Methods for the Determination of Transition Metal Impurities in Cyclotron-Produced Radiometals
Abstract
:1. Introduction
2. Results and Discussion
2.1. Post-Column Derivatization with PAR
2.2. Pre-Column Derivatization with PAR
2.3. Colorimetric Metal Determination
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. HPLC Equipment and Conditions
3.2.1. Post-Column Complexation
3.2.2. Pre-Column Complexation
3.2.3. Colorimetry
3.3. Production and Purification of Scandium-44 and Gallium-68 Radionuclides
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pandey, M.K.; DeGrado, T.R. Cyclotron Production of PET Radiometals in Liquid Targets: Aspects and Prospects. Curr. Radiopharm. 2020, 13, 325–339. [Google Scholar] [CrossRef] [PubMed]
- Rodnick, M.E.; Sollert, C.; Stark, D.; Clark, M.; Katsifis, A.; Hockley, B.G.; Parr, D.C.; Frigell, J.; Henderson, B.D.; Abghari-Gerst, M.; et al. Cyclotron-Based Production of 68Ga, [68Ga]GaCl3, and [68Ga]Ga-PSMA-11 from a Liquid Target. EJNMMI Radiopharm. Chem. 2020, 5, 25. [Google Scholar] [CrossRef] [PubMed]
- Alves, V.; do Carmo, S.; Alves, F.; Abrunhosa, A. Automated Purification of Radiometals Produced by Liquid Targets. Instruments 2018, 2, 17. [Google Scholar] [CrossRef] [Green Version]
- Siikanen, J.; Jussing, E.; Milton, S.; Steiger, C.; Ulin, J.; Jonsson, C.; Samén, E.; Tran, T.A. Cyclotron-Produced 68Ga from Enriched 68Zn Foils. Appl. Radiat. Isot. 2021, 176, 109825. [Google Scholar] [CrossRef]
- Thisgaard, H.; Kumlin, J.; Langkjær, N.; Chua, J.; Hook, B.; Jensen, M.; Kassaian, A.; Zeisler, S.; Borjian, S.; Cross, M.; et al. Multi-Curie Production of Gallium-68 on a Biomedical Cyclotron and Automated Radiolabelling of PSMA-11 and DOTATATE. EJNMMI Radiopharm. Chem. 2021, 6, 1. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.; Paolillo, V.; Ta, R.T.; Damasco, J.; Rojo, R.D.; Carl, J.C.; Melancon, M.P.; Ravizzini, G.C.; Le, D.B.; Santos, E.B. Fully Automated Preparation of 68Ga-PSMA-11 at Curie Level Quantity Using Cyclotron-Produced 68Ga for Clinical Applications. Appl. Radiat. Isot. 2020, 155, 108936. [Google Scholar] [CrossRef]
- Nelson, B.J.B.; Wilson, J.; Richter, S.; Duke, M.J.M.; Wuest, M.; Wuest, F. Taking Cyclotron 68Ga Production to the next Level: Expeditious Solid Target Production of 68Ga for Preparation of Radiotracers. Nucl. Med. Biol. 2020, 80–81, 24–31. [Google Scholar] [CrossRef]
- Svedjehed, J.; Pärnaste, M.; Gagnon, K. Demystifying Solid Targets: Simple and Rapid Distribution-Scale Production of [68Ga]GaCl3 and [68Ga]Ga-PSMA-11. Nucl. Med. Biol. 2022, 104–105, 1–10. [Google Scholar] [CrossRef]
- Becker, K.V.; Chernysheva, M.; Barnhart, T.E.; Gagnon, K.; Engle, J.W. A Review of Accelerator-Produced Ga-68 with Solid Targets. Curr. Radiopharm. 2021, 14, 315–324. [Google Scholar] [CrossRef]
- Lin, M.; Waligorski, G.J.; Lepera, C.G. Production of Curie Quantities of 68Ga with a Medical Cyclotron via the 68Zn(p,n)68Ga Reaction. Appl. Radiat. Isot. 2018, 133, 1–3. [Google Scholar] [CrossRef]
- Talip, Z.; Favaretto, C.; Geistlich, S.; van der Meulen, N.P. A Step-by-Step Guide for the Novel Radiometal Production for Medical Applications: Case Studies with 68Ga, 44Sc, 177Lu and 161Tb. Molecules 2020, 25, 966. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Umbricht, C.A.; Benešová, M.; Schmid, R.M.; Türler, A.; Schibli, R.; van der Meulen, N.P.; Müller, C. 44Sc-PSMA-617 for Radiotheragnostics in Tandem with 177Lu-PSMA-617—Preclinical Investigations in Comparison with 68Ga-PSMA-11 and 68Ga-PSMA-617. EJNMMI Res. 2017, 7, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krajewski, S.; Cydzik, I.; Abbas, K.; Bulgheroni, A.; Simonelli, F.; Holzwarth, U.; Bilewicz, A. Cyclotron Production of 44Sc for Clinical Application. Radiochim. Acta 2013, 101, 333–338. [Google Scholar] [CrossRef]
- Pandey, M.K.; Byrne, J.F.; Jiang, H.; Packard, A.B.; DeGrado, T.R. Cyclotron Production of 68Ga via the 68Zn(p,n)68Ga Reaction in Aqueous Solution. Am. J. Nucl. Med. Mol. Imaging 2014, 4, 303–310. [Google Scholar] [PubMed]
- Mikolajczak, R.; Huclier-Markai, S.; Alliot, C.; Haddad, F.; Szikra, D.; Forgacs, V.; Garnuszek, P. Production of Scandium Radionuclides for Theranostic Applications: Towards Standardization of Quality Requirements. EJNMMI Radiopharm. Chem. 2021, 6, 19. [Google Scholar] [CrossRef]
- Pyrzyńska, K.; Kilian, K.; Pęgier, M. Separation and Purification of Scandium: From Industry to Medicine. Sep. Purif. Rev. 2019, 48, 65–77. [Google Scholar] [CrossRef]
- Kilian, K.; Cheda, Ł.; Sitarz, M.; Szkliniarz, K.; Choiński, J.; Stolarz, A. Separation of 44Sc from Natural Calcium Carbonate Targets for Synthesis of 44Sc-DOTATATE. Molecules 2018, 23, 1787. [Google Scholar] [CrossRef] [Green Version]
- Oehlke, E.; Le, V.S.; Lengkeek, N.; Pellegrini, P.; Jackson, T.; Greguric, I.; Weiner, R. Influence of Metal Ions on the 68Ga-Labeling of DOTATATE. Appl. Radiat. Isot. 2013, 82, 232–238. [Google Scholar] [CrossRef]
- Pharmeuropa 30.4. Reference: PA/PH/Exp. 14/T (18) 13 ANP.
- U.S. Environmental Protection Agency’s (EPA’s) Office of Water (OW). Method 1640 Determination of Trace Elements in Water by Preconcentration and Inductively Coupled Plasma-Mass Spectrometry; U.S. Environmental Protection Agency: Washington, DC, USA, 1997. [Google Scholar]
- Rodushkin, I.; Engström, E.; Baxter, D.C. Sources of Contamination and Remedial Strategies in the Multi-Elemental Trace Analysis Laboratory. Anal. Bioanal. Chem. 2010, 396, 365–377. [Google Scholar] [CrossRef]
- Geary, W.J.; Nickless, G.; Polland, F.H. The Metal Complexes of Some Azo and Azomethine Dyestuffs. Anal. Chim. Acta 1962, 26, 575–582. [Google Scholar] [CrossRef]
- Sommer, L. Complexation of Aluminium, Yttrium, Lanthanum and Lanthanides with 4-(2-Pyridylazo)Resorcinol (Par). Talanta 1967, 14, 457–471. [Google Scholar] [CrossRef]
- Ding, X.; Mou, S.; Liu, K.; Siriraks, A.; Riviello, J. Ion Chromatography of Heavy and Transition Metals by On- and Post-Column Derivatizations. Anal. Chim. Acta 2000, 407, 319–326. [Google Scholar] [CrossRef]
- Horstkotte, B.; Jarošová, P.; Chocholouš, P.; Sklenářová, H.; Solich, P. Sequential Injection Chromatography with Post-Column Reaction/Derivatization for the Determination of Transition Metal Cations in Natural Water Samples. Talanta 2015, 136, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.H.; El Gammal, E.M. Chromatographic Separation and Determination of Some Heavy Metals and the Sum of the Rare Earth Elements in Some Geological Materials. Chem. Pap. 2020, 74, 3491–3501. [Google Scholar] [CrossRef]
- Hu, Q.; Yang, G.; Yang, J.; Yin, J. Study on Determination of Iron, Cobalt, Nickel, Copper, Zinc and Manganese in Drinking Water by Solid-Phase Extraction and RP-HPLC with 2-(2-Quinolinylazo)-5-Diethylaminophenol as Precolumn Derivatizing Reagent. J. Environ. Monitor. 2002, 4, 956–959. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Yang, G.; Wang, B.; Jiang, C.; Yin, J. Determination of Transition Metal Ions in Tobacco as Their 2-(2-Quinolinylazo)-5-Dimethylaminophenol Derivatives Using Reversed-Phase Liquid Chromatography with UV–VIS Detection. J. Chromatogr. A 2002, 971, 243–248. [Google Scholar] [CrossRef]
- Zhang, C.; Miura, J.; Nagaosa, Y. Determination of Cadmium, Zinc, Nickel and Cobalt in Tobacco by Reversed-Phase High-Performance Liquid Chromatography with 2-(8-Quinolylazo)-4,5-Diphenylimidazole as a Chelating Reagent. Anal. Sci. 2005, 21, 1105–1110. [Google Scholar] [CrossRef] [Green Version]
- Burakham, R.; Srijaranai, S.; Grudpan, K. High-Performance Liquid Chromatography with Sequential Injection for Online Precolumn Derivatization of Some Heavy Metals. J. Sep. Sci. 2007, 30, 2614–2619. [Google Scholar] [CrossRef]
- Roston, D.A. Precolumn Chelation with 4-(2-Pyridylazo)Resorcinol for Simultaneous Determination of Metal Ions by Liquid Chromatography. Anal. Chem. 1984, 56, 241–244. [Google Scholar] [CrossRef]
- Martínez-Zepeda, D.L.; Meza-González, B.; Álvarez-Hernández, M.L.; Bazany-Rodríguez, I.J.; Vilchis Néstor, A.R.; Cortés-Guzmán, F.; Gómez-Espinosa, R.M.; Valdes-García, J.; Dorazco-González, A. Efficient Naked Eye Sensing of Tartrate/Malate Based on a Zn-Xylenol Orange Complex in Water and Membrane-Based Test Strips. Dye. Pigment. 2021, 188, 109239. [Google Scholar] [CrossRef]
- Študlar, K.; Janoušek, I. The Photometric Determination of Zinc with Xylenol Orange. Talanta 1961, 8, 203–208. [Google Scholar] [CrossRef]
- Smith, F.E.; Herbert, J.; Gaudin, J.; Hennessy, D.J.; Reid, G.R. Serum Iron Determination Using Ferene Triazine. Clin. Biochem. 1984, 17, 306–310. [Google Scholar] [CrossRef]
- Dahlén, J.; Karlsson, S. Determination of Iron(II) in Natural Waters by Capillary Zone Electrophoresis Using on-Capillary Complexation with 2,4,6-Tri(2′-Pyridyl)-1,3,5-Triazine. J. Chromatogr. A 1999, 848, 491–502. [Google Scholar] [CrossRef]
- Kennedy, J.A.; Powell, H.K.J. Colorimetric Determination of Aluminium(III) with Chrome Azurol S and the Reactivity of Hydrolysed Aluminium Species. Anal. Chim. Acta 1986, 184, 329–333. [Google Scholar] [CrossRef]
- Pakalns, P. Spectrophotometric Determination of Aluminium with Chrome Azurol s. Anal. Chim. Acta 1965, 32, 57–63. [Google Scholar] [CrossRef]
- Kim, D.; Jo, A.; Seo, B.-K.; Lee, K.-W.; Park, W.H.; Lee, T.S. Colorimetric Detection of Transition Metal Ions with Azopyridine-Based Probing Molecule in Aqueous Solution and in PMMA Film. Fibers Polym. 2013, 14, 1993–1998. [Google Scholar] [CrossRef]
- McCall, K.A.; Fierke, C.A. Colorimetric and Fluorimetric Assays to Quantitate Micromolar Concentrations of Transition Metals. Anal. Biochem. 2000, 284, 307–315. [Google Scholar] [CrossRef]
- Thermo Scientific Technical Note 10 Ic-Transition-Metals Tn72544. Available online: https://assets.thermofisher.com/TFS-Assets/CMD/Technical-Notes/tn-10-ic-transition-metals-tn72544-en.pdf (accessed on 20 January 2022).
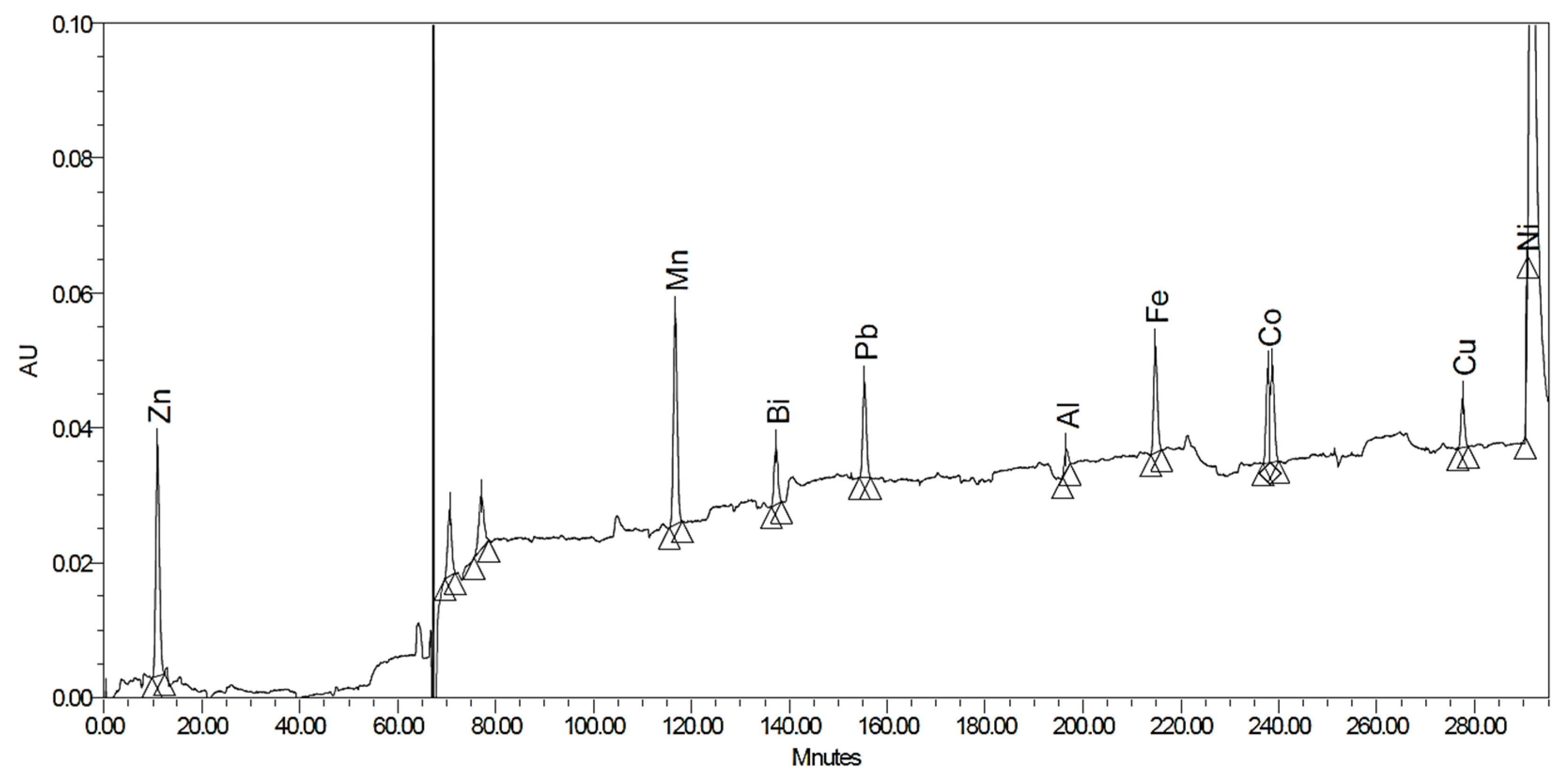
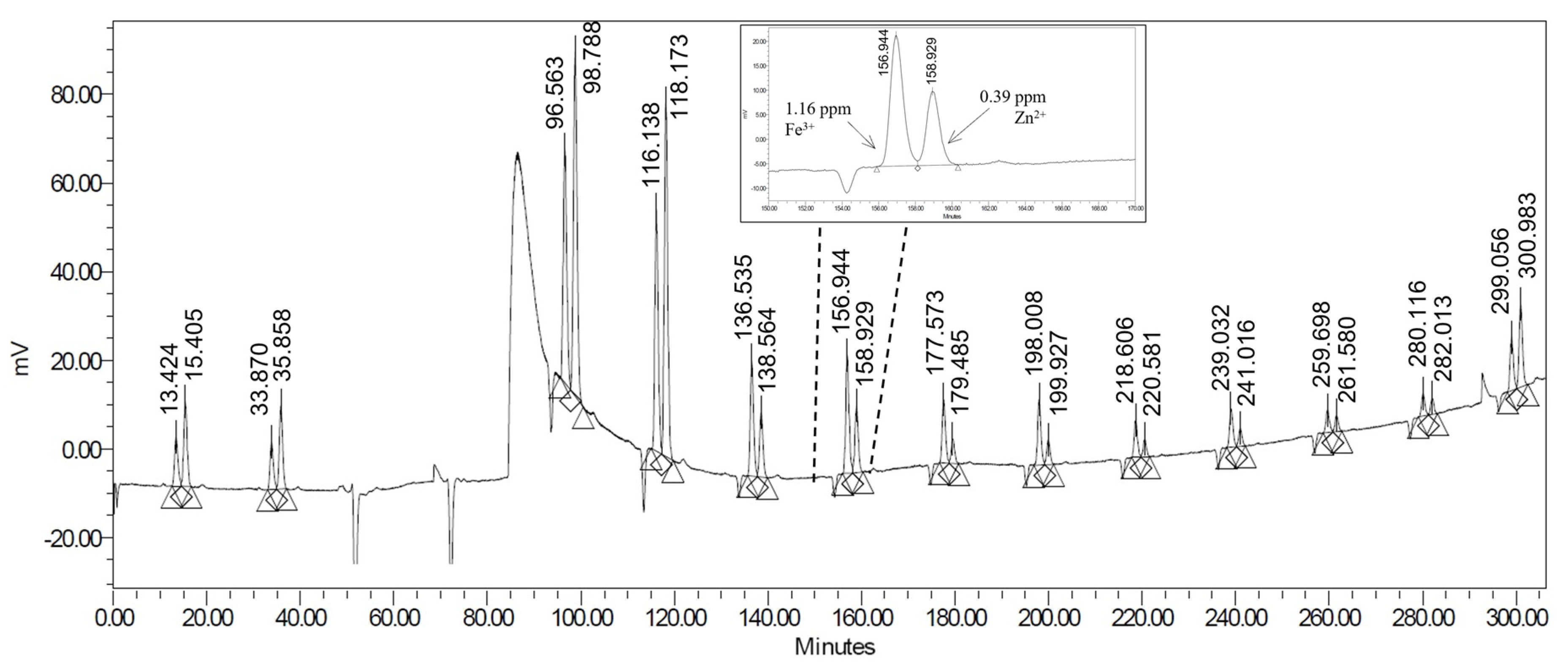
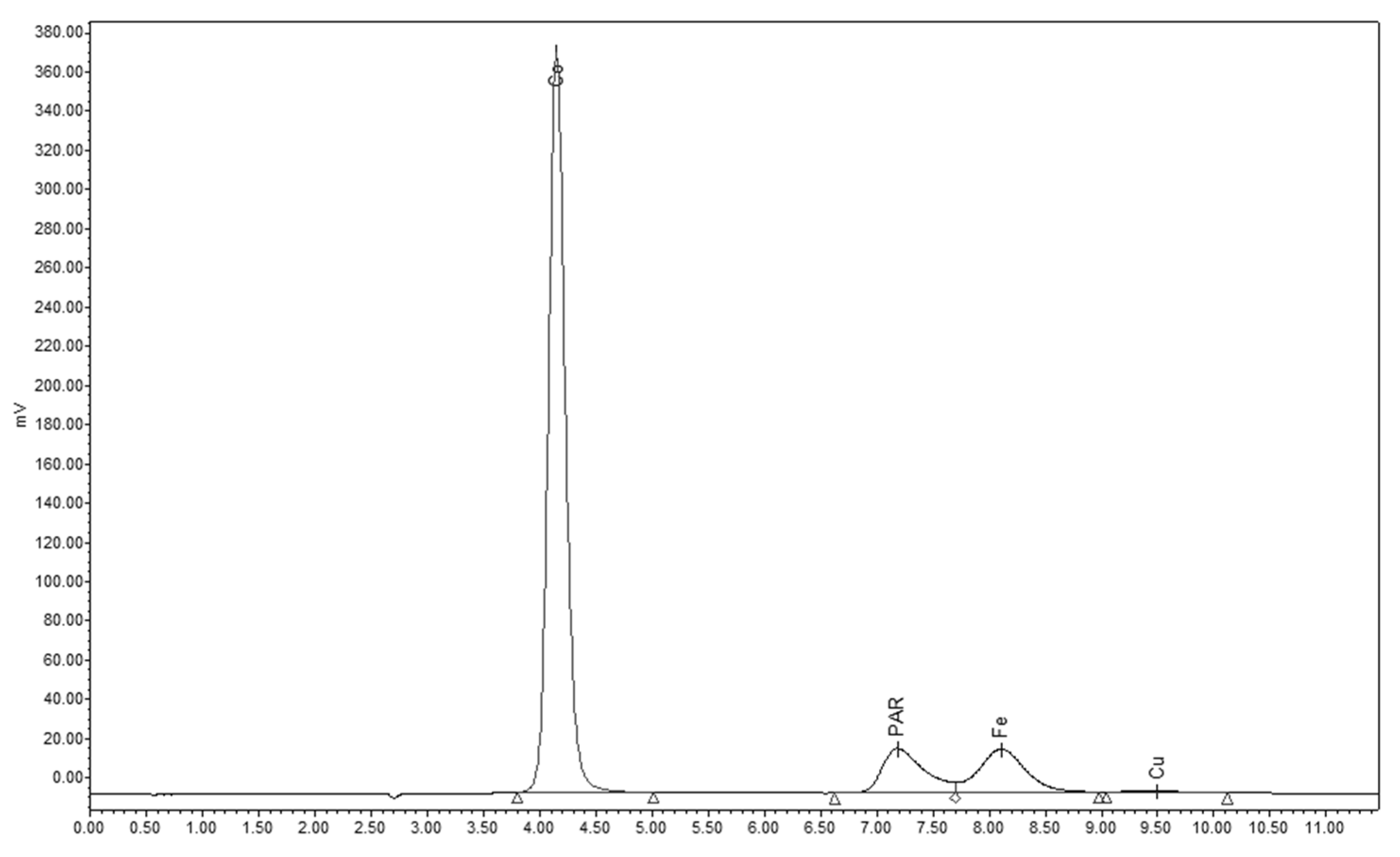

| Metal Ions | LOQ (ppm) | tR (min.) | c (ppm) | Rs |
|---|---|---|---|---|
| Bi3+ | 8.85 | 5.32 | 1.0 | - |
| Fe3+ | 0.26 | 6.56 | 0.5 | 0.89 |
| Zn2+ | 0.16 | 8.52 | 0.5 | 1.43 |
| Cu2+ | 0.13 | 8.87 | 0.3 | 0.26 |
| Ni2+ | 1.39 | 9.48 | 1.0 | 0.80 |
| Al3+ | 1.17 | 9.69 | 1.0 | 0.25 |
| Pb2+ | 0.31 | 9.75 | 1.0 | 0.04 |
| Co2+ | 0.13 | 9.96 | 1.0 | 0.15 |
| Cd2+ | 0.33 | 10.41 | 0.3 | 0.33 |
| Mn2+ | 0.10 | 17.57 | 1.0 | 5.17 |
| Metal | LOQ (ppm) | tR (min.) | c (ppm) | Rs |
|---|---|---|---|---|
| Co2+ | 0.004 | 4.14 | 4.91 | - |
| PAR | - | 7.19 | - | 6.54 |
| Fe3+ | 0.1 | 8.12 | 3.3 | 1.33 |
| Cu2+ | 22.33 | 9.50 | 52.96 | 1.78 |
| Samples | c(Fe3+) ppm | |
|---|---|---|
| ICP | HPLC Pre-Column Derivatization with PAR | |
| 07.02 | 0.39 | 0.41 |
| 07.01/2 | 0.41 | 0.30 |
| 07.01/1 | 0.39 | 0.24 |
| Reagent | Original Method 1 | Sample Preparation for HPLC Detection |
|---|---|---|
| PAR | - | Reagent: 6 mg PAR was dissolved in 3.5 mL methanol and 6.5 mL buffer 2. Sample preparation: 5 µL of PAR solution, 1465 µL of buffer 1 and 30 µL sample. |
| Xylenol orange | - | Reagent: 14.3 mg xylenol orange was dissolved in 10 mL buffer 1. Sample preparation: 10 µL of XO solution, 1460 µL of buffer 1 and 30 µL sample. |
| Zinc test | 5 mL of sample, 4 drops of reagent 1 (160 µL), 1 dosing spoon of reagent 2 (188 mg) and 1 microspoon of reagent 3 (10.3 mg). Leave to stand for exactly 5 min (reaction time). And add 4 drops of reagent 4 (160 µL). | 45 µL of reagent 1, 53 mg of reagent 2, 9 mg of reagent 3, 1380 µL of buffer 1, 30 µL sample, leave to stand for exactly 5 min (reaction time), and 45 µL of reagent 4. |
| Iron test | 20 mL of sample and 5 drops of reagent 1 (200 µL). Leave to stand for 3 min (reaction time). | 15 µL of reagent, 1455 µL of buffer 1 and 30 µL sample. Leave to stand for 3 min (reaction time). |
| Aluminum test | 5 mL of sample, 1 microspoon of reagent 1 (147.6 mg), 1.2 mL of reagent 2 and 4 drops of reagent 3 (160 µL). Leave to stand for 7 min (reaction time). | 34.8 mg of reagent 1, 282 µL of reagent 2, 1150 µL of buffer 1, 30 µL sample and 38 µL of reagent 3. Leave to stand for 7 min (reaction time). |
| Reagents | Methods | Metal Determined | Range (ppm) | LOQ (ppm) | Interfering Metal Ions |
|---|---|---|---|---|---|
| PAR | post-column | Fe3+ | 0.5–1.0 | 0.08 | - |
| pre-column | Fe3+ | 1.95–10.0 | 0.10 | - | |
| colorimetry | Fe3+ | 1.50–11.0 | 0.21 | Cu2+, Ni2+, Ga3+, Bi3+, Co2+, Cd2+ | |
| Triazine derivate (Merck) | colorimetry | Fe3+ | 1.50–13.0 | 0.62 | Co2+, Cr3+, Cu2+, Ni2+, Pb2+ |
| Xylenol Orange | colorimetry | Zn2+ | 1.50–20.0 | 0.61 | Cu2+, Ni2+, Fe3+, Co2+, Al3+ |
| Thiocyanate (Merck) | colorimetry | Zn2+ | 1.0–20.0 | 0.20 | Cu2+, Fe3+, Ni2+, Pb2+, |
| Chromazurol S (Merck) | colorimetry | Al3+ | - | 0.04 | Ag+, Co2+, Cr3+, Cu2+, Fe3+, Mn2+, Pb2+, Sn2+, Zn2+ |
| System | Column | Eluent | Flow Rate | Reagent | Detection |
|---|---|---|---|---|---|
| Post-column complexation | Dionex IonPac CS5A (2 × 250 mm) | Eluent A: 7.0 mM PDCA, 66 mM Potassium hydroxide, 74 mM Formic acid, 5.6 mM Potassium sulfate Eluent B: 0.5 mM PAR, 1.0 M 2-dimethylaminoethanol, 0.50 M ammonium hydroxide and 0.30 M sodium bicarbonate | A: 0.3 mL/min B: 0.15 mL/min | PAR | 530 nm |
| Pre-column complexation | LiChrospher 100 RP18 column (75 × 4 mm, 5 µm) | 65% 0.1 M; pH 6.5 NH4H2PO4/(NH4)2HPO4 buffer and 35% methanol | 0.8 mL/min | PAR | 530 nm |
| Colorimetry | - | water | 0.8 mL/min | PAR | 490 nm |
| XO | 570 nm | ||||
| Zinc test | 435 nm | ||||
| Iron test | 560 nm | ||||
| Aluminium test | 590 nm |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Forgács, V.; Fekete, A.; Gyuricza, B.; Szücs, D.; Trencsényi, G.; Szikra, D. Methods for the Determination of Transition Metal Impurities in Cyclotron-Produced Radiometals. Pharmaceuticals 2022, 15, 147. https://doi.org/10.3390/ph15020147
Forgács V, Fekete A, Gyuricza B, Szücs D, Trencsényi G, Szikra D. Methods for the Determination of Transition Metal Impurities in Cyclotron-Produced Radiometals. Pharmaceuticals. 2022; 15(2):147. https://doi.org/10.3390/ph15020147
Chicago/Turabian StyleForgács, Viktória, Anikó Fekete, Barbara Gyuricza, Dániel Szücs, György Trencsényi, and Dezső Szikra. 2022. "Methods for the Determination of Transition Metal Impurities in Cyclotron-Produced Radiometals" Pharmaceuticals 15, no. 2: 147. https://doi.org/10.3390/ph15020147
APA StyleForgács, V., Fekete, A., Gyuricza, B., Szücs, D., Trencsényi, G., & Szikra, D. (2022). Methods for the Determination of Transition Metal Impurities in Cyclotron-Produced Radiometals. Pharmaceuticals, 15(2), 147. https://doi.org/10.3390/ph15020147








