Human Cryptic Host Defence Peptide GVF27 Exhibits Anti-Infective Properties against Biofilm Forming Members of the Burkholderia cepacia Complex
Abstract
:1. Introduction
2. Results
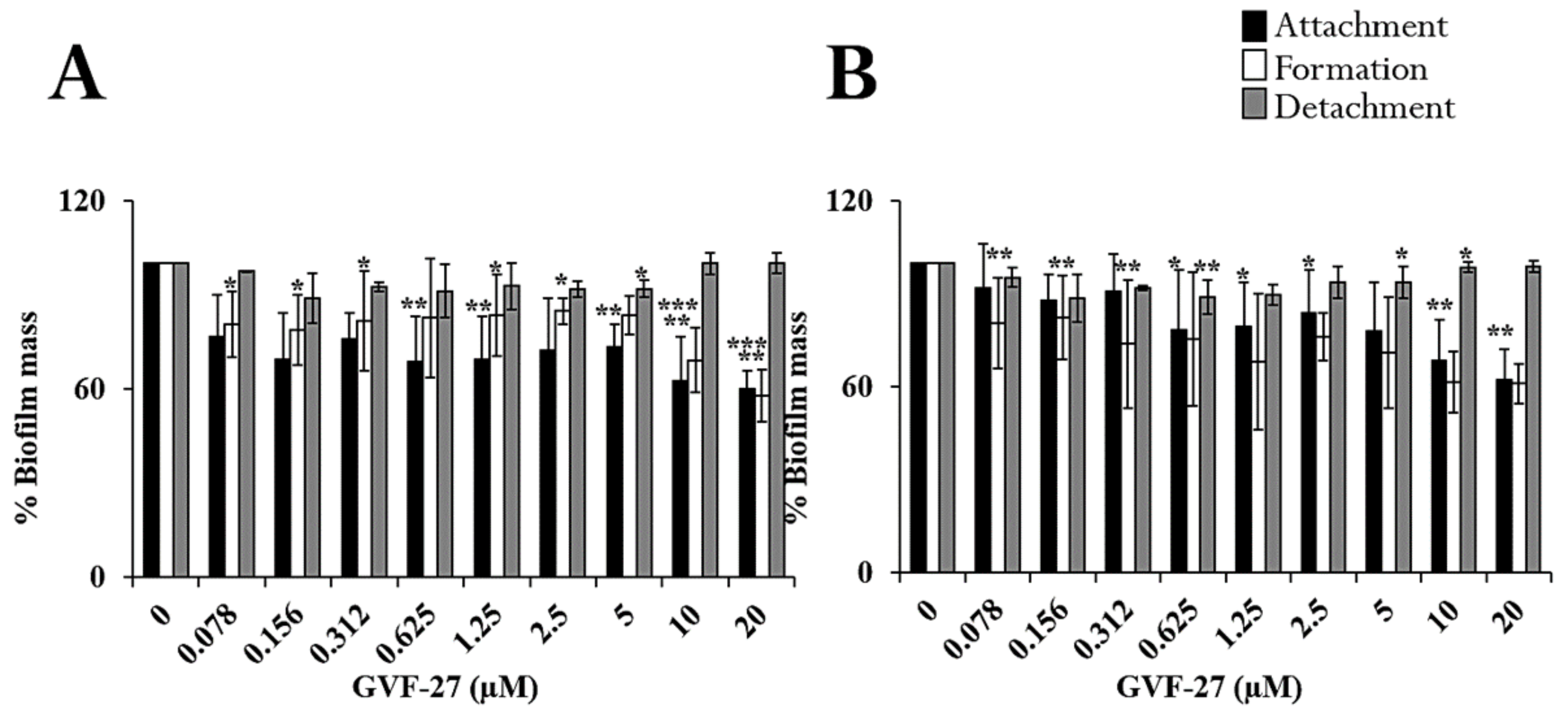
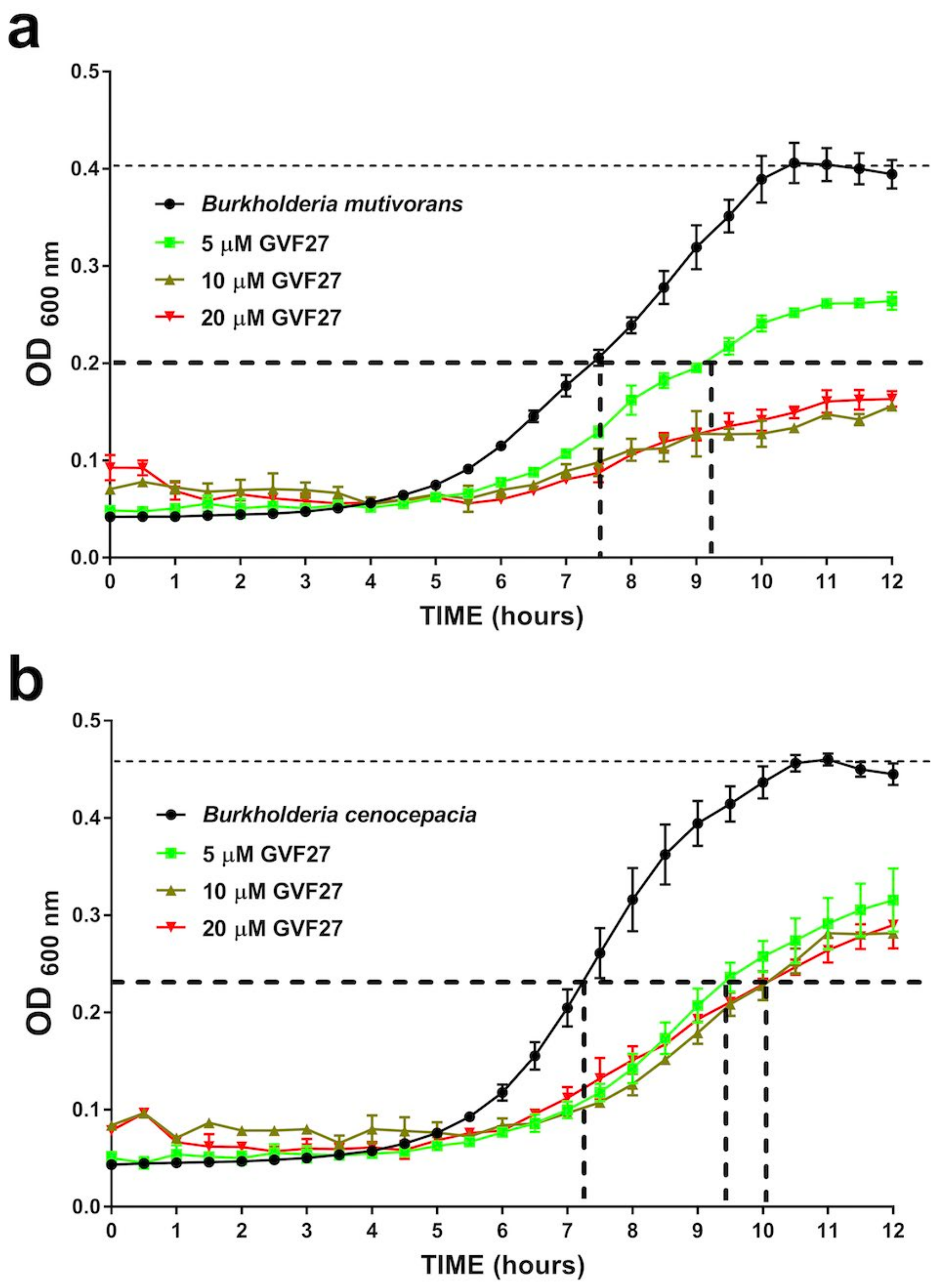
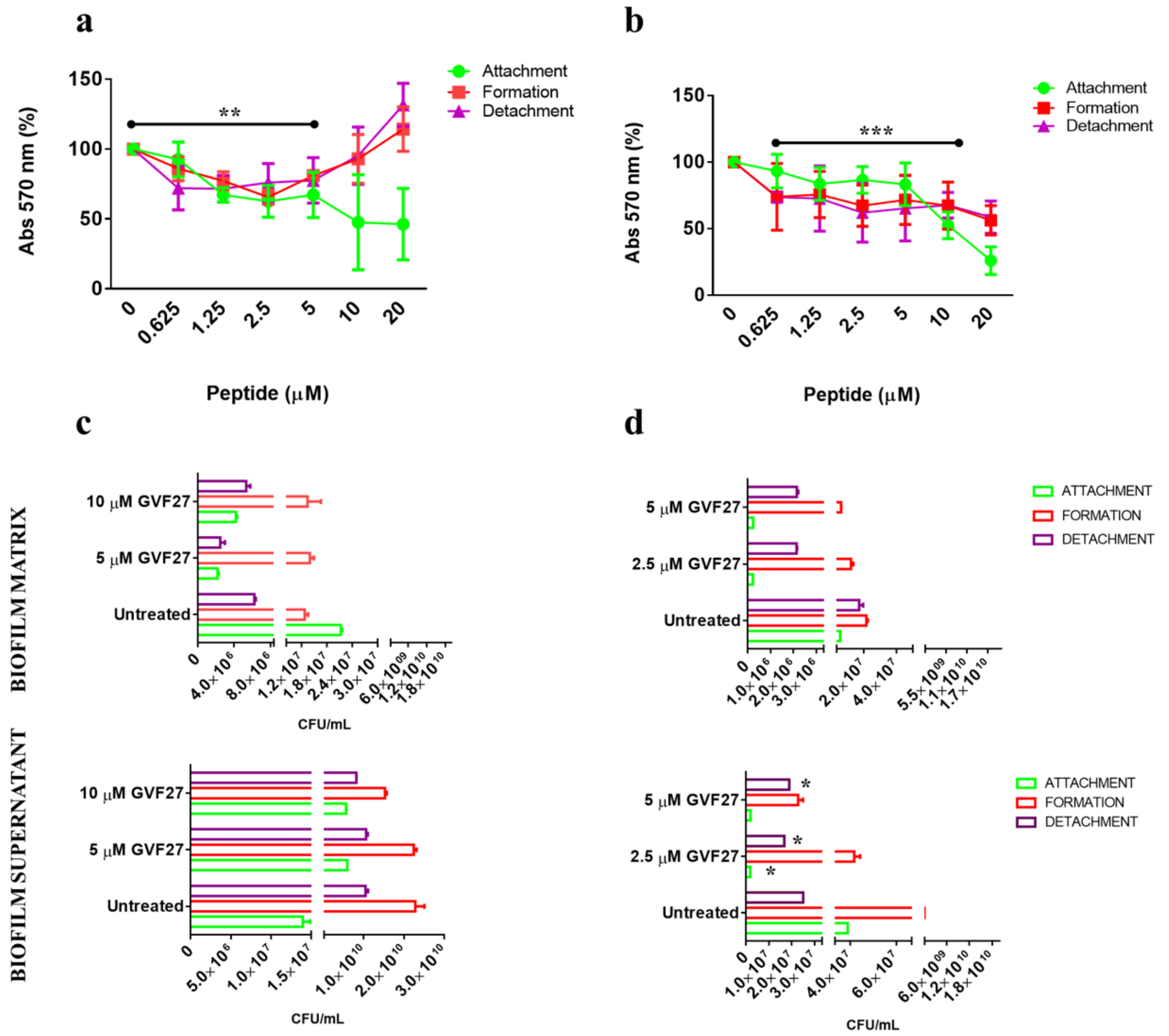
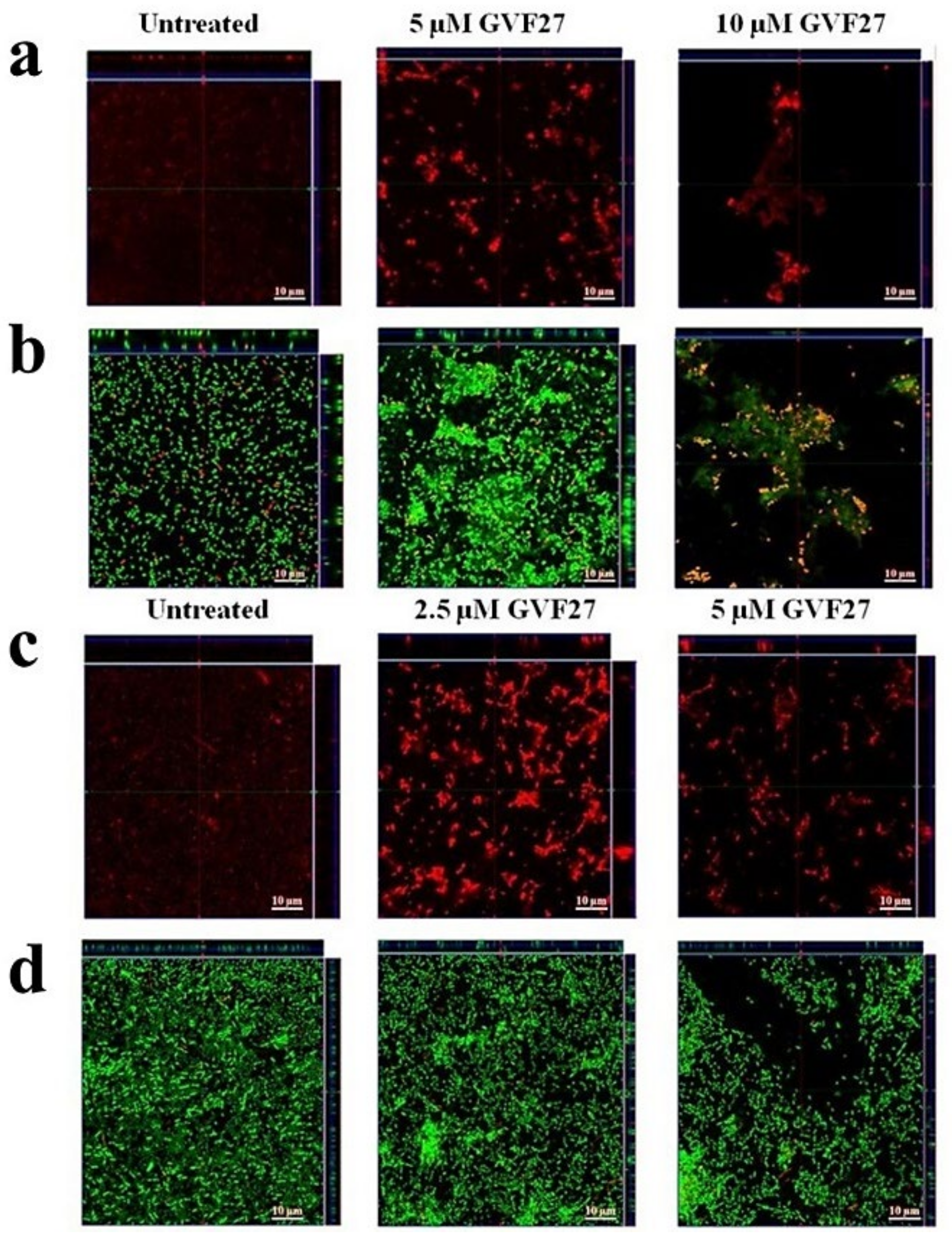
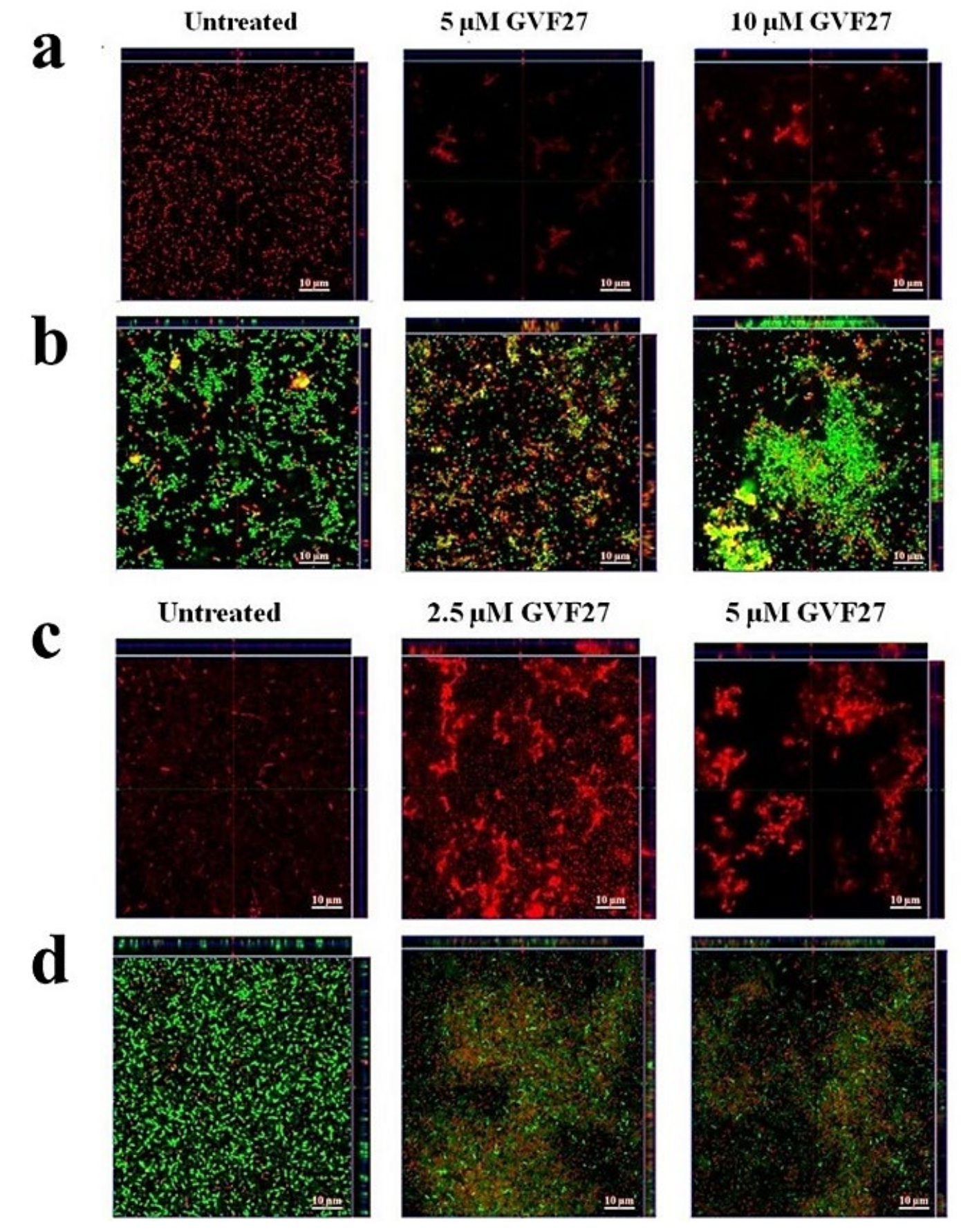
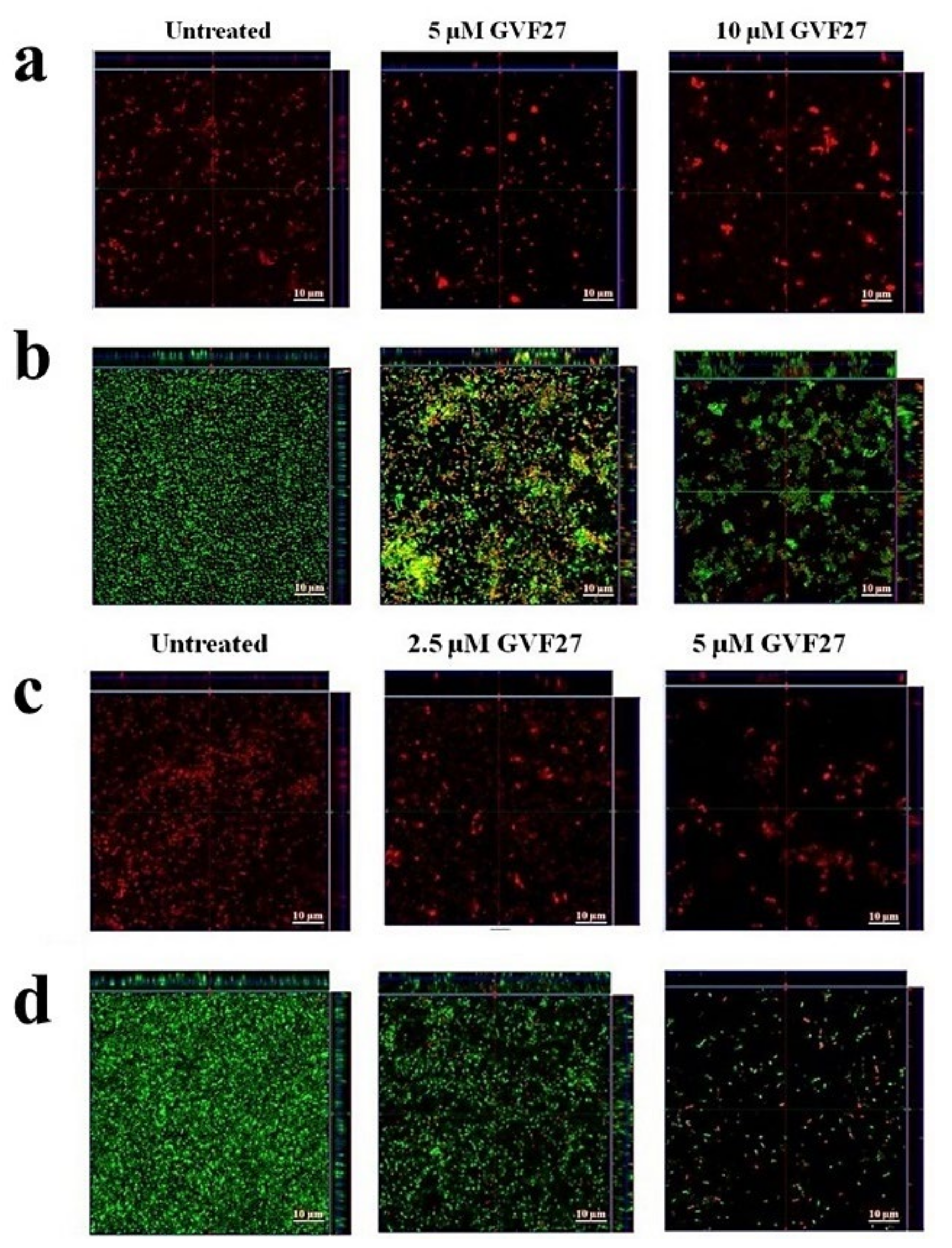
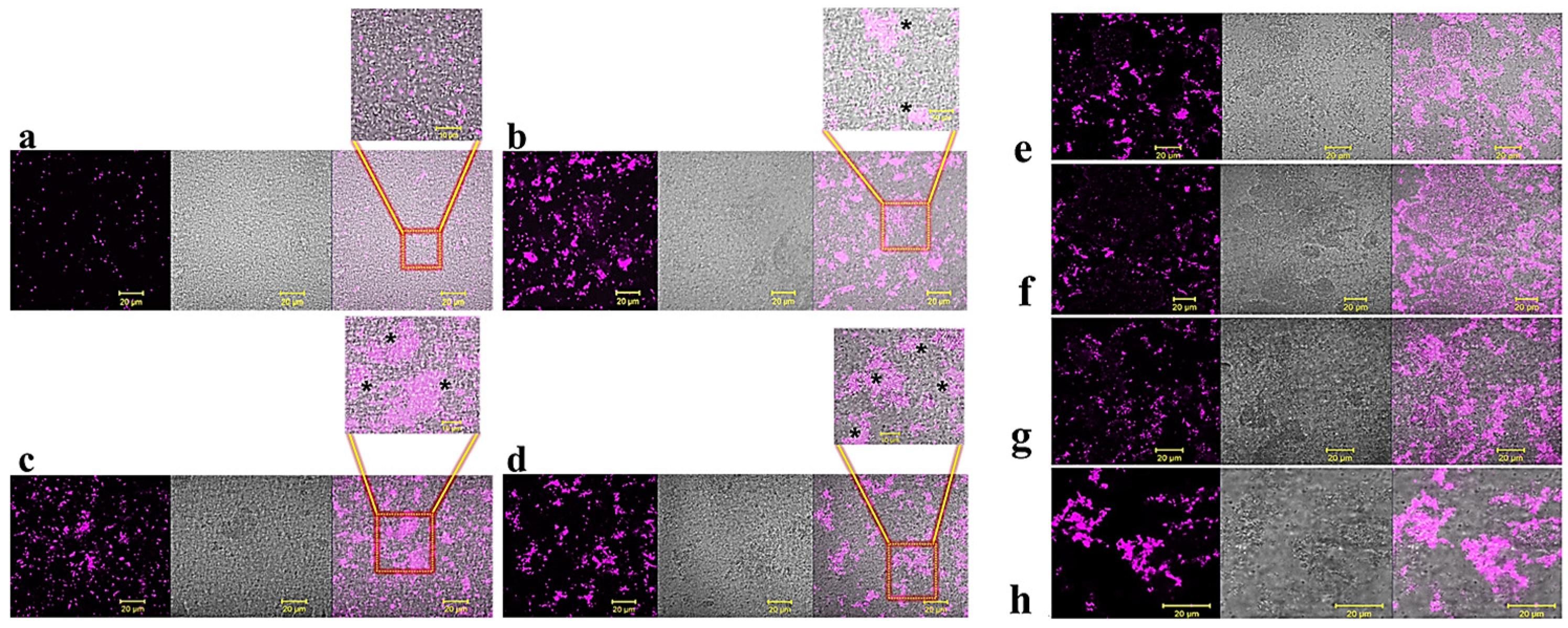
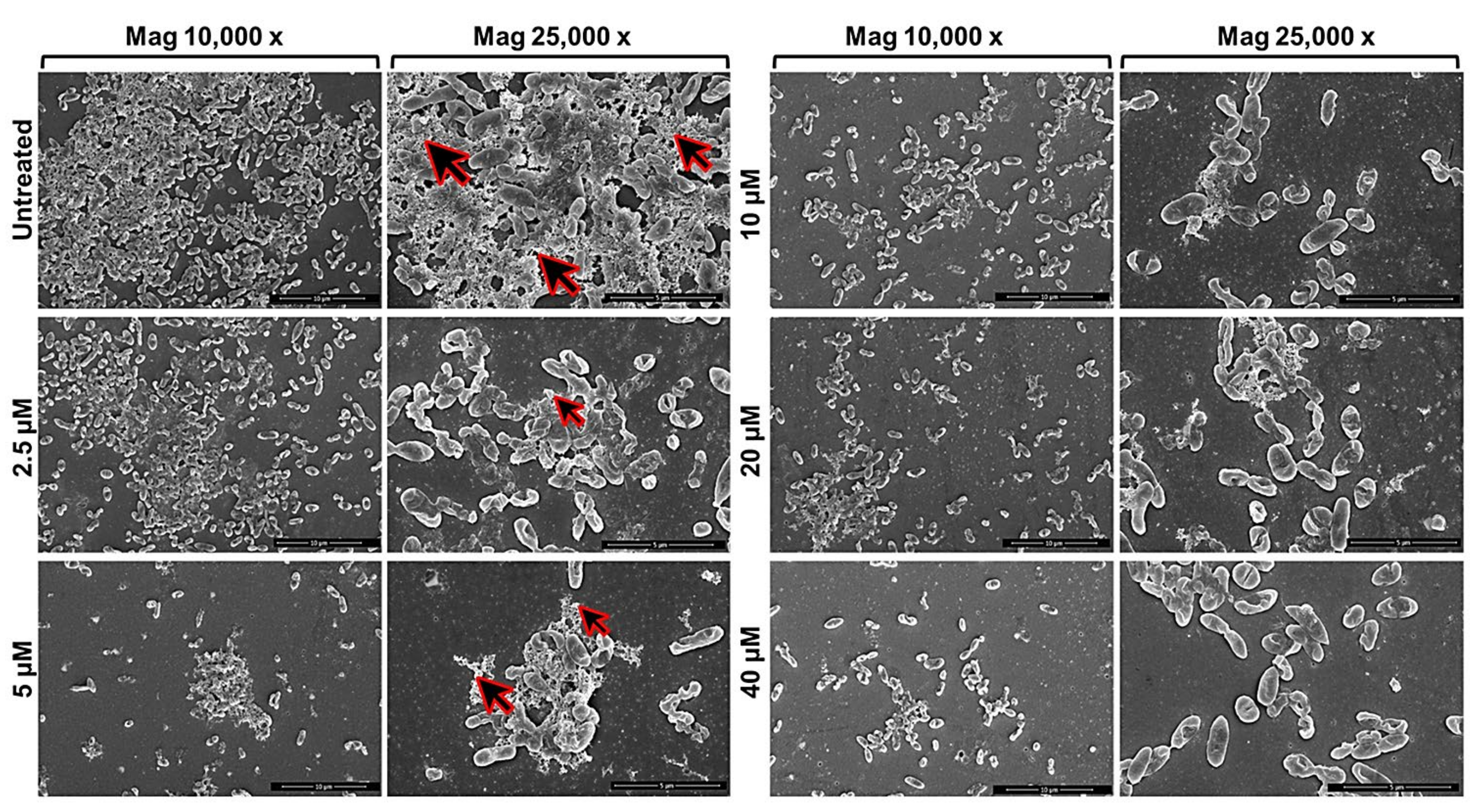
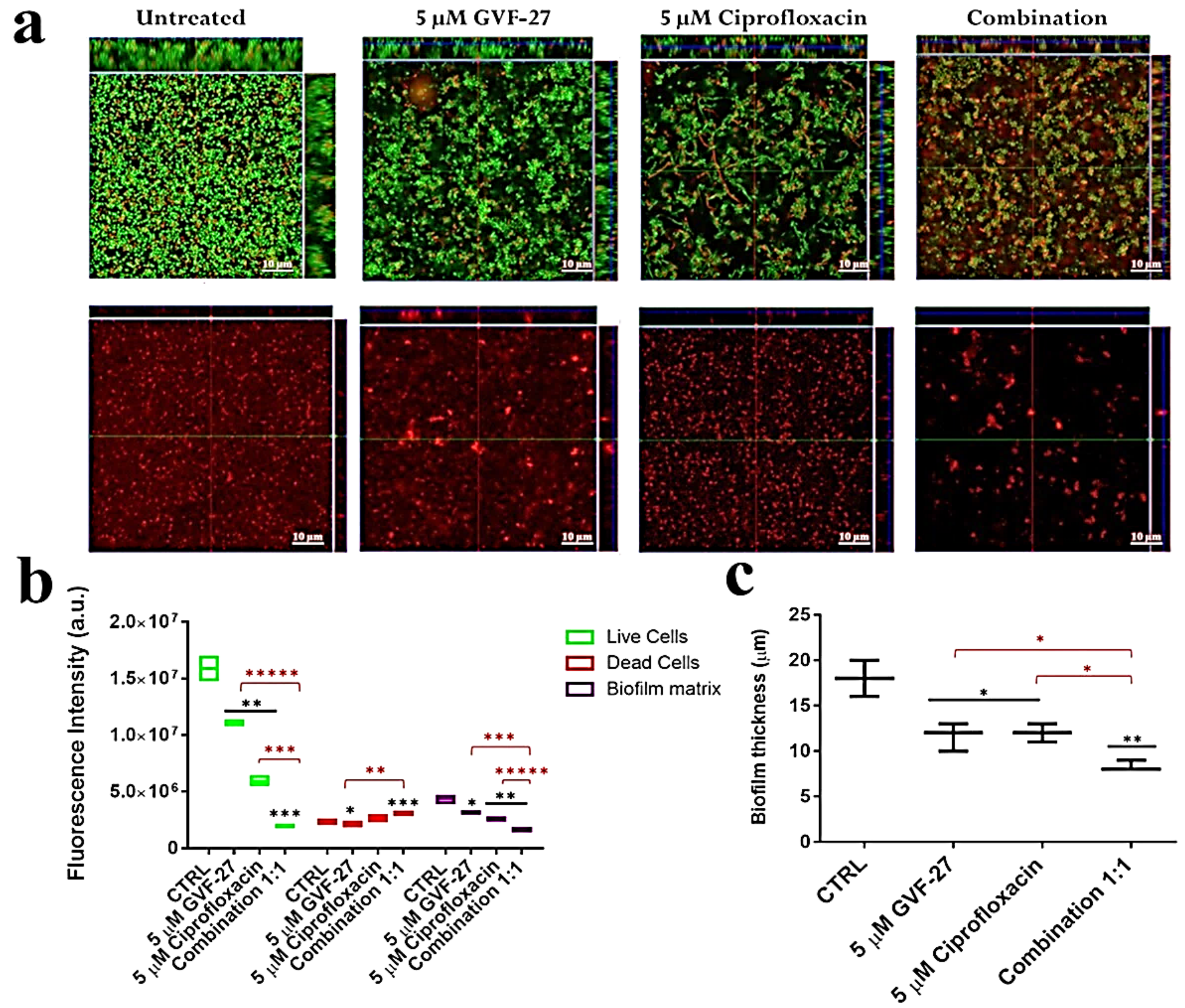
2.1. Anti-Biofilm Activity of GVF27 on Bcc Clinical Isolates
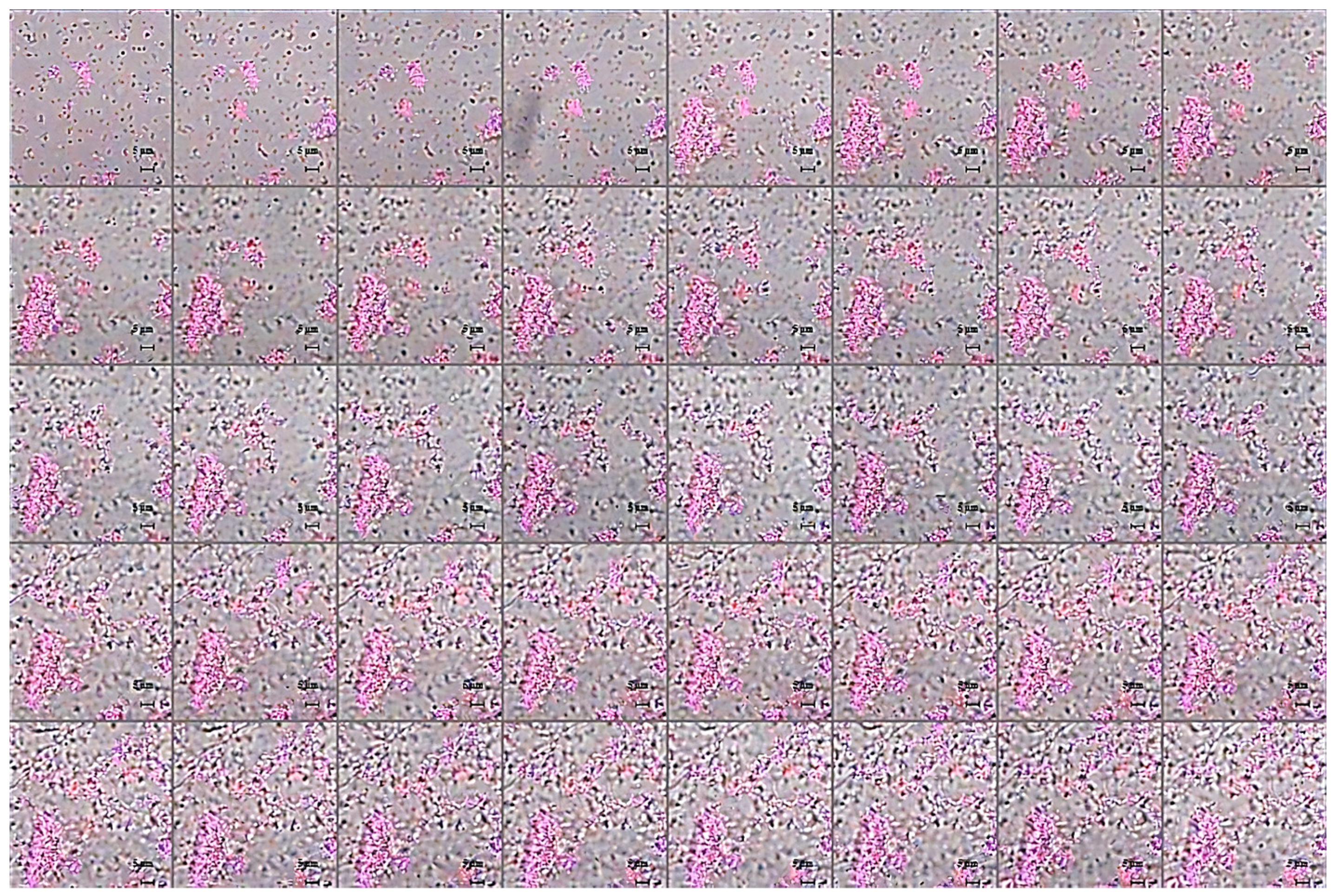
2.2. Studies of Agglutinating Activity
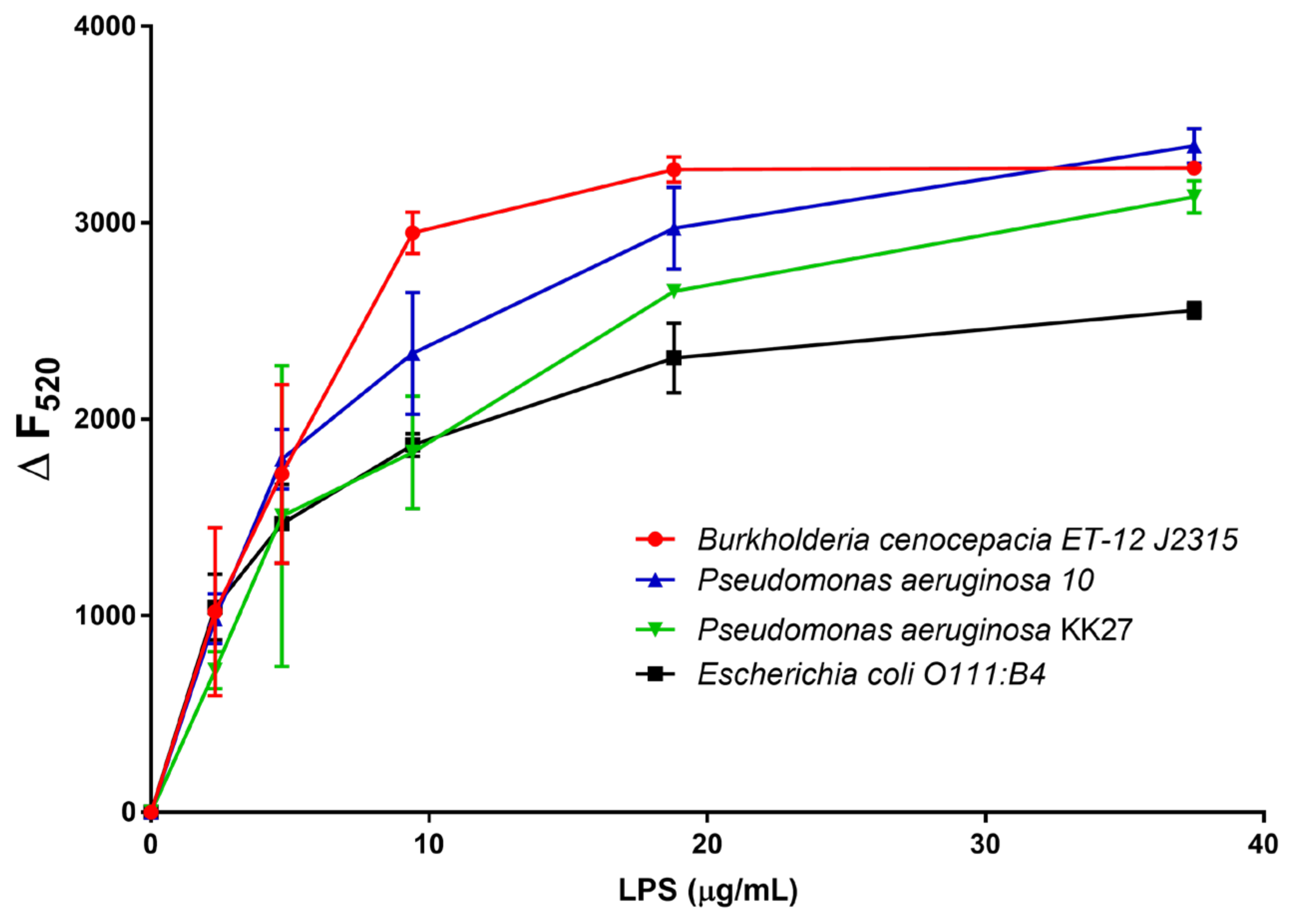
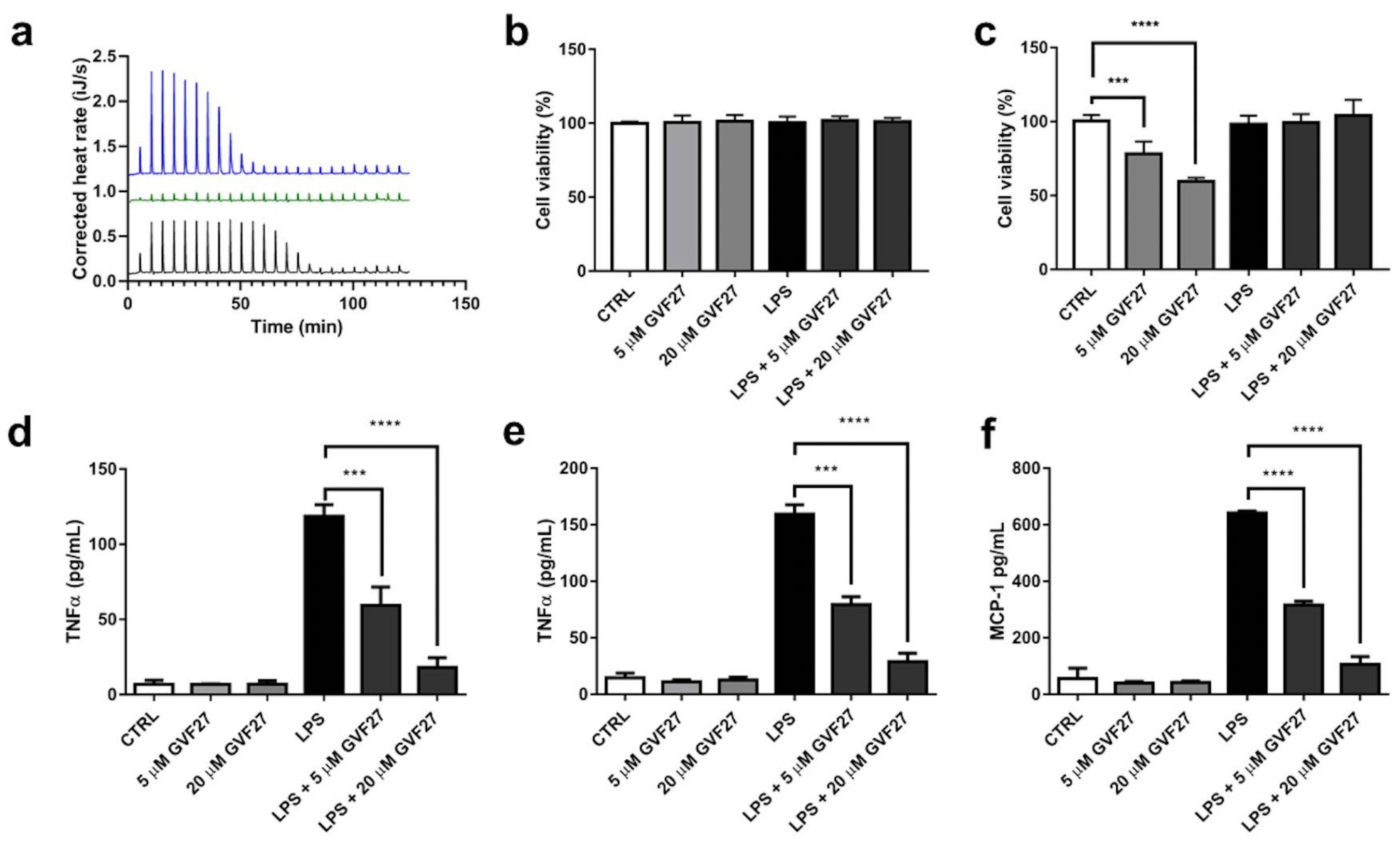
2.3. Interaction Studies of GVF27 with LPS and Immunomodulation Assays on THP-1 Cells
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Peptide Synthesis
4.3. Bacterial Strains and Growth Conditions
4.4. Antimicrobial Activity
4.5. Cell Culture and Differentiation
4.6. Cytotoxicity Assays
4.7. Anti-Biofilm Activity Assays
4.8. Minimal Agglutination Concentration (MAC)
4.9. Scanning Electron Microscopy
4.10. LPS Isolation
4.11. Isothermal Titration Calorimetry
4.12. Fluorescence Displacement Assay
4.13. LPS Neutralisation Assay by ELISA
4.14. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Prestinaci, F.; Pezzotti, P.; Pantosti, A. Antimicrobial resistance: A global multifaceted phenomenon. Pathog. Glob. Health 2015, 109, 309–318. [Google Scholar] [CrossRef] [Green Version]
- Hutchings, M.; Truman, A.; Wilkinson, B. Antibiotics: Past, present and future. Curr. Opin. Microbiol. 2019, 51, 72–80. [Google Scholar] [CrossRef]
- Payne, D.J.; Miller, L.F.; Findlay, D.; Anderson, J.; Marks, L. Time for a change: Addressing R&D and commercialization challenges for antibacterials. Philos. Trans. R. Soc. B Biol. Sci. 2015, 370, 20140086. [Google Scholar] [CrossRef] [Green Version]
- Hancock, R.E.W.; Haney, E.F.; Gill, E.E. The immunology of host defence peptides: Beyond antimicrobial activity. Nat. Rev. Immunol. 2016, 16, 321–334. [Google Scholar] [CrossRef] [PubMed]
- Fjell, C.D.; Hiss, J.A.; Hancock, R.E.W.; Schneider, G. Designing antimicrobial peptides: Form follows function. Nat. Rev. Drug Discov. 2011, 11, 37–51. [Google Scholar] [CrossRef]
- Hemshekhar, M.; Anaparti, V.; Mookherjee, N. Functions of cationic host defense peptides in immunity. Pharmaceuticals 2016, 9, 40. [Google Scholar] [CrossRef] [Green Version]
- Mangoni, M.L.; Mcdermott, A.M.; Zasloff, M. Antimicrobial peptides and wound healing: Biological and therapeutic considerations. Exp. Dermatol. 2016, 25, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.J.; Lee, S.K.; Jung, Y.S.; Lee, M.; Lee, H.Y.; Kim, S.D.; Park, J.S.; Koo, J.H.; Hwang, J.S.; Bae, Y.S. Promotion of formyl peptide receptor 1-mediated neutrophil chemotactic migration by antimicrobial peptides isolated from the centipede Scolopendra subspinipes mutilans. BMB Rep. 2016, 49, 520–525. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Veldhuizen, E.J.A.; Schneider, V.A.F.; Agustiandari, H.; Van Dijk, A.; Tjeerdsma-van Bokhoven, J.L.M.; Bikker, F.J.; Haagsman, H.P. Antimicrobial and immunomodulatory activities of PR-39 derived peptides. PLoS ONE 2014, 9, e95939. [Google Scholar] [CrossRef]
- Niyonsaba, F.; Ushio, H.; Nakano, N.; Ng, W.; Sayama, K.; Hashimoto, K.; Nagaoka, I.; Okumura, K.; Ogawa, H. Antimicrobial peptides human β-defensins stimulate epidermal keratinocyte migration, proliferation and production of proinflammatory cytokines and chemokines. J. Investig. Dermatol. 2007, 127, 594–604. [Google Scholar] [CrossRef] [Green Version]
- Oppenheim, J.J.; Biragyn, A.; Kwak, L.W.; Yang, D. Roles of antimicrobial peptides such as defensins in innate and adaptive immunity. Ann. Rheum. Dis. 2003, 62, ii17–ii21. [Google Scholar] [CrossRef] [Green Version]
- Lewies, A.; Du Plessis, L.H.; Wentzel, J.F. Antimicrobial Peptides: The Achilles’ Heel of Antibiotic Resistance? Probiotics Antimicrob. Proteins 2018, 11, 370–381. [Google Scholar] [CrossRef]
- Sun, E.; Belanger, C.R.; Haney, E.F.; Hancock, R.E.W. Host defense (antimicrobial) peptides. In Peptide Applications in Biomedicine, Biotechnology and Bioengineering; Woodhead Publishing: Sawston, UK, 2018; ISBN 9780081007426. [Google Scholar]
- Drevinek, P.; Mahenthiralingam, E. Burkholderia cenocepacia in cystic fibrosis: Epidemiology and molecular mechanisms of virulence. Clin. Microbiol. Infect. 2010, 16, 821–830. [Google Scholar] [CrossRef] [Green Version]
- Mahenthiralingam, E.; Urban, T.A.; Goldberg, J.B. The multifarious, multireplicon Burkholderia cepacia complex. Nat. Rev. Genet. 2005, 3, 144–156. [Google Scholar] [CrossRef] [PubMed]
- Melo Coutinho, H. Burkholderia cepacia complex: Virulence characteristics, importance and relationship with cystic fibrosis. Indian J. Med. Sci. 2007, 61, 422–429. [Google Scholar] [CrossRef]
- Rhodes, K.A.; Schweizer, H.P. Antibiotic resistance in Burkholderia species. Drug Resist. Updat. 2016, 28, 82–90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Podnecky, N.L.; Rhodes, K.A.; Schweizer, H.P. Efflux pump-mediated drug resistance in Burkholderia. Front. Microbiol. 2015, 6, 305-305. [Google Scholar] [CrossRef] [Green Version]
- Shommu, N.S.; Vogel, H.J.; Storey, D.G. Potential of metabolomics to reveal Burkholderia cepacia complex pathogenesis and antibiotic resistance. Front. Microbiol. 2015. [Google Scholar] [CrossRef] [PubMed]
- Bellich, B.; Jou, I.A.; Buriola, C.; Ravenscroft, N.; Brady, J.W.; Fazli, M.; Tolker-Nielsen, T.; Rizzo, R.; Cescutti, P. The biofilm of Burkholderia cenocepacia H111 contains an exopolysaccharide composed of L-rhamnose and L-mannose: Structural characterization and molecular modelling. Carbohydr. Res. 2021, 499, 108231. [Google Scholar] [CrossRef]
- Terán, L.C.; Distefano, M.; Bellich, B.; Petrosino, S.; Bertoncin, P.; Cescutti, P.; Sblattero, D. Proteomic studies of the biofilm matrix including outer membrane vesicles of Burkholderia multivorans c1576, a strain of clinical importance for cystic fibrosis. Microorganisms 2020, 8, 1826. [Google Scholar] [CrossRef]
- McClean, S.; Callaghan, M. Burkholderia cepacia complex: Epithelial cell-pathogen confrontations and potential for therapeutic intervention. J. Med. Microbiol. 2009, 58 Pt 1, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kooi, C.; Subsin, B.; Chen, R.; Pohorelic, B.; Sokol, P.A. Burkholderia cenocepacia ZmpB is a broad-specificity zinc metalloprotease involved in virulence. Infect. Immun. 2006, 74, 4083–4093. [Google Scholar] [CrossRef] [Green Version]
- Mullen, T.; Markey, K.; Murphy, P.; McClean, S.; Callaghan, M. Role of lipase in Burkholderia cepacia complex (Bcc) invasion of lung epithelial cells. Eur. J. Clin. Microbiol. Infect. Dis. 2007, 26, 869–877. [Google Scholar] [CrossRef] [PubMed]
- Ganesan, S.; Sajjan, U.S. Host evasion by Burkholderia cenocepacia. Front. Cell. Infect. Microbiol. 2012, 1, 25. [Google Scholar] [CrossRef] [Green Version]
- Shaw, D.; Poxton, I.R.; Govan, J.R.W. Biological activity of Burkholderia (Pseudomonas) cepacia lipopolysaccharide. FEMS Immunol. Med. Microbiol. 1995, 11, 99–106. [Google Scholar] [CrossRef]
- De Soyza, A.; Silipo, A.; Lanzetta, R.; Govan, R.J.; Molinaro, A. Chemical and biological features of Burkholderia cepacia complex lipopolysaccharides. Innate Immun. 2008, 14, 127–144. [Google Scholar] [CrossRef]
- Hollaus, R.; Ittig, S.; Hofinger, A.; Haegman, M.; Beyaert, R.; Kosma, P.; Zamyatina, A. Chemical synthesis of burkholderia lipid a modified with glycosyl phosphodiester-linked 4-amino-4-deoxy-β-L-arabinose and its immunomodulatory potential. Chem. A Eur. J. 2015, 21, 4102–4114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pizzo, E.; Cafaro, V.; Di Donato, A.; Notomista, E. Cryptic antimicrobial peptides: Identification methods and current knowledge of their immunomodulatory properties. Curr. Pharm. Des. 2018, 24, 1054–1066. [Google Scholar] [CrossRef]
- Bosso, A.; Di Maro, A.; Cafaro, V.; Di Donato, A.; Notomista, E.; Pizzo, E. Enzymes as a Reservoir of Host Defence Peptides. Curr. Top. Med. Chem. 2020, 20, 1310–1323. [Google Scholar] [CrossRef]
- Pane, K.; Durante, L.; Crescenzi, O.; Cafaro, V.; Pizzo, E.; Varcamonti, M.; Zanfardino, A.; Izzo, V.; Di Donato, A.; Notomista, E. Antimicrobial potency of cationic antimicrobial peptides can be predicted from their amino acid composition: Application to the detection of “cryptic” antimicrobial peptides. J. Theor. Biol. 2017, 419, 254–265. [Google Scholar] [CrossRef] [PubMed]
- Bosso, A.; Pirone, L.; Gaglione, R.; Pane, K.; Del Gatto, A.; Zaccaro, L.; Di Gaetano, S.; Diana, D.; Fattorusso, R.; Pedone, E.; et al. A new cryptic host defense peptide identified in human 11-hydroxysteroid dehydrogenase-1 β-like: From in silico identification to experimental evidence. Biochim. Biophys. Acta Gen. Subj. 2017, 1861, 2342–2353. [Google Scholar] [CrossRef] [PubMed]
- Yakandawala, N.; Gawande, P.V.; LoVetri, K.; Cardona, S.T.; Romeo, T.; Nitz, M.; Madhyastha, S. Characterization of the poly-β-1,6-N-acetylglucosamine polysaccharide component of Burkholderia biofilms. Appl. Environ. Microbiol. 2011, 77, 8303–8309. [Google Scholar] [CrossRef] [Green Version]
- Di Lorenzo, F.; Kubik, Ł.; Oblak, A.; Lorè, N.I.; Cigana, C.; Lanzetta, R.; Parrilli, M.; Hamad, M.A.; De Soyza, A.; Silipo, A.; et al. Activation of Human Toll-like receptor 4 (TLR4)·myeloid differentiation factor 2 (MD-2) by hypoacylated lipopolysaccharide from a clinical isolate of Burkholderia cenocepacia. J. Biol. Chem. 2015, 290, 21305–21319. [Google Scholar] [CrossRef] [Green Version]
- Speert, D.P.; Henry, D.; Vandamme, P.; Corey, M.; Mahenthiralingam, E. Epidemiology of Burkholderia cepacia complex in patients with cystic fibrosis, Canada. Emerg. Infect. Dis. 2002, 8, 181–187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loutet, S.A.; Valvano, M.A. Extreme Antimicrobial Peptide and Polymyxin B Resistance in the Genus Burkholderia. Front. Microbiol. 2011, 2, 159. [Google Scholar] [CrossRef] [PubMed]
- Loutet, S.A.; Valvano, M.A. A decade of Burkholderia cenocepacia virulence determinant research. Infect. Immun. 2010, 78, 4088–4100. [Google Scholar] [CrossRef] [Green Version]
- Van Acker, H.; Sass, A.; Bazzini, S.; De Roy, K.; Udine, C.; Messiaen, T.; Riccardi, G.; Boon, N.; Nelis, H.J.; Mahenthiralingam, E.; et al. Biofilm-Grown Burkholderia cepacia Complex Cells Survive Antibiotic Treatment by Avoiding Production of Reactive Oxygen Species. PLoS ONE 2013, 8, e58943. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Srikantha, T.; Daniels, K.J.; Pujol, C.; Kim, E.; Soll, D.R. Identification of genes upregulated by the transcription factor bcr1 that are involved in impermeability, impenetrability, and drug resistance of Candida albicans a/α biofilms. Eukaryot. Cell 2013, 12, 875–888. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoffman, L.R.; D’Argenio, D.A.; MacCoss, M.J.; Zhang, Z.; Jones, R.A.; Miller, S.I. Aminoglycoside antibiotics induce bacterial biofilm formation. Nature 2005, 436, 1171–1175. [Google Scholar] [CrossRef] [PubMed]
- Linares, J.F.; Gustafsson, I.; Baquero, F.; Martinez, J.L. Antibiotics as intermicrobiol signaling agents instead of weapons. Proc. Natl. Acad. Sci. USA 2006, 103, 19484–19489. [Google Scholar] [CrossRef] [Green Version]
- Sharafutdinov, I.S.; Ozhegov, G.D.; Sabirova, A.E.; Novikova, V.V.; Lisovskaya, S.A.; Khabibrakhmanova, A.M.; Kurbangalieva, A.R.; Bogachev, M.I.; Kayumov, A.R. Increasing Susceptibility of Drug-Resistant Candida albicans to Fluconazole and Terbinafine by 2(5H)-Furanone Derivative. Molecules 2020, 25, 642. [Google Scholar] [CrossRef] [Green Version]
- Cunha, M.V.; Leitão, J.H.; Mahenthiralingam, E.; Vandamme, P.; Lito, L.; Barreto, C.; Salgado, M.J.; Sá-Correia, I. Molecular analysis of Burkholderia cepacia complex isolates from a Portuguese cystic fibrosis center: A 7-year study. J. Clin. Microbiol. 2003, 41, 4113–4120. [Google Scholar] [CrossRef] [Green Version]
- Wiegand, I.; Hilpert, K.; Hancock, R.E.W. Agar and broth dilution methods to determine the minimal inhibitory concentration (MIC) of antimicrobial substances. Nat. Protoc. 2008, 3, 163–175. [Google Scholar] [CrossRef] [PubMed]
- Fratianni, F.; d’Acierno, A.; Ombra, M.N.; Amato, G.; De Feo, V.; Ayala-Zavala, J.F.; Coppola, R.; Nazzaro, F. Fatty Acid Composition, Antioxidant, and in vitro Anti-inflammatory Activity of Five Cold-Pressed Prunus Seed Oils, and Their Anti-biofilm Effect Against Pathogenic Bacteria. Front. Nutr. 2021, 8, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Gaglione, R.; Dell’Olmo, E.; Bosso, A.; Chino, M.; Pane, K.; Ascione, F.; Itri, F.; Caserta, S.; Amoresano, A.; Lombardi, A.; et al. Novel human bioactive peptides identified in Apolipoprotein B: Evaluation of their therapeutic potential. Biochem. Pharmacol. 2017, 130, 34–50. [Google Scholar] [CrossRef]
- Kittelberger, R.; Hilbink, F. Sensitive silver-staining detection of bacterial lipopolysaccharides in polyacrylamide gels. J. Biochem. Biophys. Methods 1993, 26, 81–86. [Google Scholar] [CrossRef]
- Tsai, C.M.; Frasch, C.E. A sensitive silver stain for detecting lipopolysaccharides in polyacrylamide gels. Anal. Biochem. 1982, 119, 115–119. [Google Scholar] [CrossRef]
- De Castro, C.; Parrilli, M.; Holst, O.; Molinaro, A. Microbe-associated molecular patterns in innate immunity. Extraction and chemical analysis of gram-negative bacterial lipopolysaccharides. Methods Enzymol. 2010, 480, 89–115. [Google Scholar]
- Mohanram, H.; Bhattacharjya, S. Resurrecting inactive antimicrobial peptides from the lipopolysaccharide trap. Antimicrob. Agents Chemother. 2014, 58, 1987–1996. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| MIC (μM) | ||||
|---|---|---|---|---|
| Bcc Strain | GVF27 | FAM-GVF27 | LL-37 | Ciprofloxacin |
| Burkholderia cenocepacia LMG 18863 | 40 | 40 | 5 | 2.5 |
| Burkholderia multivorans LMG 17582 | 10 | 10 | 10 | 1.2 |
| Peptide | Sequence |
|---|---|
| GVF27 | 1 GVFYPWRFRLLCLLRRWLPRPRAWFIR27 |
| LL-37 | 1 LLGDFFRKSKEKIGKEFKRIVQRIKDFLRNLVPRTES37 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bosso, A.; Gaglione, R.; Di Girolamo, R.; Veldhuizen, E.J.A.; García-Vello, P.; Fusco, S.; Cafaro, V.; Monticelli, M.; Culurciello, R.; Notomista, E.; et al. Human Cryptic Host Defence Peptide GVF27 Exhibits Anti-Infective Properties against Biofilm Forming Members of the Burkholderia cepacia Complex. Pharmaceuticals 2022, 15, 260. https://doi.org/10.3390/ph15020260
Bosso A, Gaglione R, Di Girolamo R, Veldhuizen EJA, García-Vello P, Fusco S, Cafaro V, Monticelli M, Culurciello R, Notomista E, et al. Human Cryptic Host Defence Peptide GVF27 Exhibits Anti-Infective Properties against Biofilm Forming Members of the Burkholderia cepacia Complex. Pharmaceuticals. 2022; 15(2):260. https://doi.org/10.3390/ph15020260
Chicago/Turabian StyleBosso, Andrea, Rosa Gaglione, Rocco Di Girolamo, Edwin J. A. Veldhuizen, Pilar García-Vello, Salvatore Fusco, Valeria Cafaro, Maria Monticelli, Rosanna Culurciello, Eugenio Notomista, and et al. 2022. "Human Cryptic Host Defence Peptide GVF27 Exhibits Anti-Infective Properties against Biofilm Forming Members of the Burkholderia cepacia Complex" Pharmaceuticals 15, no. 2: 260. https://doi.org/10.3390/ph15020260
APA StyleBosso, A., Gaglione, R., Di Girolamo, R., Veldhuizen, E. J. A., García-Vello, P., Fusco, S., Cafaro, V., Monticelli, M., Culurciello, R., Notomista, E., Arciello, A., & Pizzo, E. (2022). Human Cryptic Host Defence Peptide GVF27 Exhibits Anti-Infective Properties against Biofilm Forming Members of the Burkholderia cepacia Complex. Pharmaceuticals, 15(2), 260. https://doi.org/10.3390/ph15020260