Alipour, F.; Shafieian, R.; Hassanzadeh, Z.; Edalatmanesh, M.A.; Sadeghnia, H.R.; Hosseini, M. The effects of Nigella sativa on neural damage after pentylenetetrazole induced seizures in rats. J. Tradit. Complement. Med. 2016, 6, 262–268. [Google Scholar] [CrossRef] [Green Version]
- Hajipour, S.; Farbood, Y.; Dianat, M.; Rashno, M.; Khorsandi, L.S.; Sarkaki, A. Thymoquinone improves cognitive and hippocampal long-term potentiation deficits due to hepatic encephalopathy in rats. Iran. J. Basic Med. Sci. 2021, 24, 881–891. [Google Scholar]
- Akgül, B.; Aycan, İ.Ö.; Hidişoğlu, E.; Afşar, E.; Yıldırım, S.; Tanrıöver, G.; Coşkunfırat, N.; Sanlı, S.; Aslan, M. Alleviation of prilocaine-induced epileptiform activity and cardiotoxicity by thymoquinone. DARU J. Pharm. Sci. 2021, 29, 85–99. [Google Scholar] [CrossRef]
- Ustunova, S.; Kilic, A.; Bulut, H.; Gurel-Gurevin, E.; Eris, A.H.; Meral, I. Impaired memory by hippocampal oxidative stress in rats exposed to 900 MHz electromagnetic fields is ameliorated by thymoquinone. Toxicol. Environ. Chem. 2022. preprint. [Google Scholar] [CrossRef]
- Meral, I.; Esrefoglu, M.; Dar, K.; Ustunova, S.; Aydin, M.; Demirtas, M.; Arifoglu, Y. Effects of Nigella sativa on apoptosis and GABA A receptor density in cerebral cortical and hippocampal neurons in pentylenetetrazol induced kindling in rats. Biotech. Histochem. 2016, 91, 493–500. [Google Scholar] [CrossRef]
- Abdollahzade Fard, A.; Saboory, E.; Tahmazi, Y.; Rasmi, Y.; Dindarian, S.; Parsamanesh, N. Effect of orally-administrated thymoquinone during pregnancy on litter size, pentylenetetrazol-induced seizure, and body weight in rat offspring. Iran. J. Basic Med. Sci. 2021, 24, 30–37. [Google Scholar] [PubMed]
- Oskouei, Z.; Mehri, S.; Kalalinia, F.; Hosseinzadeh, H. Evaluation of the effect of thymoquinone in d-galactose-induced memory impairments in rats: Role of MAPK, oxidative stress, and neuroinflammation pathways and telomere length. Phytother. Res. 2021, 35, 2252–2266. [Google Scholar] [CrossRef] [PubMed]
- Sahak, M.K.A.; Kabir, N.; Abbas, G.; Draman, S.; Hashim, N.H.; Hasan Adli, D.S. The Role of Nigella sativa and Its Active Constituents in Learning and Memory. Evid. Based Complement. Altern. Med. 2016, 2016, 6075679. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khader, M.; Bresgen, N.; Eckl, P.M. In vitro toxicological properties of thymoquinone. Food Chem. Toxicol. 2009, 47, 129–133. [Google Scholar] [CrossRef]
- Badary, O.A.; Al-Shabanah, O.A.; Nagi, M.N.; Al-Bekairi, A.M.; Elmazar, M.M.A. Acute and subchronic toxicity of thymoquinone in mice. Drug Dev. Res. 1998, 44, 56–61. [Google Scholar] [CrossRef]
- Ali, B.H.; Blunden, G. Pharmacological and toxicological properties of Nigella sativa. Phytother. Res. 2003, 17, 299–305. [Google Scholar] [CrossRef]
| Disease | Animal Model | Treatment | Tissue Sample | Result | References |
|---|---|---|---|---|---|
| AD | 48 male albino rats | Lipopolysaccharide with a dose of 0.8 mg/kg was given as an injection into the peritoneum for one dose. Group III was treated by a TQ 10 mg/kg injection into the peritoneum. Group IV was treated by PNU-120596 1 mg/kg injection into the peritoneum. | frontal lobe | More effective using TQ or α7 nAChR agonist and PAM. | [1] |
| AD | Male rats | D-gal dose of (60 mg/kg day) and AlCl3 dose of (10 mg/kg day) administered through the peritoneum (i.p.) once daily for 42 days, and after 4 weeks, TQ was administered intragastrically (i.g.) as a dose of (20 mg/kg/day) once daily for 14 days. | whole brain | Increased potential protective effect of TQ. | [2] |
| AD | Twelve-week-old male Wistar rats | Group (1) is the Control group received (saline). group (2) received LPS (1 mg/kg i.p.), groups (3–5) received 2, 5, or 10 mg/kg TQ treatment. | hippocampal and cortical tissues | Improved the impairment of learning and memory. | [33] |
| AD | Amyloid beta- (Aβ-) induced neurotoxicity | The intervention group received Aβ1–42 and TQ as a treatment simultaneously for 72 h. | hippocampal and cortical neurons | Efficient attenuation of Aβ1–42-induced neurotoxicity | [47] |
| AD | Adult female rats injected by STZ (3 mg/kg) | TQ dose of 20 mg/kg/day was given to rats for 15 days; on the 15th day, STZ injection was given. | hippocampus | Noticeable decrease in STZ-induced neurodegeneration. | [48] |
| AD | Thirty adult male Sprague Dawley albino rats | (Control group, Group 2 is people with AD): induced by oral AlCl3 (17 mg/kg/day) for 4 weeks. Group 3 (TQ/AD): treated with oral TQ (10 mg/kg/day) and AlCl3 (17 mg/kg/day) for period of 4 weeks. | hippocampus | Protective effects against neurodegeneration. | [49] |
| AD | Adult female rats injected with aggregated Aβ1–42 | TQ dosage of (10 mg/kg) was given. The other group received a TQ dose of 20 mg/kg) for 15 days. | hippocampal tissue | Reduced neurotoxicity by removing Aβ plaques and restoring neuron viability. | [50] |
| Disease | Animal Model | Treatment | Tissue Sample | Result | References |
|---|---|---|---|---|---|
| PD | PD mouse model. | TQ (10 mg/kg was given for 1 week before administration of MPTP (25 mg/kg). | Striatal region | Inhibition effect against α-synuclein aggregation and cellular death. | [3] |
| PD | Primary dopaminergic cell culture neurons. | dopaminergic neurons tissue was received TQ (0.01, 0.1, 1, and 10 μM) on day 6 i.v. for 6 days. | NA | Protective effects against MPP+ and rotenone. | [56] |
| PD | Embryonic mouse mesencephala at gestation day 14. | Four groups: group 1 control group, group 2 received TQ on the 8th day for 4 days, group 3: received 1-methyl-4-phenylpyridinium (MPP+) on the 10th for 48 h, group 4:co-treated with TQ and MPP+. | NA | Protective effects on the dopaminergic neurons and inhibition of their apoptosis. | [61] |
| PD | 6-hydroxydopamine (6-OHDA)-lesioned rats. | Oral TQ at different doses of 5 and/or 10 mg/kg administered 3 times daily for 1 week. | Substantia nigra pars compacta and midbrain | Protective effect against 6-OHDA neurotoxicity. | [62] |
| PD | Male Wistar rats (8–10 months) received rotenone. | TQ (7.5 and 15 mg/kg/day, po) given as pretreatment for one hour before administration of rotenone injection. | Substantia nigra (SN) and striatum (ST) | Protection and antioxidant effects against rotenone. | [63] |
| PD | Adult Wistar rats of either sex, CPZ dosing for 21 days to induce Parkinson’s. | Extracts of Nigella sativa at 200 and 400 mg/kg doses were given orally. | Whole-brain | Increased anti-Parkinson’s activity | [64] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pottoo, F.H.; Ibrahim, A.M.; Alammar, A.; Alsinan, R.; Aleid, M.; Alshehhi, A.; Alshehri, M.; Mishra, S.; Alhajri, N. Thymoquinone: Review of Its Potential in the Treatment of Neurological Diseases. Pharmaceuticals 2022, 15, 408. https://doi.org/10.3390/ph15040408
Pottoo FH, Ibrahim AM, Alammar A, Alsinan R, Aleid M, Alshehhi A, Alshehri M, Mishra S, Alhajri N. Thymoquinone: Review of Its Potential in the Treatment of Neurological Diseases. Pharmaceuticals. 2022; 15(4):408. https://doi.org/10.3390/ph15040408
Chicago/Turabian StylePottoo, Faheem Hyder, Abdallah Mohammad Ibrahim, Ali Alammar, Rida Alsinan, Mahdi Aleid, Ali Alshehhi, Muruj Alshehri, Supriya Mishra, and Noora Alhajri. 2022. "Thymoquinone: Review of Its Potential in the Treatment of Neurological Diseases" Pharmaceuticals 15, no. 4: 408. https://doi.org/10.3390/ph15040408
APA StylePottoo, F. H., Ibrahim, A. M., Alammar, A., Alsinan, R., Aleid, M., Alshehhi, A., Alshehri, M., Mishra, S., & Alhajri, N. (2022). Thymoquinone: Review of Its Potential in the Treatment of Neurological Diseases. Pharmaceuticals, 15(4), 408. https://doi.org/10.3390/ph15040408






