Abstract
Antimicrobial resistance (AMR) is a leading cause of treatment failure for many infectious diseases worldwide. Improper overdosing and the misuse of antibiotics contributes significantly to the emergence of drug-resistant bacteria. The co-contamination of heavy metals and antibiotic compounds existing in the environment might also be involved in the spread of AMR. The current study was designed to test the efficacy of heavy metals (arsenic) induced AMR patterns in clinically isolated extended-spectrum β-lactamase (ESBL) producing bacteria. A total of 300 clinically isolated ESBL-producing bacteria were collected from a tertiary care hospital in Lahore, Pakistan, with the demographic characteristics of patients. After the collection of bacterial isolates, these were reinoculated on agar media for reidentification purposes. Direct antimicrobial sensitivity testing (AST) for bacterial isolates by disk diffusion methods was used to determine the AST patterns with and without heavy metal. The heavy metal was concentrated in dilutions of 1.25 g/mL. The collected bacterial isolates were isolated from wounds (n = 63, 21%), urine (n = 112, 37.3%), blood (n = 43, 14.3%), pus (n = 49, 16.3%), and aspirate (n = 33, 11%) samples. From the total 300 bacterial isolates, n = 172 were Escherichia coli (57.3%), 57 were Klebsiella spp. (19%), 32 were Pseudomonas aeruginosa (10.6%), 21 were Proteus mirabilis (7%) and 18 were Enterobacter spp. (6%). Most of the antibiotic drugs were found resistant to tested bacteria. Colistin and Polymyxin-B showed the highest sensitivity against all tested bacteria, but when tested with heavy metals, these antibiotics were also found to be significantly resistant. We found that heavy metals induced the resistance capability in bacterial isolates, which leads to higher AMR patterns as compared to without heavy metal tested isolates. The results of the current study explored the heavy metal as an inducer of AMR and may contribute to the formation and spread of AMR in settings that are contaminated with heavy metals.
1. Introduction
The rising cases of antimicrobial resistance (AMR) in bacteria are threatening the potency of antibiotics, which have revolutionized therapies, and threaten millions of lives [1]. The AMR has reached the generation of antibiotics after the emergence of the first resistance cases against penicillin and other drugs [2]. The AMR has been linked to misuse and improper use of such drugs, in addition to a shortage of novel treatment products by the biopharmaceutical sector, as a result of diminished financial remuneration and difficult compliance standards [3,4]. The Centers for Disease Control and Prevention (CDC) has identified a variety of pathogens as severe and pose alarming risks, several of which are already imposing a major interventional and economic impact on the United States (US) healthcare system, patients, and their communities [5].
Bacteria are more likely to acquire AMR as antibiotics are used more frequently. As a result, antibiotics will be ineffective when we need them in the future [6]. If we reduce the use of antibiotics, there might be a chance to reduce the prevalence of high AMR rates [4,7]. By the use of antibiotics, certain bacteria die, while resistant bacteria thrive and ultimately multiply. Antibiotic excessive use raises the prevalence of resistant bacteria [8]. The World Health Organization (WHO) survey presumed that the issue was connected with the pervasiveness and abundance of resistant microorganisms and genes in bacteria [9].
The extended-spectrum β-lactamase (ESBL) are the enzymes that express by genes located on the plasmids [10]. They show strong hydrolytic activity against aztreonam, cephalosporins and penicillin and play a vital role in multi-resistant (MDR) gram-negative bacteria [11]. The ESBLs are classified into three major groups such as CTX-M, SHV and TEM types [10]. Escherichia coli [12], Klebsiella spp. [12], Pseudomonas spp. [13] Proteus and Enterobacter spp. [14] are highly reported ESBL-producing bacteria worldwide. Nosocomial infections, often known as infections linked to hospitals or medical clinics, are thought to be the most common adverse event that endangers patient safety and has negative economic and societal repercussions. Nosocomial infection caused by Pseudomonas spp., Klebsiella spp., Escherichia coli, and Staphylococcus spp. [15].
The prevalence of beta-lactamases is being reported worldwide. β-lactams are commonly used broad-spectrum antibiotics with high efficacy, cost-effectiveness, easy delivery, and low adverse effects [16]. The risks of post-antimicrobial therapy have provoked policymakers to recognize the critical alert to human health and commit extra funding, progressively driving a resurgence of interest in antimicrobial discovery and improvement. The increased use of antibiotics can raise AMR against organisms, whereas multiple AMR has turned into a significant medical problem [17].
Additionally, hazardous metals from agrochemicals, industrial wastewater, and gas and coal mining industries can contaminate aquatic environments [18]. Because they accumulate via the food chain and pose risks to the environment, toxic metals are dangerous. The heavy metals are absorbed into enzymes and cofactors, making them necessary micronutrients for bacteria [1]. The aim of the current study was to see the effects of heavy metals on antibiotic susceptibility patterns of clinically isolated ESBL-producing bacteria of nosocomial origin.
2. Results
The sum of n = 300 clinical isolates was collected randomly from the microbiology laboratory of a tertiary care hospital in Lahore, Pakistan. These bacterial isolates were isolated from different types of clinical samples, as shown in Table 1. Most of the bacterial isolates were isolated from patients with urinary tract infections. The frequency of bacterial isolates is shown in Table 1. The type of specimen for these bacterial isolates and the demographic characteristics of infected patients has been shown in Table 1.

Table 1.
Distribution of study variables.
The Phenotypic Confirmatory Test for the Synthesis of Extended-Spectrum Beta-Lactamase Was as Follows
The antibiotics susceptibility pattern of ESBL-producing bacteria has been seen in the disk diffusion test, as shown in Figure 1.
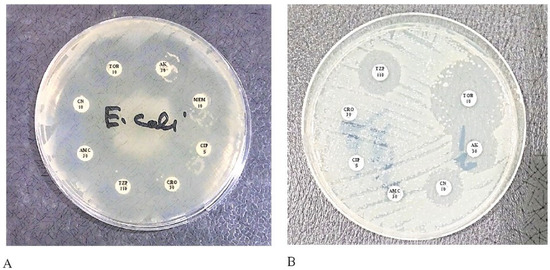
Figure 1.
The transformation between ESBL and Non-ESBL bacteria. Ceftriaxone (CRO) antibiotic is a third-generation cephalosporin that is reactive to non-ESBL strains but Non-reactive to ESBL strains; (A) is a non-ESBL strain so susceptible to CRO; (B) is an ESBL strain and CRO resistant.
The test was considered ESBL positive when the bacteria were less sensitive to cefotaxime, and there was a clear increase in the inhibition zone of ceftriaxone in front of the Clavulanate-containing disc (Figure 2, left). This often creates a shape called a champagne cork or a keyhole, as shown in Figure 2 (right).
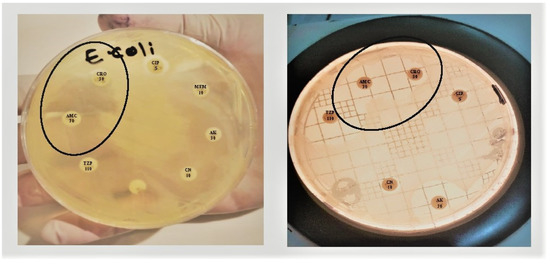
Figure 2.
Double disc synergy test (DDST): A decreased susceptibility to ceftriaxone (CRO) is combined with a clear-cut enhancement of the ZOI in front of the Amoxicillin Clavulanate (AMC) containing disk as showed in the circle, often resulting in a characteristic shape-zone referred to as ‘champagne-cork’ or ‘keyhole.’.
Most of the drugs were resistant, such as that enlisted by the CLSI-2020 for ESBL-producing bacteria, while, Colistin and Polymyxin-B were the only drugs that showed good efficacy against all isolated ESBL organisms. The direct antibiogram of tested bacterial isolates has been shown in Table 2.

Table 2.
Percentage resistance profile of clinically isolated bacteria.
ESBL-producing bacteria were analyzed as thick growth against Arsenic dilution 1.25 g/mL after incubation at 37 °C for 24 h. The heavy metals have significantly increased the resistance rate of antibiotics, as shown in Table 3.

Table 3.
Heavy metals (Arsenic) induced resistance against the CLSI-approved ESBL antibiogram panel.
3. Discussion
AMR is a major issue that might put the world in another pandemic. The misuse of antibiotics, excessive intake, and improper use are the major risk factors that contribute to the emergence of AMR [19]. The is a significant association between the misuse of antibiotics and the spread of AMR [20]. Bacteria’s genetic makeup may pass the AMR mechanisms within or between the bacterial family members or may also acquire via transportable genetic elements like plasmids from other spp. The AMR may spread between different bacterial strains as a result of horizontal gene transfer. Moreover, mutagenesis may also be responsible for causing AMR. Globally, antimicrobial drugs are widely prescribed drugs to treat nosocomial bacterial infections [21]. Apart from these factors, the presence of heavy metals in the environment may also play an important role in the emergence of MDR bacterial strains. Keeping in mind the scenario, the current study was conducted to see the prevalence of AMR strains responsible for infection of nosocomial origin in the heavy metal-containing bacterial growth agar medium. The AMR patterns of bacterial strains on with and without heavy metal treated agar were compared with each other.
The multiplication of ESBLs in recent years has significantly increased. The predominance of ESBL-producing Klebsiella spp. differs from country to country. In an overview of research facilities in the Netherlands under 1.5% of E. coli also, K. pneumoniae strains had an ESBL pattern [22]. While in France and Italy, ceftazidime obstruction was seen in as many as 45% of types of K. pneumoniae [9,23].
Antibiotic susceptibility and identification of the AMR agents implicated in the human body are critical for empirical treatment and the escaping of resistant bacteria [24]. Qamar et al. (2020) documented that antibiotics have enabled great advancements in healthcare systems, but they are under threat from rapidly evolving resistant microbes [25]. Only a few studies have been conducted in Pakistan to check the prevalence of metal-resistant bacteria and the antimicrobial sensitivity profiles of those bacteria [26,27,28]. The relationship between sensitivity and resistance patterns has also been studied previously [6]. One previous study used the double disc synergy test that verified the phenotypic resistance, which was the legitimate cause of the development of resistance to commercially available antibiotics [25]. The findings from previous studies indicated that heavy metal rates, which were elevated in the river tyne basin as a consequence of earlier industrial and mining activities, were related to the high rates of AMR [29,30].
The double-disc synergy test (DDST) was used in the current study to examine the isolates on Mueller-Hinton agar plates with 30 g/disc (containing 10 g of clavulanate) for possible patterns of the ESBLs. When compared to Pseudomonas spp., Enterobacter spp., Proteus mirabilis, and Klebsiella spp., the E. coli showed 20% isolates as ESBL-producing bacteria, while it was discovered that in total, around 50% of these bacteria produced the ESBLs. A study by Becerra-Castro et al. (2015) stated that the supplementation of metals in the culture medium reduced the culturability of E. coli by 95 and 98% [31]. Another study by Deredjianet al. (2011) showed that the strains showing strong resistance to antibiotics were the least resistant to metals [32]. However, the results of the current study showed that the metals significantly increased the AMR rates among tested bacteria. Heavy metals may enter the environment naturally or move there via anthropogenic contamination from both indirect and direct sources. Heavy metals may be released directly into the water, soil, and the environment through industrial activities, including mining, processing, and smelting.
A previous study from Canada elaborated that the health consequences of AMR include increased morbidity and mortality rates due to delays in starting effective treatments or treatment failure. People infected with antibacterial-resistant bacteria are two times more likely to be hospitalized and have long durations of hospital stay as compared to patients infected with susceptible strains [33]. Results of the current study showed that the tested bacterial isolates were 100% sensitive to certain antibiotics like colistin and polymyxin-B when tested without heavy metals. However, when tested on the heavy metals containing agar, these antibiotics also showed resistance. The possible key mechanisms for the possible link between the microbial acquisition of AMR and metal resistance are co-resistance and cross-resistance mechanisms.
The most commonly studied microorganisms with documented co-occurrences of antibiotic and heavy metal resistance are Pseudomonas aeruginosa and E. coli [34]. In contrast to water reservoirs, soils and sediments in various reservoirs have higher levels of heavy metals and antibiotic resistance. Abiotic variables like pH may also have an impact on the solubility of heavy metals for bacterial pathogens [5,34]. In the current study, when the organisms were tested for AST using heavy metal-containing agar, the P. aeruginosa isolates showed the highest resistance against tested antibiotics. P. mirabilis isolates were the second most common resistant strains. The possible factors that contribute to heavy metal pollution in environmental reservoirs include municipal wastewater, sludges, agricultural runoff, as well as industrial and pharmaceutical wastes.
The co-existence of heavy metal and AMR genes among different bacteria makes the management of bacterial infections more challenging. Due to their usage in feed and as environmental contaminants, heavy metals are abundant using in livestock and livestock production systems, which has enabled many bacteria to acquire metal resistance [35]. A previous study by Pandit et al. (2020) has shown a significant correlation between high AMR rates and AMR genes [24]. Few studies have also reported that environmental factors could also be essential in helping bacteria to acquire AMR [9,36]. A study conducted by Abrar et al. (2019) has demonstrated that the virulent and AMR genes are usually associated with transposons or large plasmids. In addition, these plasmids usually carry AMR or other pathogenic factors such as toxins [19]. Verschuuren et al., (2021) have identified that the genes coding for AMR and enterotoxin were present on the same plasmid [22]. Hence, it is important to understand the relationship between heavy metals and AMR in various environmental reservoirs because environmental reservoirs are among the main channels by which antibacterial-resistant bacteria and antibacterial-resistant genes transmission to humans may occur and because of the complexity of AMR movement between and within these reservoirs.
4. Materials and Methods
The current study was conducted from January 2018 to July 2018 by the Department of Microbiology, University of Central Punjab, Lahore, Pakistan, under ID: L1F16MSMR0008. ESBL-producing samples were collected from a tertiary care hospital in Lahore. To re-identify and purify the collected bacterial spp., they were re-inoculated on selective media such as blood agar and MacConkey agar and incubated for 24–48 h at temperatures between 35 and 37 °C.
4.1. Collection of Bacterial Isolates
The bacterial isolates were collected from a tertiary care hospital in Lahore. The ESBL-producing organisms, including Escherichia coli, Klebsiella spp., Pseudomonas aeruginosa, Proteus spp., and Enterobacter spp., were collected, which were counter-identified later as ESBL-producing bacteria using the double disc synergy test.
4.2. Isolation and Re-Identification of Bacterial Isolates
All of the bacterial isolates were reinoculated on the cysteine electrolyte deficient (CLED) agar and MacConkey agar (Thermo Fisher Scientific, Inc., Waltham, MA, USA) and incubated at 37 °C for 18–24 h. After the incubation period, the bacterial colonies were evaluated for growth morphology and Gram staining characteristics. The final confirmation of bacterial isolates was done using biochemical tests-based identification. The biochemical tests, including citrate, indole, oxidase and analytical profile index 20E (API 20E) (BioMérieux, Marcy-l’Etoile, France), were used. The API 20E results were evaluated using the API website (https://apiweb.biomerieux.com/login) (accessed from 1 January 2018 to 31 December 2019).
4.3. Antibiotic Susceptibility Testing (AST) by the Kirby Bauer Disk Diffusion Method
The AST of bacterial isolated was done using Kirby Bauer disk diffusion methods as per the standard protocol from clinical laboratory standard institute (CLSI) guidelines 2020 [37]. Muller Hinton agar (MHA) (Thermo Fisher Scientific, Inc., Waltham, MA, USA) was used to perform the AST using a 0.5 MacFarland standard. The sterilized wire loop was used to pick the isolated 2–4 bacterial colonies from the culture plate. E. coli, Proteus mirabilis, Klebsiella Spp., and Pseudomonas aeruginosa single and identical colonies were chosen and moved into the MacFarland. A calibrated digital MacFarland meter was used to measure the turbidity of a microbiological cell in comparison to the supplied sample according to the standardized MacFarland method. After the preparation of MacFarland, it was lawned on the MHA plates, and then the antibiotic disks were dispensed on them. After the inoculation of plates and dispensing of antibiotics, these were incubated at 37 °C for 18–24 h. After the incubation period, the plates were checked for zones of inhibition (ZOIs). Results were noted as resistant (R), sensitive (S) and Intermediate (I).
The antibiotic disk (Thermo Fisher Scientific Inc., Waltham, MA, USA for ampicillin, amikacin, ceftriaxone, ceftriaxone, cefuroxime, chloramphenicol, ciprofloxacin, gentamicin, imipenem, meropenem, tetracycline, levofloxacin, tobramycin, fusidic acid, cefixime, colistin and polymyxin-B were tested.
4.4. The Double Disc Synergy Test (Phenotypic Confirmation Test)
ESBL-producing isolates were confirmed by the double disc synergy test (DDST). This was done in accordance with the instructions provided by the CLSI guidelines. The bacterial isolates were inoculated on MHA plates while simultaneously placing a disc of ceftriaxone (30 µg) and a disc of amoxicillin-clavulanate (10 µg) at a distance of 1 cm from each other. This allowed the test isolates to be exposed to both antibiotics at the same time (center to center). After overnight incubation at 37 °C, the plates were examined for phenotypic evidence of ESBL production. This was done by searching for an increase in the zone of inhibition of at least 5 mm between the cephalosporin discs and the amoxicillin-clavulanate discs corresponding to each of the cephalosporin discs.
4.5. Heavy Metals Susceptibility Pattern
Heavy metals (Arsenic) were purchased in the form of sodium arsenate (Disodium hydrogen arsenate heptahydrate) from Sigma-Aldrich, Massachusetts, United States. Generally, heavy metals are very toxic for living things, and these metals could be poisonous too. Heavy metals have the ability to degrade or inhibit the growth of certain microorganisms. Different materials were used to prepare a stock solution of arsenic salts, such as distilled water and flasks. The stock solution of arsenic (1.25 g/mL) was prepared as needed for the experimental procedures. The 10 g of sodium arsenate salt was added to 100 mL of autoclaved distilled water in a flask. This solution was mixed with 100 mL of MHA, and after this, the 20 mL of solution was poured onto Petri dishes. To check the AST of bacteria on heavy metal containing MHA plates at a concentration of 1.25 g/mL, the same procedure was repeated as mentioned above. After 18–24 h of incubation period at 37 °C, the ZOIs were measured to determine the antibiotic susceptibility pattern [38].
4.6. Statistical Analysis
The data was entered in SPSS version 26.0 (IBM, New York, NY, USA). At first, the descriptive analysis was applied to check the frequency (n), percentage (%), mean, and standard deviation (SD). The chi-square test was run to see the difference among the studied variable. A p-value of <0.05 was considered statistically significant.
5. Conclusions
AMR is a worldwide health-related issue these days. The effect of antibiotics becomes lesser due to resistant mechanisms developed by bacteria. The over-administration, misuse and wrongly prescribed antibiotics lead to a worsening situation for human beings. It was found in the current study that a significant frequency of ESBL-producing bacteria was discovered in clinical isolates, and these bacteria had a high ratio of resistance to tested antibiotics. MDR-ESBL has created a great threat under the edge of the high AMR rates. Furthermore, the excessive rate of heavy metal-induced AMR has increased the risk of getting worse the situation of AMR. Current findings confirm that heavy metals contribute significantly to the rise in AMR rate. These heavy metals may also be present in the environment also, which may pose a serious risk of higher AMR rates. To further understand the exposure-response linkages between heavy metals and AMR in various environmental media, more research studies using statistical data are required. It is recommended that culture-based and molecular-based approaches be used together in future research to learn more about how bacteria can be resistant to both heavy metals and antibiotics.
Author Contributions
Conceptualization, N.A. and S.Z.; methodology, N.A., K.T., S.A. and S.Z.; software, A.A.R. and S.A.T.; validation, M.G., M.A.H., M.A. and B.R.A.S.; formal analysis, N.A., K.T., S.A. and S.Z.; investigation, N.A., K.T., S.A. and S.Z.; resources, A.A.R., S.A.T., M.G., M.A.H., M.A. and B.R.A.S.; data curation, N.A. and S.Z.; writing—original draft preparation, A.A.S., M.A.A., S.A.A., R.A.A. and G.H.A.-A.; writing—review and editing, S.M.C., C.Y.Y., A.A.R. and N.A.; visualization, N.A.; supervision, A.A.R. and C.Y.Y.; project administration, N.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Institutional Review Board (Human Research Ethics Committee) of the Faculty of Life Sciences, the University of Central Punjab, under student ID: L1F16MSMR0008 approved on 15 October 2017.
Informed Consent Statement
As the current study does not involve direct patient dealing, informed consent was obtained from the institution involved in the study. Written informed consent has also been obtained to publish this paper.
Data Availability Statement
The data relating to the current study can be accessed upon reasonable request to the corresponding author.
Acknowledgments
The current study would like to acknowledge Waqas Saleem for his exclusive guidelines in the isolation and identification of microbes in the current study. Naveed Ahmed would like to acknowledge Graduate Research Assistance Scheme of Universiti Sains Malaysia.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Hutchings, M.I.; Truman, A.W.; Wilkinson, B. Antibiotics: Past, present and future. Curr. Opin. Microbiol. 2019, 51, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Zeb, S.; Mushtaq, M.; Ahmad, M.; Saleem, W.; Rabaan, A.A.; Naqvi, B.S.Z.; Garout, M.; Aljeldah, M.; Al Shammari, B.R.; Al Faraj, N.J. Self-Medication as an Important Risk Factor for Antibiotic Resistance: A Multi-Institutional Survey among Students. Antibiotics 2022, 11, 842. [Google Scholar] [CrossRef] [PubMed]
- Pachori, P.; Gothalwal, R.; Gandhi, P. Emergence of antibiotic resistance Pseudomonas aeruginosa in intensive care unit; a critical review. Genes & diseases 2019, 6, 109–119. [Google Scholar]
- Rabaan, A.A.; Alhumaid, S.; Mutair, A.A.; Garout, M.; Abulhamayel, Y.; Halwani, M.A.; Alestad, J.H.; Bshabshe, A.A.; Sulaiman, T.; AlFonaisan, M.K. Application of Artificial Intelligence in Combating High Antimicrobial Resistance Rates. Antibiotics 2022, 11, 784. [Google Scholar] [CrossRef] [PubMed]
- Kovalakova, P.; Cizmas, L.; McDonald, T.J.; Marsalek, B.; Feng, M.; Sharma, V.K. Occurrence and toxicity of antibiotics in the aquatic environment: A review. Chemosphere 2020, 251, 126351. [Google Scholar] [CrossRef]
- Ahmed, N.; Khan, M.; Saleem, W.; Karobari, M.I.; Mohamed, R.N.; Heboyan, A.; Rabaan, A.A.; Mutair, A.A.; Alhumaid, S.; Alsadiq, S.A. Evaluation of bi-lateral co-infections and antibiotic resistance rates among COVID-19 patients. Antibiotics 2022, 11, 276. [Google Scholar] [CrossRef]
- Catalano, A.; Iacopetta, D.; Ceramella, J.; Scumaci, D.; Giuzio, F.; Saturnino, C.; Aquaro, S.; Rosano, C.; Sinicropi, M.S. Multidrug resistance (MDR): A widespread phenomenon in pharmacological therapies. Molecules 2022, 27, 616. [Google Scholar] [CrossRef]
- Ben, Y.; Fu, C.; Hu, M.; Liu, L.; Wong, M.H.; Zheng, C. Human health risk assessment of antibiotic resistance associated with antibiotic residues in the environment: A review. Environ. Res. 2019, 169, 483–493. [Google Scholar] [CrossRef]
- Chokshi, A.; Sifri, Z.; Cennimo, D.; Horng, H. Global contributors to antibiotic resistance. J. Glob. Infect. Dis. 2019, 11, 36. [Google Scholar]
- Ahmed, N.; Khalid, H.; Mushtaq, M.; Basha, S.; Rabaan, A.A.; Garout, M.; Halwani, M.A.; Al Mutair, A.; Alhumaid, S.; Al Alawi, Z. The Molecular Characterization of Virulence Determinants and Antibiotic Resistance Patterns in Human Bacterial Uropathogens. Antibiotics 2022, 11, 516. [Google Scholar] [CrossRef]
- Bergšpica, I.; Kaprou, G.; Alexa, E.A.; Prieto, M.; Alvarez-Ordóñez, A. Extended spectrum β-lactamase (ESBL) producing Escherichia coli in pigs and pork meat in the European Union. Antibiotics 2020, 9, 678. [Google Scholar] [CrossRef]
- Widodo, A.; Effendi, M.H.; Khairullah, A.R. Extended-spectrum beta-lactamase (ESBL)-producing Eschericia coli from livestock. Sys. Rev. Pharm. 2020, 11, 382–392. [Google Scholar]
- Tamma, P.D.; Aitken, S.L.; Bonomo, R.A.; Mathers, A.J.; van Duin, D.; Clancy, C.J. Infectious Diseases Society of America 2022 Guidance on the Treatment of Extended-Spectrum β-lactamase Producing Enterobacterales (ESBL-E), Carbapenem-Resistant Enterobacterales (CRE), and Pseudomonas aeruginosa with Difficult-to-Treat Resistance (DTR-P. aeruginosa). Clin. Infect. Dis. 2022, 72, e169–e183. [Google Scholar]
- Davin-Regli, A.; Lavigne, J.-P.; Pagès, J.-M. Enterobacter spp.: Update on taxonomy, clinical aspects, and emerging antimicrobial resistance. Clin. Microbiol. Rev. 2019, 32, e00002–e00019. [Google Scholar] [CrossRef]
- Palmeira, J.D.; Ferreira, H.M.N. Extended-spectrum beta-lactamase (ESBL)-producing Enterobacteriaceae in cattle production–a threat around the world. Heliyon 2020, 6, e03206. [Google Scholar] [CrossRef]
- Carcione, D.; Siracusa, C.; Sulejmani, A.; Leoni, V.; Intra, J. Old and new beta-lactamase inhibitors: Molecular structure, mechanism of action, and clinical Use. Antibiotics 2021, 10, 995. [Google Scholar] [CrossRef]
- Safavi, M.; Bostanshirin, N.; Hajikhani, B.; Yaslianifard, S.; van Belkum, A.; Goudarzi, M.; Hashemi, A.; Darban-Sarokhalil, D.; Dadashi, M. Global genotype distribution of human clinical isolates of New Delhi metallo-β-lactamase-producing Klebsiella pneumoniae; A systematic review. J. Glob. Antimicrob. Resist. 2020, 23, 420–429. [Google Scholar] [CrossRef]
- Kadri, S.S. Key takeaways from the US CDC’s 2019 antibiotic resistance threats report for frontline providers. Crit. Care Med. 2020. [Google Scholar] [CrossRef]
- Abrar, S.; Ain, N.U.; Liaqat, H.; Hussain, S.; Rasheed, F.; Riaz, S. Distribution of blaCTX− M, blaTEM, blaSHV and blaOXA genes in extended-spectrum-β-lactamase-producing clinical isolates: A three-year multi-center study from Lahore, Pakistan. Antimicrob. Resist. Infect. Control 2019, 8, 1–10. [Google Scholar] [CrossRef]
- Hayat, K.; Jamshed, S.; Rosenthal, M.; Haq, N.U.; Chang, J.; Rasool, M.F.; Malik, U.R.; Rehman, A.U.; Khan, K.M.; Fang, Y. Understanding of Pharmacy Students towards Antibiotic Use, Antibiotic Resistance and Antibiotic Stewardship Programs: A Cross-Sectional Study from Punjab, Pakistan. Antibiotics 2021, 10, 66. [Google Scholar] [CrossRef]
- Khan, F.; Mallhi, T.; Khan, F.; Hayat, K.; Rehman, A.; Shah, S.; Khan, Z.; Khan, Y.; Ahmad, T.; Gudi, S. Evaluation of Consumers Perspective on the Consumption of Antibiotics, Antibiotic Resistance, and Recommendations to Improve the Rational use of Antibiotics: An Exploratory Qualitative Study From Post-Conflicted Region of Pakistan. Front. Pharmacol. 2022, 13, 881243. [Google Scholar] [CrossRef] [PubMed]
- Verschuuren, T.; Van Hout, D.; Arredondo-Alonso, S.; Fluit, A.; Reuland, E.; Top, J.; Schürch, A.; Bosch, T.; Bonten, M.; Kluytmans, J. Comparative genomics of ESBL-producing Escherichia coli (ESBL-Ec) reveals a similar distribution of the 10 most prevalent ESBL-Ec clones and ESBL genes among human community faecal and extra-intestinal infection isolates in the Netherlands (2014–17). J. Antimicrob. Chemother. 2021, 76, 901–908. [Google Scholar] [CrossRef] [PubMed]
- Saravanan, M.; Ramachandran, B.; Barabadi, H. The prevalence and drug resistance pattern of extended spectrum β–lactamases (ESBLs) producing Enterobacteriaceae in Africa. Microb. Pathog. 2018, 114, 180–192. [Google Scholar] [CrossRef] [PubMed]
- Pandit, R.; Awal, B.; Shrestha, S.S.; Joshi, G.; Rijal, B.P.; Parajuli, N.P. Extended-spectrum β-lactamase (ESBL) genotypes among multidrug-resistant uropathogenic Escherichia coli clinical isolates from a teaching hospital of Nepal. Interdiscip. Perspect. Infect. Dis. 2020, 2020, 6525826. [Google Scholar] [CrossRef] [PubMed]
- Qamar, A.; Ismail, T.; Akhtar, S. Prevalence and antibiotic resistance of Salmonella spp. in South Punjab-Pakistan. PLoS ONE 2020, 15, e0232382. [Google Scholar] [CrossRef]
- Khan, G.A.; Berglund, B.; Khan, K.M.; Lindgren, P.-E.; Fick, J. Occurrence and abundance of antibiotics and resistance genes in rivers, canal and near drug formulation facilities–a study in Pakistan. PLoS ONE 2013, 8, e62712. [Google Scholar] [CrossRef]
- Sair, A.T.; Khan, Z.A. Prevalence of antibiotic and heavy metal resistance in Gram negative bacteria isolated from rivers in northern P akistan. Water Environ. J. 2018, 32, 51–57. [Google Scholar] [CrossRef]
- Shah, S.Q.; Colquhoun, D.J.; Nikuli, H.L.; Sørum, H. Prevalence of antibiotic resistance genes in the bacterial flora of integrated fish farming environments of Pakistan and Tanzania. Environ. Sci. Technol. 2012, 46, 8672–8679. [Google Scholar] [CrossRef]
- Hubeny, J.; Harnisz, M.; Korzeniewska, E.; Buta, M.; Zieliński, W.; Rolbiecki, D.; Giebułtowicz, J.; Nałęcz-Jawecki, G.; Płaza, G. Industrialization as a source of heavy metals and antibiotics which can enhance the antibiotic resistance in wastewater, sewage sludge and river water. PLoS ONE 2021, 16, e0252691. [Google Scholar] [CrossRef]
- Baquero, F.; Martínez, J.-L.; Cantón, R. Antibiotics and antibiotic resistance in water environments. Curr. Opin. Biotechnol. 2008, 19, 260–265. [Google Scholar] [CrossRef]
- Becerra-Castro, C.; Machado, R.A.; Vaz-Moreira, I.; Manaia, C.M. Assessment of copper and zinc salts as selectors of antibiotic resistance in Gram-negative bacteria. Sci. Total Environ. 2015, 530, 367–372. [Google Scholar] [CrossRef]
- Deredjian, A.; Colinon, C.; Brothier, E.; Favre-Bonté, S.; Cournoyer, B.; Nazaret, S. Antibiotic and metal resistance among hospital and outdoor strains of Pseudomonas aeruginosa. Res. Microbiol. 2011, 162, 689–700. [Google Scholar] [CrossRef]
- Dandachi, I.; Chaddad, A.; Hanna, J.; Matta, J.; Daoud, Z. Understanding the epidemiology of multi-drug resistant gram-negative bacilli in the Middle East using a one health approach. Front. Microbiol. 2019, 10, 1941. [Google Scholar] [CrossRef]
- Nguyen, C.C.; Hugie, C.N.; Kile, M.L.; Navab-Daneshmand, T. Association between heavy metals and antibiotic-resistant human pathogens in environmental reservoirs: A review. Front. Environ. Sci. Eng. 2019, 13, 1–17. [Google Scholar] [CrossRef]
- Dweba, C.C.; Zishiri, O.T.; El Zowalaty, M.E. Methicillin-resistant Staphylococcus aureus: Livestock-associated, antimicrobial, and heavy metal resistance. Infect. Drug Resist. 2018, 11, 2497. [Google Scholar] [CrossRef]
- Subramaniam, G.; Girish, M. Antibiotic resistance—A cause for reemergence of infections. Indian J. Pediatrics 2020, 87, 937–944. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing, 28th ed.; CLSI Supplement M100; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2020. [Google Scholar]
- Akinbowale, O.L.; Peng, H.; Grant, P.; Barton, M.D. Antibiotic and heavy metal resistance in motile aeromonads and pseudomonads from rainbow trout (Oncorhynchus mykiss) farms in Australia. Int. J. Antimicrob. Agents 2007, 30, 177–182. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).