Microneedle-Based Natural Polysaccharide for Drug Delivery Systems (DDS): Progress and Challenges
Abstract
:1. Introduction
2. Microneedle and Materials
2.1. Microneedle Systems
2.1.1. Silicon
2.1.2. Metals
2.1.3. Ceramic
2.1.4. Silica Glass
2.1.5. Carbohydrate
2.1.6. Polymers
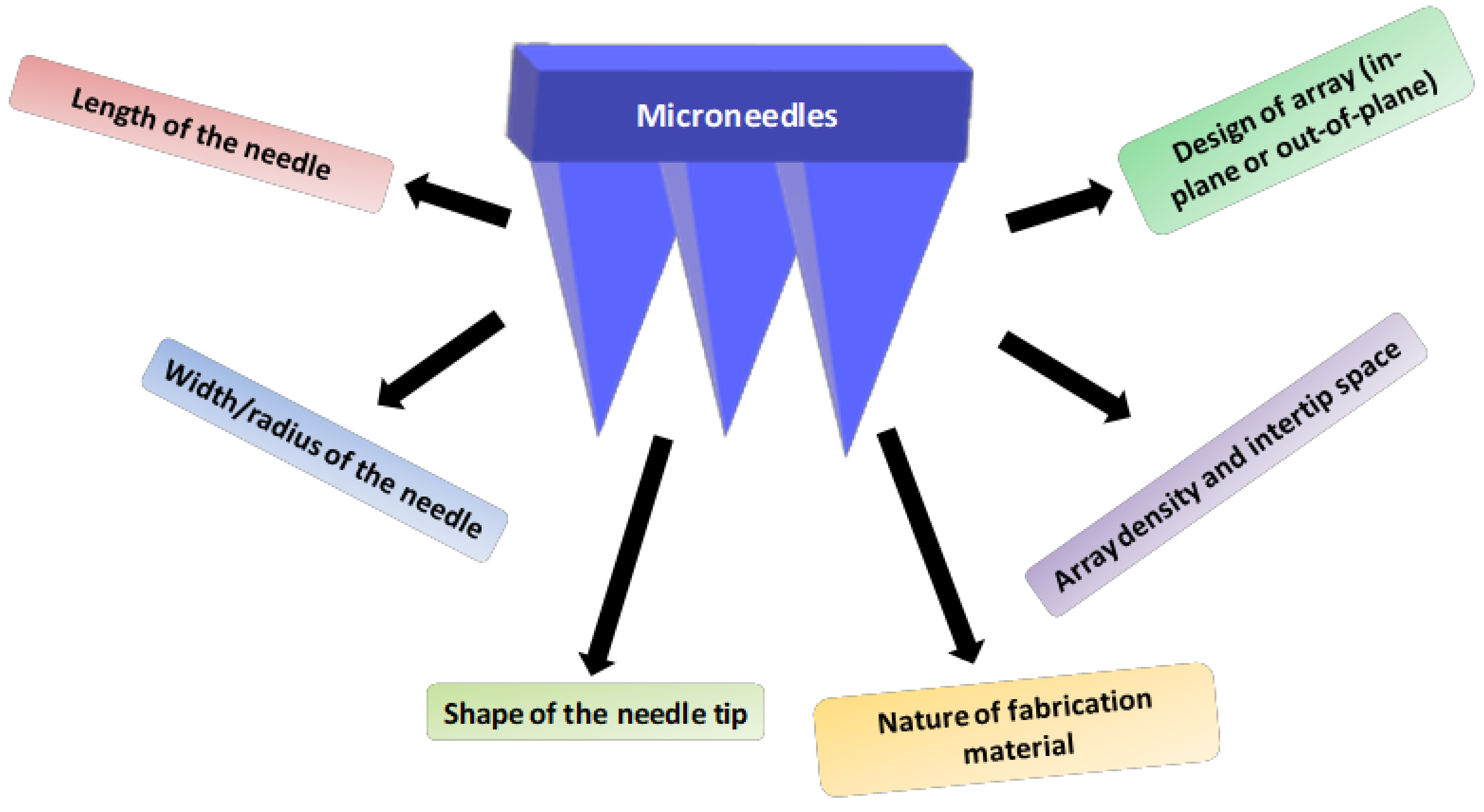
2.2. Characteristics and Geometry of Microneedles
2.3. Fabrication Techniques
2.3.1. Laser Cutting
2.3.2. Laser Ablation
2.3.3. Photolithography
2.3.4. Etching
2.3.5. Dry Etching
2.3.6. Wet Etching
2.3.7. Three-Dimensional Printing
2.3.8. Micro-Stereolithography
2.3.9. Continuous Liquid Phase Production
2.3.10. Two-Photon Polymerization
2.4. Mechanical Properties of Natural Microneedles
2.5. Advantages of Natural Microneedles
3. Types of Microneedles
3.1. Solid Microneedles
| Microneedle | Material | Technique Employed | Approach | Type of Product | Improvements | References |
|---|---|---|---|---|---|---|
| Solid Microneedles | Silicon | Dry and Wet Etching | Poke and Patch | Docetaxel Liposomes | Skin permeation | [75] |
| Derma-roller | NA | Topical 5-FU | Invitro and in vivo anti-tumor activity | [76] | ||
| MNs coated with ZnONanowires | Photolithography | Paclitaxel | 10% increase in reduction of tumor size compared to conventional method | [77] | ||
| Stainless Steel | - | Combinational (Mesoporous Nano Particles) Therapy of Phthalocyanine, Dabrafenib, Trametinib | Inhibited cell proliferation and anti-tumor activity by reactive oxygen species | [78] | ||
| Coated Microneedles | Stainless Steel | Infrared Laser Cutting, Ink-jet Printing | Coat and Poke | 5-FU, Curcumin and Cisplatin | Ink-jet printing on SS Microneedles | [79] |
| Stainless Steel | Wet Etching | PLGA Nanoparticles of DOX | Effective local delivery for oral cancer | [80] | ||
| Stainless Steel | Manual Coating | Octa-Arginine siRNA Nanocomplexes | Induced BRAF gene, which is responsible for melanoma development, induce tumor apoptosis and proliferation | [81] | ||
| Polycarbonate | Dip Coating | Immunotherapy using DNA Polyplexes and Poly Adjuvant | Induced humoral and cellular immunity facilitated targeting and activation of skin | [82] | ||
| Hallow Microneedles | Nickel | - | Poke and Flow | DOX | Increased drug diffusion coefficient | [83] |
| Stainless Steel | - | 5-FU | Effective against gastric cancer cells | [84] | ||
| Silicon | Manual Coating | HPV 16 E6 siRNA | Targeted delivery and inhibited tumor progression and observed no major adverse reactions | [85] | ||
| Dissolving Microneedles | Polyvinyl Alcohol (PVA) | Micro Molding | Poke and Dissolve | DOX | Improved permeation | [37] |
| Zein | Micro Molding | Tamoxifen and Gemcitabine | No improvement for tamoxifen, observed great permeation in gemcitabine | [86] | ||
| Sodium CMC | Micro Molding | Lipid-XoatedCisplatin Nanoparticles | Enhanced cytotoxicity and reduced tumor size | [87] | ||
| Pluronic F127 | Micro Molding | Cancer Vaccination for EG7-OVA Tumor | Improved antigen-specific humoral and cellular immunity | [88] | ||
| Hydrogel Microneedles | PLGA | Multiple Casting | Poke and Swell | Amphotericin | Controlled, prolonged release of drug for a week | [37,89] |
| Ethylene Glycol Methylvinylether-co-maleic acid | Molding | Metformin HCl | Sustained release | [39] | ||
| PEG-PMVE/MA | Micro Molding | Anti-Microbial | No microbial invasion through skin | [90] |
3.2. Dissolving Microneedle
3.3. Coated Microneedle
3.4. Hydrogel Forming Microneedle
3.5. Hollow Microneedles
4. Mechanism of Drug Delivery with Microneedles
5. Natural Polysaccharides Used in Microneedles
5.1. Hyaluronic Acid (HA)-Based MNs
5.2. Chondroitin Sulfate-Based MNs
5.3. Cellulose-Based MNs
5.4. Chitin and Chitosan(CS)-Based MNs
5.5. Starch-Based Microneedle
5.6. Sodium Alginate (SA)-Based Microneedle
5.7. Xanthan Gum (XG)-Based Microneedle
5.8. Pullulan-Based Microneedle
5.9. Bletilla Striata (BS)-Based Microneedle
| Polysaccharide | Source | Monosaccharide Units | Type of Microneedle Fabricated | Inference | Reference |
|---|---|---|---|---|---|
| Chitosan | Derived from chitin (natural sources of crustacean family) | d-glucosamineand N-acetyl-d-glucosamine | Hollow–solid, dissolving, and coated layer-by-layer microneedles | Possess good mechanical strength and also availed for its adjuvant and antibacterial property | [129,149,150,151] |
| Hyaluronic acid | Rooster combs, shark skin | d-glucuronic acid and N-acetyl-d-glucosamine | Hollow, dissolving and hydrogel microneedle | Self-dissolving ability and good penetration | [109,110,111] |
| Chondroitin sulfate | Cartilage, porcine skin and bovine trachea | N-acetyl-galactosamine and d-glucuronic acid | Dissolving microneedle | Good penetration | [117,118] |
| Alginate | Brown algae | α-l-guluronic acid and β-d-mannuronic acid | Dissolving microneedle | High mechanical strength when combined with maltose | [136,137] |
| Xanthan gum | Xanthomonas campestris | β-(1,4)-d-glucopyranose glucan as a backbone with (3,1)-α-linked d-Mann pyranose-(2,1)-β-d-glucuronic acid-(4,1)-β-d-Mann pyranose as side chains | Coated microneedles | Used as viscosity enhancer for coated microneedles | [139,140,152] |
| Starch | Corn or potato | Glucose | Dissolving microneedle | Owing to its brittleness blended with gelatin | [133,153] |
| Pullulan | Aureobasidiumpullulans | Maltose | Dissolving microneedle | Exhibited good mechanical properties | [142,154] |
| Bletilla striata | Bletilla striata | α-mannose, β-mannose, and β-glucose | Dissolving microneedle | Good mechanical strength and sufficient penetrating ability | [146] |
| Panaxnotoginseng | Panaxnotoginseng | Backbone of→4)-α-d-GalAp-(1→4-β-l-Rhap-1 →4)-β-d-Galp-(1→residues, with a branch of α-l-Araf-1→5)-α-l-Araf-(1→ | Dissolving microneedle | Good loading capacity and compatible with hydrophilic and lipophilic molecules, producing sustained and stable drug release | [148] |
6. The Benefits of Microneedles
6.1. Low Cost
6.2. Flexibility

6.3. Biodegradability, Biocompatibility and Stability
7. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent statement:
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hasnain, M.S.; Nayak, A.K. (Eds.) Natural Polysaccharides in Drug Delivery and Biomedical Applications; Elsevier: Cambridge, MA, USA, 2019; ISBN 9780128170557. [Google Scholar]
- Pushpamalar, J.; Meganathan, P.; Tan, H.L.; Dahlan, N.A.; Ooi, L.-T.; Neerooa, B.N.H.M.; Essa, R.Z.; Shameli, K.; Teow, S.-Y. Development of a Polysaccharide-Based Hydrogel Drug Delivery System (DDS): An Update. Gels 2021, 7, 153. [Google Scholar] [CrossRef]
- Sarnaik, A.; Abernathy, M.H.; Han, X.; Ouyang, Y.; Xia, K.; Chen, Y.; Cress, B.; Zhang, F.; Lali, A.; Pandit, R.; et al. Metabolic engineering of cyanobacteria for photoautotrophic production of heparosan, a pharmaceutical precursor of heparin. Algal Res. 2019, 37, 57–63. [Google Scholar] [CrossRef]
- Ebhodaghe, S.O. Natural Polymeric Scaffolds for Tissue Engineering Applications. J. Biomater. Sci. Polym. Ed. 2021, 32, 2144–2194. [Google Scholar] [CrossRef] [PubMed]
- Maxwell, E.G.; Colquhoun, I.J.; Chau, H.K.; Hotchkiss, A.T.; Waldron, K.W.; Morris, V.J.; Belshaw, N.J. Modified sugar beet pectin induces apoptosis of colon cancer cells via an interaction with the neutral sugar side-chains. Carbohydr. Polym. 2016, 136, 923–929. [Google Scholar] [CrossRef]
- Kwon, M.-J.; Nam, T.-J. A polysaccharide of the marine alga Capsosiphon fulvescens induces apoptosis in AGS gastric cancer cells via an IGF-IR-mediated PI3K/Akt pathway. Cell Biol. Int. 2007, 31, 768–775. [Google Scholar] [CrossRef]
- Fan, L.; Li, J.; Deng, K.; Ai, L. Effects of drying methods on the antioxidant activities of polysaccharides extracted from Ganoderma lucidum. Carbohydr. Polym. 2012, 87, 1849–1854. [Google Scholar] [CrossRef]
- Sun, S.; Li, K.; Lei, Z.; Xiao, L.; Gao, R.; Zhang, Z. Immunomodulatory activity of polysaccharide from Helicteres angustifolia L. on 4T1 tumor-bearing mice. Biomed. Pharmacother. 2018, 101, 881–888. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.-J.; Xie, J.-H.; Nie, S.-P.; Xie, M.-Y. Review on cell models to evaluate the potential antioxidant activity of polysaccharides. Food Funct. 2017, 8, 915–926. [Google Scholar] [CrossRef]
- Kim, Y.-C.; Park, J.-H.; Prausnitz, M.R. Microneedles for drug and vaccine delivery. Adv. Drug Deliv. Rev. 2012, 64, 1547–1568. [Google Scholar] [CrossRef] [Green Version]
- Traverso, G.; Schoellhammer, C.M.; Schroeder, A.; Maa, R.; Lauwers, G.Y.; Polat, B.E.; Anderson, D.G.; Blankschtein, D.; Langer, R. Microneedles for Drug Delivery via the Gastrointestinal Tract. J. Pharm. Sci. 2015, 104, 362–367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chi, J.; Zhang, X.; Chen, C.; Shao, C.; Zhao, Y.; Wang, Y. Antibacterial and angiogenic chitosan microneedle array patch for promoting wound healing. Bioact. Mater. 2020, 5, 253–259. [Google Scholar] [CrossRef]
- Barnum, L.; Samandari, M.; Schmidt, T.A.; Tamayol, A. Microneedle arrays for the treatment of chronic wounds. Expert Opin. Drug Deliv. 2020, 17, 1767–1780. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, R.F.; Singh, T.R.R.; Woolfson, A.D. Microneedle-based drug delivery systems: Microfabrication, drug delivery, and safety. Drug Deliv. 2010, 17, 187–207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Larrañeta, E.; Lutton, R.E.M.; Woolfson, A.D.; Donnelly, R.F. Microneedle arrays as transdermal and intradermal drug delivery systems: Materials science, manufacture and commercial development. Mater. Sci. Eng. R Rep. 2016, 104, 1–32. [Google Scholar] [CrossRef] [Green Version]
- Tuan-Mahmood, T.-M.; McCrudden, M.T.C.; Torrisi, B.M.; McAlister, E.; Garland, M.J.; Singh, T.R.R.; Donnelly, R.F. Microneedles for intradermal and transdermal drug delivery. Eur. J. Pharm. Sci. 2013, 50, 623–637. [Google Scholar] [CrossRef] [Green Version]
- Grogan, S.P.; Dorthé, E.W.; Glembotski, N.E.; Gaul, F.; D’Lima, D.D. Cartilage tissue engineering combining microspheroid building blocks and microneedle arrays. Connect. Tissue Res. 2020, 61, 229–243. [Google Scholar] [CrossRef] [PubMed]
- Moldovan, N.I.; Hibino, N.; Nakayama, K. Principles of the Kenzan Method for Robotic Cell Spheroid-Based Three-Dimensional Bioprinting. Tissue Eng. Part B Rev. 2017, 23, 237–244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Waghule, T.; Singhvi, G.; Dubey, S.K.; Pandey, M.M.; Gupta, G.; Singh, M.; Dua, K. Microneedles: A smart approach and increasing potential for transdermal drug delivery system. Biomed. Pharmacother. 2019, 109, 1249–1258. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Chen, Y.; Shi, Y. Microneedles: A potential strategy in transdermal delivery and application in the management of psoriasis. RSC Adv. 2020, 10, 14040–14049. [Google Scholar] [CrossRef]
- Jung, J.H.; Jin, S.G. Microneedle for transdermal drug delivery: Current trends and fabrication. J. Pharm. Investig. 2021, 51, 503–517. [Google Scholar] [CrossRef]
- Bhatnagar, S.; Gadeela, P.R.; Thathireddy, P.; Venuganti, V.V.K. Microneedle-based drug delivery: Materials of construction. J. Chem. Sci. 2019, 131, 90. [Google Scholar] [CrossRef] [Green Version]
- Guillot, A.J.; Cordeiro, A.S.; Donnelly, R.F.; Montesinos, M.C.; Garrigues, T.M.; Melero, A. Microneedle-Based Delivery: An Overview of Current Applications and Trends. Pharmaceutics 2020, 12, 569. [Google Scholar] [CrossRef]
- Mdanda, S.; Ubanako, P.; Kondiah, P.P.D.; Kumar, P.; Choonara, Y.E. Recent Advances in Microneedle Platforms for Transdermal Drug Delivery Technologies. Polymers 2021, 13, 2405. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Liu, M.; Mo, R. Polysaccharide-Based Biomaterials for Protein Delivery. Med. Drug Discov. 2020, 7, 100031. [Google Scholar] [CrossRef]
- Bhadale, R.S.; Londhe, V.Y. A systematic review of carbohydrate-based microneedles: Current status and future prospects. J. Mater. Sci. Mater. Med. 2021, 32, 89. [Google Scholar] [CrossRef]
- Williams, A.C.; Barry, B.W. Penetration enhancers. Adv. Drug Deliv. Rev. 2012, 64, 128–137. [Google Scholar] [CrossRef]
- Khanna, P.; Luongo, K.; Strom, J.A.; Bhansali, S. Sharpening of hollow silicon microneedles to reduce skin penetration force. J. Micromech. Microeng. 2010, 20, 045011. [Google Scholar] [CrossRef]
- Li, J.; Liu, B.; Zhou, Y.; Chen, Z.; Jiang, L.; Yuan, W.; Liang, L. Fabrication of a Ti porous microneedle array by metal injection molding for transdermal drug delivery. PLoS ONE 2017, 12, e0172043. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ginebra, M.P.; Traykova, T.; Planell, J.A. Calcium phosphate cements as bone drug delivery systems: A review. J. Control. Release 2006, 113, 102–110. [Google Scholar] [CrossRef]
- Bystrova, S.; Luttge, R. Micromolding for ceramic microneedle arrays. Microelectron. Eng. 2011, 88, 1681–1684. [Google Scholar] [CrossRef]
- Wang, P.M.; Cornwell, M.; Hill, J.; Prausnitz, M.R. Precise Microinjection into Skin Using Hollow Microneedles. J. Investig. Dermatol. 2006, 126, 1080–1087. [Google Scholar] [CrossRef] [Green Version]
- Donnelly, R.F.; Morrow, D.I.J.; Singh, T.R.R.; Migalska, K.; McCarron, P.A.; O’Mahony, C.; Woolfson, A.D. Processing difficulties and instability of carbohydrate microneedle arrays. Drug Dev. Ind. Pharm. 2009, 35, 1242–1254. [Google Scholar] [CrossRef] [PubMed]
- Miyano, T.; Tobinaga, Y.; Kanno, T.; Matsuzaki, Y.; Takeda, H.; Wakui, M.; Hanada, K. Sugar Micro Needles as Transdermic Drug Delivery System. Biomed. Microdevices 2005, 7, 185–188. [Google Scholar] [CrossRef] [PubMed]
- Yalcintas, E.P.; Ackerman, D.S.; Korkmaz, E.; Telmer, C.A.; Jarvik, J.W.; Campbell, P.G.; Bruchez, M.P.; Ozdoganlar, O.B. Analysis of In Vitro Cytotoxicity of Carbohydrate-Based Materials Used for Dissolvable Microneedle Arrays. Pharm. Res. 2020, 37, 33. [Google Scholar] [CrossRef] [PubMed]
- Yuan, W.; Hong, X.; Wu, Z.; Chen, L.; Liu, Z.; Wu, F.; Wei, L. Dissolving and biodegradable microneedle technologies for transdermal sustained delivery of drug and vaccine. Drug Des. Devel. Ther. 2013, 7, 945. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, H.X.; Bozorg, B.D.; Kim, Y.; Wieber, A.; Birk, G.; Lubda, D.; Banga, A.K. Poly (vinyl alcohol) microneedles: Fabrication, characterization, and application for transdermal drug delivery of doxorubicin. Eur. J. Pharm. Biopharm. 2018, 129, 88–103. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, R.F.; Singh, T.R.R.; Alkilani, A.Z.; McCrudden, M.T.C.; O’Neill, S.; O’Mahony, C.; Armstrong, K.; McLoone, N.; Kole, P.; Woolfson, A.D. Hydrogel-forming microneedle arrays exhibit antimicrobial properties: Potential for enhanced patient safety. Int. J. Pharm. 2013, 451, 76–91. [Google Scholar] [CrossRef] [Green Version]
- Migdadi, E.M.; Courtenay, A.J.; Tekko, I.A.; McCrudden, M.T.C.; Kearney, M.-C.; McAlister, E.; McCarthy, H.O.; Donnelly, R.F. Hydrogel-forming microneedles enhance transdermal delivery of metformin hydrochloride. J. Control. Release 2018, 285, 142–151. [Google Scholar] [CrossRef]
- Donnelly, R.F.; Majithiya, R.; Singh, T.R.R.; Morrow, D.I.J.; Garland, M.J.; Demir, Y.K.; Migalska, K.; Ryan, E.; Gillen, D.; Scott, C.J.; et al. Design, Optimization and Characterisation of Polymeric Microneedle Arrays Prepared by a Novel Laser-Based Micromoulding Technique. Pharm. Res. 2011, 28, 41–57. [Google Scholar] [CrossRef] [Green Version]
- Park, J.-H.; Allen, M.G.; Prausnitz, M.R. Biodegradable polymer microneedles: Fabrication, mechanics and transdermal drug delivery. J. Control. Release 2005, 104, 51–66. [Google Scholar] [CrossRef] [PubMed]
- McAllister, D.V.; Wang, P.M.; Davis, S.P.; Park, J.-H.; Canatella, P.J.; Allen, M.G.; Prausnitz, M.R. Microfabricated needles for transdermal delivery of macromolecules and nanoparticles: Fabrication methods and transport studies. Proc. Natl. Acad. Sci. USA 2003, 100, 13755–13760. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yung, K.L.; Xu, Y.; Kang, C.; Liu, H.; Tam, K.F.; Ko, S.M.; Kwan, F.Y.; Lee, T.M.H. Sharp tipped plastic hollow microneedle array by microinjection moulding. J. Micromech. Microeng. 2012, 22, 015016. [Google Scholar] [CrossRef]
- Ashraf, M.W.; Tayyaba, S.; Afzulpurkar, N. Micro Electromechanical Systems (MEMS) Based Microfluidic Devices for Biomedical Applications. Int. J. Mol. Sci. 2011, 12, 3648–3704. [Google Scholar] [CrossRef]
- Donnelly, R.F.; Singh, T.R.R.; Garland, M.J.; Migalska, K.; Majithiya, R.; McCrudden, C.M.; Kole, P.L.; Mahmood, T.M.T.; McCarthy, H.O.; Woolfson, A.D. Hydrogel-Forming Microneedle Arrays for Enhanced Transdermal Drug Delivery. Adv. Funct. Mater. 2012, 22, 4879–4890. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.W.; Park, J.-H.; Prausnitz, M.R. Dissolving microneedles for transdermal drug delivery. Biomaterials 2008, 29, 2113–2124. [Google Scholar] [CrossRef] [Green Version]
- Banga, A.K. Microporation applications for enhancing drug delivery. Expert Opin. Drug Deliv. 2009, 6, 343–354. [Google Scholar] [CrossRef] [PubMed]
- Davidson, A.; Al-Qallaf, B.; Das, D.B. Transdermal drug delivery by coated microneedles: Geometry effects on effective skin thickness and drug permeability. Chem. Eng. Res. Des. 2008, 86, 1196–1206. [Google Scholar] [CrossRef] [Green Version]
- Chen, M.-C.; Ling, M.-H.; Lai, K.-Y.; Pramudityo, E. Chitosan Microneedle Patches for Sustained Transdermal Delivery of Macromolecules. Biomacromolecules 2012, 13, 4022–4031. [Google Scholar] [CrossRef]
- Banks, S.L.; Pinninti, R.R.; Gill, H.S.; Paudel, K.S.; Crooks, P.A.; Brogden, N.K.; Prausnitz, M.R.; Stinchcomb, A.L. Transdermal Delivery of Naltrexol and Skin Permeability Lifetime after Microneedle Treatment in Hairless Guinea Pigs. J. Pharm. Sci. 2010, 99, 3072–3080. [Google Scholar] [CrossRef] [Green Version]
- Martanto, W.; Davis, S.P.; Holiday, N.R.; Wang, J.; Gill, H.S.; Prausnitz, M.R. Transdermal Delivery of Insulin Using Microneedles in Vivo. Pharm. Res. 2004, 21, 947–952. [Google Scholar] [CrossRef]
- Gill, H.S.; Prausnitz, M.R. Coating Formulations for Microneedles. Pharm. Res. 2007, 24, 1369–1380. [Google Scholar] [CrossRef]
- Shakya, A.K.; Ingrole, R.S.J.; Joshi, G.; Uddin, M.J.; Anvari, S.; Davis, C.M.; Gill, H.S. Microneedles coated with peanut allergen enable desensitization of peanut sensitized mice. J. Control. Release 2019, 314, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Nejad, H.R.; Sadeqi, A.; Kiaee, G.; Sonkusale, S. Low-cost and cleanroom-free fabrication of microneedles. Microsyst. Nanoeng. 2018, 4, 17073. [Google Scholar] [CrossRef]
- Wilke, M.S.; Lovering, A.L.; Strynadka, N.C. β-Lactam antibiotic resistance: A current structural perspective. Curr. Opin. Microbiol. 2005, 8, 525–533. [Google Scholar] [CrossRef] [PubMed]
- Indermun, S.; Luttge, R.; Choonara, Y.E.; Kumar, P.; du Toit, L.C.; Modi, G.; Pillay, V. Current advances in the fabrication of microneedles for transdermal delivery. J. Control. Release 2014, 185, 130–138. [Google Scholar] [CrossRef]
- Yang, M.; Zahn, J.D. Microneedle Insertion Force Reduction Using Vibratory Actuation. Biomed. Microdevices 2004, 6, 177–182. [Google Scholar] [CrossRef]
- Wilke, N.; Mulcahy, A.; Ye, S.-R.; Morrissey, A. Process optimization and characterization of silicon microneedles fabricated by wet etch technology. Microelectron. J. 2005, 36, 650–656. [Google Scholar] [CrossRef]
- Pere, C.P.P.; Economidou, S.N.; Lall, G.; Ziraud, C.; Boateng, J.S.; Alexander, B.D.; Lamprou, D.A.; Douroumis, D. 3D printed microneedles for insulin skin delivery. Int. J. Pharm. 2018, 544, 425–432. [Google Scholar] [CrossRef] [Green Version]
- Asghar, W.; Li, F.; Zhou, Y.; Wu, Y.; Yu, Z.; Li, S.; Tang, D.; Han, X.; Shang, J.; Liu, Y.; et al. Piezocapacitive Flexible E-Skin Pressure Sensors Having Magnetically Grown Microstructures. Adv. Mater. Technol. 2020, 5, 1900934. [Google Scholar] [CrossRef]
- Melchels, F.P.W.; Feijen, J.; Grijpma, D.W. A review on stereolithography and its applications in biomedical engineering. Biomaterials 2010, 31, 6121–6130. [Google Scholar] [CrossRef] [Green Version]
- Dharadhar, S.; Majumdar, A.; Dhoble, S.; Patravale, V. Microneedles for transdermal drug delivery: A systematic review. Drug Dev. Ind. Pharm. 2019, 45, 188–201. [Google Scholar] [CrossRef] [PubMed]
- Krieger, K.J.; Bertollo, N.; Dangol, M.; Sheridan, J.T.; Lowery, M.M.; O’Cearbhaill, E.D. Simple and customizable method for fabrication of high-aspect ratio microneedle molds using low-cost 3D printing. Microsyst. Nanoeng. 2019, 5, 42. [Google Scholar] [CrossRef] [Green Version]
- Schmidleithner, C.; Kalaskar, D.M. Stereolithography. In 3D Printing; InTech: London, UK, 2018. [Google Scholar]
- Johnson, A.R.; Caudill, C.L.; Tumbleston, J.R.; Bloomquist, C.J.; Moga, K.A.; Ermoshkin, A.; Shirvanyants, D.; Mecham, S.J.; Luft, J.C.; DeSimone, J.M. Single-Step Fabrication of Computationally Designed Microneedles by Continuous Liquid Interface Production. PLoS ONE 2016, 11, e0162518. [Google Scholar] [CrossRef] [PubMed]
- Ito, Y.; Yoshimitsu, J.-I.; Shiroyama, K.; Sugioka, N.; Takada, K. Self-dissolving microneedles for the percutaneous absorption of EPO in mice. J. Drug Target. 2006, 14, 255–261. [Google Scholar] [CrossRef] [PubMed]
- Balmert, S.C.; Carey, C.D.; Falo, G.D.; Sethi, S.K.; Erdos, G.; Korkmaz, E.; Falo, L.D. Dissolving undercut microneedle arrays for multicomponent cutaneous vaccination. J. Control. Release 2020, 317, 336–346. [Google Scholar] [CrossRef] [PubMed]
- Park, S.-H.; Yang, D.-Y.; Lee, K.-S. Two-photon stereolithography for realizing ultraprecise three-dimensional nano/microdevices. Laser Photon. Rev. 2009, 3, 1–11. [Google Scholar] [CrossRef]
- Du, G.; Zhang, Z.; He, P.; Zhang, Z.; Sun, X. Determination of the mechanical properties of polymeric microneedles by micromanipulation. J. Mech. Behav. Biomed. Mater. 2021, 117, 104384. [Google Scholar] [CrossRef]
- Chi, Y.; Huang, Y.; Kang, Y.; Dai, G.; Liu, Z.; Xu, K.; Zhong, W. The effects of molecular weight of hyaluronic acid on transdermal delivery efficiencies of dissolving microneedles. Eur. J. Pharm. Sci. 2022, 168, 106075. [Google Scholar] [CrossRef]
- Ma, G.J.; Shi, L.T.; Wu, C.W. Biomechanical Property of a Natural Microneedle: The Caterpillar Spine. J. Med. Device 2011, 5, 034502. [Google Scholar] [CrossRef]
- Yang, J.; Liu, X.; Fu, Y.; Song, Y. Recent advances of microneedles for biomedical applications: Drug delivery and beyond. Acta Pharm. Sin. B 2019, 9, 469–483. [Google Scholar] [CrossRef]
- Aldawood, F.K.; Andar, A.; Desai, S. A Comprehensive Review of Microneedles: Types, Materials, Processes, Characterizations and Applications. Polymers 2021, 13, 2815. [Google Scholar] [CrossRef] [PubMed]
- Ita, K. Transdermal Delivery of Drugs with Microneedles—Potential and Challenges. Pharmaceutics 2015, 7, 90–105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qiu, Y.; Gao, Y.; Hu, K.; Li, F. Enhancement of skin permeation of docetaxel: A novel approach combining microneedle and elastic liposomes. J. Control. Release 2008, 129, 144–150. [Google Scholar] [CrossRef]
- Naguib, Y.W.; Kumar, A.; Cui, Z. The effect of microneedles on the skin permeability and antitumor activity of topical 5-fluorouracil. Acta Pharm. Sin. B 2014, 4, 94–99. [Google Scholar] [CrossRef] [Green Version]
- Zandi, A.; Khayamian, M.A.; Saghafi, M.; Shalileh, S.; Katebi, P.; Assadi, S.; Gilani, A.; Salemizadeh Parizi, M.; Vanaei, S.; Esmailinejad, M.R.; et al. Microneedle-Based Generation of Microbubbles in Cancer Tumors to Improve Ultrasound-Assisted Drug Delivery. Adv. Healthc. Mater. 2019, 8, 1900613. [Google Scholar] [CrossRef]
- Tham, H.P.; Xu, K.; Lim, W.Q.; Chen, H.; Zheng, M.; Thng, T.G.S.; Venkatraman, S.S.; Xu, C.; Zhao, Y. Microneedle-Assisted Topical Delivery of Photodynamically Active Mesoporous Formulation for Combination Therapy of Deep-Seated Melanoma. ACS Nano 2018, 12, 11936–11948. [Google Scholar] [CrossRef]
- Uddin, M.J.; Scoutaris, N.; Klepetsanis, P.; Chowdhry, B.; Prausnitz, M.R.; Douroumis, D. Inkjet printing of transdermal microneedles for the delivery of anticancer agents. Int. J. Pharm. 2015, 494, 593–602. [Google Scholar] [CrossRef] [Green Version]
- Ma, Y.; Boese, S.E.; Luo, Z.; Nitin, N.; Gill, H.S. Drug coated microneedles for minimally-invasive treatment of oral carcinomas: Development and in vitro evaluation. Biomed. Microdevices 2015, 17, 44. [Google Scholar] [CrossRef]
- Ruan, W.; Zhai, Y.; Yu, K.; Wu, C.; Xu, Y. Coated microneedles mediated intradermal delivery of octaarginine/BRAF siRNA nanocomplexes for anti-melanoma treatment. Int. J. Pharm. 2018, 553, 298–309. [Google Scholar] [CrossRef]
- Duong, H.T.T.; Yin, Y.; Thambi, T.; Nguyen, T.L.; Giang Phan, V.H.; Lee, M.S.; Lee, J.E.; Kim, J.; Jeong, J.H.; Lee, D.S. Smart vaccine delivery based on microneedle arrays decorated with ultra-pH-responsive copolymers for cancer immunotherapy. Biomaterials 2018, 185, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Mansoor, I.; Lai, J.; Ranamukhaarachchi, S.; Schmitt, V.; Lambert, D.; Dutz, J.; Häfeli, U.O.; Stoeber, B. A microneedle-based method for the characterization of diffusion in skin tissue using doxorubicin as a model drug. Biomed. Microdevices 2015, 17, 61. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.S.; Koo, D.-H.; Yang, J.-Y.; Lee, H.-Y.; Park, J.-H.; Park, J.H. Peri-tumor administration of 5-fluorouracil sol-gel using a hollow microneedle for treatment of gastric cancer. Drug Deliv. 2018, 25, 872–879. [Google Scholar] [CrossRef] [Green Version]
- Tang, T.; Deng, Y.; Chen, J.; Zhao, Y.; Yue, R.; Choy, K.W.; Wang, C.C.; Du, Q.; Xu, Y.; Han, L.; et al. Local administration of siRNA through Microneedle: Optimization, Bio-distribution, Tumor Suppression and Toxicity. Sci. Rep. 2016, 6, 30430. [Google Scholar] [CrossRef] [Green Version]
- Ingrole, R.S.J.; Gill, H.S. Microneedle Coating Methods: A Review with a Perspective. J. Pharmacol. Exp. Ther. 2019, 370, 555–569. [Google Scholar] [CrossRef]
- Lan, X.; She, J.; Lin, D.; Xu, Y.; Li, X.; Yang, W.; Lui, V.W.Y.; Jin, L.; Xie, X.; Su, Y. Microneedle-Mediated Delivery of Lipid-Coated Cisplatin Nanoparticles for Efficient and Safe Cancer Therapy. ACS Appl. Mater. Interfaces 2018, 10, 33060–33069. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.W.; Kim, S.-Y.; Lee, J.E.; Yin, Y.; Lee, J.H.; Lim, S.Y.; Kim, E.S.; Duong, H.T.T.; Kim, H.K.; Kim, S.; et al. Enhanced Cancer Vaccination by In Situ Nanomicelle-Generating Dissolving Microneedles. ACS Nano 2018, 12, 9702–9713. [Google Scholar] [CrossRef]
- Peng, K.; Vora, L.K.; Domínguez-Robles, J.; Naser, Y.A.; Li, M.; Larrañeta, E.; Donnelly, R.F. Hydrogel-forming microneedles for rapid and efficient skin deposition of controlled release tip-implants. Mater. Sci. Eng. C 2021, 127, 112226. [Google Scholar] [CrossRef] [PubMed]
- Turner, J.G.; White, L.R.; Estrela, P.; Leese, H.S. Hydrogel-Forming Microneedles: Current Advancements and Future Trends. Macromol. Biosci. 2021, 21, 2000307. [Google Scholar] [CrossRef]
- Garland, M.J.; Caffarel–Salvador, E.; Migalska, K.; Woolfson, A.D.; Donnelly, R.F. Dissolving polymeric microneedle arrays for electrically assisted transdermal drug delivery. J. Control. Release 2012, 159, 52–59. [Google Scholar] [CrossRef] [Green Version]
- Mansoor, I.; Liu, Y.; Häfeli, U.O.; Stoeber, B. Arrays of hollow out-of-plane microneedles made by metal electrodeposition onto solvent cast conductive polymer structures. J. Micromech. Microeng. 2013, 23, 085011. [Google Scholar] [CrossRef]
- Cormier, M.; Johnson, B.; Ameri, M.; Nyam, K.; Libiran, L.; Zhang, D.D.; Daddona, P. Transdermal delivery of desmopressin using a coated microneedle array patch system. J. Control. Release 2004, 97, 503–511. [Google Scholar] [CrossRef]
- Chong, R.H.E.; Gonzalez-Gonzalez, E.; Lara, M.F.; Speaker, T.J.; Contag, C.H.; Kaspar, R.L.; Coulman, S.A.; Hargest, R.; Birchall, J.C. Gene silencing following siRNA delivery to skin via coated steel microneedles: In vitro and in vivo proof-of-concept. J. Control. Release 2013, 166, 211–219. [Google Scholar] [CrossRef] [Green Version]
- Gill, H.S.; Prausnitz, M.R. Coated microneedles for transdermal delivery. J. Control. Release 2007, 117, 227–237. [Google Scholar] [CrossRef] [Green Version]
- Boehm, R.D.; Daniels, J.; Stafslien, S.; Nasir, A.; Lefebvre, J.; Narayan, R.J. Polyglycolic acid microneedles modified with inkjet-deposited antifungal coatings. Biointerphases 2015, 10, 011004. [Google Scholar] [CrossRef] [Green Version]
- Donnelly, R.F.; McCrudden, M.T.C.; Zaid Alkilani, A.; Larrañeta, E.; McAlister, E.; Courtenay, A.J.; Kearney, M.-C.; Singh, T.R.R.; McCarthy, H.O.; Kett, V.L.; et al. Hydrogel-Forming Microneedles Prepared from “Super Swelling” Polymers Combined with Lyophilised Wafers for Transdermal Drug Delivery. PLoS ONE 2014, 9, e111547. [Google Scholar] [CrossRef]
- Jamaledin, R.; Yiu, C.K.Y.; Zare, E.N.; Niu, L.; Vecchione, R.; Chen, G.; Gu, Z.; Tay, F.R.; Makvandi, P. Advances in Antimicrobial Microneedle Patches for Combating Infections. Adv. Mater. 2020, 32, 2002129. [Google Scholar] [CrossRef] [PubMed]
- Caffarel-Salvador, E.; Brady, A.J.; Eltayib, E.; Meng, T.; Alonso-Vicente, A.; Gonzalez-Vazquez, P.; Torrisi, B.M.; Vicente-Perez, E.M.; Mooney, K.; Jones, D.S.; et al. Hydrogel-Forming Microneedle Arrays Allow Detection of Drugs and Glucose In Vivo: Potential for Use in Diagnosis and Therapeutic Drug Monitoring. PLoS ONE 2015, 10, e0145644. [Google Scholar] [CrossRef] [Green Version]
- Wermeling, D.P.; Banks, S.L.; Hudson, D.A.; Gill, H.S.; Gupta, J.; Prausnitz, M.R.; Stinchcomb, A.L. Microneedles permit transdermal delivery of a skin-impermeant medication to humans. Proc. Natl. Acad. Sci. USA 2008, 105, 2058–2063. [Google Scholar] [CrossRef] [Green Version]
- Alkilani, A.; McCrudden, M.T.; Donnelly, R. Transdermal Drug Delivery: Innovative Pharmaceutical Developments Based on Disruption of the Barrier Properties of the Stratum Corneum. Pharmaceutics 2015, 7, 438–470. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.; Jiao, Y.; Wang, Y.; Zhou, C.; Zhang, Z. Polysaccharides-based nanoparticles as drug delivery systems. Adv. Drug Deliv. Rev. 2008, 60, 1650–1662. [Google Scholar] [CrossRef] [PubMed]
- Damiri, F.; Bachra, Y.; Bounacir, C.; Laaraibi, A.; Berrada, M. Synthesis and Characterization of Lyophilized Chitosan-Based Hydrogels Cross-Linked with Benzaldehyde for Controlled Drug Release. J. Chem. 2020, 2020, 8747639. [Google Scholar] [CrossRef]
- Fonseca, D.F.S.; Vilela, C.; Silvestre, A.J.D.; Freire, C.S.R. A compendium of current developments on polysaccharide and protein-based microneedles. Int. J. Biol. Macromol. 2019, 136, 704–728. [Google Scholar] [CrossRef]
- Yadav, H.; Karthikeyan, C. Natural polysaccharides: Structural features and properties. In Polysaccharide Carriers for Drug Delivery; Elsevier: Amsterdam, The Netherlands, 2019; pp. 1–17. [Google Scholar]
- Dicker, K.T.; Gurski, L.A.; Pradhan-Bhatt, S.; Witt, R.L.; Farach-Carson, M.C.; Jia, X. Hyaluronan: A simple polysaccharide with diverse biological functions. Acta Biomater. 2014, 10, 1558–1570. [Google Scholar] [CrossRef] [Green Version]
- Zhai, P.; Peng, X.; Li, B.; Liu, Y.; Sun, H.; Li, X. The application of hyaluronic acid in bone regeneration. Int. J. Biol. Macromol. 2020, 151, 1224–1239. [Google Scholar] [CrossRef] [PubMed]
- Saha, I.; Rai, V.K. Hyaluronic acid based microneedle array: Recent applications in drug delivery and cosmetology. Carbohydr. Polym. 2021, 267, 118168. [Google Scholar] [CrossRef]
- Liu, S.; Jin, M.; Quan, Y.; Kamiyama, F.; Katsumi, H.; Sakane, T.; Yamamoto, A. The development and characteristics of novel microneedle arrays fabricated from hyaluronic acid, and their application in the transdermal delivery of insulin. J. Control. Release 2012, 161, 933–941. [Google Scholar] [CrossRef] [PubMed]
- Du, H.; Liu, P.; Zhu, J.; Lan, J.; Li, Y.; Zhang, L.; Zhu, J.; Tao, J. Hyaluronic Acid-Based Dissolving Microneedle Patch Loaded with Methotrexate for Improved Treatment of Psoriasis. ACS Appl. Mater. Interfaces 2019, 11, 43588–43598. [Google Scholar] [CrossRef]
- Liu, S.; Jin, M.; Quan, Y.; Kamiyama, F.; Kusamori, K.; Katsumi, H.; Sakane, T.; Yamamoto, A. Transdermal delivery of relatively high molecular weight drugs using novel self-dissolving microneedle arrays fabricated from hyaluronic acid and their characteristics and safety after application to the skin. Eur. J. Pharm. Biopharm. 2014, 86, 267–276. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Dong, L.; Du, H.; Mao, J.; Xie, Y.; Wang, H.; Lan, J.; Lou, Y.; Fu, Y.; Wen, J.; et al. 5-Aminolevulinic Acid-Loaded Hyaluronic Acid Dissolving Microneedles for Effective Photodynamic Therapy of Superficial Tumors with Enhanced Long-Term Stability. Adv. Healthc. Mater. 2019, 8, 1900896. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Li, X.; Zhang, P.; Wang, Y. Rapidly dissolving microneedle patch for synergistic gene and photothermal therapy of subcutaneous tumor. J. Mater. Chem. B 2020, 8, 4331–4339. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.; Chen, Y.; He, X.; Yang, F.; Han, R.; Yang, C.; Li, W.; Qian, Z. Near-infrared responsive 5-fluorouracil and indocyanine green loaded MPEG-PCL nanoparticle integrated with dissolvable microneedle for skin cancer therapy. Bioact. Mater. 2020, 5, 542–552. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.; Li, W.; Zhou, X.; Yang, F.; Qian, Z. Microneedles-Based Transdermal Drug Delivery Systems: A Review. J. Biomed. Nanotechnol. 2017, 13, 1581–1597. [Google Scholar] [CrossRef] [PubMed]
- Abdallah, M.M.; Fernández, N.; Matias, A.A.; do Rosário Bronze, M. Hyaluronic acid and Chondroitin sulfate from marine and terrestrial sources: Extraction and purification methods. Carbohydr. Polym. 2020, 243, 116441. [Google Scholar] [CrossRef] [PubMed]
- Fukushima, K.; Ise, A.; Morita, H.; Hasegawa, R.; Ito, Y.; Sugioka, N.; Takada, K. Two-Layered Dissolving Microneedles for Percutaneous Delivery of Peptide/Protein Drugs in Rats. Pharm. Res. 2011, 28, 7–21. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Zhang, S.; Duan, Y.; Niu, Y.; Gu, H.; Zhao, Z.; Zhang, S.; Yang, Y.; Wang, X.; Gao, Y.; et al. Transcutaneous immunization of recombinant Staphylococcal enterotoxin B protein using a dissolving microneedle provides potent protection against lethal enterotoxin challenge. Vaccine 2019, 37, 3810–3819. [Google Scholar] [CrossRef] [PubMed]
- Shokri, J.; Adibki, K. Application of Cellulose and Cellulose Derivatives in Pharmaceutical Industries. In Cellulose—Medical, Pharmaceutical and Electronic Applications; InTech: London, UK, 2013. [Google Scholar]
- Falo, L.D., Jr.; Geza, E.; Burak, O. Microneedle Arrays for Cancer Therapy Applications. US Patent 20160136407A1, 19 May 2016. [Google Scholar]
- Lan, X.; Zhu, W.; Huang, X.; Yu, Y.; Xiao, H.; Jin, L.; Pu, J.J.; Xie, X.; She, J.; Lui, V.W.Y.; et al. Microneedles loaded with anti-PD-1–cisplatin nanoparticles for synergistic cancer immuno-chemotherapy. Nanoscale 2020, 12, 18885–18898. [Google Scholar] [CrossRef]
- Seetharam, A.A.; Choudhry, H.; Bakhrebah, M.A.; Abdulaal, W.H.; Gupta, M.S.; Rizvi, S.M.D.; Alam, Q.; Gowda, D.V.; Moin, A. Microneedles Drug Delivery Systems for Treatment of Cancer: A Recent Update. Pharmaceutics 2020, 12, 1101. [Google Scholar] [CrossRef]
- Ye, C.; Zhang, R. Semiconductor Microneedle Assembly Based on Gene Therapy, Manufacturing Method and Manufacturing Mold. CN Patent 106426729A, 22 February 2017. [Google Scholar]
- Park, Y.-H.; Ha, S.K.; Choi, I.; Kim, K.S.; Park, J.; Choi, N.; Kim, B.; Sung, J.H. Fabrication of degradable carboxymethyl cellulose (CMC) microneedle with laser writing and replica molding process for enhancement of transdermal drug delivery. Biotechnol. Bioprocess Eng. 2016, 21, 110–118. [Google Scholar] [CrossRef]
- Fonseca, D.F.S.; Vilela, C.; Pinto, R.J.B.; Bastos, V.; Oliveira, H.; Catarino, J.; Faísca, P.; Rosado, C.; Silvestre, A.J.D.; Freire, C.S.R. Bacterial nanocellulose-hyaluronic acid microneedle patches for skin applications: In vitro and in vivo evaluation. Mater. Sci. Eng. C 2021, 118, 111350. [Google Scholar] [CrossRef]
- Fouad, D.; Bachra, Y.; Ayoub, G.; Ouaket, A.; Bennamara, A.; Knouzi, N.; Berrada, M. A Novel Drug Delivery System Based on Nanoparticles of Magnetite Fe3O4 Embedded in an Auto Cross-Linked Chitosan. In Chitin and Chitosan—Physicochemical Properties and Industrial Applications; IntechOpen: London, UK, 2020. [Google Scholar]
- Laaraibi, A.; Moughaoui, F.; Damiri, F.; Ouakit, A.; Charhouf, I.; Hamdouch, S.; Jaafari, A.; Abourriche, A.; Knouzi, N.; Bennamara, A.; et al. Chitosan-Clay Based (CS-NaBNT) Biodegradable Nanocomposite Films for Potential Utility in Food and Environment. In Chitin-Chitosan—Myriad Functionalities in Science and Technology; IntechOpen: London, UK, 2018. [Google Scholar]
- Chen, M.-C.; Huang, S.-F.; Lai, K.-Y.; Ling, M.-H. Fully embeddable chitosan microneedles as a sustained release depot for intradermal vaccination. Biomaterials 2013, 34, 3077–3086. [Google Scholar] [CrossRef]
- Moreira, A.F.; Rodrigues, C.F.; Jacinto, T.A.; Miguel, S.P.; Costa, E.C.; Correia, I.J. Poly (vinyl alcohol)/chitosan layer-by-layer microneedles for cancer chemo-photothermal therapy. Int. J. Pharm. 2020, 576, 118907. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, Z.; Khan, M.I.; Siddique, M.I.; Sarwar, H.S.; Shahnaz, G.; Hussain, S.Z.; Bukhari, N.I.; Hussain, I.; Sohail, M.F. Fabrication and Characterization of Thiolated Chitosan Microneedle Patch for Transdermal Delivery of Tacrolimus. AAPS PharmSciTech 2020, 21, 68. [Google Scholar] [CrossRef]
- Chandrasekharan, A.; Hwang, Y.J.; Seong, K.-Y.; Park, S.; Kim, S.; Yang, S.Y. Acid-Treated Water-Soluble Chitosan Suitable for Microneedle-Assisted Intracutaneous Drug Delivery. Pharmaceutics 2019, 11, 209. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Wu, M.; Tan, D.; Liu, Q.; Xia, R.; Chen, M.; Liu, Y.; Xue, L.; Lei, Y. A dissolving and glucose-responsive insulin-releasing microneedle patch for type 1 diabetes therapy. J. Mater. Chem. B 2021, 9, 648–657. [Google Scholar] [CrossRef]
- Pineda-Álvarez, R.A.; Bernad-Bernad, M.J.; Rodríguez-Cruz, I.M.; Escobar-Chávez, J.J. Development and Characterization of Starch/Gelatin Microneedle Arrays Loaded with Lecithin–Gelatin Nanoparticles of Losartan for Transdermal Delivery. J. Pharm. Innov. 2020. [Google Scholar] [CrossRef]
- Serrano-Castañeda, P.; Escobar-Chavez, J.J.; Rodriguez-cruz, I.M.; Melgoza, L.M.; Martinez-Hernandez, J. Microneedles as Enhancer of Drug Absorption Through the Skin and Applications in Medicine and Cosmetology. J. Pharm. Pharm. Sci. 2018, 21, 73–93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boucelkha, A.; Petit, E.; Elboutachfaiti, R.; Molinié, R.; Amari, S.; Yahaoui, R.Z. Production of guluronate oligosaccharide of alginate from brown algae Stypocaulon scoparium using an alginate lyase. J. Appl. Phycol. 2017, 29, 509–519. [Google Scholar] [CrossRef]
- Demir, Y.K.; Akan, Z.; Kerimoglu, O. Sodium Alginate Microneedle Arrays Mediate the Transdermal Delivery of Bovine Serum Albumin. PLoS ONE 2013, 8, e63819. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Jiang, G.; Yu, W.; Liu, D.; Xu, B. Microneedles fabricated from alginate and maltose for transdermal delivery of insulin on diabetic rats. Mater. Sci. Eng. C 2018, 85, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Bonfante, G.; Lee, H.; Bao, L.; Park, J.; Takama, N.; Kim, B. Comparison of polymers to enhance mechanical properties of microneedles for bio-medical applications. Micro Nano Syst. Lett. 2020, 8, 13. [Google Scholar] [CrossRef]
- Kim, Y.-C.; Quan, F.-S.; Compans, R.W.; Kang, S.-M.; Prausnitz, M.R. Formulation of Microneedles Coated with Influenza Virus-like Particle Vaccine. AAPS PharmSciTech 2010, 11, 1193–1201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, H.-J.; Song, J.-M.; Bondy, B.J.; Compans, R.W.; Kang, S.-M.; Prausnitz, M.R. Effect of Osmotic Pressure on the Stability of Whole Inactivated Influenza Vaccine for Coating on Microneedles. PLoS ONE 2015, 10, e0134431. [Google Scholar] [CrossRef] [Green Version]
- Singh, R.S.; Saini, G.K.; Kennedy, J.F. Pullulan: Microbial sources, production and applications. Carbohydr. Polym. 2008, 73, 515–531. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, D.F.S.; Costa, P.C.; Almeida, I.F.; Dias-Pereira, P.; Correia-Sá, I.; Bastos, V.; Oliveira, H.; Duarte-Araújo, M.; Morato, M.; Vilela, C.; et al. Pullulan microneedle patches for the efficient transdermal administration of insulin envisioning diabetes treatment. Carbohydr. Polym. 2020, 241, 116314. [Google Scholar] [CrossRef]
- Chen, Z.; Cheng, L.; He, Y.; Wei, X. Extraction, characterization, utilization as wound dressing and drug delivery of Bletilla striata polysaccharide: A review. Int. J. Biol. Macromol. 2018, 120, 2076–2085. [Google Scholar] [CrossRef]
- Peng, Q.; Li, M.; Xue, F.; Liu, H. Structure and immunobiological activity of a new polysaccharide from Bletilla striata. Carbohydr. Polym. 2014, 107, 119–123. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Zhao, Y.; Zhang, M.; Yang, X.; Yue, P.; Tang, D.; Wei, X. Structural characterization and antioxidant activity of a new polysaccharide from Bletilla striata fibrous roots. Carbohydr. Polym. 2020, 227, 115362. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Liao, Z.; Hu, Q.; Maffucci, K.G.; Qu, Y. Novel Bletilla striata polysaccharide microneedles: Fabrication, characterization, and in vitro transcutaneous drug delivery. Int. J. Biol. Macromol. 2018, 117, 928–936. [Google Scholar] [CrossRef]
- Xu, D.; Pan, Y.; Chen, J. Chemical Constituents, Pharmacologic Properties, and Clinical Applications of Bletilla striata. Front. Pharmacol. 2019, 10, 10. [Google Scholar] [CrossRef]
- Wang, C.; Liu, S.; Xu, J.; Gao, M.; Qu, Y.; Liu, Y.; Yang, Y.; Cui, X. Dissolvable microneedles based on Panax notoginseng polysaccharide for transdermal drug delivery and skin dendritic cell activation. Carbohydr. Polym. 2021, 268, 118211. [Google Scholar] [CrossRef] [PubMed]
- Sadeqi, A.; Nejad, H.R.; Kiaee, G.; Sonkusale, S. Cost-effective Fabrication of Chitosan Microneedles for Transdermal Drug Delivery. In Proceedings of the 2018 40th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Honolulu, HI, USA, 17–21 July 2018; pp. 5737–5740. [Google Scholar]
- Schipper, P.; van der Maaden, K.; Groeneveld, V.; Ruigrok, M.; Romeijn, S.; Uleman, S.; Oomens, C.; Kersten, G.; Jiskoot, W.; Bouwstra, J. Diphtheria toxoid and N -trimethyl chitosan layer-by-layer coated pH-sensitive microneedles induce potent immune responses upon dermal vaccination in mice. J. Control. Release 2017, 262, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; He, Y.; Deng, L.; Zhang, Z.-R.; Lin, Y. A fast-dissolving microneedle array loaded with chitosan nanoparticles to evoke systemic immune responses in mice. J. Mater. Chem. B 2020, 8, 216–225. [Google Scholar] [CrossRef]
- Bachra, Y.; Grouli, A.; Damiri, F.; Talbi, M.; Berrada, M. A Novel Superabsorbent Polymer from Crosslinked Carboxymethyl Tragacanth Gum with Glutaraldehyde: Synthesis, Characterization, and Swelling Properties. Int. J. Biomater. 2021, 2021, 5008833. [Google Scholar] [CrossRef]
- Ling, M.-H.; Chen, M.-C. Dissolving polymer microneedle patches for rapid and efficient transdermal delivery of insulin to diabetic rats. Acta Biomater. 2013, 9, 8952–8961. [Google Scholar] [CrossRef] [PubMed]
- Vora, L.K.; Courtenay, A.J.; Tekko, I.A.; Larrañeta, E.; Donnelly, R.F. Pullulan-based dissolving microneedle arrays for enhanced transdermal delivery of small and large biomolecules. Int. J. Biol. Macromol. 2020, 146, 290–298. [Google Scholar] [CrossRef]
- Chen, Y.; Xian, Y.; Carrier, A.J.; Youden, B.; Servos, M.; Cui, S.; Luan, T.; Lin, S.; Zhang, X. A simple and cost-effective approach to fabricate tunable length polymeric microneedle patches for controllable transdermal drug delivery. RSC Adv. 2020, 10, 15541–15546. [Google Scholar] [CrossRef] [Green Version]
- Dathathri, E.; Lal, S.; Mittal, M.; Thakur, G.; De, S. Fabrication of low-cost composite polymer-based micro needle patch for transdermal drug delivery. Appl. Nanosci. 2020, 10, 371–377. [Google Scholar] [CrossRef]
- Woodhouse, I.; Nejati, S.; Selvamani, V.; Jiang, H.; Chittiboyina, S.; Grant, J.; Mutlu, Z.; Waimin, J.; Abutaleb, N.S.; Seleem, M.N.; et al. Flexible Microneedle Array Patch for Chronic Wound Oxygenation and Biofilm Eradication. ACS Appl. Bio Mater. 2021, 4, 5405–5415. [Google Scholar] [CrossRef] [PubMed]
- Mir, M.; Permana, A.D.; Ahmed, N.; Khan, G.M.; ur Rehman, A.; Donnelly, R.F. Enhancement in site-specific delivery of carvacrol for potential treatment of infected wounds using infection responsive nanoparticles loaded into dissolving microneedles: A proof of concept study. Eur. J. Pharm. Biopharm. 2020, 147, 57–68. [Google Scholar] [CrossRef] [PubMed]
- Ren, L.; Xu, S.; Gao, J.; Lin, Z.; Chen, Z.; Liu, B.; Liang, L.; Jiang, L. Fabrication of Flexible Microneedle Array Electrodes for Wearable Bio-Signal Recording. Sensors 2018, 18, 1191. [Google Scholar] [CrossRef] [Green Version]
- Avcil, M.; Çelik, A. Microneedles in Drug Delivery: Progress and Challenges. Micromachines 2021, 12, 1321. [Google Scholar] [CrossRef] [PubMed]
- Vora, L.K.; Moffatt, K.; Tekko, I.A.; Paredes, A.J.; Volpe-Zanutto, F.; Mishra, D.; Peng, K.; Raj Singh Thakur, R.; Donnelly, R.F. Microneedle array systems for long-acting drug delivery. Eur. J. Pharm. Biopharm. 2021, 159, 44–76. [Google Scholar] [CrossRef] [PubMed]
- Yadav, P.R.; Munni, M.N.; Campbell, L.; Mostofa, G.; Dobson, L.; Shittu, M.; Pattanayek, S.K.; Uddin, M.J.; Das, D.B. Translation of Polymeric Microneedles for Treatment of Human Diseases: Recent Trends, Progress, and Challenges. Pharmaceutics 2021, 13, 1132. [Google Scholar] [CrossRef] [PubMed]
- Jamaledin, R.; Di Natale, C.; Onesto, V.; Taraghdari, Z.; Zare, E.; Makvandi, P.; Vecchione, R.; Netti, P. Progress in Microneedle-Mediated Protein Delivery. J. Clin. Med. 2020, 9, 542. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| MicroneedleType | Solid Microneedle | Coated Microneedle | Dissolving Microneedle | Hydrogel Microneedle | |
|---|---|---|---|---|---|
| DecisionParameter | |||||
| Drug dose | 🞅 High | ✖ Low ▲ (If several patches are used) | ✖ Low ▲ (If several patches are used) | 🞅 High | |
| Onset of action (Pharmacokinetics/ pharmacodynamics) | ✖ Slow release by diffusion | 🞅 Rapid dissolution | 🞅 Dependent on the formulation | ✖ Slow release by diffusion | |
| Delivery period | ▲ Several hours (agents that keep the pores open longer are additionally needed) | ✖ Several minutes | 🞅 Several minutes to weeks (depending on the formulation) | ▲ Several hours | |
| Delivery efficiency (Expensive drugs require high delivery efficiency) | ✖ Some drug remains in the patch or formulation | 🞅 | 🞅 | ✖ Some drug remains in the patch | |
| Sharp waste generation | 🞅 | 🞅 | ✖ No sharp waste | ▲ Swollen hydrogel microneedle tip | |
| Packaging | ▲ Separate packaging for microneedles and formulation | 🞅 | 🞅 | 🞅 | |
| Patch-wearing time | ✖ Several hours | 🞅 Several minutes | 🞅 Several minutes | ✖ Several hours | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Damiri, F.; Kommineni, N.; Ebhodaghe, S.O.; Bulusu, R.; Jyothi, V.G.S.S.; Sayed, A.A.; Awaji, A.A.; Germoush, M.O.; Al-malky, H.S.; Nasrullah, M.Z.; et al. Microneedle-Based Natural Polysaccharide for Drug Delivery Systems (DDS): Progress and Challenges. Pharmaceuticals 2022, 15, 190. https://doi.org/10.3390/ph15020190
Damiri F, Kommineni N, Ebhodaghe SO, Bulusu R, Jyothi VGSS, Sayed AA, Awaji AA, Germoush MO, Al-malky HS, Nasrullah MZ, et al. Microneedle-Based Natural Polysaccharide for Drug Delivery Systems (DDS): Progress and Challenges. Pharmaceuticals. 2022; 15(2):190. https://doi.org/10.3390/ph15020190
Chicago/Turabian StyleDamiri, Fouad, Nagavendra Kommineni, Samuel Ogbeide Ebhodaghe, Raviteja Bulusu, Vaskuri G. S. Sainaga Jyothi, Amany A. Sayed, Aeshah A. Awaji, Mousa O. Germoush, Hamdan S. Al-malky, Mohammed Z. Nasrullah, and et al. 2022. "Microneedle-Based Natural Polysaccharide for Drug Delivery Systems (DDS): Progress and Challenges" Pharmaceuticals 15, no. 2: 190. https://doi.org/10.3390/ph15020190
APA StyleDamiri, F., Kommineni, N., Ebhodaghe, S. O., Bulusu, R., Jyothi, V. G. S. S., Sayed, A. A., Awaji, A. A., Germoush, M. O., Al-malky, H. S., Nasrullah, M. Z., Rahman, M. H., Abdel-Daim, M. M., & Berrada, M. (2022). Microneedle-Based Natural Polysaccharide for Drug Delivery Systems (DDS): Progress and Challenges. Pharmaceuticals, 15(2), 190. https://doi.org/10.3390/ph15020190










