Apremilast Improves Endothelial Glycocalyx Integrity, Vascular and Left Ventricular Myocardial Function in Psoriasis
Abstract
:1. Introduction
2. Results
2.1. Association of PASI with Endothelial Glycocalyx, Vascular and LV Myocardial Function
2.2. Interrelation of Endothelial Glycocalyx, Vascular and LV Myocardial Function
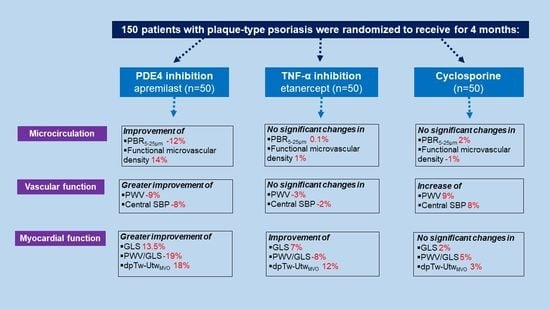
2.3. Effects of Treatment on Endothelial Glycocalyx, Vascular and LV Myocardial Deformation
2.3.1. Endothelial Glycocalyx and Microvascular Perfusion
2.3.2. Vascular Function
2.3.3. Myocardial Function
2.4. Association of the ΔPASI ≥ 75% with Markers of Vascular and Myocardial Function
2.5. Association between Changes of Endothelial Glycocalyx Markers with Changes in Vascular and LV Myocardial Function Post-Treatment
3. Discussion
3.1. Effects of Treatment on Endothelial Glycocalyx and Microvascular Perfusion
3.2. Effects of Treatment in Vascular and Myocardial Function
3.3. Limitations
4. Materials and Methods
4.1. Study Protocol
4.2. Primary and Secondary Endpoints
4.3. Imaging of Microcirculation
4.4. Arterial Stiffness
4.5. Echocardiography
4.6. Two-Dimensional Strain Measurements
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Makavos, G.; Ikonomidis, I.; Andreadou, I.; Varoudi, M.; Kapniari, I.; Loukeri, E.; Theodoropoulos, K.; Pavlidis, G.; Triantafyllidi, H.; Thymis, J.; et al. Effects of interleukin 17A inhibition on myocardial deformation and vascular function in psoriasis. Can. J. Cardiol. 2020, 36, 100–111. [Google Scholar] [CrossRef]
- Greb, J.E.; Goldminz, A.M.; Elder, J.T.; Lebwohl, M.G.; Gladman, D.D.; Wu, J.J.; Mehta, N.N.; Finlay, A.Y.; Gottlieb, A.B. Psoriasis. Nat. Rev. Dis. Primers 2016, 2, 16082. [Google Scholar] [CrossRef]
- Garshick, M.S.; Ward, N.L.; Krueger, J.G.; Berger, J.S. Cardiovascular risk in patients with psoriasis: JACC Review Topic of the Week. J. Am. Coll. Cardiol. 2021, 77, 1670–1680. [Google Scholar] [CrossRef]
- Lekakis, J.; Abraham, P.; Balbarini, A.; Blann, A.; Boulanger, C.M.; Cockcroft, J.; Cosentino, F.; Deanfield, J.; Gallino, A.; Ikonomidis, I.; et al. Methods for evaluating endothelial function: A position statement from the European Society of Cardiology Working Group on Peripheral Circulation. Eur. J. Cardiovasc. Prev. Rehabil. 2011, 18, 775–789. [Google Scholar] [CrossRef]
- Yilmaz, O.; Afsar, B.; Ortiz, A.; Kanbay, M. The role of endothelial glycocalyx in health and disease. Clin. Kidney J. 2019, 12, 611–619. [Google Scholar] [CrossRef]
- Nieuwdorp, M.; Meuwese, M.C.; Vink, H.; Hoekstra, J.B.; Kastelein, J.J.; Stroes, E.S. The endothelial glycocalyx: A potential barrier between health and vascular disease. Curr. Opin. Lipidol. 2005, 16, 507–511. [Google Scholar] [CrossRef]
- Mulders, T.A.; Nieuwdorp, M.; Stroes, E.S.; Vink, H.; Pinto-Sietsma, S.J. Non-invasive assessment of microvascular dysfunction in families with premature coronary artery disease. Int. J. Cardiol. 2013, 168, 5026–5028. [Google Scholar] [CrossRef]
- Valerio, L.; Peters, R.J.; Zwinderman, A.H.; Pinto-Sietsma, S.J. Sublingual endothelial glycocalyx and atherosclerosis. A cross-sectional study. PLoS ONE 2019, 14, e0213097. [Google Scholar] [CrossRef]
- Ikonomidis, I.; Thymis, J.; Simitsis, P.; Koliou, G.A.; Katsanos, S.; Triantafyllou, C.; Kousathana, F.; Pavlidis, G.; Kountouri, A.; Polyzogopoulou, E.; et al. Impaired endothelial glycocalyx predicts adverse outcome in subjects without overt cardiovascular disease: A 6-year follow-up study. J. Cardiovasc. Transl. Res. 2021, 1–13. [Google Scholar] [CrossRef]
- Chiricozzi, A.; Caposiena, D.; Garofalo, V.; Cannizzaro, M.V.; Chimenti, S.; Saraceno, R. A new therapeutic for the treatment of moderate-to-severe plaque psoriasis: Apremilast. Expert Rev. Clin. Immunol. 2016, 12, 237–249. [Google Scholar] [CrossRef]
- Torres, T.; Puig, L. Apremilast: A novel oral treatment for psoriasis and psoriatic arthritis. Am. J. Clin. Dermatol. 2018, 19, 23–32. [Google Scholar] [CrossRef]
- Radi, G.; Campanati, A.; Diotallevi, F.; Rizzetto, G.; Martina, E.; Bobyr, I.; Giannoni, M.; Offidani, A. Long-term efficacy and safety of apremilast in the treatment of plaques psoriasis: A real-world, single-center experience. Dermatol. Ther. 2021, 34, e15179. [Google Scholar] [CrossRef]
- Schafer, P.H.; Parton, A.; Gandhi, A.K.; Capone, L.; Adams, M.; Wu, L.; Bartlett, J.B.; Loveland, M.A.; Gilhar, A.; Cheung, Y.F.; et al. Apremilast, a cAMP phosphodiesterase-4 inhibitor, demonstrates anti-inflammatory activity in vitro and in a model of psoriasis. Br. J. Pharmacol. 2010, 159, 842–855. [Google Scholar] [CrossRef] [Green Version]
- Raker, V.K.; Becker, C.; Steinbrink, K. The cAMP pathway as therapeutic target in autoimmune and inflammatory diseases. Front. Immunol. 2016, 7, 123. [Google Scholar] [CrossRef] [Green Version]
- Mugheddu, C.; Pizzatti, L.; Sanna, S.; Atzori, L.; Rongioletti, F. COVID-19 pulmonary infection in erythrodermic psoriatic patient with oligodendroglioma: Safety and compatibility of apremilast with critical intensive care management. J. Eur. Acad. Dermatol. Venereol. 2020, 34, e376–e378. [Google Scholar] [CrossRef]
- Olisova, O.Y.; Anpilogova, E.M.; Svistunova, D.A. Apremilast as a potential treatment option for COVID-19: No symptoms of infection in a psoriatic patient. Dermatol. Ther. 2020, 33, e13668. [Google Scholar] [CrossRef]
- Santaniello, A.; Vigone, B.; Beretta, L. Letter to the editor: Immunomodulation by phosphodiesterase-4 inhibitor in COVID-19 patients. Metabolism 2020, 110, 154300. [Google Scholar] [CrossRef]
- Kearns, D.G.; Uppal, S.; Chat, V.S.; Wu, J.J. Assessing the risk of apremilast use for psoriasis during the COVID-19 pandemic. J. Drugs Dermatol. 2021, 20, 582–583. [Google Scholar]
- Lytvyn, Y.; Georgakopoulos, J.R.; Mufti, A.; Devani, A.R.; Gooderham, M.J.; Jain, V.; Lansang, P.; Vender, R.; Prajapati, V.H.; Yeung, J. Incidence and prognosis of COVID-19 in patients with psoriasis on apremilast: A multicentre retrospective cohort study. J. Eur. Acad. Dermatol. Venereol. 2021, 36, e94–e95. [Google Scholar] [CrossRef]
- Armstrong, A.W.; Read, C. Pathophysiology, clinical presentation, and treatment of psoriasis: A review. JAMA 2020, 323, 1945–1960. [Google Scholar] [CrossRef]
- Schafer, P.H.; Parton, A.; Capone, L.; Cedzik, D.; Brady, H.; Evans, J.F.; Man, H.W.; Muller, G.W.; Stirling, D.I.; Chopra, R. Apremilast is a selective PDE4 inhibitor with regulatory effects on innate immunity. Cell. Signal. 2014, 26, 2016–2029. [Google Scholar] [CrossRef] [Green Version]
- Ikonomidis, I.; Pavlidis, G.; Lambadiari, V.; Rafouli-Stergiou, P.; Makavos, G.; Thymis, J.; Kostelli, G.; Varoudi, M.; Katogiannis, K.; Theodoropoulos, K.; et al. Endothelial glycocalyx and microvascular perfusion are associated with carotid intima-media thickness and impaired myocardial deformation in psoriatic disease. J. Hum. Hypertens. 2021, 1–8. [Google Scholar] [CrossRef]
- Chelazzi, C.; Villa, G.; Mancinelli, P.; De Gaudio, A.R.; Adembri, C. Glycocalyx and sepsis-induced alterations in vascular permeability. Crit. Care 2015, 19, 26. [Google Scholar] [CrossRef] [Green Version]
- Tanaka, T.; Narazaki, M.; Kishimoto, T. Immunotherapeutic implications of IL-6 blockade for cytokine storm. Immunotherapy 2016, 8, 959–970. [Google Scholar] [CrossRef]
- Rovas, A.; Osiaevi, I.; Buscher, K.; Sackarnd, J.; Tepasse, P.R.; Fobker, M.; Kühn, J.; Braune, S.; Göbel, U.; Thölking, G.; et al. Microvascular dysfunction in COVID-19: The MYSTIC study. Angiogenesis 2021, 24, 145–157. [Google Scholar] [CrossRef]
- Otto, M.; Dorn, B.; Grasmik, T.; Doll, M.; Meissner, M.; Jakob, T.; Hrgovic, I. Apremilast effectively inhibits TNFα-induced vascular inflammation in human endothelial cells. J. Eur. Acad. Dermatol. Venereol. 2021, 36, 237–246. [Google Scholar] [CrossRef]
- Wang, H.; Yang, G.; Zhang, Q.; Liang, X.; Liu, Y.; Gao, M.; Guo, Y.; Chen, L. Apremilast ameliorates ox-LDL-induced endothelial dysfunction mediated by KLF6. Aging 2020, 12, 19012–19021. [Google Scholar] [CrossRef]
- Dalamaga, M.; Karampela, I.; Mantzoros, C.S. Commentary: Phosphodiesterase 4 inhibitors as potential adjunct treatment targeting the cytokine storm in COVID-19. Metabolism 2020, 109, 154282. [Google Scholar] [CrossRef]
- Ikonomidis, I.; Pavlidis, G.; Katsimbri, P.; Lambadiari, V.; Parissis, J.; Andreadou, I.; Tsoumani, M.; Boumpas, D.; Kouretas, D.; Iliodromitis, E. Tocilizumab improves oxidative stress and endothelial glycocalyx: A mechanism that may explain the effects of biological treatment on COVID-19. Food Chem. Toxicol. 2020, 145, 111694. [Google Scholar] [CrossRef]
- Mazzilli, S.; Lanna, C.; Chiaramonte, C.; Cesaroni, G.M.; Zangrilli, A.; Palumbo, V.; Cosio, T.; Dattola, A.; Gaziano, R.; Galluzzo, M.; et al. Real life experience of apremilast in psoriasis and arthritis psoriatic patients: Preliminary results on metabolic biomarkers. J. Dermatol. 2020, 47, 578–582. [Google Scholar] [CrossRef]
- Puig, L.; Korman, N.; Greggio, C.; Cirulli, J.; Teng, L.; Chandran, V.; Khraishi, M.; Paris, M.; Mehta, N. Long-term hemoglobin A1c changes with apremilast in patients with psoriasis and psoriatic arthritis: Pooled analysis of phase 3 ESTEEM and PALACE trials and phase 3b LIBERATE trial. J. Am. Acad. Dermatol. 2019, 81, AB89. [Google Scholar]
- Ikonomidis, I.; Pavlidis, G.; Lambadiari, V.; Kousathana, F.; Varoudi, M.; Spanoudi, F.; Maratou, E.; Parissis, J.; Triantafyllidi, H.; Dimitriadis, G.; et al. Early detection of left ventricular dysfunction in first-degree relatives of diabetic patients by myocardial deformation imaging: The role of endothelial glycocalyx damage. Int. J. Cardiol. 2017, 233, 105–112. [Google Scholar] [CrossRef]
- Ferguson, L.D.; Cathcart, S.; Rimmer, D.; Semple, G.; Brooksbank, K.; Paterson, C.; Brown, R.; Harvie, J.; Gao, X.; Radjenovic, A.; et al. Effect of the phosphodiesterase 4 inhibitor apremilast on cardiometabolic outcomes in psoriatic disease-results of the IMAPA study. Rheumatology 2021, keab474. [Google Scholar] [CrossRef]
- Ferro, C.J.; Savage, T.; Pinder, S.J.; Tomson, C.R. Central aortic pressure augmentation in stable renal transplant recipients. Kidney Int. 2002, 62, 166–171. [Google Scholar] [CrossRef] [Green Version]
- Imam, F.; Al-Harbi, N.O.; Al-Harbi, M.M.; Ansari, M.A.; Almutairi, M.M.; Alshammari, M.; Almukhlafi, T.S.; Ansari, M.N.; Aljerian, K.; Ahmad, S.F. Apremilast reversed carfilzomib-induced cardiotoxicity through inhibition of oxidative stress, NF-κB and MAPK signaling in rats. Toxicol. Mech. Methods 2016, 26, 700–708. [Google Scholar] [CrossRef]
- Imam, F.; Al-Harbi, N.O.; Al-Harbi, M.M.; Ansari, M.A.; Al-Asmari, A.F.; Ansari, M.N.; Al-Anazi, W.A.; Bahashwan, S.; Almutairi, M.M.; Alshammari, M.; et al. Apremilast prevent doxorubicin-induced apoptosis and inflammation in heart through inhibition of oxidative stress mediated activation of NF-κB signaling pathways. Pharmacol. Rep. 2018, 70, 993–1000. [Google Scholar] [CrossRef]
- Totani, L.; Piccoli, A.; Dell’Elba, G.; Concetta, A.; Di Santo, A.; Martelli, N.; Federico, L.; Pamuklar, Z.; Smyth, S.S.; Evangelista, V. Phosphodiesterase type 4 blockade prevents platelet-mediated neutrophil recruitment at the site of vascular injury. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 1689–1696. [Google Scholar] [CrossRef] [Green Version]
- Wu, C.; Rajagopalan, S. Phosphodiesterase-4 inhibition as a therapeutic strategy for metabolic disorders. Obes. Rev. 2016, 17, 429–441. [Google Scholar] [CrossRef]
- Langley, R.G.; Ellis, C.N. Evaluating psoriasis with Psoriasis Area and Severity Index, Psoriasis Global Assessment, and Lattice System Physician’s Global Assessment. J. Am. Acad. Dermatol. 2004, 51, 563–569. [Google Scholar] [CrossRef]
- Lee, D.H.; Dane, M.J.; van den Berg, B.M.; Boels, M.G.; van Teeffelen, J.W.; de Mutsert, R.; den Heijer, M.; Rosendaal, F.R.; van der Vlag, J.; van Zonneveld, A.J.; et al. Deeper penetration of erythrocytes into the endothelial glycocalyx is associated with impaired microvascular perfusion. PLoS ONE 2014, 9, e96477. [Google Scholar] [CrossRef] [Green Version]

| All Patients (n = 150) | Apremilast (n = 50) | Etanercept (n = 50) | Cyclosporine (n = 50) | p-Value | |
|---|---|---|---|---|---|
| Age, years | 51 ± 12 | 51 ± 11 | 51 ± 13 | 50 ± 10 | 0.820 |
| Sex (male/female), n (%) | 90/60 (60/40) | 30/20 (60/40) | 29/21 (58/42) | 31/19 (62/38) | 0.684 |
| BMI, kg/m2 | 30 ± 5 | 30 ± 4 | 31 ± 6 | 30 ± 5 | 0.992 |
| Duration of disease, years | 16 ± 11 | 17 ± 12 | 16 ± 11 | 14 ± 9 | 0.528 |
| PASI | 12 ± 2.3 | 12 ± 2.4 | 13 ± 2.6 | 12 ± 2.1 | 0.922 |
| Risk factors, n (%) | |||||
| Hypertension | 54 (36) | 19 (38) | 18 (36) | 17 (34) | 0.918 |
| Dyslipidemia | 53 (35) | 18 (36) | 19 (38) | 16 (32) | 0.467 |
| Diabetes Mellitus | 23 (15) | 8 (16) | 7 (14) | 8 (16) | 0.843 |
| Current smoking | 80 (53) | 26 (52) | 26 (52) | 28 (56) | 0.598 |
| Family history CAD | 21 (14) | 8 (16) | 6 (12) | 7 (14) | 0.693 |
| Μedication, n (%) | |||||
| Beta blockers | 28 (19) | 10 (20) | 9 (18) | 9 (18) | 0.923 |
| CCBs | 45 (30) | 16 (32) | 16 (32) | 13 (26) | 0.634 |
| ACEI/ARBs | 48 (32) | 17 (34) | 16 (32) | 15 (30) | 0.847 |
| Diuretics | 30 (20) | 10 (20) | 11 (22) | 9 (18) | 0.899 |
| Statins | 53 (35) | 18 (36) | 19 (38) | 16 (32) | 0.467 |
| Fibrate | 5 (3) | 2 (4) | 2 (4) | 1 92) | 0.988 |
| Antidiabetic agents | 23 (15) | 8 (16) | 7 (14) | 8 (16) | 0.843 |
| All Patients (n = 150) | Apremilast (n = 50) | Etanercept (n = 50) | Cyclosporine (n = 50) | ||
|---|---|---|---|---|---|
| ΔPASI75, % | 44 | 46 | 44 | 42 | |
| ΔPASI90, % | 28 | 32 | 28 | 26 | |
| BMI, Kg/m2 | Baseline | 30 ± 5 | 30 ± 4 | 31 ± 6 | 30 ± 5 |
| 4 months | 30 ± 5 | 29 ± 4 | 31 ± 5 | 31 ± 5 | |
| Δ% | −1 | −3 | −1 | +3 | |
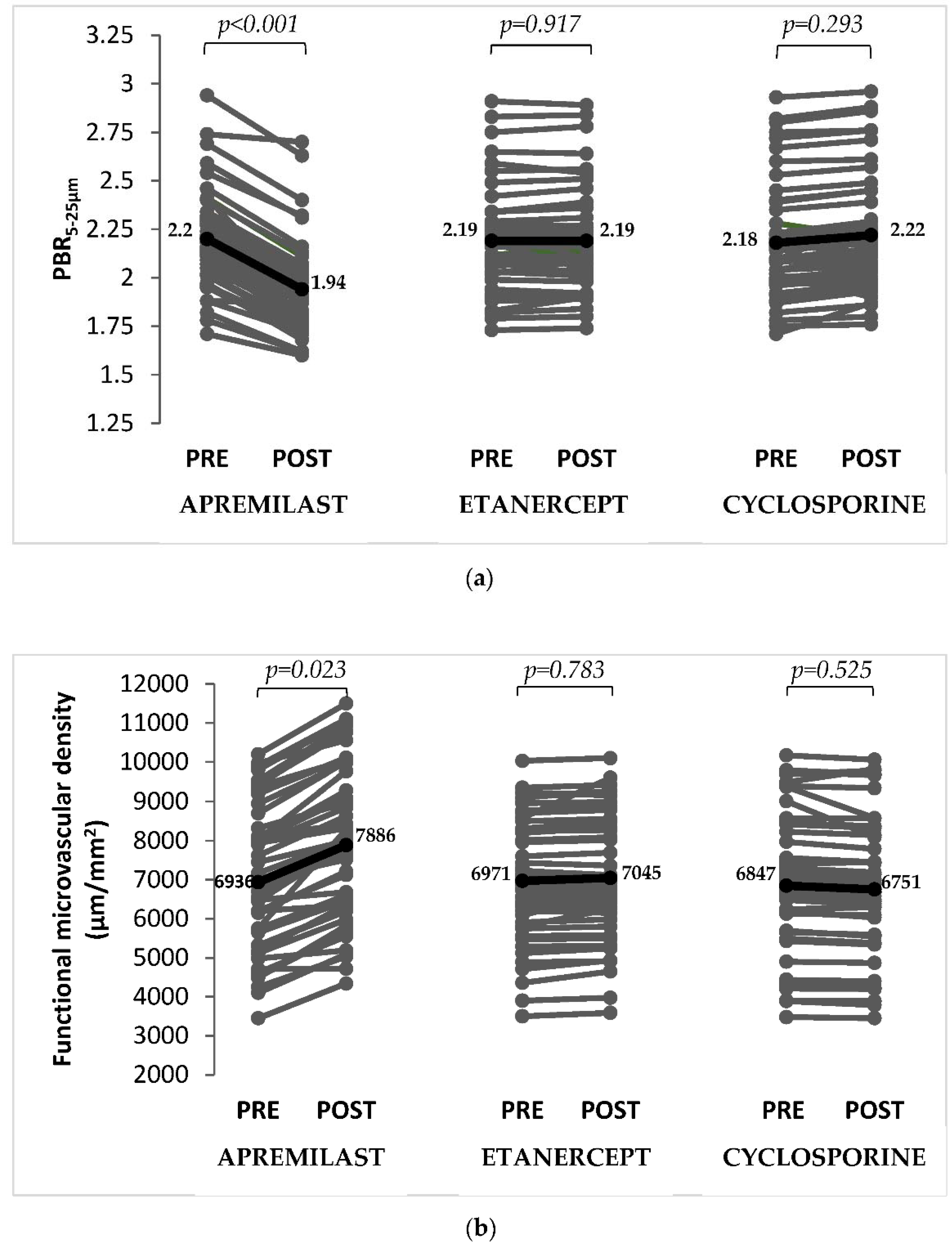
| PBR5–25 μm | Baseline | 2.19 ± 0.25 | 2.20 ± 0.25 | 2.19 ± 0.25 | 2.18 ± 0.26 |
| 4 months | 2.12 ± 0.25 | 1.94 ± 0.24 ‡ | 2.19 ± 0.27 | 2.22 ± 0.25 † | |
| Δ% | −3 | −12 | +0.1 | +2 | |
| Functional microvascular density, μm/mm2 | Baseline | 6918 ± 2413 | 6936 ± 2448 | 6971 ± 2259 | 6847 ± 2532 |
| 4 months | 7227 ± 2034 | 7886 ± 2161 ‡ | 7045 ± 2114 | 6751 ± 1828 † | |
| Δ% | +4 | +14 | +1 | −1 | |
| PWV, m/s | Baseline | 10.2 ± 2.4 | 10.4 ± 3 | 10.3 ± 2.1 | 9.9 ± 2 |
| 4 months | 10.1 ± 2.2 | 9.5 ± 2.4 ‡ | 10 ± 2.2 | 10.8 ± 1.9 †‡ | |
| Δ% | −1 | −9 | −3 | +9 | |
| cSBP, mmHg | Baseline | 133 ± 26 | 132 ± 31 | 135 ± 29 | 132 ± 19 |
| 4 months | 132 ± 22 | 122 ± 20 ‡ | 132 ± 22 | 143 ± 23 †‡ | |
| Δ% | −1 | −8 | −2 | +8 | |
| GLS, % | Baseline | −17.2 ± 4 | −17.1 ± 3 | −17.3 ± 4 | −17.2 ± 3 |
| 4 months | −18.5 ± 4 § | −19.4 ± 3 § | −18.5 ± 3 ‡ | −17.5 ± 4 * | |
| Δ% | +7 | +13.5 | +7 | +2 | |
| PWV/GLS, m/s% | Baseline | −0.59 ± 0.20 | −0.61 ± 0.21 | −0.59 ± 0.20 | 0.58 ± 0.19 |
| 4 months | −0.55 ± 0.19 | −0.49 ± 0.17 ‡ | −0.54 ± 0.19 | 0.61 ± 0.22 * | |
| Δ% | −7 | −19 | −8 | +5 | |
| pTw, ° | Baseline | 14.6 ± 6 | 14.9 ± 6.1 | 14.3 ± 5.4 | 14.8 ± 6.3 |
| 4 months | 16.5 ± 6.2 ‡ | 18.2 ± 6.8 ‡ | 16.3 ± 5.6 ‡ | 15.2 ± 5.9 * | |
| Δ% | +13 | +18 | +14 | +3 | |
| UtwMVO, ° | Baseline | 9.8 ± 4.6 | 9.8 ± 4.8 | 9.7 ± 4.3 | 9.8 ± 4.7 |
| 4 months | 10.5 ± 4.8 ‡ | 11 ± 5.2 ‡ | 10.5 ± 4.5 ‡ | 9.9 ± 4.6 * | |
| Δ% | +7 | +12 | +8 | +1 | |
| dpTw-UtwMVO, % | Baseline | 33 ± 10 | 34 ± 12 | 32 ± 10 | 34 ± 9 |
| 4 months | 37 ± 10 ‡ | 41 ± 8 ‡ | 36 ± 10 ‡ | 35 ± 11 * | |
| Δ% | +12 | +18 | +12 | +3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ikonomidis, I.; Pavlidis, G.; Kadoglou, N.; Makavos, G.; Katogiannis, K.; Kountouri, A.; Thymis, J.; Kostelli, G.; Kapniari, I.; Theodoropoulos, K.; et al. Apremilast Improves Endothelial Glycocalyx Integrity, Vascular and Left Ventricular Myocardial Function in Psoriasis. Pharmaceuticals 2022, 15, 172. https://doi.org/10.3390/ph15020172
Ikonomidis I, Pavlidis G, Kadoglou N, Makavos G, Katogiannis K, Kountouri A, Thymis J, Kostelli G, Kapniari I, Theodoropoulos K, et al. Apremilast Improves Endothelial Glycocalyx Integrity, Vascular and Left Ventricular Myocardial Function in Psoriasis. Pharmaceuticals. 2022; 15(2):172. https://doi.org/10.3390/ph15020172
Chicago/Turabian StyleIkonomidis, Ignatios, George Pavlidis, Nikolaos Kadoglou, George Makavos, Konstantinos Katogiannis, Aikaterini Kountouri, John Thymis, Gavriella Kostelli, Irini Kapniari, Konstantinos Theodoropoulos, and et al. 2022. "Apremilast Improves Endothelial Glycocalyx Integrity, Vascular and Left Ventricular Myocardial Function in Psoriasis" Pharmaceuticals 15, no. 2: 172. https://doi.org/10.3390/ph15020172
APA StyleIkonomidis, I., Pavlidis, G., Kadoglou, N., Makavos, G., Katogiannis, K., Kountouri, A., Thymis, J., Kostelli, G., Kapniari, I., Theodoropoulos, K., Parissis, J., Katsimbri, P., Papadavid, E., & Lambadiari, V. (2022). Apremilast Improves Endothelial Glycocalyx Integrity, Vascular and Left Ventricular Myocardial Function in Psoriasis. Pharmaceuticals, 15(2), 172. https://doi.org/10.3390/ph15020172