Benefits of Black Cohosh (Cimicifuga racemosa) for Women Health: An Up-Close and In-Depth Review
Abstract
1. Introduction
2. Phytochemicals Constituent of CR
2.1. Triterpenoids
2.2. Phenolic Constituents
2.3. Flavonoids
2.4. Alkaloid
3. Structure of Biologically Active Compounds
4. Quality Control
5. Pharmacology and Biochemistry of CR Extract/Biological Characterization of CR
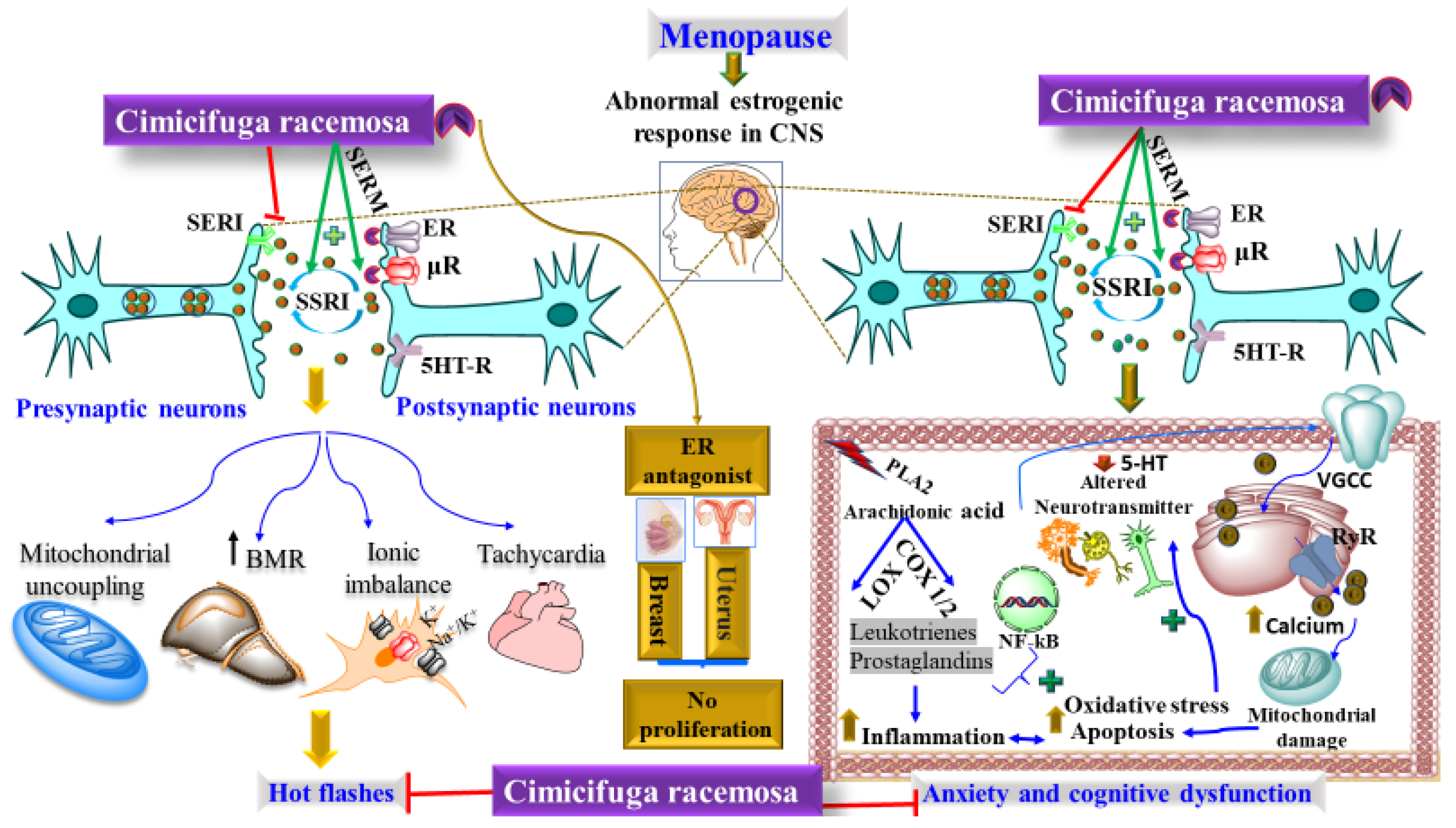
5.1. Management of Menopausal Syndrome
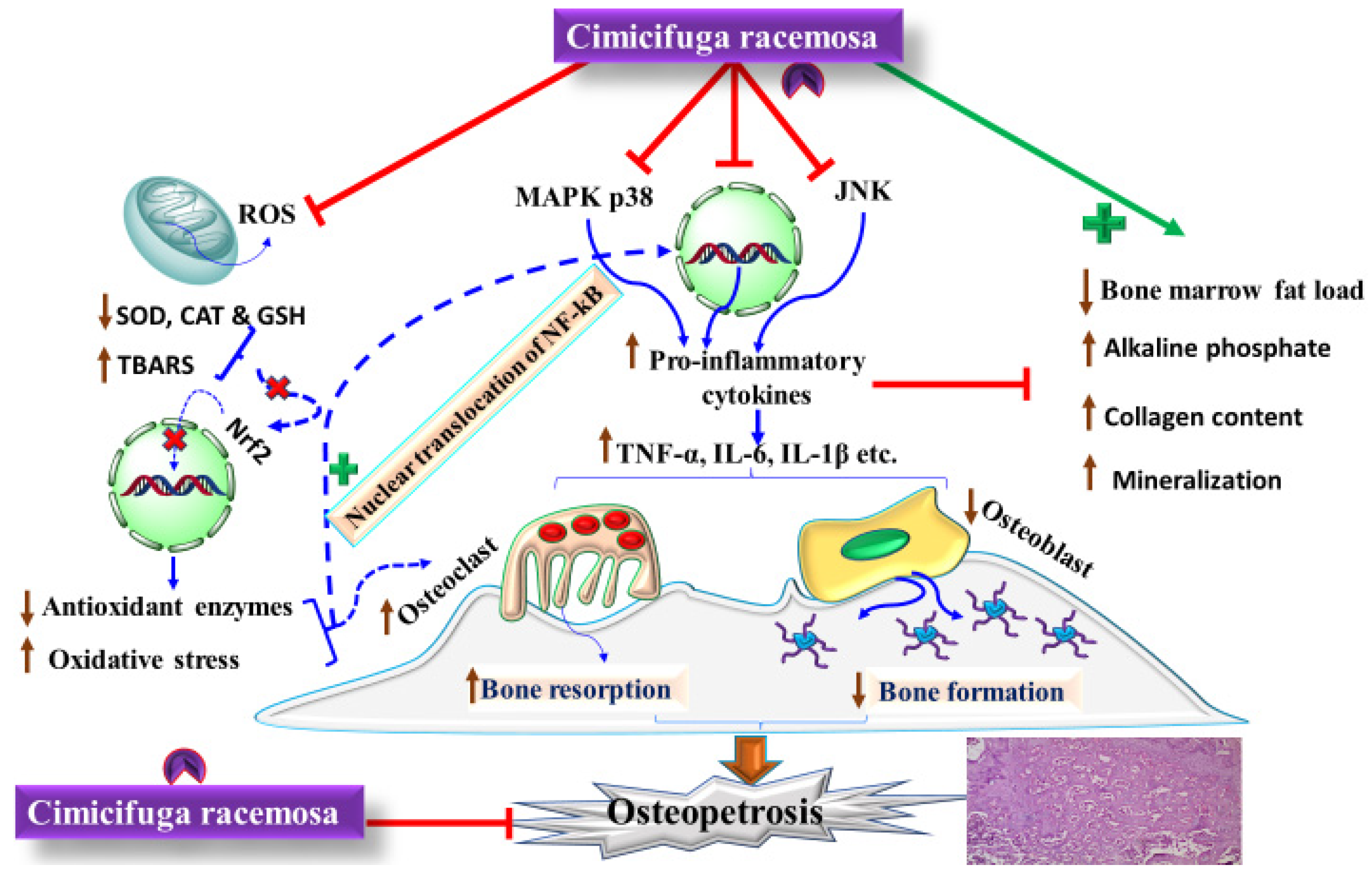
5.2. Management of Postmenopausal Osteoporosis
5.3. Adjuvant Treatment in Mammary Cancer
5.4. Management of Other Diseases
6. Pharmacokinetics
7. Health Risks
8. Future Prospective
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Reid, R.; Abramson, B.L.; Blake, J.; Desindes, S.; Dodin, S.; Johnston, S.; Rowe, T.; Sodhi, N.; Wilks, P.; Wolfman, W.; et al. Managing Menopause. J. Obstet. Gynaecol. Can. 2014, 36, 830–833. [Google Scholar] [CrossRef]
- Hill, K. The demography of menopause. Maturitas 1996, 23, 113–127. [Google Scholar] [CrossRef]
- Academic Committee of the Korean Society of Menopause; Lee, S.R.; Cho, M.K.; Cho, Y.J.; Chun, S.; Hong, S.-H.; Hwang, K.R.; Jeon, G.-H.; Kil Joo, J.; Kim, S.K.; et al. The 2020 Menopausal Hormone Therapy Guidelines. J. Menopausal Med. 2020, 26, 69–98. [Google Scholar] [CrossRef] [PubMed]
- Krieger, N.; Löwy, I.; Aronowitz, R.; Bigby, J.; Dickersin, K.; Garner, E.; Gaudillière, J.-P.; Hinestrosa, C.; Hubbard, R.; Johnson, P.A.; et al. Hormone replacement therapy, cancer, controversies, and women’s health: Historical, epidemiological, biological, clinical, and advocacy perspectives. J. Epidemiol. Community Health 2005, 59, 740–748. [Google Scholar] [CrossRef] [PubMed]
- Sjögren, L.L.; Mørch, L.S.; Løkkegaard, E. Hormone replacement therapy and the risk of endometrial cancer: A systematic review. Maturitas 2016, 91, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Woyka, J. Consensus statement for non-hormonal-based treatments for menopausal symptoms. Post Reprod. Health 2017, 23, 71–75. [Google Scholar] [CrossRef] [PubMed]
- Wuttke, W.; Jarry, H.; Haunschild, J.; Stecher, G.; Schuh, M.; Seidlova-Wuttke, D. The non-estrogenic alternative for the treatment of climacteric complaints: Black cohosh (Cimicifuga or Actaea racemosa). J. Steroid Biochem. Mol. Biol. 2014, 139, 302–310. [Google Scholar] [CrossRef] [PubMed]
- Gardner, Z.E.; McGuffin, M. American Herbal Products Association’s Botanical Safety Handbook, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2013; ISBN 9781466516953. [Google Scholar]
- The Plant List. Available online: http://www.theplantlist.org/tpl/search?q= (accessed on 22 January 2022).
- Predny, M.L.; De Angelis, P.; Chamberlain, J.L. Black Cohosh (Actaea racemosa): An Annotated Bibliography; General Technical, Report; SRS–97; U.S. Department of Agriculture Forest Service, Southern Research Station: Asheville, NC, USA, 2006; p. 99. [Google Scholar]
- Borrelli, F.; Ernst, E. Cimicifuga racemosa: A systematic review of its clinical efficacy. Eur. J. Clin. Pharmacol. 2002, 58, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Wuttke, W.; Seidlová-Wuttke, D.; Gorkow, C. The Cimicifuga preparation BNO 1055 vs. conjugated estrogens in a double-blind placebo-controlled study: Effects on menopause symptoms and bone markers. Maturitas 2003, 44, S67–S77. [Google Scholar] [CrossRef]
- Liske, E. Therapeutic efficacy and safety of Cimicifuga racemosa for gynecologic disorders. Adv. Ther. 1997, 15, 45–53. [Google Scholar]
- Leach, M.J.; Moore, V. Black cohosh (Cimicifuga spp.) for menopausal symptoms. Cochrane Database Syst. Rev. 2012, 2012, CD007244. [Google Scholar] [CrossRef]
- Smith, T.; Gillespie, M.; Eckl, V.; Knepper, J.; Reynolds, C.M. Herbal supplement sales in US increase by 9.4% in 2018. Herbalgram 2019, 123, 62–73. [Google Scholar]
- Davis, J. Available online: https://content.ces.ncsu.edu/black-cohosh-actaea-racemosa-l (accessed on 22 January 2022).
- Castelo-Branco, C.; Gambacciani, M.; Cano, A.; Minkin, M.J.; Rachoń, D.; Ruan, X.; Beer, A.M.; Schnitker, J.; Henneicke-von Zepelin, H.H.; Pickartz, S. Review & meta-analysis: Isopropanolic black cohosh extract iCR for menopausal symptoms—An update on the evidence. Climacteric 2021, 24, 109–119. [Google Scholar] [PubMed]
- Medicine; Dietary Supplement Label Database; National Institutes of Health. A Joint Effort of the Office of Dietary Supplements and the National Library of Medicine. Available online: https://dsld.od.nih.gov/dsld/rptQSearch.jsp?item=black+cohosh&db=adsld (accessed on 22 January 2022).
- Rotblatt, M. Herbal Medicine: Expanded Commission E Monographs. Ann. Intern. Med. 2000, 133, 487. [Google Scholar] [CrossRef]
- World Health Organization. WHO Monographs on Selected Medicinal Plants; World Health Organization: Geneva, Switzerland, 2003; Volume 2, ISBN 9241545178. [Google Scholar]
- European Medicines Agency. Assessment Report on Cimicifuga racemosa (L.) Nutt., Rhizoma; Committee on Herbal Medicinal Products (HMPC) European Medicines Agency: Amsterdam, The Netherlands, 2017. [Google Scholar]
- Health Canada. Natural Health Products Ingredients Database; Health Canada: Edmonton, AB, Canada, 2009. [Google Scholar]
- National Institutes of Health. NIH Dietary Supplement Fact Sheets; National Institutes of Health: Bethesda, MD, USA, 2009. [Google Scholar]
- Sahreen, S.; Khan, M.R.; Khan, R.A. Hepatoprotective effects of methanol extract of Carissa opaca leaves on CCl4-induced damage in rat. BMC Complement. Altern. Med. 2011, 11, 48. [Google Scholar] [CrossRef] [PubMed]
- Jan, B.; Parveen, R.; Zahiruddin, S.; Khan, M.U.; Mohapatra, S.; Ahmad, S. Nutritional constituents of mulberry and their potential applications in food and pharmaceuticals: A review. Saudi J. Biol. Sci. 2021, 28, 3909–3921. [Google Scholar] [CrossRef]
- Kruse, S.O.; Löhning, A.; Pauli, G.F.; Winterhoff, H.; Nahrstedt, A. Fukiic and Piscidic Acid Esters from the Rhizome of Cimicifuga racemose and the in vitro Estrogenic Activity of Fukinolic Acid. Planta Med. 1999, 65, 763–764. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Chen, S.-N.; Fabricant, D.; Angerhofer, C.K.; Fong, H.H.; Farnsworth, N.R.; Fitzloff, J.F. High-performance liquid chromatographic analysis of Black Cohosh (Cimicifuga racemosa) constituents with in-line evaporative light scattering and photodiode array detection. Anal. Chim. Acta 2002, 471, 61–75. [Google Scholar] [CrossRef]
- Liby, K.; Yore, M.M.; Sporn, M.B. Triterpenoids and rexinoids as multifunctional agents for the prevention and treatment of cancer. Nat. Cancer 2007, 7, 357–369. [Google Scholar] [CrossRef]
- Burdette, J.E.; Chen, S.-N.; Lu, Z.-Z.; Xu, H.; White, B.E.P.; Fabricant, D.S.; Liu, J.; Fong, H.H.S.; Farnsworth, N.R.; Constantinou, A.; et al. Black Cohosh (Cimicifuga racemosa L.) Protects against Menadione-Induced DNA Damage through Scavenging of Reactive Oxygen Species: Bioassay-Directed Isolation and Characterization of Active Principles. J. Agric. Food Chem. 2002, 50, 7022–7028. [Google Scholar] [CrossRef] [PubMed]
- Liao, X.; Zhang, Q.; Xu, L.; Zhang, H. Potential Targets of Actein Identified by Systems Chemical Biology Methods. ChemMedChem 2020, 15, 473–480. [Google Scholar] [CrossRef] [PubMed]
- Çiçek, S.S.; Girreser, U.; Zidorn, C. Quantification of the total amount of black cohosh cycloartanoids by integration of one specific 1H NMR signal. J. Pharm. Biomed. Anal. 2018, 155, 109–115. [Google Scholar] [CrossRef]
- Bentham Science Publisher. Cimicifugae Rhizoma: From Origins, Bioactive Constituents to Clinical Outcomes. Curr. Med. Chem. 2006, 13, 2927–2951. [Google Scholar] [CrossRef] [PubMed]
- Avula, B.; Wang, Y.-H.; Smillie, T.; Khan, I. Quantitative Determination of Triterpenoids and Formononetin in Rhizomes of Black Cohosh (Actaea racemosa) and Dietary Supplements by Using UPLC-UV/ELS Detection and Identification by UPLC-MS. Planta Med. 2009, 75, 381–386. [Google Scholar] [CrossRef] [PubMed]
- He, K.; Pauli, G.F.; Zheng, B.; Wang, H.; Bai, N.; Peng, T.; Roller, M.; Zheng, Q. Cimicifuga species identification by high performance liquid chromatography–photodiode array/mass spectrometric/evaporative light scattering detection for quality control of black cohosh products. J. Chromatogr. A 2006, 1112, 241–254. [Google Scholar] [CrossRef] [PubMed]
- Actaea racemosa (Root and Rhizome). AHPA Botanical Identity Reference Compendium. Available online: Http://www.botanicalauthentication.org/index.php/Actaea_racemosa_(root_and_rhizome) (accessed on 22 January 2022).
- Severin, N. Fast Analysis of Triterpene Glycosides in CR Using Agilent 1290 Infinity LC System and Agilent Poroshell 120 SB c-18 2.7µm Column; Application Notes; Charles Sturt University: Wagga Wagga, Australia, 2017. [Google Scholar]
- Roman, M. Determination of Triterpene Glycosides in Cimicifuga racemosa (Black Cohosh) by HPLC-CAD; Tampa Bay Analytical Research: Clearwater, FL, USA, 2011. [Google Scholar]
- Verbitski, S.M.; Gourdin, G.T.; Ikenouye, L.M.; McChesney, J.D.; Hildreth, J. Detection of Actaea racemosa Adulteration by Thin-Layer Chromatography and Combined Thin-Layer Chromatography-Bioluminescence. J. AOAC Int. 2008, 91, 268–275. [Google Scholar] [CrossRef]
- Shimadzu Excellence in Science. The HPLC Analysis of Black Cohosh Using an INA Method; Shimadzu Excellence in Science: Kyoto, Japan, 2020. [Google Scholar]
- Chen, S.-N.; Li, W.; Fabricant, D.S.; Santarsiero, B.D.; Mesecar, A.; Fitzloff, J.F.; Fong, H.H.S.; Farnsworth, N.R. Isolation, Structure Elucidation, and Absolute Configuration of 26-Deoxyactein from Cimicifuga racemosa and Clarification of Nomenclature Associated with 27-Deoxyactein. J. Nat. Prod. 2002, 65, 601–605. [Google Scholar] [CrossRef] [PubMed]
- Jiang, B.; Lyles, J.T.; Reynertson, K.A.; Kronenberg, F.; Kennelly, E.J. Stability Evaluation of Selected Polyphenols and Triterpene Glycosides in Black Cohosh. J. Agric. Food Chem. 2008, 56, 9510–9519. [Google Scholar] [CrossRef] [PubMed]
- He, K.; Zheng, B.; Kim, C.H.; Rogers, L.; Zheng, Q. Direct Analysis and Identification of Triterpene Glycosides by LC/MS in Black Cohosh, Cimicifuga racemosa, and in Several Commercially Available Black Cohosh Products. Planta Med. 2000, 66, 635–640. [Google Scholar] [CrossRef]
- Polymer Laboratories Inc. HPLC Analysis of Triterpene Glycosides in Black Cohosh Formulations Using the PL-ELS 2100; Polymer Laboratories Inc.: Amherst, MA, USA, 2014. [Google Scholar]
- Jiang, B.; Ma, C.; Motley, T.; Kronenberg, F.; Kennelly, E.J. Phytochemical fingerprinting to thwart black cohosh adulteration: A 15 Actaea species analysis. Phytochem. Anal. 2011, 22, 339–351. [Google Scholar] [CrossRef]
- Jiang, B.; Kronenberg, F.; Nuntanakorn, P.; Qiu, M.-H.; Kennelly, E.J. Evaluation of the Botanical Authenticity and Phytochemical Profile of Black Cohosh Products by High-Performance Liquid Chromatography with Selected Ion Monitoring Liquid Chromatography−Mass Spectrometry. J. Agric. Food Chem. 2006, 54, 3242–3253. [Google Scholar] [CrossRef] [PubMed]
- Sharaf, M.; Yuk, J.; Yu, K.; Wrona, M.; Isaac, G. Chemical profiling of Actaea species and commercial products using UPLC-QTof-MS. Planta Med. 2016, 81, S1–S381. [Google Scholar] [CrossRef]
- Jiang, B.; Yang, H.; Nuntanakorn, P.; Balick, M.; Kronenberg, F.; Kennelly, E. The value of plant collections in ethnopharmacology: A case study of an 85-year-old black cohosh (Actaea racemosa L.) sample. J. Ethnopharmacol. 2005, 96, 521–528. [Google Scholar] [CrossRef]
- He, K.; Zheng, B.L.; Kim, C.H.; Rogers, L.-L.; Zheng, Q.Y. Species Identification of Black Cohosh by LC-MS for Quality Control. ACS Symp. Ser. 2002, 803, 90–100. [Google Scholar]
- Nuntanakorn, P.; Jiang, B.; Einbond, L.S.; Yang, H.; Kronenberg, F.; Weinstein, I.B.; Kennelly, E.J. Polyphenolic Constituents of Actaea racemosa. J. Nat. Prod. 2006, 69, 314–318. [Google Scholar] [CrossRef]
- Jiang, B.; Kronenberg, F.; Balick, M.J.; Kennelly, E.J. Stability of black cohosh triterpene glycosides and polyphenols: Potential clinical relevance. Phytomedicine 2013, 20, 564–569. [Google Scholar] [CrossRef] [PubMed]
- Struck, D.; Tegtmeier, M.; Harnischfeger, G. Flavones in Extracts of Cimicifuga racemosa. Planta Med. 1997, 63, 289. [Google Scholar] [CrossRef] [PubMed]
- Jiang, B.; Kronenberg, F.; Balick, M.; Kennelly, E. Analysis of formononetin from black cohosh (Actaea racemosa). Phytomedicine 2006, 13, 477–486. [Google Scholar] [CrossRef]
- Jarry, P.D.D.H.; Gorkow, C.; Wuttke, W. Treatment of Menopausal Symptoms with Extracts of Cimicifuga racemosa: In vivo and in vitro Evidence for Estrogenic Activity. In Phytopharmaka in Forschung und klinischer Anwendung; Springer: Berlin/Heidelberg, Germany, 1995; pp. 99–112. [Google Scholar]
- Panossian, A.; Danielyan, A.; Mamikonyan, G.; Wikman, G. Methods of phytochemical standardisation of rhizome Cimicifugae racemosae. Phytochem. Anal. 2004, 15, 100–108. [Google Scholar] [CrossRef]
- Kennelly, E.; Baggett, S.; Nuntanakorn, P.; Ososki, A.; Mori, S.; Duke, J.; Coleton, M.; Kronenberg, F. Analysis of thirteen populations of Black Cohosh for formononetin. Phytomedicine 2002, 9, 461–467. [Google Scholar] [CrossRef]
- Nikolić, D.; Lankin, D.C.; Cisowska, T.; Chen, S.-N.; Pauli, G.F.; van Breemen, R.B. Nitrogen-Containing Constituents of Black Cohosh: Chemistry, Structure Elucidation, and Biological Activities. In The Formation, Structure and Activity of Phytochemicals; Springer: Berlin/Heidelberg, Germany, 2015; Volume 45, pp. 31–75. [Google Scholar]
- Powell, S.L.; Gödecke, T.; Nikolic, D.; Chen, S.-N.; Ahn, S.; Dietz, B.; Farnsworth, N.R.; van Breemen, R.B.; Lankin, D.C.; Pauli, G.F.; et al. In Vitro Serotonergic Activity of Black Cohosh and Identification of N ω-Methylserotonin as a Potential Active Constituent. J. Agric. Food Chem. 2008, 56, 11718–11726. [Google Scholar] [CrossRef]
- Burdette, J.E.; Liu, J.; Chen, S.-N.; Fabricant, D.S.; Piersen, C.E.; Barker, E.L.; Pezzuto, J.M.; Mesecar, A.; van Breemen, R.B.; Farnsworth, A.N.R.; et al. Black Cohosh Acts as a Mixed Competitive Ligand and Partial Agonist of the Serotonin Receptor. J. Agric. Food Chem. 2003, 51, 5661–5670. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Sha, C.; Liu, W.; Gai, Y.; Zhang, H.; Qu, H.; Wang, W. Simultaneous determination of cimicifugoside H-2, cimicifugoside H-1, 23-epi-26-deoxyactein, cimigenol xyloside and 25-O-acetylcimigenoside in beagle dog plasma by LC–MS/MS. J. Pharm. Biomed. Anal. 2012, 62, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.-N.; Fabricant, D.S.; Lu, Z.-Z.; Fong, H.H.S.; Farnsworth, N.R. Cimiracemosides I−P, New 9,19-Cyclolanostane Triterpene Glycosides from Cimicifuga racemosa. J. Nat. Prod. 2002, 65, 1391–1397. [Google Scholar] [CrossRef]
- Shao, Y.; Harris, A.; Wang, M.; Zhang, H.; Cordell, G.A.; Bowman, M.; Lemmo, E. Triterpene Glycosides from Cimicifuga racemosa. J. Nat. Prod. 2000, 63, 905–910. [Google Scholar] [CrossRef] [PubMed]
- Findeis, M.A.; Schroeder, F.; McKee, T.D.; Yager, D.; Fraering, P.C.; Creaser, S.P.; Austin, W.F.; Clardy, J.; Wang, R.; Selkoe, D.; et al. Discovery of a Novel Pharmacological and Structural Class of Gamma Secretase Modulators Derived from the Extract of Actaea racemosa. ACS Chem. Neurosci. 2012, 3, 941–951. [Google Scholar] [CrossRef] [PubMed]
- Jamróz, M.K.; Jamróz, M.H.; Dobrowolski, J.C.; Gliński, J.A.; Gleńsk, M. One new and six known triterpene xylosides from Cimicifuga racemosa: FT-IR, Raman and NMR studies and DFT calculations. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2012, 93, 10–18. [Google Scholar] [CrossRef]
- Nikolić, D.; Gödecke, T.; Chen, S.-N.; White, J.; Lankin, D.C.; Pauli, G.F.; van Breemen, R.B. Mass spectrometric dereplication of nitrogen-containing constituents of black cohosh (Cimicifuga racemosa L.). Fitoterapia 2012, 83, 441–460. [Google Scholar] [CrossRef]
- Chen, S.-N.; Fabricant, D.S.; Lu, Z.-Z.; Zhang, H.; Fong, H.H.S.; Farnsworth, N.R. Cimiracemates A—D, Phenylpropanoid Esters from the Rhizomes of Cimicifuga racemosa. Phytochemistry 2002, 61, 409–413. [Google Scholar] [CrossRef]
- Xu, H.; Fabricant, D.; Piersen, C.; Bolton, J.; Pezzuto, J.; Fong, H.; Totura, S.; Farnsworth, N.; Constantinou, A. A preliminary RAPD-PCR analysis of Cimicifuga species and other botanicals used for women’s health. Phytomedicine 2002, 9, 757–762. [Google Scholar] [CrossRef]
- Zerega, N.; A Mori, S.; Lindqvist, C.; Zheng, Q.; Motley, T.J. Using Amplified Fragment Length Polymorphisms (AFLP) to Identify Black Cohosh (Actaea racemosa). Econ. Bot. 2002, 56, 154–164. [Google Scholar] [CrossRef]
- Wang, H.-K.; Sakurai, N.; Shih, C.Y.; Lee, K.-H. LC/TIS-MS Fingerprint Profiling of Cimicifuga Species and Analysis of 23-Epi-26-deoxyactein in Cimicifuga racemosa Commercial Products. J. Agric. Food Chem. 2005, 53, 1379–1386. [Google Scholar] [CrossRef] [PubMed]
- Ankli, A.; Reich, E.; Steiner, M. Rapid High-Performance Thin-Layer Chromatographic Method for Detection of 5 Adulteration of Black Cohosh with Cimicifuga foetida, C. heracleifolia, C. dahurica, or C. americana. J. AOAC Int. 2008, 91, 1257–1264. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Kavalier, A.R.; Jiang, B.; Kennelly, E.J. Metabolic profiling of Actaea species extracts using high performance liquid chromatography coupled with electrospray ionization time-of-flight mass spectrometry. J. Chromatogr. A 2011, 1218, 1461–1476. [Google Scholar] [CrossRef] [PubMed]
- Baker, D.A.; Stevenson, D.W.; LittLe, D.P. DNA Barcode Identification of Black Cohosh Herbal Dietary Supplements. J. AOAC Int. 2012, 95, 1023–1034. [Google Scholar] [CrossRef] [PubMed]
- Harnly, J.; Chen, P.; Sun, J.; Huang, H.; Colson, K.L.; Yuk, J.; McCoy, J.-A.H.; Reynaud, D.T.H.; Harrington, P.B.; Fletcher, E.J. Comparison of Flow Injection MS, NMR, and DNA Sequencing: Methods for Identification and Authentication of Black Cohosh (Actaea racemosa). Planta Med. 2015, 82, 250–262. [Google Scholar] [CrossRef] [PubMed]
- Masada, S. Authentication of the botanical origin of Western herbal products using Cimicifuga and Vitex products as examples. J. Nat. Med. 2016, 70, 361–375. [Google Scholar] [CrossRef] [PubMed]
- Mohapatra, S.; Iqbal, Z.; Ahmad, S.; Kohli, K.; Farooq, U.; Padhi, S.; Kabir, M.; Panda, A.K. Menopausal Remediation and Quality of Life (QoL) Improvement: Insights and Perspectives. Endocr. Metab. Immune Disord. Drug Targets 2020, 20, 1624–1636. [Google Scholar] [CrossRef] [PubMed]
- Winterhoff, H.; Spengler, B.; Christoffel, V.; Butterweck, V.; Löhning, A. Cimicifuga extract BNO 1055: Reduction of hot flushes and hints on antidepressant activity. Maturitas 2003, 44, S51–S58. [Google Scholar] [CrossRef]
- Qiu, S.X.; Dan, C.; Ding, L.-S.; Peng, S.; Chen, S.-N.; Farnsworth, N.R.; Nolta, J.; Gross, M.L.; Zhou, P. A Triterpene Glycoside from Black Cohosh that Inhibits Osteoclastogenesis by Modulating RANKL and TNFα Signaling Pathways. Chem. Biol. 2007, 14, 860–869. [Google Scholar] [CrossRef] [PubMed]
- Ruhlen, R.L.; Haubner, J.; Tracy, J.K.; Zhu, W.; Ehya, H.; Lamberson, W.R.; Rottinghaus, G.E.; Sauter, E.R. Black Cohosh Does Not Exert an Estrogenic Effect on the Breast. Nutr. Cancer 2007, 59, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Seidlová-Wuttke, D.; Jarry, H.; Becker, T.; Christoffel, V.; Wuttke, W. Pharmacology of Cimicifuga racemosa extract BNO 1055 in rats: Bone, fat and uterus. Maturitas 2003, 44, S39–S50. [Google Scholar] [CrossRef]
- Wuttke, W.; Gorkow, C.; Seidlová-Wuttke, D. Effects of black cohosh (Cimicifuga racemosa) on bone turnover, vaginal mucosa, and various blood parameters in postmenopausal women. Menopause 2006, 13, 185–196. [Google Scholar] [CrossRef]
- Nappi, R.E.; Malavasi, B.; Brundu, B.; Facchinetti, F. Efficacy of Cimicifuga racemose on climacteric complaints: A randomized study versus low-dose transdermal estradiol. Gynecol. Endocrinol. 2005, 20, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Szmyd, M.; Lloyd, V.; Hallman, K.; Aleck, K.; Mladenovik, V.; McKee, C.; Morse, M.; Bedgood, T.; Dinda, S. The effects of black cohosh on the regulation of estrogen receptor (ERα) and progesterone receptor (PR) in breast cancer cells. Breast Cancer Targets Ther. 2018, 10, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Hui, Z.; Xiaoyan, M.; Mukun, Y.; Ke, W.; Liyuan, Y.; Sainan, Z.; Jing, J.; Lihua, Q.; Wenpei, B. Effects of black cohosh and estrogen on the hypothalamic nuclei of ovariectomized rats at different temperatures. J. Ethnopharmacol. 2012, 142, 769–775. [Google Scholar] [CrossRef]
- Nadaoka, I.; Yasue, M.; Sami, M.; Kitagawa, Y. Oral administration of Cimicifuga racemosa extract affects immobilization stress-induced changes in murine cerebral monoamine metabolism. Biomed. Res. 2012, 33, 133–137. [Google Scholar] [CrossRef]
- Borrelli, F.; Izzo, A.; Ernst, E. Pharmacological effects of Cimicifuga racemosa. Life Sci. 2003, 73, 1215–1229. [Google Scholar] [CrossRef]
- Reame, N.E.; Lukacs, J.L.; Padmanabhan, V.; Eyvazzadeh, A.D.; Smith, Y.R.; Zubieta, J.-K. Black cohosh has central opioid activity in postmenopausal women. Menopause 2008, 15, 832–840. [Google Scholar] [CrossRef]
- Nikolić, D.; Li, J.; van Breemen, R. Metabolism of Nω-methylserotonin, a serotonergic constituent of black cohosh (Cimicifuga racemosa, L. (Nutt.)), by human liver microsomes. Biomed. Chromatogr. 2014, 28, 1647–1651. [Google Scholar] [CrossRef]
- Hedlund, P.B.; Sutcliffe, J.G. Functional, molecular and pharmacological advances in 5-HT 7 receptor research. Trends Pharmacol. Sci. 2004, 25, 481–486. [Google Scholar] [CrossRef] [PubMed]
- Rhyu, M.-R.; Lu, J.; Webster, D.E.; Fabricant, D.S.; Farnsworth, N.R.; Wang, Z.J. Black Cohosh (Actaea racemosa, Cimicifuga racemosa) Behaves as a Mixed Competitive Ligand and Partial Agonist at the Human μ Opiate Receptor. J. Agric. Food Chem. 2006, 54, 9852–9857. [Google Scholar] [CrossRef] [PubMed]
- Da, Y.-M.; Niu, K.-Y.; Liu, S.-Y.; Wang, K.; Wang, W.-J.; Jia, J.; Qin, L.-H.; Bai, W.-P. Does Cimicifuga racemosa have the effects like estrogen on the sublingual gland in ovariectomized rats? Biol. Res. 2017, 50, 11. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Niu, K.; Da, Y.; Liu, Y.; Zhang, J.; Wang, W.; Zhang, Y.; Jiang, H.; Wang, K.; Bai, W.; et al. Effects of standardized isopropanolic black cohosh and estrogen on salivary function in ovariectomized rats. Biomed. Pharmacother. 2018, 97, 1438–1444. [Google Scholar] [CrossRef]
- Gaube, F.; Wolfl, S.; Pusch, L.; Kroll, T.C.; Hamburger, M. Gene expression profiling reveals effects of Cimicifuga racemosa (L.) NUTT. (black cohosh) on the estrogen receptor positive human breast cancer cell line MCF-7. BMC Pharmacol. 2007, 7, 11. [Google Scholar] [CrossRef]
- Löser, B.; Kruse, S.O.; Melzig, M.F.; Nahrstedt, A. Inhibition of Neutrophil Elastase Activity by Cinnamic Acid Derivatives from Cimicifuga racemosa. Planta Med. 2000, 66, 751–753. [Google Scholar] [CrossRef]
- Einbond, L.S.; Su, T.; Wu, H.-A.; Friedman, R.; Wang, X.; Jiang, B.; Hagan, T.; Kennelly, E.J.; Kronenberg, F.; Weinstein, I.B. Gene expression analysis of the mechanisms whereby black cohosh inhibits human breast cancer cell growth. Anticancer Res. 2007, 27, 697–712. [Google Scholar]
- Ruhlen, R.L.; Sun, G.Y.; Sauter, E.R. Black Cohosh: Insights into its Mechanism(s) of Action. Integr. Med. Insights 2008, 3, 21–32. [Google Scholar] [CrossRef]
- Liske, E.; Hänggi, W.; Henneicke-von Zepelin, H.-H.; Boblitz, N.; Wüstenberg, P.; Rahlfs, V. Physiological Investigation of a Unique Extract of Black Cohosh (Cimicifugae racemosae rhizoma): A 6-Month Clinical Study Demonstrates No Systemic Estrogenic Effect. J. Women’s Health Gender-Based Med. 2002, 11, 163–174. [Google Scholar] [CrossRef]
- Frei-Kleiner, S.; Schaffner, W.; Rahlfs, V.; Bodmer, C.; Birkhäuser, M. Cimicifuga racemosa dried ethanolic extract in menopausal disorders: A double-blind placebo-controlled clinical trial. Maturitas 2005, 51, 397–404. [Google Scholar] [CrossRef]
- Newton, K.M.; Reed, S.; Lacroix, A.Z.; Grothaus, L.C.; Ehrlich, K.; Guiltinan, J. Treatment of Vasomotor Symptoms of Menopause with Black Cohosh, Multibotanicals, Soy, Hormone Therapy, or Placebo: A randomized trial. Ann. Intern. Med. 2006, 145, 869–879. [Google Scholar] [CrossRef] [PubMed]
- Hirschberg, A.L.; Edlund, M.; Svane, G.; Azavedo, E.; Skoog, L.; von Schoultz, B. An isopropanolic extract of black cohosh does not increase mammographic breast density or breast cell proliferation in postmenopausal women. Menopause 2007, 14, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Geller, S.E.; Shulman, L.P.; van Breemen, R.; Banuvar, S.; Zhou, Y.; Epstein, G.; Hedayat, S.; Nikolic, D.; Krause, E.C.; Piersen, C.E.; et al. Safety and efficacy of black cohosh and red clover for the management of vasomotor symptoms. Menopause 2009, 16, 1156–1166. [Google Scholar] [CrossRef] [PubMed]
- Bebenek, M.; Kemmler, W.; von Stengel, S.; Engelke, K.; Kalender, W.A. Effect of exercise and Cimicifuga racemosa (CR BNO 1055) on bone mineral density, 10-year coronary heart disease risk, and menopausal complaints: The randomized controlled Training and Cimicifuga racemosa Erlangen (TRACE) study. Menopause 2010, 17, 791–800. [Google Scholar] [CrossRef]
- Lundström, E.; Hirschberg, A.; Söderqvist, G. Digitized assessment of mammographic breast density—Effects of continuous combined hormone therapy, tibolone and black cohosh compared to placebo. Maturitas 2011, 70, 361–364. [Google Scholar] [CrossRef]
- Rostock, M.; Fischer, J.; Mumm, A.; Stammwitz, U.; Saller, R.; Bartsch, H.H. Black cohosh (Cimicifuga racemosa) in tamoxifen-treated breast cancer patients with climacteric complaints—A prospective observational study. Gynecol. Endocrinol. 2010, 27, 844–848. [Google Scholar] [CrossRef]
- Ross, S.M. Menopause: A standardized isopropanolic black cohosh extract (remifemin) is found to be safe and effective for menopausal symptoms. Holist. Nurs. Pract. 2012, 26, 58–61. [Google Scholar] [CrossRef]
- Mohammad-Alizadeh-Charandabi, S.; Shahnazi, M.; Nahaee, J.; Bayatipayan, S. Efficacy of black cohosh (Cimicifuga racemosa L.) in treating early symptoms of menopause: A randomized clinical trial. Chin. Med. 2013, 8, 20. [Google Scholar] [CrossRef]
- Huang, Y.-X.; Song, L.; Zhang, X.; Lun, W.-W.; Pan, C.; Huang, Y.S. Clinical study of combined treatment of remifemin and paroxetine for perimenopausal depression. Zhonghua Yi Xue Za Zhi 2013, 93, 600–602. [Google Scholar]
- Jiang, G.; Chen, J.; Gao, H.; Li, Q.; Cong, J.; Wu, J.; Pu, D. Efficacy and Safety of Remifemin on Peri-Menopausal Symptoms Induced by Post-Operative GnRH-a Therapy for Endometriosis: A Randomized Study versus Tibolone. Med. Sci. Monit. 2014, 20, 1950–1957. [Google Scholar] [CrossRef][Green Version]
- Jiang, K.; Jin, Y.; Huang, L.; Feng, S.; Hou, X.; Du, B.; Zheng, J.; Li, L. Black cohosh improves objective sleep in postmenopausal women with sleep disturbance. Climacteric 2015, 18, 559–567. [Google Scholar] [CrossRef] [PubMed]
- Tanmahasamut, P.; Vichinsartvichai, P.; Rattanachaiyanont, M.; Techatraisak, K.; Dangrat, C.; Sardod, P. Cimicifuga racemose extract for relieving menopausal symptoms: A randomized controlled trial. Climacteric 2014, 18, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Huang, Q.; Liang, C.-L.; Zhang, Y.-W.; Deng, D.-H.; Yu, Y.; Chen, D.-B.; Yang, H.-J.; Yu, X.-F. Effect of Cimicifuga racemosa on menopausal syndrome caused by LHRH-a in breast cancer. J. Ethnopharmacol. 2019, 238, 111840. [Google Scholar] [CrossRef] [PubMed]
- Friederichsen, L.; Nebel, S.; Zahner, C.; Bütikofer, L.; Stute, P. Effect of Cimicifuga racemosa on metaBOLIC parameters in women with menopausal symptoms: A retrospective observational study (CIMBOLIC). Arch. Gynecol. Obstet. 2019, 301, 517–523. [Google Scholar] [CrossRef] [PubMed]
- Seidlova-Wuttke, D.; Stecher, G.; Kammann, M.; Haunschild, J.; Eder, N.; Stahnke, V.; Wessels, J.; Wuttke, W. Osteoprotective effects of Cimicifuga racemosa and its triterpene-saponins are responsible for reduction of bone marrow fat. Phytomedicine 2012, 19, 855–860. [Google Scholar] [CrossRef] [PubMed]
- Cui, G.; Leng, H.; Wang, K.; Wang, J.; Zhu, S.; Jia, J.; Chen, X.; Zhang, W.; Qin, L.; Bai, W. Effects of Remifemin Treatment on Bone Integrity and Remodeling in Rats with Ovariectomy-Induced Osteoporosis. PLoS ONE 2013, 8, e82815. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Liu, J.; He, C.; Yu, Z.; Du, Y.; Kadota, S.; Seto, H. Triterpenoids from Cimicifugae rhizoma, a novel class of inhibitors on bone resorption and ovariectomy-induced bone loss. Maturitas 2007, 58, 59–69. [Google Scholar] [CrossRef]
- Lee, Y.S.; Choi, E.M. Actein Isolated from Black Cohosh Promotes the Function of Osteoblastic MC3T3-E1 Cells. J. Med. Food 2014, 17, 414–423. [Google Scholar] [CrossRef]
- Choi, E.M. Deoxyactein stimulates osteoblast function and inhibits bone-resorbing mediators in MC3T3-E1 cells. J. Appl. Toxicol. 2013, 33, 190–195. [Google Scholar] [CrossRef]
- Suh, K.S.; Chon, S.; Choi, E.M. Actein protects against methylglyoxal-induced oxidative damage in osteoblastic MC3T3-E1 cells. J. Sci. Food Agric. 2017, 97, 207–214. [Google Scholar] [CrossRef]
- Zakir, F.; Ahmad, A.; Farooq, U.; Mirza, M.A.; Tripathi, A.; Singh, D.; Shakeel, F.; Mohapatra, S.; Ahmad, F.; Kohli, K. Design and development of a commercially viable in situ nanoemulgel for the treatment of postmenopausal osteoporosis. Nanomedicine 2020, 15, 1167–1187. [Google Scholar] [CrossRef] [PubMed]
- Einbond, L.S.; Soffritti, M.; Degli Esposti, D.; Tibaldi, E.; Lauriola, M.; Bua, L.; He, K.; Genovese, G.; Su, T.; Huggins, L.; et al. Chemopreventive potential of black cohosh on breast cancer in Sprague-Dawley rats. Anticancer Res. 2012, 32, 21–30. [Google Scholar] [PubMed]
- Fritz, H.; Seely, D.; McGowan, J.; Skidmore, B.; Fernandes, R.; Kennedy, D.A.; Cooley, K.; Wong, R.; Sagar, S.; Balneaves, L.G.; et al. Black cohosh and breast cancer: A systematic review. Integr. Cancer Ther. 2014, 13, 12–29. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Gödecke, T.; Chen, S.-N.; Imai, A.; Lankin, D.C.; Farnsworth, N.R.; Pauli, G.F.; Van Breemen, R.B.; Nikolic, D. In vitro metabolic interactions between black cohosh (Cimicifuga racemosa) and tamoxifen via inhibition of cytochromes P450 2D6 and 3A4. Xenobiotica 2011, 41, 1021–1030. [Google Scholar] [CrossRef]
- Ruan, X.; Mueck, A.O.; Beer, A.-M.; Naser, B.; Pickartz, S. Benefit–risk profile of black cohosh (isopropanolic Cimicifuga racemosa extract) with and without St John’s wort in breast cancer patients. Climacteric 2019, 22, 339–347. [Google Scholar] [CrossRef]
- Freudenstein, J.; Dasenbrock, C.; Nißlein, T. Lack of promotion of estrogen-dependent mammary gland tumors in vivo by an isopropanolic Cimicifuga racemosa extract. Cancer Res. 2002, 62, 3448–3452. [Google Scholar]
- Bodinet, C.; Freudenstein, J. Influence of Cimicifuga racemosa on the Proliferation of Estrogen Receptor-Positive Human Breast Cancer Cells. Breast Cancer Res. Treat. 2002, 76, 1–10. [Google Scholar] [CrossRef]
- Singh, M.; Mohapatra, S.; Sanskriti; Kaur, N.; Mushtaq, A.; Zahid, S.; Pandith, A.A.; Mansoor, S.; Iqbal, Z. Harnessing the Potential of Phytochemicals for Breast Cancer Treatment. In Dietary Phytochemicals; Springer: Berlin/Heidelberg, Germany, 2021. [Google Scholar]
- Crone, M.; Hallman, K.; Lloyd, V.; Szmyd, M.; Badamo, B.; Morse, M.; Dinda, S. The antiestrogenic effects of black cohosh on BRCA1 and steroid receptors in breast cancer cells. Breast Cancer Targets Ther. 2019, 11, 99–110. [Google Scholar] [CrossRef]
- Poschner, S.; Wackerlig, J.; Dobusch, D.; Pachmann, B.; Banh, S.J.; Thalhammer, T.; Jäger, W. Actaea racemosa L. extract inhibits steroid sulfation in human breast cancer cells: Effects on androgen formation. Phytomedicine 2020, 79, 153357. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, J.; Liu, S.; Wang, W.; Chen, X.; Jiang, H.; Li, J.; Wang, K.; Bai, W.; Zhang, H.; et al. Effects of oestrogen and Cimicifuga racemosa on the cardiac noradrenaline pathway of ovariectomized rats. Exp. Physiol. 2017, 102, 974–984. [Google Scholar] [CrossRef]
- Kim, C.D.; Lee, W.; Lee, M.; Cho, H.S.; Lee, Y.K.; Roh, S. Inhibition of Mast Cell-Dependent Allergy Reaction by Extract of Black Cohosh (Cimicifuga racemosa). Immunopharmacol. Immunotoxicol. 2004, 26, 299–308. [Google Scholar] [CrossRef] [PubMed]
- Boonen, G. Novel Pharmacological Use of Cimicifuga Extract. WO Patent Application No. 2014006104A1, 9 January 2014. [Google Scholar]
- Einbond, L.S.; Soffritti, M.; Kolaja, K.L.; Brennan, R.J.; Weinstein, I.B.; Weinstein, J.; Weinstein, T.; Weinstein, C.; Weinstein, M. Anti-Neoplastic Compositions Comprising Extracts of Black Cohosh. U.S. Patent Application No. 7407675B2, 5 August 2008. [Google Scholar]
- Wuttke, W.; Seidlová-Wuttke, D. Arzneimittel Enthaltend Cimicifuga zur Anwendung Bei der Behandlung von Sarkopenie und Herzmuskelschwäche. WO Patent Application No. 2013113772A1, 8 August 2013. [Google Scholar]
- Nadaoka, I.; Sugiyama, H. Anti-Obesity Agent. U.S. Patent Application No. 20060210659A1, 21 September 2006. [Google Scholar]
- Van Breemen, R.B.; Liang, W.; Banuvar, S.; Shulman, L.P.; Pang, Y.; Tao, Y.; Nikolic, D.; Krock, K.M.; Fabricant, D.S.; Chen, S.-N.; et al. Pharmacokinetics of 23-Epi-26-Deoxyactein in Women After Oral Administration of a Standardized Extract of Black Cohosh. Clin. Pharmacol. Ther. 2009, 87, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Disch, L.; Forsch, K.; Siewert, B.; Drewe, J.; Fricker, G. In Vitro and In Situ Characterization of Triterpene Glycosides from Cimicifuga racemosa Extract. J. Pharm. Sci. 2017, 106, 3642–3650. [Google Scholar] [CrossRef] [PubMed]
- Mahady, G.B.; Dog, T.L.; Barrett, M.L.; Chavez, M.L.; Gardiner, P.; Ko, R.; Marles, R.; Pellicore, L.S.; Giancaspro, G.I.; Sarma, D.N. United States Pharmacopeia review of the black cohosh case reports of hepatotoxicity. Menopause 2008, 15, 628–638. [Google Scholar] [CrossRef]
- Nasr, A.; Nafeh, H. Influence of black cohosh (Cimicifuga racemosa) use by postmenopausal women on total hepatic perfusion and liver functions. Fertil. Steril. 2009, 92, 1780–1782. [Google Scholar] [CrossRef]
- Naser, B.; Schnitker, J.; Minkin, M.J.; de Arriba, S.G.; Nolte, K.-U.; Osmers, R. Suspected black cohosh hepatotoxicity: No evidence by meta-analysis of randomized controlled clinical trials for isopropanolic black cohosh extract. Menopause 2011, 18, 366–375. [Google Scholar] [CrossRef]
- Teschke, R.; Schwarzenboeck, A.; Schmidt-Taenzer, W.; Wolff, A.; Hennermann, K.-H. Herb induced liver injury presumably caused by black cohosh: A survey of initially purported cases and herbal quality specifications. Ann. Hepatol. 2011, 10, 249–259. [Google Scholar] [CrossRef]
- Sarri, G.; Pedder, H.; Dias, S.; Guo, Y.; Lumsden, M.A. Vasomotor symptoms resulting from natural menopause: A systematic review and network meta-analysis of treatment effects from the National Institute for Health and Care Excellence guideline on menopause. BJOG Int. J. Obstet. Gynaecol. 2017, 124, 1514–1523. [Google Scholar] [CrossRef]
| Sl. No | Products | Serving Size | Amount (mg) of CR Extract/1 Serving Size | Amount of Triterpenes/1 Serving Size (mg) |
|---|---|---|---|---|
| 1 | Remifemin | 1 tablet | - | 40 |
| 2 | Enzymatic Therapy Remifemin | 1 tablet | - | 20 |
| 3 | XYMOGEN MenoFem | 1.0 Capsule(s) | 50 | - |
| 4 | Vitanica Women’s Passage | 1.0 Capsule(s) | 40 | - |
| 5 | Estroven Extra Strength Estroven | 2.0 Caplet(s) | 40 | - |
| 6 | Irwin Naturals EstroPause | 4.0 Liquid Softgel(s) | 80 | - |
| 7 | Lydia E. 8Pinkham Herbal Liquid Supplement with Vitamins C and E | 1.0 Tablespoon(s) | - | - |
| 8 | Gaia Herbs Single Herbs Black Cohosh | 1.0 Capsule(s) | 400 | 2 |
| 9 | Oregon’s Wild Harvest Black Cohosh | 1.0 Capsule(s) | 300 | - |
| 10 | Natrol Black Cohosh Extract 80 mg | 2.0 Capsule(s)) | 160 | 4 |
| 11 | Major Black Cohosh 40 mg | 1.0 Capsule(s) | - | 40 |
| 12 | Bluebonnet Standardized Black Cohosh Root Extract | 1.0 Capsule(s) | 250 | 6.25 |
| 13 | Gaia Herbs SystemSupport Women’s Balance | 1.0 Capsule(s) | 200 | 1 |
| 14 | Nature’s Way Black Cohosh Root | 3.0 Capsule(s) | 540 | - |
| 15 | Oregon’s Wild Harvest Black Cohosh | 1.0 mL | 500 | - |
| 16 | NOW Red Clover/Black Cohosh 225 mg/40 mg | Serving size: 1.0 Vcap(s)(R) | 40 | - |
| 17 | Vitamin World Extra Strength Black Cohosh 40 mg | 1.0 Softgel(s) | 40 | 1 |
| 18 | Terry Naturally Menopause Relief plus | 2 capsule(s) | - | - |
| 19 | Botanic Choice Black Cohosh Root | 1.0 mL | 35 | - |
| Title of the Manuscript | System and Detector | Plate or Column Used | Solvent System/Mobile Phase | Advantage | Reference |
|---|---|---|---|---|---|
| “Actaea racemosa (root and rhizome)” | HPTLC-anisaldehyde reagent | TLC plate having coating of Silica gel 60, F254 | Toluen:ethyl formate:formic acid (5:3:2, v/v/v) | Simple method | [35] |
| “Fast analysis of Triterpene glycosides in CR using Agilent 1290 Infinity LC system and Agilent Poroshell 120 SB c-18 2.7 µm” | 1290 infinity LC system ELSD | Poroshell 120 SB C-18 2.7 µm column. Agilent 385 ELSD, Model-G4 261 A. | A Solvent: 1% formic acid in water B Solvent: Acetonitrile | This is a revised method for the established USP method of analysis of CR triterpenes. A more efficient approach than USP condition in terms of time and solvent consumption. Here, all the conditions (mobile phase, detector setting and column temp) are the same except for column | [36] |
| “Determination of Triterpene Glycosides in Cimicifuga racemosa (Black Cohosh) by HPLC-CAD” | HPLC-CAD | C18 HPLC column, 4.6 × 150 mm, 2.7 μm particle size | A Solvent: 0.1% formic acid in water B Solvent: Acetonitrile | Here, the calibration curves and signal-to-noise ratios for ELSD and charged aerosol detection of the triterpene glycoside (27-deoxyactein) in a black cohosh extract are compared. It can be concluded that the Thermo Scientific Dionex Corona™ CAD™ Charged Aerosol detector has ↑ sensitivity, calibration linearity, and reproducibility over ELSD | [37] |
| “Detection of Actaea racemosa Adulteration by Thin-Layer Chromatography and Combined Thin-Layer Chromatography-Bioluminescence” | HPTLC and HPTLC Bioluminescence (For identification of adulterants)—5% H2SO4–anisaldehyde reagent, Vibrio fischeri culture | Bioluminex silica gel 60 F254 HPTLC plates | Toluene:ethyl formate:formic acid (5 + 3 + 2, v/v/v). | An efficient, economical, and effective technique that helps to identify common adulterants in black cohosh. Unknown contaminants that were not identified by standard identification techniques were easily identified by this | [38] |
| “The HPLC Analysis of CR Using an INA Method” | HPLC-ELSD | Phenomenex Prodigy ODS-3, 5 μm, 250 × 4.6 mm | A Solvent:0.1% formic acid B Solvent: Acetonitrile | --- | [39] |
| “Isolation, structure elucidation, and absolute configuration of 26-deoxyactein from Cimicifuga racemosa and clarification of nomenclature associated with 27-deoxyactein” | HPLC-ELSD | YMC ODS-AQ RP-18 column (5 μm, 120 Å, 4.6 × 250 mm) | Solvent A: water containing 0.05% TFA, Solvent B: acetonitrile, Solvent C: water | Helps to isolate 3 triterpenes from the roots/rhizomes of CR | [40] |
| “Stability Evaluation of Selected Polyphenols and Triterpene Glycosides in CR” | HPLC-PDA | 125 × 4.0 mm i.d. Hypersil ODS column-quantitative 250 × 4.6 mm i.d., 5 μm Aqua C18 column-qualitative | A Solvent: 5% (v/v) acetonitrile B Solvent: Water | Evaluates the stability of the triterpene glycosides present in CR (plant material, extracts, and encapsulated commercial extract). With an HPLC-PDA method, 3 triterpene glycosides in CR were quantitatively measured for a specific period and were found stable at the tested conditions | [41] |
| “Quantitative determination of triterpenoids and formononetin in rhizomes of black cohosh (Actaea racemosa) and dietary supplements by using UPLC-UV/ELS detection and identification by UPLC-MS” | UPLC-UV/ELS | UPLC BEH C18 column (100 mm × 2.1 mm i.d., 1.7 μm | Gradient elution with water and acetonitrile: methanol (7:3) at a constant flow rate of 0.3 mL/min n, 55% A/45% B; in the next 7 min, 35% A/65% B using a slightly concave gradient profile | Successfully used to examine the various commercial product of CR along with differentiating between 2 other Actaea species. It has been concluded that there was significant inconsistency in the quantities of the selected triterpenes for different products of black cohosh | [33] |
| “Direct analysis and identification of triterpene glycosides by LC/MS in black cohosh, Cimicifuga racemosa, and in several commercially available black cohosh products” | HPLC-PDA | Hypersil ODS column-(5 μm, 4ID × 125 mm | A Solvent: water B Solvent: acetonitrile | Used to differentiate CR products from different plant species for quality control reasons | [42] |
| “HPLC Analysis of Triterpene Glycosides in Black Cohosh Formulations using the PL-ELS 2100” | PL-ELS 2100 (neb = 30 °C, evap = 50 °C, gas = 1.4 SLM) UV-Vis @ 230 nm | Inertsil C18 5 µm, 150 × 4.6 mm | A Solvent: 0.1% Formic Acid in Water B Solvent: Acetonitrile | To certify the potency of commercially available black cohosh tablets | [43] |
| “Phytochemical Fingerprinting to Thwart Black Cohosh Adulteration: a 15 Actaea Species Analysis” | HPLC-PDA-LCMS | Triterpene glycosides were performed with a 125 × 4.0 mm i.d. Hypersil ODS column (Agilent, Santa Clara, CA, USA | Step gradient starting with 5% (v/v) acetonitrile (A) in water (B) and ↑ to 100% acetonitrile over 60 min | A practical, reliable method for authenticating black cohosh and differentiating it from adulterants | [44] |
| “Evaluation of the Botanical Authenticity and Phytochemical Profile of Black Cohosh Products by High-Performance Liquid Chromatography with Selected Ion Monitoring Liquid Chromatography−Mass Spectrometry” | HPLC-PDA -LCMS (APCI) | 150 mm × 3.9 mm i.d., 5 µm, Waters C18 column | Step gradient starting with 5% (v/v) acetonitrile (solvent A) in water (Solvent B) | 11 black cohosh products were analyzed for triterpene glycosides and other constituents by using HPLC-PDA and a newly selected ion monitoring LC-MS method. The study concluded that the product contained Asian Actaea as a replacement for black cohosh | [45] |
| “Chemical profiling of Actaea species and commercial products using UPLC-QTof-MS” | UPLC-QTof-MS | Acquity UPLC HSS T3 2.1 × 100 mm,1.8 μm 40 °C | Gradient elution with Solvent A: 0.1% formic acid in water Solvent B: acetonitrile | Helps to recognize useful marker compounds such as cimifugin derivatives, triterpenes, and alkaloids that distinguish between Actaea species based on exact mass precursor ion, theoretical isotopic distribution, and high-energy fragment ion data | [46] |
| “The value of plant collections in ethnopharmacology: a case study of 85-year-old black cohosh (Actaea racemosa L.) sample” | HPLC- PDA- LCMS | C18 column (3.9 mm × 150 mm, 5 m) | Solvent A: water Solvent B: acetonitrile | A comparative study to confirm stability between the ingredients of the 85-year-old plant sample with that of a new collection of Actaea racemosa by quantitative study. Both plant samples have comparable quantities of the 4 major triterpene glycosides, thus concluding the similarity of both samples and confirming the stability of the older sample | [47] |
| “Species Identification of Black Cohosh by LC-MS for Quality Control” | Reversed-phase liquid chromatography with positive atmospheric pressure chemical ionization mass spectrometry (LC/(+)APCIMS | A fast and accurate method for analyzing the 4 triterpene: actein, 27-deoxyactein, cimicifugoside M and cimicifugoside from CR for quality control purposes | [48] |
| Source | Compound Class and Name | Part of the Plant | Reference |
|---|---|---|---|
| Cimicifuga racemosa | Cimigenol-3-O-β-D-xyloside (Cimigenoside) | Rhizome | [59] |
| 25-O-Acetylcimigenol-3-O-β-Dxyloside (25-O-Acetylcimigenol xyloside | Rhizome | [60] | |
| Cimiracemoside A | Rhizome | [44,61] | |
| (24S)-24-O-Acetylhydroshengmanol- 3-O-β-D-xyl-Δ16,17-enol ether | Root and rhizome | [62] | |
| 23-epi-26-Deoxyactei | Root and rhizome | [63] | |
| 26-Deoxyactein (=27-Deoxyactein) | Root and rhizome | [40] | |
| Actein | Root and rhizome | [40,63] | |
| Caffeic acid | Root and rhizome | [64] | |
| Cimiracemate A | Rhizome | [60] | |
| Cimiracemate B | Rhizome | [65] |
| Name of the Constituent | Chemical Structure |
|---|---|
| Cimiracemate A | 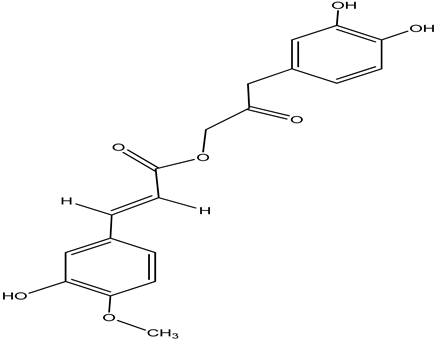 |
| Cimiracemate B | 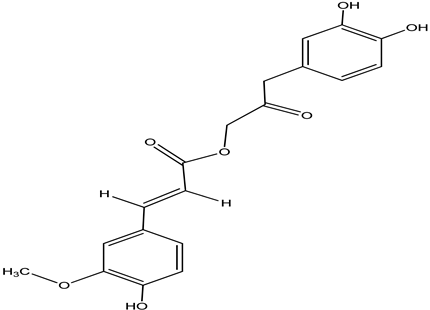 |
| Caffeic acid | 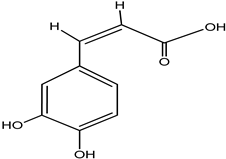 |
| n-methyl serotonin | 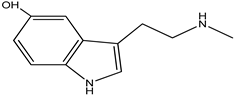 |
| isoferulic acid | 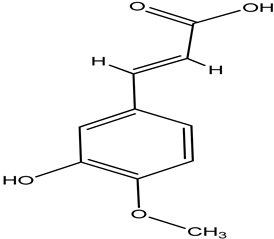 |
| Actein | 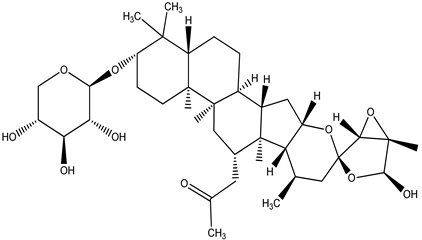 |
| 26-Deoxyactein | 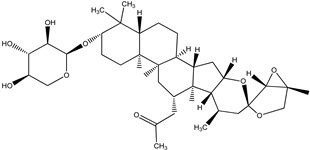 |
| 23-epi-26-Deoxyactei | 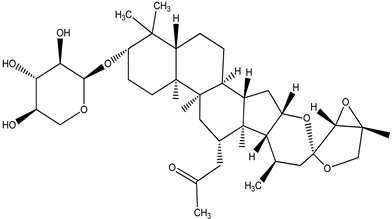 |
| Cimigenol-3-O-β-D-xyloside (Cimigenoside) | 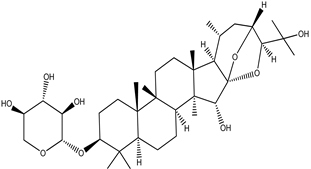 |
| 25-O-Acetylcimigenol-3-O-β-Dxyloside (25-O-Acetylcimigenol xyloside | 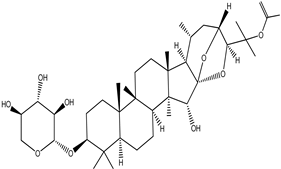 |
| 25-O-Anhydrocimigenol-3-O-β-Dxyloside (25-Anhydrocimigenol xyloside | 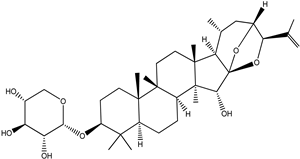 |
| Cimiracemoside A | 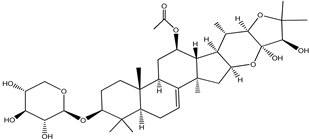 |
| Cimiracemoside B | 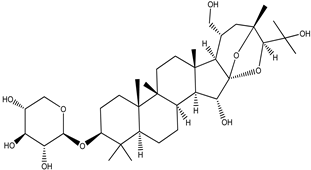 |
| Cimiracemoside C | 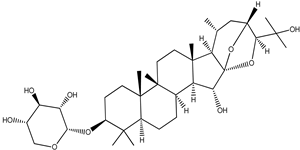 |
| Cimiracemoside D | 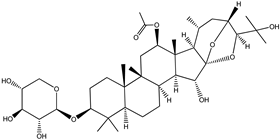 |
| Cimiracemoside E | 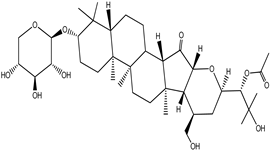 |
| Cimiracemoside F | 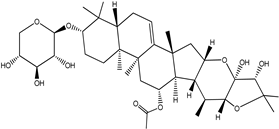 |
| Cimiracemoside G | 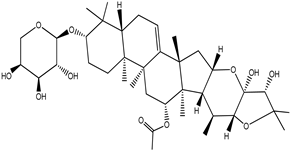 |
| Cimiracemoside H | 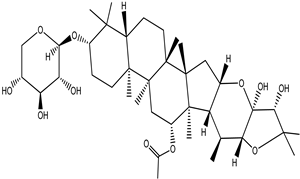 |
| Cimiracemoside M | 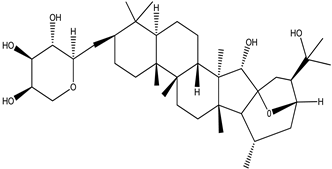 |
| Sl. No. | Purpose | Analytical Method Used | Conclusion | Reference |
|---|---|---|---|---|
| 1 | A quality control method of CR products used to differentiate CR products from among other plant species | Employed reversed-phase LC-MS to identifies triterpene glycosides | Identified several triterpenes, such as 27-deoxyactein, actein, cimicfugoside M, and cimicifugoside, from CR; also identified a chromone, cimifugin, from C. foetida. Study can conclude that Cimicifugoside M and cimifugin can act precisely as a marker for the identification of species | [42] |
| 2 | Used to distinguish species of Cimicifuga and other botanicals for women’s health issues. This methodology can be used to recognize species of commercial value without considering collection time or geographic area | Employed random amplified polymorphic DNA (RAPD) analysis | The study identified species-specific DNA fragments and concluded that Cimicifuga species collected from diverse geographical locations have the same profiles but not matched DNA | [66] |
| 3 | Analysis of the methanolic extract of CR root and its methanolysis products | Employed GC-MS method | Identified the marker that is most specific for the identification of this plant: 2-Hexylcyclopropaneoctanoic acid (9,10-methylenehexadecanoic acid). Moreover, developed a validated method for the quantitative analysis of different compounds such as isoferulic acid, formononetin, and total triterpene glycosides in CR root | [54] |
| 4 | This method is used to observe the chromatography or fingerprint profile of 7 CR herbs and 6 CR commercial samples | LC/turbo ion spray (TIS)- MS method | Provides a reliable and reproducible method that can be used for botanical identification of CR plants and the inspection and validation of CR commercial samples. Different chromatograms patterns were developed for different species of CR, such as Cimicifuga racemosa, Cimicifuga dahurica, Cimicifuga foetida, Cimicifuga heracleifolia, Cimicifuga japonica, Cimicifuga acerina, and Cimicifuga simplex, by using LC/MS. 23-Epi-26-deoxyactein was present only in CR, C. dahurica, and C. foetida and were quantified by LC/MS/MS method | [68] |
| 5 | Method used for the botanical standardization and quality control of CR products | HPLC-PDA/MS/ELSD | Developed and validated using 10 Cimicifuga species. The triterpenes cimifugin, cimigenol-3-O-arabinoside, and cimifugin-3-O-glucoside were identified as appropriate species-specific markers for the identification of CR from the other species of Cimicifuga. It offers the identification as well as perception into chemical inter conversion methods occurring among the various triterpenes in CR | [34] |
| 6 | This is used for the identification of CR and recognition of its common adulterants by fingerprint profiles | HPTLC method | A practical, fast, and reliable method with specific derivatization reagents. This allows the detection of mixtures of CR with a minimum of 5% of the adulterants. Can be used for quality control of CR raw material in a current good manufacturing practices environment | [69] |
| 7 | Phytochemicals method was developed to differentiate four different groups of Actaea | HPLC-TOF-ESI-MS technique and principal component analysis | Used for metabolic profiling to distinguish CR from related species of Actaea. It identified 15 chemical markers where 3 markers were recognized using reliable standards, and 12 marker compounds were tentatively recognized by comparing the fragmentation patterns with past information. The occurrence of these marker compounds is critical for the identification of 4 groups of closely associated plants | [70] |
| 8 | This method is used for polyphenols and triterpene glycosides | HPLC and LC-MS fingerprints | Showed different patterns that make CR distinguishable from several other Actaea species. Cimifugin and cimiracemoside F are the two marker compounds, which were found to be essential to differentiate CR from most Asian species of Actaea | [44] |
| 9 | Identified 2 matK nucleotides that constantly discriminate CR from other correlated species and correctly identified the CR samples | Employed nucleotides | Out of 36 dietary supplements sequenced, 75% had a sequence that accurately paired with CR and the remaining 25% had a sequence identical to that of 3 Asian Actaea species (A. cimicifuga, A. dahurica, and A. simplex) | [71] |
| 10 | Proposed 2 metabolic fingerprinting methods to identify and validate Actaea species | DNA sequencing in combination with flow injection MS and proton NMR spectrometry | Single-source CR samples were differentiated from other species based on key component analysis, soft independent modelling of class analogies of flow injection MS and proton NMR spectrometry metabolic fingerprints. DNA sequence information from 2 independent gene regions confirmed the metabolic fingerprinting by DNA sequencing. Moreover, the combined CR samples were distinguishable from commercial root samples and commercial products | [72] |
| 11 | Tried to establish an analytical method to evaluate the authentication of the plant materials and finished products | Polymerase chain reaction-restriction fragment length polymorphism method and a multiplex amplification refractory mutation system | Described a genome-based confirmation method and LCMS-based authentication for CR products | [73] |
| Year | No. of Subjects | Study Length | Extract/Formulation/Dosage Form | Study Design | Status | Study Outcome | Reference |
|---|---|---|---|---|---|---|---|
| 2002 | 152 Perimenopausal and postmenopausal females | 6 months | 2 different doses (39 mg and 127.3 mg) of CR preparation | A controlled, randomized, double-blinded parallel group study | Completed | CR extract helps in ↓ the menopause symptoms without showing estrogen-like effects and also supports the 40 mg/day standard dose of the isopropanolic aqueous CR extract over the higher dose | [95] |
| 2003 | 62 postmenopausal women | 3 months | With CR BNO 1055 (Klimadynon/Menofem): daily dose corresponding to 40 mg herbal drug, 0.6 mg conjugated estrogens, or matching placebo | A double-blind, randomized, multi-centre study | Completed | The results of the study concerning climacteric complaints and on bone metabolism indicate an equipotent effect of CR BNO 1055 in comparison to 0.6 mg CE/day. It is expected that CR BNO 1055 has ingredients with SERM activity | [12] |
| 2005 | 64 postmenopausal women | 3 Months | Either isopropanolic aqueous CR extract (40 mg daily) or transdermal estradiol (25 μ every 7 days) + dihydrogesterone (10 mg/day) for the last 12 days of the 3-month estradiol treatment | A randomized clinical study | Completed | CR (40 mg/day) may be a valid substitute to low-dose transdermal estradiol in managing the climacteric complaints of women who cannot be treated with conventional approaches | [80] |
| 2005 | 122 menopausal women | 12 weeks | CR extract | A multi-center, randomized, placebo-controlled, double-blind, parallel-group study | Completed | CR extract is superior in comparison to placebo in patients having menopausal syndromes of modest strength | [96] |
| 2006 | 351 women | 12 months | Black cohosh (160 mg daily); multi-botanical having black cohosh, (200 mg daily) with 9 other ingredients | A randomized, double-blind, placebo-controlled trial | Completed | Black cohosh used singly, or as a component of a multi-botanical treatment, reveals little potential as a vital remedy for vasomotor problems | [97] |
| 2007 | 74 women | 6 months | Black cohosh (40 mg daily) | A prospective, open, uncontrolled drug safety study | Completed | There are no harmful effects of the isopropanolic extract of black cohosh on breast tissue and also no indication of any endometrial or general safety concerns in the course of treatment | [98] |
| 2009 | 88 women | 12 months | Black cohosh (128 mg/day) | A randomized, four-arm, double-blind clinical trial | Completed | Black cohosh and red clover did not ↓ the number of vasomotor signs compared with placebo but standardized extracts of black cohosh and red clover were safe biologically and chemically during daily administration for the total period | [99] |
| 2010 | 128 women | 10 weeks | Black cohosh (40 mg/day) | A randomized, controlled trial | Completed | Adjuvant supplementation of black cohosh did not ↑ our exercise schedule, positively affect bone, menopausal symptoms, lean body mass, or to a lesser degree, 10-year CHD risk in early postmenopausal females | [100] |
| 2011 | 65 women | 6 months | Black cohosh | A prospective, double-blind, placebo-controlled study | Completed | Black cohosh does not affect mammographic breast density | [101] |
| 2011 | 50 Tamoxifen treated breast cancer patient | 6 months | Isopropanolic extract of black cohosh (1–4 tablets, 2.5 mg) | A prospective observational study | Completed | Breast cancer patients treated with tamoxifen, mainly having psychovegetative indications can be reasonably treated with black cohosh extract | [102] |
| 2012 | 304 women | Standardized isopropanolic extract of black cohosh (Remifemin) | A randomized, double-blind, placebo-controlled, multicenter clinical study | Completed | Remifemin is efficacious and tolerable in the management of menopausal symptoms, specifically hot flashes | [103] | |
| 2013 | 84 women | 8 weeks | Dried extract of Black cohosh roots (6.5 mg daily orally). | A randomized, double-blind, placebo-controlled clinical trial | Completed | Black cohosh reduced the Greene climacteric scale (GCS) total score, and all GCS subscale scores (vasomotor, psychiatric, physical, and sexual symptoms) during the period of treatment. | [104] |
| 2013 | 120 women | 2 months | Remifemin (one tablet twice daily) and Paroxetine (20 mg once daily) | A randomized, controlled trial | Completed | The combined treatment of Remifemin and Paroxetine can ↑ the efficacy of perimenopausal depression and also has a ↑ safety profile with lesser side effects | [105] |
| 2014 | 116 women | 12 weeks | Remifemin (20 mg) and Tibolone (2.5 mg) | A randomized study | Completed | Remifemin had a parallel clinical efficacy as compared to Tibolone and was safer for the peri-menopausal symptoms induced by GnRH-a in endometriosis patients | [106] |
| 2015 | 48 women | 6 months | Black cohosh extract (Oral) | A randomized, double-blind and placebo-controlled research | Completed | Black cohosh extract effectively ↑ sleep in early postmenopausal females with major sleep complaints and might be a safe measure in managing menopausal sleep disturbance | [107] |
| 2015 | 54 women | Black cohosh extract (40 mg/day) | A randomized, double-blind, placebo-controlled clinical trial | Completed | Black cohosh extract was not more efficient than placebo for alleviating moderate to serious menopausal symptoms or ↑ quality-of-life scores in Thai women | [108] | |
| 2019 | 85 women | 12 weeks | Remifemin, the commercialized product of CR extract, combined with LHRH-a. | A perspective randomized-design study | Completed | CR is efficient, reliable and safe for the management of menopausal syndrome caused by luteinizing-hormone releasing hormone analogue in breast malignancy | [109] |
| 2020 | 174 women | 12 months | CR extract, Ze 450 and menopausal hormone therapy | A monocentric retrospective cohort study | Completed | Menopausal symptoms ↑ significantly in both groups (MHT and CR), without altering the serum metabolic parameters and body weight | [110] |
| Patent No. | Title | Disease/Problem | Proof of Concept |
|---|---|---|---|
| US20040202736A1 United States 2004 | Method of ameliorating side effects of SERMs | Side effects of selective estrogen receptor modulators (SERMs). | The present invention narrates a method of treatment and/or prevention of side effects such as hot flashes caused by SERMs such as tamoxifen, by administering an effective amount of a standardized dry extract of CR over a particular period |
| US6713097B2 United States | Use of preparation of Cimicifuga racemosa | Females having urinary incontinence following an ovariohysterectomy/hysterectomy/menopause | The present invention describes a specific preparation from the rhizome of CR which can be used for the successful treatment of urinary incontinence in females following an ovariohysterectomy. Positive results can also be expected for females having a hysterectomy or after menopause |
| WO1998026791A1 WIPO (PCT) | The use of a CR extract | Estrogen-dependent tumors | This innovation relates to the use of an extract of CR for the treatment of estrogen-dependent tumors. This invention describes that the simultaneous administration of a CR extract (doses of 5 to 500 mg/day) with an anti-estrogenic active substance will enhance its action on estrogen-dependent tumors |
| US20120071501A1 United States 2012 | Use of extracts of the genus cimicifuga as organo selective medicines for treating diseases of the genitourinary tract caused by sex hormones. | Genitourinary tract infection is caused by sex hormones. | Describes CR extracts suitable for making a ready-formulated drug for the selective treatment and/or prevention of sexual hormone-related ailments of the urogenital tract, post-menopausal urinary bladder infections and for the treatment of benign and malignant prostate hyperplasia |
| EP2545932A1 European Patent Office 2013 | Selected cimicifuga fractions for the treatment of osteoporosis | Osteoporosis | The invention relates to the methods for producing cimicifuga fractions from a Cimicifug extract, for the prevention and treatment of osteoporosis in humans and animals |
| US20060210659A1 United States | Anti-obesity agent | Obesity | The present invention provides the use of a CR plant in order to prepare an active substance which has an anti-obesity effect such as a blood triglyceride-lowering agent, a cholesterol-lowering agent, a body fat storage suppressive agent, an anti-obesity agent and an anti-lipemic agent comprises a cycloartane-type triterpene or glycoside thereof. Additionally, the present invention provides a beverage, food, and quasi-drug comprising of said agents |
| WO2020144588A1 WIPO (PCT) | Compositions for treatment of menopause, osteopenia, and osteoporosis, and menopause/related metabolic and vascular disorders. | Menopause, osteopenia and osteoporosis, and menopause/related metabolic and vascular disorders | The present invention discloses a composition comprising of CR and Ferula extracts, and optionally other phytotherapeutic extracts, vitamins, and oligo-elements for the treatment of symptoms related to menopause |
| US20030224068A1 United States 2003 | Compounds for hormonal therapy | Hormonal therapy | The present invention offers a therapeutic composition consisting of a therapeutically effective amount of 27-deoxyactein. It also describes a multi-step process for the isolation of 27-deoxyactein from CR by chromatographic suitable techniques |
| Regulatory Agencies/Organizations | Recommendations/Conclusions | Reference |
|---|---|---|
| Australian Department of Health (Therapeutic Goods Administration) 2007 | They reviewed the existing regulatory controls on CR and concluded that there is a link between the use of CR and liver injury; however, it is very unusual. They determined that although CR shows rare liver-damaging properties, it is still suitable for use in complementary medicines, with proper warning statements such as: “Warning: CR may harm the liver in some individuals. Use under the supervision of a healthcare professional” on the product label | [129] |
| Health Canada | Health Canada suggests that consumers using CR products should use them with caution and if they have any concerns regarding its use they should refer to a physician. If the consumers have a weakness, loss of appetite, rare fatigue, or if they develop signs indicative of liver damages such as in the whites of the eyes or the yellowing of the skin, abdominal pain or dark urine, they should immediately terminate the use of the product and refer to a physician | [130] |
| Medicine and Healthcare products Regulatory Agency (MHRA), UK | MHRA suggests that warnings must be included in the product info for CR, relating to the rare harmful reactions in the liver for both registered and unregistered goods. The liver toxicity concern of the CR should be observed carefully, and further evidence should be collected on the quality of CR products and their composition accessible in the UK market. Furthermore, the potential mechanism of CR products associated with liver injury should be studied | [131] |
| The U.S. Pharmacopeia, 2008 | USP recommends CR products must be labelled with a cautionary statement such as: “Discontinue use and consult a healthcare practitioner if you have a liver disorder or develop symptoms of liver trouble, such as abdominal pain, dark urine, or jaundice”. Nevertheless, the U.S. FDA does not need such caution on labels of CR products | [125] |
| The American Herbal Products Association, 2013 | They recommend that CR should be avoided in pregnant women apart from females under the care of their healthcare professional | [8] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohapatra, S.; Iqubal, A.; Ansari, M.J.; Jan, B.; Zahiruddin, S.; Mirza, M.A.; Ahmad, S.; Iqbal, Z. Benefits of Black Cohosh (Cimicifuga racemosa) for Women Health: An Up-Close and In-Depth Review. Pharmaceuticals 2022, 15, 278. https://doi.org/10.3390/ph15030278
Mohapatra S, Iqubal A, Ansari MJ, Jan B, Zahiruddin S, Mirza MA, Ahmad S, Iqbal Z. Benefits of Black Cohosh (Cimicifuga racemosa) for Women Health: An Up-Close and In-Depth Review. Pharmaceuticals. 2022; 15(3):278. https://doi.org/10.3390/ph15030278
Chicago/Turabian StyleMohapatra, Sradhanjali, Ashif Iqubal, Mohammad Javed Ansari, Bisma Jan, Sultan Zahiruddin, Mohd Aamir Mirza, Sayeed Ahmad, and Zeenat Iqbal. 2022. "Benefits of Black Cohosh (Cimicifuga racemosa) for Women Health: An Up-Close and In-Depth Review" Pharmaceuticals 15, no. 3: 278. https://doi.org/10.3390/ph15030278
APA StyleMohapatra, S., Iqubal, A., Ansari, M. J., Jan, B., Zahiruddin, S., Mirza, M. A., Ahmad, S., & Iqbal, Z. (2022). Benefits of Black Cohosh (Cimicifuga racemosa) for Women Health: An Up-Close and In-Depth Review. Pharmaceuticals, 15(3), 278. https://doi.org/10.3390/ph15030278







