Targeted Drug Delivery to the Central Nervous System Using Extracellular Vesicles
Abstract
:1. Introduction
2. BBB Structure, Drug Transportation, and Administration
3. Use of Various Artificial/Synthetical Nanoparticles for Targeted Drug Delivery to CNS and Their Limitations
4. EV Category & Benefits of EVs in Drug Delivery System and Drug Loading Methods
4.1. Endogenous Drug Loading
4.2. Exogenous Drug Loading
5. Clinical Trials Evaluating Peripherally Targeted Extracellular Vesicle-Based Therapeutics
6. Drug Delivery to the CNS Cells Using EVs: Promising Drug Delivery Vehicles to the CNS Cells
7. Approaches to Engineer the EVs to Achieve Targeted Delivery to the CNS
8. Challenges in Targeting EVs Research, Clinical Trials, and Commercial Launch
9. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pardridge, W.M. Alzheimer’s disease drug development and the problem of the blood-brain barrier. Alzheimers Dement. 2009, 5, 427–432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bertrand, L.; Nair, M.; Toborek, M. Solving the Blood-Brain Barrier Challenge for the Effective Treatment of HIV Replication in the Central Nervous System. Curr. Pharm. Des. 2016, 22, 5477–5486. [Google Scholar] [CrossRef]
- Davis, T.P.; Sanchez-Covarubias, L.; Tome, M.E. P-glycoprotein trafficking as a therapeutic target to optimize CNS drug delivery. Adv. Pharmacol. 2014, 71, 25–44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ronaldson, P.T.; Davis, T.P. Targeting blood-brain barrier changes during inflammatory pain: An opportunity for optimizing CNS drug delivery. Ther. Deliv. 2011, 2, 1015–1041. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saeedi, M.; Eslamifar, M.; Khezri, K.; Dizaj, S.M. Applications of nanotechnology in drug delivery to the central nervous system. Biomed. Pharmacother. 2019, 111, 666–675. [Google Scholar] [CrossRef] [PubMed]
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; Rodriguez-Torres, M.D.P.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S.; et al. Nano based drug delivery systems: Recent developments and future prospects. J. Nanobiotechnol. 2018, 16, 71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gong, Y.; Chowdhury, P.; Nagesh, P.K.B.; Cory, T.J.; Dezfuli, C.; Kodidela, S.; Singh, A.; Yallapu, M.M.; Kumar, S. Nanotechnology approaches for delivery of cytochrome P450 substrates in HIV treatment. Expert Opin. Drug Deliv. 2019, 16, 869–882. [Google Scholar] [CrossRef] [PubMed]
- Belfiore, L.; Saunders, D.N.; Ranson, M.; Thurecht, K.J.; Storm, G.; Vine, K.L. Towards clinical translation of ligand-functionalized liposomes in targeted cancer therapy: Challenges and opportunities. J. Control. Release 2018, 277, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bulbake, U.; Doppalapudi, S.; Kommineni, N.; Khan, W. Liposomal Formulations in Clinical Use: An Updated Review. Pharmaceutics 2017, 9, 12. [Google Scholar] [CrossRef] [PubMed]
- Deshpande, P.P.; Biswas, S.; Torchilin, V.P. Current trends in the use of liposomes for tumor targeting. Nanomedicine 2013, 8, 1509–1528. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Castro Bravo, K.M.; Liu, J. Targeted liposomal drug delivery: A nanoscience and biophysical perspective. Nanoscale Horiz. 2021, 6, 78–94. [Google Scholar] [CrossRef]
- Zolnik, B.S.; González-Fernández, A.; Sadrieh, N.; Dobrovolskaia, M.A. Nanoparticles and the immune system. Endocrinology 2010, 151, 458–465. [Google Scholar] [CrossRef] [PubMed]
- Ishida, T.; Ichihara, M.; Wang, X.; Yamamoto, K.; Kimura, J.; Majima, E.; Kiwada, H. Injection of PEGylated liposomes in rats elicits PEG-specific IgM, which is responsible for rapid elimination of a second dose of PEGylated liposomes. J. Control. Release 2006, 112, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Ishida, T.; Wang, X.; Shimizu, T.; Nawata, K.; Kiwada, H. PEGylated liposomes elicit an anti-PEG IgM response in a T cell-independent manner. J. Control. Release 2007, 122, 349–355. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Ishida, T.; Kiwada, H. Anti-PEG IgM elicited by injection of liposomes is involved in the enhanced blood clearance of a subsequent dose of PEGylated liposomes. J. Control. Release 2007, 119, 236–244. [Google Scholar] [CrossRef] [PubMed]
- Win-Shwe, T.T.; Fujimaki, H. Nanoparticles and neurotoxicity. Int. J. Mol. Sci. 2011, 12, 6267–6280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, Y.L.; Gao, J.Q. Potential neurotoxicity of nanoparticles. Int. J. Pharm. 2010, 394, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Teleanu, D.M.; Chircov, C.; Grumezescu, A.M.; Teleanu, R.I. Neurotoxicity of Nanomaterials: An Up-to-Date Overview. Nanomaterials 2019, 9, 96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bunggulawa, E.J.; Wang, W.; Yin, T.; Wang, N.; Durkan, C.; Wang, Y.; Wang, G. Recent advancements in the use of exosomes as drug delivery systems. J. Nanobiotechnol. 2018, 16, 81. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giannini, A.J.; Sorger, L.G.; Martin, D.M.; Bates, L. Impaired reception of nonverbal cues in women with premenstrual tension syndrome. J. Psychol. 1988, 122, 591–596. [Google Scholar] [CrossRef]
- Elsharkasy, O.M.; Nordin, J.Z.; Hagey, D.W.; de Jong, O.G.; Schiffelers, R.M.; Andaloussi, S.E.; Vader, P. Extracellular vesicles as drug delivery systems: Why and how? Adv. Drug Deliv. Rev. 2020, 159, 332–343. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.-S. The potential of exosomes as theragnostics in various clinical situations. Exosomes 2020, 467–486. [Google Scholar] [CrossRef]
- Yuan, D.; Zhao, Y.; Banks, W.A.; Bullock, K.M.; Haney, M.; Batrakova, E.; Kabanov, A.V. Macrophage exosomes as natural nanocarriers for protein delivery to inflamed brain. Biomaterials 2017, 142, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Haney, M.J.; Klyachko, N.L.; Zhao, Y.; Gupta, R.; Plotnikova, E.G.; He, Z.; Patel, T.; Piroyan, A.; Sokolsky, M.; Kabanov, A.V.; et al. Exosomes as drug delivery vehicles for Parkinson’s disease therapy. J. Control. Release 2015, 207, 18–30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klyachko, N.L.; Polak, R.; Haney, M.J.; Zhao, Y.; Gomes Neto, R.J.; Hill, M.C.; Kabanov, A.V.; Cohen, R.E.; Rubner, M.F.; Batrakova, E.V. Macrophages with cellular backpacks for targeted drug delivery to the brain. Biomaterials 2017, 140, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Richardson, J.J.; Ejima, H. Surface Engineering of Extracellular Vesicles through Chemical and Biological Strategies. Chem. Mater. 2019, 31, 2191–2201. [Google Scholar] [CrossRef]
- Ramasubramanian, L.; Kumar, P.; Wang, A. Engineering Extracellular Vesicles as Nanotherapeutics for Regenerative Medicine. Biomolecules 2019, 10, 48. [Google Scholar] [CrossRef] [Green Version]
- Salunkhe, S.; Dheeraj; Basak, M.; Chitkara, D.; Mittal, A. Surface functionalization of exosomes for target-specific delivery and in vivo imaging & tracking: Strategies and significance. J. Control. Release 2020, 326, 599–614. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Erviti, L.; Seow, Y.; Yin, H.; Betts, C.; Lakhal, S.; Wood, M.J.A. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat. Biotechnol. 2011, 29, 341–345. [Google Scholar] [CrossRef] [PubMed]
- Shahjin, F.; Chand, S.; Yelamanchili, S.V. Extracellular Vesicles as Drug Delivery Vehicles to the Central Nervous System. J. Neuroimmune Pharmacol. 2020, 15, 443–458. [Google Scholar] [CrossRef] [PubMed]
- Abbott, N.J.; Patabendige, A.A.; Dolman, D.E.; Yusof, S.R.; Begley, D.J. Structure and function of the blood-brain barrier. Neurobiol. Dis. 2010, 37, 13–25. [Google Scholar] [CrossRef] [PubMed]
- Banks, W.A. From blood–brain barrier to blood–brain interface: New opportunities for CNS drug delivery. Nature Reviews Drug Discovery 2016, 15, 275–292. [Google Scholar] [CrossRef] [PubMed]
- Dong, X. Current Strategies for Brain Drug Delivery. Theranostics 2018, 8, 1481–1493. [Google Scholar] [CrossRef] [PubMed]
- Pardridge, W.M. Drug transport across the blood-brain barrier. J. Cereb Blood Flow Metab 2012, 32, 1959–1972. [Google Scholar] [CrossRef] [PubMed]
- Kadry, H.; Noorani, B.; Cucullo, L. A blood-brain barrier overview on structure, function, impairment, and biomarkers of integrity. Fluids Barriers CNS 2020, 17, 69. [Google Scholar] [CrossRef] [PubMed]
- Daneman, R.; Prat, A. The blood-brain barrier. Cold Spring Harb. Perspect Biol. 2015, 7, a020412. [Google Scholar] [CrossRef] [Green Version]
- Banks, W.A.; Sharma, P.; Bullock, K.M.; Hansen, K.M.; Ludwig, N.; Whiteside, T.L. Transport of Extracellular Vesicles across the Blood-Brain Barrier: Brain Pharmacokinetics and Effects of Inflammation. Int. J. Mol. Sci. 2020, 21, 4407. [Google Scholar] [CrossRef] [PubMed]
- Jain, K.K. An Overview of Drug Delivery Systems; Springer: New York, NY, USA, 2020; pp. 1–54. [Google Scholar] [CrossRef]
- Gupta, S.; Kesarla, R.; Chotai, N.; Misra, A.; Omri, A. Systematic Approach for the Formulation and Optimization of Solid Lipid Nanoparticles of Efavirenz by High Pressure Homogenization Using Design of Experiments for Brain Targeting and Enhanced Bioavailability. BioMed Res. Int. 2017, 2017, 5985014. [Google Scholar] [CrossRef] [PubMed]
- Rip, J.; Chen, L.; Hartman, R.; van den Heuvel, A.; Reijerkerk, A.; van Kregten, J.; van der Boom, B.; Appeldoorn, C.; de Boer, M.; Maussang, D.; et al. Glutathione PEGylated liposomes: Pharmacokinetics and delivery of cargo across the blood-brain barrier in rats. J. Drug Target. 2014, 22, 460–467. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gray, S.J.; Woodard, K.T.; Samulski, R.J. Viral vectors and delivery strategies for CNS gene therapy. Ther. Deliv. 2010, 1, 517–534. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov. 2021, 20, 101–124. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.W.; Hsu, F.F.; Qiu, J.T.; Chern, G.J.; Lee, Y.A.; Chang, C.C.; Huang, Y.T.; Sung, Y.C.; Chiang, C.C.; Huang, R.L.; et al. Highly efficient and tumor-selective nanoparticles for dual-targeted immunogene therapy against cancer. Sci. Adv. 2020, 6, eaax5032. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krishnaswamy, K.; Orsat, V. Chapter 2—Sustainable Delivery Systems Through Green Nanotechnology. In Nano- and Microscale Drug Delivery Systems; Grumezescu, A.M., Ed.; Elsevier: Amsterdam, The Netherlands, 2017. [Google Scholar] [CrossRef]
- Xu, C.; Nam, J.; Hong, H.; Xu, Y.; Moon, J.J. Positron Emission Tomography-Guided Photodynamic Therapy with Biodegradable Mesoporous Silica Nanoparticles for Personalized Cancer Immunotherapy. ACS Nano 2019, 13, 12148–12161. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Meng, S.; Ding, J.; Peng, Q.; Yu, Y. Transition metal-coordinated graphitic carbon nitride dots as a sensitive and facile fluorescent probe for β-amyloid peptide detection. Analyst 2019, 144, 504–511. [Google Scholar] [CrossRef] [PubMed]
- Wagner, A.M.; Knipe, J.M.; Orive, G.; Peppas, N.A. Quantum dots in biomedical applications. Acta Biomater. 2019, 94, 44–63. [Google Scholar] [CrossRef] [PubMed]
- Povsic, T.J.; Lawrence, M.G.; Lincoff, A.M.; Mehran, R.; Rusconi, C.P.; Zelenkofske, S.L.; Huang, Z.; Sailstad, J.; Armstrong, P.W.; Steg, P.G.; et al. Pre-existing anti-PEG antibodies are associated with severe immediate allergic reactions to pegnivacogin, a PEGylated aptamer. J. Allergy Clin. Immunol. 2016, 138, 1712–1715. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schwartz, S., Jr. Unmet needs in developing nanoparticles for precision medicine. Nanomedicine 2017, 12, 271–274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- von Roemeling, C.; Jiang, W.; Chan, C.K.; Weissman, I.L.; Kim, B.Y. Breaking down the barriers to precision cancer nanomedicine. Trends Biotechnol. 2017, 35, 159–171. [Google Scholar] [CrossRef]
- Saraiva, C.; Praça, C.; Ferreira, R.; Santos, T.; Ferreira, L.; Bernardino, L. Nanoparticle-mediated brain drug delivery: Overcoming blood–brain barrier to treat neurodegenerative diseases. J. Control. Release 2016, 235, 34–47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lower, E.E.; Drosick, D.R.; Blau, R.; Brennan, L.; Danneman, W.; Hawley, D.K. Increased rate of brain metastasis with trastuzumab therapy not associated with impaired survival. Clin. Breast Cancer 2003, 4, 114–119. [Google Scholar] [CrossRef]
- Yang, Q.; Lai, S.K. Anti-PEG immunity: Emergence, characteristics, and unaddressed questions. WIREs Nanomed. Nanobiotechnol. 2015, 7, 655–677. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Q.; Jacobs, T.M.; McCallen, J.D.; Moore, D.T.; Huckaby, J.T.; Edelstein, J.N.; Lai, S.K. Analysis of Pre-existing IgG and IgM Antibodies against Polyethylene Glycol (PEG) in the General Population. Anal. Chem. 2016, 88, 11804–11812. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.-M.; Su, Y.-C.; Chang, C.-J.; Burnouf, P.-A.; Chuang, K.-H.; Chen, C.-H.; Cheng, T.-L.; Chen, Y.-T.; Wu, J.-Y.; Roffler, S.R. Measurement of Pre-Existing IgG and IgM Antibodies against Polyethylene Glycol in Healthy Individuals. Anal. Chem. 2016, 88, 10661–10666. [Google Scholar] [CrossRef] [PubMed]
- Wilhelm, S.; Tavares, A.J.; Dai, Q.; Ohta, S.; Audet, J.; Dvorak, H.F.; Chan, W.C.W. Analysis of nanoparticle delivery to tumours. Nat. Rev. Mater. 2016, 1, 16014. [Google Scholar] [CrossRef]
- Goodridge, H.S.; Underhill, D.M.; Touret, N. Mechanisms of Fc receptor and dectin-1 activation for phagocytosis. Traffic 2012, 13, 1062–1071. [Google Scholar] [CrossRef] [PubMed]
- Wolfram, J.; Zhu, M.; Yang, Y.; Shen, J.; Gentile, E.; Paolino, D.; Fresta, M.; Nie, G.; Chen, C.; Shen, H.; et al. Safety of Nanoparticles in Medicine. Curr. Drug Targets 2015, 16, 1671–1681. [Google Scholar] [CrossRef] [Green Version]
- Johnstone, R.M.; Adam, M.; Hammond, J.R.; Orr, L.; Turbide, C. Vesicle formation during reticulocyte maturation. Association of plasma membrane activities with released vesicles (exosomes). J. Biol. Chem. 1987, 262, 9412–9420. [Google Scholar] [CrossRef]
- Al-Nedawi, K.; Meehan, B.; Micallef, J.; Lhotak, V.; May, L.; Guha, A.; Rak, J. Intercellular transfer of the oncogenic receptor EGFRvIII by microvesicles derived from tumour cells. Nat. Cell Biol. 2008, 10, 619–624. [Google Scholar] [CrossRef] [PubMed]
- Théry, C.; Boussac, M.; Véron, P.; Ricciardi-Castagnoli, P.; Raposo, G.; Garin, J.; Amigorena, S. Proteomic analysis of dendritic cell-derived exosomes: A secreted subcellular compartment distinct from apoptotic vesicles. J. Immunol. 2001, 166, 7309–7318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Géminard, C.; De Gassart, A.; Blanc, L.; Vidal, M. Degradation of AP2 during reticulocyte maturation enhances binding of hsc70 and Alix to a common site on TFR for sorting into exosomes. Traffic 2004, 5, 181–193. [Google Scholar] [CrossRef]
- Thakur, B.K.; Zhang, H.; Becker, A.; Matei, I.; Huang, Y.; Costa-Silva, B.; Zheng, Y.; Hoshino, A.; Brazier, H.; Xiang, J.; et al. Double-stranded DNA in exosomes: A novel biomarker in cancer detection. Cell Res. 2014, 24, 766–769. [Google Scholar] [CrossRef] [Green Version]
- Kahlert, C.; Melo, S.A.; Protopopov, A.; Tang, J.; Seth, S.; Koch, M.; Zhang, J.; Weitz, J.; Chin, L.; Futreal, A.; et al. Identification of double-stranded genomic DNA spanning all chromosomes with mutated KRAS and p53 DNA in the serum exosomes of patients with pancreatic cancer. J. Biol. Chem. 2014, 289, 3869–3875. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valadi, H.; Ekström, K.; Bossios, A.; Sjöstrand, M.; Lee, J.J.; Lötvall, J.O. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat. Cell Biol. 2007, 9, 654–659. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doyle, L.M.; Wang, M.Z. Overview of Extracellular Vesicles, Their Origin, Composition, Purpose, and Methods for Exosome Isolation and Analysis. Cells 2019, 8, 727. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Liu, Y.; Liu, H.; Tang, W.H. Exosomes: Biogenesis, biologic function and clinical potential. Cell Biosci. 2019, 9, 19. [Google Scholar] [CrossRef]
- Wiklander, O.P.B.; Brennan, M.Á.; Lötvall, J.; Breakefield, X.O.; El Andaloussi, S. Advances in therapeutic applications of extracellular vesicles. Sci. Transl. Med. 2019, 11, eaav8521. [Google Scholar] [CrossRef] [PubMed]
- Yáñez-Mó, M.; Siljander, P.R.M.; Andreu, Z.; Zavec, A.B.; Borràs, F.E.; Buzas, E.I.; Buzas, K.; Casal, E.; Cappello, F.; Carvalho, J.; et al. Biological properties of extracellular vesicles and their physiological functions. J. Extracell Vesicles 2015, 4, 27066. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Becker, A.; Thakur, B.K.; Weiss, J.M.; Kim, H.S.; Peinado, H.; Lyden, D. Extracellular Vesicles in Cancer: Cell-to-Cell Mediators of Metastasis. Cancer Cell 2016, 30, 836–848. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murphy, D.E.; de Jong, O.G.; Brouwer, M.; Wood, M.J.; Lavieu, G.; Schiffelers, R.M.; Vader, P. Extracellular vesicle-based therapeutics: Natural versus engineered targeting and trafficking. Exp. Mol. Med. 2019, 51, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Rankin-Turner, S.; Vader, P.; O’Driscoll, L.; Giebel, B.; Heaney, L.M.; Davies, O.G. A call for the standardised reporting of factors affecting the exogenous loading of extracellular vesicles with therapeutic cargos. Adv. Drug Deliv. Rev. 2021, 173, 479–491. [Google Scholar] [CrossRef] [PubMed]
- Luan, X.; Sansanaphongpricha, K.; Myers, I.; Chen, H.; Yuan, H.; Sun, D. Engineering exosomes as refined biological nanoplatforms for drug delivery. Acta Pharmacol. Sin. 2017, 38, 754–763. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herrmann, I.K.; Wood, M.J.A.; Fuhrmann, G. Extracellular vesicles as a next-generation drug delivery platform. Nat. Nanotechnol. 2021, 16, 748–759. [Google Scholar] [CrossRef] [PubMed]
- Walker, S.; Busatto, S.; Pham, A.; Tian, M.; Suh, A.; Carson, K.; Quintero, A.; Lafrence, M.; Malik, H.; Santana, M.X.; et al. Extracellular vesicle-based drug delivery systems for cancer treatment. Theranostics 2019, 9, 8001–8017. [Google Scholar] [CrossRef] [PubMed]
- De Jong, O.G.; Van Balkom, B.W.; Schiffelers, R.M.; Bouten, C.V.; Verhaar, M.C. Extracellular vesicles: Potential roles in regenerative medicine. Front. Immunol. 2014, 5, 608. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.-S.; Lin, E.-Y.; Chiou, T.-W.; Harn, H.-J. Exosomes in clinical trial and their production in compliance with good manufacturing practice. Ci Ji Yi Xue Za Zhi 2019, 32, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Bernardi, S.; Balbi, C. Extracellular Vesicles: From Biomarkers to Therapeutic Tools. Biology 2020, 9, 258. [Google Scholar] [CrossRef] [PubMed]
- Pitt, J.M.; André, F.; Amigorena, S.; Soria, J.-C.; Eggermont, A.; Kroemer, G.; Zitvogel, L. Dendritic cell-derived exosomes for cancer therapy. J. Clin. Investig. 2016, 126, 1224–1232. [Google Scholar] [CrossRef] [PubMed]
- Maumus, M.; Rozier, P.; Boulestreau, J.; Jorgensen, C.; Noël, D. Mesenchymal Stem Cell-Derived Extracellular Vesicles: Opportunities and Challenges for Clinical Translation. Front. Bioeng. Biotechnol. 2020, 8, 997. [Google Scholar] [CrossRef] [PubMed]
- Gowen, A.; Shahjin, F.; Chand, S.; Odegaard, K.E.; Yelamanchili, S.V. Mesenchymal Stem Cell-Derived Extracellular Vesicles: Challenges in Clinical Applications. Front. Cell Dev. Biol. 2020, 8, 149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allan, D.; Tieu, A.; Lalu, M.; Burger, D. Mesenchymal stromal cell-derived extracellular vesicles for regenerative therapy and immune modulation: Progress and challenges toward clinical application. Stem Cells Transl. Med. 2020, 9, 39–46. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Bonacquisti, E.E.; Brown, A.D.; Nguyen, J. Boosting the Biogenesis and Secretion of Mesenchymal Stem Cell-Derived Exosomes. Cells 2020, 9, 660. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Huang, R.; Xu, Q.; Zheng, G.; Qiu, G.; Ge, M.; Shu, Q.; Xu, J. Mesenchymal Stem Cell-Derived Extracellular Vesicles Alleviate Acute Lung Injury Via Transfer of miR-27a-3p. Crit. Care Med. 2020, 48, e599–e610. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Sun, J.; Dong, C.; Zhao, M.; Hu, Y.; Jin, F. Extracellular Vesicles Derived from Adipose Mesenchymal Stem Cells Alleviate PM2.5-Induced Lung Injury and Pulmonary Fibrosis. Med. Sci Monit 2020, 26, e922782. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, Q.; Wang, D.; Wen, X.; Tang, X.; Qi, D.; He, J.; Zhao, Y.; Deng, W.; Zhu, T. Adipose-derived exosomes protect the pulmonary endothelial barrier in ventilator-induced lung injury by inhibiting the TRPV4/Ca2+ signaling pathway. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2020, 318, L723–L741. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, R.; Qin, C.; Wang, J.; Hu, Y.; Zheng, G.; Qiu, G.; Ge, M.; Tao, H.; Shu, Q.; Xu, J. Differential effects of extracellular vesicles from aging and young mesenchymal stem cells in acute lung injury. Aging 2019, 11, 7996–8014. [Google Scholar] [CrossRef] [PubMed]
- Silva, J.D.; de Castro, L.L.; Braga, C.L.; Oliveira, G.P.; Trivelin, S.A.; Barbosa-Junior, C.M.; Morales, M.A.-O.; Dos Santos, C.C.; Weiss, D.J.; Lopes-Pacheco, M.A.-O.; et al. Mesenchymal Stromal Cells Are More Effective Than Their Extracellular Vesicles at Reducing Lung Injury Regardless of Acute Respiratory Distress Syndrome Etiology. Stem Cells Int. 2019, 2019, 8262849. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, C.; Guo, F.; Chang, M.; Zhou, Z.; Yi, L.; Gao, C.; Huang, X.; Huan, J. Exosome-delivered syndecan-1 rescues acute lung injury via a FAK/p190RhoGAP/RhoA/ROCK/NF-κB signaling axis and glycocalyx enhancement. Exp. Cell Res. 2019, 384, 111596. [Google Scholar] [CrossRef] [PubMed]
- Harper, L.; Kalfa, N.; Beckers, G.M.A.; Kaefer, M.; Nieuwhof-Leppink, A.J.; Fossum, M.; Herbst, K.W.; Bagli, D.; Committee, E.R. The impact of COVID-19 on research. J. Pediatr. Urol. 2020, 16, 715–716. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.-y.; Zhou, W.-m.; Wang, Y.-q.; Guo, Q.-r.; Zhao, F.-x.; Zhu, Z.-y.; Xing, Y.-x.; Zhang, H.-y.; Aljofan, M.; Jarrahi, A.M.; et al. The Potential Role of Extracellular Vesicles in COVID-19 Treatment: Opportunity and Challenge. Front. Mol. Biosci. 2021, 8, 9929. [Google Scholar] [CrossRef]
- Shi, M.M.; Yang, Q.Y.; Monsel, A.; Yan, J.Y.; Dai, C.X.; Zhao, J.Y.; Shi, G.C.; Zhou, M.; Zhu, X.M.; Li, S.K.; et al. Preclinical efficacy and clinical safety of clinical-grade nebulized allogenic adipose mesenchymal stromal cells-derived extracellular vesicles. J. Extracell Vesicles 2021, 10, e12134. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, V.; Sengupta, S.; Lazo, A.; Woods, P.; Nolan, A.; Bremer, N. Exosomes Derived from Bone Marrow Mesenchymal Stem Cells as Treatment for Severe COVID-19. Stem Cells Dev. 2020, 29, 747–754. [Google Scholar] [CrossRef] [PubMed]
- Deng, S.X.; Dos Santos, A.; Gee, S. Therapeutic Potential of Extracellular Vesicles for the Treatment of Corneal Injuries and Scars. Transl. Vis. Sci. Technol. 2020, 9, 1. [Google Scholar] [CrossRef] [PubMed]
- Mead, B.; Tomarev, S. Extracellular vesicle therapy for retinal diseases. Prog. Retin. Eye Res. 2020, 79, 100849. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Chen, P.; Xu, J.; Liu, Y.; Li, H.; Wang, L.; Di, G. hADSCs derived extracellular vesicles inhibit NLRP3inflammasome activation and dry eye. Sci. Rep. 2020, 10, 14521. [Google Scholar] [CrossRef]
- Weng, J.; He, C.; Lai, P.; Luo, C.; Guo, R.; Wu, S.; Geng, S.; Xiangpeng, A.; Liu, X.; Du, X. Mesenchymal stromal cells treatment attenuates dry eye in patients with chronic graft-versus-host disease. Mol. Ther. 2012, 20, 2347–2354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silachev, D.N.; Goryunov, K.V.; Shpilyuk, M.A.; Beznoschenko, O.S.; Morozova, N.Y.; Kraevaya, E.E.; Popkov, V.A.; Pevzner, I.B.; Zorova, L.D.; Evtushenko, E.A.; et al. Effect of MSCs and MSC-Derived Extracellular Vesicles on Human Blood Coagulation. Cells 2019, 8, 258. [Google Scholar] [CrossRef] [PubMed]
- Chance, T.C.; Rathbone, C.; Kamucheka, R.M.; Peltier, G.C.; Cap, A.P.; Bynum, J.A. The effects of cell type and culture condition on the procoagulant activity of human mesenchymal stromal cell-derived extracellular vesicles. J. Trauma Acute Care Surg. 2019, 87, 74–82. [Google Scholar] [CrossRef]
- Fiedler, T.; Rabe, M.; Mundkowski, R.G.; Oehmcke-Hecht, S.; Peters, K. Adipose-derived mesenchymal stem cells release microvesicles with procoagulant activity. Int. J. Biochem. Cell Biol. 2018, 100, 49–53. [Google Scholar] [CrossRef] [PubMed]
- Abbade, L.P.F.; Frade, M.A.C.; Pegas, J.R.P.; Dadalti-Granja, P.; Garcia, L.C.; Bueno Filho, R.; Parenti, C.E.F. Consensus on the diagnosis and management of chronic leg ulcers—Brazilian Society of Dermatology. An. Bras. Dermatol. 2020, 95, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Shinkuma, S. Dystrophic epidermolysis bullosa: A review. Clin. Cosmet. Investig. Dermatol. 2015, 8, 275–284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fadus, M.C.; Lau, C.; Bikhchandani, J.; Lynch, H.T. Curcumin: An age-old anti-inflammatory and anti-neoplastic agent. J. Tradit. Complementary Med. 2017, 7, 339–346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yallapu, M.M.; Nagesh, P.K.; Jaggi, M.; Chauhan, S.C. Therapeutic Applications of Curcumin Nanoformulations. AAPS J. 2015, 17, 1341–1356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caruso Bavisotto, C.; Scalia, F.; Marino Gammazza, A.; Carlisi, D.; Bucchieri, F.; Conway de Macario, E.; Macario, A.J.L.; Cappello, F.; Campanella, C. Extracellular Vesicle-Mediated Cell⁻Cell Communication in the Nervous System: Focus on Neurological Diseases. Int. J. Mol. Sci. 2019, 20, 434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghidoni, R.; Paterlini, A.; Albertini, V.; Glionna, M.; Monti, E.; Schiaffonati, L.; Benussi, L.; Levy, E.; Binetti, G. Cystatin C is released in association with exosomes: A new tool of neuronal communication which is unbalanced in Alzheimer’s disease. Neurobiol. Aging 2011, 32, 1435–1442. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhuang, X.; Xiang, X.; Grizzle, W.; Sun, D.; Zhang, S.; Axtell, R.C.; Ju, S.; Mu, J.; Zhang, L.; Steinman, L.; et al. Treatment of Brain Inflammatory Diseases by Delivering Exosome Encapsulated Anti-inflammatory Drugs From the Nasal Region to the Brain. Mol. Ther. 2011, 19, 1769–1779. [Google Scholar] [CrossRef] [PubMed]
- Qu, M.; Lin, Q.; Huang, L.; Fu, Y.; Wang, L.; He, S.; Fu, Y.; Yang, S.; Zhang, Z.; Zhang, L.; et al. Dopamine-loaded blood exosomes targeted to brain for better treatment of Parkinson’s disease. J. Control. Release 2018, 287, 156–166. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Zhang, X.; Chen, X.; Wang, L.; Yang, G. Exosome Mediated Delivery of miR-124 Promotes Neurogenesis after Ischemia. Mol. Ther.-Nucleic Acids 2017, 7, 278–287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, T.; Fogarty, B.; LaForge, B.; Aziz, S.; Pham, T.; Lai, L.; Bai, S. Delivery of Small Interfering RNA to Inhibit Vascular Endothelial Growth Factor in Zebrafish Using Natural Brain Endothelia Cell-Secreted Exosome Nanovesicles for the Treatment of Brain Cancer. AAPS J. 2017, 19, 475–486. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Martin, P.; Fogarty, B.; Brown, A.; Schurman, K.; Phipps, R.; Yin, V.P.; Lockman, P.; Bai, S. Exosome Delivered Anticancer Drugs Across the Blood-Brain Barrier for Brain Cancer Therapy in Danio Rerio. Pharm Res. 2015, 32, 2003–2014. [Google Scholar] [CrossRef] [PubMed]
- Jurgielewicz, B.J.; Yao, Y.; Stice, S.L. Kinetics and Specificity of HEK293T Extracellular Vesicle Uptake using Imaging Flow Cytometry. Nanoscale Res. Lett. 2020, 15, 170. [Google Scholar] [CrossRef] [PubMed]
- Richter, M.; Vader, P.; Fuhrmann, G. Approaches to surface engineering of extracellular vesicles. Adv. Drug Deliv. Rev. 2021, 173, 416–426. [Google Scholar] [CrossRef] [PubMed]
- Qiao, L.; Hu, S.; Huang, K.; Su, T.; Li, Z.; Vandergriff, A.; Cores, J.; Dinh, P.U.; Allen, T.; Shen, D.; et al. Tumor cell-derived exosomes home to their cells of origin and can be used as Trojan horses to deliver cancer drugs. Theranostics 2020, 10, 3474–3487. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Altinoglu, S.; Takeda, Y.S.; Xu, Q. Integrating Protein Engineering and Bioorthogonal Click Conjugation for Extracellular Vesicle Modulation and Intracellular Delivery. PLoS ONE 2015, 10, e0141860. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Lee, H.; Goh, U.; Kim, J.; Jeong, M.; Lee, J.; Park, J.-H. Cellular Engineering with Membrane Fusogenic Liposomes to Produce Functionalized Extracellular Vesicles. ACS Appl. Mater. Interfaces 2016, 8, 6790–6795. [Google Scholar] [CrossRef] [PubMed]
- Piffoux, M.; Silva, A.K.A.; Wilhelm, C.; Gazeau, F.; Tareste, D. Modification of Extracellular Vesicles by Fusion with Liposomes for the Design of Personalized Biogenic Drug Delivery Systems. ACS Nano 2018, 12, 6830–6842. [Google Scholar] [CrossRef] [PubMed]
- Choi, E.S.; Song, J.; Kang, Y.Y.; Mok, H. Mannose-Modified Serum Exosomes for the Elevated Uptake to Murine Dendritic Cells and Lymphatic Accumulation. Macromol. Biosci. 2019, 19, e1900042. [Google Scholar] [CrossRef] [PubMed]
- Lawson, L.J.; Perry, V.H.; Gordon, S. Turnover of resident microglia in the normal adult mouse brain. Neuroscience 1992, 48, 405–415. [Google Scholar] [CrossRef]
- Guo, M.; Hao, Y.; Feng, Y.; Li, H.; Mao, Y.; Dong, Q.; Cui, M. Microglial Exosomes in Neurodegenerative Disease. Front. Mol. Neurosci. 2021, 14, 808. [Google Scholar] [CrossRef]
- Casella, G.; Colombo, F.; Finardi, A.; Descamps, H.; Ill-Raga, G.; Spinelli, A.; Podini, P.; Bastoni, M.; Martino, G.; Muzio, L.; et al. Extracellular Vesicles Containing IL-4 Modulate Neuroinflammation in a Mouse Model of Multiple Sclerosis. Mol. Ther. 2018, 26, 2107–2118. [Google Scholar] [CrossRef] [Green Version]
- Ye, Z.; Zhang, T.; He, W.; Jin, H.; Liu, C.; Yang, Z.; Ren, J. Methotrexate-Loaded Extracellular Vesicles Functionalized with Therapeutic and Targeted Peptides for the Treatment of Glioblastoma Multiforme. ACS Appl. Mater. Interfaces 2018, 10, 12341–12350. [Google Scholar] [CrossRef]
- Kopecka, J.; Campia, I.; Olivero, P.; Pescarmona, G.; Ghigo, D.; Bosia, A.; Riganti, C. A LDL-masked liposomal-doxorubicin reverses drug resistance in human cancer cells. J. Control. Release 2011, 149, 196–205. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Sun, X.; Mei, H.; Wang, Y.; Liao, Z.; Chen, J.; Zhang, Q.; Hu, Y.; Pang, Z.; Jiang, X. LDLR-mediated peptide-22-conjugated nanoparticles for dual-targeting therapy of brain glioma. Biomaterials 2013, 34, 9171–9182. [Google Scholar] [CrossRef] [PubMed]
- Tian, T.; Zhang, H.-X.; He, C.-P.; Fan, S.; Zhu, Y.-L.; Qi, C.; Huang, N.-P.; Xiao, Z.-D.; Lu, Z.-H.; Tannous, B.A.; et al. Surface functionalized exosomes as targeted drug delivery vehicles for cerebral ischemia therapy. Biomaterials 2018, 150, 137–149. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Wang, X.; Wang, C.; Feng, L.; Li, Y.; Liu, Z. Drug-Induced Self-Assembly of Modified Albumins as Nano-theranostics for Tumor-Targeted Combination Therapy. ACS Nano 2015, 9, 5223–5233. [Google Scholar] [CrossRef] [PubMed]
- Shuhendler, A.J.; Prasad, P.; Leung, M.; Rauth, A.M.; DaCosta, R.S.; Wu, X.Y. A Novel Solid Lipid Nanoparticle Formulation for Active Targeting to Tumor αvβ3 Integrin Receptors Reveals Cyclic RGD as A Double-Edged Sword. Adv. Healthc. Mater. 2012, 1, 600–608. [Google Scholar] [CrossRef] [PubMed]
- Kamerkar, S.; LeBleu, V.S.; Sugimoto, H.; Yang, S.; Ruivo, C.F.; Melo, S.A.; Lee, J.J.; Kalluri, R. Exosomes facilitate therapeutic targeting of oncogenic KRAS in pancreatic cancer. Nature 2017, 546, 498–503. [Google Scholar] [CrossRef] [PubMed]
- Lamparski, H.G.; Metha-Damani, A.; Yao, J.Y.; Patel, S.; Hsu, D.H.; Ruegg, C.; Le Pecq, J.B. Production and characterization of clinical grade exosomes derived from dendritic cells. J. Immunol. Methods 2002, 270, 211–226. [Google Scholar] [CrossRef]
- Qi, H.; Liu, C.; Long, L.; Ren, Y.; Zhang, S.; Chang, X.; Qian, X.; Jia, H.; Zhao, J.; Sun, J.; et al. Blood Exosomes Endowed with Magnetic and Targeting Properties for Cancer Therapy. ACS Nano 2016, 10, 3323–3333. [Google Scholar] [CrossRef] [PubMed]
- Morales-Kastresana, A.; Telford, B.; Musich, T.A.; McKinnon, K.; Clayborne, C.; Braig, Z.; Rosner, A.; Demberg, T.; Watson, D.C.; Karpova, T.S.; et al. Labeling Extracellular Vesicles for Nanoscale Flow Cytometry. Sci. Rep. 2017, 7, 1878. [Google Scholar] [CrossRef] [PubMed]
- US Food and Drug Administration. Definition of the Term “Biological Product”; 21 CFR 600; US Food and Drug Administration: Washinton, DC, USA, 2020.
- US Food and Drug Administration. Liposome Drug Products Chemistry, Manufacturing, and Controls; Human Pharmacokinetics and Bioavailability; and Labeling Documentation. Guidance for Industry. (CDER); US Food and Drug Administration: Washington, DC, USA, 2018.
- Zhi, K.; Kumar, A.; Raji, B.; Kochat, H.; Kumar, S. Formulation, manufacturing and regulatory strategies for extracellular vesicles-based drug products for targeted therapy of central nervous system diseases. Expert Rev. Precis. Med. Drug Dev. 2020, 5, 469–481. [Google Scholar] [CrossRef]
- Kumar, A.; Zhou, L.; Zhi, K.; Raji, B.; Pernell, S.; Tadrous, E.; Kodidela, S.; Nookala, A.; Kochat, H.; Kumar, S. Challenges in Biomaterial-Based Drug Delivery Approach for the Treatment of Neurodegenerative Diseases: Opportunities for Extracellular Vesicles. Int. J. Mol. Sci. 2020, 22, 138. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.B.; Liu, P.; Shao, F.M.; Miao, Q.L. Formulation and evaluation of PLGA nanoparticles loaded capecitabine for prostate cancer. Int. J. Clin. Exp. Med. 2015, 8, 19670–19681. [Google Scholar] [PubMed]
- Yallapu, M.M.; Gupta, B.K.; Jaggi, M.; Chauhan, S.C. Fabrication of curcumin encapsulated PLGA nanoparticles for improved therapeutic effects in metastatic cancer cells. J. Colloid Interface Sci. 2010, 351 1, 19–29. [Google Scholar] [CrossRef]
- Cai, K.; He, X.; Song, Z.; Yin, Q.; Zhang, Y.; Uckun, F.M.; Jiang, C.; Cheng, J. Dimeric Drug Polymeric Nanoparticles with Exceptionally High Drug Loading and Quantitative Loading Efficiency. J. Am. Chem. Soc. 2015, 137, 3458–3461. [Google Scholar] [CrossRef] [PubMed]
- Zucker, D.; Marcus, D.; Barenholz, Y.; Goldblum, A. Liposome drugs’ loading efficiency: A working model based on loading conditions and drug’s physicochemical properties. J. Control. Release 2009, 139, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Laridi, R.; Kheadr, E.E.; Benech, R.O.; Vuillemard, J.C.; Lacroix, C.; Fliss, I. Liposome encapsulated nisin Z: Optimization, stability and release during milk fermentation. Int. Dairy J. 2003, 13, 325–336. [Google Scholar] [CrossRef]
- Sangsuriyawong, A.; Limpawattana, M.; Siriwan, D.; Klaypradit, W. Properties and bioavailability assessment of shrimp astaxanthin loaded liposomes. Food Sci. Biotechnol. 2019, 28, 529–537. [Google Scholar] [CrossRef] [PubMed]
- Chotphruethipong, L.; Battino, M.; Benjakul, S. Effect of stabilizing agents on characteristics, antioxidant activities and stability of liposome loaded with hydrolyzed collagen from defatted Asian sea bass skin. Food Chem. 2020, 328, 127127. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, R.; Aucamp, M.; Ebrahim, N.; Samsodien, H. Supramolecular assembly of rifampicin and PEGylated PAMAM dendrimer as a novel conjugate for tuberculosis. J. Drug Deliv. Sci. Technol. 2021, 66, 102773. [Google Scholar] [CrossRef]
- Tawfik, M.A.; Tadros, M.I.; Mohamed, M.I. Polyamidoamine (PAMAM) dendrimers as potential release modulators and oral bioavailability enhancers of vardenafil hydrochloride. Pharm. Dev. Technol. 2019, 24, 293–302. [Google Scholar] [CrossRef]
- Alfei, S.; Spallarossa, A.; Lusardi, M.; Zuccari, G. Successful Dendrimer and Liposome-Based Strategies to Solubilize an Antiproliferative Pyrazole Otherwise Not Clinically Applicable. Nanomaterials 2022, 12, 233. [Google Scholar] [CrossRef] [PubMed]
- Lv, S.; Wu, Y.; Cai, K.; He, H.; Li, Y.; Lan, M.; Chen, X.; Cheng, J.; Yin, L. High Drug Loading and Sub-Quantitative Loading Efficiency of Polymeric Micelles Driven by Donor–Receptor Coordination Interactions. J. Am. Chem. Soc. 2018, 140, 1235–1238. [Google Scholar] [CrossRef] [PubMed]
- Miller, T.; van Colen, G.; Sander, B.; Golas, M.M.; Uezguen, S.; Weigandt, M.; Goepferich, A. Drug loading of polymeric micelles. Pharm. Res. 2013, 30, 584–595. [Google Scholar] [CrossRef] [PubMed]
- Teo, J.Y.; Chin, W.; Ke, X.; Gao, S.; Liu, S.; Cheng, W.; Hedrick, J.L.; Yang, Y.Y. pH and redox dual-responsive biodegradable polymeric micelles with high drug loading for effective anticancer drug delivery. Nanomed. Nanotechnol. Biol. Med. 2017, 13, 431–442. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Rashid, R.S.; Helal, D.A.; Alaa-Eldin, A.A.; Abdel-Monem, R. Polymeric versus lipid nanocapsules for miconazole nitrate enhanced topical delivery: In vitro and ex vivo evaluation. Drug Deliv. 2022, 29, 294–304. [Google Scholar] [CrossRef] [PubMed]
- Matoso Sombra, F.; Richter, A.R.; de Araújo, A.R.; de Oliveira Silva Ribeiro, F.; de Fátima Souza Mendes, J.; Dos Santos Fontenelle, R.O.; da Silva, D.A.; Beserra de Paula, H.C.; Pessoa de Andrade Feitosa, J.; Martín Goycoolea, F.; et al. Nanocapsules of Sterculia striata acetylated polysaccharide as a potential monomeric amphotericin B delivery matrix. Int. J. Biol. Macromol. 2019, 130, 655–663. [Google Scholar] [CrossRef]
- Youm, I.; Yang, X.; Murowchick, J.B.; Youan, B.-B.C. Encapsulation of docetaxel in oily core polyester nanocapsules intended for breast cancer therapy. Nanoscale Res. Lett. 2011, 6, 630. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Almutairy, B.K.; Alshetaili, A.; Alali, A.S.; Ahmed, M.M.; Anwer, M.K.; Aboudzadeh, M.A. Design of Olmesartan Medoxomil-Loaded Nanosponges for Hypertension and Lung Cancer Treatments. Polymers 2021, 13, 2272. [Google Scholar] [CrossRef] [PubMed]
- Moin, A.; Roohi, N.K.F.; Rizvi, S.M.D.; Ashraf, S.A.; Siddiqui, A.J.; Patel, M.; Ahmed, S.M.; Gowda, D.V.; Adnan, M. Design and formulation of polymeric nanosponge tablets with enhanced solubility for combination therapy. RSC Adv. 2020, 10, 34869–34884. [Google Scholar] [CrossRef]
- Zhao, S.; Li, J.; Zhou, Y.; Huang, L.; Li, Y.; Xu, J.; Fu, C.; Guo, X.; Yang, J. Lipid Nanoparticles-Encapsulated YF4: A Potential Therapeutic Oral Peptide Delivery System for Hypertension Treatment. Front. Pharmacol. 2019, 10, 102. [Google Scholar] [CrossRef] [PubMed]
| Sr No | Clinical Trial | Status | Phase | Subject | Indication | EV Source | Target Sites | EV Manipulation | Reference |
|---|---|---|---|---|---|---|---|---|---|
| 1. | Evaluation of Safety and Efficiency of Method of Exosome Inhalation in SARS-CoV-2 Associated Pneumonia. (COVID-19EXO) | Completed | 1/2 | 30 | COVID-19 | MSC 1-derived | Lungs | NA | NCT04491240 |
| 2. | A Pilot Clinical Study on Inhalation of Mesenchymal Stem Cells Exosomes Treating Severe Novel Coronavirus Pneumonia | Completed | 1 | 24 | COVID-19 | Allogenic adipose MSC 1-derived | Lungs | NA | NCT04276987 |
| 3. | Safety and Efficiency of Method of Exosome Inhalation in COVID-19 Associated Pneumonia (COVID-19EXO2) | Enrolling by invitation | 2 | 90 | COVID-19 | MSC 1-derived | Lungs | NA | NCT04602442 |
| 4. | COVID-19 Specific T Cell Derived Exosomes (CSTC-Exo) | Active, not recruiting | 1 | 60 | COVID-19 | COVID-19 specific T-cells derived | Lungs | NA | NCT04389385 |
| 5. | Extracellular Vesicle Infusion Treatment for COVID-19 Associated ARDS (EXIT-COVID19) | Completed | 2 | 120 | COVID-19 Associated ARDS | Bone marrow derived | Lungs | NA | NCT04493242 |
| 6. | A Clinical Study of Mesenchymal Progenitor Cell Exosomes Nebulizer for The Treatment of Pulmonary Infection | Recruiting | 1/2 | 60 | Drug resistant pulmonary infection | MPC 2-derived | Lungs | NA | NCT04544215 |
| 7. | A Tolerance Clinical Study on Aerosol Inhalation of Mesenchymal Stem Cells Exosomes in Healthy Volunteers | Completed | 1 | 24 | Safety and tolerance | Allogenic adipose MSC 1-derived | Lungs | NA | NCT04313647 |
| 8. | A Clinical Study of Mesenchymal Stem Cell Exosomes Nebulizer for the Treatment of ARDS | Not yet recruiting | 1/2 | 169 | Acute Respiratory Distress Syndrome | Allogeneic human MSC 1-derived | Lungs | NA | NCT04602104 |
| 9. | Effect of UMSCs Derived Exosomes on Dry Eye in Patients With cGVHD | Recruiting | 1/2 | 27 | Dry Eye | Umbilical MSC 1-derived | Eyes | NA | NCT04213248 |
| 10. | MSC 1-Exos Promote Healing of MHs | Active, not recruiting | Early Phase 1 | 44 | Macular Holes | MSC 1-derived | Retina-Eyes | NA | NCT03437759 |
| 11. | Evaluation of Adipose Derived Stem Cells Exo. in Treatment of Periodontitis (exosomes) | Recruiting | Early Phase 1 | 10 | Periodontitis | Adipose- stem-cell-derived | Gums-oral cavity | NA | NCT04270006 |
| 12. | Edible Plant Exosome Ability to Prevent Oral Mucositis Associated with Chemoradiation Treatment of Head and Neck Cancer | Active, not recruiting | 1 | 60 | Oral Mucositis in Head and Neck Cancer | Grape derived | Oral cavity | NA | NCT01668849 |
| 13. | MSC 1 EVs in Dystrophic Epidermolysis Bullosa | Not yet recruiting | 1–2 | 10 | Dystrophic Epidermolysis Bullosa | Allogeneic MSC 1-derived | Integument | NA | NCT04173650 |
| 14. | Effect of Plasma Derived Exosomes on Cutaneous Wound Healing | Unknown | Early Phase 1 | 5 | Intractable cutaneous ulcers | Plasma derived | Integument | NA | NCT02565264 |
| 15. | Use of Autologous Plasma Rich in Platelets and Extracellular Vesicles in the Surgical Treatment of Chronic Middle Ear Infections | Recruiting | 2–3 | 100 | Otitis Media | Plasma derived | Middle ear | NA | NCT04761562 |
| 16. | Effect Of Microvesicles and Exosomes Therapy on Β-Cell Mass in Type I Diabetes Mellitus (T1DM) | Unknown | 2/3 | 20 | Diabetes Mellitus Type 1 | Umbilical cord-blood derived MSC 1-derived | Pancreas | NA | NCT02138331 |
| 17. | iExosomes in Treating Participants with Metastatic Pancreas Cancer with KrasG12D Mutation | Recruiting | 1 | 28 | Metastatic Pancreatic Adenocarcinoma, Pancreatic Ductal Adenocarcinoma | MSC 1-derived | Metastatic Pancreatic cancer cells | loaded with siRNA against KrasG12D | NCT03608631 |
| 18. | Study Investigating the Ability of Plant Exosomes to Deliver Curcumin to Normal and Colon Cancer Tissue | Recruiting | 1 | 35 | Colon Cancer | Plant derived | Colon | Loaded with curcumin | NCT01294072 |
| EVs Source | Encapsulated Agents | Target | Goal | Outcome |
|---|---|---|---|---|
| EL-4 T cells [107] | 1. Curcumin 2. JSI-124 (cucurbitacin I) | Microglial cells | To show that the intranasal administration of curcumin and JSI-124 encapsulated in exosomes can pass the BBB and prevent microglial cell activation induced by lipopolysaccharide, delay experimental autoimmune encephalomyelitis disease, and inhibit tumor progression in vivo. | Intranasal administration of curcumin and JSI-124 encapsulated in exosomes showed a rapid uptake by microglial cells and provided neuroprotection. This approach has the potential to be a non-invasive treatment option in brain inflammatory-related diseases |
| Mouse macrophage cell line (Raw 264.7) [24] | Catalase (antioxidant) | Neurovascular unit: 1. Endothelial cells 2. Neurons 3. Astrocytes | To show that intranasal administration of exosomes loaded with catalase may protect catalase enzymatic activity, decrease immunogenicity, and extend blood flow time in a Parkinson’s Disease mouse model. | Intranasal administration of exosomes loaded with catalase showed significant neuroprotective effects (in vitro and in vivo) and thus potentially an applicable treatment strategy for inflammatory and neurodegenerative disorders. |
| Blood of Kunming mice [108] | Dopamine | Brain epithelial cells | Show how loading blood derived exosomes with dopamine may increase distribution past the BBB and thus a more effective drug delivery approach compared to conventional treatment options. | Blood derived exosomes were delivered across the BBB via the transferrin-TfR interaction, thus dopamine distribution increased >15-fold and toxicity significantly decreased compared to free dopamine. |
| Bone marrow-derived mesenchymal stem cell modified with rabies virus glycoprotein (RVG) [109] | microRNA-124 | The ischemic cortex of brain | Investigate if loading miR-124 into RVG-modified exosomes can safeguard against cortical ischemia. | miR124 shown to be neuroprotective and to lead neuron remodeling via promotion of neurogenesis, thus can be considered a promising gene therapy approach for ischemic injury. |
| Brain endothelial bEND.3 cells [110] | Vascular endothelial growth factor small interfering RNA (VEGF siRNA) | Neuronal glioblastoma-astrocytoma U-87 malignant glioma cells | Given siRNA’s therapeutic potential, to test if brain endothelial cell-derived exosomes can cross the BBB in zebrafish with U-87 malignant gliomas (MG) glioblastoma to deliver siRNA. | In glioblastoma-astrocytoma U-87 MG cells expression of vascular endothelial growth factor (VEGF) RNA and protein levels were inhibited by the exosomal delivery of siRNA. |
| 1. Brain neuronal glioblastoma-astrocytoma U-87 MG cells 2. Brain endothelial bEND.3 cells 3. Neuroectodermal tumor PFSK-1 cells 4. Glioblastoma A-172 cells [111] | 1. Rhodamine 123 2. Paclitaxel 3. Doxorubicin | Neuronal glioblastoma-astrocytoma U-87 malignant glioma cells | Evaluate drug delivery across the BBB based on particle size, morphology, total protein, and transmembrane protein markers. | Brain endothelial bEND.3 exosome drug delivery performed best compared to the others. bEND.3 exosome success was attributed to the high expression levels of CD63. |
| Engineering Method | Target Site | Outcomes | Potential Application on CNS Targeting | Reference |
| Engineering EV Parent Cells before the Isolation of EVs | ||||
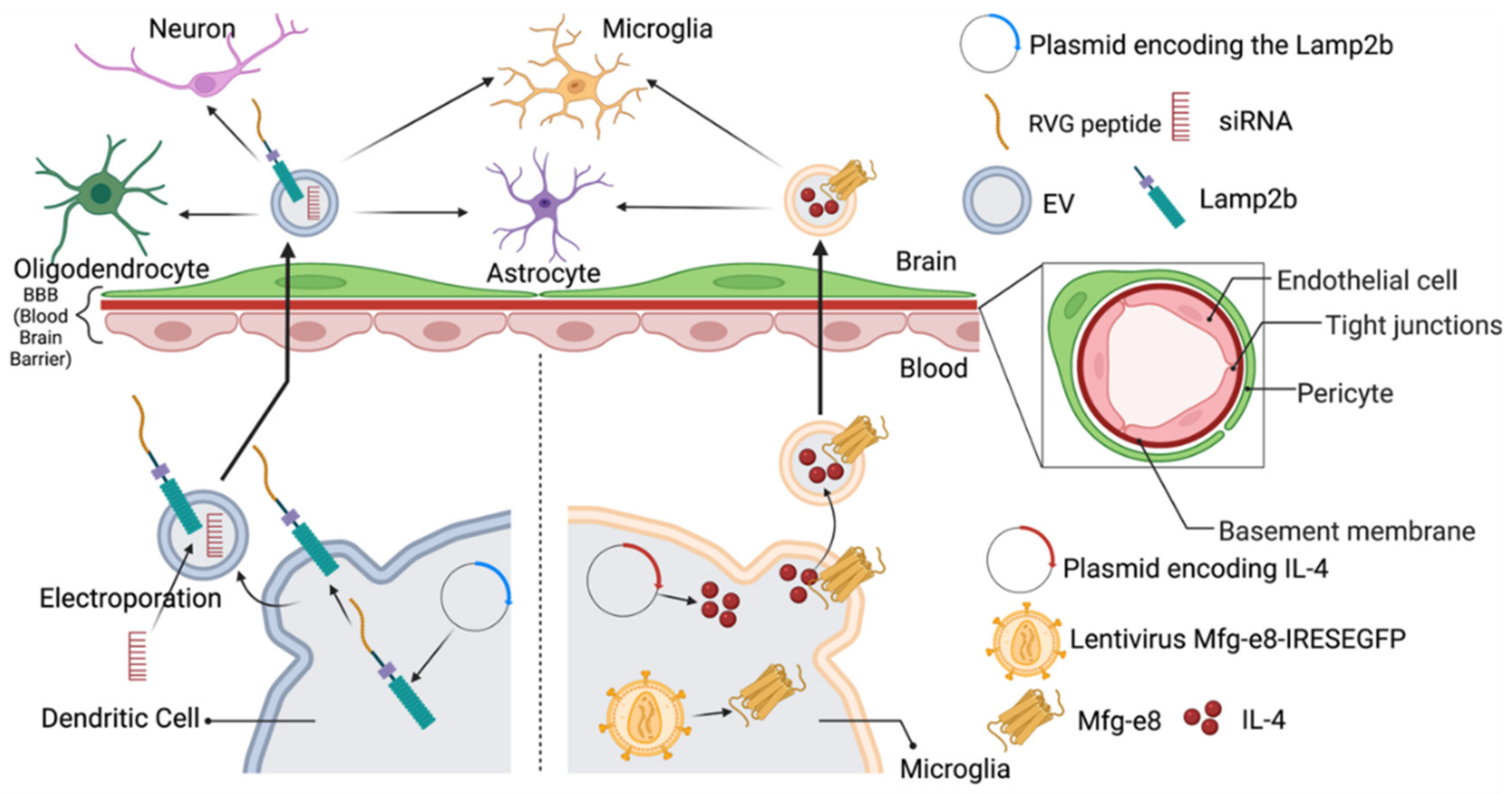
| Lamb2b plasmid was transfected into dendritic cells 4 days before EVs isolation. RVG peptides were cloned into extra-exosomal N terminus of Lamp2b. After EV isolation, load EVs with BACE1 siRNA via electroporation | Acetylcholine receptor in brain | Intravenous (IV) injection of RVG-targeted EVs loaded with BACE1 siRNA can knockdown mRNA (60%) and protein (62%) expression of BACE1 in the brain. Uptake was not observed in other off-target organs in mice. | Delivery of gene therapy in CNS for neurodegenerative diseases | [29] |
| EVs were generated by BV-2 microglia cells infected with the lentivirus Mfg-e8-IRES-EGFP to overexpress Mfg-e8 and transfected with a lentiviral plasmid coding for IL-4. | Phagocytes in brain | After cisterna magna injection of IL-4+Mfg-e8+ EVs into mice, EVs could target phagocytes and anti-inflammatory markers. Chitinase 3-like 3 (ym1) and arginase-1 (arg1) were upregulated in the CNS, which decrease neuroinflammation and brain damage. | Engineer anti-inflammatory molecules to treat neuroinflammatory diseases | [121] |
| Engineering EV after EV isolation | ||||
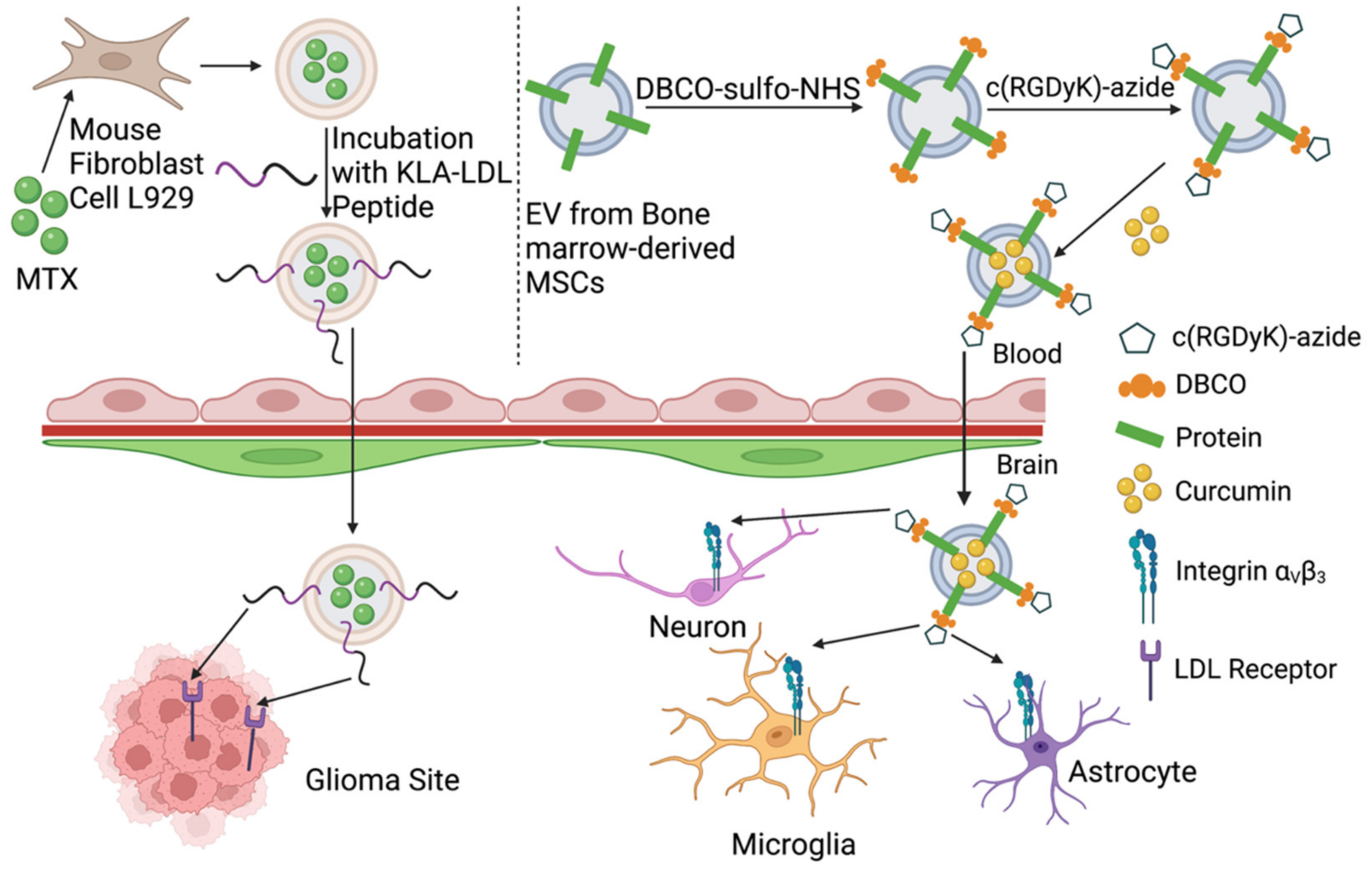
| Conjugate cyclo(Arg-Gly-Asp-D-Tyr-Lys) peptide [c(RGDyK)] onto EVs derived from mesenchymal stromal cell (MSC) surface using click chemistry. Curcumin(cur) was incorporated in the cRGD-Exo for 5 min at RT | Target site: cerebral vascular endothelial cells in the brain | IV administration of cRGD-EXO-cur could successfully suppress inflammation and cellular apoptosis in the ischemic brain in mice | Load therapeutic agents into cRGD-Exo to target the lesion region of the brain | [125] |
| EVs derived from L929 cells were loaded with methotrexate and conjugated with [Lys-Leu-Ala (KLA)], containing an ApoA-I mimetic sequence, and [low-density lipoprotein (LDL)], phospholipids, by agitation at room temperature for 3 h. | Glioma spheroid in the brain | EVs-KLA-LDL were injected intravenously. They crossed the BBB more efficiently than the control EV and an inhibition of glioma spheroid growth after administration of EVs-KLA-LDL was observed, resulting in improved survival in mice models. | Conjugation of peptides onto EVs surface during post-isolation modification can improve penetration across the BBB of EVs and their target binding for brain tumor tissue, which improves the therapeutic effect of drugs. | [122] |
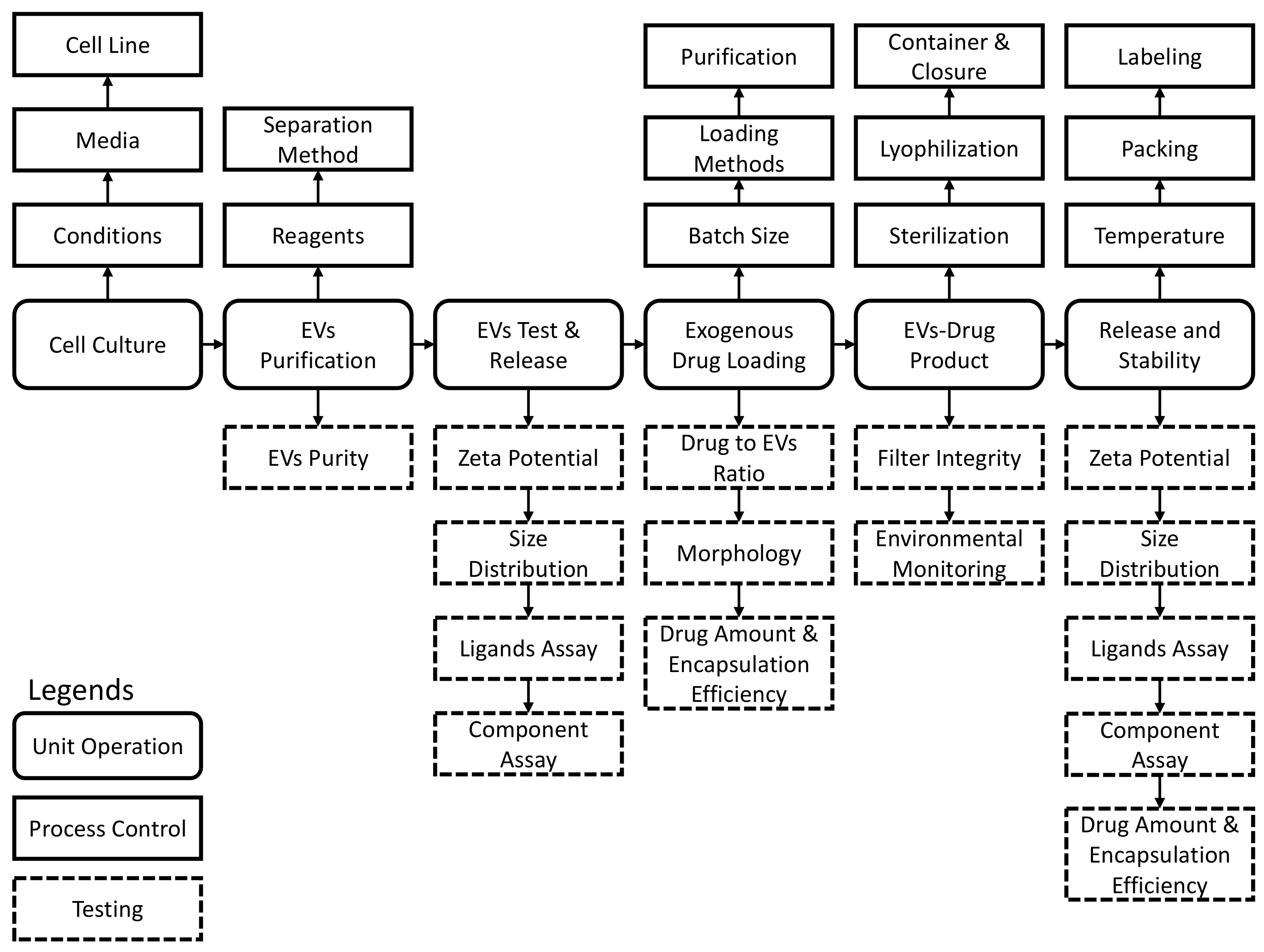
| Purpose | USP Chapter | Test |
|---|---|---|
| Release | N/A | EVs and ligands assay |
| N/A | Drug substance assay | |
| <71> | Sterility | |
| <785> | Osmolality | |
| <467> | Residual organic solvents * | |
| <281> | Residue on ignition | |
| <731, 921> | Loss on drying for lyophilized products | |
| <790> | Visible particulate inspection | |
| <61> | Microbial enumeration | |
| <791> | pH | |
| <85> | Bacterial endotoxins | |
| <788> | Particulate matter for injection | |
| <1207> | Uniformity of dosages |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, L.; Kodidela, S.; Godse, S.; Thomas-Gooch, S.; Kumar, A.; Raji, B.; Zhi, K.; Kochat, H.; Kumar, S. Targeted Drug Delivery to the Central Nervous System Using Extracellular Vesicles. Pharmaceuticals 2022, 15, 358. https://doi.org/10.3390/ph15030358
Zhou L, Kodidela S, Godse S, Thomas-Gooch S, Kumar A, Raji B, Zhi K, Kochat H, Kumar S. Targeted Drug Delivery to the Central Nervous System Using Extracellular Vesicles. Pharmaceuticals. 2022; 15(3):358. https://doi.org/10.3390/ph15030358
Chicago/Turabian StyleZhou, Lina, Sunitha Kodidela, Sandip Godse, Stacey Thomas-Gooch, Asit Kumar, Babatunde Raji, Kaining Zhi, Harry Kochat, and Santosh Kumar. 2022. "Targeted Drug Delivery to the Central Nervous System Using Extracellular Vesicles" Pharmaceuticals 15, no. 3: 358. https://doi.org/10.3390/ph15030358
APA StyleZhou, L., Kodidela, S., Godse, S., Thomas-Gooch, S., Kumar, A., Raji, B., Zhi, K., Kochat, H., & Kumar, S. (2022). Targeted Drug Delivery to the Central Nervous System Using Extracellular Vesicles. Pharmaceuticals, 15(3), 358. https://doi.org/10.3390/ph15030358








