Mineralocorticoid Receptor Antagonist (Potassium Canrenoate) Does Not Influence Outcome in the Treatment of COVID-19-Associated Pneumonia and Fibrosis—A Randomized Placebo Controlled Clinical Trial
Abstract
:1. Introduction
2. Results
2.1. Study Population
2.2. The Effect of Treatment on Survival, Mechanical Ventilation and Passive Oxygenation Time
2.3. The Effect of Treatment on Lung Imaging and Physical Performance
2.4. Adverse Events
3. Discussion
3.1. Effect of Potassium Canrenoate on the Lung Fibrosis Process
3.2. The Effect of Potassium Canrenoate on Mortality
3.3. Safety of Potassium Canrenoate
3.4. Limitations
4. Materials and Methods
4.1. Ethics
4.2. Study Population
4.3. Inclusion Criteria
- Patients of both sexes, 18–90 years of age.
- Patient requiring oxygen therapy, blood oxygen saturation level <94%.
- Confirmed COVID-19 infection (rt-PCR).
- At least one risk factor for increased mortality during COVID-19 currently published in the literature e.g., smoking, hypertension, diabetes, cardiovascular disease.
- Documented informed consent according to ICH-GCP and national regulations.
4.4. Exclusion Criteria
- Chronic bronchitis, emphysema, interstitial lung disease or other history of lung disease.
- Contraindications to the use of spironolactone.
- Hypersensitivity to spironolactone or any of the excipients.
- Pregnant patients (pregnancy test will be performed in every patient of reproductive age) and during lactation.
- Patients with mental illness or dementia who are unable to give informed consent to the examination.
- ARDS caused by another viral infection (SARS-CoV-2 negative).
- ARDS from other causes/trauma.
- Ionic disorders: hyperkalemia, hyponatremia.
- Adrenal crisis.
- Acute and chronic renal failure, creatinine clearance less than 30 mL/min.
- Anuria.
- Porphyria.
- Chronic use of MRA drugs from spironolactone group.
4.5. Clinical Experiment Measures
4.6. Outcome Measures
4.6.1. Primary Outcome Measures
- Duration of invasive mechanical ventilation via endotracheal intubation or tracheotomy (observation time 30 days).
- Duration of passive oxygen therapy (Observation time 30 days).
4.6.2. Secondary Outcome Measures
- Intensive Care Unit length of stay (LOS) (time frame 30 days).
- Total hospital length of stay (LOS) (time frame 90 days).
- Assessment of the dynamics of recovery of changes in lung ultrasound at 7 days.
- Assessment of the dynamics of recovery of changes in lung ultrasound at 30 days.
- Assessment of the dynamics of recovery of changes in chest computed tomography (CT) at 3 months (90 days).
- Assessment of mortality at 30 days.
- Assessment of mortality at 90 days.
- Six-minute walk test (6MWT) at 30 days.
- Six-minute walk test (6MWT) at 90 days.
4.7. Lung Ultrasound (LUS), Lung CT Evaluation and 6-min Walk Test
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, D.; Hu, B.; Hu, C.; Zhu, F.; Liu, X.; Zhang, J.; Wang, B.; Xiang, H.; Cheng, Z.; Xiong, Y.; et al. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA 2020, 323, 1061–1069. [Google Scholar] [CrossRef]
- Chen, N.; Zhou, M.; Dong, X.; Qu, J.; Gong, F.; Han, Y.; Qiu, Y.; Wang, J.; Liu, Y.; Wei, Y.; et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: A descriptive study. Lancet 2020, 395, 507–513. [Google Scholar] [CrossRef] [Green Version]
- Kucewicz-Czech, E.; Damps, M. Triage during the COVID-19 pandemic. Anaesthesiol. Intensive Ther. 2021, 52, 312–315. [Google Scholar] [CrossRef] [PubMed]
- Lian, J.; Jin, X.; Hao, S.; Jia, H.; Cai, H.; Zhang, X.; Hu, J.; Zheng, L.; Wang, X.; Zhang, S.; et al. Epidemiological, clinical, and virological characteristics of 465 hospitalized cases of coronavirus disease 2019 (COVID-19) from Zhejiang province in China. Influenza Other Respi. Viruses 2020, 14, 564–574. [Google Scholar] [CrossRef] [PubMed]
- Kotfis, K.; Williams Roberson, S.; Wilson, J.; Pun, B.; Ely, E.W.; Jeżowska, I.; Jezierska, M.; Dabrowski, W. COVID-19: What do we need to know about ICU delirium during the SARS-CoV-2 pandemic? Anaesthesiol. Intensive Ther. 2020, 52, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Huang, L.; Wang, Y.; Li, X.; Ren, L.; Gu, X.; Kang, L.; Guo, L.; Liu, M.; Zhou, X.; et al. 6-month consequences of COVID-19 in patients discharged from hospital: A cohort study. Lancet 2021, 397, 220–232. [Google Scholar] [CrossRef]
- Hui, D.S.; Wong, K.T.; Ko, F.W.; Tam, L.S.; Chan, D.P.; Woo, J.; Sung, J.J.Y. The 1-year impact of severe acute respiratory syndrome on pulmonary function, exercise capacity, and quality of life in a cohort of survivors. Chest 2005, 128, 2247–2261. [Google Scholar] [CrossRef] [Green Version]
- Ngai, J.C.; Ko, F.W.; Ng, S.S.; To, K.-W.; Tong, M.; Hui, D.S. The long-term impact of severe acute respiratory syndrome on pulmonary function, exercise capacity and health status. Respirology 2010, 15, 543–550. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization WHO Coronavirus (COVID-19) Dashboard. Available online: https://covid19.who.int (accessed on 6 January 2022).
- Lechowicz, K.; Drożdżal, S.; Machaj, F.; Rosik, J.; Szostak, B. COVID-19: The potential treatment of pulmonary fibrosis associated with SARS-CoV-2 infection. J. Clin. Med. 2020, 9, 1917. [Google Scholar] [CrossRef]
- Wu, X.; Liu, X.; Zhou, Y.; Yu, H.; Li, R.; Zhan, Q.; Ni, F.; Fang, S.; Lu, Y.; Ding, X.; et al. 3-month, 6-month, 9-month, and 12-month respiratory outcomes in patients following COVID-19-related hospitalisation: A prospective study. Lancet Respir. Med. 2021, 9, 747–754. [Google Scholar] [CrossRef]
- WHO TEAM. Summary of Probable SARS Cases with Onset of Illness from 1 November 2002 to 31 July 2003; WHO: Geneva, Switzerland, 2015. [Google Scholar]
- Zhang, P.; Li, J.; Liu, H.; Han, N.; Ju, J.; Kou, Y.; Chen, L.; Jiang, M.; Pan, F.; Zheng, Y.; et al. Long-term bone and lung consequences associated with hospital-acquired severe acute respiratory syndrome: A 15-year follow-up from a prospective cohort study. Bone Res. 2020, 8, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barut, F.; Ozacmak, V.H.; Turan, I.; Sayan-Ozacmak, H.; Aktunc, E. Reduction of Acute Lung Injury by Administration of Spironolactone After Intestinal Ischemia and Reperfusion in Rats. Clin. Invest. Med. 2016, 39, E15–E24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yavas, G.; Yavas, C.; Celik, E.; Sen, E.; Ata, O.; Afsar, R.E. The impact of spironolactone on the lung injury induced by concomitant trastuzumab and thoracic radiotherapy. Int. J. Radiat. Res. 2019, 17, 87–95. [Google Scholar] [CrossRef] [Green Version]
- Zannad, F.; Alla, F.; Dousset, B.; Perez, A.; Pitt, B. Limitation of excessive extracellular matrix turnover may contribute to survival benefit of spironolactone therapy in patients with congestive heart failure: Insights from the randomized aldactone evaluation study (RALES). Rales Investigators. Circulation 2000, 102, 2700–2706. [Google Scholar] [CrossRef] [Green Version]
- Kumar, N.; Zuo, Y.; Yalavarthi, S.; Hunker, K.L.; Knight, J.S.; Kanthi, Y.; Obi, A.T.; Ganesh, S.K. SARS-CoV-2 Spike Protein S1-Mediated Endothelial Injury and Pro-Inflammatory State Is Amplified by Dihydrotestosterone and Prevented by Mineralocorticoid Antagonism. Viruses 2021, 13, 2209. [Google Scholar] [CrossRef]
- Edwards, C.; Klekot, O.; Halugan, L.; Korchev, Y. Follow Your Nose: A Key Clue to Understanding and Treating COVID-19. Front. Endocrinol. (Lausanne) 2021, 12, 12. [Google Scholar] [CrossRef]
- Cannavo, A.; Bencivenga, L.; Liccardo, D.; Elia, A.; Marzano, F.; Gambino, G.; D’Amico, M.L.; Perna, C.; Ferrara, N.; Rengo, G.; et al. Aldosterone and Mineralocorticoid Receptor System in Cardiovascular Physiology and Pathophysiology. Oxid. Med. Cell. Longev. 2018, 2018, 1–10. [Google Scholar] [CrossRef]
- Khalifa, S.A.M.; Yosri, N.; El-Mallah, M.F.; Ghonaim, R.; Guo, Z.; Musharraf, S.G.; Du, M.; Khatib, A.; Xiao, J.; Saeed, A.; et al. Screening for natural and derived bio-active compounds in preclinical and clinical studies: One of the frontlines of fighting the coronaviruses pandemic. Phytomedicine 2021, 85, 153311. [Google Scholar] [CrossRef]
- Lieber, G.B.; Fernandez, X.; Mingo, G.G.; Jia, Y.; Caniga, M.; Gil, M.A.; Keshwani, S.; Woodhouse, J.D.; Cicmil, M.; Moy, L.Y.; et al. Mineralocorticoid receptor antagonists attenuate pulmonary inflammation and bleomycin-evoked fibrosis in rodent models. Eur. J. Pharmacol. 2013, 718, 290–298. [Google Scholar] [CrossRef]
- Ji, W.-J.; Ma, Y.-Q.; Zhou, X.; Zhang, Y.-D.; Lu, R.-Y.; Guo, Z.-Z.; Sun, H.-Y.; Hu, D.-C.; Yang, G.-H.; Li, Y.-M.; et al. Spironolactone attenuates bleomycin-induced pulmonary injury partially via modulating mononuclear phagocyte phenotype switching in circulating and alveolar compartments. PLoS ONE 2013, 8, e81090. [Google Scholar] [CrossRef]
- Kotfis, K.; Lechowicz, K.; Drożdżal, S.; Niedźwiedzka-Rystwej, P.; Wojdacz, T.K.; Grywalska, E.; Biernawska, J.; Wiśniewska, M.; Parczewski, M. COVID-19—The Potential Beneficial Therapeutic Effects of Spironolactone during SARS-CoV-2 Infection. Pharmaceuticals 2021, 14, 71. [Google Scholar] [CrossRef]
- Atalay, C.; Dogan, N.; Aykan, S.; Gundogdu, C.; Keles, M.S. The efficacy of spironolactone in the treatment of acute respiratory distress syndrome-induced rats. Singap. Med. J. 2010, 51, 501–505. [Google Scholar]
- Maleszka, P.; Kruszewski, J. Comparative evaluation of inhaling a single dose of furosemide or spironolactone on bronchial hyperreactivity of patients with atopic bronchial asthma. Pol. Tyg. Lek. 1994, 49, 415–418. [Google Scholar] [PubMed]
- Zaafan, M.A.; Haridy, A.R.; Abdelhamid, A.M. Amitriptyline attenuates bleomycin-induced pulmonary fibrosis: Modulation of the expression of NF-κβ, iNOS, and Nrf2. Naunyn-Schmiedebergs Arch. Pharmacol. 2019, 392, 279–286. [Google Scholar] [CrossRef]
- Loas, G.; Le Corre, P. Update on Functional Inhibitors of Acid Sphingomyelinase (FIASMAs) in SARS-CoV-2 Infection. Pharmaceuticals 2021, 14, 691. [Google Scholar] [CrossRef] [PubMed]
- Carpinteiro, A.; Edwards, M.J.; Hoffmann, M.; Kochs, G.; Gripp, B.; Weigang, S.; Adams, C.; Carpinteiro, E.; Gulbins, A.; Keitsch, S.; et al. Pharmacological Inhibition of Acid Sphingomyelinase Prevents Uptake of SARS-CoV-2 by Epithelial Cells. Cell Rep. Med. 2020, 1, 100142. [Google Scholar] [CrossRef] [PubMed]
- Adams, C.; Icheva, V.; Deppisch, C.; Lauer, J.; Herrmann, G.; Graepler-Mainka, U.; Heyder, S.; Gulbins, E.; Riethmueller, J. Long-Term Pulmonal Therapy of Cystic Fibrosis-Patients with Amitriptyline. Cell. Physiol. Biochem. 2016, 39, 565–572. [Google Scholar] [CrossRef]
- Wilcox, C.S.; Pitt, B. Is Spironolactone the Preferred Renin–Angiotensin–Aldosterone Inhibitor for Protection Against COVID-19? J. Cardiovasc. Pharmacol. 2021, 77, 323–331. [Google Scholar] [CrossRef]
- Yu, M.; Liu, Y.; Xu, D.; Zhang, R.; Lan, L.; Xu, H. Prediction of the development of pulmonary fibrosis using serial thin-section ct and clinical features in patients discharged after treatment for COVID-19 pneumonia. Korean J. Radiol. 2020, 21, 746–755. [Google Scholar] [CrossRef]
- Wynn, T.A. Integrating mechanisms of pulmonary fibrosis. J. Exp. Med. 2011, 208, 1339–1350. [Google Scholar] [CrossRef] [Green Version]
- Xu, X.; Zhang, J.; Dai, H. IL-25/IL-33/TSLP contributes to idiopathic pulmonary fibrosis: Do alveolar epithelial cells and (myo)fibroblasts matter? Exp. Biol. Med. 2020, 245, 897–901. [Google Scholar] [CrossRef]
- Crosby, L.M.; Waters, C.M. Epithelial repair mechanisms in the lung. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2010, 298, L715–L731. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jover, E.; Matilla, L.; Garaikoetxea, M.; Fernández-Celis, A.; Muntendam, P.; Jaisser, F.; Rossignol, P.; López-Andrés, N. Beneficial effects of mineralocorticoid receptor pathway blockade against endothelial inflammation induced by sars-COV-2 spike protein. Biomedicines 2021, 9, 639. [Google Scholar] [CrossRef]
- Umemura, Y.; Mitsuyama, Y.; Minami, K.; Nishida, T.; Watanabe, A.; Okada, N.; Yamakawa, K.; Nochioka, K.; Fujimi, S. Efficacy and safety of nintedanib for pulmonary fibrosis in severe pneumonia induced by COVID-19: An interventional study. Int. J. Infect. Dis. 2021, 108, 454–460. [Google Scholar] [CrossRef] [PubMed]
- Combet, M.; Pavot, A.; Savale, L.; Humbert, M.; Monnet, X. Rapid onset honeycombing fibrosis in spontaneously breathing patient with COVID-19. Eur. Respir. J. 2020, 56, 2001808. [Google Scholar] [CrossRef] [PubMed]
- Ahmad Alhiyari, M.; Ata, F.; Islam Alghizzawi, M.; Bint I Bilal, A.; Salih Abdulhadi, A.; Yousaf, Z. Post COVID-19 fibrosis, an emerging complicationof SARS-CoV-2 infection. IDCases 2021, 23, e01041. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.; Bruen, C.; Schnaus, M.; Zhang, J.; Ali, S.; Lind, A.; Stoecker, Z.; Stauderman, K.; Hebbar, S. Auxora versus standard of care for the treatment of severe or critical COVID-19 pneumonia: Results from a randomized controlled trial. Crit. Care 2020, 24, 502. [Google Scholar] [CrossRef]
- Jeon, D.; Son, M.; Choi, J. Effect of Spironolactone on COVID-19 in Patients With Underlying Liver Cirrhosis: A Nationwide Case-Control Study in South Korea. Front. Med. 2021, 8, 629176. [Google Scholar] [CrossRef]
- Thille, A.W.; Esteban, A.; Fernández-Segoviano, P.; Rodriguez, J.-M.; Aramburu, J.-A.; Vargas-Errázuriz, P.; Martín-Pellicer, A.; Lorente, J.A.; Frutos-Vivar, F. Chronology of histological lesions in acute respiratory distress syndrome with diffuse alveolar damage: A prospective cohort study of clinical autopsies. Lancet. Respir. Med. 2013, 1, 395–401. [Google Scholar] [CrossRef]
- Soldati, G.; Smargiassi, A.; Inchingolo, R.; Buonsenso, D.; Perrone, T.; Briganti, D.F.; Perlini, S.; Torri, E.; Mariani, A.; Mossolani, E.E.; et al. Proposal for International Standardization of the Use of Lung Ultrasound for Patients With COVID-19. J. Ultrasound Med. 2020, 39, 1413–1419. [Google Scholar] [CrossRef] [Green Version]
- Manivel, V.; Lesnewski, A.; Shamim, S.; Carbonatto, G.; Govindan, T. CLUE: COVID-19 lung ultrasound in emergency department. EMA-Emerg. Med. Australas. 2020, 32, 694–696. [Google Scholar] [CrossRef] [PubMed]
- Fraser, E.; St Noble, V.; Hoyles, R.K.; Benamore, R.; Ho, L.P. Readily accessible CT scoring method to quantify fibrosis in IPF. BMJ Open Respir. Res. 2020, 7, e000584. [Google Scholar] [CrossRef] [PubMed]

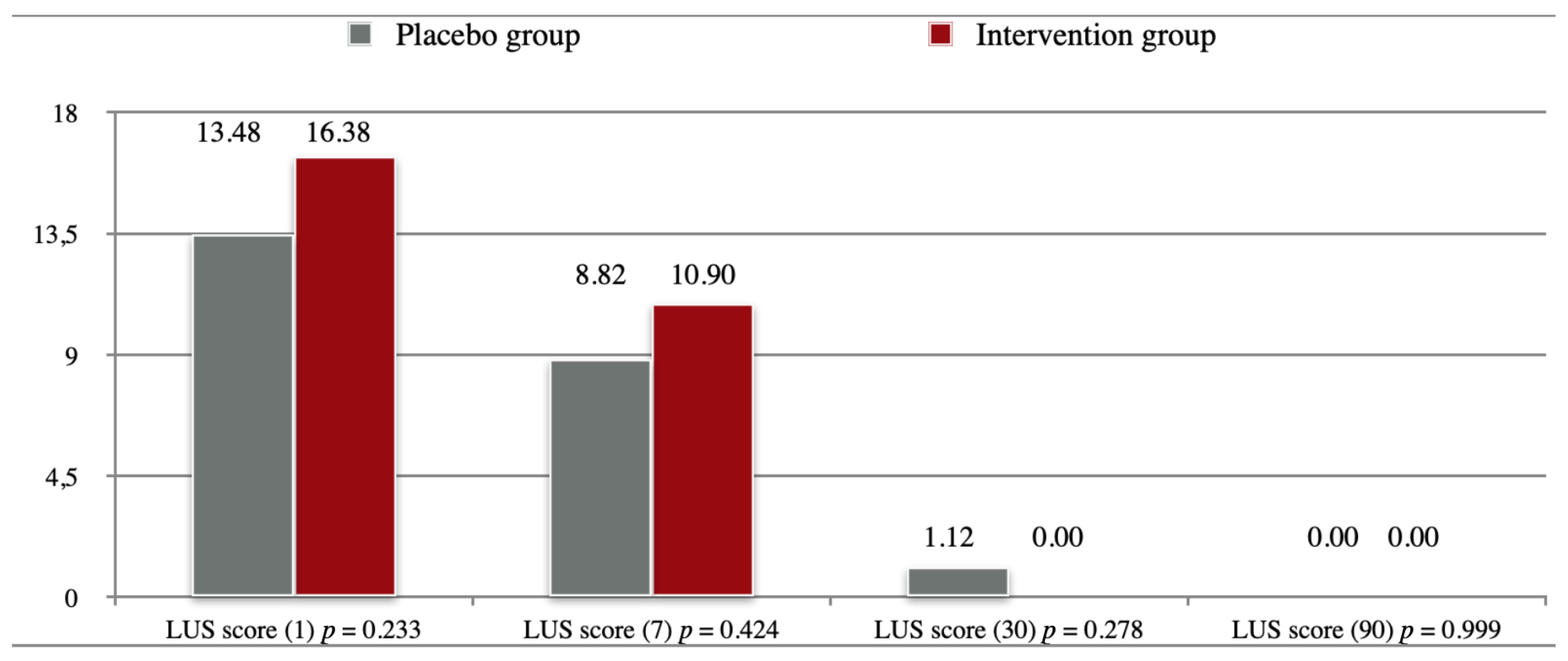
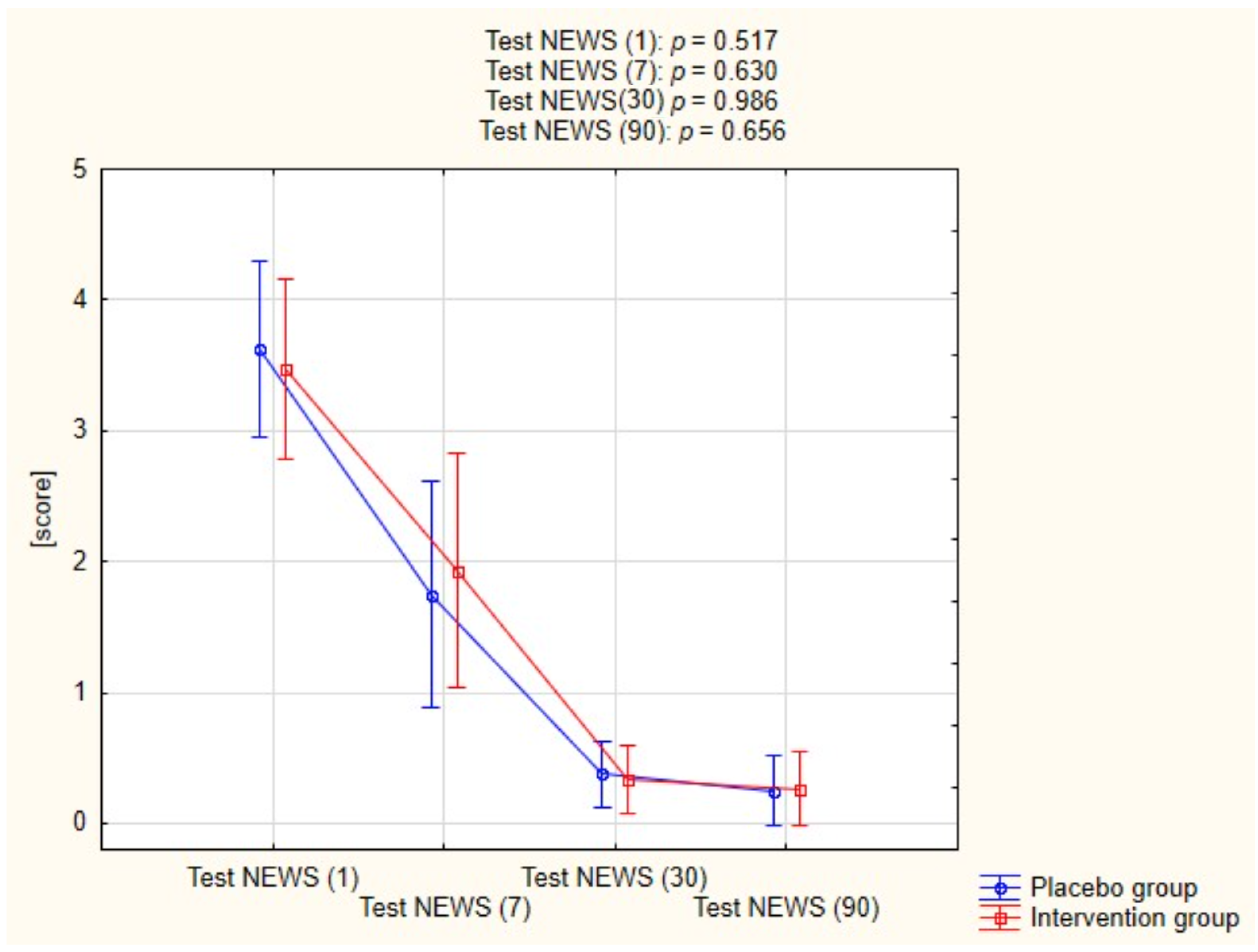

| Variables | Placebo Group (n = 25) | Intervention Group (n = 24) | p-Value | |
|---|---|---|---|---|
| Age [years], mean ± SD; Me | 63.84 ± 14.75; 66.00 | 61.54 ± 9.06; 64.00 | 0.513 | |
| Gender [male], n (%) | 16 (64.00) | 10 (41.67) | 0.200 | |
| BMI [kg/m2], mean ± SD; Me | 30.57 ± 4.63; 29.05 | 30.92 ± 4.10; 30.78 | 0.780 | |
| Smoking, n (%) | No | 12 (48.00) | 14 (58.33) | 0.204 |
| Yes | 3 (12.00) | 0 (0.00) | ||
| Quit >1 month | 10 (40.00) | 10 (41.67) | ||
| Alcohol use, n (%) | No | 7 (29.17) | 9 (37.50) | 0.734 |
| Yes | 2 (8.33) | 1 (4.17) | ||
| Occasionally | 15 (62.50) | 14 (58.33) | ||
| CFS [1,2,3,4,5,6,7], (mean ± SD; Me) | 3.76 ± 1.01; 4.00 | 3.17 ± 0.70; 3.00 | 0.034 | |
| Co-Morbidities | Placebo Group (n = 25) | Intervention Group (n = 24) | p-Value |
|---|---|---|---|
| Arterial hypertension, n (%) | 16 (64.00) | 15 (62.50) | 0.851 |
| Ischemic heart disease, n (%) | 7 (28.00) | 0 (0.00) | 0.017 |
| Myocardial infarction, n (%) | 4 (16.00) | 0 (0.00) | 0.128 |
| Chronic heart failure, n (%) | 3 (12.00) | 0 (0.00) | 0.248 |
| Atrial Fibrillation, n (%) | 4 (16.00) | 0 (0.00) | 0.128 |
| Hypercholesterolemia, n (%) | 8 (32.00) | 3 (12.50) | 0.196 |
| TIA, n (%) | 1 (4.00) | 0 (0.00) | 0.984 |
| Diabetes, n (%) | 4 (16.00) | 10 (41.67) | 0.095 |
| Peripheral vascular disease, n (%) | 5 (20.00) | 1 (4.17) | 0.209 |
| Peptic ulcer disease, n (%) | 1 (4.00) | 0 (0.00) | 0.984 |
| Thyroid disease, n (%) | 4 (16.00) | 4 (16.67) | 0.746 |
| Active NPL, n (%) | 2 (8.00) | 2 (8.33) | 0.632 |
| Depression, n (%) | 1 (4.00) | 0 (0.00) | 0.984 |
| Medications | Placebo Group (n = 25) | Intervention Group (n = 24) | p-Value |
|---|---|---|---|
| Aspirin, n (%) | 7 (29.17) | 1 (4.17) | 0.053 |
| ADP Inhibitors, n (%) | 2 (8.33) | 0 (0.00) | 0.470 |
| NOAC, n (%) | 1 (4.17) | 0 (0.00) | 0.984 |
| Beta-blockers, n (%) | 10 (40.00) | 6 (25.00) | 0.415 |
| ACE-I/Sartans, n (%) | 12 (50.00) | 11 (45.83) | 0.999 |
| Ca-blockers, n (%) | 6 (25.00) | 3 (12.50) | 0.459 |
| Statins, n (%) | 6 (25.00) | 2 (8.33) | 0.245 |
| Nitrates, n (%) | 1 (4.17) | 0 (0.00) | 0.999 |
| Diuretics, n (%) | 7 (28.00) | 8 (33.33) | 0.924 |
| Bronchodilators, n (%) | 1 (4.00) | 0 (0.00) | 0.984 |
| Oral hypoglycemic drugs, n (%) | 2 (8.00) | 10 (41.67) | 0.016 |
| Insulin, n (%) | 1 (4.00) | 2 (8.33) | 0.971 |
| Levothyroxine, n (%) | 4 (16.00) | 3 (12.50) | 0.953 |
| Opioids, n (%) | 0 (0.00) | 1 (4.17) | 0.984 |
| Variables | Placebo Group (n = 25) | Intervention Group (n = 24) | p |
|---|---|---|---|
| Mean ± SD; Me | Mean ± SD; Me | ||
| White blood cells [G/L] | 7.52 ± 3.06; 7.59 | 8.19 ± 3.00; 8.41 | 0.440 |
| Neutrophils [G/L] | 6.02 ± 2.84; 5.74 | 6.64 ± 2.98; 6.69 | 0.461 |
| Lymphocytes [G/L] | 0.95 ± 0.32; 0.90 | 1.05 ± 0.30; 0.98 | 0.266 |
| Red blood cells [T/L] | 4.20 ± 0.63; 4.27 | 4.30 ± 0.41; 4.20 | 0.523 |
| Platelets [G/L] | 260.08 ± 93.54; 245,00 | 317.04 ± 132.56; 265.00 | 0.091 |
| Hemoglobin [mmol/L] | 7.90 ± 0.99; 7.90 | 7.97 ± 1.11; 7.90 | 0.836 |
| Hematocrit [l/l] | 0.37 ± 0.05; 0.38 | 0.38 ± 0.04; 0.37 | 0.574 |
| C-reactive protein [mg/dL] | 71.08 ± 44.78; 76.04 | 95.58 ± 65.37; 80.14 | 0.135 |
| Interleukin-6 [pg/mL] | 46.68 ± 56.79; 24.90 | 64.97 ± 72.52; 41.00 | 0.332 |
| Procalcitonin [ng/mL] | 0.15 ± 0.12; 0.12 | 0.23 ± 0.35; 0.09 | 0.327 |
| AST [U/L] | 67.72 ± 89.11; 48.00 | 48.29 ± 20.78; 44.0 | 0.298 |
| ALT [U/L] | 52.88 ± 46.5; 35.00 | 48.79 ± 36.9; 38.0 | 0.734 |
| LDH [U/L] | 460.61 ± 174.88; 403.00 | 460.39 ± 154.07; 472.00 | 0.996 |
| D-Dimer [ng/mL] | 1799.32 ± 1902.33; 1158.00 | 2329.58 ± 2695.07; 1016.00 | 0.432 |
| Ferritin [µg/L] | 1262.88 ± 866.89; 983.50 | 948.83 ± 570.37; 835.00 | 0.223 |
| K+ [mmol/L] | 4.07 ± 0.54; 4.10 | 4.05 ± 0.51; 4.20 | 0.915 |
| Na+ [mmol/L] | 139.96 ± 3.32; 141.00 | 139.38 ± 3.88; 139.00 | 0.574 |
| Cl- [mmol/L] | 102.44 ± 3.96; 102.00 | 100.17 ± 4.73; 101.00 | 0.075 |
| Variables | Placebo Group | Intervention Group | p |
|---|---|---|---|
| Mean ± SD; Me | Mean ± SD; Me | ||
| Length of hospital stay [days] | 13.52 ± 5.84; 11.00 | 14.42 ± 6.57; 12.00 | 0.617 |
| Length of ICU stay [h] | 166.07 ± 88.89; 139.00 | 238.67 ± 217.01; 189.00 | 0.471 |
| Passive oxygenation [days] | 7.76 ± 4.48; 7.00 | 7.08 ± 5.61; 6.00 | 0.644 |
| HFNOT [h] | 90.13 ± 60.14; 88.00 | 112.31 ± 92.46; 88.50 | 0.580 |
| Mechanical ventilation [h] | 102.00 ± 59.06; 95.00 | 270.20 ± 224.39; 238.00 | 0.171 |
| PO + HFNOT [days] | 8.96 ± 4.54; 8.00 | 8.64 ± 6.90; 6.50 | 0.850 |
| PO + HFNOT + MV [days] | 10.15 ± 5.77; 8.00 | 10.99 ± 8.02; 8.50 | 0.678 |
| Variables | Placebo Group | Intervention Group | p |
|---|---|---|---|
| n (%) | n (%) | ||
| ICU admission | 7 (28.00%) | 6 (25.00%) | 0.932 |
| Death in hospital | 5 (20.00%) | 4 (16.67%) | 0.945 |
| Death after 90 days | 5 (20.83%) | 4 (18.18%) | 0.884 |
| Secondary infection | 7 (28.00%) | 5 (20.83%) | 0.802 |
| Pneumothorax | 0 (0.00%) | 3 (12.50%) | 0.219 |
| Hypotension (SBP < 100 mmHg) | 5 (20.00%) | 8 (33.33%) | 0.463 |
| Thromboembolic events | 2 (8.00%) | 3 (12.50%) | 0.962 |
| Hyperkalemia | 4 (16.00%) | 8 (33.33%) | 0.281 |
| Hypernatremia | 2 (8.00%) | 1 (4.17%) | 0.971 |
| Hypokalemia | 3 (12.00%) | 3 (12.50%) | 0.702 |
| Hyponatremia | 1 (4.00%) | 2 (8.33%) | 0.971 |
| Variables | Placebo Group | Intervention Group | p |
|---|---|---|---|
| Mean ± SD; Me | Mean ± SD; Me | ||
| Total Honeycombing | 7.11 ± 20.50; 0.00 | 0.00 ± 0.00; 0.00 | 0.148 |
| Total Reticulation | 26.53 ± 38.30; 15.00 | 26.94 ± 34.42; 7.00 | 0.972 |
| Total Traction Bronchiectasis | 5.32 ± 10.92; 0.00 | 2.00 ± 3.82; 0.00 | 0.226 |
| Total Ground Glass Opacification | 2.63 ± 11.47; 0.00 | 0.00 ± 0.00; 0.00 | 0.331 |
| TFS | 41.58 ± 74.07; 15.00 | 28.94 ± 36.39; 8.50 | 0.513 |
| Variables | Placebo Group | Intervention Group | p |
|---|---|---|---|
| Mean ± SD; Me | Mean ± SD; Me | ||
| White blood cells [G/L] | 10.88 ± 5.77; 10.09 | 9.90 ± 4.19; 9.27 | 0.512 |
| Neutrophils [G/L] | 8.36 ± 5.81; 6.12 | 7.49 ± 4.41; 6.93 | 0.574 |
| Lymphocytes [G/L] | 1.57 ± 0.72; 1.44 | 1.60 ± 0.62; 1.78 | 0.875 |
| Red blood cells [T/L] | 4.31 ± 0.55; 4.33 | 4.26 ± 0.43; 4.19 | 0.714 |
| Platelets [G/L] | 372.70 ± 119.06; 365.00 | 385.87 ± 112.78; 380.00 | 0.702 |
| Hemoglobin [mmol/L] | 8.08 ± 0.77; 8.10 | 7.89 ± 0.97; 7.80 | 0.463 |
| Hematocrit [l/l] | 0.39 ± 0.04; 0.39 | 0.38 ± 0.04; 0.38 | 0.815 |
| C-reactive protein [mg/dL] | 25.71 ± 41.52; 7.60 | 28.35 ± 45.98; 10.50 | 0.841 |
| Interleukin-6 [pg/mL] | 60.56 ± 152.47; 11.00 | 24.20 ± 69.38; 5.30 | 0.317 |
| Procalcitonin [ng/mL] | 5.43 ± 23.14; 0.07 | 0.20 ± 0.49; 0.06 | 0.337 |
| AST [U/L] | 37.61 ± 29.22; 29.00 | 32.35 ± 14.63; 29.00 | 0.452 |
| ALT [U/L] | 65.22 ± 32.54; 67.00 | 59.05 ± 38.78; 49.00 | 0.579 |
| LDH [U/L] | 340.44 ± 164.33; 269.00 | 313.95 ± 100.17; 301.00 | 0.579 |
| D-Dimer [ng/mL] | 1719.48 ± 1826.09; 1105.00 | 1782.45 ± 1607.25; 1312.00 | 0.903 |
| Ferritin [µg/L] | 1495.19 ± 2205.69; 857.00 | 923.11 ± 816.19; 678.50 | 0.343 |
| K+ [mmol/L] | 4.45 ± 0.39; 4.50 | 4.66 ± 0.65; 4.65 | 0.198 |
| Na+ [mmol/L] | 139.22 ± 5.33; 138.00 | 139.41 ± 3.32; 139.50 | 0.885 |
| Cl- [mmol/L] | 102.05 ± 4.39; 100.50 | 100.95 ± 2.76; 101.50 | 0.350 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kotfis, K.; Karolak, I.; Lechowicz, K.; Zegan-Barańska, M.; Pikulska, A.; Niedźwiedzka-Rystwej, P.; Kawa, M.; Sieńko, J.; Szylińska, A.; Wiśniewska, M. Mineralocorticoid Receptor Antagonist (Potassium Canrenoate) Does Not Influence Outcome in the Treatment of COVID-19-Associated Pneumonia and Fibrosis—A Randomized Placebo Controlled Clinical Trial. Pharmaceuticals 2022, 15, 200. https://doi.org/10.3390/ph15020200
Kotfis K, Karolak I, Lechowicz K, Zegan-Barańska M, Pikulska A, Niedźwiedzka-Rystwej P, Kawa M, Sieńko J, Szylińska A, Wiśniewska M. Mineralocorticoid Receptor Antagonist (Potassium Canrenoate) Does Not Influence Outcome in the Treatment of COVID-19-Associated Pneumonia and Fibrosis—A Randomized Placebo Controlled Clinical Trial. Pharmaceuticals. 2022; 15(2):200. https://doi.org/10.3390/ph15020200
Chicago/Turabian StyleKotfis, Katarzyna, Igor Karolak, Kacper Lechowicz, Małgorzata Zegan-Barańska, Agnieszka Pikulska, Paulina Niedźwiedzka-Rystwej, Miłosz Kawa, Jerzy Sieńko, Aleksandra Szylińska, and Magda Wiśniewska. 2022. "Mineralocorticoid Receptor Antagonist (Potassium Canrenoate) Does Not Influence Outcome in the Treatment of COVID-19-Associated Pneumonia and Fibrosis—A Randomized Placebo Controlled Clinical Trial" Pharmaceuticals 15, no. 2: 200. https://doi.org/10.3390/ph15020200
APA StyleKotfis, K., Karolak, I., Lechowicz, K., Zegan-Barańska, M., Pikulska, A., Niedźwiedzka-Rystwej, P., Kawa, M., Sieńko, J., Szylińska, A., & Wiśniewska, M. (2022). Mineralocorticoid Receptor Antagonist (Potassium Canrenoate) Does Not Influence Outcome in the Treatment of COVID-19-Associated Pneumonia and Fibrosis—A Randomized Placebo Controlled Clinical Trial. Pharmaceuticals, 15(2), 200. https://doi.org/10.3390/ph15020200







