Treatment of Central Nervous System Infection Caused by Multidrug-Resistant Klebsiella pneumoniae with Colistin Sulfate Intravenously and Intrathecally: A Case Report
Abstract
1. Introduction
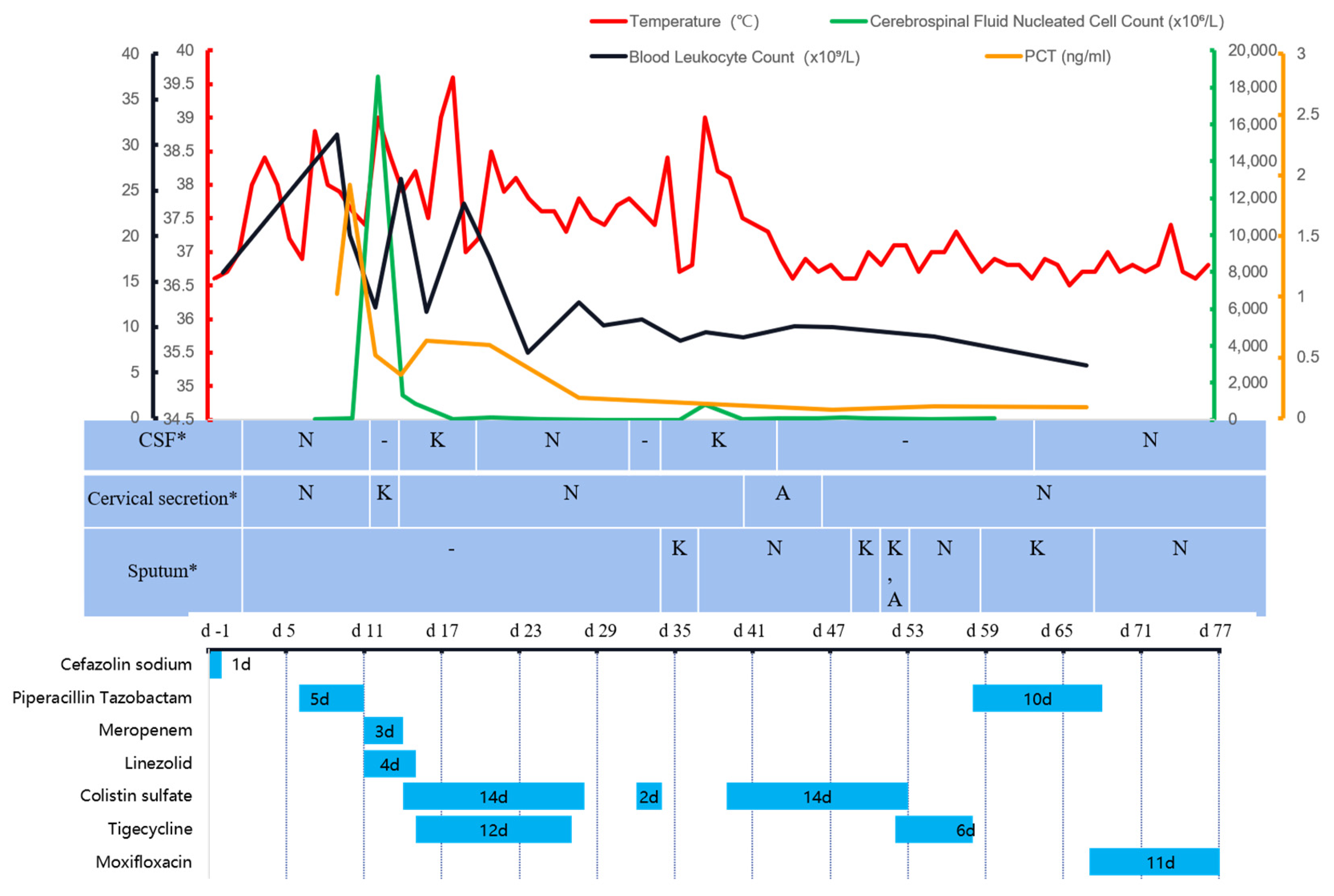
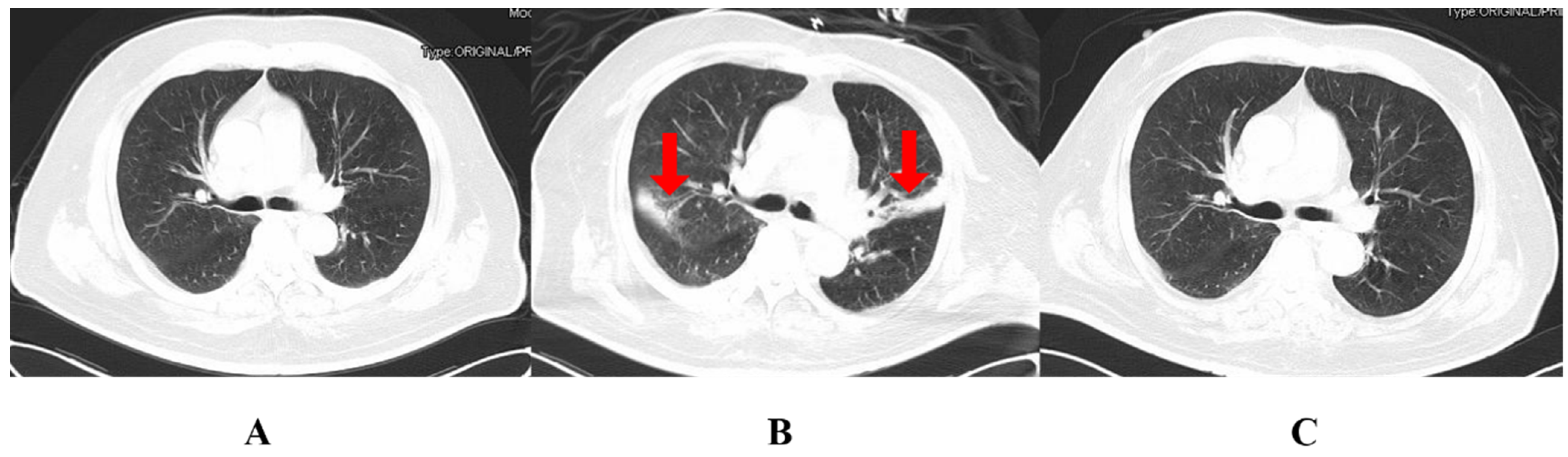
2. Case Report
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tunkel, A.R.; Hasbun, R.; Bhimraj, A.; Byers, K.; Kaplan, S.L.; Scheld, W.M.; Zunt, J.R. Infectious Diseases Society of America’s Clinical Practice Guidelines for Healthcare-Associated Ventriculitis and Meningitis. Clin. Infect. Dis. 2017, 64, e34–e65. [Google Scholar] [CrossRef]
- Karvouniaris, M.; Brotis, A.; Tsiakos, K.; Palli, E.; Koulenti, D. Current Perspectives on the Diagnosis and Management of Healthcare-Associated Ventriculitis and Meningitis. Infect. Drug Resist. 2022, 15, 697–721. [Google Scholar] [CrossRef] [PubMed]
- Nation, R.L.; Li, J.; Cars, O.; Couet, W.; Dudley, M.N.; Kaye, K.S.; Mouton, J.W.; Paterson, D.L.; Tam, V.H.; Theuretzbacher, U.; et al. Framework for optimisation of the clinical use of colistin and polymyxin B: The Prato polymyxin consensus. Lancet Infect. Dis. 2015, 15, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Xing, H.; Cheng, C.; Zhang, Y.; Cai, Y.; Wang, X.; Deng, D.; Xu, L.; Xu, M.; Chen, J. Successful Treatment With Intrathecal and Intravenous Polymyxin B-Based Combination Against MDR Acinetobacter baumannii Meningitis in Pediatric Patient: A Case Report. Front. Pediatr. 2021, 9, 564991. [Google Scholar] [CrossRef]
- Karaiskos, I.; Galani, L.; Baziaka, F.; Giamarellou, H. Intraventricular and intrathecal colistin as the last therapeutic resort for the treatment of multidrug-resistant and extensively drug-resistant Acinetobacter baumannii ventriculitis and meningitis: A literature review. Int. J. Antimicrob. Agents 2013, 41, 499–508. [Google Scholar] [CrossRef]
- Ng, J.; Gosbell, I.B.; Kelly, J.A.; Boyle, M.J.; Ferguson, J.K. Cure of multiresistant Acinetobacter baumannii central nervous system infections with intraventricular or intrathecal colistin: Case series and literature review. J. Antimicrob. Chemother. 2006, 58, 1078–1081. [Google Scholar] [CrossRef]
- National Medical Products Administration Data Search. Available online: https://www.nmpa.gov.cn/datasearch/search-info.html?nmpa=aWQ9Yjg4YmJjOTY2ZmY2NGFhOTNhMGZiM2JkYzY1NjY0MjYmaXRlbUlkPWZmODA4MDgxODNjYWQ3NTAwMTg0MDg4MWY4NDgxNzlm (accessed on 5 November 2022).
- Yang, Q.; Ma, X.; Hu, F.; Zhang, J.; Sun, T.; Chen, B.; Xu, Y.; Liu, Y. Expert Consensus on Polymyxin Antimicrobial Susceptibility Testing and Clinical Interpretation. Chin. Med. Sci. J. 2020, 11, 559–570. [Google Scholar]
- Pogue, J.M.; Jones, R.N.; Bradley, J.S.; Andes, D.R.; Bhavnani, S.M.; Drusano, G.L.; Dudley, M.N.; Flamm, R.K.; Rodvold, K.A.; Ambrose, P.G. Polymyxin Susceptibility Testing and Interpretive Breakpoints: Recommendations from the United States Committee on Antimicrobial Susceptibility Testing (USCAST). Antimicrob. Agents Chemother. 2020, 64, e01495-19. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Qu, X.; Zhou, J. Chinese expert consensus on the diagnosis and treatment of central nervous system infections in neurosurgery (2021 edition). Chin. J. Neurosurg. 2021, 37, 2–15. [Google Scholar]
- Yu, Y. Expert consensus on the clinical application of β-lactam antibiotic/β-lactamase inhibitor combination preparations (2020 edition). Natl. Med. J. China 2020, 100, 738–747. [Google Scholar]
- Tzouvelekis, L.S.; Markogiannakis, A.; Psichogiou, M.; Tassios, P.T.; Daikos, G.L. Carbapenemases in Klebsiella pneumoniae and Other Enterobacteriaceae: An Evolving Crisis of Global Dimensions. Clin. Microbiol. Rev. 2012, 25, 682–707. [Google Scholar] [CrossRef] [PubMed]
- Chiș, A.A.; Rus, L.L.; Morgovan, C.; Arseniu, A.M.; Frum, A.; Vonica-Țincu, A.L.; Dobrea, C.M. Microbial Resistance to Antibiotics and Effective Antibiotherapy. Biomedicines 2022, 10, 1121. [Google Scholar] [CrossRef] [PubMed]
- Kadri, S.S.; Adjemian, J.; Lai, Y.L.; Spaulding, A.B.; Ricotta, E.; Prevots, D.R.; National Institutes of Health Antimicrobial Resistance Outcomes Research Initiative (NIH–ARORI). Difficult-to-Treat Resistance in Gram-negative Bacteremia at 173 US Hospitals: Retrospective Cohort Analysis of Prevalence, Predictors, and Outcome of Resistance to All First-line Agents. Clin. Infect. Dis. 2018, 67, 1803–1814. [Google Scholar] [CrossRef] [PubMed]
- Sipahi, O.R.; Mermer, S.; Demirdal, T.; Ulu, A.C.; Fillatre, P.; Ozcem, S.B.; Kaya, Ş.; Şener, A.; Bulut, C.; Tekin, R.; et al. Tigecycline in the treatment of multidrug-resistant Acinetobacter baumannii meningitis: Results of the Ege study. Clin. Neurol. Neurosurg. 2018, 172, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Li, L.-M.; Zheng, W.-J.; Shi, S.-W. Spinal arachnoiditis followed by intrathecal tigecycline therapy for central nervous system infection by extremely drug-resistant Acinetobacter baumannii. J. Int. Med. Res. 2020, 48, 0300060520920405. [Google Scholar] [CrossRef]
- Yu, Y.; Liu, Y.; Wang, M. Expert consensus on the diagnosis, treatment and prevention and control of carbapenem-resistant Enterobacteriaceae bacterial infections in China. Natl. Med. J. China 2021, 101, 11. [Google Scholar]
- Falcone, M.; Paterson, D. Spotlight on ceftazidime/avibactam: A new option for MDR Gram-negative infections. J. Antimicrob. Chemother. 2016, 71, 2713–2722. [Google Scholar] [CrossRef]
- Bialvaei, A.Z.; Samadi Kafil, H. Colistin, mechanisms and prevalence of resistance. Curr. Med. Res. Opin. 2015, 31, 707–721. [Google Scholar] [CrossRef]
- Yu, X.; Huang, Y.; Zhang, X.; Wang, Y.; Shi, D.; Zhang, C.; Chen, J.; Wang, X.; Lin, G. Intraventricular colistin sulphate as a last resort therapy in a patient with multidrug-resistant Acinetobacter baumannii induced post-neurosurgical ventriculitis. Br. J. Clin. Pharmacol. 2022, 88, 3490–3494. [Google Scholar] [CrossRef]
- Cheng, G.-Y.; Wang, D.-X.; Zhou, Q.; Deng, A.-P. Pharmacists participated in the multi-approach application of colistin sulfate in the treatment of CRAB associated postoperative neurosurgical ventriculitis. Chin. J. Hosp. Pharm. 2022, 42, 2300–2303. [Google Scholar]
- Yu, X.-B.; Zhang, X.-S.; Wang, Y.-X.; Wang, Y.-Z.; Zhou, H.-M.; Xu, F.-M.; Yu, J.-H.; Zhang, L.-W.; Dai, Y.; Zhou, Z.-Y.; et al. Population Pharmacokinetics of Colistin Sulfate in Critically Ill Patients: Exposure and Clinical Efficacy. Front. Pharmacol. 2022, 13, 915958. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, B.T.; Pogue, J.M.; Zavascki, A.P.; Paul, M.; Daikos, G.L.; Forrest, A.; Giacobbe, D.R.; Viscoli, C.; Giamarellou, H.; Karaiskos, I.; et al. International Consensus Guidelines for the Optimal Use of the Polymyxins: Endorsed by the American College of Clinical Pharmacy (ACCP), European Society of Clinical Microbiology and Infectious Diseases (ESCMID), Infectious Diseases Society of America (IDSA), International Society for Anti-infective Pharmacology (ISAP), Society of Critical Care Medicine (SCCM), and Society of Infectious Diseases Pharmacists (SIDP). Pharmacother. J. Hum. Pharmacol. Drug Ther. 2019, 39, 10–39. [Google Scholar]
| Antibiotics | Methods | MICs | Results |
|---|---|---|---|
| STC | KB | + | + |
| AMO/CLA | MIC | ≥32 | R |
| Amikacin | MIC | ≥64 | R |
| Ampicillin | MIC | ≥32 | R |
| Aztreonam | MIC | ≥64 | R |
| Ceftazidime | MIC | ≥64 | R |
| Cefoxitin | MIC | ≥64 | R |
| Ciprofloxacin | MIC | ≥4 | R |
| Cefpodoxime | MIC | ≥8 | R |
| Ceftriaxone | MIC | ≥64 | R |
| CPZ/SBT | KB | 7 | R |
| Cefotaxime | MIC | ≥64 | R |
| Cefuroxime | MIC | ≥64 | R |
| CAZ/AVI | KB | 21 | S |
| Cefazolin | MIC | ≥64 | R |
| Donipenem | MIC | ≥8 | R |
| Gentamicin | MIC | ≥16 | R |
| Imipenem | MIC | ≥16 | R |
| Levofloxacin | MIC | ≥8 | R |
| Meropenem | MIC | ≥16 | R |
| Moxifloxacin | MIC | ≥8 | R |
| Minocycline | KB | 11 | R |
| Polymyxin B | MIC | 0.5 | WT |
| AMP/SBT | MIC | ≥32 | R |
| SMZ | MIC | ≥16/304 | R |
| TIC/CLA | MIC | ≥128 | R |
| Tetracycline | MIC | ≥16 | R |
| Tigecycline | MIC | 1 | S |
| Ticarcillin | MIC | ≥128 | R |
| Tobramycin | MIC | ≥16 | R |
| PIP/TAZ | MIC | ≥128 | R |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, X.; Zhong, C.; Chen, H.; Xie, X.; Lv, X. Treatment of Central Nervous System Infection Caused by Multidrug-Resistant Klebsiella pneumoniae with Colistin Sulfate Intravenously and Intrathecally: A Case Report. Pharmaceuticals 2022, 15, 1482. https://doi.org/10.3390/ph15121482
Lu X, Zhong C, Chen H, Xie X, Lv X. Treatment of Central Nervous System Infection Caused by Multidrug-Resistant Klebsiella pneumoniae with Colistin Sulfate Intravenously and Intrathecally: A Case Report. Pharmaceuticals. 2022; 15(12):1482. https://doi.org/10.3390/ph15121482
Chicago/Turabian StyleLu, Xin, Cejun Zhong, Haifeng Chen, Xiaoqi Xie, and Xiaoju Lv. 2022. "Treatment of Central Nervous System Infection Caused by Multidrug-Resistant Klebsiella pneumoniae with Colistin Sulfate Intravenously and Intrathecally: A Case Report" Pharmaceuticals 15, no. 12: 1482. https://doi.org/10.3390/ph15121482
APA StyleLu, X., Zhong, C., Chen, H., Xie, X., & Lv, X. (2022). Treatment of Central Nervous System Infection Caused by Multidrug-Resistant Klebsiella pneumoniae with Colistin Sulfate Intravenously and Intrathecally: A Case Report. Pharmaceuticals, 15(12), 1482. https://doi.org/10.3390/ph15121482






