Gut Microbiota-Assisted Synthesis, Cellular Interactions and Synergistic Perspectives of Equol as a Potent Anticancer Isoflavone
Abstract
1. Introduction
2. Biosynthesis, Absorption to Metabolism
3. Structure-Activity Relationship of Equol
4. Equol Production at a Laboratory and Industrial Scale
5. Equol as a Potent Anticancer Agent
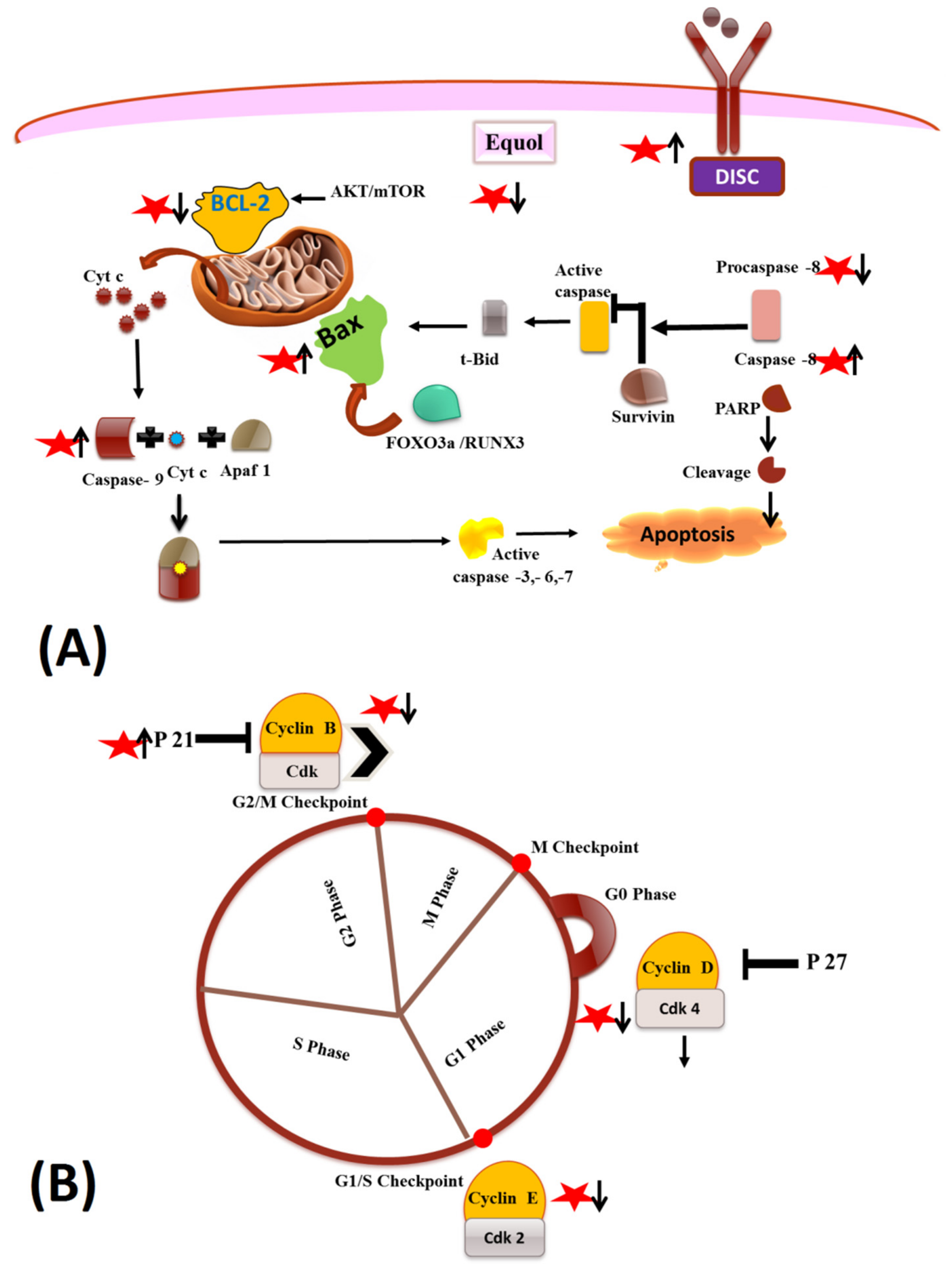
5.1. Apoptotic and Cell Cycle Arrest Mechanisms
5.2. Antiestrogenic Action
5.3. Anti-Angiogenic and Anti-Metastasis Activities
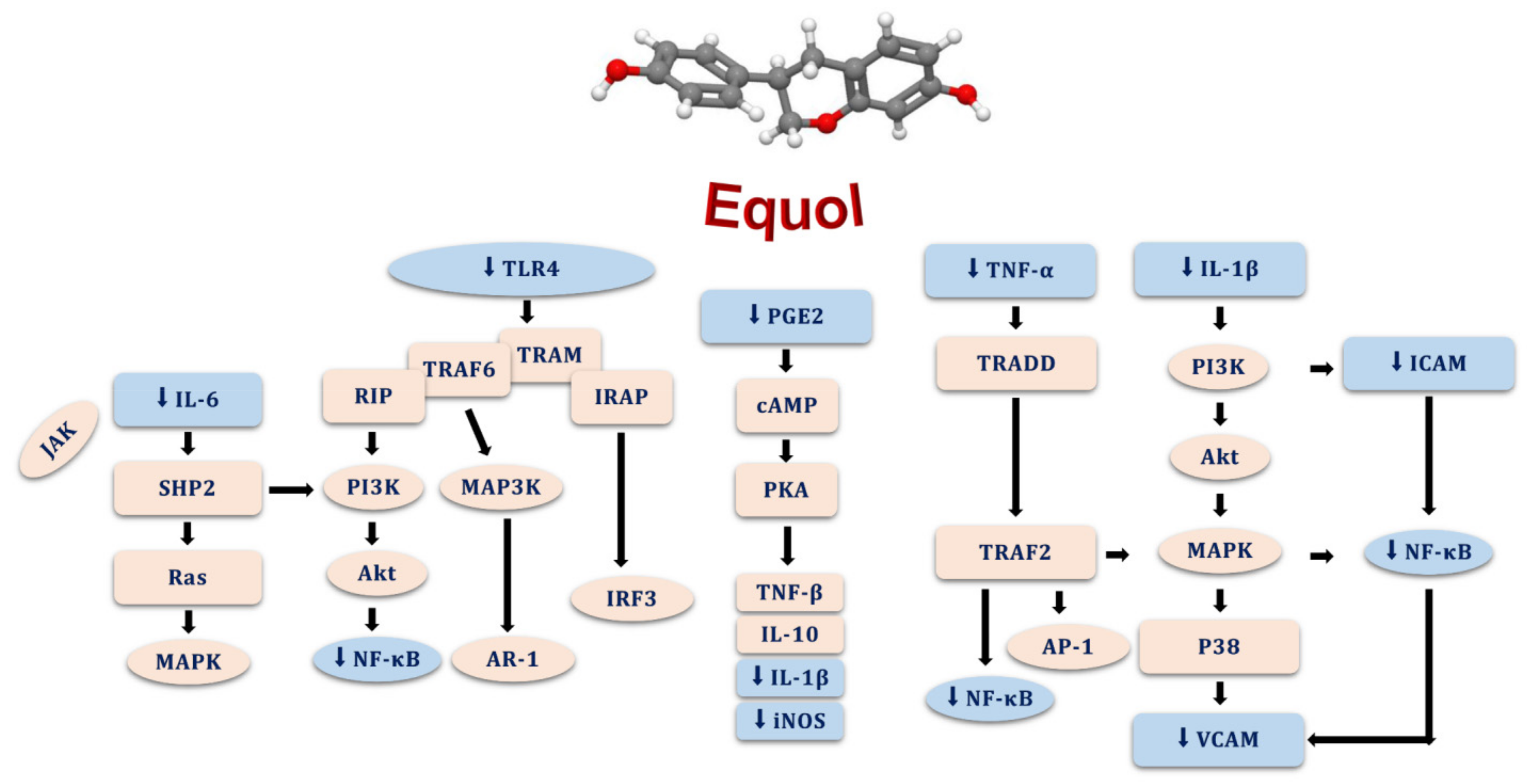
5.4. Antioxidant and Anti-Inflammatory Effects of Equol
6. Synergism with Anti-Cancer Agents
7. Role of Nanotechnology and Clinical Studies Using Equol
8. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Feng, X.; Li, Y.; Brobbey Oppong, M.; Qiu, F. Insights into the Intestinal Bacterial Metabolism of Flavonoids and the Bioactivities of Their Microbe-Derived Ring Cleavage Metabolites. Drug Metab. Rev. 2018, 50, 343–356. [Google Scholar] [CrossRef] [PubMed]
- Mayo, B.; Vázquez, L.; Flórez, A.B. Equol: A Bacterial Metabolite from the Daidzein Isoflavone and Its Presumed Beneficial Health Effects. Nutrients 2019, 11, 2231. [Google Scholar] [CrossRef] [PubMed]
- Rafii, F. The Role of Colonic Bacteria in the Metabolism of the Natural Isoflavone Daidzin to Equol. Metabolites 2015, 5, 56–73. [Google Scholar] [CrossRef] [PubMed]
- Horiuchi, H.; Harada, N.; Adachi, T.; Nakano, Y.; Inui, H.; Yamaji, R. S-Equol Enantioselectively Activates CAMP-Protein Kinase A Signaling and Reduces Alloxan-Induced Cell Death in INS-1 Pancreatic b-Cells. J. Nutr. Sci. Vitaminol. 2014, 60, 291–296. [Google Scholar] [CrossRef] [PubMed]
- Yemelyanov, A.; Czwornog, J.; Gera, L.; Joshi, S.; Chatterton, R.T., Jr.; Budunova, I. Novel steroid receptor phyto-modulator compound a inhibits growth and survival of prostate cancer cells. Cancer Res. 2008, 68, 4763–4773. [Google Scholar] [CrossRef]
- Thulasidasan, A.K.; Retnakumari, A.P.; Shankar, M.; Vijayakurup, V.; Anwar, S.; Thankachan, S.; Pillai, K.S.; Pillai, J.J.; Nandan, C.D.; Alex, V.V.; et al. Folic acid conjugation improves the bioavailability and chemosensitizing efficacy of curcumin-encapsulated PLGA-PEG nanoparticles towards paclitaxel chemotherapy. Oncotarget 2017, 12, 107374. [Google Scholar] [CrossRef]
- Rahman, M.S.; Cao, J. Estrogen receptors in gastric cancer: Advances and perspectives. World J. Gastroenterol. 2016, 28, 2475. [Google Scholar] [CrossRef]
- Michikawa, T.; Inoue, M.; Sawada, N.; Tanaka, Y.; Yamaji, T.; Iwasaki, M.; Shimazu, T.; Sasazuki, S.; Mizokami, M.; Tsugane, S.; et al. Plasma Isoflavones and Risk of Primary Liver Cancer in Japanese Women and Men with Hepatitis Virus Infection: A Nested Case–Control StudyPlasma Isoflavones and Primary Liver Cancer Risk. Cancer Epidemiol. Biomark. Prev. 2015, 24, 532–537. [Google Scholar] [CrossRef]
- Jeong, H.; Phan, A.N.H.; Choi, J.W. Anti-Cancer Effects of Polyphenolic Compounds in Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitor-Resistant Non-Small Cell Lung Cancer. Pharm. Mag. 2017, 13, 595–599. [Google Scholar] [CrossRef]
- Zhang, J.; Ren, L.; Yu, M.; Liu, X.; Ma, W.; Huang, L.; Li, X.; Ye, X. S-Equol Inhibits Proliferation and Promotes Apoptosis of Human Breast Cancer MCF-7 cells via Regulating MiR-10a-5p and PI3K/AKT Pathway. Arch. Biochem. Biophys. 2019, 672, 108064. [Google Scholar] [CrossRef]
- Kim, E.Y.; Shin, J.Y.; Park, Y.J.; Kim, A.K. Equol Induces Mitochondria-Mediated Apoptosis of Human Cervical Cancer Cells. Anticancer Res. 2014, 34, 4985–4992. [Google Scholar]
- Fares, J.; Fares, M.Y.; Khachfe, H.H.; Salhab, H.A.; Fares, Y. Molecular principles of metastasis: A hallmark of cancer revisited. Signal Transduct. Target. Ther. 2020, 5, 28. [Google Scholar] [CrossRef]
- Fatima, A.; Khan, M.S.; Ahmad, M.d.W. Therapeutic Potential of Equol: A Comprehensive Review. Curr. Pharm. Des. 2020, 26, 5837–5843. [Google Scholar] [CrossRef]
- Hüser, S.; Guth, S.; Joost, H.G.; Soukup, S.T.; Köhrle, J.; Kreienbrock, L.; Diel, P.; Lachenmeier, D.W.; Eisenbrand, G.; Vollmer, G.; et al. Effects of Isoflavones on Breast Tissue and the Thyroid Hormone System in Humans: A Comprehensive Safety Evaluation. Arch. Toxicol. 2018, 92, 2703–2748. [Google Scholar] [CrossRef]
- Zhu, Y.; Yang, Z.; Xie, Y.; Yang, M.; Zhang, Y.; Deng, Z.; Cai, L. Investigation of Inhibition Effect of Daidzein on Osteosarcoma Cells Based on Experimental Validation and Systematic Pharmacology Analysis. PeerJ 2021, 9, e12072. [Google Scholar] [CrossRef]
- Han, B.-J.; Li, W.; Jiang, G.-B.; Lai, S.-H.; Zhang, C.; Zeng, C.-C.; Liu, Y.-J. Effects of Daidzein in Regards to Cytotoxicity in Vitro, Apoptosis, Reactive Oxygen Species Level, Cell Cycle Arrest and the Expression of Caspase and Bcl-2 Family Proteins. Oncol. Rep. 2015, 34, 1115–1120. [Google Scholar] [CrossRef]
- Liang, Y.-S.; Qi, W.-T.; Guo, W.; Wang, C.-L.; Hu, Z.-B.; Li, A.-K. Genistein and Daidzein Induce Apoptosis of Colon Cancer Cells by Inhibiting the Accumulation of Lipid Droplets. Food Nutr. Res. 2018, 62, 1–9. [Google Scholar] [CrossRef]
- Cai, Y.F.; Zhang, H.M.; Niu, W.Y.; Zou, Y.Q.; Ma, D.F. Effects of Equol on Colon Cancer Cell Proliferation. J. Peking Univ. Health Sci. 2017, 49, 383–387. [Google Scholar]
- Kumar, V.; Chauhan, S.S. Daidzein Induces Intrinsic Pathway of Apoptosis along with ER α/β Ratio Alteration and ROS Production. Asian Pac. J. Cancer Prev. 2021, 22, 603–610. [Google Scholar] [CrossRef]
- Montalesi, E.; Cipolletti, M.; Cracco, P.; Fiocchetti, M.; Marino, M. Divergent Effects of Daidzein and Its Metabolites on Estrogen-Induced Survival of Breast Cancer Cells. Cancers 2020, 12, 167. [Google Scholar] [CrossRef]
- Guo, J.; Wang, Q.; Zhang, Y.; Sun, W.; Zhang, S.; Li, Y.; Wang, J.; Bao, Y. Functional Daidzein Enhances the Anticancer Effect of Topotecan and Reverses BCRP-Mediated Drug Resistance in Breast Cancer. Pharm. Res 2019, 147, 104387. [Google Scholar] [CrossRef] [PubMed]
- Bao, C.; Namgung, H.; Lee, J.; Park, H.-C.; Ko, J.; Moon, H.; Ko, H.W.; Lee, H.J. Daidzein Suppresses Tumor Necrosis Factor-α Induced Migration and Invasion by Inhibiting Hedgehog/Gli1 Signaling in Human Breast Cancer Cells. J. Agric. Food Chem. 2014, 62, 3759–3767. [Google Scholar] [CrossRef] [PubMed]
- Charalambous, C.; Pitta, C.A.; Constantinou, A.I. Equol Enhances Tamoxifen’s Anti-Tumor Activity by Induction of Caspase-Mediated Apoptosis in MCF-7 Breast Cancer Cells. BMC Cancer 2013, 13, 238. [Google Scholar] [CrossRef] [PubMed]
- Choi, E.J.; Kim, G.-H. Daidzein Causes Cell Cycle Arrest at the G1 and G2/M Phases in Human Breast Cancer MCF-7 and MDA-MB-453 Cells. Phytomedicine 2008, 15, 683–690. [Google Scholar] [CrossRef] [PubMed]
- Kaushik, S.; Shyam, H.; Sharma, R.; Balapure, A.K. Dietary Isoflavone Daidzein Synergizes Centchroman Action via Induction of Apoptosis and Inhibition of PI3K/Akt Pathway in MCF-7/MDA MB-231 Human Breast Cancer Cells. Phytomedicine 2018, 40, 116–124. [Google Scholar] [CrossRef]
- Rigalli, J.P.; Scholz, P.N.; Tocchetti, G.N.; Ruiz, M.L.; Weiss, J. The Phytoestrogens Daidzein and Equol Inhibit the Drug Transporter BCRP/ABCG2 in Breast Cancer Cells: Potential Chemosensitizing Effect. Eur. J. Nutr. 2019, 58, 139–150. [Google Scholar] [CrossRef]
- Ono, M.; Ejima, K.; Higuchi, T.; Takeshima, M.; Wakimoto, R.; Nakano, S. Equol Enhances Apoptosis-Inducing Activity of Genistein by Increasing Bax/Bcl-XL Expression Ratio in MCF-7 Human Breast Cancer Cells. Nutr. Cancer 2017, 69, 1300–1307. [Google Scholar] [CrossRef]
- de la Parra, C.; Borrero-Garcia, L.D.; Cruz-Collazo, A.; Schneider, R.J.; Dharmawardhane, S. Equol, an Isoflavone Metabolite, Regulates Cancer Cell Viability and Protein Synthesis Initiation via c-Myc and EIF4G. J. Biol. Chem. 2015, 290, 6047–6057. [Google Scholar] [CrossRef]
- Magee, P.J.; Allsopp, P.; Samaletdin, A.; Rowland, I.R. Daidzein, R-(+)Equol and S-(−)Equol Inhibit the Invasion of MDA-MB-231 Breast Cancer Cells Potentially via the down-Regulation of Matrix Metalloproteinase-2. Eur. J. Nutr. 2014, 53, 345–350. [Google Scholar] [CrossRef]
- Jin, S.; Zhang, Q.Y.; Kang, X.M.; Wang, J.X.; Zhao, W.H. Daidzein Induces MCF-7 Breast Cancer Cell Apoptosis via the Mitochondrial Pathway. Ann. Oncol. 2010, 21, 263–268. [Google Scholar] [CrossRef]
- Ju, Y.H.; Fultz, J.; Allred, K.F.; Doerge, D.R.; Helferich, W.G. Effects of Dietary Daidzein and Its Metabolite, Equol, at Physiological Concentrations on the Growth of Estrogen-Dependent Human Breast Cancer (MCF-7) Tumors Implanted in Ovariectomized Athymic Mice. Carcinogenesis 2006, 27, 856–863. [Google Scholar] [CrossRef]
- Guo, S.; Wang, Y.; Li, Y.; Li, Y.; Feng, C.; Li, Z. Daidzein-Rich Isoflavones Aglycone Inhibits Lung Cancer Growth through Inhibition of NF-ΚB Signaling Pathway. Immunol. Lett. 2020, 222, 67–72. [Google Scholar] [CrossRef]
- Spagnuolo, C.; Russo, G.L.; Orhan, I.E.; Habtemariam, S.; Daglia, M.; Sureda, A.; Nabavi, S.F.; Devi, K.P.; Loizzo, M.R.; Tundis, R.; et al. Genistein and cancer: Current status, challenges, and future directions. Adv. Nutr. 2015, 6, 408–419. [Google Scholar] [CrossRef]
- Yang, Z.-P.; Zhao, Y.; Huang, F.; Chen, J.; Yao, Y.-H.; Li, J.; Wu, X.-N. Equol Inhibits Proliferation of Human Gastric Carcinoma Cells via Modulating Akt Pathway. World J. Gastroenterol. 2015, 21, 10385–10399. [Google Scholar] [CrossRef]
- Tang, S.; Hu, J.; Meng, Q.; Dong, X.; Wang, K.; Qi, Y.; Chu, C.; Zhang, X.; Hou, L. Daidzein Induced Apoptosis via Down-Regulation of Bcl-2/Bax and Triggering of the Mitochondrial Pathway in BGC-823 Cells. Cell Biochem. Biophys. 2013, 65, 197–202. [Google Scholar] [CrossRef]
- Gao, L.; Wang, K.-X.; Zhang, N.-N.; Li, J.-Q.; Qin, X.-M.; Wang, X.-L. 1H Nuclear Magnetic Resonance Based Metabolomics Approach Reveals the Metabolic Mechanism of (-)-5-Hydroxy-Equol against Hepatocellular Carcinoma Cells in Vitro. J. Proteome. Res. 2018, 17, 1833–1843. [Google Scholar] [CrossRef]
- Liang, X.-L.; Li, M.; Li, J.; Wang, X.-L. Equol Induces Apoptosis in Human Hepatocellular Carcinoma SMMC-7721 Cells through the Intrinsic Pathway and the Endoplasmic Reticulum Stress Pathway. Anticancer Drugs 2014, 25, 633–640. [Google Scholar] [CrossRef]
- Park, H.J.; Jeon, Y.K.; You, D.H.; Nam, M.J. Daidzein Causes Cytochrome C-Mediated Apoptosis via the Bcl-2 Family in Human Hepatic Cancer Cells. Food Chem. Toxicol. 2013, 60, 542–549. [Google Scholar] [CrossRef]
- Guo, J.-M.; Xiao, B.-X.; Dai, D.-J.; Liu, Q.; Ma, H.-H. Effects of Daidzein on Estrogen-Receptor-Positive and Negative Pancreatic Cancer Cells in Vitro. World J. Gastroenterol. 2004, 10, 860–863. [Google Scholar] [CrossRef]
- Salama, A.A.A.; Allam, R.M. Promising Targets of Chrysin and Daidzein in Colorectal Cancer: Amphiregulin, CXCL1, and MMP-9. Eur. J. Pharm. 2021, 892, 173763. [Google Scholar] [CrossRef]
- Zou, Y.; Wang, Y.; Cai, Y.; Ma, D. Effects of Equol on Proliferation of Colorectal Cancer HCT-15 Cell. Wei Sheng Yan Jiu 2019, 48, 803–806. [Google Scholar] [PubMed]
- He, Y.; Wu, X.; Cao, Y.; Hou, Y.; Chen, H.; Wu, L.; Lu, L.; Zhu, W.; Gu, Y. Daidzein Exerts Anti-Tumor Activity against Bladder Cancer Cells via Inhibition of FGFR3 Pathway. Neoplasma 2016, 63, 523–531. [Google Scholar] [CrossRef] [PubMed]
- Ranjithkumar, R.; Saravanan, K.; Balaji, B.; Hima, S.; Sreeja, S.; Timane, S.R.; Ram Pravin Kumar, M.; Kabilan, S.; Ramanathan, M. Novel Daidzein Molecules Exhibited Anti-Prostate Cancer Activity through Nuclear Receptor ERβ Modulation, in Vitro and in Vivo Studies. J. Chemother. 2021, 33, 582–594. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Zhou, R.; Kong, Y.; Wang, J.; Xia, W.; Guo, J.; Liu, J.; Sun, H.; Liu, K.; Yang, J.; et al. S-Equol, a Secondary Metabolite of Natural Anticancer Isoflavone Daidzein, Inhibits Prostate Cancer Growth In Vitro and In Vivo, Though Activating the Akt/FOXO3a Pathway. Curr. Cancer Drug Targets 2016, 16, 455–465. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Zhang, Y.; Ma, D.; Shi, Y.; Liu, C.; Wang, P. (±)Equol Inhibits Invasion in Prostate Cancer DU145 Cells Possibly via down-Regulation of Matrix Metalloproteinase-9, Matrix Metalloproteinase-2 and Urokinase-Type Plasminogen Activator by Antioxidant Activity. J. Clin. Biochem. Nutr. 2012, 51, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Zhang, Y.; Ma, D.; Li, G.; Wang, P. Anti-Invasion Effects of R- and S-Enantiomers of Equol on Prostate Cancer PC3, DU145 Cells. Wei Sheng Yan Jiu 2011, 40, 423–425. [Google Scholar]
- Dai, W.; Wang, F.; He, L.; Lin, C.; Wu, S.; Chen, P.; Zhang, Y.; Shen, M.; Wu, D.; Wang, C.; et al. Genistein inhibits hepatocellular carcinoma cell migration by reversing the epithelial–mesenchymal transition: Partial mediation by the transcription factor NFAT1. Mol. Carcinog. 2015, 54, 301–311. [Google Scholar] [CrossRef]
- Hua, F.; Li, C.-H.; Chen, X.-G.; Liu, X.-P. Daidzein Exerts Anticancer Activity towards SKOV3 Human Ovarian Cancer Cells by Inducing Apoptosis and Cell Cycle Arrest, and Inhibiting the Raf/MEK/ERK Cascade. Int. J. Mol. Med. 2018, 41, 3485–3492. [Google Scholar] [CrossRef]
- Goris, T.; Cuadrat, R.R.C.; Braune, A. Flavonoid-Modifying Capabilities of the Human Gut Microbiome—An in Silico Study. Nutrients 2021, 13, 2688. [Google Scholar] [CrossRef]
- Soukup, S.T.; Stoll, D.A.; Danylec, N.; Schoepf, A.; Kulling, S.E.; Huch, M. Metabolism of Daidzein and Genistein by Gut Bacteria of the Class Coriobacteriia. Foods 2021, 10, 2741. [Google Scholar] [CrossRef]
- Xiaobin, L.; Jinglong, X.; Fang, Z.; Chenchen, W.; Kailun, Y. Effect of the HXBM408 Bacteria on Rat Intestinal Bacterial Diversity and the Metabolism of Soybean Isoflavones. PLoS ONE 2021, 16, e0253728. [Google Scholar] [CrossRef]
- Wada, K.; Ueno, T.; Uchiyama, S.; Abiru, Y.; Tsuji, M.; Konishi, K.; Mizuta, F.; Goto, Y.; Tamura, T.; Shiraki, M.; et al. Relationship of Equol Production between Children Aged 5–7 Years and Their Mothers. Eur. J. Nutr. 2017, 56, 1911–1917. [Google Scholar] [CrossRef]
- Wang, Q.; Spenkelink, B.; Boonpawa, R.; Rietjens, I.M.C.M.; Beekmann, K. Use of Physiologically Based Kinetic Modeling to Predict Rat Gut Microbial Metabolism of the Isoflavone Daidzein to S-Equol and Its Consequences for ERα Activation. Mol. Nutr. Food Res. 2020, 64, 1900912. [Google Scholar] [CrossRef]
- Iino, C.; Shimoyama, T.; Iino, K.; Yokoyama, Y.; Chinda, D.; Sakuraba, H.; Fukuda, S.; Nakaji, S. Daidzein Intake Is Associated with Equol Producing Status through an Increase in the Intestinal Bacteria Responsible for Equol Production. Nutrients 2019, 11, 433. [Google Scholar] [CrossRef]
- Zhu, A.; Sunagawa, S.; Mende, D.R.; Bork, P. Inter-Individual Differences in the Gene Content of Human Gut Bacterial Species. Genome Biol. 2015, 16, 82. [Google Scholar] [CrossRef]
- Yuan, J.P.; Wang, J.H.; Liu, X. Metabolism of Dietary Soy Isoflavones to Equol by Human Intestinal Microflora—Implications for Health. Mol. Nutr. Food Res. 2007, 51, 765–781. [Google Scholar] [CrossRef]
- Kim, I.-S. Current Perspectives on the Beneficial Effects of Soybean Isoflavones and Their Metabolites for Humans. Antioxidants 2021, 10, 1064. [Google Scholar] [CrossRef]
- Cho, H.W.; Gim, H.J.; Li, H.; Subedi, L.; Kim, S.Y.; Ryu, J.-H.; Jeon, R. Structure–Activity Relationship of Phytoestrogen Analogs as ERα/β Agonists with Neuroprotective Activities. Chem. Pharm. Bull. 2021, 69, 99–105. [Google Scholar] [CrossRef]
- Tanaka, H.; Ito, S.; Ojika, M.; Nishimaki-Mogami, T.; Kondo, K.; Wakamatsu, K. The Oxidation of Equol by Tyrosinase Produces a Unique Di-Ortho-Quinone: Possible Implications for Melanocyte Toxicity. Int. J. Mol. Sci. 2021, 22, 9145. [Google Scholar] [CrossRef]
- Yu, Z.T.; Yao, W.; Zhu, W.Y. Isolation and Identification of Equol-Producing Bacterial Strains from Cultures of Pig Faeces. FEMS Microbiol. Lett. 2008, 282, 73–80. [Google Scholar] [CrossRef]
- Vázquez, L.; Flórez, A.B.; Rodríguez, J.; Mayo, B. Heterologous Expression of Equol Biosynthesis Genes from Adlercreutzia Equolifaciens. FEMS Microbiol. Lett. 2021, 368, fnab082. [Google Scholar] [CrossRef] [PubMed]
- Heng, Y.; Kim, M.J.; Yang, H.J.; Kang, S.; Park, S. Lactobacillus Intestinalis Efficiently Produces Equol from Daidzein and Chungkookjang, Short-Term Fermented Soybeans. Arch. Microbiol. 2019, 201, 1009–1017. [Google Scholar] [CrossRef]
- KAWADA, Y.; YOKOYAMA, S.; YANASE, E.; NIWA, T.; SUZUKI, T. The production of S-equol from daidzein is associated with a cluster of three genes in Eggerthella sp. YY7918. Biosci. Microbiota. Food Health 2016, 35, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Mustafa, S.E.; Mustafa, S.; Abas, F.; Manap, M.Y.A.B.D.; Ismail, A.; Amid, M.; Elzen, S. Optimization of Culture Conditions of Soymilk for Equol Production by Bifidobacterium Breve 15700 and Bifidobacterium Longum BB536. Food Chem. 2019, 278, 767–772. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.-G.; Lee, S.-H.; Kim, J.; Kim, E.-J.; Choi, K.-Y.; Kim, B.-G. Polymeric Solvent Engineering for Gram/Liter Scale Production of a Water-Insoluble Isoflavone Derivative, (S)-Equol. Appl. Microbiol. Biotechnol. 2018, 102, 6915–6921. [Google Scholar] [CrossRef]
- Nazzaro, F.; Fratianni, F.; d’Acierno, A.; Coppola, R. Gut Microbiota and Polyphenols: A Strict Connection Enhancing Human Health. In Advances in Food Biotechnology; John Wiley & Sons Ltd.: Chichester, UK, 2015; pp. 335–350. [Google Scholar]
- Mahalingam, S.; Gao, L.; Gonnering, M.; Helferich, W.; Flaws, J.A. Equol Inhibits Growth, Induces Atresia, and Inhibits Steroidogenesis of Mouse Antral Follicles in Vitro. Toxicol. Appl. Pharm. 2016, 295, 47–55. [Google Scholar] [CrossRef]
- Iglesias-Aguirre, C.E.; Cortés-Martín, A.; Ávila-Gálvez, M.Á.; Giménez-Bastida, J.A.; Selma, M.V.; González-Sarrías, A.; Espín, J.C. Main drivers of (poly) phenol effects on human health: Metabolite production and/or gut microbiota-associated metabotypes? Food Funct. 2021, 12, 10324–10355. [Google Scholar] [CrossRef]
- Shi, J.; Ji, A.; Cao, Z.; Cao, R.; Li, D.; Yang, R.; Wang, F. Equol Induced Apoptosis of Human Breast Cancer MDA-MB-231 Cell by Inhibiting the Expression of Nuclear Factor-KappaB. Wei Sheng Yan Jiu 2011, 40, 95–98. [Google Scholar]
- Hong, S.H.; Cha, H.J.; Hwang-Bo, H.; Kim, M.Y.; Kim, S.Y.; Ji, S.Y.; Cheong, J.; Park, C.; Lee, H.; Kim, G.Y.; et al. Anti-proliferative and pro-apoptotic effects of Licochalcone A through ROS-mediated cell cycle arrest and apoptosis in human bladder cancer cells. Int. J. Mol. Sci. 2019, 20, 3820. [Google Scholar] [CrossRef]
- Kang, H.-B.; Zhang, Y.-F.; Yang, J.-D.; Lu, K.-L. Study on Soy Isoflavone Consumption and Risk of Breast Cancer and Survival. Asian Pac. J. Cancer Prev. 2012, 13, 995–998. [Google Scholar] [CrossRef]
- Choi, E.J.; Ahn, W.S.; Bae, S.M. Equol Induces Apoptosis through Cytochrome C-Mediated Caspases Cascade in Human Breast Cancer MDA-MB-453 Cells. Chem. Biol. Interact. 2009, 177, 7–11. [Google Scholar] [CrossRef]
- Taghizadeh, B.; Ghavami, L.; Nikoofar, A.; Goliaei, B. Equol as a Potent Radiosensitizer in Estrogen Receptor-Positive and -Negative Human Breast Cancer Cell Lines. Breast Cancer 2015, 22, 382–390. [Google Scholar] [CrossRef]
- Setchell, K.D.R.; Clerici, C. Equol: Pharmacokinetics and Biological Actions. J. Nutr. 2010, 140, 1363S–1368S. [Google Scholar] [CrossRef]
- Majeed, R.; Hamid, A.; Sangwan, P.L.; Chinthakindi, P.K.; Koul, S.; Rayees, S.; Singh, G.; Mondhe, D.M.; Mintoo, M.J.; Singh, S.K.; et al. Inhibition of phosphotidylinositol-3 kinase pathway by a novel naphthol derivative of betulinic acid induces cell cycle arrest and apoptosis in cancer cells of different origin. Cell Death Dis. 2014, 5, e1459. [Google Scholar] [CrossRef]
- Itsumi, M.; Shiota, M.; Takeuchi, A.; Kashiwagi, E.; Inokuchi, J.; Tatsugami, K.; Kajioka, S.; Uchiumi, T.; Naito, S.; Eto, M.; et al. Equol Inhibits Prostate Cancer Growth through Degradation of Androgen Receptor by S-Phase Kinase-Associated Protein 2. Cancer Sci. 2016, 107, 1022–1028. [Google Scholar] [CrossRef]
- Lund, T.D.; Blake, C.; Bu, L.; Hamaker, A.N.; Lephart, E.D. IEquol an Isoflavonoid: Potential for Improved Prostate Health, in Vitro and in Vivo Evidence. Reprod. Biol. Endocrinol. 2011, 9, 4. [Google Scholar] [CrossRef]
- Hod, R.; Maniam, S.; Mohd Nor, N.H. A systematic review of the effects of equol (soy metabolite) on breast cancer. Molecules 2021, 26, 1105. [Google Scholar] [CrossRef]
- Onoda, A.; Ueno, T.; Uchiyama, S.; Hayashi, S.-I.; Kato, K.; Wake, N. Effects of S-Equol and Natural S-Equol Supplement (SE5-OH) on the Growth of MCF-7 in Vitro and as Tumors Implanted into Ovariectomized Athymic Mice. Food Chem. Toxicol. 2011, 49, 2279–2284. [Google Scholar] [CrossRef]
- Mitchell, J.H.; Duthie, S.J.; Collins, A.R. Effects of phytoestrogens on growth and DNA integrity in human prostate tumor cell lines: PC-3 and LNCaP. Nutr. Cancer 2000, 38, 223–228. [Google Scholar] [CrossRef]
- Thibodeau, P.A.; Kachadourian, R.; Lemay, R.; Bisson, M.; Day, B.J.; Paquette, B. In Vitro Pro-and Antioxidant Properties of Estrogens. J. Steroid Biochem. Mol. Biol. 2002, 81, 227–236. [Google Scholar] [CrossRef]
- Caldon, C.E. Estrogen signaling and the DNA damage response in hormone dependent breast cancers. Front. Oncol. 2014, 14, 106. [Google Scholar] [CrossRef] [PubMed]
- Cavalieri, E.; Chakravarti, D.; Guttenplan, J.; Hart, E.; Ingle, J.; Jankowiak, R.; Muti, P.; Rogan, E.; Russo, J.; Santen, R.; et al. Catechol Estrogen Quinones as Initiators of Breast and Other Human Cancers: Implications for Biomarkers of Susceptibility and Cancer Prevention. Biochim. Biophys. Acta Rev. Cancer 2006, 1766, 63–78. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, C.; Frankenfeld, C.L.; Lampe, J.W. Gut bacterial metabolism of the soy isoflavone daidzein: Exploring the relevance to human health. Exp. Biol. Med. 2005, 230, 155–170. [Google Scholar] [CrossRef] [PubMed]
- Nettleton, J.A.; Greany, K.A.; Thomas, W.; Wangen, K.E.; Adlercreutz, H.; Kurzer, M.S. The Effect of Soy Consumption on the Urinary 2:16-Hydroxyestrone Ratio in Postmenopausal Women Depends on Equol Production Status but Is Not Influenced by Probiotic Consumption 1. J. Nutr. 2005, 135, 603–608. [Google Scholar] [CrossRef] [PubMed]
- Cassidy, A.; Brown, J.E.; Hawdon, A.; Faughnan, M.S.; King, L.J.; Millward, J.; Zimmer-Nechemias, L.; Wolfe, B.; Setchell, K.D.R. Nutrient Physiology, Metabolism, and Nutrient-Nutrient Interactions Factors Affecting the Bioavailability of Soy Isoflavones in Humans after Ingestion of Physiologically Relevant Levels from Different Soy Foods 1. J. Nutr. 2006, 136, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Rowland, I.R.; Wiseman, H.; Sanders, T.A.B.; Adlercreutz, H.; Bowey, E.A. Interindividual Variation in Metabolism of Soy Isoflavones and Lignans: Influence of Habitual Diet on Equol Production by the Gut Microflora. Nutr. Cancer 2000, 36, 27–32. [Google Scholar] [CrossRef]
- Dewi, F.N.; Wood, C.E.; Lampe, J.W.; Hullar, M.A.J.; Franke, A.A.; Golden, D.L.; Adams, M.R.; Cline, J.M. Endogenous and Exogenous Equol Are Antiestrogenic in Reproductive Tissues of Apolipoprotein E-Null Mice. J. Nutr. 2012, 142, 1829–1835. [Google Scholar] [CrossRef]
- Uchiyama, S. New Insights into “Equol”, a Novel Ingredient Derived from Soy. Nippon Shokuhin Kagaku Kogaku Kaishi 2015, 62, 356–363. [Google Scholar] [CrossRef]
- Lathrop, K.I.; Kaklamani, V.G.; Brenner, A.J.; Li, R.; Nazarullah, A.; Hackman, S.; Thomas, C.; Gelfond, J.; Rodriguez, M.; Elledge, R. Novel Estrogen Receptor Beta Agonist S-Equol Decreases Tumor Proliferation in Patients with Triple Negative Breast Cancer (TNBC). J. Clin. Oncol. 2020, 38, 560–560. [Google Scholar] [CrossRef]
- Setchell, K.D.R.; Zhao, X.; Jha, P.; Heubi, J.E.; Brown, N.M. The Pharmacokinetic Behavior of the Soy Isoflavone Metabolite S-(−)Equol and Its Diastereoisomer R-(+)Equol in Healthy Adults Determined by Using Stable-Isotope-Labeled Tracers. Am. J. Clin. Nutr. 2009, 90, 1029–1037. [Google Scholar] [CrossRef]
- Setchell, K.D.; Clerici, C.; Lephart, E.D.; Cole, S.J.; Heenan, C.; Castellani, D.; Wolfe, B.E.; Nechemias-Zimmer, L.; Brown, N.M.; Lund, T.D.; et al. S-Equol, a Potent Ligand for Estrogen Receptor β, Is the Exclusive Enantiomeric Form of the Soy Isoflavone Metabolite Produced by Human Intestinal Bacterial Flora. Am. J. Clin. Nutr. 2005, 81, 1072–1079. [Google Scholar] [CrossRef]
- Muthyala, R.S.; Ju, Y.H.; Sheng, S.; Williams, L.D.; Doerge, D.R.; Katzenellenbogen, B.S.; Helferich, W.G.; Katzenellenbogen, J.A. Equol, a Natural Estrogenic Metabolite from Soy Isoflavones: Convenient Preparation and Resolution of R- and S-Equols and Their Differing Binding and Biological Activity through Estrogen Receptors Alpha and Beta. Bioorg. Med. Chem. 2004, 12, 1559–1567. [Google Scholar] [CrossRef]
- Xu, W.H.; Zheng, W.; Xiang, Y.B.; Ruan, Z.X.; Cheng, J.R.; Dai, Q.; Gao, Y.T.; Shu, X.O. Soya Food Intake and Risk of Endometrial Cancer among Chinese Women in Shanghai: Population Based Case-Control Study. Br. Med. J. 2004, 328, 1285–1288. [Google Scholar] [CrossRef]
- Horn-Ross, P.L.; John, E.M.; Canchola, A.J.; Stewart, S.L.; Lee, M.M. Phytoestrogen Intake and Endometrial Cancer Risk. J. Natl. Cancer Inst. 2003, 95, 1158–1164. [Google Scholar] [CrossRef]
- Bandera, E.V.; Williams, M.G.; Sima, C.; Bayuga, S.; Pulick, K.; Wilcox, H.; Soslow, R.; Zauber, A.G.; Olson, S.H. Phytoestrogen Consumption and Endometrial Cancer Risk: A Population-Based Case-Control Study in New Jersey. Cancer Causes Control 2009, 20, 1117–1127. [Google Scholar] [CrossRef]
- Shah, S.C.; Kayamba, V.; Peek, R.M.; Heimburger, D. Cancer Control in Low-and Middle-Income Countries: Is It Time to Consider Screening? J. Glob. Oncol. 2019, 5, 1–8. [Google Scholar] [CrossRef]
- Aspriţoiu, V.M.; Stoica, I.; Bleotu, C.; Diaconu, C.C. Epigenetic Regulation of Angiogenesis in Development and Tumors Progression: Potential Implications for Cancer Treatment. Front. Cell Dev. Biol. 2021, 9, 2462. [Google Scholar] [CrossRef]
- Akbarian, M.; Bertassoni, L.E.; Tayebi, L. Biological Aspects in Controlling Angiogenesis: Current Progress. Cell. Mol. Life Sci. 2022, 79, 349. [Google Scholar] [CrossRef]
- Zhong, H.; Sun, X. Contribution of Interleukin-17A to Retinal Degenerative Diseases. Front. Immunol. 2022, 13, 847937. [Google Scholar] [CrossRef]
- Singh, P.G.; Basalingappa, K.M.; Gopenath, T.S.; Sushma, B.V. Tumour Angiogenesis in Breast Cancer. In Tumor Angiogenesis and Modulators; IntechOpen: London, UK, 2022. [Google Scholar]
- Sadhukhan, S.; Dey, S. Biology, Chemistry, and Physics of Cancer Cell Motility and Metastasis. In Cancer Diagnostics and Therapeutics; Springer Singapore: Singapore, 2022; pp. 81–109. [Google Scholar]
- Unterleuthner, D.; Neuhold, P.; Schwarz, K.; Janker, L.; Neuditschko, B.; Nivarthi, H.; Crncec, I.; Kramer, N.; Unger, C.; Hengstschläger, M.; et al. Cancer-Associated Fibroblast-Derived WNT2 Increases Tumor Angiogenesis in Colon Cancer. Angiogenesis 2020, 23, 159–177. [Google Scholar] [CrossRef]
- Aguilar-Cazares, D.; Chavez-Dominguez, R.; Carlos-Reyes, A.; Lopez-Camarillo, C.; Hernadez de la Cruz, O.N.; Lopez-Gonzalez, J.S. Contribution of Angiogenesis to Inflammation and Cancer. Front. Oncol. 2019, 9, 1399. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.S.; Yoon, M. Regulation of Obesity by Antiangiogenic Herbal Medicines. Molecules 2020, 25, 4549. [Google Scholar] [CrossRef] [PubMed]
- Fallah, A.; Sadeghinia, A.; Kahroba, H.; Samadi, A.; Heidari, H.R.; Bradaran, B.; Zeinali, S.; Molavi, O. Therapeutic Targeting of Angiogenesis Molecular Pathways in Angiogenesis-Dependent Diseases. Biomed. Pharmacother. 2019, 110, 775–785. [Google Scholar] [CrossRef] [PubMed]
- Cohen, R.B.; Oudard, S. Antiangiogenic Therapy for Advanced Renal Cell Carcinoma: Management of Treatment-Related Toxicities. Investig. New Drugs 2012, 30, 2066–2079. [Google Scholar] [CrossRef]
- Bhise, N.S.; Shmueli, R.B.; Sunshine, J.C.; Tzeng, S.Y.; Green, J.J. Drug Delivery Strategies for Therapeutic Angiogenesis and Antiangiogenesis. Expert Opin. Drug Deliv. 2011, 8, 485–504. [Google Scholar] [CrossRef]
- Fridlender, M.; Kapulnik, Y.; Koltai, H. Plant Derived Substances with Anti-Cancer Activity: From Folklore to Practice. Front. Plant Sci. 2015, 6, 799. [Google Scholar] [CrossRef]
- Miura, A.; Sugiyama, C.; Sakakibara, H.; Simoi, K.; Goda, T. Bioavailability of isoflavones from soy products in equol producers and non-producers in Japanese women. J. Nutr. Intermed. Metab. 2016, 6, 41–47. [Google Scholar] [CrossRef]
- Lu, K.; Bhat, M.; Basu, S. Plants and Their Active Compounds: Natural Molecules to Target Angiogenesis. Angiogenesis 2016, 19, 287–295. [Google Scholar] [CrossRef]
- Bellou, S.; Karali, E.; Bagli, E.; Al-Maharik, N.; Morbidelli, L.; Ziche, M.; Adlercreutz, H.; Murphy, C.; Fotsis, T. The Isoflavone Metabolite 6-Methoxyequol Inhibits Angiogenesis and Suppresses Tumor Growth. Mol. Cancer 2012, 11, 35. [Google Scholar] [CrossRef]
- Yang, Z.; Zhao, Y.; Yao, Y.; Li, J.; Wang, W.; Wu, X. Equol induces mitochondria-dependent apoptosis in human gastric cancer cells via the sustained activation of ERK1/2 pathway. Mol. Cells 2016, 39, 742–749. [Google Scholar] [CrossRef]
- Leiva, B.; Carrasco, I.; Montenegro, I.; Gaete, L.; Lemus, I.; Tchernitchin, A.; Bustamante, R.; Párraga, M.; Villena, J. Boletín Latinoamericano y Del Caribe de Equol and Daidzein Decrease Migration, Invasion and Matrix Metalloproteinase (MMPs) Gene Expression in Prostate Cancer Cell Lines, DU-145 and PC-3. Bol. Latinoam. Caribe Plant Med. Aromat. 2015, 14, 251–262. [Google Scholar]
- Lu, C.; Gao, R.; Zhang, Y.; Jiang, N.; Chen, Y.; Sun, J.; Wang, Q.; Fan, B.; Liu, X.; Wang, F. S-equol, a metabolite of dietary soy isoflavones, alleviates lipopolysaccharide-induced depressive-like behavior in mice by inhibiting neuroinflammation and enhancing synaptic plasticity. Food Funct. 2021, 12, 5770–5778. [Google Scholar]
- Setchell, K.D.R.; Clerici, C. Equol: History, Chemistry, and Formation. J. Nutr. 2010, 140, 1355S–1362S. [Google Scholar] [CrossRef]
- Mitchell, J.H.; Gardner, P.T.; Mcphail, D.B.; Morrice, P.C.; Collins, A.R.; Duthie, G.G. Antioxidant Efficacy of Phytoestrogens in Chemical and Biological Model Systems. Arch. Biochem. Biophys. 1998, 360, 142–148. [Google Scholar] [CrossRef]
- Ma, Y.; Sullivan, J.C.; Schreihofer, D.A. Dietary Genistein and Equol (4=, 7 Isoflavandiol) Reduce Oxidative Stress and Protect Rats against Focal Cerebral Ischemia. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2010, 299, 871–877. [Google Scholar] [CrossRef]
- Liu, T.H.; Tsai, T.Y. Effects of Equol on Deoxycorticosterone Acetate Salt-Induced Hypertension and Associated Vascular Dementia in Rats. Food Funct. 2016, 7, 3444–3457. [Google Scholar] [CrossRef]
- Jackman, K.A.; Woodman, O.L.; Chrissobolis, S.; Sobey, C.G. Vasorelaxant and Antioxidant Activity of the Isoflavone Metabolite Equol in Carotid and Cerebral Arteries. Brain Res. 2007, 1141, 99–107. [Google Scholar] [CrossRef]
- Nam, J.K.; Ki, W.L.; Rogozin, E.A.; Cho, Y.Y.; Heo, Y.S.; Bode, A.M.; Hyong, J.L.; Dong, Z. Equol, a Metabolite of the Soybean Isoflavone Daidzein, Inhibits Neoplastic Cell Transformation by Targeting the MEK/ERK/P90RSK/Activator Protein-1 Pathway. J. Biol. Chem. 2007, 282, 32856–32866. [Google Scholar] [CrossRef]
- Hong, S.; Ryu, K.S.; Oh, M.S.; Ji, I.; Ji, T.H. Roles of Transmembrane Prolines and Proline-Induced Kinks of the Lutropin/Choriogonadotropin Receptor. J. Biol. Chem. 1997, 272, 4166–4171. [Google Scholar] [CrossRef]
- Avantaggiato, A.; Bertuzzi, G.; Vitiello, U.; Iannucci, G.; Pasin, M.; Pascali, M.; Cervelli, V.; Carinci, F. Role of Antioxidants in Dermal Aging: An In Vitro Study by q-RT-PCR. Aesthetic Plast Surg. 2014, 38, 1011–1016. [Google Scholar] [CrossRef]
- Pandel, R.; Poljšak, B.; Godic, A.; Dahmane, R. Skin Photoaging and the Role of Antioxidants in Its Prevention. ISRN Derm. 2013, 2013, 1–11. [Google Scholar] [CrossRef]
- Velarde, M.C.; Flynn, J.M.; Day, N.U.; Melov, S.; Campisi, J. Mitochondrial Oxidative Stress Caused by Sod2 Deficiency Promotes Cellular Senescence and Aging Phenotypes in the Skin. Aging 2012, 4, 3–12. [Google Scholar] [CrossRef]
- Cheng, C.; Wang, X.; Weakley, S.M.; Kougias, P.; Lin, P.H.; Yao, Q.; Chen, C. The Soybean Isoflavonoid Equol Blocks Ritonavir-Induced Endothelial Dysfunction in Porcine Pulmonary Arteries and Human Pulmonary Artery Endothelial Cells. J. Nutr. 2010, 140, 12–17. [Google Scholar] [CrossRef]
- Chung, J.E.; Kim, S.Y.; Jo, H.H.; Hwang, S.J.; Chae, B.; Kwon, D.J.; Lew, Y.O.; Lim, Y.T.; Kim, J.H.; Kim, E.J.; et al. Antioxidant Effects of Equol on Bovine Aortic Endothelial Cells. Biochem. Biophys. Res. Commun. 2008, 375, 420–424. [Google Scholar] [CrossRef]
- Kamiyama, M.; Kishimoto, Y.; Tani, M.; Utsunomiya, K.; Kondo, K. Effects of Equol on Oxidized Low-Density Lipoprotein-Induced Apoptosis in Endothelial Cells. J. Atheroscler. Thromb. 2009, 16, 239–249. [Google Scholar] [CrossRef] [PubMed]
- Bar-Or, D.; Bar-Or, R.; Rael, L.T.; Brody, E.N. Oxidative Stress in Severe Acute Illness. Redox. Biol. 2015, 4, 340–345. [Google Scholar] [CrossRef] [PubMed]
- Khansari, N.; Shakiba, Y.; Mahmoudi, M. Chronic inflammation and oxidative stress as a major cause of age-related diseases and cancer. Recent Pat. Inflamm. Allergy Drug Discov. 2009, 3, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Landskron, G.; De la Fuente, M.; Thuwajit, P.; Thuwajit, C.; Hermoso, M.A. Chronic inflammation and cytokines in the tumor microenvironment. J. Immunol. Res. 2014, 2014, 149185. [Google Scholar] [CrossRef]
- Reuter, S.; Gupta, S.C.; Chaturvedi, M.M.; Aggarwal, B.B. Oxidative Stress, Inflammation, and Cancer: How Are They Linked? Free Radic. Biol. Med. 2010, 49, 1603–1616. [Google Scholar] [CrossRef]
- Gupta, S.C.; Sundaram, C.; Reuter, S.; Aggarwal, B.B. Inhibiting NF-ΚB Activation by Small Molecules as a Therapeutic Strategy. Biochim. Biophys. Acta Gene Regul. Mech. 2010, 1799, 775–787. [Google Scholar] [CrossRef]
- Hayden, M.S.; Ghosh, S. NF-ΚB, the First Quarter-Century: Remarkable Progress and Outstanding Questions. Genes Dev. 2012, 26, 203–234. [Google Scholar] [CrossRef]
- Lephart, E.D. Protective Effects of Equol and Their Polyphenolic Isomers against Dermal Aging: Microarray/Protein Evidence with Clinical Implications and Unique Delivery into Human Skin. Pharm. Biol. 2013, 51, 1393–1400. [Google Scholar] [CrossRef]
- Kang, J.S.; Yoon, Y.D.; Han, M.H.; Han, S.B.; Lee, K.; Kang, M.R.; Moon, E.Y.; Jeon, Y.J.; Park, S.K.; Kim, H.M. Estrogen Receptor-Independent Inhibition of Tumor Necrosis Factor-α Gene Expression by Phytoestrogen Equol Is Mediated by Blocking Nuclear Factor-ΚB Activation in Mouse Macrophages. Biochem. Pharm. 2005, 71, 136–143. [Google Scholar] [CrossRef]
- Kang, J.S.; Yoon, Y.D.; Han, M.H.; Han, S.B.; Lee, K.; Park, S.K.; Kim, H.M. Equol Inhibits Nitric Oxide Production and Inducible Nitric Oxide Synthase Gene Expression through Down-Regulating the Activation of Akt. Int. Immunopharmacol. 2007, 7, 491–499. [Google Scholar] [CrossRef]
- Blay, M.; Espinel, A.E.; Delgado, M.A.; Baiges, I.; Bladé, C.; Arola, L.; Salvadó, J. Isoflavone Effect on Gene Expression Profile and Biomarkers of Inflammation. J. Pharm. Biomed. Anal. 2010, 51, 382–390. [Google Scholar] [CrossRef]
- Gopaul, R.; Knaggs, H.E.; Lephart, E.D. Biochemical Investigation and Gene Analysis of Equol: A Plant and Soy-Derived Isoflavonoid with Antiaging and Antioxidant Properties with Potential Human Skin Applications. BioFactors 2012, 38, 44–52. [Google Scholar] [CrossRef]
- Johnson, S.L.; Kirk, R.D.; Dasilva, N.A.; Ma, H.; Seeram, N.P.; Bertin, M.J. Polyphenol Microbial Metabolites Exhibit Gut and Blood–Brain Barrier Permeability and Protect Murine Microglia against Lps-Induced Inflammation. Metabolites 2019, 9, 78. [Google Scholar] [CrossRef]
- Subedi, L.; Ji, E.; Shin, D.; Jin, J.; Yeo, J.H.; Kim, S.Y. Equol, a Dietary Daidzein Gut Metabolite Attenuates Microglial Activation and Potentiates Neuroprotection in Vitro. Nutrients 2017, 9, 207. [Google Scholar] [CrossRef]
- Moriyama, M.; Hashimoto, A.; Satoh, H.; Kawabe, K.; Ogawa, M.; Takano, K.; Nakamura, Y. S-Equol, a Major Isoflavone from Soybean, Inhibits Nitric Oxide Production in Lipopolysaccharide-Stimulated Rat Astrocytes Partially via the GPR30-Mediated Pathway. Int. J. Inflam. 2018, 2018, 8496973. [Google Scholar] [CrossRef]
- Lephart, E.D. Equol’s Anti-Aging Effects Protect against Environmental Assaults by Increasing Skin Antioxidant Defense and ECM Proteins While Decreasing Oxidative Stress and Inflammation. Cosmetics 2018, 5, 16. [Google Scholar] [CrossRef]
- Kim, E.Y.; Kim, A.K. Combination Effect of Equol and TRAIL against Human Cervical Cancer Cells. Anticancer Res. 2013, 33, 903–912. [Google Scholar]
- Adam, V.; Ekblad, M.; Sweeney, K.; Müller, H.; Busch, K.H.; Johnsen, C.T.; Kang, N.R.; Lemoine, N.R.; Halldén, G. Synergistic and Selective Cancer Cell Killing Mediated by the Oncolytic Adenoviral Mutant AdΔΔ and Dietary Phytochemicals in Prostate Cancer Models. Hum. Gene 2012, 23, 1003–1015. [Google Scholar] [CrossRef]
- Teng, H.; Zheng, Y.; Cao, H.; Huang, Q.; Xiao, J.; Chen, L. Enhancement of Bioavailability and Bioactivity of Diet-Derived Flavonoids by Application of Nanotechnology: A Review. Crit. Rev. Food Sci. Nutr. 2021, 19, 1–16. [Google Scholar] [CrossRef]
- Aiello, P.; Consalvi, S.; Poce, G.; Raguzzini, A.; Toti, E.; Palmery, M.; Biava, M.; Bernardi, M.; Kamal, M.A.; Perry, G.; et al. Dietary Flavonoids: Nano Delivery and Nanoparticles for Cancer Therapy. Semin. Cancer Biol. 2021, 69, 150–165. [Google Scholar] [CrossRef]
- Maan, G.; Sikdar, B.; Kumar, A.; Shukla, R.; Mishra, A. Role of Flavonoids in Neurodegenerative Diseases: Limitations and Future Perspectives. Curr. Top Med. Chem. 2020, 20, 1169–1194. [Google Scholar] [CrossRef]
- Mishra, P.K. Nano-Engineered Flavonoids for Cancer Protection. Front. Biosci. 2019, 24, 4771. [Google Scholar] [CrossRef]
- Yang, P.; Zhang, J.; Xiang, S.; Jin, Z.; Zhu, F.; Wang, T.; Duan, G.; Liu, X.; Gu, Z.; Li, Y. Green Nanoparticle Scavengers against Oxidative Stress. ACS Appl. Mater. Interfaces 2021, 13, 39126–39134. [Google Scholar] [CrossRef] [PubMed]
- Turuvekere Vittala Murthy, N.; Agrahari, V.; Chauhan, H. Polyphenols against Infectious Diseases: Controlled Release Nano-Formulations. Eur. J. Pharm. Biopharm. 2021, 161, 66–79. [Google Scholar] [CrossRef]
- Yoshikata, R.; Myint, K.Z.Y.; Ohta, H.; Ishigaki, Y. Effects of an Equol-Containing Supplement on Advanced Glycation End Products, Visceral Fat and Climacteric Symptoms in Postmenopausal Women: A Randomized Controlled Trial. PLoS ONE 2021, 16, e0257332. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhang, D.; Li, G.; Ho, S.C.; Chen, Y.; Ma, J.; Huang, Q.; Li, S.; Ling, W. The 6-month effect of whole soy and purified isoflavones daidzein on thyroid function—A double-blind, randomized, placebo controlled trial among Chinese equol-producing postmenopausal women. Phytother. Res. 2021, 35, 5838–5846. [Google Scholar] [CrossRef] [PubMed]
- Caruso, S.; Cianci, S.; Fava, V.; Rapisarda, A.M.C.; Cutello, S.; Cianci, A. Vaginal Health of Postmenopausal Women on Nutraceutical Containing Equol. Menopause 2018, 25, 430–435. [Google Scholar] [CrossRef]
- Bosland, M.C.; Enk, E.; Schmoll, J.; Schlicht, M.J.; Randolph, C.; Deaton, R.J.; Xie, H.; Zeleniuch-Jacquotte, A.; Kato, I. Soy Protein Supplementation in Men Following Radical Prostatectomy: A 2-Year Randomized, Placebo-Controlled Clinical Trial. Am. J. Clin. Nutr. 2021, 113, 821–831. [Google Scholar] [CrossRef]
- Schneider, L.S.; Hernandez, G.; Zhao, L.; Franke, A.A.; Chen, Y.-L.; Pawluczyk, S.; Mack, W.J.; Brinton, R.D. Safety and Feasibility of Estrogen Receptor-β Targeted PhytoSERM Formulation for Menopausal Symptoms: Phase 1b/2a Randomized Clinical Trial. Menopause 2019, 26, 874–884. [Google Scholar] [CrossRef]
- Ribeiro, A.E.; Monteiro, N.E.S.; de Moraes, A.V.G.; Costa-Paiva, L.H.; Pedro, A.O. Can the Use of Probiotics in Association with Isoflavone Improve the Symptoms of Genitourinary Syndrome of Menopause? Results from a Randomized Controlled Trial. Menopause 2019, 26, 643–652. [Google Scholar] [CrossRef]
- Furlong, O.N.; Parr, H.J.; Hodge, S.J.; Slevin, M.M.; Simpson, E.E.; McSorley, E.M.; McCormack, J.M.; Magee, P.J. Consumption of a Soy Drink Has No Effect on Cognitive Function but May Alleviate Vasomotor Symptoms in Post-Menopausal Women; a Randomised Trial. Eur. J. Nutr. 2020, 59, 755–766. [Google Scholar] [CrossRef]
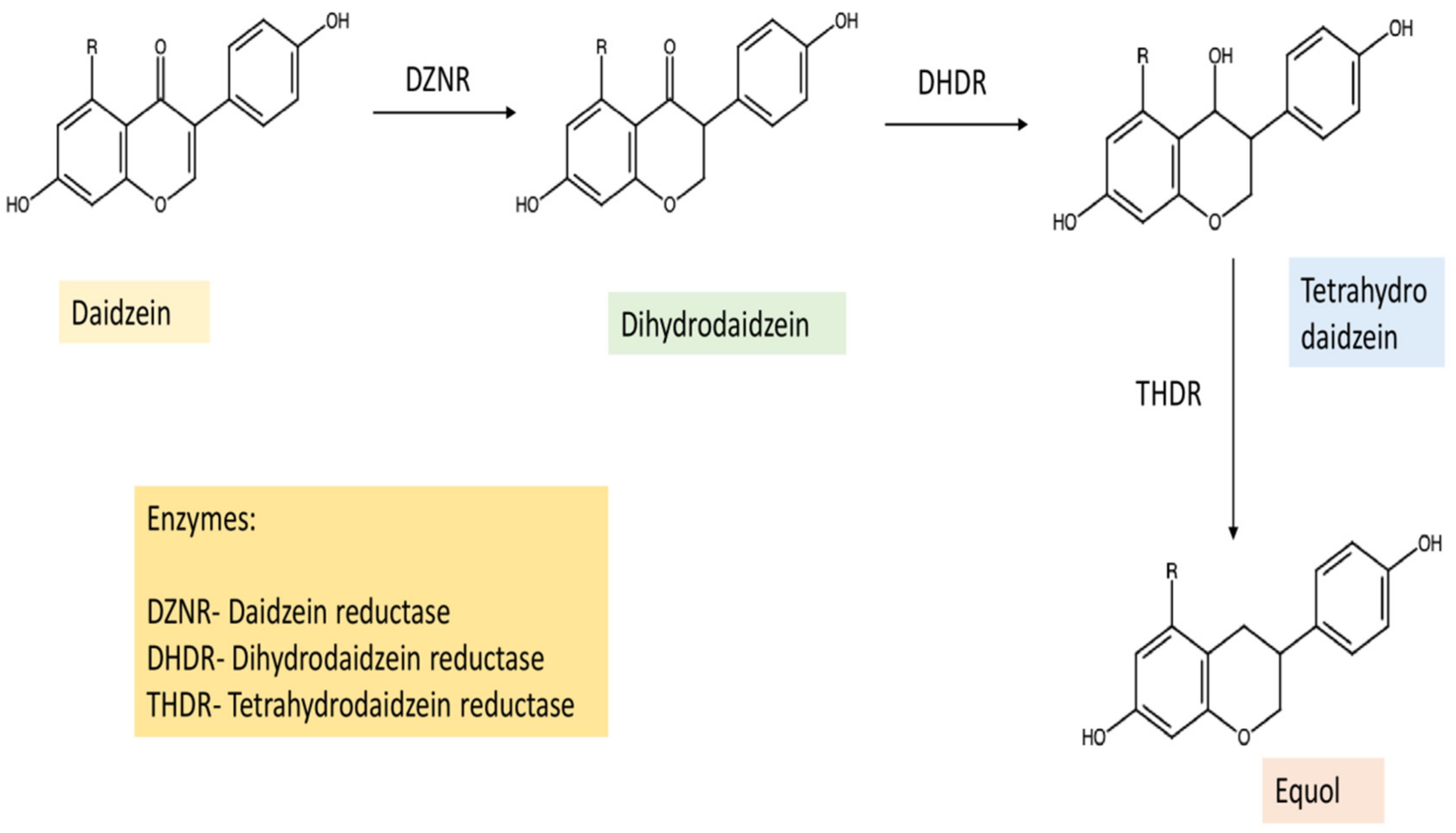
 star indicated the Equol.
star indicated the Equol.
 star indicated the Equol.
star indicated the Equol.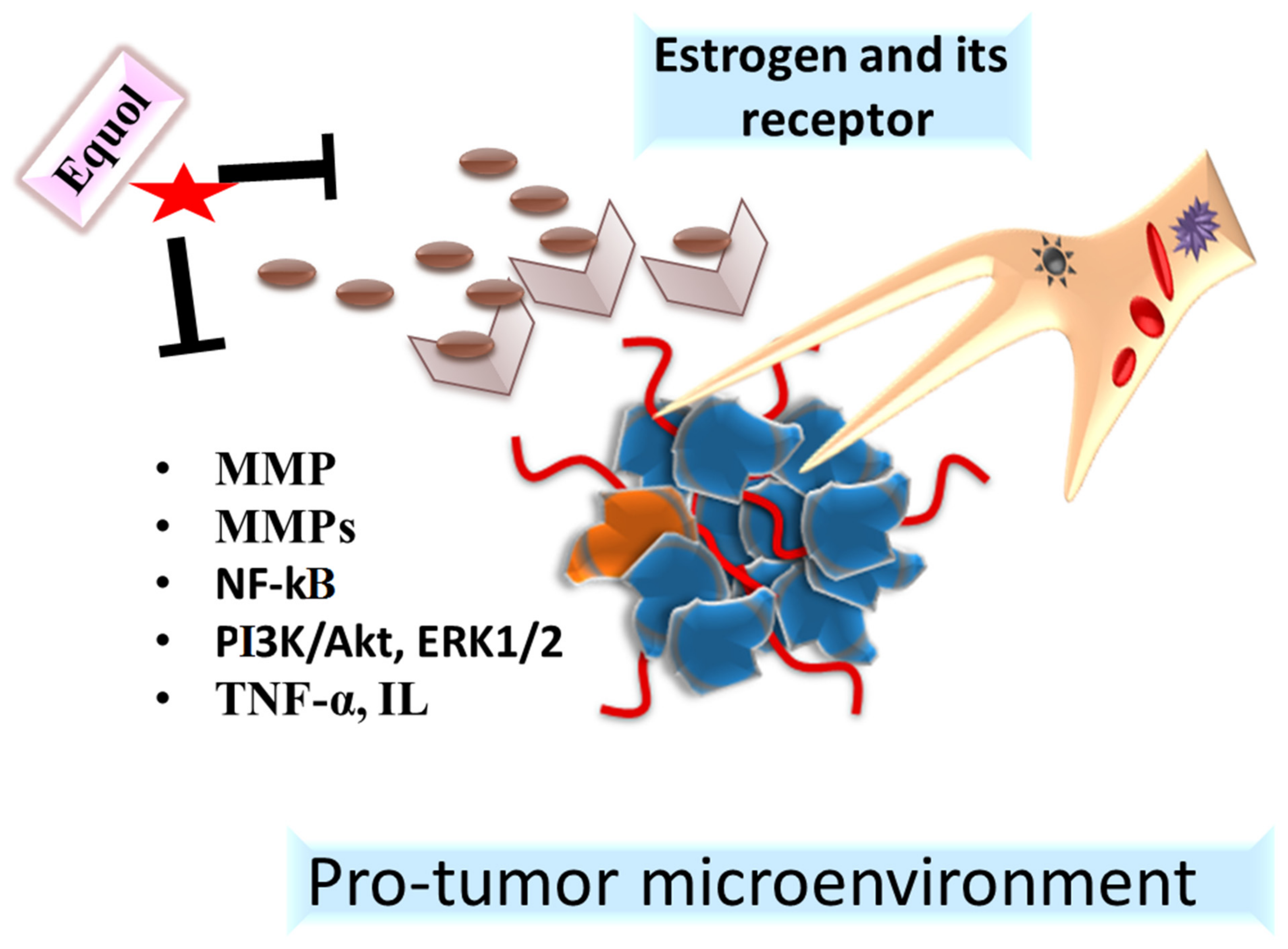
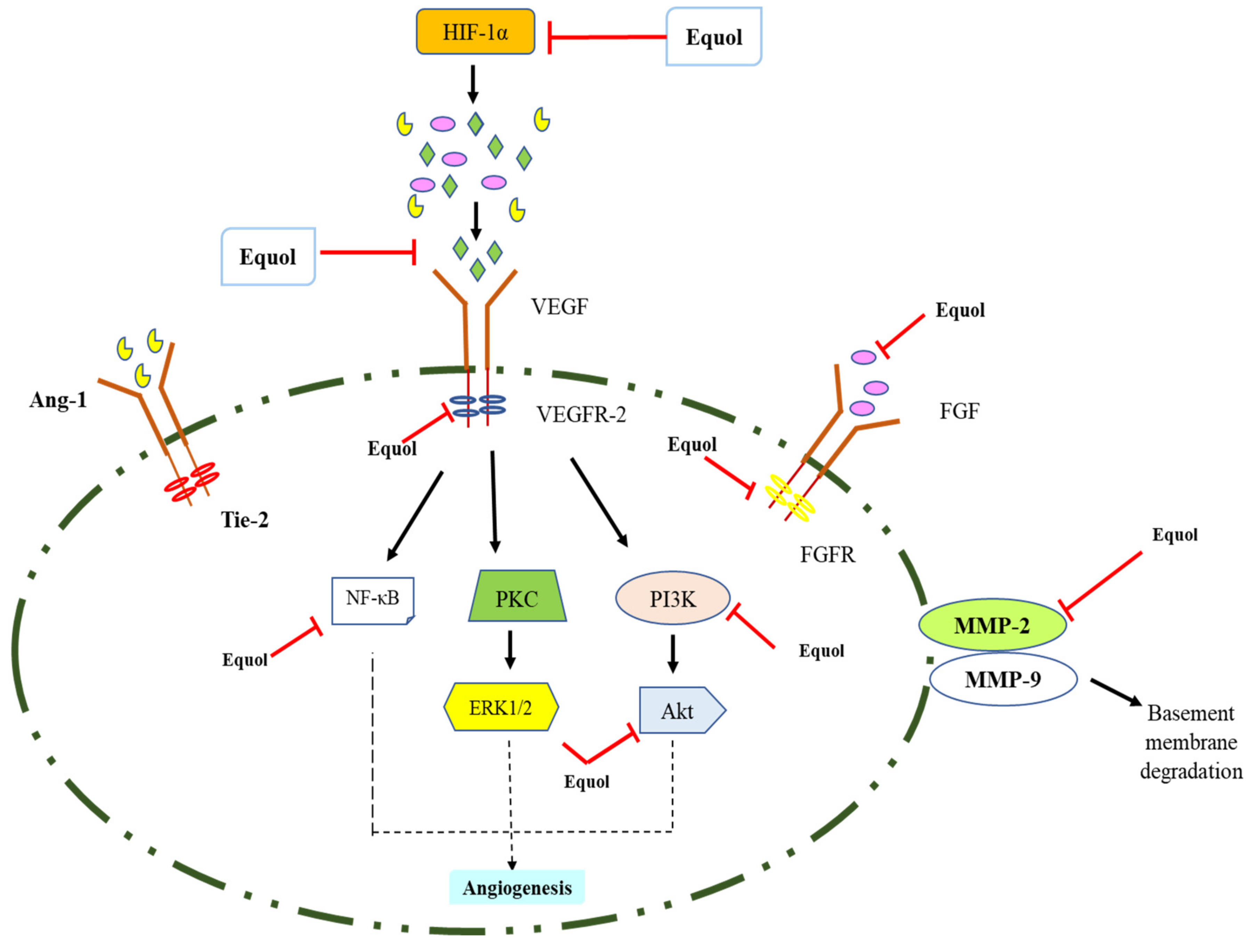
| Type of Cancer | Cell Lines | Effects | Mechanisms | Concentration | References |
|---|---|---|---|---|---|
| Osteosarcoma | 143B and U2OS | Induces apoptosis | ↓ proliferation and migration of 143B and U2OS osteosarcoma cells, ↑ %age of S phase cells, ↓ %age of G0/G1 phase cells, ↓ p-Src-ERK, ↓ p-Src, ↓ p-ERK, No change in expression levels of Src, JNK, p-JNK, ERK, p38 and p-p38 | Daidzein—0, 10, 20, 50, 100, 200 or 500 µM | [15] |
| MG-63 | Induces apoptosis | ↑ ROS, ↓ mitochondrial membrane potential, ↑ apoptosis rate, ↑ cell cycle arrest at the G2/M phase, ↓ Bcl-2, ↓ Bcl-x and ↓ Baid proteins, ↑ Bim protein | Daidzein—IC50 value of 59.7 µM | [16] | |
| Colon | HT-29 | Induces apoptosis | ↓ growth of cancer cells, significant increase in cells in the G0/G1 phase, ↓ Lipid droplets accumulation, ↓ Perilipin-1, ↓ ADRP and↓ Tip-47 family proteins, ↓ vimentin, ↑ PPAR, ↑ Fas, ↑ FABP, ↑ GPAT3, ↑MTTP, ↓ UCP2. ↓ PI3K, ↑ FOXO3a, ↑ caspase-8 | Genistein and Daidzein—0, 25, 50, 100, 200, and 400 μM | [17] |
| DLD1, HCT15, COLO205, LOVO, SW480 | Induces apoptosis | ↓ growth of HCT-15 cells with the expression of ERα and ERβ, ↓ growth of LOVO, and SW480 cells with the ERβ expression, ↑ ERα and ERβ in HCT-15. ↑ ERα and ERβ, ↑ Nrf2 | Equol—0, 0.5, 1, 5, 10 μM | [18] | |
| Breast | MCF-7 | Induces apoptosis | ↑ % age of apoptotic cells, ↑ Caspase 3/7 activity, ↑ Bax, ↓ Bcl2, ↑ ROS, ↓ ERα, ↑ ERβ | Daidzein—IC50—50 µM | [19] |
| MCF-7 and T47D | Induces apoptosis | ↑ cytotoxic effects towards cancer cells, ↓ NGB, ↑p- AKT, ↑ p38 phosphorylation, ↑ cleaved PARP-1 | Daidzein—1–10 µM and Equol 1 μM | [20] | |
| MCF7 and MCF7/ADR | Enhances the anticancer effect of topotecan (tpt) and reverses BCRP-mediated drug resistance | ↑ anti-proliferative effect with TPT on MCF7 and MCF7/ADR cells, ↑ inhibitory effect of TPT on Topo Ⅰ activity, ↑ inhibition of TPT on the catalytic activity of Topo Ⅰ, ↑ cells arresting at the G2/M phase, ↑ apoptosis rate, ↓ resistance of MCF7/ADR cells to TPT, ↓ ERα and BCRP, ↑ TPT accumulation intracellularly | Daidzein—0, 2.13, 6.25, 12.5, 25, 50, 100, 200 and 400 µM and Topotecan 0, 0.78, 1.56, 3.13, 6.25, 12.5, 25, 50 and 100 µM | [21] | |
| MCF10DCIS.com | Induces apoptosis | ↓ TNF-α induced cell migration and invasion, no effect on IκBα expression and NF-κB p65 phosphorylation, ↓ Gli1, ↓ MMP-9 | Daidzein—0, 5, 10, 30 and 50 µM and Equol—10 μM | [22] | |
| MCF-7 | Induces apoptosis | ↓ MCF-7 viability, ↑ %age of apoptotic cells, ↑ % age cells sub-G1 phase, ↓ % age cells in G0/G1, S and G2/M phase, ↑ p53, ↑ p21, ↑ PARP cleavage, ↑ α-fodrin proteolysis, ↑ pro-caspase-7 and pro-caspase-9 cleavage, ↓ Bcl-2, ↑ cytochrome-c release to the cytosol, ↓ Bcl-2: Bax ratio, ↑ tamoxifen’s anti-tumor activity | Equol—0, 25, 50 and 100 μM and 4-OHT 0, 0.01, 0.1, 1.0, 10.0 μM | [23] | |
| MCF-7 and MDA-MB-453 | Induces apoptosis | ↓ cell proliferation of cancer cells, ↑ cell cycle arrest in the G1 and G2/M phases, ↑ %age cells in sub-G0 phase, ↓ cyclin D, ↓ CDK2, ↓ CDK4, No Change in the expression of CDK6 and cyclin E, ↓ CDK 1, ↑ p21Cip1 and ↑ p57Kip2, No change in p27Kip1 | Daidzein—1–100 μM | [24] | |
| MCF-7/MDA MB-231 | Induces apoptosis | ↓ viability of MCF-7 and MDA MB-231 cell lines, no significant growth inhibition was observed in MCF-10A cells, ↑ no of rounded cells due to shrinkage and condensation of cytoplasm, ↑ apoptotic cells, ↑ tunnel +live cells, ↑ ROS, ↓ ∆ψm, ↓ Bcl-xL, ↑ BAX, ↑ Caspase 3/7/9, ↑ cleaved PARP, ↓ PI3K, ↓ p-Akt, ↓ p-mTOR, ↑ affectivity of Centchroman | Centchroman—1–30 µM and Daidzein 10–200 µM | [25] | |
| MCF-7 and MDA-MB-231 | Induces apoptosis | ↑ MRP2, ↓ MRP1, ↓ ABCC2 and ABCC1 mRNA | Daidzein—0.05, 0.5 and 5 µM, R-equol and S-equol—0.1, 1 and 10 µM | [26] | |
| MCF-7 | Enhances apoptosis-inducing activity of genistein | ↑ cytotoxicity of genistein, ↑ G2/M phase cells, ↓ G1/S blockade and G2/M progression ↑ sub-G0/G1 population ↑ apoptosis rate, ↑ Bax/Bcl-xL expression ratio, No change in activities of Akt and mTOR, ↑ c-PARP | Genistein—0–100 µM, Equol—0–100 µM | [27] | |
| MDA-MB-435 (ER) | Induces apoptosis | ↑ eIF4GI, ↑ c-Myc, ↑ Cyclin D ↑Bcl-XL ↑ p120 catenin | (R, S) Equol—25 μM | [28] | |
| MDA-MB-231 | Inhibit metastasis | ↓ invasive capacity, ↓ MMP-2, No Change on n the expression levels of MMP- 9, TIMP-1 or TIMP-2 | Daidzein, R—and S-Equol—0, 2.5, 10, 50 µM | [29] | |
| MCF-7 | Induces apoptosis | ↑ ROS, ↓ Bcl-2, ↑ Bax, ↑ release of cytochrome C from the mitochondria into the cytosol, ↑ caspase-9, ↑ caspase-7 | Daidzein—25–100 µM | [30] | |
| MCF-7 | -- | ↑ antiproliferative effects, ↑ pS2 mRNA | Daidzein and (±)-equol 0.001 to 50 µM | [31] | |
| Lung | A594 and 95D | Induces apoptosis | ↓ proliferation and colony formation property of cancer cells ↓ IL-6, ↓IL-8, ↓ p65-NFκB expression and activation, ↓ level of p65-NFκB upregulation induced by C/EBPβ | Daidzein—0, 5, 10, and 25 μM | [32] |
| A549, HepG-2 | Induces apoptosis | ↑ ROS, ↓ mitochondrial membrane potential, ↑ apoptosis rate, ↑ cell cycle arrest at the G2/M phase, ↓ Bcl-2, ↓ Bcl-x and ↓ Baid proteins, ↑ Bim protein | Daidzein—IC50 value of 59.7 µM | [33] | |
| Gastric | MGC-803 | Induces apoptosis | ↓ viability of MGC-803 cells, ↑ G0/G1 cell cycle arrest, ↓ CDK2/4, ↓ Cyclin D1/Cyclin E1 ↑ P21WAF1, ↑ apoptosis frequency, ↑ cleaved PARP, ↑ caspase-3. ↑ P-Akt (Ser473 and Thr308) | Equol—5, 10, 20, 40, or 80 μM | [34] |
| BGC-823 | Induces apoptosis | ↓ growth and proliferation of gastric carcinoma cells, ↓ mitochondrial transmembrane potential ↑ cleaved PARP, ↑ cleaved caspase-9, ↑ cleaved caspase-3, ↑ Bax, ↓ Bcl-2, ↓ Bcl-2/Bax | Daidzein—0, 20, 40, and 80 µM | [35] | |
| Hepatocellular | SMMC-7721 and HepG2 | Induces apoptosis | ↓ proliferation, migration and invasion of cancer cells, ↓ concentrations of pyruvate, glutamate and glucose, ↓ activities of hexokinase, phosphofructokinase and pyruvate kinase, ↓ pyruvate kinase M2, ↑ levels of glycerophosphocholine, ethanolamine, taurine, fumarate, leucine, acetate, ↓ levels of pyruvate, glutamate, glutamine, adenosine monophosphate, creatine, glycine | (−)—5-hydroxy Equol—0, 10, 20, 30, 40 and 50 µM | [36] |
| SMMC-7721 | Induces apoptosis | ↓ proliferation of SMMC-7721 cells, ↑ apoptosis frequency, ↑ S-phase cell cycle arrest, ↑ p21, ↓ cyclin A2; No change in expression of cyclin D1 and H2AX, ↑ caspase-9, ↑ caspase-3, ↑ c-PARP, ↑ Bax ↓ Bcl-2, ↑ caspase-8, ↑ caspase-12, ↑ Chop, ↑ Bip | (±)—Equol, R-(+)-Equol, and S-(–)-Equol—0, 5, 10, 20, 50, and 100 µM | [37] | |
| SK-HEP-1 | Induces apoptosis | ↓ cell proliferation of cancer cells, ↑ Prdx-3, ↑ Bak, ↓ Bcl-2, ↓ Bcl-xL, ↑ release of mitochondrial cytochrome c to cytosol, ↑ APAF-1, ↑ caspase 9, ↑ caspase 3 | Daidzein—0, 200, 400 and 600 µM | [38] | |
| Pancreatic | MiaPaCa-2 and PANC-1 | Induces apoptosis | ↓ growth and proliferation of pancreatic cancer cells, inhibitory effects on both ER positive and negative pancreatic cancer cells | Daidzein—0.1, 1, 10, 25, 50, 75 and 100 µmol/L | [39] |
| Colorectal | SW620 | Anti-proliferative effects | ↓ p-ERK/ERK, ↓ p-AKT/AKT | Chrysin IC50 values 70 µM and Daidzein IC50 values 23.5 µM | [40] |
| HCT-15 | Induces apoptosis | Racemic equol ↓ proliferation of HCT-15 cells, whereas(S) equol had no effect on the proliferation of HCT-15 cells. Racemic equol ↓ ERβ and ↓ Nrf2, while (R) equol ↓ Nrf2 | Racemic equol and equol enantiomers—0, 0. 5, 1, 5 and 10 μM | [41] | |
| Bladder | RT112, RT4 and SW780 | Induces apoptosis | ↓ cell viability, Impaired colony formation, ↑ G1/S cell cycle arrest, ↑ apoptosis frequency, ↓ FGFR3 signaling pathway, ↓ p-FGFR3, ↓ p-Akt, p-ERK | Daidzein—0, 0.5, 1, 2.5, 5, 7.5, 10, 50 and 100 μM | [42] |
| Prostate | DU145, LNCaP and PC3 | Induces apoptosis | ↑ cytotoxic activity, ↑ ERβ binding activity, ↑ ERβ gene expression, ↓ cMYC, ↓ Cyclin D1 genes, ↑ caspase 3 and 9, No change in uterotropic and anti-androgenic activities | Novel daidzein molecules—1, 5, 10, 50, 100, 200, 300, 400, 500 µM | [43] |
| LNCaP, DU145 and PC3 | Induces apoptosis | ↑ cell cycle arrest in the G2/M phase↓ Cyclin B1 ↓ CDK1, ↑ p21 and p27, ↑ apoptosis rate, ↑ FasL ↑ Bim. ↑ FOXO3a, ↓ p-FOXO3a, ↑ nuclear stability of FOXO3a, ↓ MDM2 | S-Equol—0, 0.5, 1, 5, 10 μM | [44] | |
| DU145 | Induces apoptosis | ↓ cell migration and invasion, ↓ MMP-, ↓ u-PA, ↓ secreted MMP-2 and MMP-9, ↑ SOD, ↑ Nrf2, ↑ PTEN | (±) Equol 5, 10, 50 µM, Daidzein and Genistein—0.5, 1 and 5 µM | [45] | |
| PC3, DU145 cells | Induces apoptosis | ↓ MMP-2, ↓ MMP-9, ↑ ERγ, No change in Erβ | Equol—0, 0.5, 1, 5, 10 μM | [46] | |
| Choriocarcinoma | JAR and JEG-3 | Induces apoptosis | ↓ cell viability, ↑ early and late apoptotic cells, ↑ apoptosis frequency, ↑ caspase-9, ↑ caspase-3, ↑ c-PARP, ↓ Bcl-2/Bax | Daidzein—0, 25, 50 or 100 µM | [29] |
| Cervix | BEL-7402, HeLa, | Induces apoptosis | ↑ ROS, ↓ mitochondrial membrane potential, ↑ apoptosis rate, ↑ cell cycle arrest at the G2/M phase, ↓ Bcl-2, ↓ Bcl-x and ↓ Baid proteins, ↑ Bim protein | Daidzein—IC50 value of 59.7 µM | [47] |
| Ovarian | caov-3, OVAcAR-3, SKOV3 and A2780 | Induces apoptosis | ↑ antiproliferative effects on SKVO3 cells, SKOV3 cancer cells became rounder, shrunken and detached from the substratum, ↑ apoptotic cells, ↑ release of cytochrome c into the cytoplasm, ↑ cytosolic levels of cyt c, ↑ Bax, ↑ cleaved caspase-3 and -9, ↑ cleaved PARP, ↑ G2 phase cells leading to G2/M cell cycle phase arrest, ↓ pcdc25c (Ser216), ↓ cdc25c, ↓ pcdc2 (Tyr15), ↓ cdc2, ↓ cyclin B1, ↑ p21, ↓ migratory capability of cancer cells, ↓ MMP-9, ↓ MMP-2, ↓ p-MEK, ↓ p-ERK | Daidzein—0, 10, 20 and 40 µM | [48] |
| Type of Cancer | Animal Models | Effects | Mechanisms | Dosage | Duration | References |
|---|---|---|---|---|---|---|
| Osteosarcoma | BALB/c nude mice xenografted with 143B (1 × 107) cells | Inhibited Tumor Growth | ↓ volume and weight of the tumors, ↑ number of necrotic cells, no systemic toxicity | Daidzein—20 mg/kg | 16 days | [15] |
| Breast | Athymic nude mice xenografted with MCF7 cells (5 × 106 cells) | Inhibited Tumor Growth | No obvious damage found in visceral organs of MCF7 xenograft nude mice, ↓ tumor volume, ↑ tumor inhibition rate of the combination group, ↑ Bax, ↑ p53 and ↑ p21 in the combination group than in the TPT monotherapy group, ↓ Bcl2 | Topotecan—3 mg/ kg and Daidzein—5 mg/kg. | 15 days | [21] |
| MCF-7 cells implanted in ovariectomized athymic mice | Inhibited Tumor Growth | No significant difference was observed in uterine weight, No significant induction of pS2 mRNA (an estrogen responsive marker) in tumors | Daidzein—125, 250, 500 and 1000 p.p.m and (±)-Equol—250, 500 and 1000 p.p.m | 7 days | [31] | |
| Lung | Balb/c nude mice xenografted with A549 cells | Inhibited Tumor Growth | ↓ Ki-67 and ↓ p65-NF-κB, ↓ tumor volume | Daidzein—5 mg/kg. | 21 days | [32] |
| Bladder | Nude mice xenografted with RT112 cells (1 × 106 cells) | Inhibited Tumor Growth | ↓ tumor volume and weight and size, ↓ tumor volume toxicity of daidzein to normal cells, | Daidzein—10 mg/kg and 20 mg/kg | 27 days | [42] |
| Colorectal | Albino rats subcutaneous injected with DMH (40 mg/kg) | Inhibited Tumor Growth | ↓ NO, ↓ MDA, ↑ GSH, ↓ CYP2E1 colon content, ↓ CXCL1, ↓ AREG level, ↓ colon content of MMP-9↓ DMH+DSS induced histopathological changes at both doses | Chrysin—125 and 250 mg/kg and Daidzein—5 and 10 mg/kg | 56 days | [40] |
| Prostate | C57B1/6 male mice xenografted with PC3 cell (5 × 104) | Inhibited Tumor Growth | ↑ doxorubicin anti-tumor activity, ↑ number of necrotic cells, shows hyperplastic acini lined by simple columnar epithelium and basal cells, ↑ recovery from prostate cancer | The novel metabolites 1 and 2–30 mg/ kg and Doxorubicin—6 mg/kg | 21 days | [43] |
| BALB/c nude mice xenografted with PC3 cells | Inhibited Tumor Growth | ↓ volume and weight of the tumors, ↑ no. number of necrotic cells, ↓ p-FOXO3a and ↑ nuclear stability of FOXO3a | Daidzein | -- | [44] | |
| Ovary | Nude mice xenografted with SKVO3 cells (5 × 106 cells). | Inhibited Tumor Growth | ↓ Ki-67 ↑ cleaved caspase-3 | Daidzein—10, 20 and 40 µg/kg | 27 days | [48] |
| Agent Administered, Dosage and Duration | Volunteers | Design | Outcome | Conclusion | Reference |
|---|---|---|---|---|---|
| 10 mg/day supplement containing 98% equol, for 3 months | 57 post-menopausal women | Single center, randomized, controlled clinical trial | Reduction in visceral fat, as well as the levels of LDL and total cholesterol, some indications of delayed skin ageing | Equol supplementation may be used for the management of excess visceral fat, and may be used for the alleviation of climacteric symptoms as well as metabolic disorders | [152] |
| 40 g low-fat milk powder + 63 mg daidzein, for a period of 6 months | 270 post-menopausal, equol-producing women | Double-blind, randomized, placebo controlled clinical trial | Lowering of T4 levels following daidzein supplementation, no disruption of thyroid functioning | Daidzein supplementation was found to be safe and did not hamper the levels of key markers of thyroid functioning, thereby establishing its safety profile | [153] |
| Supplement containing 10 mg equol + 10 mg resveratrol + 150 mg quercetin + 178 mg Passiflora, administered up to 8 months | 126 post-menopausal women | Clinical trial | Improved vaginal health index, stabilization of pH, improvement of dyspareunia | Equol supplementation may be used for relieving post-menopausal symptoms | [154] |
| 20 g/day soy isolate supplementation, containing 41 mg of isoflavones | 44–75-year-old men predisposed to prostate cancer recurrence, following prostatectomy | Randomized, placebo-controlled clinical trial | Slight improvement in hemoglobin and hematocrit levels, reduction in blood pressure in non-producers of equol | The observation from the trial helped to establish the relationship between the equol-producing status of volunteers and soy supplementation, suggesting that certain effects may be observed in each sub-type that may be different from the other, and may be used to enhance therapeutic outcomes | [155] |
| 50 and 100 mg supplementation of phytoSERM, containing genistein, daidzein and S-equol, for 12 weeks | 71 peri-menopausal women | Double-blinded, randomized, placebo-controlled clinical trial | Good tolerance of the formulation in volunteers, mild adverse effects | Potentials for the usage of equol in the management of post-menopausal symptoms, as well as mild improvement vasomotor and cognitive functioning | [156] |
| Oral isoflavone administration (150 mg extract), alone or in conjunction with probiotics or hormonal therapy | 60 post-menopausal women | Randomized, controlled clinical trial | Alleviation of urogenital complications, increase in the formation of metabolic intermediates, overall improvement of vaginal health | Isoflavone administration may be used in the management of urogenital symptoms and administration with probiotics may be linked with improvement of the synthesis of products of metabolism of isoflavones, which have been established to have therapeutic benefits | [157] |
| Oral isoflavone administration (consumption of a soy drink providing a dosage of 10–60 mg/day), over 12 weeks | 101 post-menopausal women | Randomized, controlled clinical trial | Reduction in the severity of vasomotor symptoms associated with post-menopausal complications | Soy drink supplementation, containing isoflavones, may be used in the management of vasomotor symptoms and may be provided as a natural therapeutic agent | [158] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tuli, H.S.; Kumar, A.; Sak, K.; Aggarwal, D.; Gupta, D.S.; Kaur, G.; Vashishth, K.; Dhama, K.; Kaur, J.; Saini, A.K.; et al. Gut Microbiota-Assisted Synthesis, Cellular Interactions and Synergistic Perspectives of Equol as a Potent Anticancer Isoflavone. Pharmaceuticals 2022, 15, 1418. https://doi.org/10.3390/ph15111418
Tuli HS, Kumar A, Sak K, Aggarwal D, Gupta DS, Kaur G, Vashishth K, Dhama K, Kaur J, Saini AK, et al. Gut Microbiota-Assisted Synthesis, Cellular Interactions and Synergistic Perspectives of Equol as a Potent Anticancer Isoflavone. Pharmaceuticals. 2022; 15(11):1418. https://doi.org/10.3390/ph15111418
Chicago/Turabian StyleTuli, Hardeep Singh, Ajay Kumar, Katrin Sak, Diwakar Aggarwal, Dhruv Sanjay Gupta, Ginpreet Kaur, Kanupriya Vashishth, Kuldeep Dhama, Jagjit Kaur, Adesh K. Saini, and et al. 2022. "Gut Microbiota-Assisted Synthesis, Cellular Interactions and Synergistic Perspectives of Equol as a Potent Anticancer Isoflavone" Pharmaceuticals 15, no. 11: 1418. https://doi.org/10.3390/ph15111418
APA StyleTuli, H. S., Kumar, A., Sak, K., Aggarwal, D., Gupta, D. S., Kaur, G., Vashishth, K., Dhama, K., Kaur, J., Saini, A. K., Varol, M., Capanoglu, E., & Haque, S. (2022). Gut Microbiota-Assisted Synthesis, Cellular Interactions and Synergistic Perspectives of Equol as a Potent Anticancer Isoflavone. Pharmaceuticals, 15(11), 1418. https://doi.org/10.3390/ph15111418












