Update on COVID-19 Therapy in Pediatric Age
Abstract
:1. Introduction
2. Anti-SARS-CoV-2 Measures Presently Authorized
2.1. Remdesivir
2.2. Nirmatrelvir plus Ritonavir
2.3. Bebtelovimab
2.4. Molnupiravir
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cui, X.; Zhao, Z.; Zhang, T.; Guo, W.; Guo, W.; Zheng, J. A systematic review and meta-analysis of children with coronavirus disease 2019 (COVID-19). J. Med. Virol. 2021, 93, 1057–1069. [Google Scholar] [CrossRef] [PubMed]
- Tsankov, B.K.; Allaire, J.M.; Irvine, M.A.; Lopez, A.A.; Sauve, L.J.; Vallance, B.A.; Jacobson, K. Severe COVID-19 Infection and Pediatric Comorbidities: A Systematic Review and Meta-Analysis. Int. J. Infect. Dis. 2021, 103, 246–256. [Google Scholar] [CrossRef]
- Garazzino, S.; Montagnani, C.; Donà, D.; Meini, A.; Felici, E.; Vergine, G.; Bernardi, S.; Giacchero, R.; Lo Vecchio, A.; Marchisio, P.; et al. Multicentre Italian study of SARS-CoV-2 infection in children and adolescents, preliminary data as at 10 April 2020. Euro Surveill. 2020, 25, 2000600. [Google Scholar] [CrossRef] [PubMed]
- American Academy of Pediatrics and the Children’s Hospital Association. Children and COVID-19: State Data Report. Available online: https://downloads.aap.org/AAP/PDF/AAP%20and%20CHA%20-%20Children%20and%20COVID-19%20State%20Data%20Report%209.29.22%20FINAL.pdf?_ga=2.170767266.309218142.1664893746-995825483.1664893746 (accessed on 6 October 2022).
- Food and Drug Administration. FDA Authorizes Pfizer-BioNTech COVID-19 Vaccine for Emergency Use in Children 5 through 11 Years of Age. Available online: https://www.fda.gov/news-events/press-announcements/fda-authorizes-pfizer-biontech-covid-19-vaccine-emergency-use-children-5-through-11-years-age (accessed on 10 October 2022).
- Food and Drug Administration Coronavirus (COVID-19) Update: FDA Authorizes Moderna and Pfizer-BioNTech COVID-19 Vaccines for Children Down to 6 Months of Age. Available online: https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-moderna-and-pfizer-biontech-covid-19-vaccines-children (accessed on 10 October 2022).
- Zimmermann, P.; Uka, A.; Buettcher, M.; Fougère, Y.; Plebani, M.; Relly, C.; Schmid, H.; Ritz, N.; Swiss Paediatric Surveillance Unit (SPSU). Neonates with SARS-CoV-2 infection: Spectrum of disease from a prospective nationwide observational cohort study. Swiss. Med. Wkly. 2022, 152, w30185. [Google Scholar] [PubMed]
- Esposito, S.; Giordano, R.; Paini, G.; Puntoni, M.; Principi, N.; Caminiti, C. Can we get out of the COVID pandemic without adequate vaccination coverage in the pediatric population? Ital. J. Pediatr. 2022, 48, 150. [Google Scholar] [CrossRef] [PubMed]
- Marks, K.J.; Whitaker, M.; Agathis, N.T.; Anglin, O.; Milucky, J.; Patel, K.; Pharm, H.; Kirley, P.D.; Kawasaki, B.; Milucky, J.; et al. Hospitalization of Infants and Children Aged 0–4 Years with Laboratory-Confirmed COVID-19—COVID-NET, 14 States, March 2020–February 2022. MMWR Morb. Mortal. Wkly Rep. 2022, 71, 429–436. [Google Scholar] [CrossRef]
- Molloy, E.J.; Nakra, N.; Gale, C.; Dimitriades, V.R.; Lakshminrusimha, S. Multisystem inflammatory syndrome in children (MIS-C) and neonates (MIS-N) associated with COVID-19: Optimizing definition and management. Pediatr. Res. 2022, 1–10. [Google Scholar] [CrossRef]
- Esposito, S.; Principi, N. Multisystem Inflammatory Syndrome in Children Related to SARS-CoV-2. Paediatr. Drugs 2021, 23, 119–129. [Google Scholar] [CrossRef]
- More, K.; Aiyer, S.; Goti, A.; Parikh, M.; Sheikh, S.; Patel, G.; Kallem, V.; Soni, R.; Kumar, P. Multisystem inflammatory syndrome in neonates (MIS-N) associated with SARS-CoV2 infection: A case series. Eur. J. Pediatr. 2022, 181, 1883–1898. [Google Scholar] [CrossRef]
- Pawar, R.; Gavade, V.; Patil, N.; Mali, V.; Girwalkar, A.; Tarkasband, V.; Loya, S.; Chavan, A.; Nanivadekar, N.; Shinde, R.; et al. Neonatal Multisystem Inflammatory Syndrome (MIS-N) Associated with Prenatal Maternal SARS-CoV-2: A Case Series. Children 2021, 8, 572. [Google Scholar] [CrossRef]
- Stephenson, T.; Shafran, R.; Ladhani, S.N. Long COVID in children and adolescents. Curr. Opin. Infect. Dis. 2022, 35, 461–467. [Google Scholar] [CrossRef] [PubMed]
- Esposito, S.; Principi, N.; Azzari, C.; Cardinale, F.; Di Mauro, G.; Galli, L.; Gattinara, G.C.; Fainardi, V.; Guarino, A.; Lancella, L.; et al. Italian intersociety consensus on management of long covid in children. Ital. J. Pediatr. 2022, 48, 42. [Google Scholar] [CrossRef] [PubMed]
- Fainardi, V.; Meoli, A.; Chiopris, G.; Motta, M.; Skenderaj, K.; Grandinetti, R.; Bergomi, A.; Antodaro, F.; Zona, S.; Esposito, S. Long COVID in Children and Adolescents. Life 2022, 12, 285. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Leon, S.; Wegman-Ostrosky, T.; Ayuzo Del Valle, N.C.; Perelman, C.; Sepulveda, R.; Rebolledo, P.A.; Cuapio, A.; Villapol, S. Long-COVID in children and adolescents: A systematic review and meta-analyses. Sci. Rep. 2022, 12, 9950. [Google Scholar] [CrossRef]
- Gatti, E.; Piotto, M.; Lelii, M.; Pensabene, M.; Madini, B.; Cerrato, L.; Hassan, V.; Aliberti, S.; Bosis, S.; Marchisio, S.; et al. Therapeutic Strategies for COVID-19 Lung Disease in Children. Front. Pediatr. 2022, 10, 829521. [Google Scholar] [CrossRef]
- European Medicines Agency. Comirnaty. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/comirnaty#authorisation-details-section (accessed on 1 November 2022).
- Principi, N.; Esposito, S. Is the Immunization of Pregnant Women against COVID-19 Justified? Vaccines 2021, 9, 970. [Google Scholar] [CrossRef]
- Regan, A.K.; Kaur, R.; Nosek, M.; Swathi, P.A.; Gu, N.Y. COVID-19 vaccine acceptance and coverage among pregnant persons in the United States. Prev. Med. Rep. 2022, 29, 101977. [Google Scholar] [CrossRef]
- American Academy of Pediatrics. Children and COVID-19 Vaccination Trends. Available online: https://www.aap.org/en/pages/2019-novel-coronavirus-covid-19-infections/children-and-covid-19-vaccination-trends/ (accessed on 10 October 2022).
- Kokic, G.; Hillen, H.S.; Tegunov, D.; Dienemann, C.; Seitz, F.; Schmitzova, J.; Farnung, L.; Siewert, A.; Höbartner, C.; Cramer, P. Mechanism of SARS-CoV-2 polymerase stalling by remdesivir. Nat. Commun. 2021, 12, 279. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration. Coronavirus (COVID-19) Update: FDA Approves First COVID-19 Treatment for Young Children. Available online: https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-approves-first-covid-19-treatment-young-children (accessed on 10 October 2022).
- Chan-Tack, K.; Sampson, M.; Earp, J.; Arya, V.; Yao, L.; Alexander, J.; Hausman, E.; Belew, Y.; Birnkrant, E.; Struble, K.; et al. Considerations and Challenges in the Remdesivir COVID-19 Pediatric Development Program. J. Clin. Pharmacol. 2022. [Google Scholar] [CrossRef]
- Goldman, D.L.; Aldrich, M.L.; Hagmann, S.H.F.; Bamford, A.; Camacho-Gonzalez, A.; Lapadula, G.; Lee, P.; Bonfanti, P.; Carter, C.C.; Zhao, Y.; et al. Compassionate Use of Remdesivir in Children with Severe COVID-19. Pediatrics 2021, 147, e2020047803. [Google Scholar] [CrossRef]
- La Tessa, A.; Motisi, M.A.; Marseglia, G.L.; Cardinale, F.; Licari, A.; Manti, S.; Tosca, M.; Del Giudice, M.M.; de Filippo, M.; Galli, L.; et al. Use of remdesivir in children with COVID-19 infection: A quick narrative review. Acta Biomed. 2021, 92, e2021524. [Google Scholar] [PubMed]
- Beigel, J.; Tomashek, K.M.; Dodd, L.E.; Mehta, A.K.; Zingman, B.S.; Kalil, A.C.; Hohmann, E.; Chu, H.Y.; Luetkemeyer, A.; Kline, S.; et al. Remdesivir for the treatment of COVID-19—Final report. N. Engl. J. Med. 2020, 383, 1813–1826. [Google Scholar] [CrossRef]
- Goldman, J.D.; Lye, D.C.B.; Hui, D.S.; Marks, K.M.; Bruno, R.; Montejano, R.; Spinner, C.D.; Galli, M.; Ahn, M.-Y.; Nahass, R.-G.; et al. Remdesivir for 5 or 10 days in patients with severe COVID-19. N. Engl. J. Med. 2020, 383, 1827–1837. [Google Scholar] [CrossRef] [PubMed]
- Spinner, C.D.; Gottlieb, R.L.; Criner, G.J.; Arribas López, J.R.; Cattelan, A.M.; Ogbuagu, O.; Malhotra, P.; Mullane, K.M.; Castagna, A.; Chai, L.Y.A.; et al. Effect of remdesivir vs. standard care on clinical status at 11 days in patients with moderate COVID-19: A randomized clinical trial. JAMA 2020, 24, 1048–1057. [Google Scholar] [CrossRef] [PubMed]
- Shang, W.; Dai, W.; Yao, C.; Xu, L.; Tao, X.; Su, H.; Li, J.; Xie, X.; Xu, Y.; Hu, M.; et al. In vitro and in vivo evaluation of the main protease inhibitor FB2001 against SARS-CoV-2. Antivir. Res. 2022. [Google Scholar] [CrossRef] [PubMed]
- Gottlieb, R.L.; Vaca, C.E.; Paredes, R.; Mera, J.; Webb, B.J.; Perez, G.; Oguchi, G.; Pyan, P.; Nielsen, B.U.; Brown, M.; et al. Early Remdesivir to Prevent Progression to Severe COVID-19 in Outpatients. N. Engl. J. Med. 2022, 386, 305–315. [Google Scholar] [CrossRef]
- Gilead. Phase 2/3 Interim Data Evaluating the Safety, Tolerability and Clinical Outcomes of Veklury® (Remdesivir) in Pediatric Patients with COVID-19 Presented at CROI. 2022. Available online: https://www.gilead.com/news-and-press/press-room/press-releases/2022/2/phase-23-interim-data-evaluating-the-safety-tolerability-and-clinical-outcomes-of-veklury-remdesivir-in-pediatric-patients-with-covid19-present (accessed on 10 October 2022).
- Gomez, R.A.; Sequeira Lopez, M.L.; Fernandez, L.; Cherñavvsky, D.R.; Norwood, V.F. The maturing kidney: Development and susceptibility. Ren. Fail. 1999, 21, 283–291. [Google Scholar] [CrossRef]
- Adamsick, M.L.; Gandhi, R.G.; Bidell, M.R.; Elshaboury, R.H.; Bhattacharyya, R.P.; Kim, A.Y.; Nigwekar, S.; Rhee, E.P.; Sise, M.E. Remdesivir in Patients with Acute or Chronic Kidney Disease and COVID-19. J. Am. Soc. Nephrol. 2020, 31, 1384–1386. [Google Scholar] [CrossRef]
- FDA. Fact Sheet for Healthcare Providers: Emergency Use Authorization for Paxlovid. Available online: https://www.fda.gov/media/155050/download (accessed on 3 November 2022).
- Lewnard, J.A.; Malden, D.; Hong, V.; Puzniak, L.; Kim, J.S.; Shaw, S.F.; Takhar, H.; Jodar, L.; McLaughlin, J.M.; Tartof, S.Y. Effectiveness of nirmatrelvir-ritonavir against hospital admission: A matched cohort study in a large US healthcare system. medRxiv 2022. [Google Scholar] [CrossRef]
- Hammond, J.; Leister-Tebbe, H.; Gardner, A.; Abreu, P.; Bao, W.; Wisemandle, W.; Baniecki, M.; Hendrick, V.M.; Damle, B.; Simon-Campos, A.; et al. Oral Nirmatrelvir for High-Risk, Nonhospitalized Adults with COVID-19. N. Engl. J. Med. 2022, 386, 1397–1408. [Google Scholar] [CrossRef]
- Zheng, Q.; Ma, P.; Wang, M.; Cheng, Y.; Zhou, M.; Ye, L.; Feng, Z.; Zhang, C. Efficacy and safety of Paxlovid for COVID-19: A meta-analysis. J. Infect. 2022, S163-4453, 00557-6. [Google Scholar] [CrossRef] [PubMed]
- Dai, E.Y.; Lee, K.A.; Nathanson, A.B.; Leonelli, A.T.; Petros, B.A.; Brock-Fisher, T.; Dobbins, S.T.; Maclnns, B.L.; Capone, A.; Littlehale, N.; et al. Viral Kinetics of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Omicron Infection in mRNA-Vaccinated Individuals Treated and Not Treated with Nirmatrelvir-Ritonavir. medRxiv 2022. [Google Scholar] [CrossRef]
- Wang, L.; Berger, N.A.; Davis, P.B.; Kaelber, D.C.; Volkow, N.D.; Xu, R. COVID-19 rebound after Paxlovid and Molnupiravir during January–June 2022. medRxiv 2022. [Google Scholar] [CrossRef]
- Li, H.; Gao, M.; You, H.; Zhang, P.; Pan, Y.; Li, N.; Qin, L.; Wang, H.; Li, D.; Li, L.; et al. Association of nirmatrelvir/ritonavir treatment on upper respiratory SARS-CoV-2 RT-PCR negative conversion rates among high-risk patients with COVID-19. Clin. Infect. Dis. 2022, ciac600. [Google Scholar] [CrossRef]
- Yan, G.; Zhou, J.; Zhu, H.; Chen, Y.; Lu, Y.; Zhang, T.; Hui, Y.; Wang, L.; Xu, H.; Wang, Z.; et al. The feasibility, safety, and efficacy of Paxlovid treatment in SARS-CoV-2-infected children aged 6–14 years: A cohort study. Ann. Transl. Med. 2022, 10, 619. [Google Scholar] [CrossRef] [PubMed]
- Kearns, G.L.; Abdel-Rahman, S.M.; Alander, S.W.; Blowey, D.L.; Leeder, J.S.; Kauffman, R.E. Developmental pharmacology--drug disposition, action, and therapy in infants and children. N. Engl. J. Med. 2003, 349, 1157–1167. [Google Scholar] [CrossRef]
- Larkin, H.D. Paxlovid Drug Interaction Screening Checklist Updated. JAMA 2022, 328, 1290. [Google Scholar] [CrossRef]
- Westendorf, K.; Žentelis, S.; Wang, L.; Foster, D.; Vaillancourt, P.; Wiggin, M.; Lovett, E.; van der Lee, R.; Hendle, J.; Pustilnik, A.; et al. LY-CoV1404 (bebtelovimab) potently neutralizes SARS-CoV-2 variants. Cell Rep. 2022, 39, 110812. [Google Scholar] [CrossRef]
- Food and Drug Administration. Fact sheet for healthcare providers: Emergency use authorization for Bebtelovimab. Available online: https://www.fda.gov/media/156152/download (accessed on 10 October 2022).
- Chigutsa, E.; Jordie, E.; Riggs, M.; Nirula, A.; Elmokadem, A.; Knab, T.; Chien, J.Y. A Quantitative Modeling and Simulation Framework to Support Candidate and Dose Selection of Anti-SARS-CoV-2 Monoclonal Antibodies to Advance Bamlanivimab into a First-in-Human Clinical Trial. Clin. Pharmacol. Ther. 2022, 111, 595–604. [Google Scholar] [CrossRef]
- National Institute of Health. Anti-SARS-CoV-2 Monoclonal Antibodies. Available online: https://www.covid19treatmentguidelines.nih.gov/therapies/anti-sars-cov-2-antibody-products/anti-sars-cov-2-monoclonal-antibodies/ (accessed on 10 October 2020).
- Dougan, M.; Azizad, M.; Chen, P.; Feldman, B.; Frieman, M.; Igbinadolor, A.; Kumar, P.; Morris, J.; Potts, J.; Baracco, L. Bebtelovimab, alone or together with bamlanivimab and etesevimab, as a broadly neutralizing monoclonal antibody treatment for mild to moderate, ambulatory COVID-19. medRxiv 2022. [Google Scholar] [CrossRef]
- Gordon, C.J.; Tchesnokov, E.P.; Schinazi, R.F.; Götte, M. Molnupiravir promotes SARS-CoV-2 mutagenesis via the RNA template. J. Biol. Chem. 2021, 297, 100770. [Google Scholar] [CrossRef] [PubMed]
- Food and Drug Administration. Emergency Use Authorization. Available online: https://www.fda.gov/media/155053/download (accessed on 10 October 2022).
- European Medicines Agency. Assessment Report. Use of Molnupiravir for the Treatment of COVID-19. Available online: https://www.ema.europa.eu/en/documents/referral/lagevrio-also-known-molnupiravir-mk-4482-covid-19-article-53-procedure-assessment-report_en.pdf (accessed on 10 October 2022).
- Jayk Bernal, A.; Gomes da Silva, M.M.; Musungaie, D.B.; Kovalchuk, E.; Gonzalez, A.; Reyes, V.D.; Martín-Quirós, A.; Caraco, Y.; Williams-Diaz, A.; Brown, M.L.; et al. MOVe-OUT Study Group. Molnupiravir for oral treatment of COVID-19 in nonhospitalized patients. N. Engl. J. Med. 2022, 386, 509–520. [Google Scholar] [CrossRef] [PubMed]
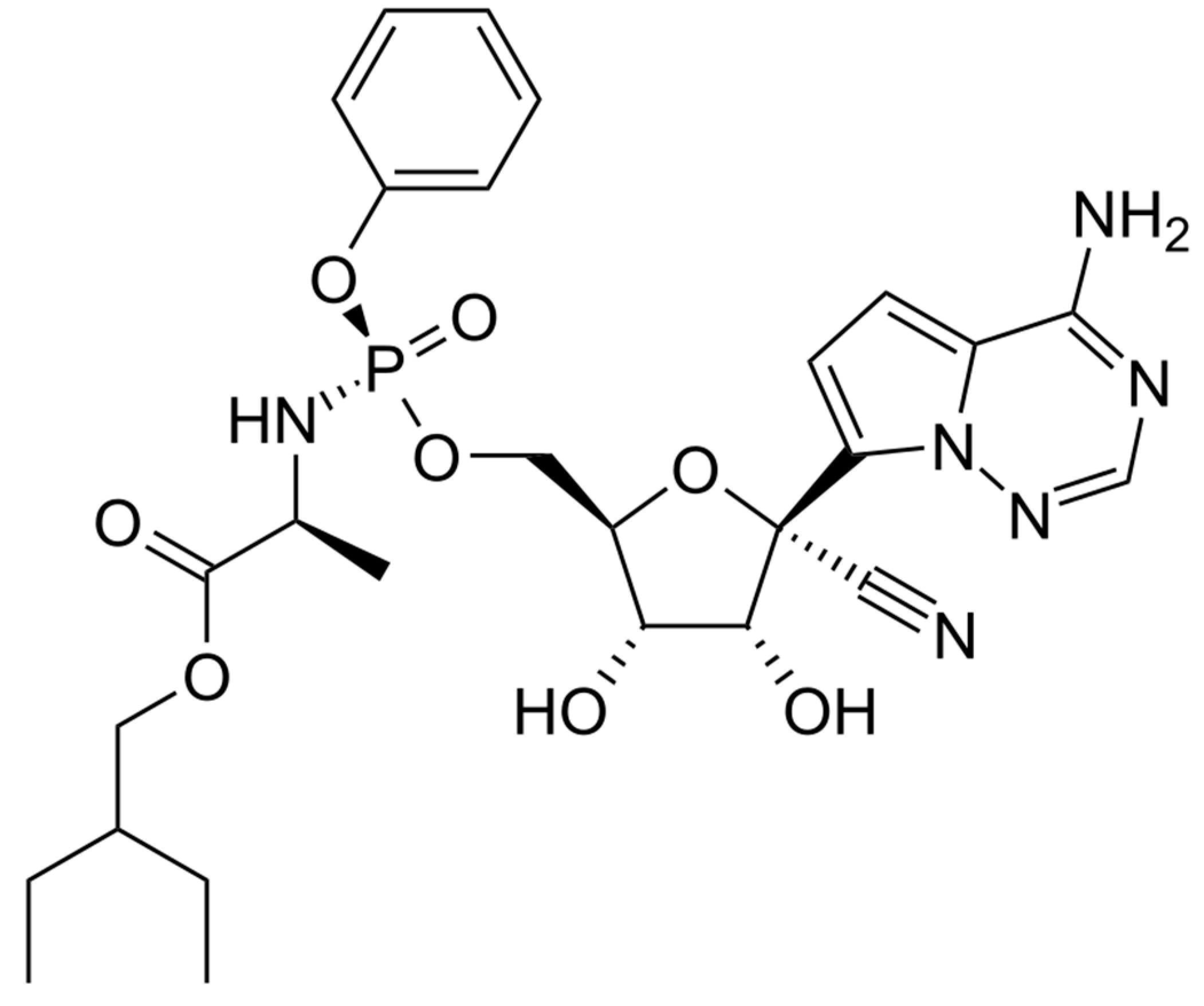
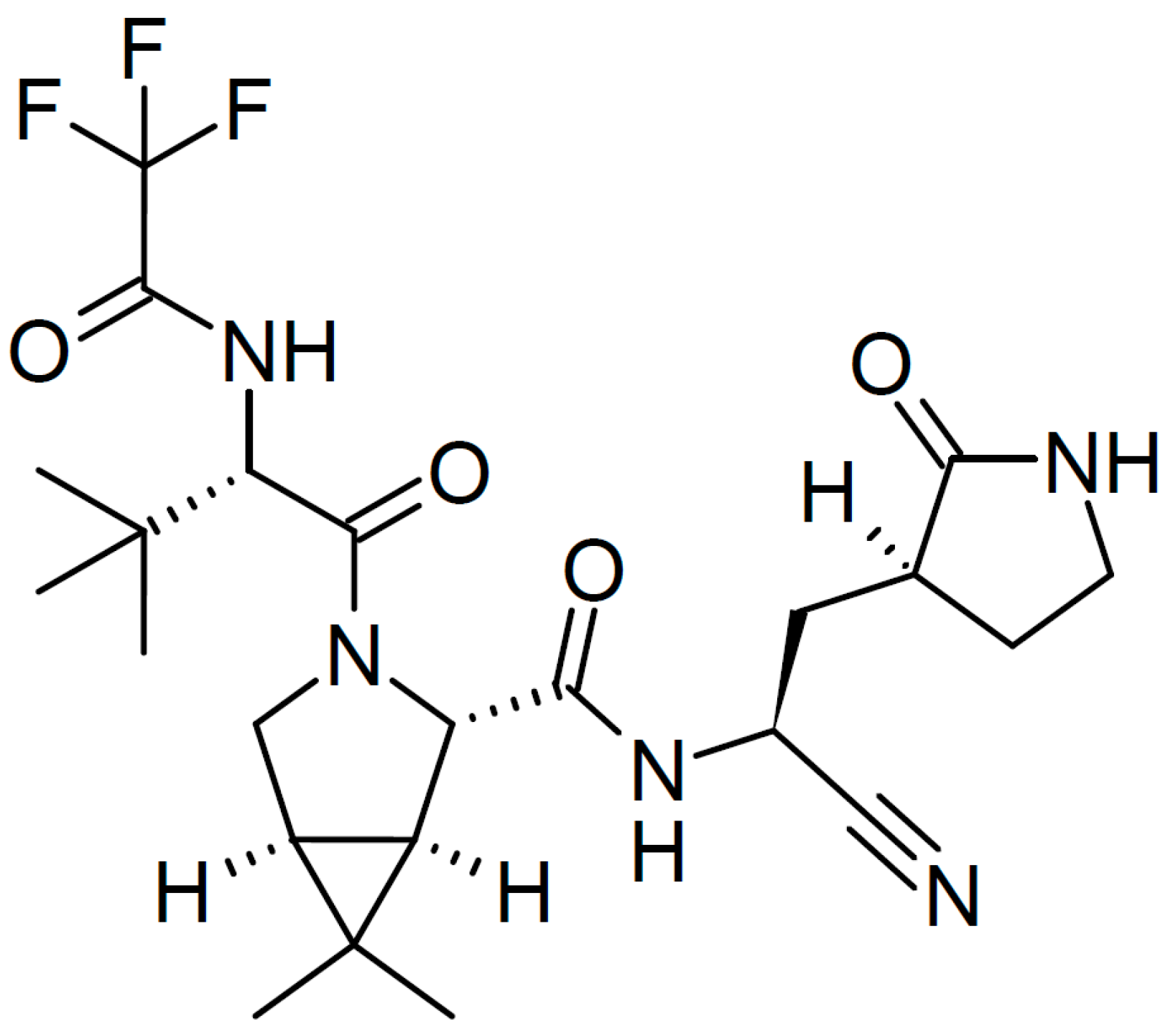
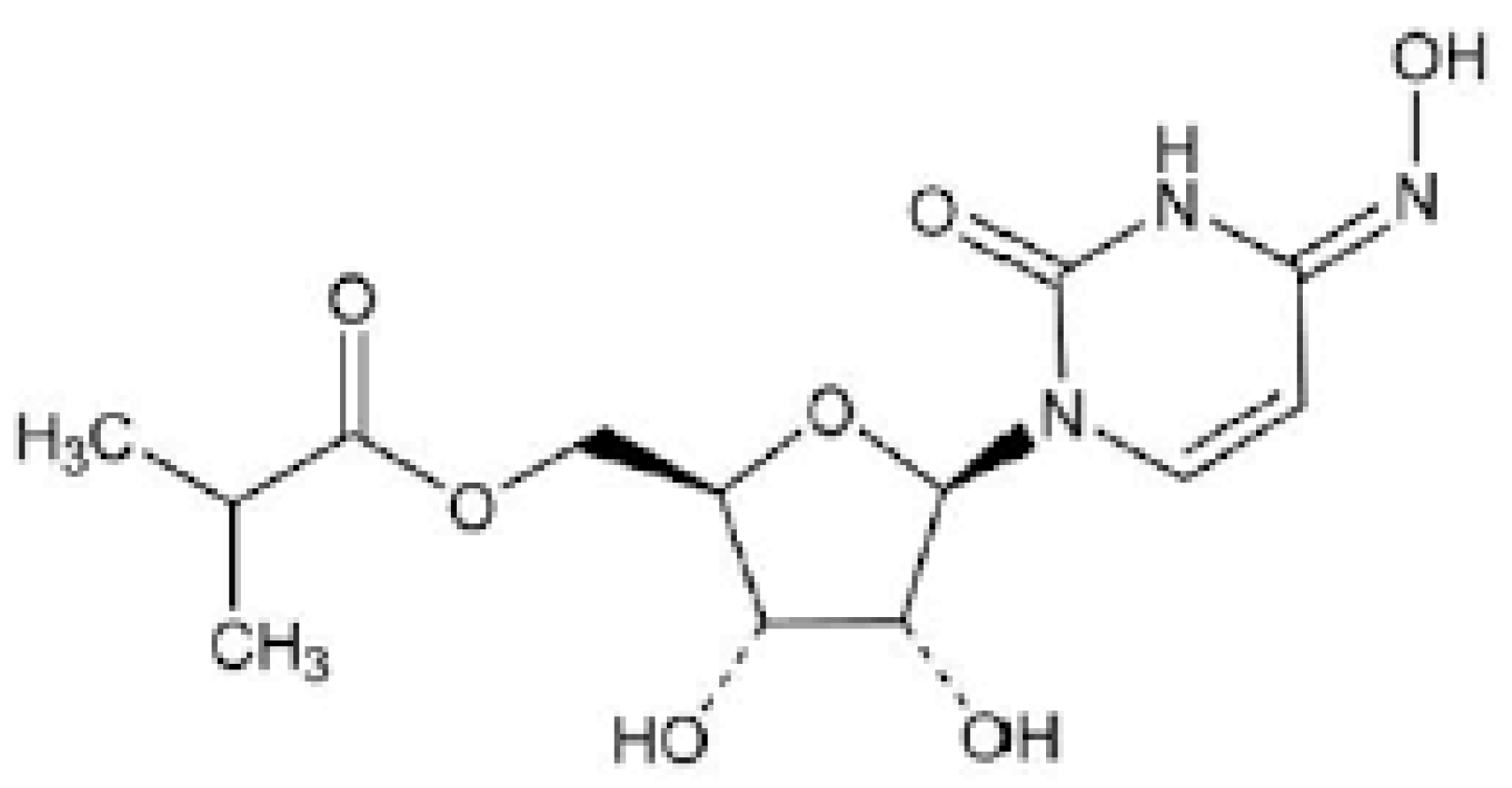
| Dosages According to Age and Weight |
|---|
| Neonates weighing < 3.5 kg: Loading dose 2.5 to 5 mg/kg IV on day 1, followed by 1.25 mg/kg/dose IV once daily on day 2–5. Neonates weighing ≥ 3.5 kg: Loading dose 5 mg/kg IV on day 1, followed by 2.5 mg/kg/dose IV once daily on day 2–5. 3 to <40 kg: Loading dose: 5 mg/kg/dose IV on day 1, followed by 2.5 mg/kg/dose IV once daily on day 2–5. ≥40 kg: Loading dose: 200 mg IV on day 1, followed by 100 mg IV once daily on day 2–5. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Esposito, S.; Autore, G.; Argentiero, A.; Ramundo, G.; Perrone, S.; Principi, N. Update on COVID-19 Therapy in Pediatric Age. Pharmaceuticals 2022, 15, 1512. https://doi.org/10.3390/ph15121512
Esposito S, Autore G, Argentiero A, Ramundo G, Perrone S, Principi N. Update on COVID-19 Therapy in Pediatric Age. Pharmaceuticals. 2022; 15(12):1512. https://doi.org/10.3390/ph15121512
Chicago/Turabian StyleEsposito, Susanna, Giovanni Autore, Alberto Argentiero, Greta Ramundo, Serafina Perrone, and Nicola Principi. 2022. "Update on COVID-19 Therapy in Pediatric Age" Pharmaceuticals 15, no. 12: 1512. https://doi.org/10.3390/ph15121512
APA StyleEsposito, S., Autore, G., Argentiero, A., Ramundo, G., Perrone, S., & Principi, N. (2022). Update on COVID-19 Therapy in Pediatric Age. Pharmaceuticals, 15(12), 1512. https://doi.org/10.3390/ph15121512







