Gene Expression Profiling and Protein Analysis Reveal Suppression of the C-Myc Oncogene and Inhibition JAK/STAT and PI3K/AKT/mTOR Signaling by Thymoquinone in Acute Myeloid Leukemia Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Line and Growth Media
2.2. TQ Treatment
2.3. RNA Extraction
2.4. Next-Generation Sequencing for mRNA Expression Profiling
2.5. DAVID Database Analysis
2.6. Reverse Transcription Quantitative Polymerase Chain Reaction (RT-qPCR)
2.7. Protein Analysis Using Jess Simple Western Analysis
2.8. Statistical Analysis
3. Results
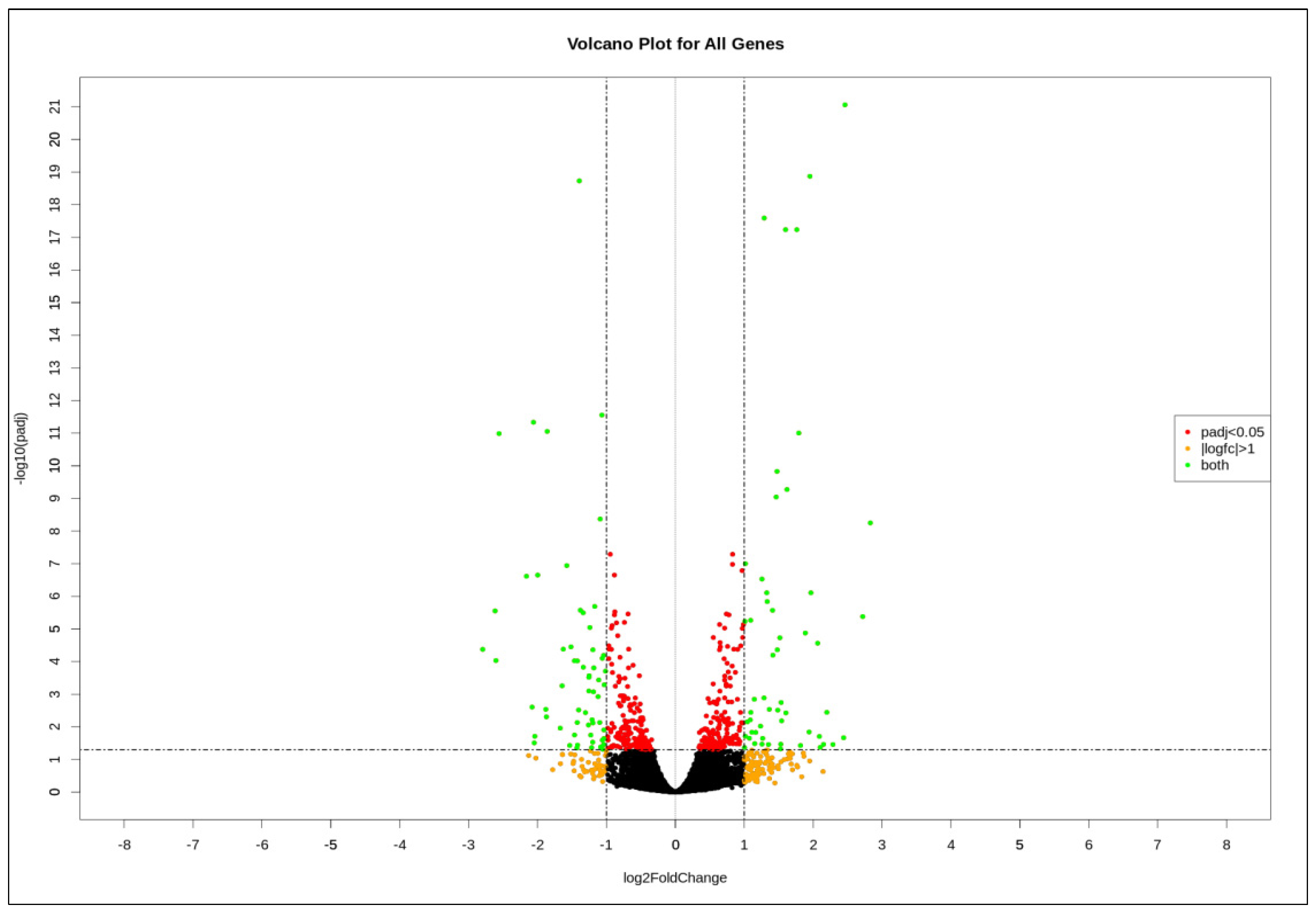
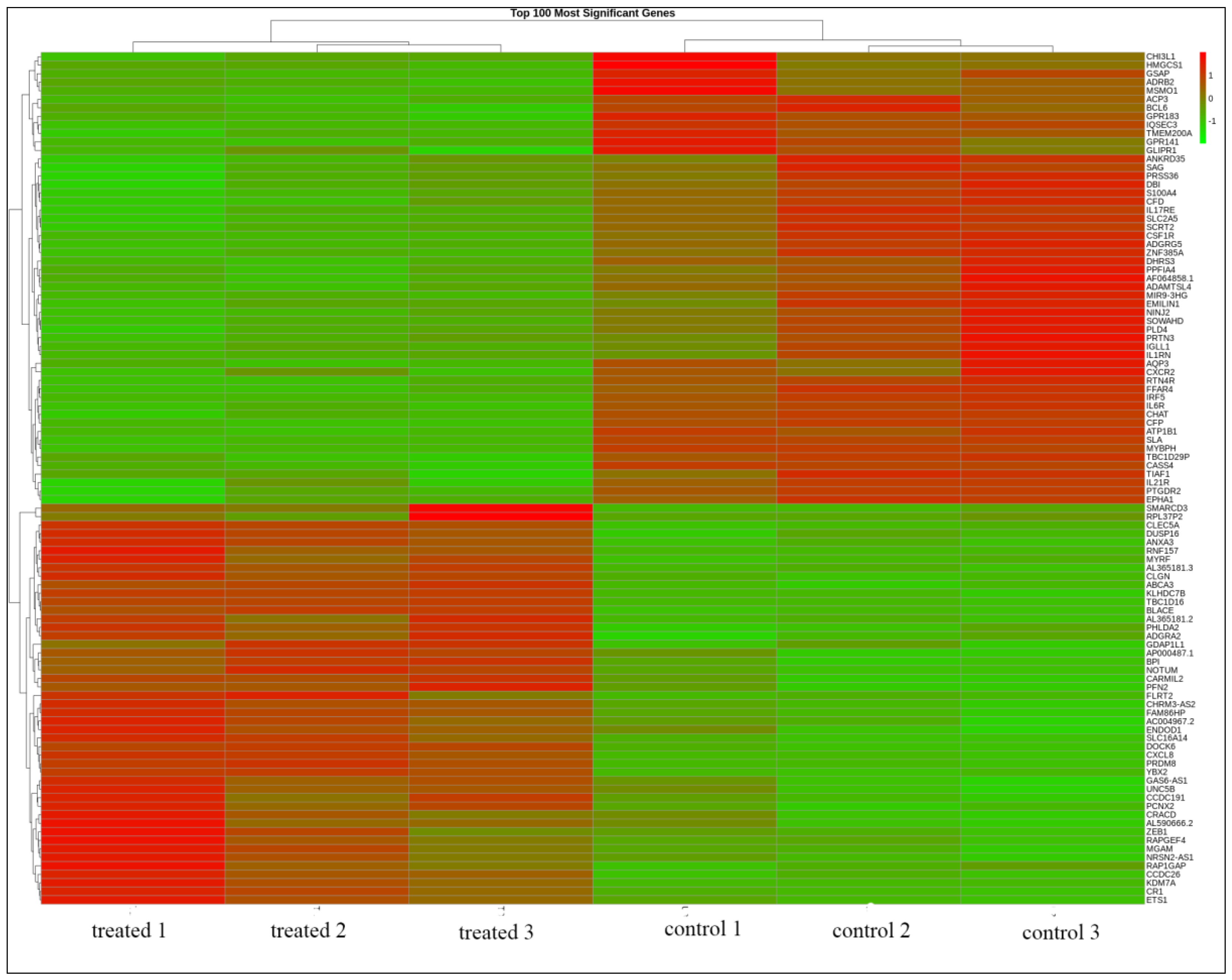
3.1. Thymoquinone Induced Differentially Expressed Genes in HL60 Cells
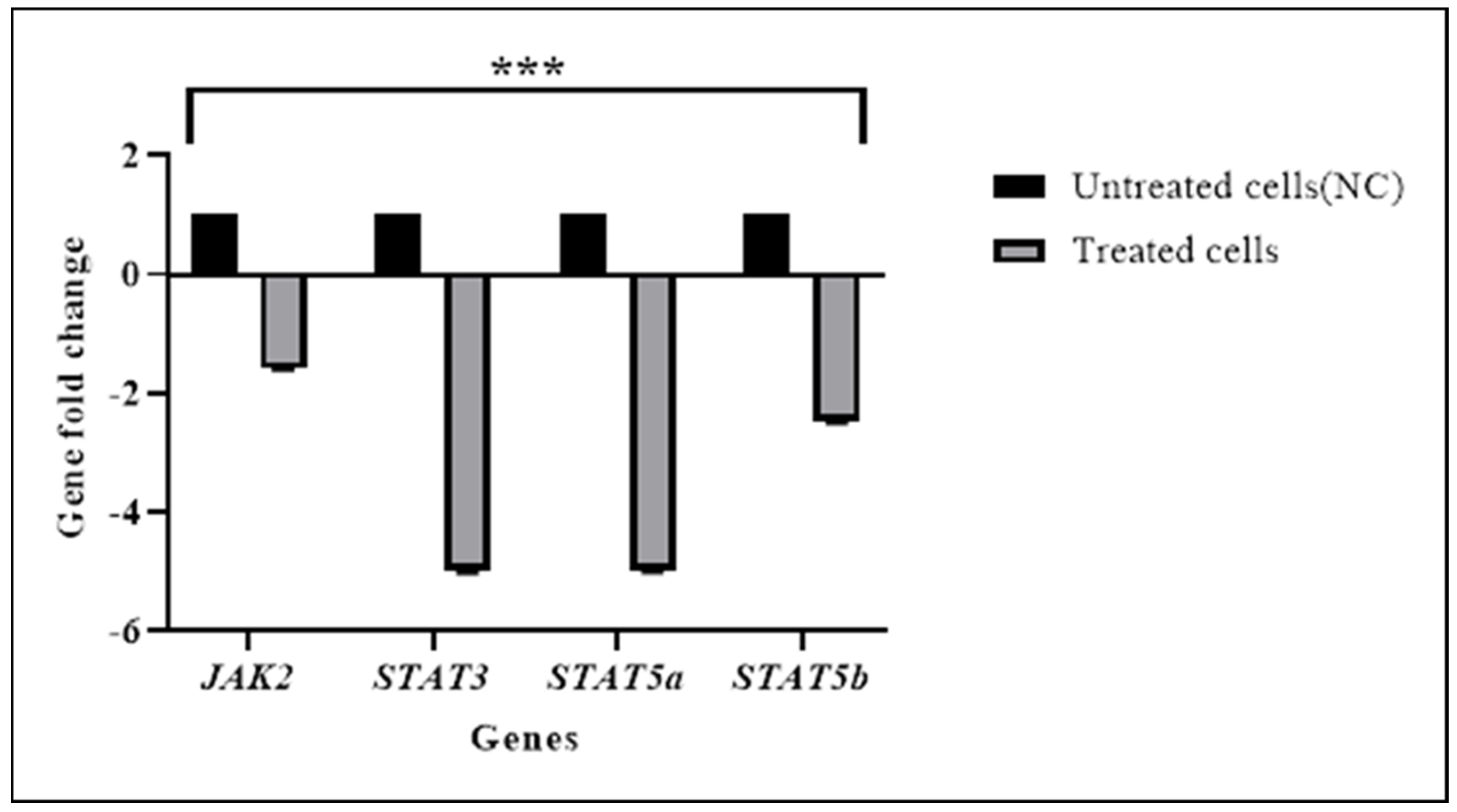
3.2. TQ Downregulated the Expression of JAK/STAT Pathways Genes
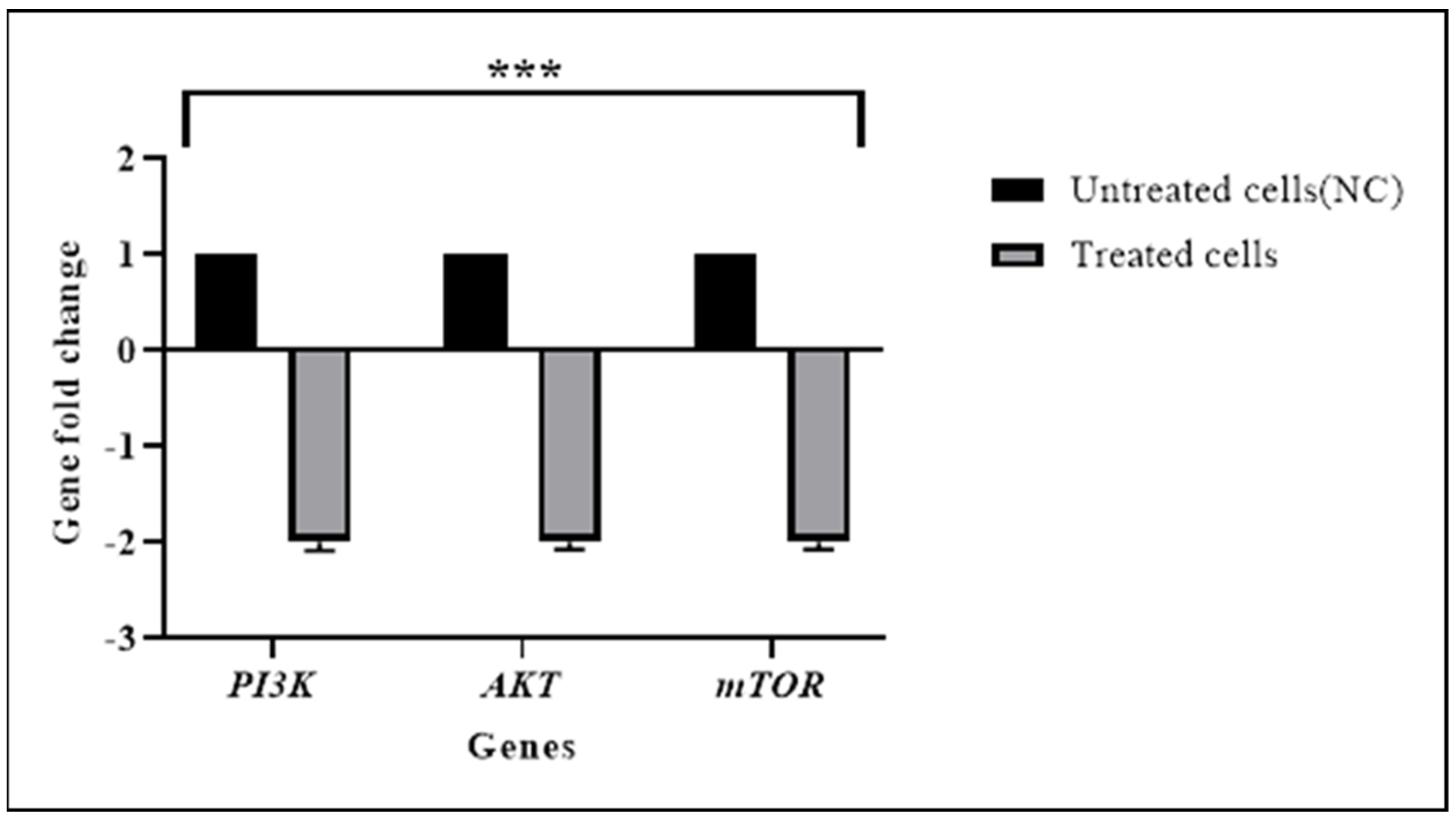
3.3. TQ Downregulated the Expression of the PI3K/AKT Pathway Genes
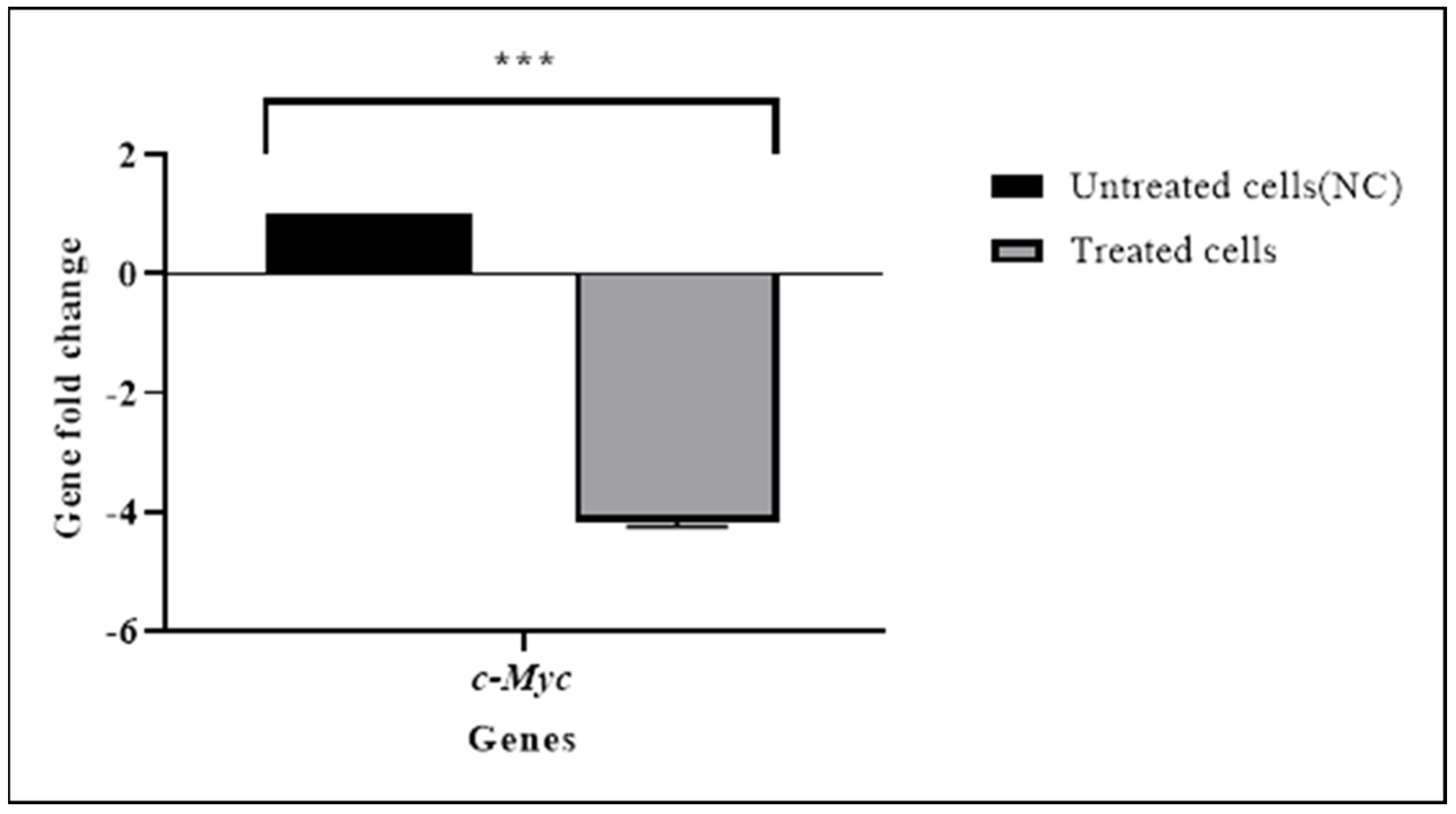
3.4. TQ Downregulated the Expression of the c-Myc Gene
3.5. TQ Inhibited JAK/STAT Signaling
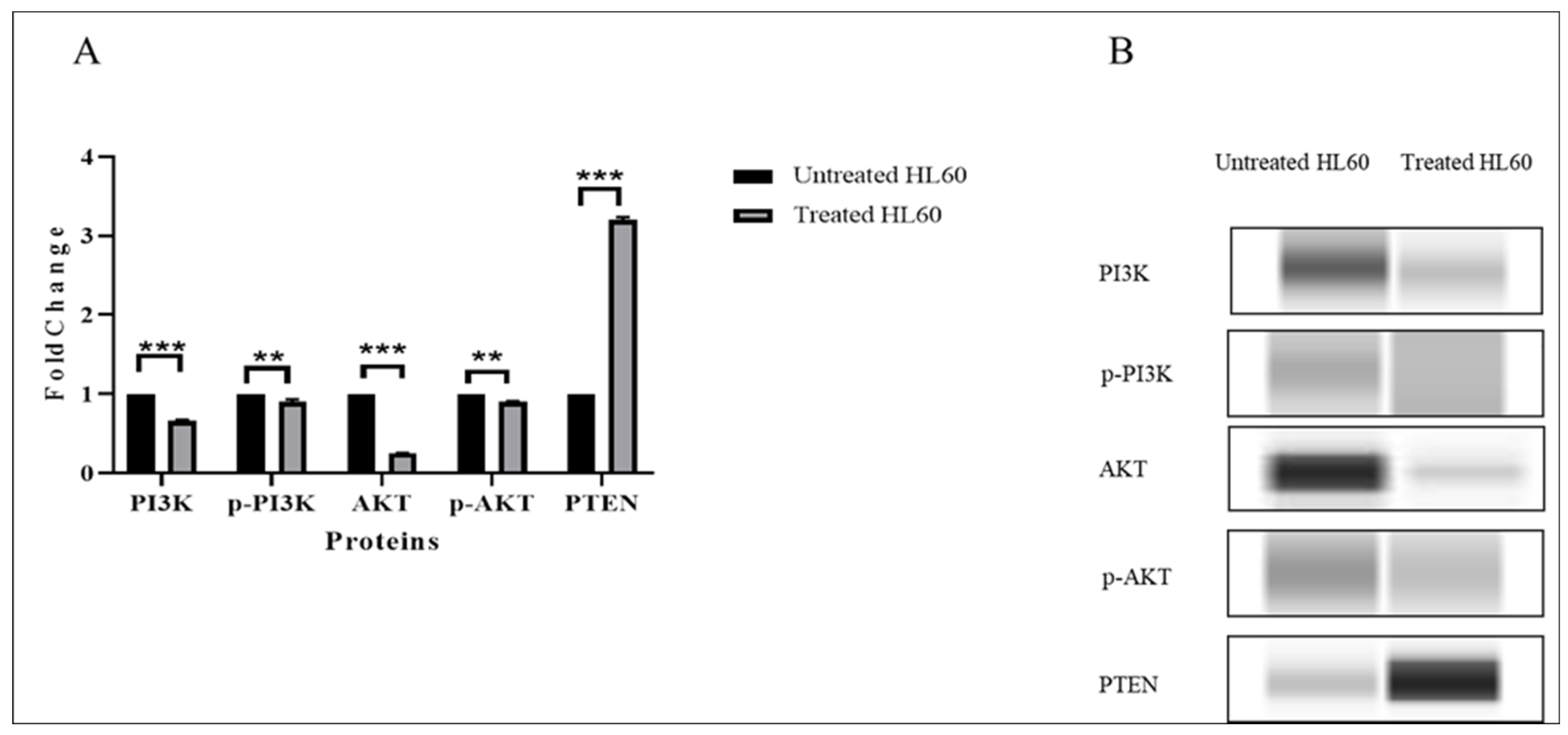
3.6. TQ Inhibited PI3K/AKT Signaling
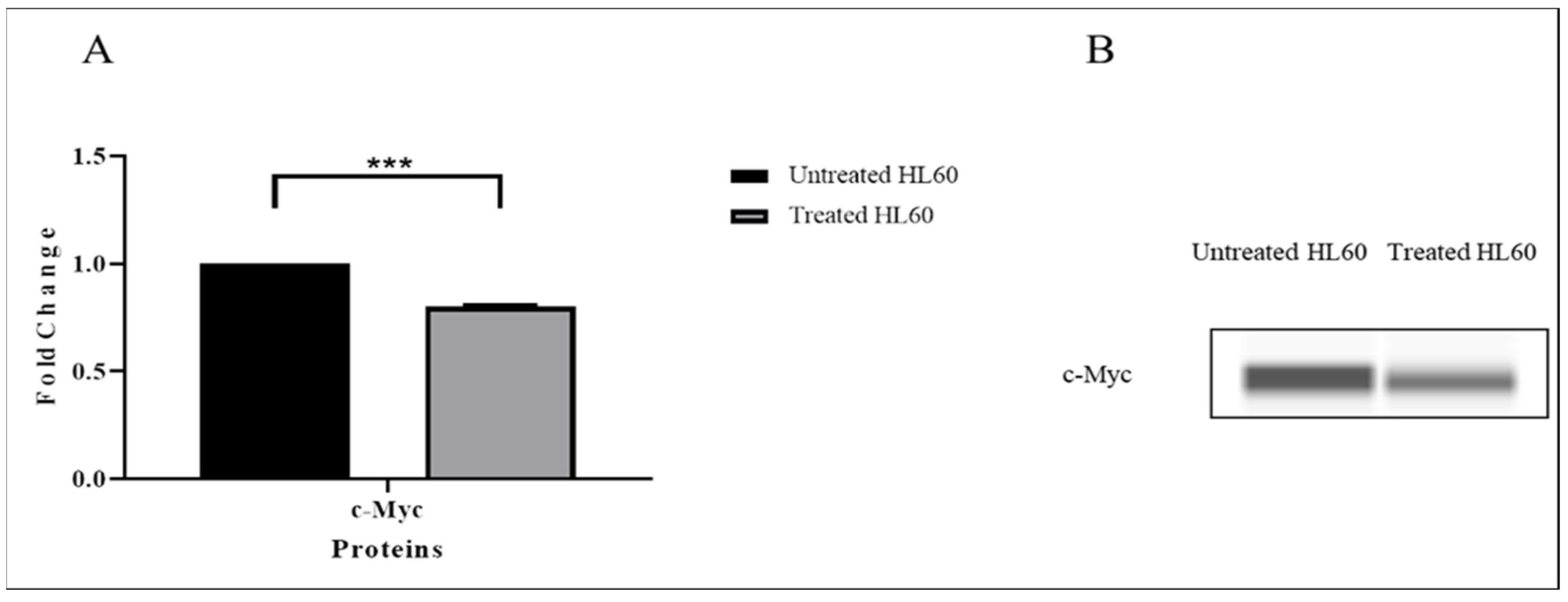
3.7. TQ Inhibited c-Myc Protein Expression
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yamamoto, J.-F.; Goodman, M.-T. Patterns of leukemia incidence in the United States by subtype and demographic characteristics, 1997–2002. Cancer Causes Control 2008, 19, 379–390. [Google Scholar] [CrossRef] [PubMed]
- Bullinger, L.; Döhner, K.; Döhner, H. Genomics of acute myeloid leukemia diagnosis and pathways. J. Clin. Oncol. 2017, 35, 934–946. [Google Scholar] [CrossRef] [PubMed]
- Maniwa, Y.; Kasukabe, T.; Kumakura, S. Vitamin K2 and cotylenin A synergistically induce monocytic differentiation and growth arrest along with the suppression of c-MYC expression and induction of cyclin G2 expression in human leukemia HL-60 cells. Int. J. Oncol. 2015, 47, 473–480. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Letellier, E.; Haan, S. SOCS2: Physiological and pathological functions. Front. Biosci. 2016, 8, 189–204. [Google Scholar]
- Song, W.; Liu, M.-G.; Zhang, J.-B.; Zhang, J.-J.; Sun, M.-M.; Yu, Q.-K. Mechanism of action of EBV, Bcl-2, p53, c-Myc and Rb in non-Hodgkin’s lymphoma. Eur. Rev. Med. Pharmacol. Sci. 2016, 20, 1093–1097. [Google Scholar] [PubMed]
- Chen, H.; Liu, H.; Qing, G. Targeting oncogenic Myc as a strategy for cancer treatment. Signal Transduct. Target. Ther. 2018, 3, 5. [Google Scholar] [CrossRef] [Green Version]
- Reddy, D.; Kumavath, R.; Ghosh, P.; Barh, D. Lanatoside C induces G2/M cell cycle arrest and suppresses cancer cell growth by attenuating MAPK, Wnt, JAK-STAT, and PI3K/AKT/mTOR signaling pathways. Biomolecules 2019, 9, 792. [Google Scholar] [CrossRef] [Green Version]
- Elekofehinti, O.-O.; Oyedokun, V.-O.; Iwaloye, O.; Lawal, A.-O.; Ejelonu, O.-C. Momordica charantia silver nanoparticles modulate S OCS/JAK/STAT and P13K/Akt/PTEN signalling pathways in the kidney of streptozotocin-induced diabetic rats. J. Diabetes Metab. Disord. 2021, 20, 245–260. [Google Scholar] [CrossRef]
- McCubrey, J.-A.; Steelman, L.-S.; Abrams, S.-L.; Bertrand, F.-E.; Ludwig, D.-E.; Bäsecke, J.; Libra, M.; Stivala, F.; Milella, M.; Tafuri, A. Targeting survival cascades induced by activation of Ras/Raf/MEK/ERK, PI3K/PTEN/Akt/mTOR and Jak/STAT pathways for effective leukemia therapy. Leukemia 2008, 22, 708–722. [Google Scholar] [CrossRef] [Green Version]
- Steelman, L.S.; Pohnert, S.C.; Shelton, J.G.; Franklin, R.A.; Bertrand, F.E.; McCubrey, J.A. JAK/STAT, Raf/MEK/ERK, PI3K/Akt and BCR-ABL in cell cycle progression and leukemogenesis. Leukemia 2004, 18, 189–218. [Google Scholar] [CrossRef] [Green Version]
- Shah, A.; Andersson, T.; Rachet, B.; Björkholm, M.; Lambert, P.-C. Survival and cure of acute myeloid leukaemia in E ngland, 1971–2006: A population–based study. Br. J. Haematol. 2013, 162, 509–516. [Google Scholar] [CrossRef]
- Almajali, B.; Al-Jamal, H.A.N.; Taib, W.R.W.; Ismail, I.; Johan, M.F.; Doolaanea, A.A.; Ibrahim, W.N. Thymoquinone, as a Novel Therapeutic Candidate of Cancers. Pharmaceuticals 2021, 14, 369. [Google Scholar] [CrossRef] [PubMed]
- AlGhamdi, A.-A.; Mohammed, M.-R.; Zamzami, M.-A.; Al-Malki, A.-L.; Qari, M.-H.; Khan, M.-I.; Choudhry, H. Untargeted Metabolomics Identifies Key Metabolic Pathways Altered by Thymoquinone in Leukemic Cancer Cells. Nutrients 2020, 12, 1792. [Google Scholar] [CrossRef]
- Almajali, B.; Al-Jamal, H.A.N.; Taib, W.R.W.; Ismail, I.; Johan, M.F.; Doolaanea, A.-A.; Ibrahim, W.-N.; Tajudin, S.-A. Thymoquinone Suppresses Cell Proliferation and Enhances Apoptosis of HL60 Leukemia Cells through Re-Expression of JAK/STAT Negative Regulators. Asian Pac. J. Cancer Prev. 2021, 22, 879–885. [Google Scholar] [CrossRef] [PubMed]
- Network, Cancer Genome Atlas Research. Genomic and epigenomic landscapes of adult de novo acute myeloid leukemia. N. Engl. J. Med. 2013, 368, 2059–2074. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Papaemmanuil, E.; Gerstung, M.; Bullinger, L.; Gaidzik, V.-I.; Paschka, P.; Roberts, N.-D.; Potter, N.-E.; Heuser, M.; Thol, F.; Bolli, N. Genomic classification and prognosis in acute myeloid leukemia. N. Engl. J. Med. 2016, 374, 2209–2221. [Google Scholar] [CrossRef]
- Gatt, L.; Saliba, D.G.; Schembri-Wismayer, P.; Zammit-Mangion, M. Tyrosol, at the Concentration Found in Maltese Extra Virgin Olive Oil, Induces HL-60 Differentiation towards the Monocyte lineage. Appl. Sci. 2021, 11, 10199. [Google Scholar] [CrossRef]
- Chen, S.; Zhou, Y.; Chen, Y.; Gu, J. fastp: An ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 2018, 34, i884–i890. [Google Scholar] [CrossRef]
- Dobin, A.; Davis, C.-A.; Schlesinger, F.; Drenkow, J.; Zaleski, C.; Jha, S.; Batut, P.; Chaisson, M.; Gingeras, T.-R. STAR: Ultrafast universal RNA-seq aligner. Bioinformatics 2013, 29, 15–21. [Google Scholar] [CrossRef]
- Liao, Y.; Smyth, G.-K.; Shi, W. FeatureCounts: An efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics 2014, 30, 923–930. [Google Scholar] [CrossRef] [Green Version]
- Love, M.-I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sherman, B.-T.; Tan, Q.; Collins, J.-R.; Alvord, W.-G.; Roayaei, J.; Stephens, R.; Baseler, M.-W.; Lane, H.-C.; Lempicki, R.-A. The DAVID Gene Functional Classification Tool: A novel biological module-centric algorithm to functionally analyze large gene lists. Genome Biol. 2007, 8, R183. [Google Scholar]
- Livak, K.-J.; Schmittgen, T.-D. Analysis of relative gene expression data using real-time quantitative PCR and the 2− ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Gupta, M.; Han, J.; Stenson, M.; Maurer, M.; Wellik, L.; Hu, G.; Ziesmer, S.; Dogan, A.; Witzig, T.-E. Elevated serum IL-10 levels in diffuse large B-cell lymphoma: A mechanism of aberrant JAK2 activation. Am. J. Hematol. 2012, 119, 2844–2853. [Google Scholar] [CrossRef] [PubMed]
- Bellon, M.; Moles, R.; Chaib-Mezrag, H.; Pancewicz, J.; Nicot, C. JAG1 overexpression contributes to Notch1 signaling and the migration of HTLV-1-transformed ATL cells. J. Hematol. Oncol. 2018, 11, 119. [Google Scholar] [CrossRef]
- Rahat, B.; Thakur, S.; Bagga, R.; Kaur, J. Epigenetic regulation of STAT5A and its role as fetal DNA epigenetic marker during placental development and dysfunction. Placenta 2016, 44, 46–53. [Google Scholar] [CrossRef]
- Loaiza Cantù, C.; Ierardi, R.; Alborelli, I.; Fugazza, C.; Cassinelli, L.; Piconese, S.; Bose, F.; Ottolenghi, S.; Ferrari, G.; Ronchi, A. Sox6 enhances erythroid differentiation in human erythroid progenitors. Am. J. Hematol. 2011, 117, 3669–3679. [Google Scholar]
- Chen, M.; Lu, J.; Wei, W.; Lv, Y.; Zhang, X.; Yao, Y.; Wang, L.; Ling, T.; Zou, X. Effects of proton pump inhibitors on reversing multidrug resistance via downregulating V-ATPases/PI3K/Akt/mTOR/HIF-1α signaling pathway through TSC1/2 complex and Rheb in human gastric adenocarcinoma cells in vitro and in vivo. OncoTargets Ther. 2018, 11, 6705. [Google Scholar] [CrossRef] [Green Version]
- Yi, W.; Li, D.; Guo, Y.; Zhang, Y.; Huang, B.; Li, X. Sevoflurane inhibits the migration and invasion of glioma cells by upregulating microRNA-637. Int. J. Mol. Med. 2016, 38, 1857–1863. [Google Scholar] [CrossRef] [Green Version]
- Yu, Q.; Zhou, X.; Xia, Q.; Shen, J.; Yan, J.; Zhu, J.; Li, X.; Shu, M. Long non-coding RNA CCAT1 that can be activated by c-Myc promotes pancreatic cancer cell proliferation and migration. Am. J. Transl. Res. 2016, 8, 5444. [Google Scholar]
- Darwish, W.-S.; Ikenaka, Y.; Nakayama, S.; Mizukawa, H.; Ishizuka, M. Constitutive Effects of Lead on Aryl Hydrocarbon Receptor Gene Battery and Protection by β-carotene and Ascorbic Acid in Human HepG2 Cells. J. Food Sci. 2016, 81, T275–T281. [Google Scholar] [CrossRef] [Green Version]
- Bashash, D.; Sayyadi, M.; Safaroghli-Azar, A.; Sheikh-Zeineddini, N.; Riyahi, N.; Momeny, M. Small molecule inhibitor of c-Myc 10058-F4 inhibits proliferation and induces apoptosis in acute leukemia cells, irrespective of PTEN status. Int. J. Biochem. Cell Biol. 2019, 108, 7–16. [Google Scholar] [CrossRef]
- Pan, X.-N.; Chen, J.-J.; Wang, L.-X.; Xiao, R.-Z.; Liu, L.-L.; Fang, Z.-G.; Liu, Q.; Long, Z.-J.; Lin, D.-J. Inhibition of c-Myc overcomes cytotoxic drug resistance in acute myeloid leukemia cells by promoting differentiation. PLoS ONE 2014, 9, e105381. [Google Scholar] [CrossRef]
- Al-Jamal, H.A.N.; Johan, M.F.; Jusoh, S.A.M.; Ismail, I.; Taib, W.R.W. Re-expression of bone marrow proteoglycan-2 by 5-azacytidine is associated with STAT3 inactivation and sensitivity response to imatinib in resistant CML cells. Asian Pac. J. Cancer Prev. 2018, 19, 1585. [Google Scholar]
- Zhang, B.; Zhou, P.; Li, X.; Shi, Q.; Li, D.; Ju, X. Bitterness in sugar: O-GlcNAcylation aggravates pre-B acute lymphocytic leukemia through glycolysis via the PI3K/Akt/c-Myc pathway. Am. J. Cancer Res. 2017, 7, 1337. [Google Scholar]
- Mizutani, K.; Sud, S.; McGregor, N.-A.; Martinovski, G.; Rice, B.-T.; Craig, M.-J.; Varsos, Z.-S.; Roca, H.; Pienta, K.-J. The chemokine CCL2 increases prostate tumor growth and bone metastasis through macrophage and osteoclast recruitment. Neoplasia 2009, 11, 1235–1242. [Google Scholar] [CrossRef] [Green Version]
- Rattanaburee, T.; Tipmanee, V.; Tedasen, A.; Thongpanchang, T.; Graidist, P. Inhibition of CSF1R and AKT by (±)-kusunokinin hinders breast cancer cell proliferation. Biomed. Pharmacother. 2020, 129, 110361. [Google Scholar] [CrossRef]
- Shang, F.-M.; Li, J. A small-molecule antagonist of CXCR1 and CXCR2 inhibits cell proliferation, migration and invasion in melanoma via PI3K/AKT pathway. Med. Clin. 2019, 152, 425–430. [Google Scholar] [CrossRef]
- Denley, S.-M.; Jamieson, N.-B.; McCall, P.; Oien, K.-A.; Morton, J.-P.; Carter, C.-R.; Edwards, J.; McKay, C.-J. Activation of the IL-6R/Jak/stat pathway is associated with a poor outcome in resected pancreatic ductal adenocarcinoma. J. Gastrointest. Surg. 2013, 17, 887–898. [Google Scholar] [CrossRef]
- Chen, J.; Lan, T.; Zhang, W.; Dong, L.; Kang, N.; Zhang, S.; Fu, M.; Liu, B.; Liu, K.; Zhan, Q. Feed-forward reciprocal activation of PAFR and STAT3 regulates epithelial–mesenchymal transition in non–small cell lung Cancer. Cancer Res. 2015, 75, 4198–4210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, K.-R.; Yun, H.-M.; Yoo, K.; Ham, Y.-W.; Han, S.-B.; Hong, J.-T. Chitinase 3 like 1 suppresses the stability and activity of p53 to promote lung tumorigenesis. Cell Commun. Signal. 2020, 18, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Ren, X.; Luo, W. Exploration of pro-apoptotic effect of Thymoquinone on oral squamous cell carcinoma cells through PI3K/Akt signaling pathway. Mol. Cell Biol. 2019, 65, 61–64. [Google Scholar] [CrossRef]
- Li, F.; Rajendran, P.; Sethi, G. Thymoquinone inhibits proliferation, induces apoptosis and chemosensitizes human multiple myeloma cells through suppression of signal transducer and activator of transcription 3 activation pathway. Br. J. Pharmacol. 2010, 161, 541–554. [Google Scholar] [CrossRef] [Green Version]
- Chen, Z.; Gao, W.; Pu, L.; Zhang, L.; Han, G.; Zuo, X.; Zhang, Y.; Li, X.; Shen, H.; Wu, J. PRDM8 exhibits antitumor activities toward hepatocellular carcinoma by targeting NAP1L1. Hepatology 2018, 68, 994–1009. [Google Scholar] [CrossRef] [Green Version]
- Wang, N.; Li, Y.; Wei, J.; Pu, J.; Liu, R.; Yang, Q.; Guan, H.; Shi, B.; Hou, P.; Ji, M. TBX1 functions as a tumor suppressor in thyroid cancer through inhibiting the activities of the PI3K/AKT and MAPK/ERK pathways. Thyroid 2019, 29, 378–394. [Google Scholar] [CrossRef]
- Su, W.; Guo, C.; Wang, L.; Wang, Z.; Yang, X.; Niu, F.; Tzou, D.; Yang, X.; Huang, X.; Wu, J. LncRNA MIR22HG abrogation inhibits proliferation and induces apoptosis in esophageal adenocarcinoma cells via activation of the STAT3/c-Myc/FAK signaling. Aging 2019, 11, 4587. [Google Scholar] [CrossRef]
- Chen, Y.; Han, L.; Bai, L.; Tang, H.; Zheng, A. Trichosanthin inhibits the proliferation of cervical cancer cells and downregulates STAT-5/C-myc signaling pathway. Pathol. Res. Pract. 2019, 215, 632–638. [Google Scholar] [CrossRef]
- Al-Rawashde, F.A.; Johan, M.F.; Taib, W.R.W.; Ismail, I.; Johari, S.A.T.T.; Almajali, B.; Al-Wajeeh, A.S.; Nazari, V.M.; Al-Jamal, H.A.N. Thymoquinone Inhibits Growth of Acute Myeloid Leukemia Cells through Reversal SHP-1 and SOCS-3 Hypermethylation: In Vitro and In Silico Evaluation. Pharmaceuticals 2021, 14, 1287. [Google Scholar] [CrossRef]
- Al-Rawashde, F.A.; Wan Taib, W.R.; Ismail, I.; Johan, M.F.; Al-Wajeeh, A.S.; Al-Jamal, H.A.N. Thymoquinone Induces Downregulation of BCR-ABL/JAK/STAT Pathway and Apoptosis in K562 Leukemia Cells. Asian Pac. J. Cancer Prev. 2021, 22, 3959–3965. [Google Scholar] [CrossRef]
- Zhang, H.-F.; Wu, C.; Alshareef, A.; Gupta, N.; Zhao, Q.; Xu, X.-E.; Jiao, J.-W.; Li, E.-M.; Xu, L.-Y.; Lai, R. The PI3K/AKT/c-MYC axis promotes the acquisition of cancer stem-like features in esophageal squamous cell carcinoma. Stem. Cells 2016, 34, 2040–2051. [Google Scholar] [CrossRef] [Green Version]
- Swords, R.-T.; Schenk, T.; Stengel, S.; Gil, V.-S.; Petrie, K.-R.; Perez, A.; Ana, R.; Watts, J.-M.; Vargas, F.; Elias, R. Inhibition of the PI3K/AKT/mTOR pathway leads to down-regulation of c-Myc and overcomes resistance to ATRA in acute myeloid leukemia. Blood 2015, 126, 1363. [Google Scholar] [CrossRef]
- Chen, A.; Koehler, A.-N. Transcription factor inhibition: Lessons learned and emerging targets. Trends. Mol. Med. 2020, 26, 508–518. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.-Q.; Wang, J.; Guo, X.-F.; Liu, Z.; Dong, W.-G. Thymoquinone inhibits proliferation in gastric cancer via the STAT3 pathway in vivo and in vitro. World J. Gastroenterol. 2016, 22, 4149. [Google Scholar] [CrossRef]
- Pang, J.; Shen, N.; Yan, F.; Zhao, N.; Dou, L.; Wu, L.-C.; Seiler, C.-L.; Yu, L.; Yang, K.; Bachanova, V. Thymoquinone exerts potent growth-suppressive activity on leukemia through DNA hypermethylation reversal in leukemia cells. Oncotarget 2017, 8, 34453. [Google Scholar] [CrossRef] [Green Version]
- Braglia, L.; Zavatti, M.; Vinceti, A.; Martelli, A.-M.; Marmiroli, S. Deregulated PTEN/PI3K/AKT/mTOR signaling in prostate cancer: Still a potential druggable target? Biochim. Biophys. Acta Mol. Cell Res. 2020, 1867, 118731. [Google Scholar] [CrossRef]
- Afrose, S.-S.; Junaid, M.-D.; Akter, Y.; Tania, M.; Zheng, M.; Khan, M.-D. Targeting kinases with thymoquinone: A molecular approach to cancer therapeutics. Drug. Discov. 2020, 25, 2294–2306. [Google Scholar] [CrossRef]
- Krygier, A.; Szmajda-Krygier, D.; Sałagacka-Kubiak, A.; Jamroziak, K.; Żebrowska-Nawrocka, M.; Balcerczak, E. Association between the CEBPA and c-MYC genes expression levels and acute myeloid leukemia pathogenesis and development. Med. Oncol. 2020, 37, 109. [Google Scholar] [CrossRef]




| Gene Name | Primer Sequence (5′ to 3′) | References |
|---|---|---|
| JAK2 | F: TGTCTTACCTCTTTGCTCAGTGGCG R: CAATGACATTTTCTCGCTCGACAGC | [24] |
| STAT3 | F: GATTGACCAGCAGTATAGCCGCTTC R: CTGCAGTCTGTAGAAGGCGTG | [25] |
| STAT5a | F: GTCCTGAAGACCCAGACCAA R: GTTGCGGGTGTTCTCATTTT | [26] |
| STAT5b | F: CATTTTCCCATTGAGGTGCG R: GGGTGGCCTTAATGTTCTCC | [27] |
| PI3K | F: TTAGCTATTCCCACGCAGGA R: CACAATAGTGTCTGTGACTC | [28] |
| AKT | F: CTGAGATTGTGTCAGCCCTG R: CACAGCCCGAAGTCTGTGATCTTA | [29] |
| mTOR | F: ATGCAGCTGTCCTGGTTCTC R: AATCAGACAGGCACGAAG | [28] |
| c-Myc | F: CCACAGCAAACCTCCTCACA R: TCCAACTTGACCCTCTTGGC | [30] |
| β-actin | F: CTGGCACCCAGGACAATG R: GCCGATCCACACGGAGTA | [31] |
| Category | Term | Similarity Score | p-Value | Genes |
|---|---|---|---|---|
| GOTERM_BP_DIRECT | Positive regulation of endothelial cell migration | 1.00 | 0.0037 | ETS1, ADGRA2, and ANXA3 |
| GOTERM_BP_DIRECT | Regulation of angiogenesis | 0.79 | 0.0037 | ETS1, ADGRA2, and ANXA3 |
| GOTERM_BP_DIRECT | Positive regulation of angiogenesis | 1.00 | 0.021 | CXCL8, ETS1, and ANXA3 |
| KEGG_PATHWAY | Pathways in cancer | 0.79 | 0.021 | CXCL8, ETS1, and ANXA3 |
| GOTERM_BP_DIRECT | Oligodendrocyte development | 1.00 | 0.044 | PRDM8 and MYRF |
| GOTERM_BP_DIRECT | Immune response | 1.00 | 0.049 | CXCL8, ETS1, and BPI |
| GOTERM_BP_DIRECT | Negative regulation of cell proliferation | 0.85 | 0.049 | CXCL8, ETS1, and BPI |
| GOTERM_MF_DIRECT | Ras GTPase binding | 1.00 | 0.029 | RAP1GAP and RAPGEF4 |
| KEGG_PATHWAY | Rap1 signaling pathway | 0.79 | 0.029 | RAP1GAP and RAPGEF4 |
| Category | Term | Similarity Score | p-Value | Genes |
|---|---|---|---|---|
| GOTERM_BP_DIRECT | Cytokine-mediated signaling pathway | 1.00 | 0.000047 | CCL2, CSF1R, IRF5, IL17RE, IL6R, and RTN4R |
| GOTERM_BP_DIRECT | Positive regulation of ERK1 and ERK2 cascade | 1.00 | 0.00019 | CCL2, GPR183, GPR55, CHI3L1, CSF1R, and FFAR4 |
| GOTERM_BP_DIRECT | Inflammatory response | 1.00 | 0.00097 | BCL6, CCL2, CXCR2, GPR68, CHI3L1, CSF1R, and IL17RE |
| UP_KEYWORDS | Inflammatory response | 0.81 | 0.00097 | BCL6, CCL2, CXCR2, GPR68, CHI3L1, CSF1R, and IL17RE |
| GOTERM_BP_DIRECT | Cell surface receptor signaling pathway | 1.00 | 0.0014 | CCL2, CXCR2, EPHA1, SAG, ADGRG5, and ADRB2 |
| GOTERM_BP_DIRECT | Immune response | 1.00 | 0.0088 | CCL2, GPR183, CFP, IGLL1, IL1RN, and PTGDR2 |
| GOTERM_BP_DIRECT | Cell adhesion | 1.00 | 0.012 | ATP1B1, CCL2, CASS4, EMILIN1, MYBPH, and NINJ2 |
| UP_KEYWORDS | Cell adhesion | 0.81 | 0.012 | ATP1B1, CCL2, CASS4, EMILIN1, MYBPH, and NINJ2 |
| GOTERM_BP_DIRECT | Positive regulation of apoptotic process | 1.00 | 0.013 | ADAMTSL4, BCL6, ARHGEF3, S100B, and IRF5 |
| GOTERM_BP_DIRECT | Positive regulation of cell proliferation | 1.00 | 0.013 | CXCR2, EPHA1, S100B, CSF1R, IL6R, and PRTN3 |
| GOTERM_BP_DIRECT | Cellular response to macrophage colony-stimulating factor stimulus | 1.00 | 0.018 | CCL2 and CSF1R |
| GOTERM_BP_DIRECT | Cell shape regulation | 0.79 | 0.018 | CCL2 and CSF1R |
| GOTERM_BP_DIRECT | G protein-coupled receptor pathway | 1.00 | 0.018 | CCL2, GPR141, GPR183, GPR55, GPR68, ADGRG5, FFAR4, and PTGDR2 |
| GOTERM_MF_DIRECT | G protein-coupled receptor activity | 0.92 | 0.018 | CCL2, GPR141, GPR183, GPR55, GPR68, ADGRG5, FFAR4, and PTGDR2 |
| UP_KEYWORDS | Transducer | 0.79 | 0.018 | CCL2, GPR141, GPR183, GPR55, GPR68, ADGRG5, FFAR4, and PTGDR2 |
| UP_KEYWORDS | G protein-coupled receptor | 0.79 | 0.018 | CCL2, GPR141, GPR183, GPR55, GPR68, ADGRG5, FFAR4, and PTGDR2 |
| GOTERM_BP_DIRECT | Relaxation of cardiac muscle | 1.00 | 0.036 | ATP1B1 and RGS2 |
| KEGG_PATHWAY | cGMP–PKG signaling pathway | 0.79 | 0.036 | ATP1B1 and RGS2 |
| GOTERM_BP_DIRECT | Protein O-linked fucosylation | 1.00 | 0.036 | ADAMTSL4 and CFP |
| GOTERM_BP_DIRECT | Complement activation, alternative pathway | 1.00 | 0.039 | CFD and CFP |
| GOTERM_BP_DIRECT | Positive regulation of angiogenesis | 1.00 | 0.048 | CXCR2, EPHA1, and CHI3L1 |
| Genes | Fold Change | ||
|---|---|---|---|
| NGS | p-Value | RT-qPCR | |
| JAK2 | –0.52 | 0.006 | −1.6 |
| STAT3 | −0.31 | 0.012 | −5.0 |
| STAT5a | −0.31 | 0.025 | −5.0 |
| STAT5b | −0.49 | 0.001 | −2.5 |
| Genes | Fold Change | ||
|---|---|---|---|
| NGS | p-Value | RT-qPCR | |
| AKT | −0.89 | 0.006 | −2.0 |
| PI3K | −0.59 | 0.003 | −2.0 |
| mTOR | −0.26 | 0.043 | −2.0 |
| Genes | Fold Change | ||
|---|---|---|---|
| NGS | p-Value | RT-qPCR | |
| c-Myc | −0.625 | 0.00001 | −4.16 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Almajali, B.; Johan, M.F.; Al-Wajeeh, A.S.; Wan Taib, W.R.; Ismail, I.; Alhawamdeh, M.; Al-Tawarah, N.M.; Ibrahim, W.N.; Al-Rawashde, F.A.; Al-Jamal, H.A.N. Gene Expression Profiling and Protein Analysis Reveal Suppression of the C-Myc Oncogene and Inhibition JAK/STAT and PI3K/AKT/mTOR Signaling by Thymoquinone in Acute Myeloid Leukemia Cells. Pharmaceuticals 2022, 15, 307. https://doi.org/10.3390/ph15030307
Almajali B, Johan MF, Al-Wajeeh AS, Wan Taib WR, Ismail I, Alhawamdeh M, Al-Tawarah NM, Ibrahim WN, Al-Rawashde FA, Al-Jamal HAN. Gene Expression Profiling and Protein Analysis Reveal Suppression of the C-Myc Oncogene and Inhibition JAK/STAT and PI3K/AKT/mTOR Signaling by Thymoquinone in Acute Myeloid Leukemia Cells. Pharmaceuticals. 2022; 15(3):307. https://doi.org/10.3390/ph15030307
Chicago/Turabian StyleAlmajali, Belal, Muhammad Farid Johan, Abdullah Saleh Al-Wajeeh, Wan Rohani Wan Taib, Imilia Ismail, Maysa Alhawamdeh, Nafe M. Al-Tawarah, Wisam Nabeel Ibrahim, Futoon Abedrabbu Al-Rawashde, and Hamid Ali Nagi Al-Jamal. 2022. "Gene Expression Profiling and Protein Analysis Reveal Suppression of the C-Myc Oncogene and Inhibition JAK/STAT and PI3K/AKT/mTOR Signaling by Thymoquinone in Acute Myeloid Leukemia Cells" Pharmaceuticals 15, no. 3: 307. https://doi.org/10.3390/ph15030307
APA StyleAlmajali, B., Johan, M. F., Al-Wajeeh, A. S., Wan Taib, W. R., Ismail, I., Alhawamdeh, M., Al-Tawarah, N. M., Ibrahim, W. N., Al-Rawashde, F. A., & Al-Jamal, H. A. N. (2022). Gene Expression Profiling and Protein Analysis Reveal Suppression of the C-Myc Oncogene and Inhibition JAK/STAT and PI3K/AKT/mTOR Signaling by Thymoquinone in Acute Myeloid Leukemia Cells. Pharmaceuticals, 15(3), 307. https://doi.org/10.3390/ph15030307









