ApoA-I Nanoparticles as Curcumin Carriers for Cerebral Endothelial Cells: Improved Cytoprotective Effects against Methylglyoxal
Abstract
:1. Introduction
2. Results
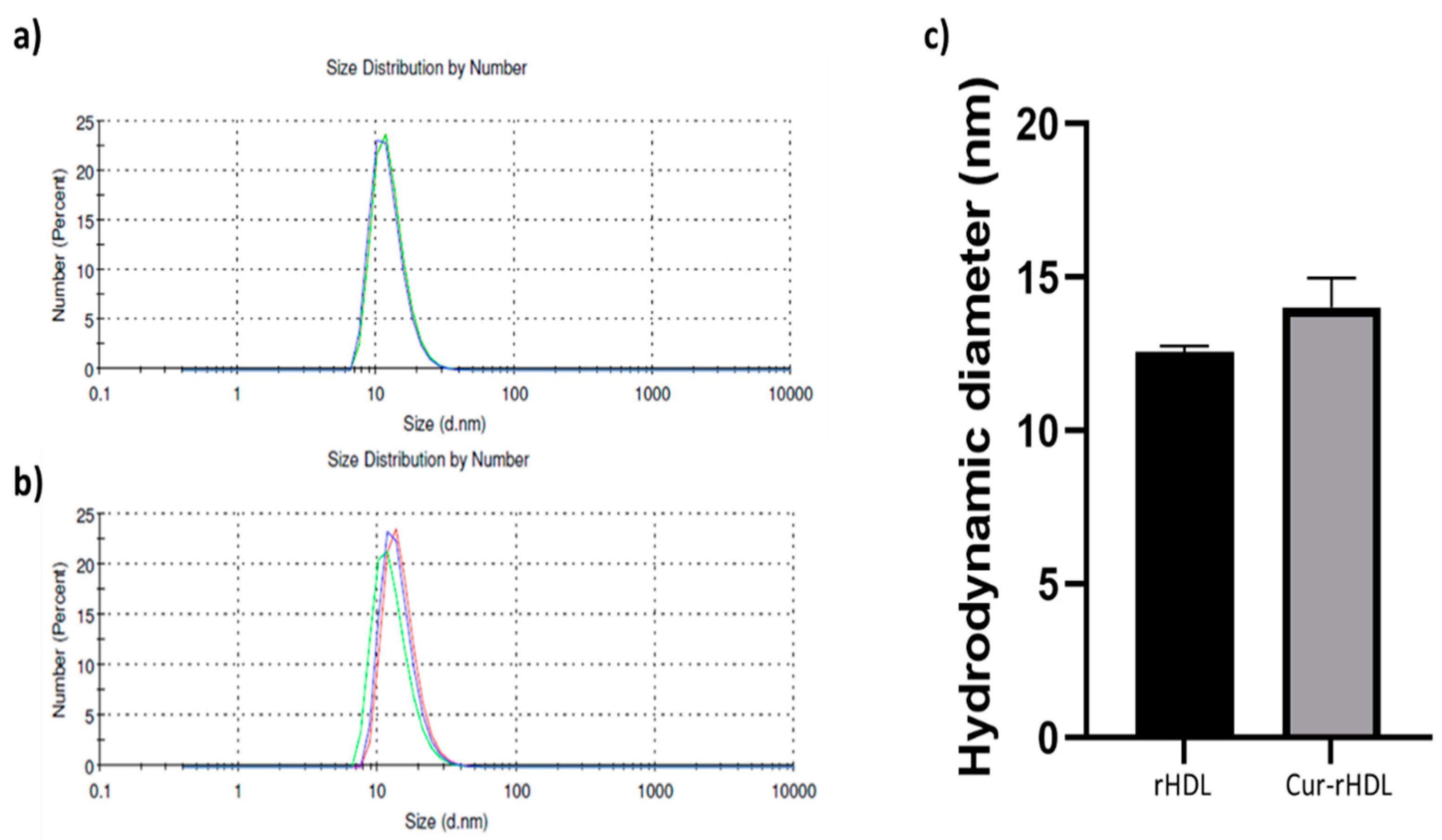
2.1. Particle Size Distribution of rHDL and Cur-rHDLs by DLS Analysis
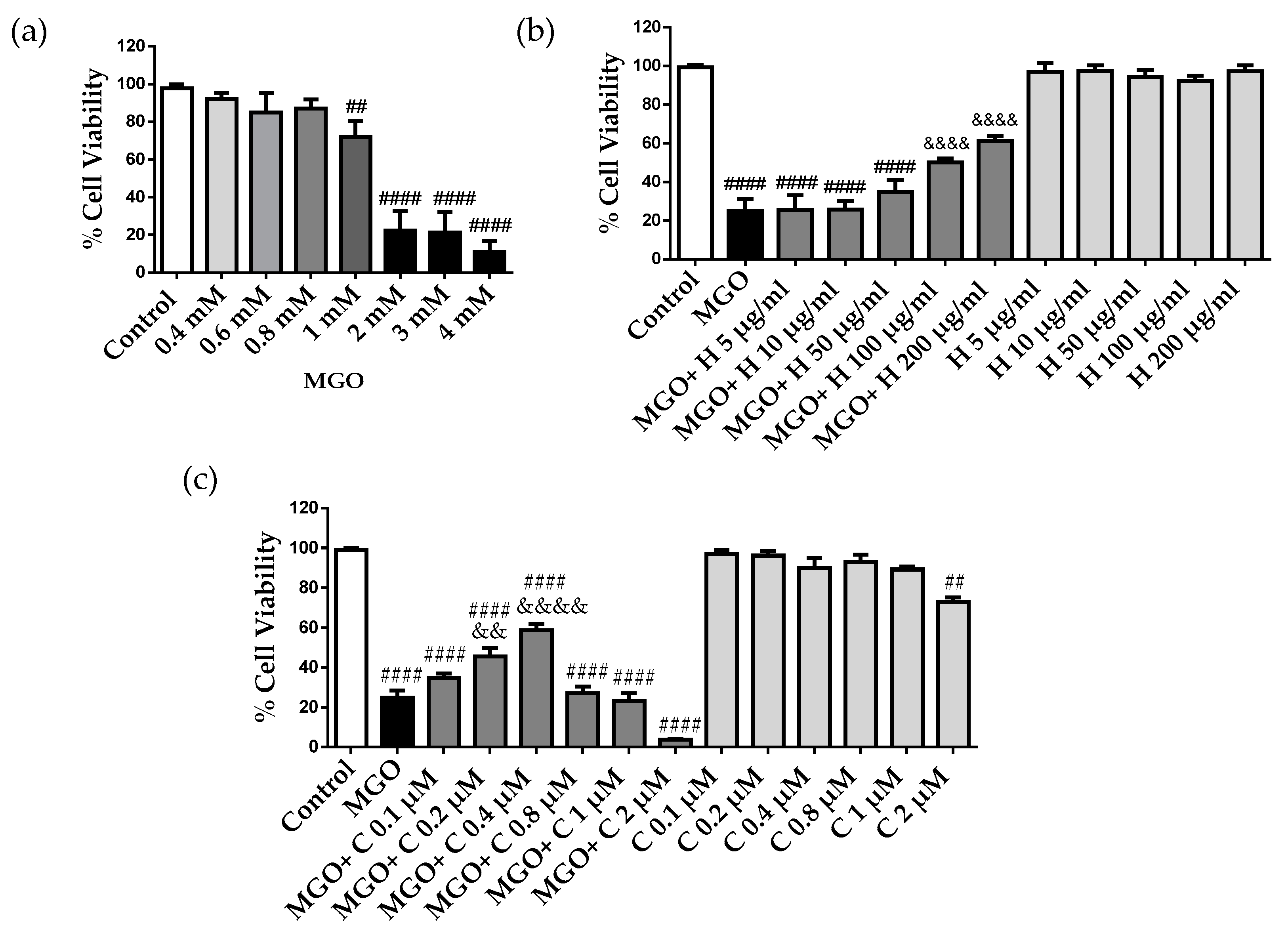
2.2. Effect of HDL and Curcumin on Cerebrovascular Endothelial Cell Cytotoxicity Induced by MGO
2.3. Effect of Curcumin-Enriched rHDLs on MGO Cytotoxicity in Cerebral Endothelial Cells
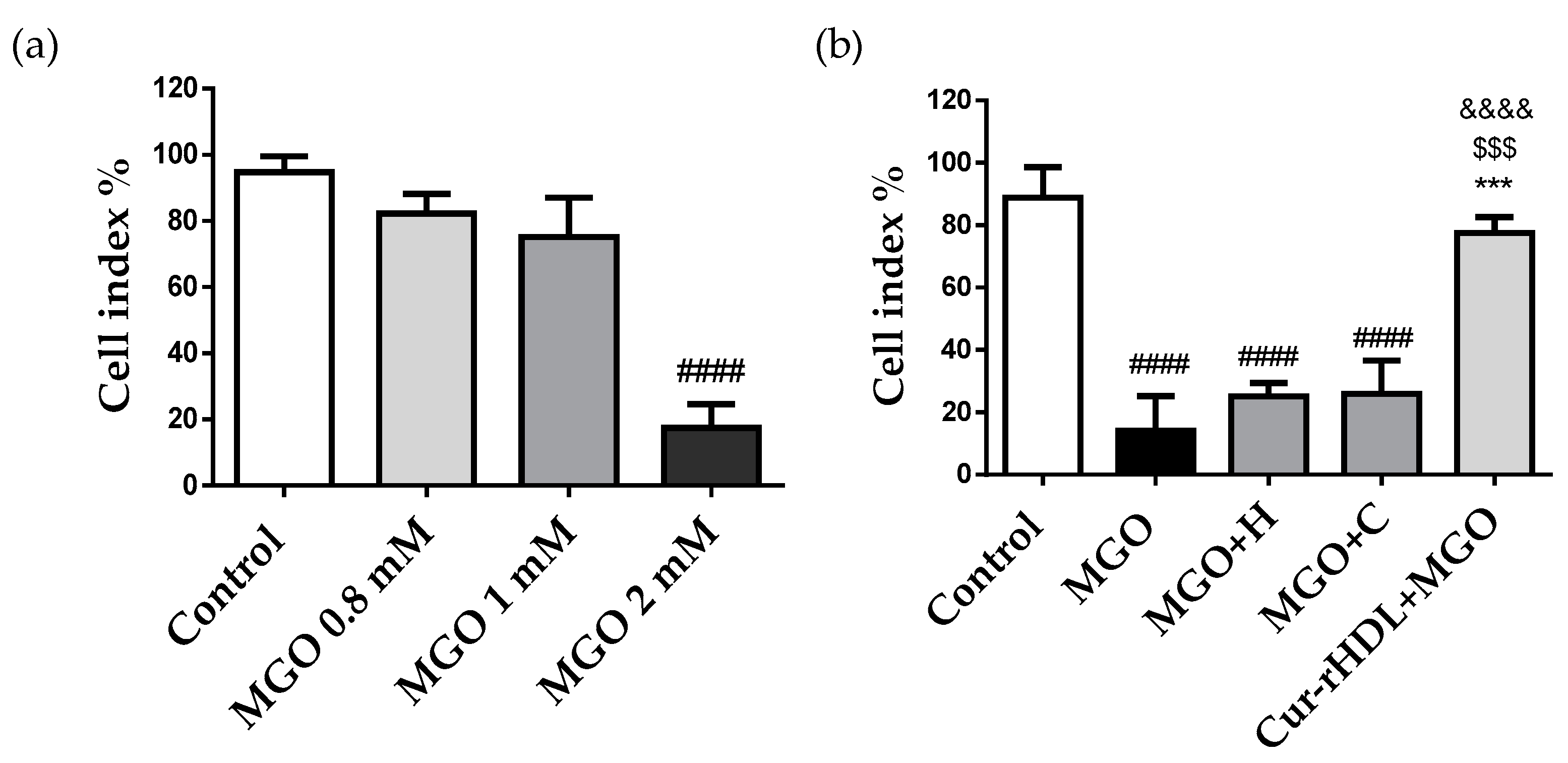
2.4. Effect of Curcumin-Enriched rHDLs on Cerebral Endothelial Layer Integrity
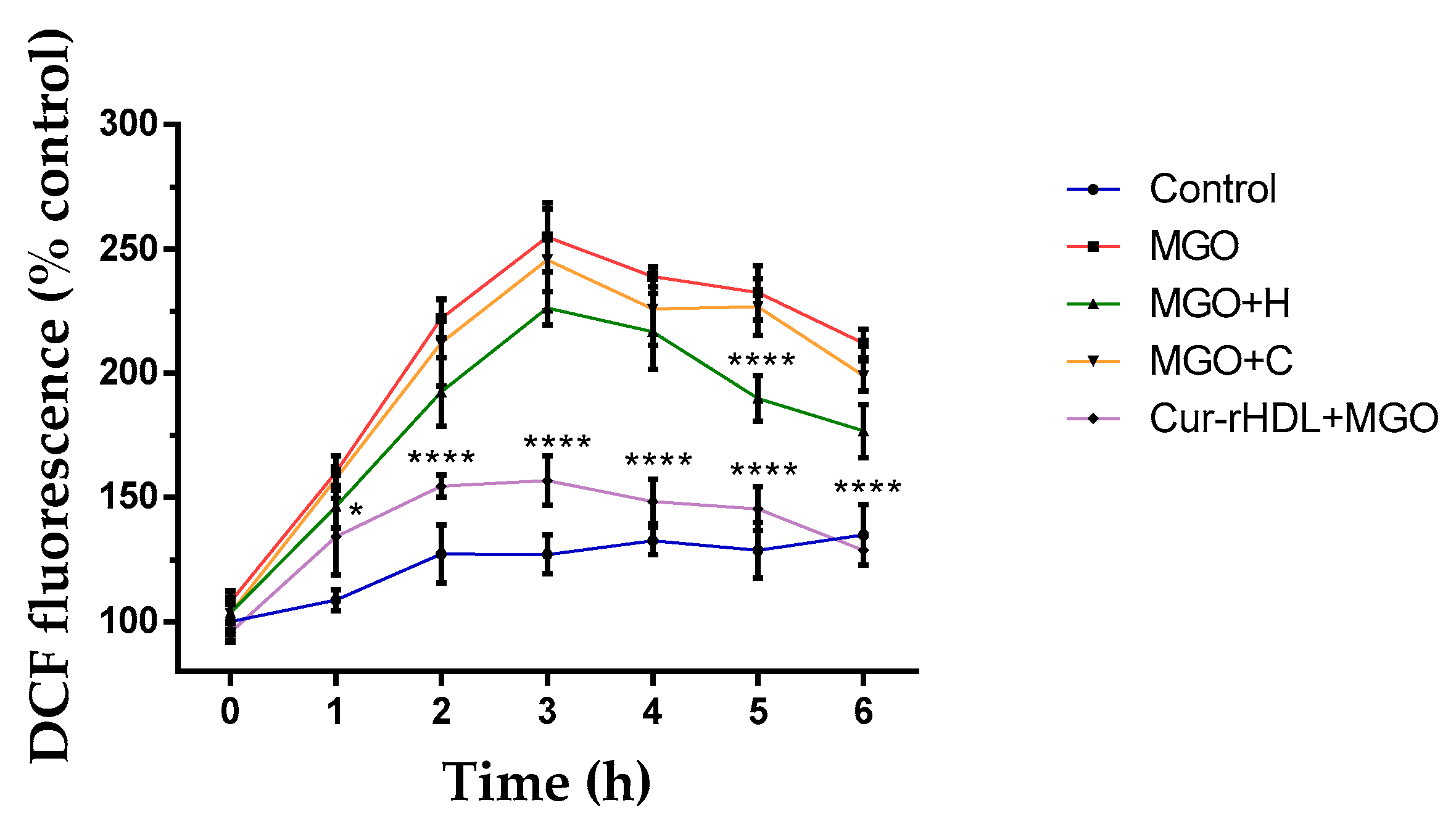
2.5. Effect of Curcumin-Enriched rHDLs on Intracellular ROS Production Induced by MGO in Cerebral Endothelial Cells
2.6. Effect of Curcumin-Enriched rHDLs on MGO-Induced Chromatin Condensation in Cerebral Endothelial Cells
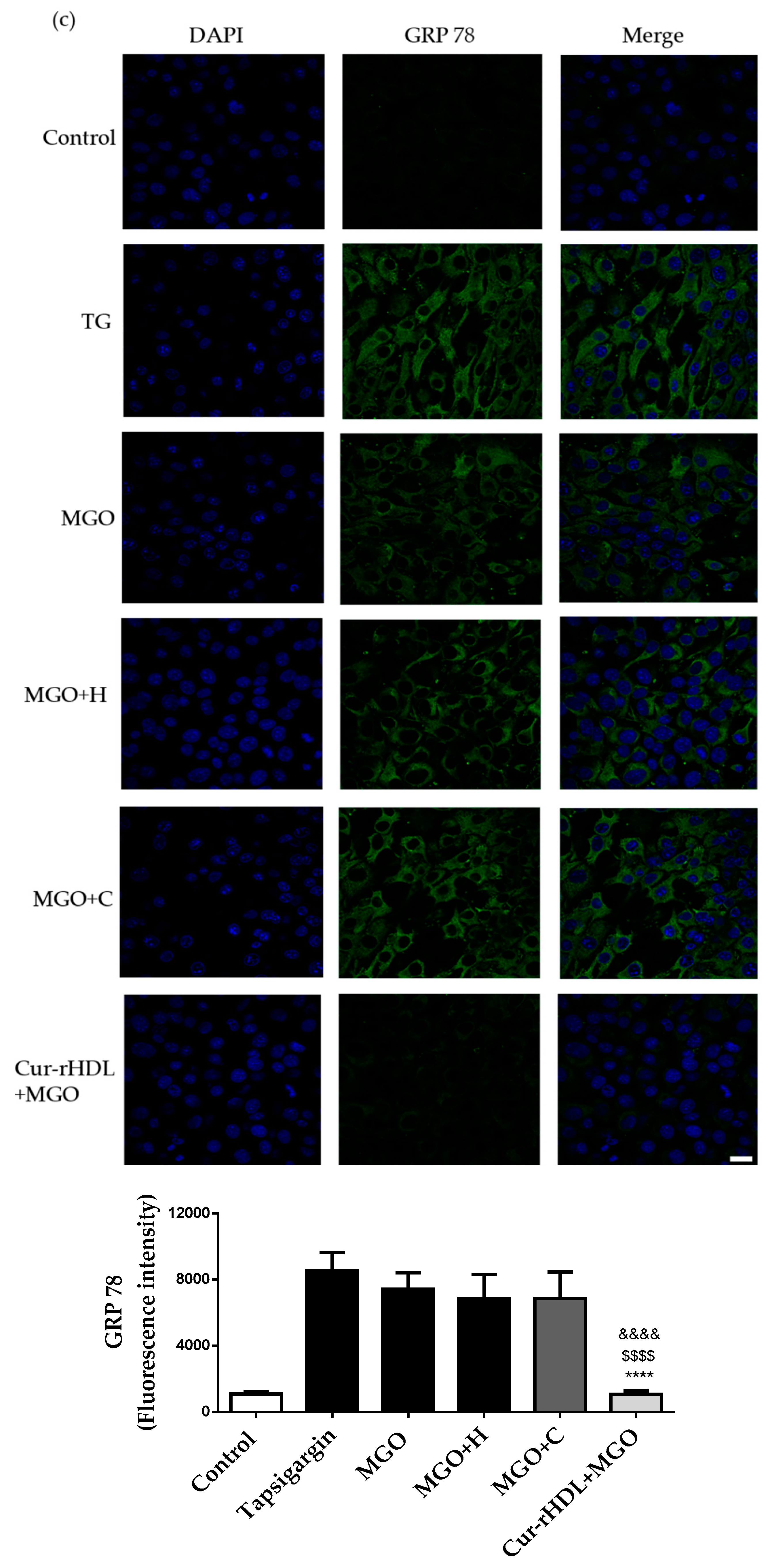
2.7. Effect of Curcumin-Enriched rHDLs on MGO-Induced ER Stress in Cerebral Endothelial Cells
2.8. Quantification of Cellular Curcumin after Incubation of bEend 3 Cells with Curcumin-Enriched rHDLs or Curcumin Alone
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Preparation of Curcumin-Enriched rHDLs (Cur-HDLs)
4.3. Quantification of Curcumin by LC–MS Analysis
4.3.1. Extraction of Curcumin from HDL-Curcumin
4.3.2. Identification and Quantification of HDL-Curcumin by LC–MS/MS
4.3.3. Preparation of Standard Solution and Calibration Curve
4.4. Determination of Particle Siz
4.5. Evaluation of Cell Viability
4.6. Evaluation of Intracellular ROS
4.7. Assessment of Endothelial Layer Integrity by Real-Time Electrical Impedance
4.8. Evaluation of Chromatin Condensation
4.9. Immunocytochemistry
4.10. Intracellular Uptake of Curcumin-Enriched rHDLs
4.11. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- McLellan, A.C.; Thornalley, P.J.; Benn, J.; Sonksen, P.H. Glyoxalase system in clinical diabetes mellitus and correlation with diabetic complications. Clin. Sci. 1994, 87, 21–29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Eupen, M.G.A.; Schram, M.T.; Colhoun, H.M.; Hanssen, N.M.J.; Niessen, H.W.M.; Tarnow, L.; Parving, H.H.; Rossing, P.; Stehouwer, C.D.A.; Schalkwijk, C.G. The methylglyoxal-derived AGE tetrahydropyrimidine is increased in plasma of individuals with type 1 diabetes mellitus and in atherosclerotic lesions and is associated with sVCAM-1. Diabetologia 2013, 56, 1845–1855. [Google Scholar] [CrossRef] [PubMed]
- Kilhovd, B.K.; Giardino, I.; Torjesen, P.; Birkeland, K.; Berg, T.; Thornalley, P.; Brownlee, M.; Hanssen, K. Increased serum levels of the specific AGE-compound methylglyoxal-derived hydroimidazolone in patients with type 2 diabetes. Metabolism 2003, 52, 163–167. [Google Scholar] [CrossRef] [PubMed]
- Hanssen, N.M.J.; Westerink, J.; Scheijen, J.L.; van der Graaf, Y.; Stehouwer, C.D.; Schalkwijk, C.G.; Algra, A.; Grobbee, R.D.; Rutten, G.E.; Visseren, F.L.; et al. Higher Plasma Methylglyoxal Levels Are Associated With Incident Cardiovascular Disease and Mortality in Individuals with Type 2 Diabetes. Diabetes Care 2018, 41, 1689–1695. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bourajjaj, M.; Stehouwer, C.; Van Hinsbergh, V.; Schalkwijk, C. Role of methylglyoxal adducts in the development of vascular complications in diabetes mellitus. Biochem. Soc. Trans. 2003, 31, 1400–1402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamawaki, H.; Saito, K.; Okada, M.; Hara, Y. Methylglyoxal mediates vascular inflammation via JNK and p38 in human endothelial cells. Am. J. Physiol. Physiol. 2008, 295, 1510–1517. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, W.; Chen, Z.; Yan, M.; He, P.; Chen, Z.; Dai, H. The protective role of isorhamnetin on human brain microvascular endothelial cells from cytotoxicity induced by methylglyoxal and oxygen-glucose deprivation. J. Neurochem. 2016, 136, 651–659. [Google Scholar] [CrossRef] [PubMed]
- Navarro, V. The Arguments against a National Health Program: Science or Ideology? Int. J. Heal. Serv. 1988, 18, 179–189. [Google Scholar] [CrossRef] [PubMed]
- Santini, S.J.; Cordone, V.; Mijit, M.; Bignotti, V.; Aimola, P.; Dolo, V.; Falone, S.; Amicarelli, F. SIRT1-Dependent Upregulation of Antiglycative Defense in HUVECs Is Essential for Resveratrol Protection against High Glucose Stress. Antioxidants 2019, 8, 346. [Google Scholar] [CrossRef] [Green Version]
- Tang, D.; Xiao, W.; Gu, W.-T.; Zhang, Z.-T.; Xu, S.-H.; Chen, Z.-Q.; Xu, Y.-H.; Zhang, L.-Y.; Wang, S.-M.; Nie, H. Pterostilbene prevents methylglyoxal-induced cytotoxicity in endothelial cells by regulating glyoxalase, oxidative stress and apoptosis. Food Chem. Toxicol. 2021, 153, 112244. [Google Scholar] [CrossRef]
- Hsuuw, Y.D.; Chang, C.-K.; Chan, W.-H.; Yu, J.-S. Curcumin prevents methylglyoxal-induced oxidative stress and apoptosis in mouse embryonic stem cells and blastocysts. J. Cell. Physiol. 2005, 205, 379–386. [Google Scholar] [CrossRef]
- Chan, W.H.; Wu, H.J.; Hsuuw, Y.D. Curcumin Inhibits ROS Formation and Apoptosis in Methylglyoxal-Treated Human Hepatoma G2 Cells. Ann. NY Acad. Sci. 2005, 1042, 372–378. [Google Scholar] [CrossRef]
- Tran-Dinh, A.; Levoye, A.; Couret, D.; Galle-Treger, L.; Moreau, M.; Delbosc, S.; Hoteit, C.; Montravers, P.; Amarenco, P.; Huby, T.; et al. High-Density Lipoprotein Therapy in Stroke: Evaluation of Endothelial SR-BI-Dependent Neuroprotective Effects. Int. J. Mol. Sci. 2021, 22, 106. [Google Scholar] [CrossRef]
- Tran-Dinh, A.; Diallo, D.; Delbosc, S.; Varela-Perez, L.M.; Dang, Q.B.; Lapergue, B.; Burillo, E.; Michel, J.B.; Levoye, A.; Martin-Ventura, J.L.; et al. HDL and endothelial protection. Br. J. Pharmacol. 2013, 169, 493–511. [Google Scholar] [CrossRef] [Green Version]
- Gonzales, C.M.; Dalmolin, L.F.; da Silva, K.A.; Slade, N.B.L.; Lopez, R.F.V.; Moreto, J.A.; Schwarz, K. New Insights of Turmeric Extract-Loaded PLGA Nanoparticles: Development, Characterization and In Vitro Evaluation of Antioxidant Activity. Mater. Veg. 2021, 76, 507–515. [Google Scholar] [CrossRef]
- Kim, D.; Kim, K.-A.; Kim, J.-H.; Kim, E.-H.; Bae, O.-N. Methylglyoxal-Induced Dysfunction in Brain Endothelial Cells via the Suppression of Akt/HIF-1α Pathway and Activation of Mitophagy Associated with Increased Reactive Oxygen Species. Antioxidants 2020, 9, 820. [Google Scholar] [CrossRef] [PubMed]
- Koike, S.; Toriumi, K.; Kasahara, S.; Kibune, Y.; Ishida, Y.-I.; Dan, T.; Miyata, T.; Arai, M.; Ogasawara, Y. Accumulation of Carbonyl Proteins in the Brain of Mouse Model for Methylglyoxal Detoxification Deficits. Antioxidants 2021, 10, 574. [Google Scholar] [CrossRef]
- Rabbani, N.; Thornalley, P.J. Glyoxalase in diabetes, obesity and related disorders. Semin. Cell Dev. Biol. 2011, 22, 309–317. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, K.A.; Shin, Y.J.; Kim, H.; Majid, A.; Bae, O.N. Methylglyoxal induced advanced glycation end products (AGE)/receptor for AGE (RAGE)-mediated angiogenic impairment in bone marrow-derived endothelial progenitor cells. J. Toxicol. Environ. Health Part A 2018, 81, 266–277. [Google Scholar] [CrossRef]
- Vulesevic, B.; McNeill, B.; Giacco, F.; Maeda, K.; Blackburn, N.J.R.; Brownlee, M.; Milne, R.W.; Suuronen, E.J. Methylglyoxal-Induced Endothelial Cell Loss and Inflammation Contribute to the Development of Diabetic Cardiomyopathy. Diabetes 2016, 65, 1699–1713. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chan, W.H.; Wu, H.J. Methylglyoxal and high glucose co-treatment induces apoptosis or necrosis in human umbilical vein endothelial cells. J. Cell. Biochem. 2008, 103, 1144–1157. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, D.; Chandra, D.; Kale, R.K. Influence of methylglyoxal on antioxidant enzymes and oxidative damage. Toxicol. Lett. 1997, 93, 141–152. [Google Scholar] [CrossRef]
- Weber, V.; Olzscha, H.; Längrich, T.; Hartmann, C.; Jung, M.; Hofmann, B.; Horstkorte, R.; Bork, K. Glycation Increases the Risk of Microbial Traversal through an Endothelial Model of the Human Blood-Brain Barrier after Use of Anesthetics. J. Clin. Med. 2020, 9, 3672. [Google Scholar] [CrossRef]
- Miyazawa, N.; Abe, M.; Souma, T.; Tanemoto, M.; Abe, T.; Nakayama, M.; Ito, S. Methylglyoxal augments intracellular oxidative stress in human aortic endothelial cells. Free Radic. Res. 2009, 44, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Maloney, R.E.; Circu, M.L.; Alexander, J.S.; Aw, T.Y. Acute carbonyl stress induces occludin glycation and brain microvascular endothelial barrier dysfunction: Role for glutathione-dependent metabolism of methylglyoxal. Free Radic. Biol. Med. 2012, 54, 51–61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zannis, V.I.; Fotakis, P.; Koukos, G.; Kardassis, D.; Ehnholm, C.; Jauhiainen, M.; Chroni, A. HDL Biogenesis, Remodeling, and Catabolism. Anxiety Anxiolytic Drugs 2014, 224, 53–111. [Google Scholar]
- Kim, S.; Kim, S.; Hwang, A.R.; Choi, H.C.; Lee, J.Y.; Woo, C.H. Apelin-13 inhibits methylglyoxal-induced unfolded protein responses and endothelial dysfunction via regulating AMPK pathway. Int. J. Mol. Sci. 2020, 21, 4069. [Google Scholar] [CrossRef] [PubMed]
- Chan, C.M.; Huang, D.-Y.; Huang, Y.-P.; Hsu, S.-H.; Kang, L.-Y.; Shen, C.-M.; Lin, W.W. Methylglyoxal induces cell death through endoplasmic reticulum stress-associated ROS production and mitochondrial dysfunction. J. Cell. Mol. Med. 2016, 20, 1749–1760. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liebner, S.; Plate, K.H. Differentiation of the brain vasculature: The answer came blowing by the Wnt. J. Angiogenesis Res. 2010, 2, 1. [Google Scholar] [CrossRef] [Green Version]
- Japp, A.G.; Newby, D.E. The apelin–APJ system in heart failure: Pathophysiologic relevance and therapeutic potential. Biochem. Pharmacol. 2008, 75, 1882–1892. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Zhu, W.; Zhang, P.; Chen, K.; Zhao, L.; Li, J.; Wei, M.; Liu, M. Apelin-13 stimulates angiogenesis by promoting cross-talk between AMP-activated protein kinase and Akt signaling in myocardial microvascular endothelial cells. Mol. Med. Rep. 2014, 9, 1590–1596. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jutooru, I.; Chadalapaka, G.; Lei, P.; Safe, S. Inhibition of NFκB and pancreatic cancer cell and tumor growth by curcumin is dependent on specificity protein down-regulation. J. Biol. Chem. 2010, 285, 25332–25344. [Google Scholar] [CrossRef] [Green Version]
- Chhunchha, B.; Fatma, N.; Kubo, E.; Rai, P.; Singh, S.P.; Singh, D.P. Curcumin abates hypoxia-induced oxidative stress based-ER stress-mediated cell death in mouse hippocampal cells (HT22) by controlling Prdx6 and NF-κB regulation. Am. J. Physiol. Cell Physiol. 2013, 304, 636–655. [Google Scholar] [CrossRef] [Green Version]
- Holder, G.M.; Plummer, J.L.; Ryan, A.J. The Metabolism and Excretion of Curcumin (1,7-Bis-(4-hydroxy-3-methoxyphenyl)-1,6-heptadiene-3,5-dione) in the Rat. Xenobiotica 1978, 8, 761–768. [Google Scholar] [CrossRef] [PubMed]
- Pan, M.H.; Huang, T.M.; Lin, J.K. Biotransformation of curcumin through reduction and glucuronidation in mice. Drug Metab. Dispos. 1999, 27, 486–494. [Google Scholar]
- Júnior, O.V.R.; Alves, B.D.S.; Barros, P.A.B.; Rodrigues, J.L.; Ferreira, S.P.; Monteiro, L.K.S.; Araújo, G.D.M.S.; Fernandes, S.S.; Vaz, G.R.; Dora, C.L.; et al. Nanoemulsion Improves the Neuroprotective Effects of Curcumin in an Experimental Model of Parkinson’s Disease. Neurotox. Res. 2021, 39, 787–799. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Mahajan, S.D.; Kutscher, H.L.; Kim, S.; Prasad, P.N. Curcumin-Pluronic Nanoparticles: A Theranostic Nanoformulation for Alzheimer’s Disease. Crit. Rev. Biomed. Eng. 2020, 48, 153–168. [Google Scholar] [CrossRef]
- Jutkova, A.; Chorvat, D.; Miskovsky, P.; Jancura, D.; Datta, S. Encapsulation of anticancer drug curcumin and co-loading with photosensitizer hypericin into lipoproteins investigated by fluorescence resonance energy transfer. Int. J. Pharm. 2019, 564, 369–378. [Google Scholar] [CrossRef] [PubMed]
- Paternò, R.; Ruocco, A.; Postiglione, A.; Hubsch, A.; Andresen, I.; Lang, M.G. Reconstituted High-Density Lipoprotein Exhibits Neuroprotection in Two Rat Models of Stroke. Cerebrovasc. Dis. 2003, 17, 204–211. [Google Scholar] [CrossRef]
- Sulliman, N.C.; Ghaddar, B.; Gence, L.; Patche, J.; Rastegar, S.; Meilhac, O.; Diotel, N. HDL biodistribution and brain receptors in zebrafish, using HDLs as vectors for targeting endothelial cells and neural progenitors. Sci. Rep. 2021, 11, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Couret, D.; Planesse, C.; Patche, J.; Diotel, N.; Nativel, B.; Bourane, S.; Meilhac, O. Lack of Neuroprotective Effects of High-Density Lipoprotein Therapy in Stroke under Acute Hyperglycemic Conditions. Molecules 2021, 26, 6365. [Google Scholar] [CrossRef]
- Hatia, S.; Septembre-Malaterre, A.; Le Sage, F.; Badiou-Bénéteau, A.; Baret, P.; Payet, B.; D’Hellencourt, C.L.; Gonthier, M.-P. Evaluation of antioxidant properties of major dietary polyphenols and their protective effect on 3T3-L1 preadipocytes and red blood cells exposed to oxidative stress. Free Radic. Res. 2014, 48, 387–401. [Google Scholar] [CrossRef] [PubMed]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Narra, S.S.; Rosanaly, S.; Rondeau, P.; Patche, J.; Veeren, B.; Gonthier, M.-P.; Viranaicken, W.; Diotel, N.; Ravanan, P.; Hellencourt, C.L.d.; et al. ApoA-I Nanoparticles as Curcumin Carriers for Cerebral Endothelial Cells: Improved Cytoprotective Effects against Methylglyoxal. Pharmaceuticals 2022, 15, 347. https://doi.org/10.3390/ph15030347
Narra SS, Rosanaly S, Rondeau P, Patche J, Veeren B, Gonthier M-P, Viranaicken W, Diotel N, Ravanan P, Hellencourt CLd, et al. ApoA-I Nanoparticles as Curcumin Carriers for Cerebral Endothelial Cells: Improved Cytoprotective Effects against Methylglyoxal. Pharmaceuticals. 2022; 15(3):347. https://doi.org/10.3390/ph15030347
Chicago/Turabian StyleNarra, Sai Sandhya, Sarah Rosanaly, Philippe Rondeau, Jessica Patche, Bryan Veeren, Marie-Paule Gonthier, Wildriss Viranaicken, Nicolas Diotel, Palaniyandi Ravanan, Christian Lefebvre d’ Hellencourt, and et al. 2022. "ApoA-I Nanoparticles as Curcumin Carriers for Cerebral Endothelial Cells: Improved Cytoprotective Effects against Methylglyoxal" Pharmaceuticals 15, no. 3: 347. https://doi.org/10.3390/ph15030347
APA StyleNarra, S. S., Rosanaly, S., Rondeau, P., Patche, J., Veeren, B., Gonthier, M.-P., Viranaicken, W., Diotel, N., Ravanan, P., Hellencourt, C. L. d., & Meilhac, O. (2022). ApoA-I Nanoparticles as Curcumin Carriers for Cerebral Endothelial Cells: Improved Cytoprotective Effects against Methylglyoxal. Pharmaceuticals, 15(3), 347. https://doi.org/10.3390/ph15030347





