Anti-Alopecia Activity of Alkaloids Group from Noni Fruit against Dihydrotestosterone-Induced Male Rabbits and Its Molecular Mechanism: In Vivo and In Silico Studies
Abstract
1. Introduction
2. Results
2.1. Standardization of Noni Fruit Extract
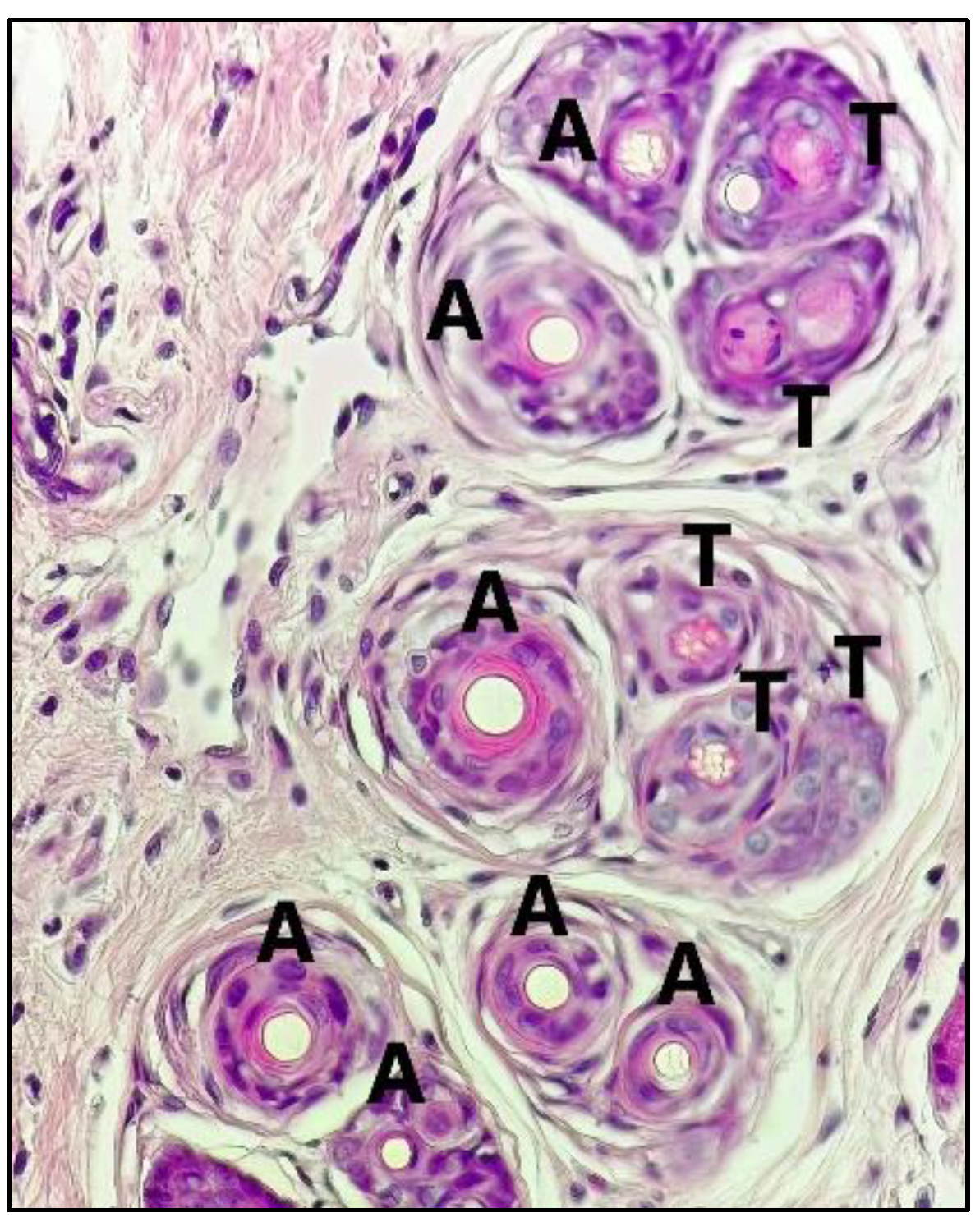
2.2. Anti-Alopecia Activity of Fraction and Sub-Fraction: In Vivo Studies
2.3. Area of Baldness in the Alopecia Rabbit Model
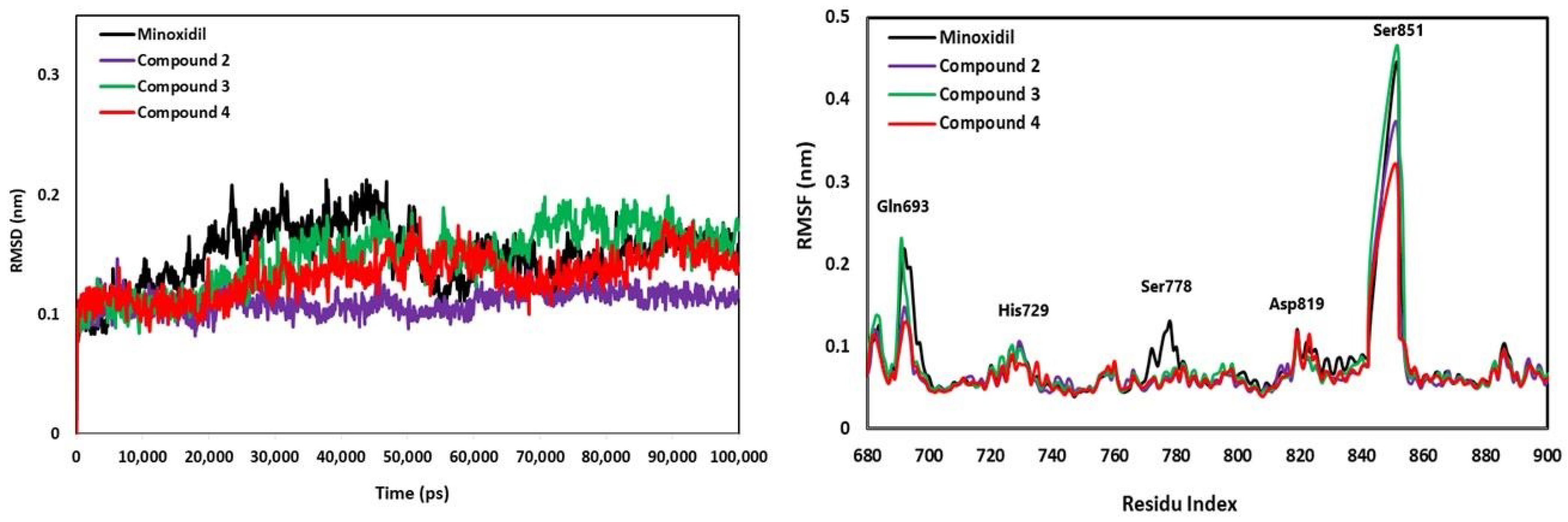
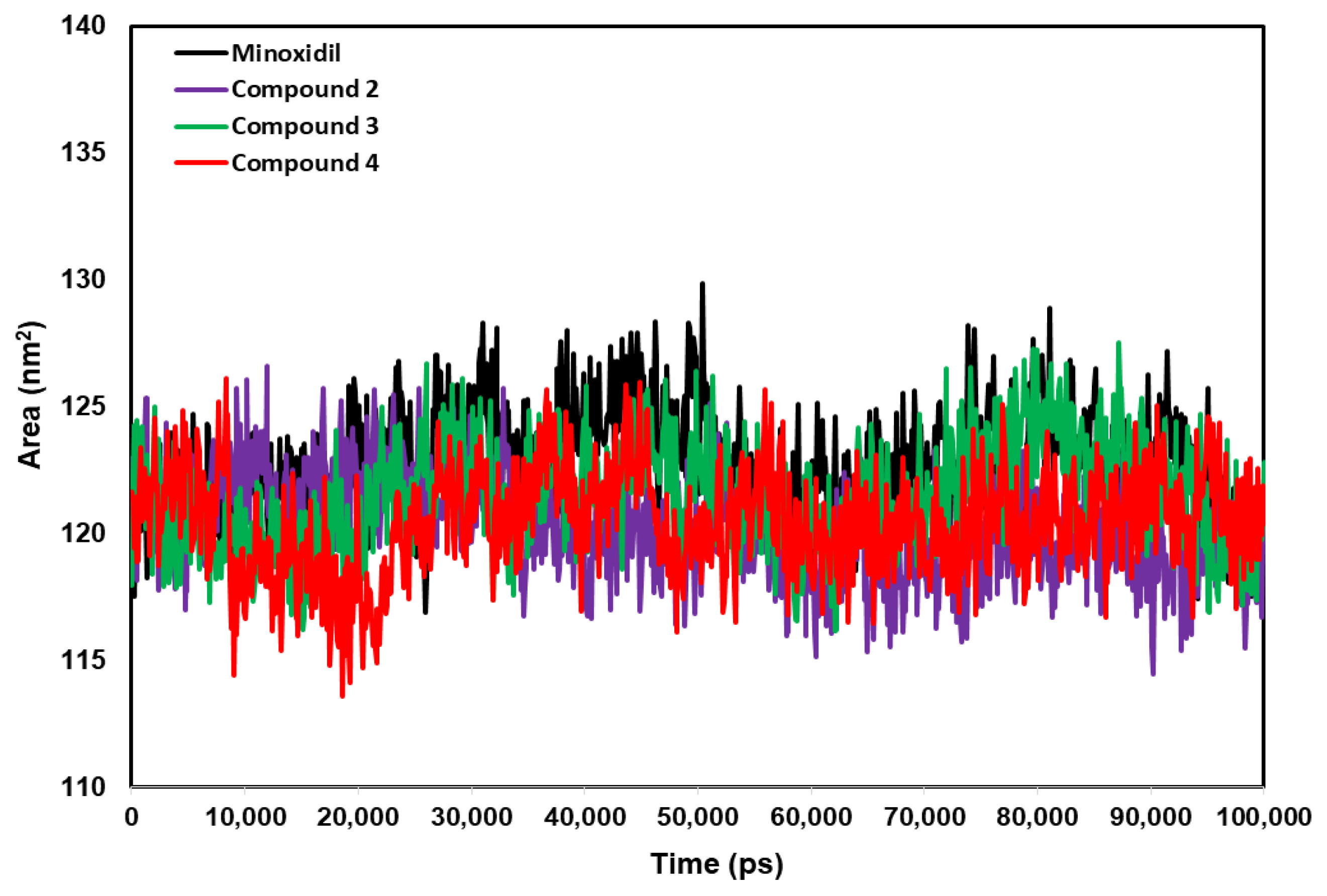
2.4. Anti-Alopecia Activity: In Silico Studies (Molecular Docking, Molecular Dynamics, ADME-Tox)
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Preparation and Standardization of Extract
4.3. Open-Column Chromatography (OPC)
4.4. Animal
4.5. Alopecia Rabbit Model
4.6. Anti-Alopecia Activity of Fraction and Sub-Fraction: In Vivo Studies
4.7. Histology Treatment
4.8. LC-MS/MS Analysis
4.9. Anti-Alopecia Activity: In Silico Studies (Molecular Docking Simulation)
4.10. Anti-Alopecia Activity: In Silico Studies (Molecular Dynamics Simulation)
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Guo, H.; Gao, W.V.; Endo, H.; McElwee, K.J. Experimental and early investigational drugs for androgenetic alopecia. Expert Opin. Investig. Drugs 2017, 26, 917–932. [Google Scholar] [CrossRef]
- Sinclair, R.D. Female pattern hair loss: A pilot study investigating combination therapy with low-dose oral minoxidil and spironolactone. Int. J. Dermatol. 2018, 57, 104–109. [Google Scholar] [CrossRef]
- Wolf, H.; Fischer, T.W.; Blume-Peytavi, U. The diagnosis and treatment of hair and scalp diseases. Dtsch. Arztebl. Int. 2016, 13, 377–386. [Google Scholar] [CrossRef]
- Škulj, Z.A.; Poljšak, N.; Glavač, N.K.; Kreft, S. Herbal preparations for the treatment of hair loss. Arch. Dermatol. Res. 2020, 312, 395–406. [Google Scholar] [CrossRef]
- McClatchey, W. From Polynesian healers to health food stores: Changing ethnopharmacology of Morinda citrifolia. Integr. Cancer Ther. 2002, 1, 110–120. [Google Scholar] [CrossRef]
- Potterat, O.; Hamburger, M. Morinda citrifolia (Noni) fruit-phytochemistry, pharmacology, safety. Planta Med. 2007, 73, 191–199. [Google Scholar] [CrossRef]
- Adriani, L.; Yeane, B. Dietary supplementation effects of noni (morinda citrifolia l.) fruit flour on uric acid and blood glucose of quails (coturnix coturnix japonica) layer phase. KnE Life Sci. 2017, 2, 80–85. [Google Scholar] [CrossRef]
- Guo, M.; Mao, B.; Sadiq, F.A.; Hao, Y.; Cui, S.; Yi, M.; Hong, Q.; Lee, Y.; Zhao, J. Effects of noni fruit and fermented noni juice against acute alcohol induced liver injury in mice. J. Funct. Foods. 2020, 70, 103995. [Google Scholar] [CrossRef]
- West, B.J.; Deng, S. Morinda citrifolia (Noni) Fruit Juice Inhibits SARS-CoV-2 Spike Protein Binding of Angiotensin-Converting Enzyme 2 (ACE2). J. Biosci. Med. 2015, 9, 42–51. [Google Scholar] [CrossRef]
- Meza-Gutiérrez, N.N.; Ulloa, J.A.; Rosas-Ulloa, P.; Balois-Morales, R.; López-Guzmán, G.G.; Berumen-Varela, G.; Escalera-Lara, A.A.; Casas-Junco, P.P.; Bautista-Rosales, P.U. Antibacterial Effect of Noni Juice In Vitro and Applied to Fresh-Cut Papaya to Control Escherichia coli O157: H7. J. Food Qual. 2022, 2022, 5543473. [Google Scholar] [CrossRef]
- Lolok, N.; Sahidin, S.; Sumiwi, S.A.; Muhtadi, A. Antidiabetes effect of Noni Fruit (Morinda citrifolia L.) on mice with Oral Glucose Tolerance Method and Streptozotocin Induction Method. Res. J. Pharm. Technol. 2021, 14, 5067–5071. [Google Scholar] [CrossRef]
- Lee, D.; Yu, J.S.; Huang, P.; Qader, M.; Manavalan, A.; Wu, X.; Kim, J.C.; Pang, C.; Cao, S.; Kang, K.S.; et al. Identification of Anti-Inflammatory Compounds from Hawaiian Noni (Morinda citrifolia L.) Fruit Juice. Molecules 2020, 25, 4968. [Google Scholar] [CrossRef] [PubMed]
- Yoshitomi, H.; Zhou, J.; Nishigaki, T.; Li, W.; Liu, T.; Wu, L.; Gao, M. Morinda citrifolia (Noni) fruit juice promotes vascular endothelium function in hypertension via glucagon-like peptide-1 receptor-CaMKKβ-AMPK-eNOS pathway. Phytother. Res. 2020, 34, 2341–2350. [Google Scholar] [CrossRef]
- Pandiselvi, P.; Manohar, M.; Thaila, M.; Sudha, A. Pharmacological Benefits of Natural Product, 1st ed.; JPS Scientific Publication: Tamil Nadu, India, 2019; Chapter 13; p. 221. ISBN 978-81-934054-2-0. [Google Scholar]
- Ambarwati; Sujono, T.A.; Sintowati, R. Uji Penghambatan Ekstrak Buah Mengkudu (Morinda citrifolia L.) Terhadap Isolat Jamur Penyebab Ketombe; Proceeding Biology Education Conference; Universitas Sebelas Maret: Surakarta, Indonesia, 2015. [Google Scholar]
- Susanti, T. Pemanfaatan Buah Mengkudu (Morinda citrifolia L.) Terhadap Penyembuhan Ketombe Kering; Universitas Negeri Padang: Padang, Indonesia, 2013. [Google Scholar]
- Mustarichie, R.; Ramdhani, D.; Iskandar, Y. Characteristics And Alopecia Activity Of Pakis Gajah (Angiopteris Evecta (G.Forst) Hoffm.) Growing In Galunggung Mountainside, West Java. Asian, J. Pharm. Clin. Res. 2017, 10, 337–340. [Google Scholar] [CrossRef]
- Dahmani, M.M.; Laoufi, R.; Selama, O.; Arab, K. Gas chromatography coupled to mass spectrometry characterization, anti-inflammatory effect, wound-healing potential, and hair growth-promoting activity of Algerian Carthamus caeruleus L. (Asteraceae). Indian J. Pharmacol. 2018, 50, 123–129. [Google Scholar] [CrossRef]
- Li, Y.; Mingnuan, H.; Pei, L.; Yanran, H.; Jie, Y.; Ronghua, Z. Hair Growth Promotion Activity and Its Mechanism of Polygonum multiflorum. Evid. Based Complement. Altern. Med. 2015, 2015, 517901. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.C.; Kang, J.I.; Kim, M.K.; Hyun, J.H.; Boo, H.J.; Park, D.B.; Lee, Y.J.; Yoo, E.S.; Kim, Y.H.; Kim, Y.H.; et al. Promotion effect of norgalanthamine, a component of Crinum asiaticum on hair growth. Eur. J. Dermatol. 2010, 20, 42–48. [Google Scholar] [CrossRef]
- Yoon, H.S.; Kang, J.I.; Kim, S.M.; Ko, A.; Koh, Y.S.; Hyun, J.W.; Yoon, S.P.; Ahn, M.J.; Kim, Y.H.; Kang, J.H.; et al. Norgalanthamine Stimulates Proliferation of Dermal Papilla Cells via Anagen-Activating Signaling Pathways. Biol. Pharm. Bull. 2019, 42, 139–143. [Google Scholar] [CrossRef] [PubMed]
- Cai, K.; Dou, R.; Lin, X.; Hu, X.; Wang, Z.; Liu, S.; Li, C.; Li, W. Changes in Phenolic Profiles and Inhibition Potential of Macrophage Foam Cell Formation during Noni (Morinda citrifolia Linn.) Fruit Juice Fermentation. Fermentation 2022, 8, 201. [Google Scholar] [CrossRef]
- Lin, S.Y.; Liao, Y.Y.; Roan, S.F.; Chen, I.Z.; Chen, P.A. Growth of Noni fruits (Morinda citrifolia L.) and accumulation of phenolic compounds during fruit development. Sci. Hortic. 2014, 178, 168–174. [Google Scholar] [CrossRef]
- Bussman, R.W.; Hennig, L.; Giannis, A.; Ortwein, J.; Kutchan, T.M.; Feng, X. Anthraquinone Content in Noni (Morinda citrifolia L.). Evid. Based Complement. Altern. Med. 2013, 2013, 208378. [Google Scholar] [CrossRef]
- Sanni, M.D.; Fatoki, T.H.; Kolawole, A.O.; Akinmoladun, A.C. Xeronine structure and function: Computational comparative mastery of its mystery. In Silico Pharmacol. 2017, 5, 8. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Liu, B.; Han, L.; Tang, X.; Deng, W.; Lai, W.; Wan, M. Dihydrotestosterone Regulates Hair Growth Through the Wnt/β-Catenin Pathway in C57BL/6 Mice and In Vitro Organ Culture. Front. Pharmacol. 2020, 10, 1528. [Google Scholar] [CrossRef] [PubMed]
- Matias, J.R.; Malloy, V.; Orentreich, N. Animal Models of Androgen-Dependent Disorders Of The pilosebaceous apparatus. The androchronogenetic alopecia (AGA) mouse as a model for male-pattern Baldness. Arch. Dermatol. Res. 1989, 281, 247–253. [Google Scholar] [CrossRef]
- Noubarani, M.; Rostamkhani, H.; Erfan, M.; Kamalinejad, M.; Eskandaria, M.R.; Babaeian, M.; Salamzadeh, J. Effect of Adiantum Capillus veneris Linn on an Animal Model of Testosterone-Induced Hair Loss. Iran. J. Pharm. Res. 2014, 13, 113–118. [Google Scholar] [PubMed]
- Patel, S.; Sharma, V.; Chauhan, N.S.; Thakur, M.; Dixit, V.K. A comparative in vivo and in vitro evaluation of hair growth potential of extracts and an isolate from petroleum ether extract of Cuscuta reflexa Roxb. Beni-Seuf Univ. J. Appl. Sci. 2014, 3, 165–171. [Google Scholar] [CrossRef]
- Manglik, A.; Lin, H.; Aryal, D.K.; McCorvy, J.D.; Dengler, D.; Corder, G.; Levit, A.; Kling, R.C.; Bernat, V.; Hübner, H.; et al. Structure-based discovery of opioid analgesics with reduced side effects. Nature 2016, 537, 185–190. [Google Scholar] [CrossRef] [PubMed]
- Jin, Z.; Du, X.; Xu, Y.; Deng, Y.; Liu, M.; Zhao, Y.; Zhang, B.; Li, X.; Zhang, L.; Peng, C.; et al. Structure of Mpro from SARS-CoV-2 and discovery of its inhibitors. Nature 2020, 582, 289–293. [Google Scholar] [CrossRef] [PubMed]
- Hollingsworth, S.A.; Dror, R.O. Molecular Dynamics Simulation for All. Neuron 2018, 99, 1129–1143. [Google Scholar] [CrossRef]
- Hospital, A.; Goñi, J.R.; Orozco, M.; Gelpi, J. Molecular dynamics simulations: Advances and applications. Adv. Appl. Bioinform. Chem. 2015, 8, 37–47. [Google Scholar]
- Adelusi, T.I.; Oyedele, A.K.; Boyenle, I.D.; Ogunlana, A.T.; Adeyemi, R.O.; Ukachi, C.D.; Idris, M.O.; Olaoba, O.T.; Adedotun, I.O.; Kolawole, O.E.; et al. Molecular modeling in drug discovery. Inform. Med. Unlocked. 2022, 29, 100880. [Google Scholar] [CrossRef]
- Wu, F.; Zhou, Y.; Li, L.; Shen, X.; Chen, G.; Wang, X.; Liang, X.; Tan, M.; Huang, Z. Computational Approaches in Preclinical Studies on Drug Discovery and Development. Front. Chem. 2020, 8, 726. [Google Scholar] [CrossRef]
- Kementerian Kesehatan Republik Indonesia. Farmakope Herbal Indonesia, 2nd ed.; Kementrian Kesehatan RI: Jakarta, Indonesia, 2017; pp. 313–315. [Google Scholar]
- Wang, N.N.; Huang, C.; Dong, J.; Yao, Z.J.; Zhu, M.F.; Deng, Z.K.; Lv, B.; Lu, A.P.; Chen, A.F.; Cao, D.S. A Predicting human intestinal absorption with modified random forest approach: A comprehensive evaluation of molecular representation, unbalanced data, and applicability domain issues. RSC Adv. 2017, 7, 19007–19018. [Google Scholar] [CrossRef]
- Chen, C.P.; Chen, C.C.; Huang, C.W.; Chang, Y.C. Evaluating Molecular Properties Involved in Transport of Small Molecules in Stratum Corneum: A Quantitative Structure-Activity Relationship for Skin Permeability. Molecules 2018, 23, 911. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Sharma, A.; Alexui, A.; Bilgrami, A.L.; Kamal, M.A.; Ashraf, G.M. DeePred-BBB: A Blood Brain Barrier Permeability Prediction Model With Improved Accuracy. Front. Neurosci. 2022, 16, 858126. [Google Scholar] [CrossRef]
- Liu, X.; Wright, M.; Hop, C.E.A. Rational Use of Plasma Protein and Tissue Binding Data in Drug Design. J. Med. Chem. 2014, 57, 8238–8248. [Google Scholar] [CrossRef]
- Sadino, A.; Muhtadi, A.; Susilawati, Y.; Charisma, S.L. Hypoglycemic Activity of Ethyl Acetate Fractions of Mengkudu Fruit (Morinda citrifolia L.) Mice Induced Alloxan. Res. J. Pharm. Technol. 2019, 12, 197–201. [Google Scholar] [CrossRef]
- Musfiroh, I.; Yusuf, N.; Moektiwardoyo, M. Hyploglycemic Activity of Ethyl Acetate Fraction Combination of Morinda Fruit and Cinnamon Bark Using Glucose-Induced in Mice. Int. J. App. Pharm. 2021, 13, 162–166. [Google Scholar] [CrossRef]
- Susanti, L.; Mustarichie, R.; Halimah, E.; Kurnia, D. Ethyl Acetate Fraction in Ethanol Extract of Noni Fruit Modified by Zeolite as Anti-seborrheic Dermatitis: In-vitro and In-silico Studies. Rasayan J. Chem. 2022, 15, 269–279. [Google Scholar] [CrossRef]
- Pandy, V.; Narasingam, M.; Vijeepallam, K.; Mohan, S.; Mani, V.; Mohamed, Z. The ethyl acetate fraction of a methanolic extract of unripe Noni (Morinda citrifolia Linn.) fruit exhibits a biphasic effect on the dopaminergic system in mice. Exp. Anim. 2017, 66, 283–291. [Google Scholar] [CrossRef]
- Kaliyadan, F.; Nambiar, A.; Vijayaraghavan, S. Update Androgenetic alopecia: An update. Indian J. Dermatol. Venerol. Leprol. 2013, 79, 613–625. [Google Scholar] [CrossRef] [PubMed]
- Sperling, C.L.; Cowper, S.E.; Knoop, E.A. An Atlas of Hair Pathology with Clinical Correlation, 2nd ed.; Informa Healthcare: London, UK, 2012; pp. 42–52. [Google Scholar]
- Patel, S.; Vikas, S.; Chauhan, N.S.; Thakur, M.; Dixit, V.K. Evaluation of Hair Growth promoting activity of Phyllanthus ninuri. Avicenna J. Phitomed. 2014, 5, 512–519. [Google Scholar]
- Kasmawati, H.; Mustarichie, R.; Halimah, E.; Ruslin. Evaluation of Hair Growth Promoting Activity of Sansevieria Trifasciata P. on Alopecia Androgenic Rabbit Male. Int. J. Pharm. Res. 2021, 13, 3703–3709. [Google Scholar]
- Salentin, S.; Haupt, V.J.; Daminelli, S.; Schroeder, M. Polypharmacology rescored: Protein– ligand interaction profiles for remote binding site similarity assessment. Prog. Biophys. Mol. Biol. 2014, 116, 174–186. [Google Scholar] [CrossRef] [PubMed]
- Ross, G.A.; Morris, G.M.; Biggin, P.C. Rapid and accurate prediction and scoring of water molecules in protein binding sites. PLoS ONE 2012, 7, e32036. [Google Scholar] [CrossRef] [PubMed]
- Brown, T.E.; LeMay, H.E.; Busten, B.E.; Murphy, C.J.; Woodward, P.M.; Stoltzfus, M.E. Chemistry—The Central Science, 13th ed.; Pearson Prentice Hall: Hoboken, NJ, USA, 2015; Chapter 6.1.1–6.1.5. [Google Scholar]
- Silverstein, T.P. The hydrophobic efect: Is water afraid, or just not that interested? ChemTexts 2020, 6, 1–26. [Google Scholar] [CrossRef]
- Kelutur, F.J.; Mustarichie, R. Molecular Docking of The Potential Compound from Cocoa Shells (Theobroma cacao L.) Against Androgen Receptor as Anti-Alopecia. J. Glob. Pharma Technol. 2020, 12, 52–60. [Google Scholar]
- Abdurrahman, S.; Ruslin; Hasanah, A.N.; Mustarichie, R. Molecular docking studies and ADME-Tox prediction of phytocompounds from Merremia peltata as a potential anti-alopecia treatment. J. Adv. Pharm. Technol. Res. 2021, 12, 132–139. [Google Scholar]
- Pandey, A.; Tripathi, S. Concept of Standardization, extraction and pre phytochemical screening strategies for herbal drug. J. Pharmacogn. Phytochem. 2014, 2, 115–119. [Google Scholar]
- Bandiola, T.M.B. Extraction and Qualitative Phytochemical Screening of Medical Plants: A Brief Summary. Int. J. Pharm. 2018, 8, 137–143. [Google Scholar]
- Forli, S.; Olson, A.J. A force field with discrete displaceable waters and desolvations entropy for hydrated ligand docking. J. Med. Chem. 2012, 55, 623–638. [Google Scholar] [CrossRef] [PubMed]
- Cosconati, S.; Forli, S.; Perryman, A.L.; Harris, R.; Goodsell, D.S.; Olson, A.J. Virtual screening with AutoDock: Theory and practice. Expert Opin. Drug Discov. 2010, 5, 597–607. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, D.; Caballero, J. Is It Reliable to Take the Molecular Docking Top Scoring Position as the Best Solution without Considering Available Structural Data? Molecules 2018, 22, 1038. [Google Scholar] [CrossRef] [PubMed]
- Park, S.J.; Lee, J.; Qi, Y.; Kern, N.R.; Lee, H.S.; Jo, S.; Joung, I.; Joo, K.; Lee, J.; Im, W. CHARMM-GUI Glycan Modeler for modeling and simulation of carbohydrates and glycoconjugates. Glycobiology 2019, 29, 320–331. [Google Scholar] [CrossRef]
- Lee, J.; Cheng, X.; Swails, J.M.; Yeom, M.S.; Eastman, P.K.; Lemkul, J.A.; Wei, S.; Buckner, J.; Jeong, J.C.; Qi, Y.; et al. CHARMM-GUI Input Generator for NAMD, GROMACS, AMBER, OpenMM, and CHARMM/OpenMM Simulations Using the CHARMM36 Additive Force Field. J. Chem Theory Comput. 2016, 12, 405–413. [Google Scholar] [CrossRef]
- Huang, J.; Rauscher, S.; Nawrocki, G.; Ran, T.; Feig, M.; de Groot, B.L.; Grubmüller, H.; MacKerell, A.D., Jr. CHARMM36m: An Improved Force Field for Folded and Intrinsically Disordered Proteins. Nat. Methods 2017, 14, 1–13. [Google Scholar] [CrossRef]
- Abraham, M.J.; Murtola, T.; Schulz, R.; Páll, S.; Smith, J.C.; Hess, B.; Lindahl, E. GROMACS: High Performance Molecular Simulations through Multi-Level Parallelism from Laptops to Supercomputers. SoftwareX 2015, 1–2, 19–25. [Google Scholar] [CrossRef]


| Specific and Non-Specific Parameters | Results | Standard [36] |
|---|---|---|
| Shapes | Viscous extract | Viscous extract |
| Color | Dark brown | Dark brown |
| Odor | Distinctive odor | Distinctive odor |
| Taste | Slightly bitter | Bitter |
| Yield (%) | 14.65 | >10 |
| Water content (%) | 8376 | ≤10 |
| Total ash content (%) | 0.7 | ≤0.8 |
| Acid insoluble ash content (%) | 0.08 | ≤0.1 |
| Phytochemical Screening | Test | FW | FEA | FH |
|---|---|---|---|---|
| Alkaloid | Dragendorf | + | + | - |
| Mayer | + | + | - | |
| Wagner | + | + | - | |
| Flavonoid | Shinoda | + | + | - |
| Phytosterols | Liebermann–Burchard | - | - | + |
| Anthraquinones | Brontager | - | - | + |
| Saponins | Foam | + | + | - |
| Tannins | Ferric Chloride | + | + | - |
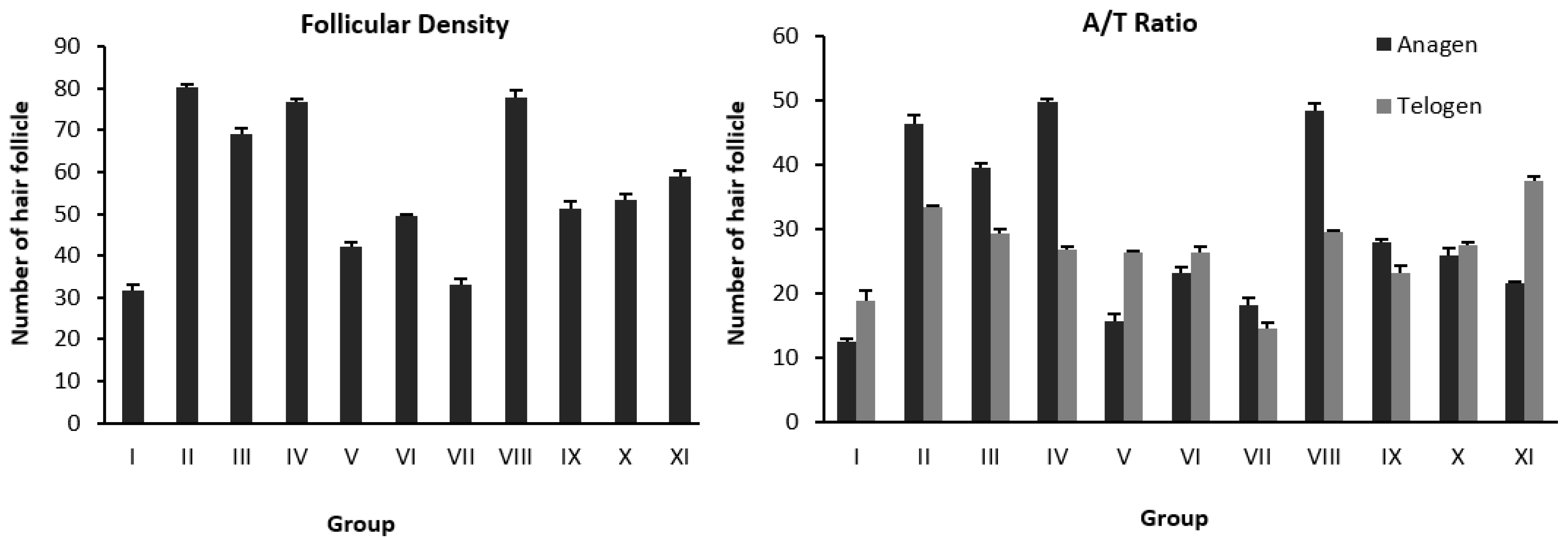
| Stage | Group | Treatment | Hair Follicular Density | Total of Hair Follicular Density | A/T Ratio | |
|---|---|---|---|---|---|---|
| Anagen | Telogen | |||||
| Fraction | I | DHT (s.c.)+vehicle (NaCMC 1%) | 12.55 ± 0.38 | 19.00 ± 1.53 | 31.55 ± 1.64 * | (1/1.50) |
| II | DHT (s.c.)+Minoxidil | 46.56 ± 1.17 | 33.56 ± 0.19 | 80.12 ± 1.02 ** | (1.39/1) | |
| III | DHT (s.c.)+FW 25% | 39.67 ± 0.67 | 29.45 ± 0.69 | 69.12 ± 1.35 ** | (1.35/1) | |
| IV | DHT (s.c.)+FEA 25% | 49.78 ± 0.51 | 27.00 ± 0.33 | 76.78 ± 0.84 ** | (1.84/1) | |
| V | DHT (s.c.)+FH 25% | 16.78 ± 0.88 | 25.33 ± 0.33 | 42.11 ± 1.07 * | (1/1.68) | |
| Sub-fraction | VI | DHT (s.c.)+FEA-1 25% | 23.33 ± 1.07 | 26.33 ± 1.00 | 49.66 ± 0.33 * | (1.13/1) |
| VII | DHT (s.c.)+FEA-2 25% | 18.22 ± 1.07 | 14.67 ± 0.88 | 32.89 ± 1.71 * | (1.24/1) | |
| VIII | DHT (s.c.)+FEA-3 25% | 48.44 ± 1.17 | 29.55 ± 0.38 | 78.00 ± 1.52 ** | (1.64/1) | |
| IX | DHT (s.c.)+FEA-4 25% | 28.00 ± 0.58 | 23.22 ± 1.26 | 51.22 ± 1.68 * | (1.21/1) | |
| X | DHT (s.c.)+FEA-5 25% | 25.89 ± 1.17 | 27.56 ± 0.51 | 53.45 ± 1.39 * | (1/1.06) | |
| XI | DHT (s.c.)+FEA-6 25% | 21.55 ± 0.38 | 37.55 ± 0.69 | 59.11 ± 1.07 * | (1/1.74) | |
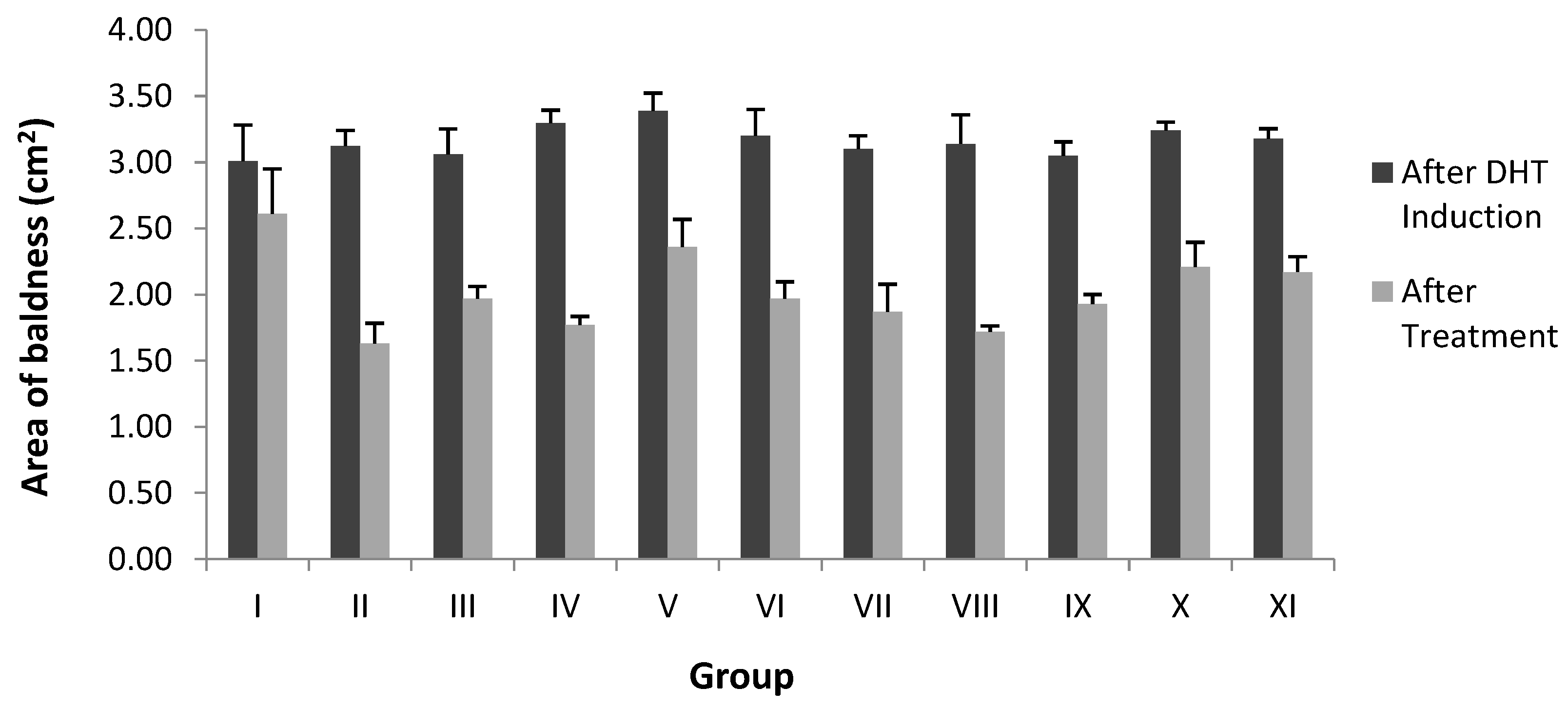
| Stage | Group | Area of Baldness (cm2) | |
|---|---|---|---|
| After DHT Induction | After Treatment of Sample | ||
| Fraction | I | 3.01 ± 0.23 | 2.61 ± 0.34 * |
| II | 3.12 ± 0.11 | 1.63 ± 0.15 ** | |
| III | 3.06 ± 0.19 | 1.97 ± 0.09 ** | |
| IV | 3.29 ± 0.09 | 1.77 ± 0.06 ** | |
| V | 3.38 ± 0.14 | 2.36 ± 0.20 ** | |
| Average | 3.18 ± 0.16 | 2.07 ± 0.17 | |
| Sub-fraction | VI | 3.20 ± 0.11 | 1.97 ± 0.12 ** |
| VII | 3.04 ± 0.09 | 1.87 ± 0.21 ** | |
| VIII | 3.10 ± 0.06 | 1.72 ± 0.04 ** | |
| IX | 3.05 ± 0.10 | 1.93 ± 0.07 ** | |
| X | 3.23 ± 0.05 | 2.21 ± 0.18 ** | |
| XI | 3.18 ± 0.07 | 2.17 ± 0.11 ** | |
| Average | 3.15 ± 0.12 | 1.97 ± 0.12 | |
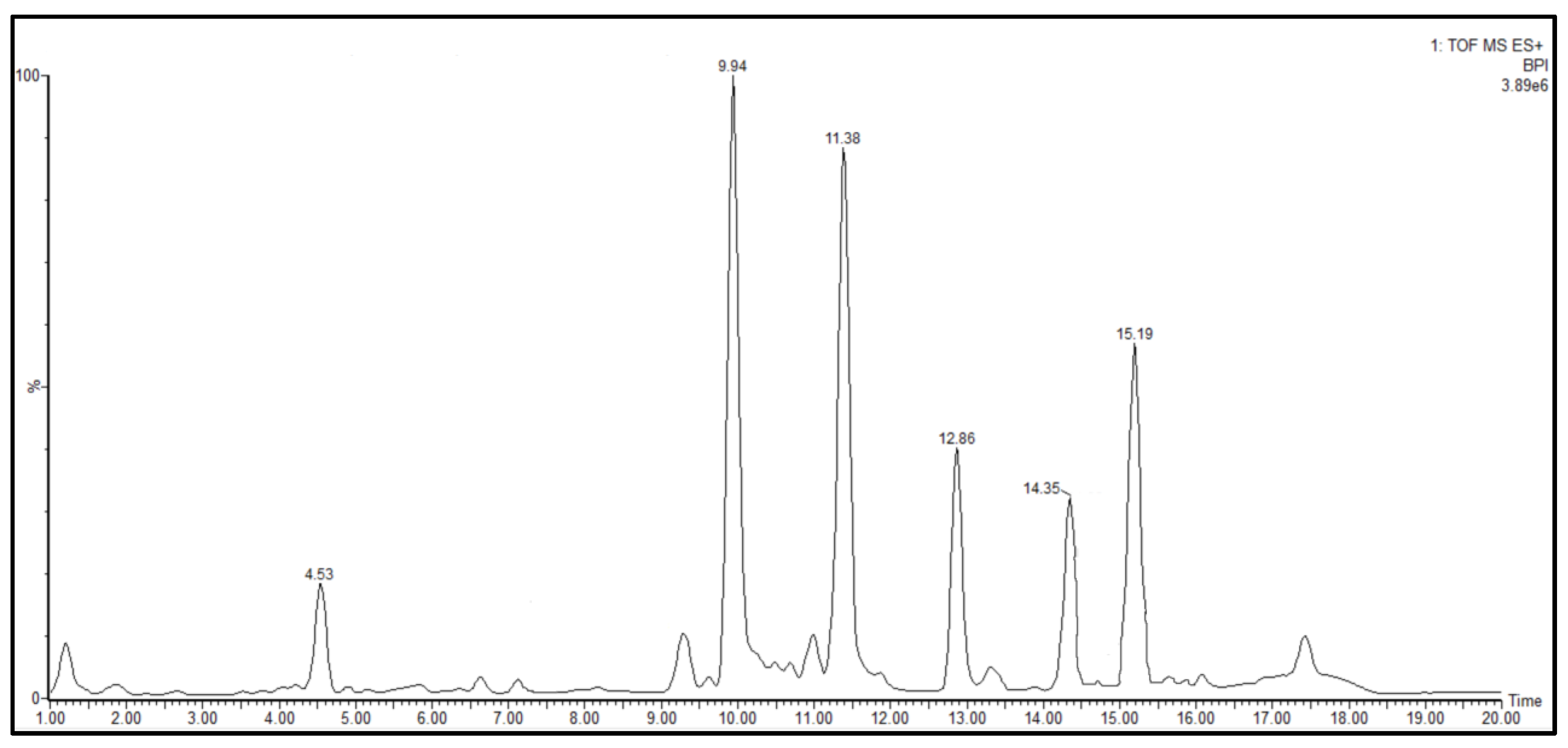
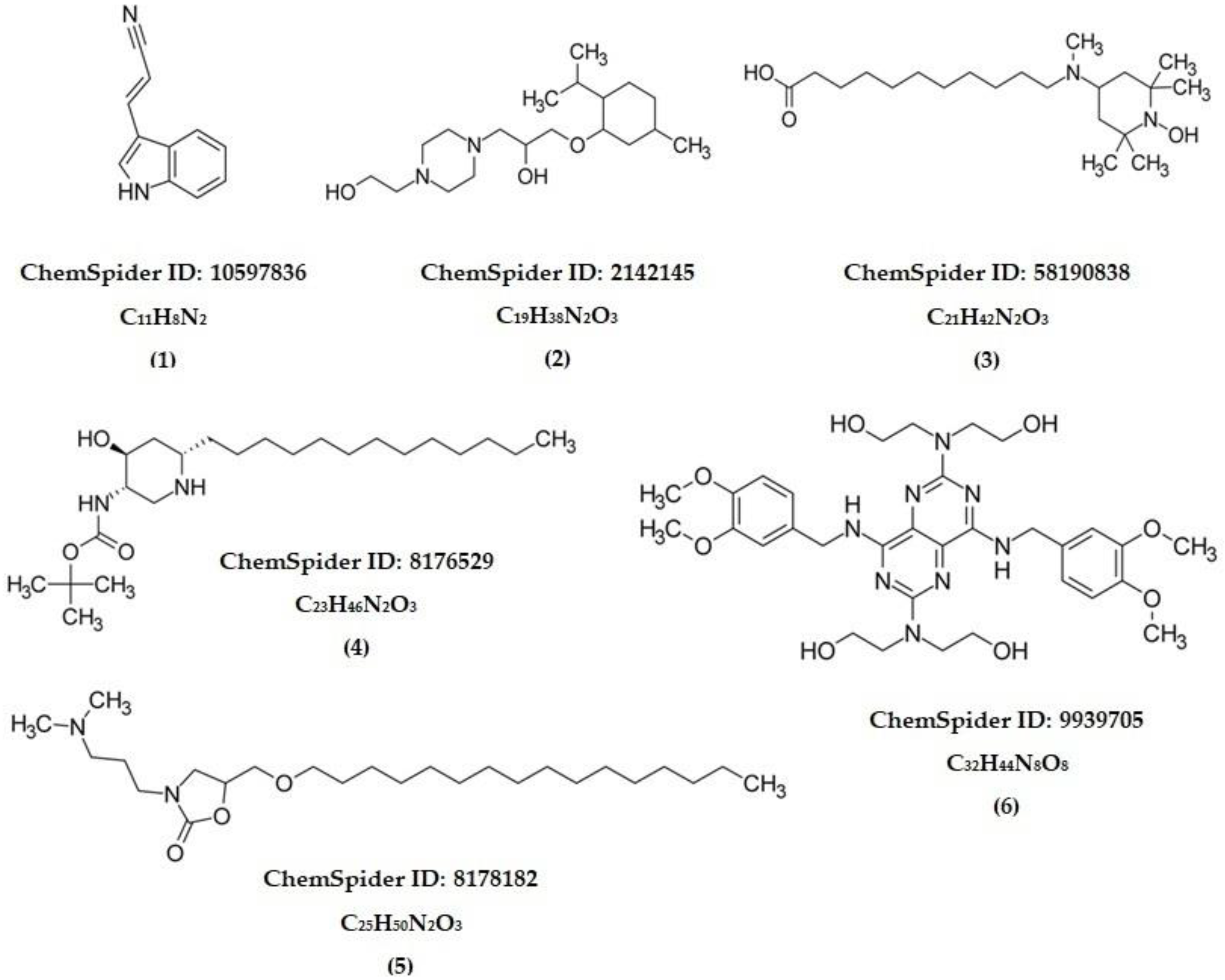
| Compound | RT (Min) | Formula | Observed m/z | Abundance |
|---|---|---|---|---|
| 1 | 4.53 | C11H8N2 | 169.0765 | + |
| 2 | 9.94 | C19H38N2O3 | 343.2945 | +++ |
| 3 | 11.38 | C21H42N2O3 | 371.3281 | +++ |
| 4 | 12.86 | C23H46N2O3 | 399.3608 | ++ |
| 5 | 14.35 | C25H50N2O3 | 427.3687 | ++ |
| 6 | 15.19 | C32H44N8O8 | 669.3326 | ++ |
| Compound | Molecule | Alkaloid Skeleton |
|---|---|---|
| 1 | (E)-3-(1H-Indol-3-yl)acrylonitrile | Indole |
| 2 | 1-[4-(2-Hydroxyethyl)-1-piperazinyl]-3-[(2-isopropyl-5-methylcyclohexyl)oxy]-2-propanol | Piperazine |
| 3 | 11-[(1-Hydroxy-2,2,6,6-tetramethyl-4-piperidinyl)(methyl)amino]undecanoic acid | Piperidine |
| 4 | 2-Methyl-2-propanyl [(3S,4S,6S)-4-hydroxy-6-tridecyl-3-piperidinyl]carbamate | Piperidine |
| 5 | 3-[3-(Dimethylamino)propyl]-5-[(hexadecyloxy)methyl]-1,3-oxazolidin-2-one | Oxazolidine |
| 6 | 2,2′,2″,2‴-({4,8-Bis[(3,4-dimethoxybenzyl)amino]pyrimido[5,4-d]pyrimidine-2,6-diyl}dinitrilo)tetraethanol | Pyrimidine |
| Standard | 3-hydroxy-2-imino-6-piperidin-1-ylpyrimidin-4-amine (Minoxidil) | Piperidine–pyrimidine |
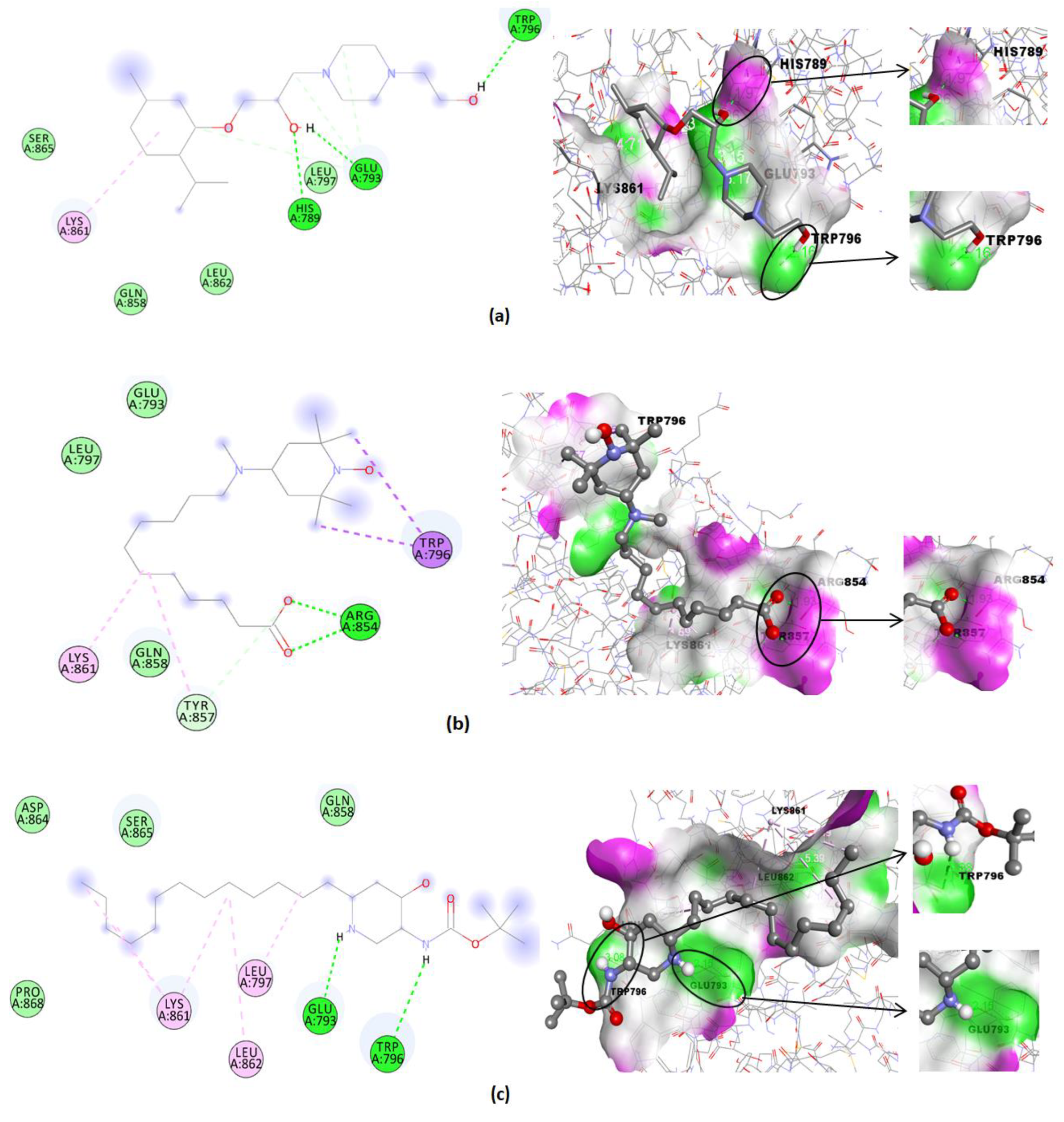
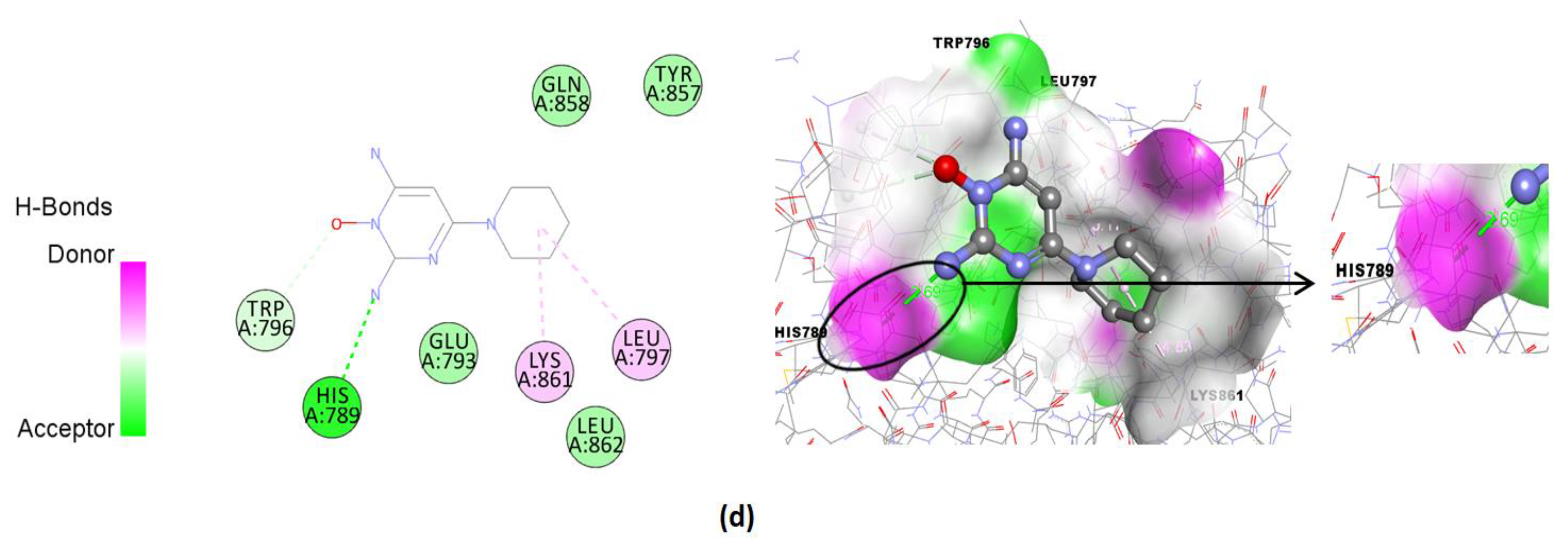
| Compound | Binding Affinity (Kcal/mol) | Hydrogen Bonds | Hydrogen Bonds Distance (Å) | Nearest Amino Acid Residues |
|---|---|---|---|---|
| 1 | −3.45 | - | - | Glu793-Gln858-Tyr857-Lys861-Leu862-Leu797 |
| 2 | −4.99 | His789-Glu793-Trp796 | 1.97; 1.86; 2.16 | Ser865-Gln858-Leu862-Leu797-Lys861 |
| 3 | −4.60 | Arg854(2) | 1.93; 1.74 | Gln858-Lys861-Tyr857-Glu793-Leu797-Trp796 |
| 4 | −4.57 | Glu793-Trp796 | 2.15; 3.08 | Asp864-Ser865-Pro868-Gln858-Lys861-Leu797-Leu862 |
| 5 | −2.76 | His789-Lys861 | 2.94; 2.27 | Glu793-Gln858-Ser865-Asp864 |
| 6 | −3.39 | Leu862-Gln858 | 3.07; 2.27 | Lys861-Glu793-Leu797-Tyr857-Arg854-Trp796 |
| Minoxidil (standard) | −4.83 | His789 | 2.69 | Gln858-Glu797-Tyr857-Leu862-Trp796-Lys861-Leu797 |
| Compound | Absorption | Distribution | Mutagenic | Carcinogenic | ||
|---|---|---|---|---|---|---|
| HIA (%) | SP (Log KP) | BBB (Log BB) | PPB (%) | |||
| 2 | 91.84 | −2.77 | 1.40 | 43.48 | yes | no |
| 3 | 95.62 | −1.255 | 1.53 | 93.21 | no | no |
| 4 | 89.07 | −0.851 | 6.87 | 95.13 | no | no |
| Minoxidil | 82.11 | −3.930 | 0.26 | 58.38 | yes | no |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Susanti, L.; Mustarichie, R.; Halimah, E.; Kurnia, D.; Setiawan, A.; Maladan, Y. Anti-Alopecia Activity of Alkaloids Group from Noni Fruit against Dihydrotestosterone-Induced Male Rabbits and Its Molecular Mechanism: In Vivo and In Silico Studies. Pharmaceuticals 2022, 15, 1557. https://doi.org/10.3390/ph15121557
Susanti L, Mustarichie R, Halimah E, Kurnia D, Setiawan A, Maladan Y. Anti-Alopecia Activity of Alkaloids Group from Noni Fruit against Dihydrotestosterone-Induced Male Rabbits and Its Molecular Mechanism: In Vivo and In Silico Studies. Pharmaceuticals. 2022; 15(12):1557. https://doi.org/10.3390/ph15121557
Chicago/Turabian StyleSusanti, Laila, Resmi Mustarichie, Eli Halimah, Dikdik Kurnia, Andi Setiawan, and Yustinus Maladan. 2022. "Anti-Alopecia Activity of Alkaloids Group from Noni Fruit against Dihydrotestosterone-Induced Male Rabbits and Its Molecular Mechanism: In Vivo and In Silico Studies" Pharmaceuticals 15, no. 12: 1557. https://doi.org/10.3390/ph15121557
APA StyleSusanti, L., Mustarichie, R., Halimah, E., Kurnia, D., Setiawan, A., & Maladan, Y. (2022). Anti-Alopecia Activity of Alkaloids Group from Noni Fruit against Dihydrotestosterone-Induced Male Rabbits and Its Molecular Mechanism: In Vivo and In Silico Studies. Pharmaceuticals, 15(12), 1557. https://doi.org/10.3390/ph15121557








