Mechanism and Prospect of Gastrodin in Osteoporosis, Bone Regeneration, and Osseointegration
Abstract
1. Introduction
2. Oxidative Stress in Menopausal Women, Elderly Patients, and Diabetes Patients with Osteoporosis
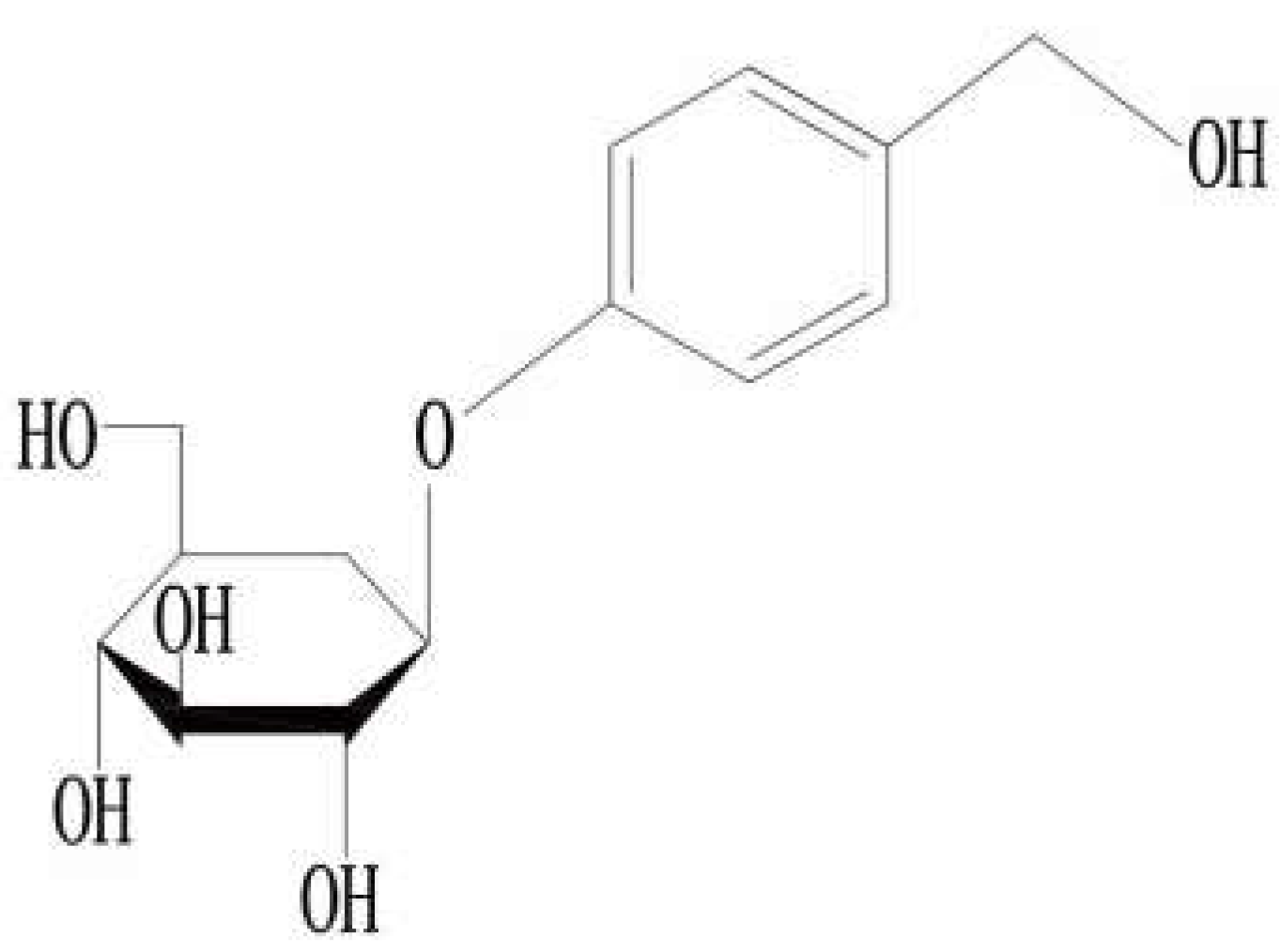
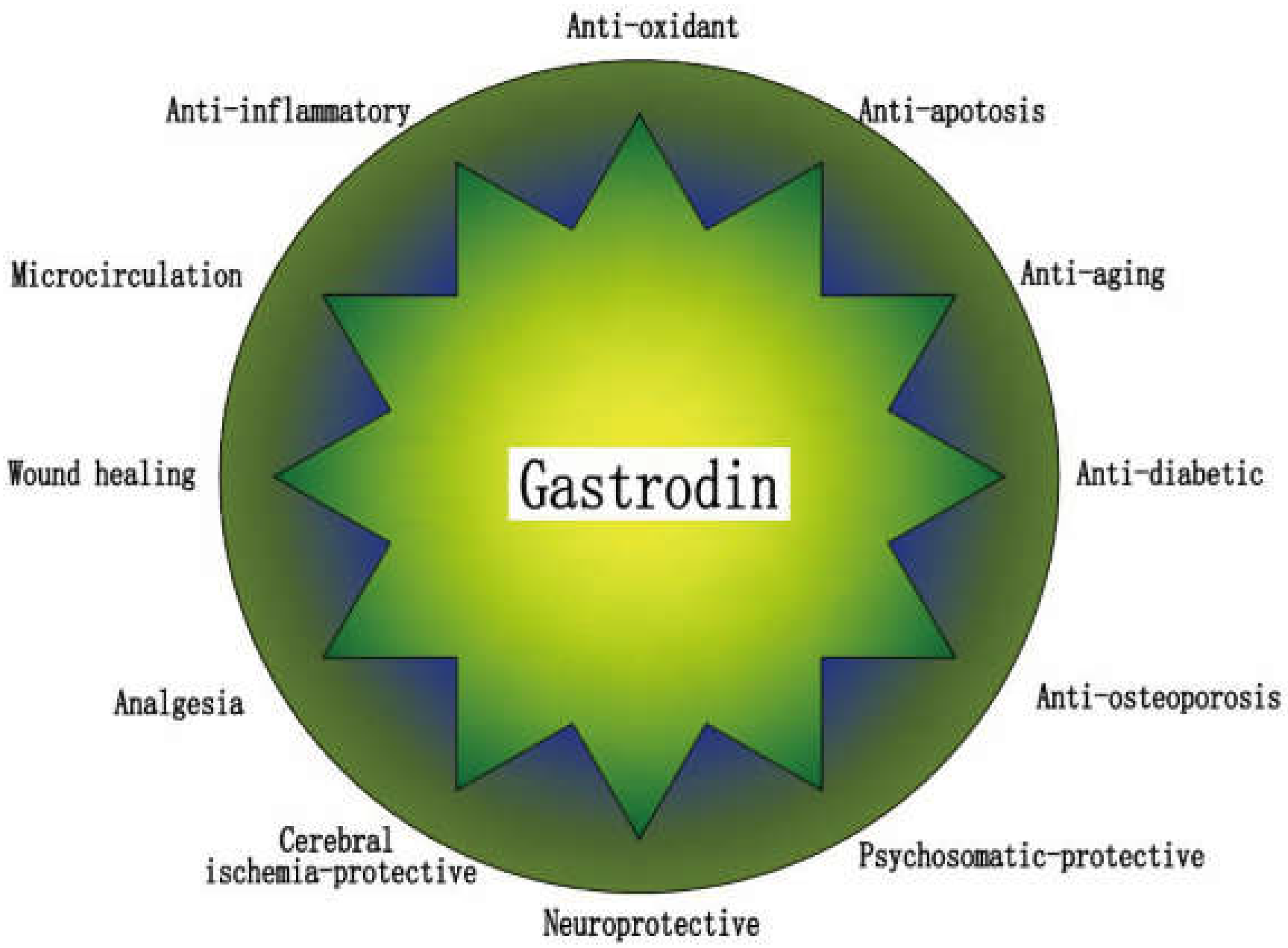
3. Gastrodin
4. Gastrodin for Osteoporosis Treatment
4.1. Protection of Osteogenesis
4.1.1. Antioxidant Effect
4.1.2. Anti-Apoptotic Effect
4.1.3. Anti-Inflammatory Effect
4.2. Inhibition of Bone Resorption
4.2.1. Gastrodin Inhibits Osteoclast Differentiation under Oxidative Stress through Antiox-Idant Effect
4.2.2. Gastrodin Inhibits Osteoclast Differentiation in Normal Environment through Antioxidant Effect
5. Gastrodin and Actin Filament in Anti-Osteoporosis
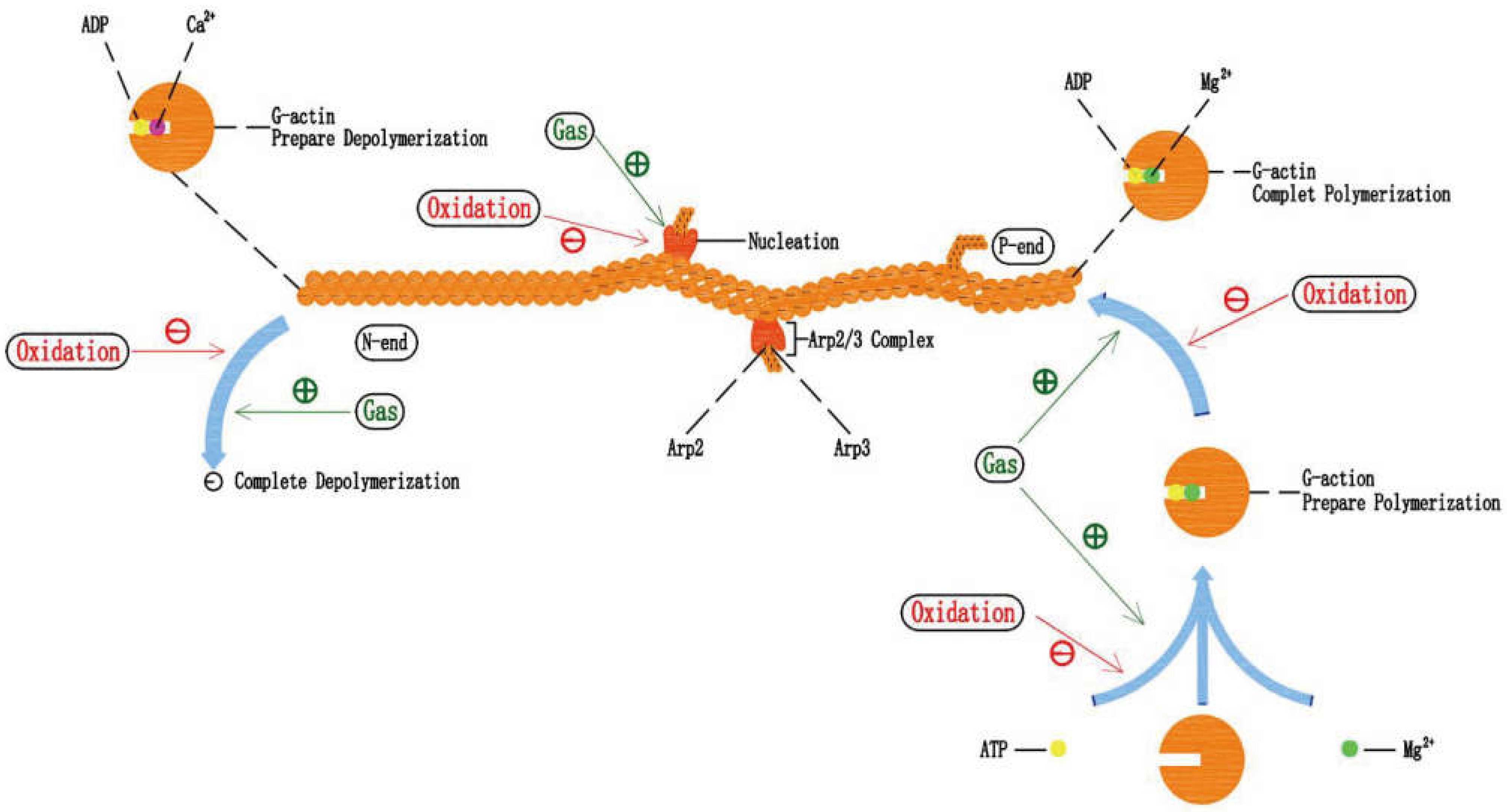
5.1. Gastrodin Can Protect Actin Filament under Oxidative Stress
5.2. Effects of Oxidative Stress on Actin Filament Polymerization-Depolymerization
5.3. The Potential Mechanism of Gastrodin Protecting Actin Filament and the Combination of Gastrodin and Mg2+
6. Gastrodin and RAS in Anti-Osteoporosis
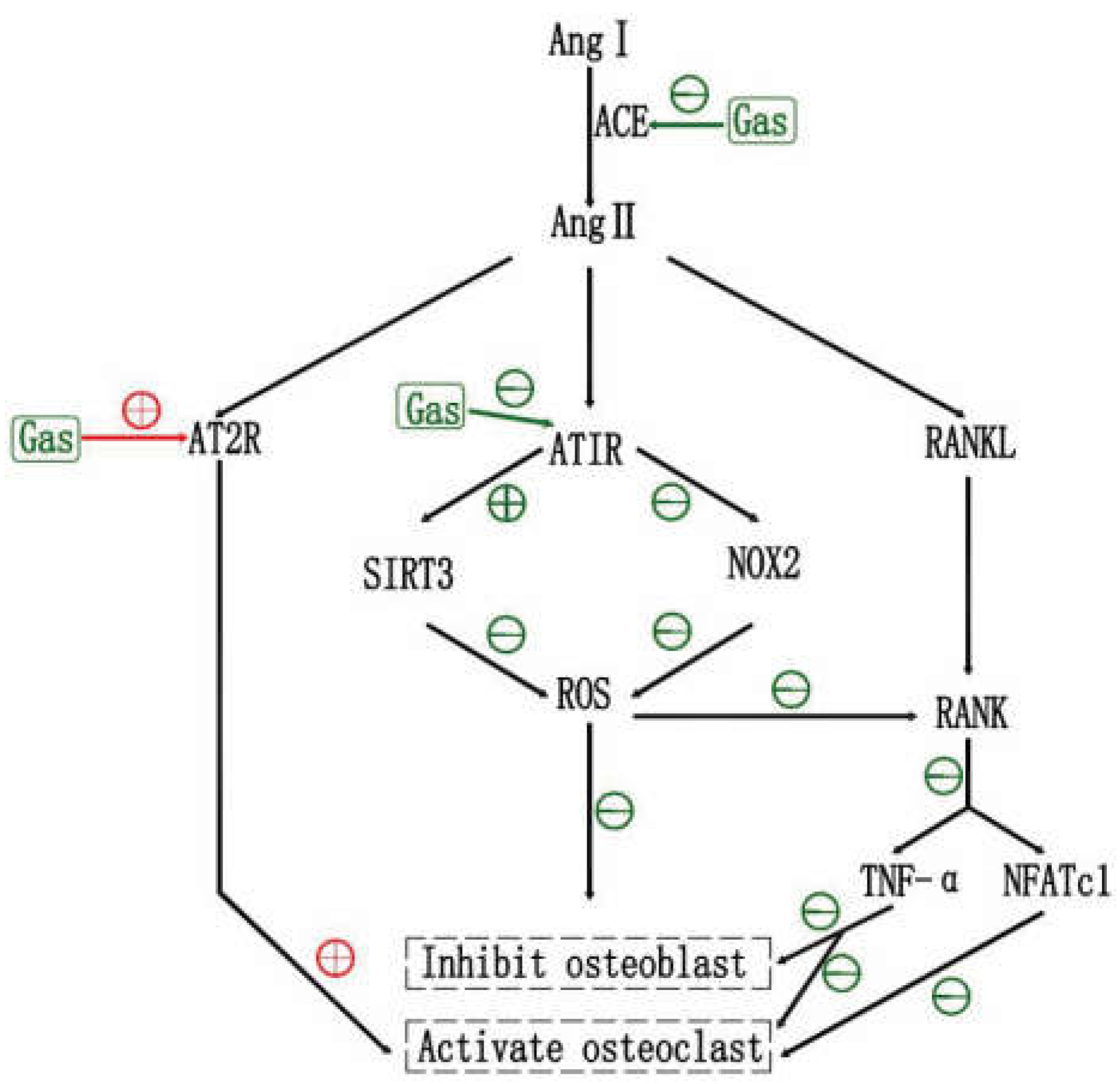
6.1. Effects of RAS and RAS Inhibtors on OP
6.2. Effects of Gastrodin on RAS
6.3. Gastrodin May Inhibit OP via RAS, and ARB2 May Be an Adjunct
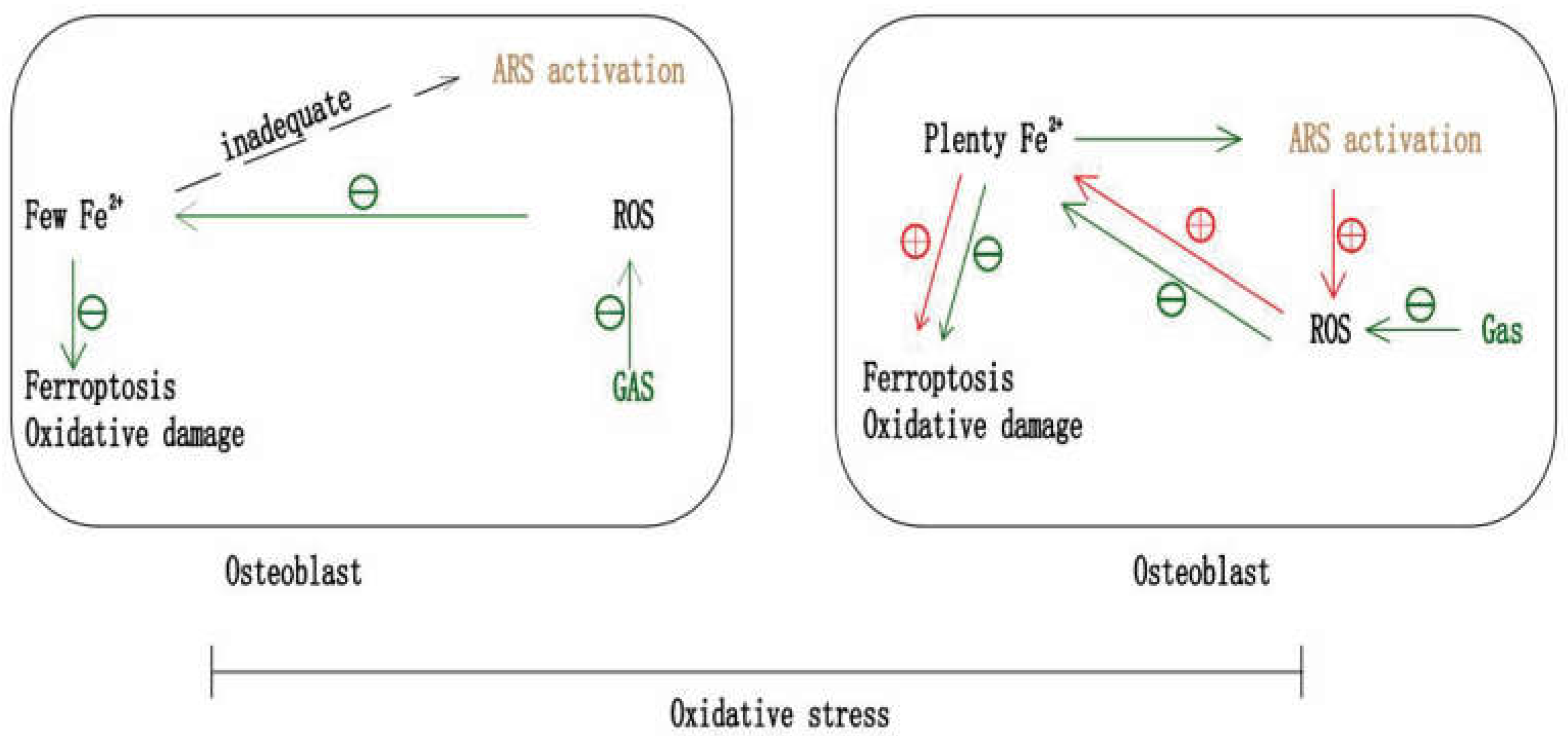
7. Gastrodin and Ferroptosis in Anti-Osteoporosis
7.1. Gastrodin May Inhibit OP by Anti-Ferroptosis
7.2. Artemisinin May Selectively Inhibit Osteoclast
7.3. The Potential of Gastrodin and ARS in Combination against OP
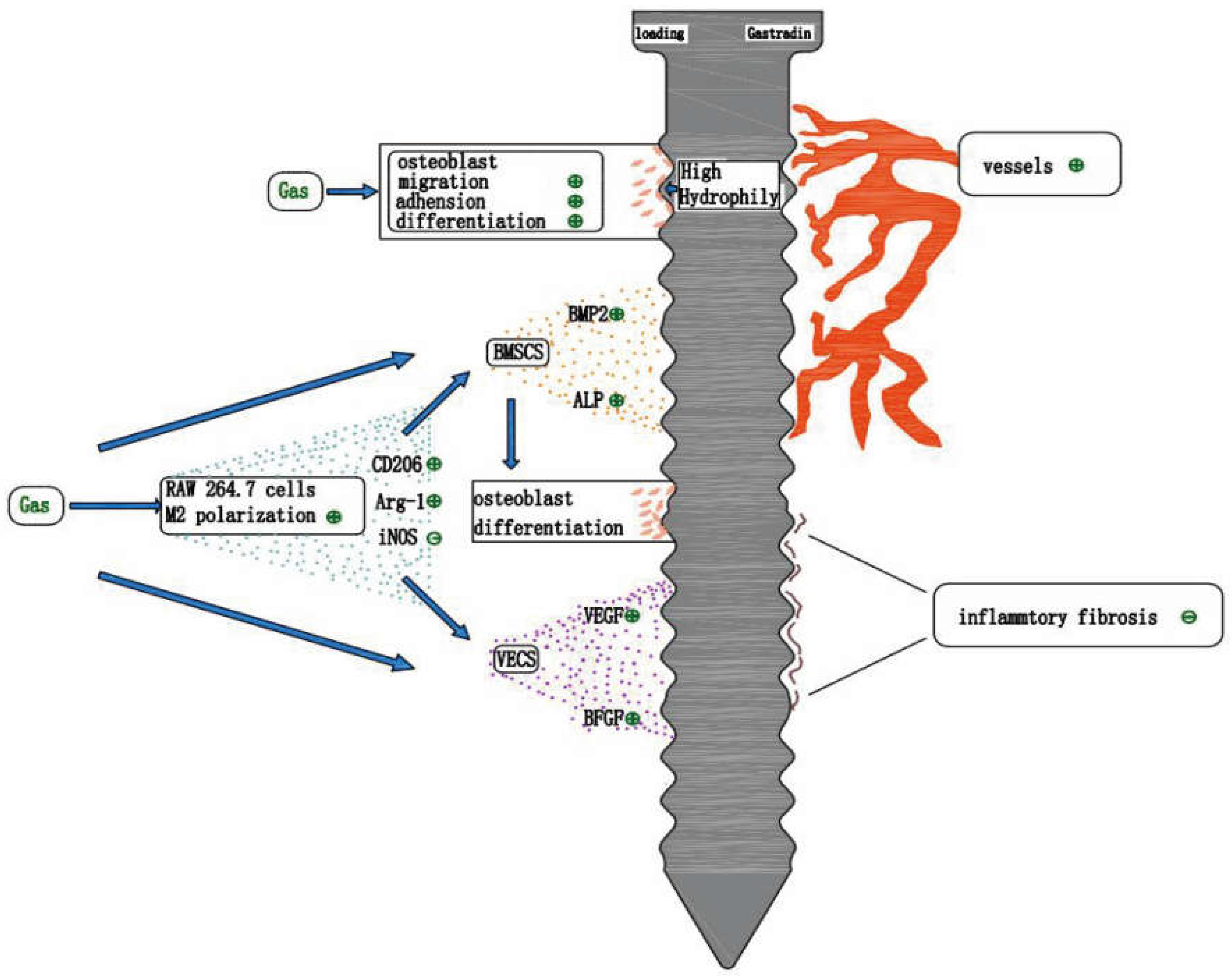
8. Gastrodin and Tissue Engineering Bone Regeneration
8.1. Gastrodin Maintains Its Inherent Antioxidant Property
8.2. Gastrodin Improves the Hydrophilicity of the Material Surface
8.3. Anti-Inflammatory Effect of Gastrodin
8.4. Gastrodin Promotes Vascular Regeneration
9. Gastrodin and Implant Osseointegration
9.1. Studies on the Promotion of Osseointegration by Gastrodin
9.2. Prospect on the Application of Gastrodin for Osseointegration
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xia, W.; Zhang, Z.; Lin, H. Guidelines for diagnosis and treatment of primary osteoporosis. Chin. J. Osteoporos. 2019, 25, 281–309. [Google Scholar]
- Sozen, T.; Ozisik, L.; Calik Basaran, N. An overview and management of osteoporosis. Eur. J. Rheumatol. 2017, 4, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Noh, J.; Yang, Y.; Jung, H. Molecular Mechanisms and Emerging Therapeutics for Osteoporosis. Int. J. Mol. Sci. 2020, 21, 7623. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Bai, S.; Chang, Y. China White Paper on Osteoporosis. Chin. J. Health Manag. 2009, 3, 148–149. [Google Scholar]
- Maraka, S.; Kennel, K.A. Bisphosphonates for the prevention and treatment of osteoporosis. BMJ 2015, 351, h3783. [Google Scholar] [CrossRef]
- Binkley, N.; Bolognese, M.; Sidorowicz-Bialynicka, A. A phase 3 trial of the efficacy and safety of oral recombinant calcitonin: The Oral Calcitonin in Postmenopausal Osteoporosis (ORACAL) trial. J. Bone Miner. Res. 2012, 27, 1821–1829. [Google Scholar] [CrossRef]
- Lim, S.Y.; Bolster, M.B. Current approaches to osteoporosis treatment. Curr. Opin. Rheumatol. 2015, 27, 216–224. [Google Scholar] [CrossRef]
- Gao, H.J.; Liu, P.F.; Li, P.W. Ligustrazine monomer against cerebral ischemia/reperfusion injury. Neural. Regen Res. 2015, 10, 832–840. [Google Scholar]
- Zheng, B.; Shi, C.; Muhammed, F.K. Gastrodin alleviates bone damage by modulating protein expression and tissue redox state. FEBS Open Bio 2020, 10, 2404–2416. [Google Scholar] [CrossRef]
- Xie, Y.; Sun, W.; Yan, F. Icariin-loaded porous scaffolds for bone regeneration through the regulation of the coupling process of osteogenesis and osteoclastic activity. Int. J. Nanomed. 2019, 14, 6019–6033. [Google Scholar] [CrossRef]
- Chen, S.; Liang, H.; Ji, Y. Curcumin Modulates the Crosstalk Between Macrophages and Bone Mesenchymal Stem Cells to Ameliorate Osteogenesis. Front. Cell Dev. Biol. 2021, 9, 634650. [Google Scholar] [CrossRef] [PubMed]
- Jiao, H.; Xiao, E.; Graves, D.T. Diabetes and Its Effect on Bone and Fracture Healing. Curr. Osteoporos. Rep. 2015, 13, 327–335. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Li, Q.; Gui, L. Sequential gastrodin release PU/n-HA composite scaffolds reprogram macrophages for improved osteogenesis and angiogenesis. Bioact. Mater. 2022, 19, 24–37. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Sun, Y.; Wu, Y. Advances in chitosan-based drug delivery vehicles. Nanoscale 2013, 5, 3103–3111. [Google Scholar] [CrossRef]
- Ahmed, T.A.; Aljaeid, B.M. Preparation, characterization, and potential application of chitosan, chitosan derivatives, and chitosan metal nanoparticles in pharmaceutical drug delivery. Drug Des. Devel. Ther. 2016, 10, 483–507. [Google Scholar] [CrossRef]
- Liu, Y.; Gao, J.; Peng, M. A Review on Central Nervous System Effects of Gastrodin. Front. Pharmacol. 2018, 9, 24. [Google Scholar] [CrossRef]
- Zheng, M.; Guo, J.; Li, Q. Syntheses and characterization of anti-thrombotic and anti-oxidative Gastrodin-modified polyurethane for vascular tissue engineering. Bioact. Mater. 2021, 6, 404–419. [Google Scholar] [CrossRef]
- Wang, F.S.; Wu, R.W.; Chen, Y.S. Biophysical Modulation of the Mitochondrial Metabolism and Redox in Bone Homeostasis and Osteoporosis: How Biophysics Converts into Bioenergetics. Antioxidants 2021, 10, 1394. [Google Scholar] [CrossRef]
- Li, Y.; Hao, W.; Guan, J. Relationship between indices of circulating blood cells and bone homeostasis in osteoporosis. Front. Endocrinol. 2022, 13, 965290. [Google Scholar] [CrossRef]
- Sheu, A.; Greenfield, J.R.; White, C.P. Assessment and treatment of osteoporosis and fractures in type 2 diabetes. Trends Endocrinol. Metab. 2022, 33, 333–344. [Google Scholar] [CrossRef]
- Ruaro, B.; Casabella, A.; Paolino, S. Correlation between bone quality and microvascular damage in systemic sclerosis patients. Rheumatology 2018, 57, 1548–1554. [Google Scholar] [CrossRef] [PubMed]
- Chaichit, S.; Sato, T.; Yu, H. Evaluation of Dexamethasone-Induced Osteoporosis In Vivo Using Zebrafish Scales. Pharmaceuticals 2021, 14, 536. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Zhang, S. Clinical characteristics, diagnosis and treatment of dental implant repair in patients with diabetes. Chin. J. Stomatol. 2021, 56, 1172–1178. [Google Scholar]
- Zhang, C.; Zhang, T.; Geng, T. Dental Implants Loaded With Bioactive Agents Promote Osseointegration in Osteoporosis: A Review. Front. Bioeng. Biotechnol. 2021, 9, 591796. [Google Scholar] [CrossRef]
- De Medeiros, F.; Kudo, G.; Leme, B.G. Dental implants in patients with osteoporosis: A systematic review with meta-analysis. Int. J. Oral. Maxillofac. Surg. 2018, 47, 480–491. [Google Scholar] [CrossRef]
- Liang, B.; Burley, G.; Lin, S. Osteoporosis pathogenesis and treatment: Existing and emerging avenues. Cell Mol. Biol. Lett. 2022, 27, 72. [Google Scholar] [CrossRef]
- Almeida, M.; Han, L.; Martin-Millan, M. Oxidative Stress Antagonizes Wnt Signaling in Osteoblast Precursors by Diverting Î2-Catenin from T Cell Factor- to Forkhead Box O-mediated Transcription. J. Biol. Chem. 2007, 282, 27298. [Google Scholar] [CrossRef]
- Lee, N.K.; Choi, Y.G.; Baik, J.Y. A crucial role for reactive oxygen species in RANKL-induced osteoclast differentiation. Blood 2005, 106, 852–859. [Google Scholar] [CrossRef]
- Arai, M.; Shibata, Y.; Pugdee, K. Effects of reactive oxygen species (ROS) on antioxidant system and osteoblastic differentiation in MC3T3-E1 cells. IUBMB Life 2007, 59, 27–33. [Google Scholar] [CrossRef]
- Sanchez-Rodriguez, M.A.; Ruiz-Ramos, M.; Correa-Munoz, E. Oxidative stress as a risk factor for osteoporosis in elderly Mexicans as characterized by antioxidant enzymes. BMC Musculoskelet. Disord. 2007, 8, 124. [Google Scholar] [CrossRef]
- Manolagas, S.C. From estrogen-centric to aging and oxidative stress: A revised perspective of the pathogenesis of osteoporosis. Endocr. Rev. 2010, 31, 266–300. [Google Scholar] [CrossRef] [PubMed]
- Kanis, J.A.; Cooper, C.; Rizzoli, R. Executive summary of the European guidance for the diagnosis and management of osteoporosis in postmenopausal women. Calcif. Tissue Int. 2019, 104, 235–238. [Google Scholar] [CrossRef] [PubMed]
- Manolagas, S.C. De-fense! De-fense! De-fense: Scavenging H2O2 While Making Cholesterol. Endocrinology 2008, 149, 3264–3266. [Google Scholar] [CrossRef] [PubMed]
- Sendur, O.F.; Turan, Y.; Tastaban, E. Antioxidant status in patients with osteoporosis: A controlled study. Jt. Bone Spine 2009, 76, 514–518. [Google Scholar] [CrossRef]
- Ozgocmen, S.; Kaya, H.; Fadillioglu, E. Role of antioxidant systems, lipid peroxidation, and nitric oxide in postmenopausal osteoporosis. Mol. Cell Biochem. 2007, 295, 45–52. [Google Scholar] [CrossRef]
- Witko-Sarsat, V.; Friedlander, M.; Capeillere-Blandin, C. Advanced oxidation protein products as a novel marker of oxidative stress in uremia. Kidney Int. 1996, 49, 1304–1313. [Google Scholar] [CrossRef]
- Huang, Q.; Shi, J.; Gao, B. Gastrodin: An ancient Chinese herbal medicine as a source for anti-osteoporosis agents via reducing reactive oxygen species. Bone 2015, 73, 132–144. [Google Scholar] [CrossRef]
- Giorgi, C.; Marchi, S.; Simoes IC, M. Mitochondria and Reactive Oxygen Species in Aging and Age-Related Diseases. Int. Rev. Cell Mol. Biol. 2018, 340, 209–344. [Google Scholar]
- Anik, M.I.; Mahmud, N.; Masud, A.A. Role of Reactive Oxygen Species in Aging and Age-Related Diseases: A Review. ACS Appl. Bio Mater. 2022, 5, 4028–4054. [Google Scholar] [CrossRef]
- Sfeir, J.G.; Drake, M.T.; Khosla, S. Skeletal Aging. Mayo. Clin. Proc. 2022, 97, 1194–1208. [Google Scholar] [CrossRef]
- Giorgio, M.; Migliaccio, E.; Orsini, F. Electron transfer between cytochrome c and p66Shc generates reactive oxygen species that trigger mitochondrial apoptosis. Cell 2005, 122, 221–233. [Google Scholar] [CrossRef]
- Zhang, P.; Li, T.; Wu, X. Oxidative stress and diabetes: Antioxidative strategies. Front. Med. 2020, 14, 583–600. [Google Scholar] [CrossRef]
- Tong Ying ZG, D.X. A Survey of Research on Anti senility of Gastrodia elata. Chin. Folk. Med. 2020, 29, 42–46. [Google Scholar]
- Liu, S.; Fang, T.; Yang, L. Gastrodin protects MC3T3-E1 osteoblasts from dexamethasone-induced cellular dysfunction and promotes bone formation via induction of the NRF2 signaling pathway. Int. J. Mol. Med. 2018, 41, 2059–2069. [Google Scholar] [CrossRef]
- Takanche, J.S.; Kim, J.E.; Han, S.H. Effect of gomisin A on osteoblast differentiation in high glucose-mediated oxidative stress. Phytomedicine 2020, 66, 153107. [Google Scholar] [CrossRef]
- Li, X.; Ma, X.Y.; Feng, Y.F. Osseointegration of chitosan coated porous titanium alloy implant by reactive oxygen species-mediated activation of the PI3K/AKT pathway under diabetic conditions. Biomaterials 2015, 36, 44–54. [Google Scholar] [CrossRef]
- Ma, H.; Wang, X.; Zhang, W. Melatonin Suppresses Ferroptosis Induced by High Glucose via Activation of the Nrf2/HO-1 Signaling Pathway in Type 2 Diabetic Osteoporosis. Oxid. Med. Cell Longev. 2020, 2020, 9067610. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Feng, Z.; Li, J. High glucose induces autophagy of MC3T3-E1 cells via ROS-AKT-mTOR axis. Mol. Cell Endocrinol. 2016, 429, 62–72. [Google Scholar] [CrossRef] [PubMed]
- An, Y.; Zhang, H.; Wang, C. Activation of ROS/MAPKs/NF-kappaB/NLRP3 and inhibition of efferocytosis in osteoclast-mediated diabetic osteoporosis. FASEB J. 2019, 33, 12515–12527. [Google Scholar] [CrossRef]
- Huang, Z.; Jiang, Z.; Zheng, Z. Methyl 3, 4-dihydroxybenzoate inhibits RANKL-induced osteoclastogenesis via Nrf2 signaling in vitro and suppresses LPS-induced osteolysis and ovariectomy-induced osteoporosis in vivo. Acta Biochim. Biophys Sin. 2022, 54, 1068–1079. [Google Scholar] [CrossRef]
- Zhou, F.; Shen, Y.; Liu, B. Gastrodin inhibits osteoclastogenesis via down-regulating the NFATc1 signaling pathway and stimulates osseointegration in vitro. Biochem. Biophys. Res. Commun. 2017, 484, 820–826. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Yang, E.; Peng, H. Gastrodin prevents steroid-induced osteonecrosis of the femoral head in rats by anti-apoptosis. Chin. Med. J. 2014, 127, 3926–3931. [Google Scholar] [PubMed]
- Zhang, Z.; Gao, Y.; Zang, P. Research progress in the mechanism of gastrodin and p-hydroxybenzyl alcohol on central nervous system. Chin. J. Tradit. Chin. Med. 2020, 45, 312–320. [Google Scholar]
- Zhu, T.; Wang, L.; Feng, Y. Classical Active Ingredients and Extracts of Chinese Herbal Medicines: Pharmacokinetics, Pharmacodynamics, and Molecular Mechanisms for Ischemic Stroke. Oxid. Med. Cell Longev. 2021, 2021, 8868941. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L. Research progress in determination methods and pharmacokinetics of gastrodin in biological samples. World Sci. Technol. Mod. Tradit. Chin. Med. 2020, 22, 1930–1939. [Google Scholar]
- Cheng, Z.; Shan, S. Research progress on phenolic components of Gastrodia elata and their central nervous pharmacological effectsJ. Pharmacol. Clin. Tradit. Chin. Med. 2019, 35, 167–174. [Google Scholar]
- Tang, C.; Wang, L.; Liu, X. Comparative pharmacokinetics of gastrodin in rats after intragastric administration of free gastrodin, parishin and Gastrodia elata extractJ. J. Ethnopharmacol. 2015, 176, 49–54. [Google Scholar] [CrossRef]
- Ju, X.H.; Shi, Y.; Liu, N. Determination and pharmacokinetics of gastrodin in human plasma by HPLC coupled with photodiode array detectorJ. J Chromatogr B Analyt. Technol. Biomed. Life Sci. 2010, 878, 1982–1986. [Google Scholar] [CrossRef]
- Jiang, L.; Dai, J.; Huang, Z. Simultaneous determination of gastrodin and puerarin in rat plasma by HPLC and the application to their interaction on pharmacokineticsJ. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2013, 915, 8–12. [Google Scholar] [CrossRef]
- Liu, K. Determination of gastrodin in plasma and its pharmacokinetics. J. Dalian Med. Coll. 1986, 36–42. [Google Scholar]
- Jiang, Z.; Zheng, X.; Gong, X. Relative tissue distribution and excretion studies of gastrodin and parishin from powder and extract of Gastrodiae Rhizoma in rat by UHPLC-ESI-MS/MSJ. Biomed. Chromatogr. 2017, 31, e3909. [Google Scholar] [CrossRef] [PubMed]
- Liu, N. Study on pharmacokinetics and tissue distribution characteristics of gastrodinJ. J. Pharm. Anal. 2015, 35, 1369–1376. [Google Scholar]
- Wu, M. Study on the function and long-term toxicity of new gastrodin derivatives in ratsJ. West. China J. Pharm. 2021, 36, 284–288. [Google Scholar]
- Yang, X. Therapeutic effect and safety evaluation of new gastrodin derivatives on cerebral ischemia/reperfusion injury in ratsJ. Res. Dev. Nat. Prod. 2018, 30, 1427–1431. [Google Scholar]
- He, X. Study on the Acute Toxicity of Gastrodia elata Component D and Its Effect on Sleep in MiceJ. Chin. J. Tradit. Chin. Med. 2012, 30, 379–381. [Google Scholar]
- Zheng, Y. Analysis of 315 Clinical Adverse Reactions/Events of GastrodinJ. Chin. J. Tradit. Chin. Med. 2015, 40, 2037–2041. [Google Scholar]
- Liu, S.; Zhou, L.; Yang, L. Gastrodin alleviates glucocorticoid induced osteoporosis in rats via activating the Nrf2 signaling pathwaysJ. Oncotarget 2018, 9, 11528–11540. [Google Scholar] [CrossRef]
- Zhang, J. Preliminary study on the effect of gastrodin on bone tissue around implants in type 2 diabetes ratsJ. Chin. J. Stomatol. 2022, 57, 938–945. [Google Scholar]
- Feng, Q. Gastrodin attenuates lipopolysaccharide-induced inflammation and oxidative stress, and promotes the osteogenic differentiation of human periodontal ligament stem cells through enhancing sirtuin3 expressionJ. Exp. Med. 2022, 23, 296. [Google Scholar] [CrossRef]
- Chen, J.; Gu, Y.T.; Xie, J.J. Gastrodin reduces IL-1beta-induced apoptosis, inflammation, and matrix catabolism in osteoarthritis chondrocytes and attenuates rat cartilage degeneration in vivoJ. Biomed Pharm. 2018, 97, 642–651. [Google Scholar] [CrossRef]
- Lee, W.; Guntur, A.R.; Long, F. Energy Metabolism of the Osteoblast: Implications for OsteoporosisJ. Endocr. Rev. 2017, 38, 255–266. [Google Scholar] [CrossRef] [PubMed]
- Shares, B.H.; Busch, M.; White, N. Active mitochondria support osteogenic differentiation by stimulating beta-catenin acetylationJ. J. Biol. Chem. 2018, 293, 16019–16027. [Google Scholar] [CrossRef] [PubMed]
- Shengye, L. Molecular mechanism of gastrodin reversing glucocorticoid induced osteoporosis in rats through Nrf2 signal pathwayD. Ph.D. Thesis, China Medical University, Shenyang, China, 2018; pp. 1–77. [Google Scholar]
- Sánchez-de-Diego, C.; Pedrazza, L.; Pimenta-Lopes, C. NRF2 function in osteocytes is required for bone homeostasis and drives osteocytic gene expressionJ. Redox biology. 2021, 40, 101845. [Google Scholar] [CrossRef] [PubMed]
- Qu, L.L.; Yu, B.; Li, Z. Gastrodin Ameliorates Oxidative Stress and Proinflammatory Response in Nonalcoholic Fatty Liver Disease through the AMPK/Nrf2 PathwayJ. Phytother. Res. 2016, 30, 402–411. [Google Scholar] [CrossRef] [PubMed]
- Lai, L. Application of traditional Chinese medicine and its chemical components based on antioxidant mechanism in osteoporosisJ. J. Nav. Med. Univ. 2022, 43, 943–950. [Google Scholar]
- Kim, W.K.; Meliton, V.; Bourquard, N. Hedgehog signaling and osteogenic differentiation in multipotent bone marrow stromal cells are inhibited by oxidative stressJ. J. Cell Biochem. 2010, 111, 1199–1209. [Google Scholar] [CrossRef] [PubMed]
- Justesen, J.; Stenderup, K.; Ebbesen, E.N. Adipocyte tissue volume in bone marrow is increased with aging and in patients with osteoporosisJ. Biogerontology 2001, 2, 165–171. [Google Scholar] [CrossRef]
- Syed, F.A.; Oursler, M.J.; Hefferanm, T.E. Effects of estrogen therapy on bone marrow adipocytes in postmenopausal osteoporotic womenJ. Osteoporos. Int. 2008, 19, 1323–1330. [Google Scholar] [CrossRef]
- Giralt, A.; Villarroya, F. SIRT3, a pivotal actor in mitochondrial functions: Metabolism, cell death and agingJ. Biochem. J. 2012, 444, 1. [Google Scholar] [CrossRef]
- Huh, J.E.; Shin, J.H.; Jang, E.S. Sirtuin 3 (SIRT3) maintains bone homeostasis by regulating AMPK-PGC-1beta axis in miceJ. Sci. Rep. 2016, 6, 22511. [Google Scholar] [CrossRef]
- Cen, L.P.; Ng, T.K.; Liang, J.J. Human Periodontal Ligament-Derived Stem Cells Promote Retinal Ganglion Cell Survival and Axon Regeneration After Optic Nerve InjuryJ. Stem. Cells 2018, 36, 844–855. [Google Scholar] [CrossRef] [PubMed]
- Rose, G.; Santoro, A.; Salvioli, S. Mitochondria and mitochondria-induced signalling molecules as longevity determinantsJ. Mech. Ageing Dev. 2017, 165, 115–128. [Google Scholar] [CrossRef] [PubMed]
- Cosentino, K.; Garcia-Saez, A.J. Bax and Bak Pores: Are We Closing the Circle? J. Trends Cell Biol. 2017, 27, 266–275. [Google Scholar] [CrossRef] [PubMed]
- Marcu, K.B.; Otero, M.; Olivotto, E. NF-kappaB signaling: Multiple angles to target OAJ. Curr. Drug Targets 2010, 11, 599–613. [Google Scholar] [CrossRef]
- Kitazawa, R.; Kimble, R.B.; Vannice, J.L. Interleukin-1 receptor antagonist and tumor necrosis factor binding protein decrease osteoclast formation and bone resorption in ovariectomized miceJ. J. Clin. Investig. 1994, 94, 2397–2406. [Google Scholar] [CrossRef]
- Yan, L. The role and application of melatonin in the prevention and treatment of osteoporosisJ. Res. Tissue Eng. China 2022, 27, 2222–2228. [Google Scholar]
- Galibert, L.; Tometsko, M.E.; Anderson, D.M. The involvement of multiple tumor necrosis factor receptor (TNFR)-associated factors in the signaling mechanisms of receptor activator of NF-kappaB, a member of the TNFR superfamilyJ. J. Biol. Chem. 1998, 273, 34120–34127. [Google Scholar] [CrossRef]
- Takayanagi, H.; Kim, S.; Koga, T. Induction and activation of the transcription factor NFATc1 (NFAT2) integrate RANKL signaling in terminal differentiation of osteoclastsJ. Dev. Cell 2002, 3, 889–901. [Google Scholar] [CrossRef]
- Zhang, J. The osteoprotective effects of artemisinin compounds and the possible mechanisms associated with intracellular iron: A review of in vivo and in vitro studies. Environ. Toxicol. Pharmacol. 2020, 76, 103358. [Google Scholar] [CrossRef]
- Rouyère, C.; Serrano, T.; Frémont, S. Oxidation and reduction of actin: Origin, impact in vitro and functional consequences in vivoJ. Eur. J. Cell Biol. 2022, 101, 151249. [Google Scholar] [CrossRef]
- Valdivia, A.; Duran, C.; San Martin, A. The role of Nox-mediated oxidation in the regulation of cytoskeletal dynamicsJ. Curr. Pharm. Des. 2015, 21, 6009. [Google Scholar] [CrossRef] [PubMed]
- Taulet, N.; Delorme-Walker, V.D.; Der Mardirossian, C. Reactive oxygen species regulate protrusion efficiency by controlling actin dynamicsJ. PLoS ONE 2012, 7, e41342. [Google Scholar] [CrossRef] [PubMed]
- DalleDonne, I.; Milzani, A.; Colombo, R. H2O2-treated actin: Assembly and polymer interactions with cross-linking proteinsJ. Biophys. J. 1995, 69, 2710–2719. [Google Scholar] [CrossRef]
- Lassing, I.; Schmitzberger, F.; Bjornstedt, M. Molecular and structural basis for redox regulation of beta-actin. J. Mol. Biol. 2007, 370, 331–348. [Google Scholar] [CrossRef]
- Lin, W.; Izu, Y.; Smriti, A. Profilin1 is expressed in osteocytes and regulates cell shape and migrationJ. J. Cell Physiol. 2018, 233, 259–268. [Google Scholar] [CrossRef]
- Lin, J.; Shi, Y.; Miao, J. Gastrodin Alleviates Oxidative Stress-Induced Apoptosis and Cellular Dysfunction in Human Umbilical Vein Endothelial Cells via the Nuclear Factor-Erythroid 2-Related Factor 2/Heme Oxygenase-1 Pathway and Accelerates Wound Healing In Vivo. Front. Pharm. 2019, 10, 1273. [Google Scholar] [CrossRef]
- Wang, J.; Wu, M. The up-regulation of miR-21 by gastrodin to promote the angiogenesis ability of human umbilical vein endothelial cells by activating the signaling pathway of PI3K/AktJ. Bioengineered 2021, 12, 5402–5410. [Google Scholar] [CrossRef]
- Zhang XL, N.; Yang, X.K.; Wu, C.Y. Gastrodin exerts protective effects in reactive TNC1 astrocytes via regulation of the Notch signaling pathwayJ. Ann. Transl. Med. 2021, 9, 1754. [Google Scholar] [CrossRef]
- Cheng, Q.Q.; Wan, Y.W.; Yang, W.M. Gastrodin protects H9c2 cardiomyocytes against oxidative injury by ameliorating imbalanced mitochondrial dynamics and mitochondrial dysfunctionJ. Acta Pharm. Sin. 2020, 41, 1314–1327. [Google Scholar] [CrossRef]
- De Oliveira, M.R.; de Bittencourt, B.F.; Furstenau, C.R. Inhibition of the Nrf2/HO-1 Axis Suppresses the Mitochondria-Related Protection Promoted by Gastrodin in Human Neuroblastoma Cells Exposed to ParaquatJ. Mol. Neurobiol. 2019, 56, 2174–2184. [Google Scholar] [CrossRef]
- De Oliveira, M.R.; Brasil, F.B.; Furstenau, C.R. Evaluation of the Mitochondria-Related Redox and Bioenergetics Effects of Gastrodin in SH-SY5Y Cells Exposed to Hydrogen PeroxideJ. J. Mol. Neurosci. 2018, 64, 242–251. [Google Scholar] [CrossRef] [PubMed]
- Zhai, Z.; Wang, X.; Ding, M. Cell BiologyM. 4; Higher Education Press: Beijing, China, 2011. [Google Scholar]
- Fremont, S.; Hammich, H.; Bai, J. Oxidation of F-actin controls the terminal steps of cytokinesis. Nat. Commun. 2017, 8, 14528. [Google Scholar] [CrossRef] [PubMed]
- Hung, R.J.; Pak, C.W.; Terman, J.R. Direct redox regulation of F-actin assembly and disassembly by MicalJ. Science 2011, 334, 1710–1713. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.C.; Lai, Y.S.; Lin, S.H. Anti-depressant effects of Gastrodia elata Blume and its compounds gastrodin and 4-hydroxybenzyl alcohol, via the monoaminergic system and neuronal cytoskeletal remodelingJ. J. Ethnopharmacol. 2016, 182, 190–199. [Google Scholar] [CrossRef] [PubMed]
- Labandeira-Garcia, J.L.; Rodríguez-Perez, A.I.; Garrido-Gil, P. Brain Renin-Angiotensin System and Microglial Polarization: Implications for Aging and NeurodegenerationJ. Front. Aging Neurosci. 2017, 9, 129. [Google Scholar] [CrossRef]
- Vaz De Castro PA, S.; Jose, P.A.; Simões ESilva, A.C. Interactions between the intrarenal dopaminergic and the renin-angiotensin systems in the control of systemic arterial pressureJ. Clin. Sci. 2022, 136, 1205–1227. [Google Scholar] [CrossRef]
- Mo, C.; Ke, J.; Zhao, D. Role of the renin-angiotensin-aldosterone system in bone metabolismJ. J. Bone Min. Metab. 2020, 38, 772–779. [Google Scholar] [CrossRef]
- Gu, S.S.; Zhang, Y.; Li, X.L. Involvement of the skeletal renin-angiotensin system in age-related osteoporosis of ageing miceJ. Biosci. Biotechnol. Biochem. 2012, 76, 1367–1371. [Google Scholar] [CrossRef]
- Yongtao, Z.; Kunzheng, W.; Jingjing, Z. Glucocorticoids activate the local renin-angiotensin system in bone: Possible mechanism for glucocorticoid-induced osteoporosisJ. Endocrine 2014, 47, 598–608. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, L.; Song, Y. Renin inhibitor aliskiren exerts beneficial effect on trabecular bone by regulating skeletal renin-angiotensin system and kallikrein-kinin system in ovariectomized miceJ. Osteoporos. Int. 2016, 27, 1083–1092. [Google Scholar] [CrossRef]
- Nakai, K.; Kawato, T.; Morita, T. Angiotensin II suppresses osteoblastic differentiation and mineralized nodule formation via AT1 receptor in ROS17/2.8 cellsJ. Arch. Med. Sci. 2015, 11, 628–637. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Wang, C.; Xu, Y. Effects of the Local Bone Renin-Angiotensin System on Titanium-Particle-Induced Periprosthetic OsteolysisJ. Front. Pharm. 2021, 12, 684375. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, H.; Nakagami, H.; Osako, M.K. Angiotensin II accelerates osteoporosis by activating osteoclastsJ. FASEB J. 2008, 22, 2465–2475. [Google Scholar] [CrossRef] [PubMed]
- Kunutsor, S.K.; Blom, A.W.; Whitehouse, M.R. Renin-angiotensin system inhibitors and risk of fractures: A prospective cohort study and meta-analysis of published observational cohort studiesJ. Eur. J. Epidemiol. 2017, 32, 947–959. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, H.; Nakagami, H.; Osako, M.K. Prevention of osteoporosis by angiotensin-converting enzyme inhibitor in spontaneous hypertensive ratsJ. Hypertens. Res. 2009, 32, 786–790. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Guan, X.; Chen, X. Angiotensin II/Angiotensin II Receptor Blockade Affects Osteoporosis via the AT1/AT2-Mediated cAMP-Dependent PKA PathwayJ. Cells Tissues Organs 2017, 204, 25–37. [Google Scholar] [CrossRef]
- Asaba, Y.; Ito, M.; Fumoto, T. Activation of renin-angiotensin system induces osteoporosis independently of hypertensionJ. J. Bone Min. Res. 2009, 24, 241–250. [Google Scholar] [CrossRef]
- Liu, S.; Liu, X.; Li, J. Gastrodin attenuates microglia activation through renin-angiotensin system and Sirtuin3 pathwayJ. Neurochem. Int. 2018, 120, 49–63. [Google Scholar] [CrossRef]
- Wu, F.; Zuo, H.J.; Ren, X.Q. Gastrodin Regulates the Notch-1 Signal Pathway via Renin-Angiotensin System in Activated MicrogliaJ. Neuromolecular. Med. 2022, 6. [Google Scholar] [CrossRef]
- Lu, J.; Ma, X.; Gao, W.C. Gastrodin Exerts Cardioprotective Action via Inhibition of Insulin-Like Growth Factor Type 2/Insulin-Like Growth Factor Type 2 Receptor Expression in Cardiac HypertrophyJ. ACS Omega 2021, 6, 16763–16774. [Google Scholar] [CrossRef]
- Liu, W.; Wang, L.; Yu, J. Gastrodin Reduces Blood Pressure by Intervening with RAAS and PPARgamma in SHRsJ. Evid. Based Complement Altern. Med. 2015, 2015, 828427. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Dong, R.; Wu, Y. Physiological Effects of Ferroptosis on Organ Fibrosis. Oxidative Med. Cell. Longev. 2022, 2022, 5295434. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Lin, Y.; Wang, M. Targeting ferroptosis suppresses osteocyte glucolipotoxicity and alleviates diabetic osteoporosis. Bone Res. 2022, 10, 26. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Xia, T.; Zhang, S. Hops extract and xanthohumol ameliorate bone loss induced by iron overload via activating Akt/GSK3beta/Nrf2 pathwayJ. J. Bone Min. Metab. 2022, 40, 375–388. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhao, L.; Shen, Y. Curculigoside Protects against Excess-Iron-Induced Bone Loss by Attenuating Akt-FoxO1-Dependent Oxidative Damage to Mice and Osteoblastic MC3T3-E1 CellsJ. Oxidative Med. Cell. Longev. 2019, 2019, 9281414–9281481. [Google Scholar] [CrossRef]
- Zhang, S.; Wu, W.; Jiao, G. MiR-455-3p activates Nrf2/ARE signaling via HDAC2 and protects osteoblasts from oxidative stressJ. Int. J. Biol. Macromol. 2018, 107, 2094–2101. [Google Scholar] [CrossRef]
- Jiang, T.; Cheng, H.; Su, J. Gastrodin protects against glutamate-induced ferroptosis in HT-22 cells through Nrf2/HO-1 signaling pathwayJ. Toxicol. Vitr. 2020, 62, 104715. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, E.; Yang, H. Gastrodin Ameliorates Cognitive Dysfunction in Vascular Dementia Rats by Suppressing Ferroptosis via the Regulation of the Nrf2/Keap1-GPx4 Signaling PathwayJ. Molecules 2022, 27, 6311. [Google Scholar] [CrossRef]
- Chen, X.; Wang, J.; He, Z. Analgesic and Anxiolytic Effects of Gastrodin and Its Influences on Ferroptosis and Jejunal Microbiota in Complete Freund’s Adjuvant-Injected MiceJ. Front. Microbiol. 2022, 13, 841662. [Google Scholar] [CrossRef]
- Lee, S.K.; Kim, H.; Park, J. Artemisia annua extract prevents ovariectomy-induced bone loss by blocking receptor activator of nuclear factor kappa-B ligand-induced differentiation of osteoclastsJ. Sci. Rep. 2017, 7, 17332. [Google Scholar] [CrossRef]
- Wei, C.M.; Liu, Q.; Song, F.M. Artesunate inhibits RANKL-induced osteoclastogenesis and bone resorption in vitro and prevents LPS-induced bone loss in vivo. J. Cell. Physiol. 2018, 233, 476–485. [Google Scholar] [CrossRef] [PubMed]
- Feng, M.; Hong, J.; Wang, Q. Dihydroartemisinin prevents breast cancer-induced osteolysis via inhibiting both breast caner cells and osteoclastsJ. Sci. Rep. 2016, 6, 19074. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Mu, W.; Xu, B. Artesunate, an Anti-Malaria Agent, Attenuates Experimental Osteoarthritis by Inhibiting Bone Resorption and CD31(hi)Emcn(hi) Vessel Formation in Subchondral BoneJ. Front. Pharm. 2019, 10, 685. [Google Scholar] [CrossRef] [PubMed]
- Ge, X.; Chen, Z.; Xu, Z. The effects of dihydroartemisinin on inflammatory bowel disease-related bone loss in a rat modelJ. Exp. Biol. Med. 2018, 243, 715–724. [Google Scholar] [CrossRef]
- Efferth, T. From ancient herb to modern drug: Artemisia annua and artemisinin for cancer therapyJ. Semin. Cancer Biol. 2017, 46, 65–83. [Google Scholar] [CrossRef]
- Ishii, K.A.; Fumoto, T.; Iwai, K. Coordination of PGC-1beta and iron uptake in mitochondrial biogenesis and osteoclast activationJ. Nat. Med. 2009, 15, 259–266. [Google Scholar] [CrossRef]
- Zhou, L.; Liu, Q.; Yang, M. Dihydroartemisinin, an Anti-Malaria Drug, Suppresses Estrogen Deficiency-Induced Osteoporosis, Osteoclast Formation, and RANKL-Induced Signaling PathwaysJ. J. Bone Min. Res. 2016, 31, 964–974. [Google Scholar] [CrossRef]
- Wu, H.; Hu, B.; Zhou, X. Artemether attenuates LPS-induced inflammatory bone loss by inhibiting osteoclastogenesis and bone resorption via suppression of MAPK signaling pathwayJ. Cell Death Dis. 2018, 9, 498. [Google Scholar] [CrossRef]
- Dou, C.; Ding, N.; Xing, J. Dihydroartemisinin attenuates lipopolysaccharide-induced osteoclastogenesis and bone loss via the mitochondria-dependent apoptosis pathwayJ. Cell Death Dis. 2016, 7, e2162. [Google Scholar] [CrossRef]
- Li, L.; Li, Q.; Yang, J. Enhancement in mechanical properties and cell activity of polyurethane scaffold derived from gastrodin. Mater. Lett. 2018, 228, 435–438. [Google Scholar] [CrossRef]
- Yang, H.; Li, Q.; Li, L. Gastrodin modified polyurethane conduit promotes nerve repair via optimizing Schwann cells functionJ. Bioact. Mater. 2022, 8, 355–367. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Li, L.; Yu, M. Elastomeric polyurethane porous film functionalized with gastrodin for peripheral nerve regenerationJ. J. Biomed. Mater Res. A 2020, 108, 1713–1725. [Google Scholar] [CrossRef] [PubMed]
- Ochsner, P.E. Osteointegration of orthopaedic devicesJ. Semin. Immunopathol. 2011, 33, 245–256. [Google Scholar] [CrossRef]
- Behary, N.; Eap, S.; Cayla, A. Nano-Structured Ridged Micro-Filaments (>/=100 microm Diameter) Produced Using a Single Step Strategy for Improved Bone Cell Adhesion and Proliferation in Textile Scaffolds. Molecules 2022, 27, 3790. [Google Scholar] [CrossRef]
- Jo, S.; Kang, S.M.; Park, S.A. Enhanced Adhesion of Preosteoblasts inside 3D PCL Scaffolds by Polydopamine Coating and MineralizationJ. Macromol. Biosci. 2013, 13, 1389–1395. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Wang, J.; Song, W. The researches on cells adhesion on the biocompatible polymer surface with superwettbility. Front. Bioeng. Biotechnol. 2022, 4, 9. Available online: https://www.frontiersin.org/10.3389/conf.FBIOE.2016.01.01348/eventabstract (accessed on 16 November 2022).
- Chen, S.; Guo, Y.; Liu, R. Tuning surface properties of bone biomaterials to manipulate osteoblastic cell adhesion and the signaling pathways for the enhancement of early osseointegrationJ. Colloids Surf. B Biointerfaces 2018, 164, 58–69. [Google Scholar] [CrossRef]
- Ren, B.; Wan, Y.; Wang, G. Morphologically modified surface with hierarchical micro-/nano-structures for enhanced bioactivity of titanium implantsJ. J. Mater. Sci. 2018, 53, 12679–12691. [Google Scholar] [CrossRef]
- Wang, T.; Wan, Y.; Liu, Z. Fabrication of hierarchical micro/nanotopography on bio-titanium alloy surface for cytocompatibility improvementJ. J. Mater. Sci. 2016, 51, 9551–9561. [Google Scholar] [CrossRef]
- Wei, W.; Lingzhou, Z.B.; Ma, A. The role of the Wnt/b-catenin pathway in the effect of implant topographyon MG63 differentiationJ. Biomaterials 2012, 32, 7993–8002. [Google Scholar]
- Yang, Y.; Zhang, T.; Jiang, M. Effect of the immune responses induced by implants in a integrated three-dimensional micro-nano topography on osseointegrationJ. J. Biomed. Mater. Research. Part A 2021, 109, 1429–1440. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zuo, Y.; Zou, Q. Hierarchical Structure and Mechanical Improvement of an n-HA/GCO–PU Composite Scaffold for Bone RegenerationJ. ACS Appl. Mater. Interfaces 2015, 7, 22618–22629. [Google Scholar] [CrossRef] [PubMed]
- Dimitriou, R.; Jones, E.; McGonagle, D. Bone regeneration: Current concepts and future directionsJ. BMC Med. 2011, 9, 66. [Google Scholar] [CrossRef] [PubMed]
- Einhorn, T.A. The Cell and Molecular Biology of Fracture HealingJ. Clin. Orthop. Relat. Res. 1998, 355, S7–S21. [Google Scholar] [CrossRef]
- He, J.; Chen, G.; Liu, M. Scaffold strategies for modulating immune microenvironment during bone regenerationJ. Mater. Sci. Eng. C 2020, 108, 110411. [Google Scholar] [CrossRef]
- Wang, Q.; Tang, Y.; Ke, Q. Magnetic lanthanum-doped hydroxyapatite/chitosan scaffolds with endogenous stem cell-recruiting and immunomodulatory properties for bone regenerationJ. J. Mater. Chem. B 2020, 8, 5280–5292. [Google Scholar] [CrossRef]
- Ji, X.; Yuan, X.; Ma, L. Mesenchymal stem cell-loaded thermosensitive hydroxypropyl chitin hydrogel combined with a three-dimensional-printed poly(ε-caprolactone) /nano-hydroxyapatite scaffold to repair bone defects via osteogenesis, angiogenesis and immunomodulationJ. Theranostics 2020, 10, 725–740. [Google Scholar] [CrossRef]
- Alaarg, A.; Perez-Medina, C.; Metselaar, J.M. Applying nanomedicine in maladaptive inflammation and angiogenesisJ. Adv. Drug Deliv. Rev. 2017, 119, 143–158. [Google Scholar] [CrossRef]
- Lee, C.; Kozaki, T.; Ginhoux, F. Studying tissue macrophages in vitro: Are iPSC-derived cells the answer? J. Nat. Rev. Immunol. 2018, 18, 716–725. [Google Scholar] [CrossRef]
- Juban, G.; Chazaud, B. Metabolic regulation of macrophages during tissue repair: Insights from skeletal muscle regenerationJ. FEBS Lett. 2017, 591, 3007–3021. [Google Scholar] [CrossRef]
- Sadowska, J.M.; Wei, F.; Guo, J. The effect of biomimetic calcium deficient hydroxyapatite and sintered beta-tricalcium phosphate on osteoimmune reaction and osteogenesisJ. Acta Biomater 2019, 96, 605–618. [Google Scholar] [CrossRef] [PubMed]
- Diomede, F.; Marconi, G.D.; Fonticoli, L. Functional Relationship between Osteogenesis and Angiogenesis in Tissue RegenerationJ. Int. J. Mol. Sci. 2020, 21, 3242. [Google Scholar] [CrossRef] [PubMed]
- Street, J.; Bao, M.; De Guzman, L. Vascular endothelial growth factor stimulates bone repair by promoting angiogenesis and bone turnoverJ. Proc. Natl. Acad. Sci. USA 2002, 99, 9656–9661. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.S.; Yang, S.S.; You, H.K. Fucoidan-induced osteogenic differentiation promotes angiogenesis by inducing vascular endothelial growth factor secretion and accelerates bone repairJ. J. Tissue Eng. Regen. Med. 2018, 12, e1311–e1324. [Google Scholar] [CrossRef] [PubMed]
- Quinlan, E.; Lopez-Noriega, A.; Thompson, E.M. Controlled release of vascular endothelial growth factor from spray-dried alginate microparticles in collagen-hydroxyapatite scaffolds for promoting vascularization and bone repairJ. J. Tissue Eng. Regen. Med. 2017, 11, 1097–1109. [Google Scholar] [CrossRef]
- Wang, S.; Nan, Y.; Zhu, W. Gastrodin improves the neurological score in MCAO rats by inhibiting inflammation and apoptosis, promoting revascularizationJ. Int. J. Clin. Exp. Pathol. 2018, 11, 5343–5350. [Google Scholar]
- Zhang, F.; Deng, C.; Huang, Y. Early Intervention of Gastrodin Improved Motor Learning in Diabetic Rats Through Ameliorating Vascular DysfunctionJ. Neurochem. Res. 2020, 45, 1769–1780. [Google Scholar] [CrossRef]
- Liu, M.; Zhao, L.; Han, L. Discovery and identification of proangiogenic chemical markers from Gastrodiae Rhizoma based on zebrafish model and metabolomics approachJ. Phytochem. Anal. 2020, 31, 835–845. [Google Scholar] [CrossRef]
- Cui, J. Progress in application of sustained release drug delivery system on implant surfaceJ. Oral Biomed. 2022, 13, 63–66. [Google Scholar]
| Model | Type | Inducer | Animal/Cell | Major Findings | RF |
|---|---|---|---|---|---|
| Oxidative stress | In vitro | DEX | MC3T3-E1 cells | ↑Cell viability, ↑Osteogenic differentiation, ↓ROS, ↑Nrf2/Keapl pathway | [44] |
| In vivo | DEX | SD rats | ↑BMD, ↑Trabecular microstructure, ↑Skeletal mechanical strength, ↓ROS, ↑Nrf2/Keapl pathway | [67] | |
| In vitro | H2O2 | HBMMSCs | ↑Cell viability, ↑Osteogenic differentiation, ↓Lipogenic differentiation, ↓ROS | [37] | |
| In vivo | OVX | BALB/c female mice | ↑Trabecular microstructure, ↑Mineral apposition rate, ↓MDA, ↑GSH | [37] | |
| In vivo | High glycolipid + Streptozocin | SD rats | ↑Trabecular microstructure, ↓MDA, ↑SOD | [68] | |
| In vivo | Sodium fluoride | Wistar rats | ↑Trabecular microstructure, ↓MDA, ↑CAT | [9] | |
| In vitro | LPS | hPDLSCs | ↑Osteogenic differentiation, ↓ROS, ↓MDA, ↓ LDH, ↑SIRT3 | [69] | |
| In vitro | H2O2 | RAW264.7 cells | ↓Osteoclastic differentiation, ↓NFATc1, ↓TRAP, ↓CTR, ↓CTSK | [37] | |
| In vivo | H2O2 | RAW264.7 cells | ↓Osteoclastic differentiation, ↓TRAP, ↓CTX-1 | [37] | |
| In vitro | -- | BMMs | ↓NFATc1, ↓TRAP, ↓CTSK, ↓DC-STAMP | [51] | |
| Apoptosis | In vitro | Sodium fluoride | MC3T3-E1 cells | ↑Cell viability, ↓Caspase 3, ↓Caspase 9, ↓Bax | [9] |
| In vivo | Steroid | Wistar rats | ↓Osteonecrosis rate, ↓Bax, ↓Caspase 3, ↑Bcl-2 | [52] | |
| In vivo | High glycolipid + Streptozocin | SD rats | ↑Trabecular microstructure, ↓Bax, ↑Bcl-2 | [68] | |
| In vitro | DEX | MC3T3-E1 cells | ↑Cell viability, ↑Osteogenic differentiation, ↓Caspase 3 | [44] | |
| In vivo | DEX | SD rats | ↑BMD,↑Trabecular microstructure, ↑Skeletal mechanical strength, ↑AIF, ↓Caspase 3, ↓Bax, ↑Bcl-2 | [67] | |
| In vitro | LPS | hPDLSCs | ↑Osteogenic differentiation, ↓Caspase 3, ↓Caspase 9, ↓Bax, ↑Bcl-2 | [69] | |
| In vitro | 1L-1β | Chondrocytes | ↑Cell viability, ↓Caspase 3, ↓Bax, ↑Bcl-2 | [70] | |
| Inflammation | In vitro | LPS | hPDLSCs | ↑Osteogenic differentiation, ↓TNF-α, ↓IL-6 | [69] |
| In vitro | 1L-1β | Chondrocytes | ↑Cell viability, ↓TNF-α, ↓IL-6, ↓NF-κB pathway | [70] | |
| In vivo | OA | SD rats | ↑Cartilage structure, ↓OARSI scores, ↓MMP3, ↓TNF-α | [70] | |
| In vitro | H2O2 | HBMMSCs | ↑Cell viability, ↑Osteogenic differentiation, ↓Lipogenic differentiation, ↓RANKL, ↓IL-6 | [37] |
| Components | Mode | Type | Tissue/Cell | Major Findings | RF |
|---|---|---|---|---|---|
| Organizational engineering | Anti- oxidation | In vitro | HUVECs (H2O2) | ↑Cell viability, ↑Nrf2, ↑HO-1 | [17] |
| Improvement of hydrophilic | In vitro | RBMSCs | ↑Adhesion, ↑Migration | [13] | |
| In vivo | Femoral condyle defect of rats | ↑Osteogenesis, ↑Angiogenesis | [13] | ||
| In vitro | HUVECs; Schwann cells; PC12 cells | ↑Adhesion, ↑Migration | [17,143,144] | ||
| In vivo | Subcutaneous pocket of rats | ↑Angiogenesis, ↑Nerve regeneration | [17,143] | ||
| Anti- inflammatory | In vitro | RAW264.7 cells, RBMSCs, HUVECs | ↑M2 polarization, ↑CD206, ↓Arg-1; ↑BMP-2, ↑ALP; ↑VEGF, ↑BFGF | [13] | |
| In vivo | Femoral condyle defect of rats | ↑Osteogenesis, ↑Angiogenesis | [13] | ||
| pro-vascular regeneration | In vitro | HUVECs | ↑Cell viability, ↑Angiogenesis; ↑VEGF | [13] | |
| In vivo | Femoral condyle defect of rats; Subcutaneous pocket of rats | ↑Osteogenesis, ↑Angiogenesis | [13,17,143] | ||
| Implant osseointegration | -- | In vitro | BMSCs | ↑Adhesion on titanium plates, ↑ALP | [51] |
| Anti- oxidation | In vivo | Rats (T2DM) | ↑Trabecular microstructure around implant, ↓MDA, ↑SOD | [68] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Li, F. Mechanism and Prospect of Gastrodin in Osteoporosis, Bone Regeneration, and Osseointegration. Pharmaceuticals 2022, 15, 1432. https://doi.org/10.3390/ph15111432
Li Y, Li F. Mechanism and Prospect of Gastrodin in Osteoporosis, Bone Regeneration, and Osseointegration. Pharmaceuticals. 2022; 15(11):1432. https://doi.org/10.3390/ph15111432
Chicago/Turabian StyleLi, Yi, and Fenglan Li. 2022. "Mechanism and Prospect of Gastrodin in Osteoporosis, Bone Regeneration, and Osseointegration" Pharmaceuticals 15, no. 11: 1432. https://doi.org/10.3390/ph15111432
APA StyleLi, Y., & Li, F. (2022). Mechanism and Prospect of Gastrodin in Osteoporosis, Bone Regeneration, and Osseointegration. Pharmaceuticals, 15(11), 1432. https://doi.org/10.3390/ph15111432






