Virological and Immunological Outcomes of an Intensified Four-Drug versus a Standard Three-Drug Antiretroviral Regimen, Both Integrase Strand Transfer Inhibitor-Based, in Primary HIV Infection
Abstract
1. Introduction
2. Results
2.1. Baseline Characteristics and Follow-Up
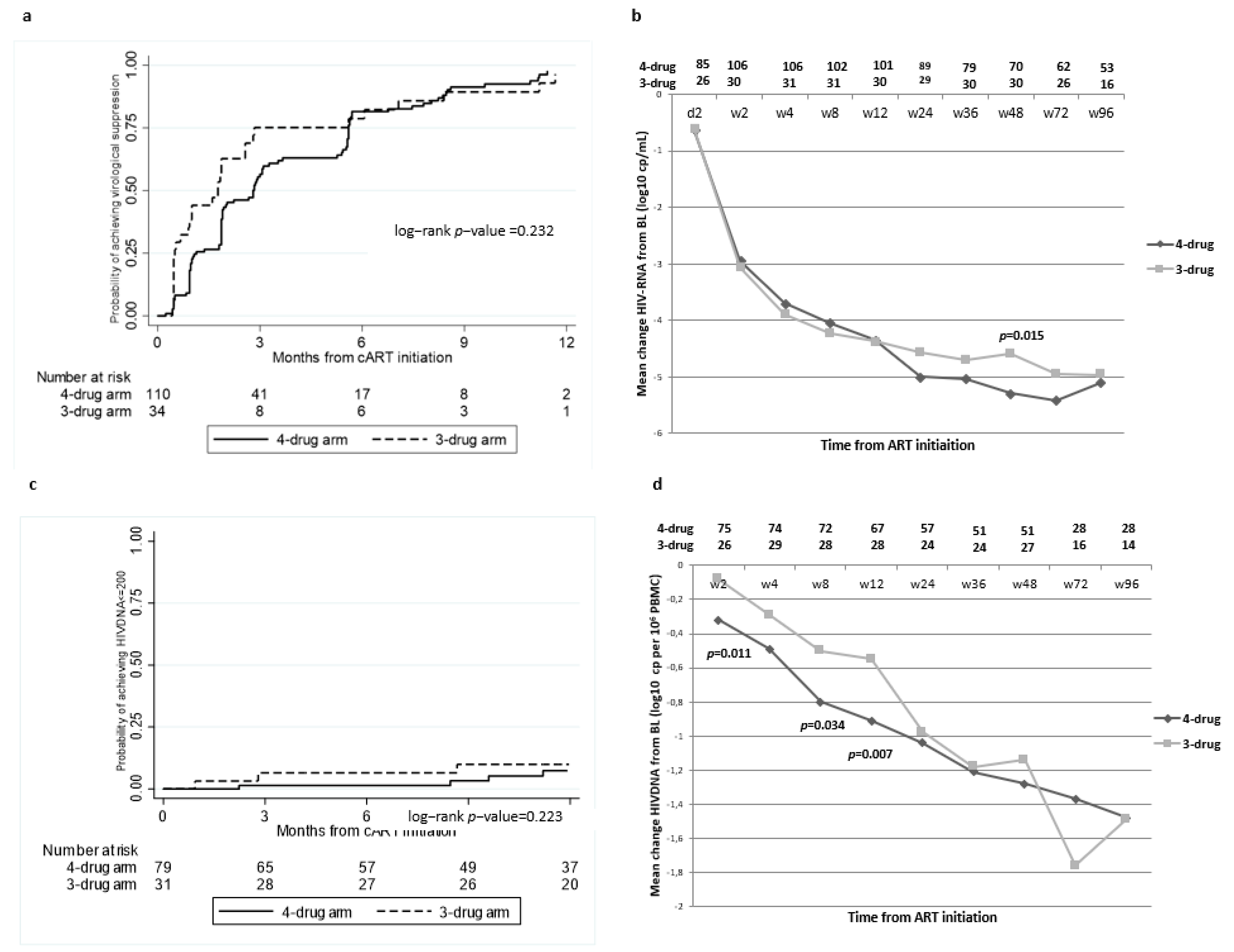
2.2. Virological Outcomes
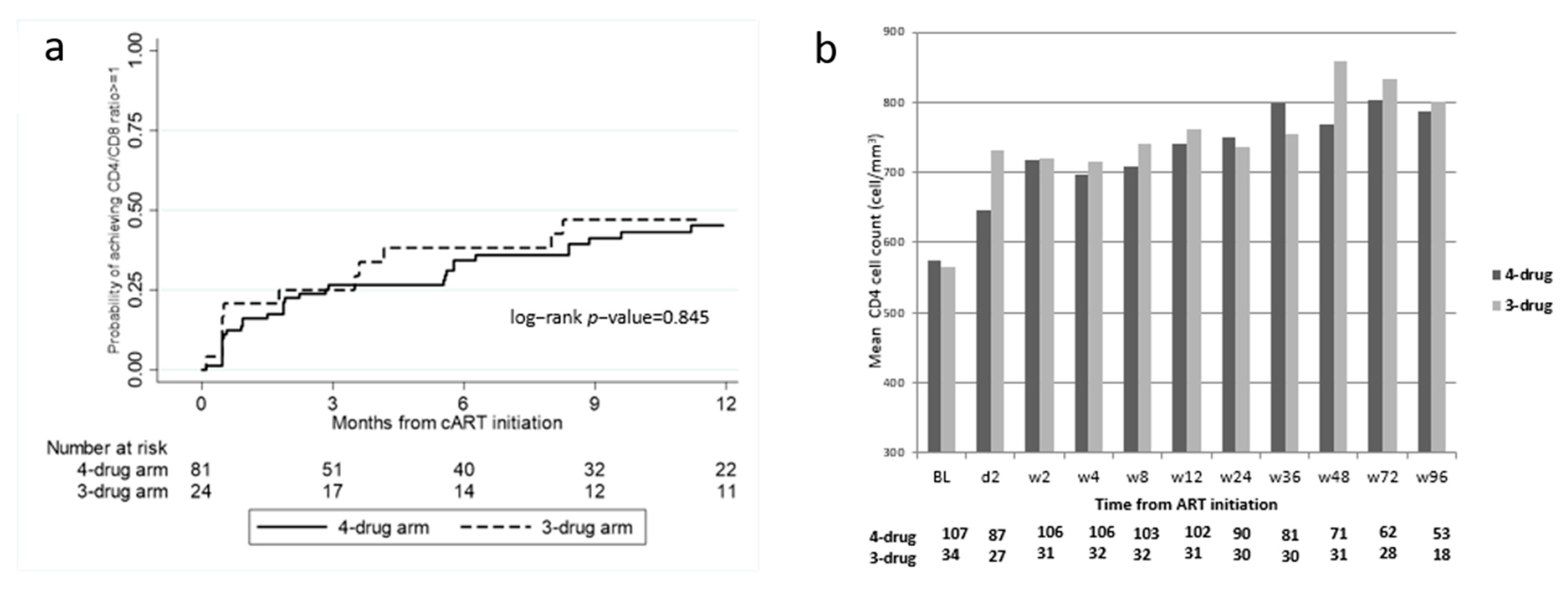
2.3. Immunological Outcomes
2.4. Adherence
3. Discussion
4. Materials and Methods
4.1. Study Population
4.2. Virological Assessment
4.3. Antiretroviral Treatments and Timing of the Evaluations
4.4. Outcomes
4.5. Definitions
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the Use of Antiretroviral Agents in HIV-1-Infected Adults and Adolescents. Department of Health and Human Services. 2020. Available online: https://clinicalinfo.hiv.gov/sites/default/files/guidelines/documents/AdultandAdolescentGL.pdf (accessed on 8 October 2020).
- European AIDS Clinical Society (EACS). Guidelines, Version 10.1, October 2020. Available online: https://www.eacsociety.org/files/guidelines-10.1.finalsept2020.pdf (accessed on 8 October 2020).
- Le, T.; Wright, E.J.; Smith, D.M.; He, W.; Catano, G.; Okulicz, J.F.; Young, J.A.; Clark, R.A.; Richman, D.D.; Little, S.J.; et al. Enhanced CD4+ T-cell recovery with earlier HIV-1 antiretroviral therapy. N. Engl. J. Med. 2013, 368, 218–230. [Google Scholar] [CrossRef] [PubMed]
- Davy-Mendez, T.; Napravnik, S.; Davy-Mendez, T.; Napravnik, S.; Zakharova, O.; Kuruc, J.; Gay, C.; Hicks, C.B.; Mcgee, K.S.; Eron, J.J. Acute HIV Infection and CD4/CD8 Ratio Normalization After Antiretroviral Therapy Initiation. J. Acquir. Immune Defic. Syndr. 2018, 79, 510–518. [Google Scholar] [CrossRef] [PubMed]
- Laanani, M.; Ghosn, J.; Essat, A.; Melard, A.; Seng, R.; Gousset, M.; Panjo, H.; Mortier, E.; Girard, P.M.; Goujard, C.; et al. Impact of the Timing of Initiation of Antiretroviral Therapy During Primary HIV-1 Infection on the Decay of Cell-Associated HIV-DNA. Clin. Infect. Dis. 2015, 60, 1715–1721. [Google Scholar] [CrossRef] [PubMed]
- Jain, V.; Hartogensis, W.; Bacchetti, P.; Hunt, P.W.; Hatano, H.; Sinclair, E.; Epling, L.; Lee, T.H.; Busch, M.P.; McCune, J.M.; et al. Antiretroviral therapy initiated within 6 months of HIV infection is associated with lower T-cell activation and smaller HIV reservoir size. J. Infect. Dis. 2013, 208, 1202–1211. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.Q.; Reddy, K.; Einkauf, K.B.; Gounder, K.; Chevalier, J.M.; Dong, K.L.; Walker, B.D.; Yu, X.G.; Ndung’u, T.; Lichter-feld, M. cHIV-1 DNA sequence diversity and evolution during acute subtype C infection. Nat. Commun. 2019, 10, 2737. [Google Scholar] [CrossRef] [PubMed]
- Cohen, M.S.; Chen, Y.Q.; McCauley, M.; Gamble, T.; Hosseinipour, M.C.; Kumarasamy, N.; Hakim, J.G.; Kumwenda, J.; Grinsztejn, B.; Pilotto, J.H.; et al. Prevention of HIV-1 infection with early antiretroviral therapy. N. Engl. J. Med. 2011, 365, 493–505. [Google Scholar] [CrossRef]
- Muscatello, A.; Nozza, S.; Fabbiani, M.; De Benedetto, I.; Ripa, M.; Dell’acqua, R.; Antinori, A.; Pinnetti, C.; Calcagno, A.; Ferrara, M.; et al. Enhanced Immunological Recovery With Early Start of Antiretroviral Therapy During Acute or Early HIV Infection-Results of Italian Network of ACuTe HIV InfectiON. (INACTION) Retrospective Study. Pathog. Immun. 2020, 5, 8–33. [Google Scholar] [CrossRef] [PubMed]
- Lama, J.R.; Ignacio, R.A.B.; Alfaro, R.; Rios, J.; Cartagena, J.G.; Valdez, R.; Bain, C.; Barbarán, K.S.; Villaran, M.V.; Pilcher, C.D.; et al. Clinical and Immunologic Outcomes after Immediate or Deferred Antiretroviral Therapy Initiation during Primary HIV Infection: The Sabes Randomized Clinical Study. Clin. Infect. Dis. 2021, 72, 1042–1050. [Google Scholar] [CrossRef] [PubMed]
- Martin, T.C.S.; Abrams, M.; Anderson, C.; Little, S.J. Rapid Antiretroviral Therapy Among Individuals with Acute and Early HIV. Clin. Infect. Dis. 2021, 73, 130–133. [Google Scholar] [CrossRef] [PubMed]
- Coffey, S.; Bacchetti, P.; Sachdev, D.; Bacon, O.; Jones, D.; Ospina-Norvell, C.; Torres, S.; Lynch, E.; Camp, C.; Mer-cer-Slomoff, R.; et al. RAPID antiretroviral therapy: High virologic suppression rates with immediate antiretroviral therapy initiation in a vulnerable urban clinic population. AIDS 2019, 33, 825–832. [Google Scholar] [CrossRef] [PubMed]
- Wada, N.I.; Jacobson, L.P.; Margolick, J.B.; Breen, E.C.; Macatangay, B.; Penugonda, S.; Martínez-Maza, O.; Bream, J.H. The effect of HAART-induced HIV suppression on circulating markers of inflammation and immune activation. AIDS 2015, 29, 463–471. [Google Scholar] [CrossRef]
- Hunt, P.W.; Lee, S.A.; Siedner, M.J. Immunologic biomarkers, morbidity, and mortality in treated HIV infection. J. Infect. Dis. 2016, 214 (Suppl. 2), S44–S50. [Google Scholar] [CrossRef]
- Sereti, I.; Krebs, S.J.; Phanuphak, N.; Fletcher, J.L.; Slike, B.; Pinyakorn, S.; O’Connell, R.J.; Rupert, A.; Chomont, N.; Valcour, V.; et al. Persistent, Albeit Reduced, Chronic Inflammation in Persons Starting Antiretroviral Therapy in Acute HIV Infection. Clin. Infect. Dis. 2017, 64, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Markowitz, M.; Evering, T.H.; Garmon, D.; Caskey, M.; La Mar, M.; Rodriguez, K.; Sahi, V.; Palmer, S.; Prada, N.; Mohri, H. A randomized open-label study of 3- versus 5-drug combination antiretroviral therapy in newly HIV-1-infected individuals. J. Acquir. Immune Defic. Syndr. 2014, 66, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Chéret, A.; Nembot, G.; Mélard, A.; Lascoux, C.; Slama, L.; Miailhes, P.; Yeni, P.; Abel, S.; Avettand-Fenoel, V.; Venet, A. Intensive five-drug antiretroviral therapy regimen versus standard triple-drug therapy during primary HIV-1 infection (OPTIPRIM-ANRS 147): A randomised, open-label, phase 3 trial. Lancet Infect. Dis. 2015, 15, 387–396. [Google Scholar] [CrossRef]
- Ananworanich, J.; Chomont, N.; Fletcher, J.L.; Pinyakorn, S.; Schuetz, A.; Sereti, I.; Rerknimitr, R.; Dewar, R.; Kroon, E.; Vandergeeten, C.; et al. Markers of HIV reservoir size and immune activation after treatment in acute HIV infection with and without raltegravir and maraviroc intensification. J. Virus Erad. 2015, 1, 116–122. [Google Scholar]
- Veil, R.; Poizot-Martin, I.; Reynes, J.; Goujard, C.; Seng, R.; Delobel, P.; Cotte, L.; Duvivier, C.; Rey, D.; Tran, L.; et al. Virological and immunological impact of integrase inhibitor-based regimens initiated during primary HIV-1 infection. AIDS 2020, 34, 493–500. [Google Scholar] [CrossRef] [PubMed]
- Girometti, N.; Lander, F.; McOwan, A.; Nwokolo, N.; Boffito, M.; Whitlock, G.; Dean Street Collaborative group. Rapid ART start in early HIV infection: Time to viral load suppression and retention in care in a London cohort. HIV Med. 2020, 21, 613–615. [Google Scholar] [CrossRef] [PubMed]
- Trezza, C.R.; Kashuba, A.D.M. Pharmacokinetics of Antiretrovirals in Genital Secretions and Anatomic Sites of HIV Transmission: Implications for HIV Prevention. Clin. Pharmacokinet. 2014, 53, 611–624. [Google Scholar] [CrossRef] [PubMed]
- Collier, A.C.; Chun, T.W.; Maenza, J.; Coombs, R.W.; Tapia, K.; Chang, M.; Stevens, C.E.; Justement, J.S.; Murray, D.; Stekler, J.D.; et al. A Pilot Study of Raltegravir Plus Combination Antiretroviral Therapy in Early Human Immunodeficiency Virus Infection: Challenges and Lessons Learned. Biores. Open Access 2016, 5, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Nozza, S.; Poli, A.; Ripa, M.; Galli, L.; Chiappetta, S.; Spagnuolo, V.; Rovelli, C.; Lazzarin, A.; Castagna, A.; Tambussi, G. Efficacy of elvitegravir/cobicistat/emtricitabine/tenofovir disoproxil fumarate as treatment for primary or recent HIV infection. J. Antimicrob. Chemother. 2017, 72, 632–633. [Google Scholar] [CrossRef]
- Ngo Bell, E.C.; Vandenhende, M.A.; Caldato, S.; Saunier, A.; Bellecave, P.; Tumiotto, C.; Avettand-Fenoel, V.; Hessamfar, M.; Morlat, P.; Bonnet, F. High decay of blood HIV reservoir when tenofovir/emtricitabine/elvitegravir/cobicistat is initiated during the acute primary HIV infection. J. Antimicrob. Chemother. 2017, 72, 2681–2683. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Puertas, M.C.; Massanella, M.; Llibre, J.M.; Ballestero, M.; Buzon, M.J.; Ouchi, D.; Esteve, A.; Boix, J.; Manzardo, C.; Miró, J.M.; et al. Intensification of a raltegravir-based regimen with maraviroc in early HIV-1 infection. AIDS 2014, 28, 325–334. [Google Scholar] [CrossRef] [PubMed]
- Taramasso, L.; Fabbiani, M.; Nozza, S.; De Benedetto, I.; Bruzzesi, E.; Mastrangelo, A.; Pinnetti, C.; Calcagno, A.; Ferrara, M.; Bozzi, G.; et al. Predictors of incomplete viral response and virologic failure in patients with acute and early HIV infection. Results of Italian Network of ACuTe HIV InfectiON. (INACTION) cohort. HIV Med. 2020, 21, 523–535. [Google Scholar] [CrossRef] [PubMed]
- Murray, J.M.; Emery, S.; Kelleher, A.D.; Law, M.; Chen, J.; Hazuda, D.J.; Nguyen, B.T.; Teppler, H.; Cooper, D.A. Antiretroviral therapy with the integrase inhibitor raltegravir alters decay kinetics of HIV, significantly reducing the second phase. AIDS 2007, 12, 2315–2321. [Google Scholar] [CrossRef] [PubMed]
- Ambrosioni, J.; Mosquera, M.M.; Miró, J.M. Comment on: Efficacy of elvitegravir/cobicistat/emtricitabine/tenofovir disoproxil fumarate as treatment for primary or recent HIV infection. J. Antimicrob. Chemother. 2017, 72, 1548–1549. [Google Scholar] [CrossRef]
- Ambrosioni, J.; Nicolás, D.; Manzardo, C.; Agüero, F.; Blanco, J.L.; Mosquera, M.M.; Peñafiel, J.; Gatell, J.M.; Marcos, M.A.; Miró, J.M. Integrase strand-transfer inhibitor polymorphic and accessory resistance substitutions in patients with acute/recent HIV infection. J. Antimicrob. Chemother. 2017, 72, 205–209. [Google Scholar] [CrossRef] [PubMed]
- Fabrizio, C.; Lepore, L.; Lagioia, A.; Punzi, G.; Saracino, A.; Angarano, G.; Monno, L. Comment on: Integrase strand-transfer inhibitor polymorphic and accessory resistance substitutions in patients with acute/recent HIV infection. J. Antimicrob. Chemother. 2017, 72, 1546–1547. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Parisi, S.G.; Andreis, S.; Mengoli, C.; Scaggiante, R.; Ferretto, R.; Manfrin, V.; Cruciani, M.; Giobbia, M.; Boldrin, C.; Basso, M.; et al. Baseline cellular HIV DNA load predicts HIV DNA decline and residual HIV plasma levels during effective antiretroviral therapy. J. Clin. Microbiol. 2012, 50, 258–263. [Google Scholar] [CrossRef] [PubMed]
- Hocqueloux, L.; Avettand-Fènoël, V.; Jacquot, S.; Prazuck, T.; Legac, E.; élard, A.; Niang, M.; Mille, C.; Le Moal, G.; Viard, J.P.; et al. Long-term antiretroviral therapy initiated during primary HIV-1 infection is key to achieving both low HIV reservoirs and normal T cell counts. J. Antimicrob. Chemother. 2013, 68, 1169–1178. [Google Scholar] [CrossRef]
- Thornhill, J.; Inshaw, J.; Kaleebu, P.; Cooper, D.; Ramjee, G.; Schechter, M.; Tambussi, G.; Fox, J.; Samuel, M.; Miro, J.M.; et al. Enhanced Normalization of CD4/CD8 Ratio With Earlier Antiretroviral Therapy at Primary HIV Infection. J. Acquir. Immune Defic. Syndr. 2016, 73, 69–73. [Google Scholar] [CrossRef] [PubMed]
- Mussini, C.; Lorenzini, P.; Cozzi-Lepri, A.; Lapadula, G.; Marchetti, G.; Nicastri, E.; Cingolani, A.; Lichtner, M.; Antinori, A.; Gori, A.; et al. CD4/CD8 ratio normalisation and non-AIDS-related events in individuals with HIV who achieve viral load suppression with antiretroviral therapy: An observational cohort study. Lancet HIV 2015, 2, e98–e106. [Google Scholar] [CrossRef]
- Fiebig, E.W.; Wright, D.J.; Rawal, B.D.; Garrett, P.E.; Schumacher, R.T.; Peddada, L.; Heldebrant, C.; Smith, R.; Conrad, A.; Kleinman, S.H.; et al. Dynamics of HIV viremia and antibody seroconversion in plasma donors: Implications for diagnosis and staging of primary HIV infection. AIDS 2003, 17, 1871–1879. [Google Scholar] [CrossRef] [PubMed]
- Suligoi, B.; Massi, M.; Galli, C.; Sciandra, M.; Di Sora, F.; Pezzotti, P.; Recchia, O.; Montella, F.; Sinicco, A.; Rezza, G. Identifying recent HIV infections using the avidity index and an automated enzyme immunoassay. J. Acquir. Immune Defic. Syndr. 2003, 32, 424–428. [Google Scholar] [CrossRef]
- Rozera, G.; Fabbri, G.; Lorenzini, P.; Mastrorosa, I.; Timelli, L.; Zaccarelli, M.; Amendola, A.; Vergori, A.; Plazzi, M.M.; Cicalini, S.; et al. Peripheral blood HIV-1 DNA dynamics in antiretroviral-treated HIV/HCV co-infected patients receiving directly-acting antivirals. PLoS ONE 2017, 12, e0187095. [Google Scholar]
- Piketty, C.; Weiss, L.; Assoumou, L.; Burgard, M.; Mélard, A.; Ragnaud, J.M.; Bentata, M.; Girard, P.M.; Rouzioux, C.; Costa-gliola, D. A high HIV DNA level in PBMCs at antiretroviral treatment interruption predicts a shorter time to treatment resumption, independently of the CD4 nadir. J. Med. Virol. 2010, 82, 1819–1828. [Google Scholar] [CrossRef] [PubMed]
- Chéret, A.; Bacchus-Souffan, C.; Avettand-Fenoël, V.; Mélard, A.; Nembot, G.; Blanc, C.; Samri, A.; Sáez-Cirión, A.; Hocqueloux, L.; Lascoux-Combe, C.; et al. Combined ART started during acute HIV infection protects central memory CD4+ T cells and can induce remission. J. Antimicrob. Chemother. 2015, 70, 2108–2120. [Google Scholar] [CrossRef] [PubMed]
- Bennett, D.E.; Camacho, R.J.; Otelea, D.; Kuritzkes, D.R.; Fleury, H.; Kiuchi, M.; Heneine, W.; Kantor, R.; Jordan, M.R.; Schapiro, J.M.; et al. Drug resistance mutations for surveillance of transmitted HIV-1 drug-resistance: 2009 update. PLoS ONE 2009, 4, e47242. [Google Scholar] [CrossRef]
- Stanford HIV Database Algorithm, Version 8.9. Available online: https://hivdb.stanford.edu/hivdb/by-mutations/ (accessed on 8 October 2020).
| 4-Drug ARM (TDF/FTC + DRV/b + RAL) n = 110 | 3-Drug ARM (TDF/FTC + DTG) n = 34 | p | Total Population n = 144 | |
|---|---|---|---|---|
| Male gender, n (%) | 107 (97.2) | 31 (91.2) | 0.120 | 138 (95.8) |
| Age years, median (IQR) | 34 (26–45) | 35 (28–39) | 0.832 | 34 (27–43) |
| Mode of HIV transmission, n (%) | 0.065 | |||
| Homosexual contact | 95 (86.4) | 23 (67.7) | 118 (81.9) | |
| Heterosexual contact | 13 (11.8) | 10 (29.4) | 23 (16.0) | |
| IDU | 1 (0.9) | 1 (2.9) | 2 (1.4) | |
| Other/Unknown | 1 (0.9) | - | 1 (0.7) | |
| Non-Italian born, n (%) | 11 (10.0) | 6 (17.7) | 0.235 | 17 (11.8) |
| Days from HIV diagnosis to ART, | ||||
| median (IQR) | 5 (2–7) | 6 (4–17) | 0.021 | 5 (3–9) |
| n (%) | ||||
| ≤7 | 83 (75.5) | 19 (55.9) | 0.050 | 102 (70.8) |
| 8–14 | 15 (13.6) | 6 (17.6) | 21 (14.6) | |
| ≥15 | 12 (10.9) | 9 (26.5) | 21 (14.6) | |
| BL CD4 count, cells/μL, median (IQR) | 557 (379–686) | 564 (383–729) | 0.946 | 557 (383–697) |
| BL CD4 count, cells/μL, n (%) | 0.516 | |||
| ≤500 cell/μL | 41 (37.3) | 16 (47.1) | 57 (39.6) | |
| >500 cell/μL | 66 (60.0) | 18 (52.9) | 84 (58.3) | |
| Missing | 3 (2.7) | - | 3 (2.1) | |
| BL CD4/CD8 ratio ≥ 1, n (%) | 25 (23.6) | 10 (29.4) | 0.495 | 35 (25.0) |
| BL HIV-RNA, log10 cp/mL, median (IQR) | 5.7 (5.0–6.5) | 5.5 (4.4–6.6) | 0.503 | 5.6 (4.8–6.6) |
| BL HIV-RNA, n (%) | ||||
| HIVRNA ≤ 500.000 cp/mL | 54 (49.1) | 14 (41.2) | 0.524 | 68 (47.2) |
| HIVRNA > 500.000 cp/mL | 55 (50.0) | 19 (55.9) | 74 (51.4) | |
| Missing | 1 (0.9) | 1 (2.9) | 2 (1.4) | |
| BL HIV-DNA, log10 cp/106PBMC, median (IQR) | 4.6 (3.8–4.9) | 4.1 (3.8–4.7) | 0.228 | 4.4 (3.8–4.8) |
| BL Fiebig stage, n (%) | 0.172 | |||
| II/III | 17 (15.4) | 5 (14.7) | 22 (15.3) | |
| IV | 35 (31.8) | 10 (29.4) | 45 (31.2) | |
| V | 35 (31.8) | 6 (17.7) | 41 (28.5) | |
| VI | 20 (18.2) | 13 (38.2) | 33 (22.9) | |
| missing | 3 (2.7) | - | 3 (2.1) | |
| Boosted PI in the regimen, n (%) | - | |||
| DRV/r | 72 (65.5) | - | 72 (50.0) | |
| DRV/c | 38 (34.5) | - | 38 (26.4) | |
| Observation time in months, median (IQR) | 19 (8–35) | 23 (17–29) | 0.839 | 21 (8.4–34) |
| Univariate | Multivariate | |||
|---|---|---|---|---|
| HR (95% CI) | p-Value | aRH (95% CI) | p-Value | |
| Age | ||||
| per 10 years older | 0.91 (0.77–1.07) | 0.260 | ||
| Mode of HIV transmission | ||||
| Homosexual | 1.00 | 1.00 | ||
| Heterosexual | 1.49 (0.94–2.36) | 0.093 | 2.14 (1.06–4.29) | 0.033 |
| IDU | 1.71 (0.23–12.45) | 0.597 | - | - |
| Baseline CD4 count, cells/mm3 | ||||
| >500 vs. ≤500 | 2.08 (1.43–3.02) | <0.001 | 1.97 (1.18–3.29) | 0.009 |
| Baseline CD4/CD8 ratio | ||||
| ≥1 vs. <1 | 1.76 (1.18–2.64) | 0.006 | 1.45 (0.72–2.92) | 0.299 |
| Baseline HIV-RNA | ||||
| per 1 log higher | 0.47 (0.38–0.57) | <0.001 | 0.68 (0.51–0.90) | 0.007 |
| Baseline HIV-DNA | ||||
| per 1 log higher | 0.49 (0.35–0.68) | <0.001 | 0.56 (0.34–0.93) | 0.026 |
| Baseline Fiebig stage | ||||
| II/III | 1.00 | |||
| IV | 0.82 (0.47–1.41) | 0.465 | ||
| V | 0.82 (0.48–1.42) | 0.487 | ||
| VI | 1.27 (0.72–2.23) | 0.411 | ||
| ART regimen | ||||
| 3-drug vs. 4-drug arm | 1.27 (0.85–1.91) | 0.244 | 1.34 (0.76–2.37) | 0.318 |
| Adherence | ||||
| VAS < 100 (time-updated) | 0.87 (0.52–1.45) | 0.597 | 1.32 (0.71–2.46) | 0.377 |
| Univariate | Multivariate | |||
|---|---|---|---|---|
| HR (95% CI) | p-Value | aRH (95% CI) | p-Value | |
| Baseline CD4 count, cells/mm3 | ||||
| >500 vs. ≤500 | 1.46 (0.38–5.64) | 0.584 | ||
| Baseline CD4/CD8 ratio | ||||
| ≥1 vs. <1 | 1.46 (0.38–5.65) | 0.582 | ||
| Baseline HIV-RNA | ||||
| per 1 log higher | 0.44 (0.26–0.74) | 0.002 | 0.62 (0.30–1.31) | 0.210 |
| Baseline HIV-DNA | ||||
| per 1 log higher | 0.20 (0.08–0.54) | 0.001 | 0.30 (0.09–1.01) | 0.052 |
| Baseline Fiebig stage | ||||
| II/III | 1.00 | 1.00 | ||
| IV | 0.11 (0.01–1.26) | 0.076 | 0.09 (0.01–1.09) | 0.058 |
| V | - | - | - | - |
| VI | 1.21 (0.25–5.81) | 0.814 | 0.23 (0.02–2.18) | 0.201 |
| ART regimen | ||||
| 3-drug vs. 4-drug arm | 2.56 (0.74–8.84) | 0.137 | 1.92 (0.51–7.17) | 0.333 |
| Univariate | Multivariate | |||
|---|---|---|---|---|
| HR (95% CI) | p-Value | aRH (95% CI) | p-Value | |
| Days from HIV diagnosis to ART start | ||||
| ≤7 | 1.00 | - | 1.00 | - |
| 8–14 | 1.51 (0.66–3.48) | 0.328 | 1.19 (0.46–3.04) | 0.718 |
| ≥15 | 1.94 (1.00–3.74) | 0.049 | 1.42 (0.68–2.98) | 0.349 |
| Baseline CD4 count, cells/mm3 | ||||
| >500 vs. ≤500 | 2.48 (1.34–4.58) | 0.004 | 1.73 (0.89–3.38) | 0.108 |
| Baseline CD4/CD8 ratio (by quartiles) | ||||
| <0.44 (Q1) | 1.00 | - | 1.00 | - |
| 0.45–0.77 (Q1–Q2) | 2.02 (0.94–4.33) | 0.007 | 2.08 (0.88–4.94) | 0.095 |
| 0.78–0.99 (Q2–Q3) | 2.70 (1.29–5.68) | 0.009 | 2.96 (1.29–6.79) | 0.011 |
| Baseline HIV-RNA | ||||
| per 1 log higher | 0.83 (0.64–1.08) | 0.164 | 1.06 (0.75–1.49) | 0.750 |
| Baseline HIV-DNA | ||||
| per 1 log higher | 0.65 (0.42–1.01) | 0.053 | 0.74 (0.45–1.21) | 0.233 |
| ART regimen | ||||
| 3-drug vs. 4-drug arm | 1.06 (0.55–2.04) | 0.856 | 0.82 (0.41–1.64) | 0.571 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mondi, A.; Pinnetti, C.; Lorenzini, P.; Plazzi, M.M.; Abbate, I.; Camici, M.; Agrati, C.; Grilli, E.; Gili, F.; Esvan, R.; et al. Virological and Immunological Outcomes of an Intensified Four-Drug versus a Standard Three-Drug Antiretroviral Regimen, Both Integrase Strand Transfer Inhibitor-Based, in Primary HIV Infection. Pharmaceuticals 2022, 15, 403. https://doi.org/10.3390/ph15040403
Mondi A, Pinnetti C, Lorenzini P, Plazzi MM, Abbate I, Camici M, Agrati C, Grilli E, Gili F, Esvan R, et al. Virological and Immunological Outcomes of an Intensified Four-Drug versus a Standard Three-Drug Antiretroviral Regimen, Both Integrase Strand Transfer Inhibitor-Based, in Primary HIV Infection. Pharmaceuticals. 2022; 15(4):403. https://doi.org/10.3390/ph15040403
Chicago/Turabian StyleMondi, Annalisa, Carmela Pinnetti, Patrizia Lorenzini, Maria Maddalena Plazzi, Isabella Abbate, Marta Camici, Chiara Agrati, Elisabetta Grilli, Francesca Gili, Rozenn Esvan, and et al. 2022. "Virological and Immunological Outcomes of an Intensified Four-Drug versus a Standard Three-Drug Antiretroviral Regimen, Both Integrase Strand Transfer Inhibitor-Based, in Primary HIV Infection" Pharmaceuticals 15, no. 4: 403. https://doi.org/10.3390/ph15040403
APA StyleMondi, A., Pinnetti, C., Lorenzini, P., Plazzi, M. M., Abbate, I., Camici, M., Agrati, C., Grilli, E., Gili, F., Esvan, R., Orchi, N., Rozera, G., Amendola, A., Forbici, F., Gori, C., Gagliardini, R., Bellagamba, R., Ammassari, A., Cicalini, S., ... Antinori, A. (2022). Virological and Immunological Outcomes of an Intensified Four-Drug versus a Standard Three-Drug Antiretroviral Regimen, Both Integrase Strand Transfer Inhibitor-Based, in Primary HIV Infection. Pharmaceuticals, 15(4), 403. https://doi.org/10.3390/ph15040403







