Central Composite Design Implemented Azilsartan Medoxomil Loaded Nanoemulsion to Improve Its Aqueous Solubility and Intestinal Permeability: In Vitro and Ex Vivo Evaluation
Abstract
1. Introduction
2. Results
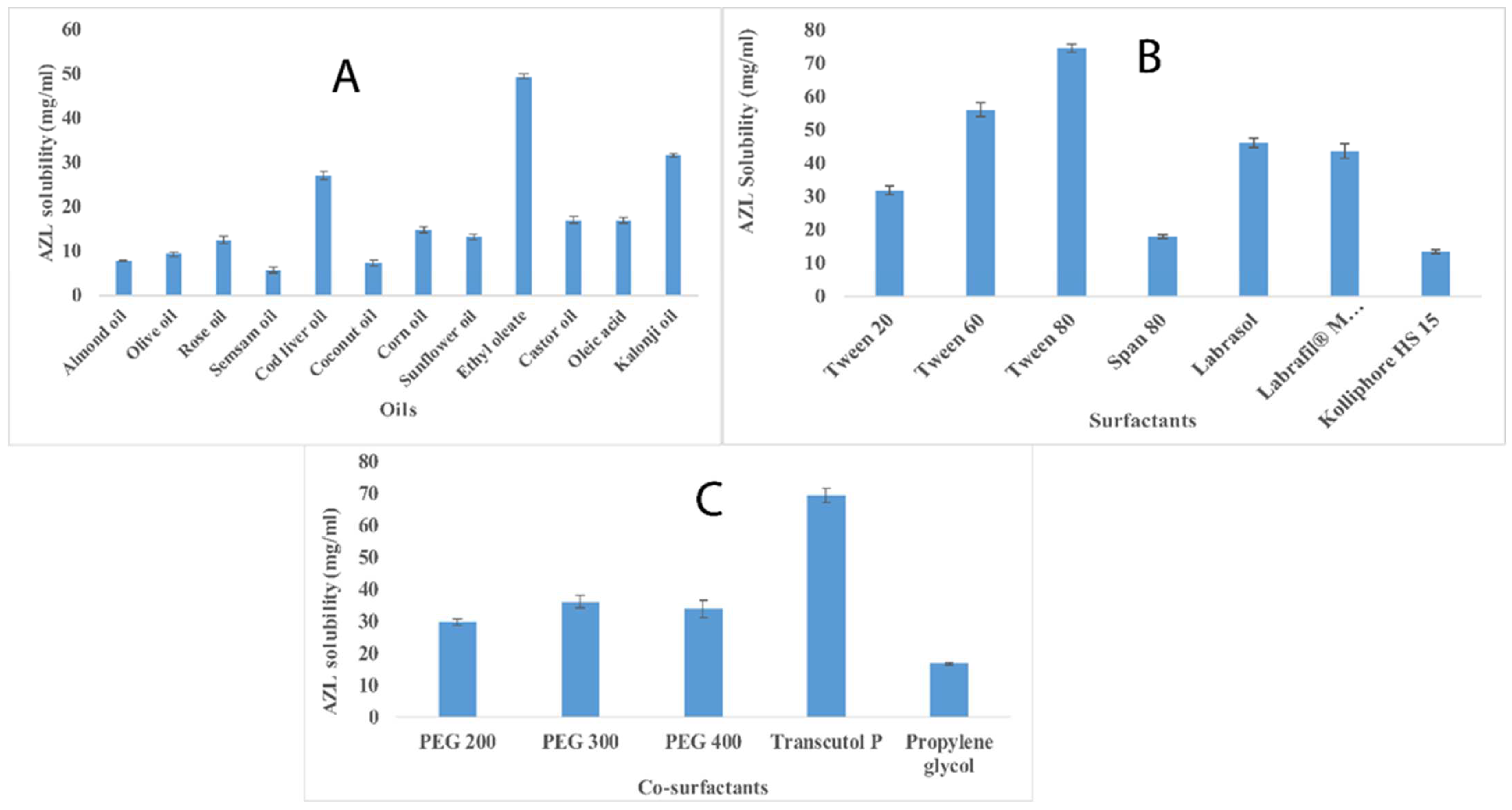
2.1. Selection of Formulation Components Using Solubility Determination
2.2. Miscibility Determination of Oil, Surfactant and Co-Surfactant
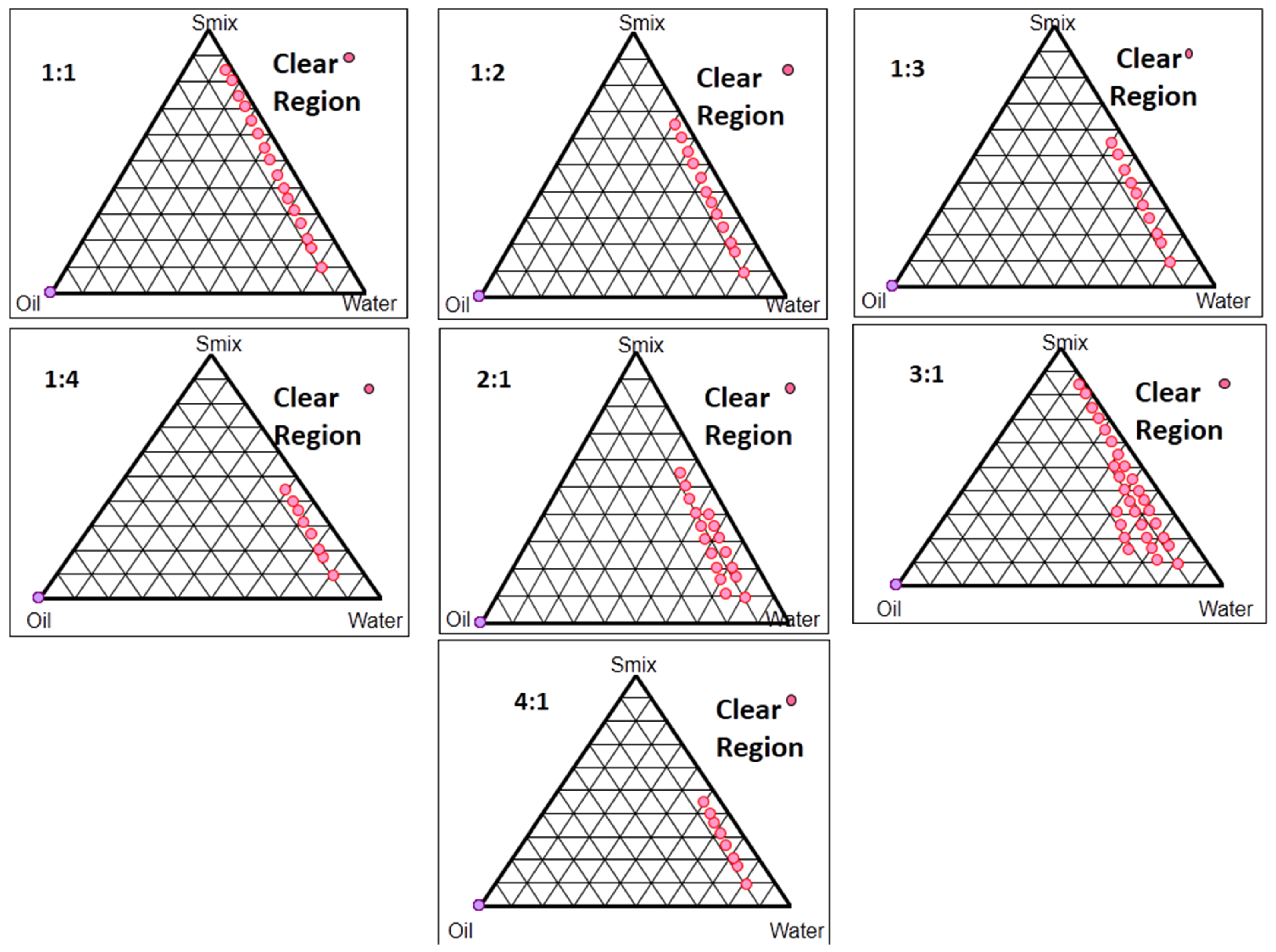
2.3. Pseudo-Ternary Phase Diagram Studies
2.4. Optimization of Formulation Components for Nanoemulsion
2.5. Characterization of Optimized Formulation
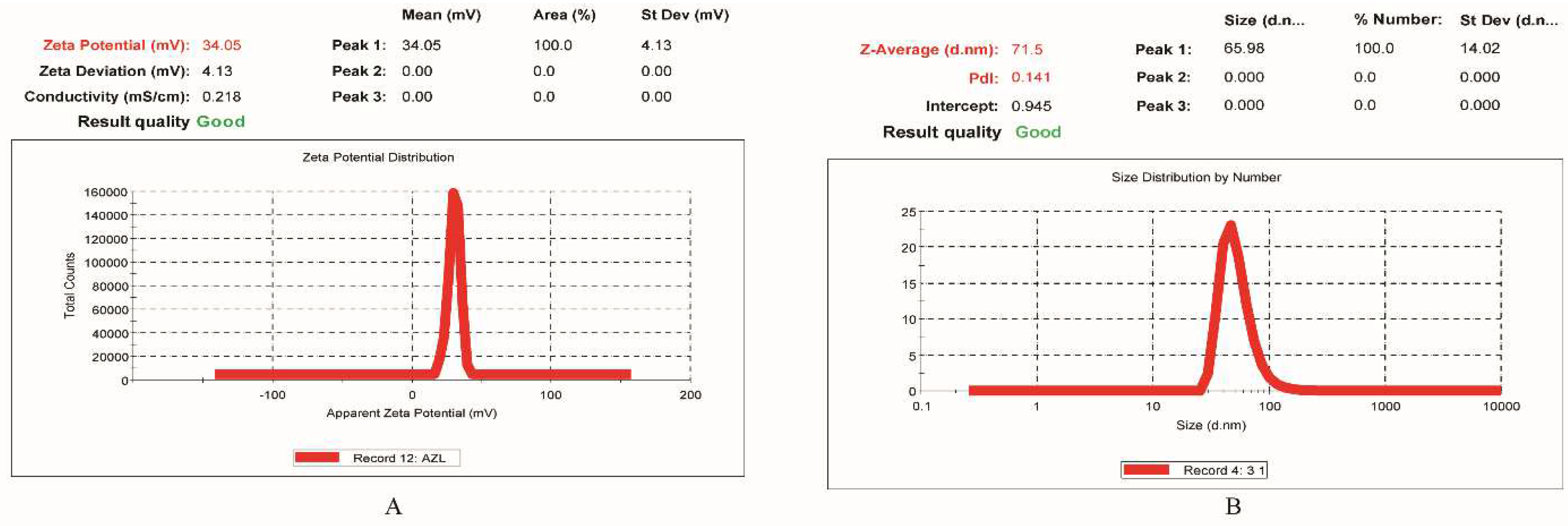
2.5.1. Droplet Size and PDI Determination
2.5.2. Surface Charge (Zeta Potential) Determination
2.5.3. pH, Viscosity, and Refractive Index Determination
2.5.4. Percentage Transmittance Determination
2.5.5. Drug Content Determination
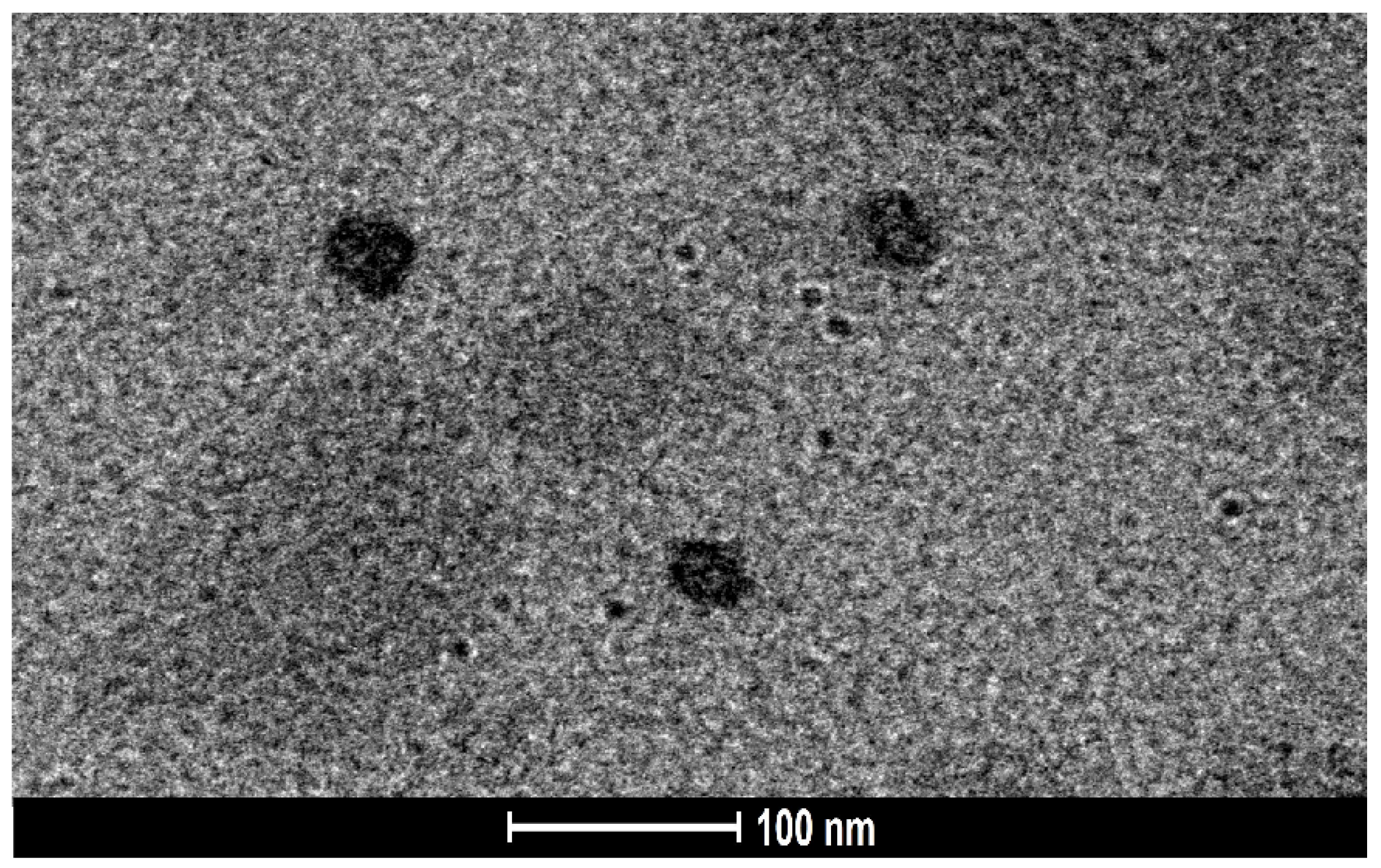
2.5.6. Surface Morphology Study Using Transmission Electron Microscopy (TEM)
2.5.7. Endorsement of Molecular Dispersion of Drug in Nanoemulsion Using Differential Scanning Calorimetry (DSC)
2.5.8. Drug–Excipient Interactions Assessment Employing Fourier Transform Infrared Spectroscopy (FTIR)
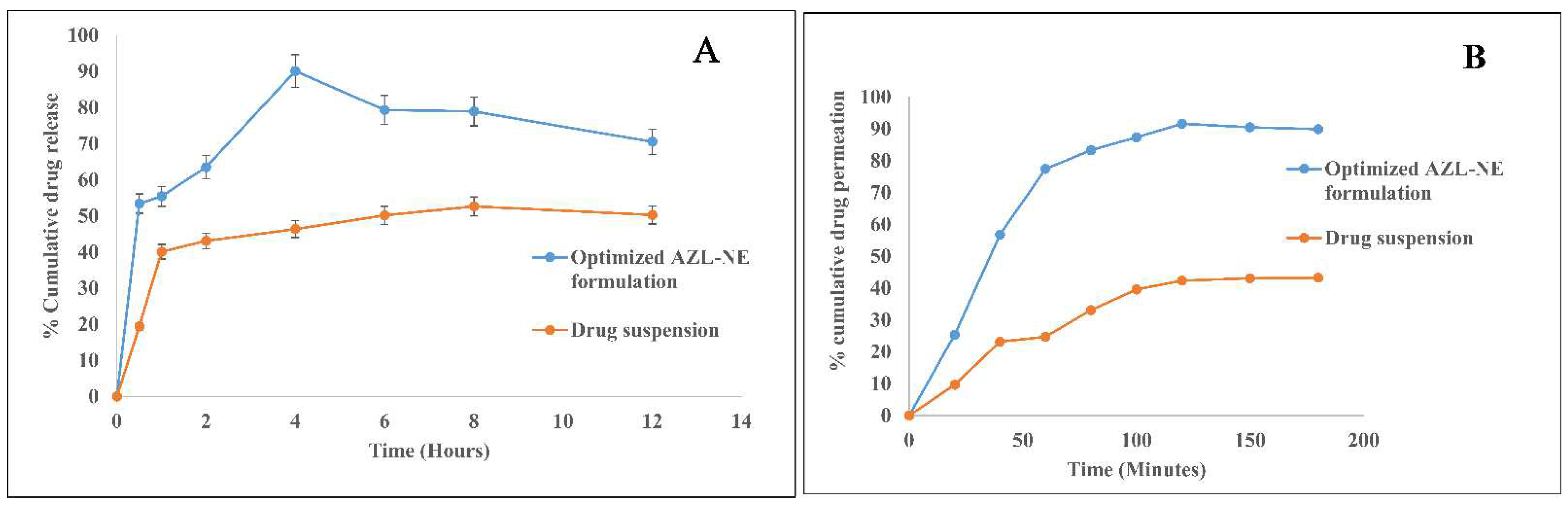
2.5.9. In Vitro Drug Release Studies Employing Dialysis Membrane
2.5.10. Ex Vivo Intestinal Permeation Studies
2.5.11. Estimation of the Depth of Permeation Using Confocal Laser Scanning Microscopy (CLSM)
2.5.12. Assessment of Stability using Thermodynamic Stability and Storage Stability
3. Materials and Methods
3.1. Materials
3.2. Methods
3.2.1. Selection of Formulation Components Using Solubility Determination
3.2.2. Miscibility Determination of Oil, Surfactant, and Co-Surfactant
3.2.3. Pseudo-Ternary Phase Diagram Studies
3.2.4. Preparation of Drug-Loaded Nanoemulsion
3.2.5. Optimization of Formulation Components for Nanoemulsion
3.2.6. Characterization of Optimized Formulation
Droplet Size and Polydispersity Index (PDI) Determination
Surface Charge (Zeta Potential) Determination
pH, Viscosity, and Refractive Index Determination
Percentage Transmittance Determination
Drug Content Determination
Surface Morphology Study Using Transmission Electron Microscopy (TEM)
Endorsement of Molecular Dispersion of Drug in Nanoemulsion Using Differential Scanning Calorimetry (DSC)
Drug–Excipient Interactions Assessment Employing Fourier Transform Infrared Spectroscopy (FTIR)
In Vitro Drug Release Studies Employing Dialysis Membrane
Ex Vivo Intestinal Permeation Studies
Estimation of the Depth of Permeation Using Confocal Laser Scanning Microscopy (CLSM)
Assessment of Stability Using Thermodynamic Stability and Storage Stability Studies
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jones, J.D.; Jackson, S.H.; Agboton, C.; Martin, T.S. Azilsartan Medoxomil (Edarbi). Pharm. Ther. 2011, 36, 634–640. [Google Scholar]
- Jassem, N.; Ayash, N. Formulation and In vitro Evaluation of azilsartan medoxomil nanosuspension. Int. J. Pharm. Pharm. Sci. 2017, 9, 110. [Google Scholar] [CrossRef][Green Version]
- Ma, J.; Yang, Y.; Sun, Y.; Sun, J. Optimization, Characterization and in Vitro/Vivo Evaluation of Azilsartan Nanocrystals. Asian J. Pharm. Sci. 2017, 12, 344–352. [Google Scholar] [CrossRef] [PubMed]
- Angeloni, E. Azilsartan Medoxomil in the Management of Hypertension: An Evidence-Based Review of Its Place in Therapy. Core Evid. 2016, 11, 1. [Google Scholar] [CrossRef]
- Chopra, D.K.; Kar, D.M.; Sahu, P.K. Lipid-Based Solid Dispersions of Azilsartan Medoxomil with Improved Oral Bioavailability: In Vitro and In Vivo Evaluation. Int. J. Curr. Res. Rev. 2020, 12, 134–139. [Google Scholar] [CrossRef]
- McClements, D.J. Advances in Edible Nanoemulsions: Digestion, Bioavailability, and Potential Toxicity. Prog. Lipid Res. 2021, 81, 101081. [Google Scholar] [CrossRef]
- Kumar, M.; Bishnoi, R.S.; Shukla, A.K.; Jain, C.P. Techniques for Formulation of Nanoemulsion Drug Delivery System: A Review. Prev. Nutr. Food Sci. 2019, 24, 225–234. [Google Scholar] [CrossRef]
- Amarachinta, P.R.; Sharma, G.; Samed, N.; Chettupalli, A.K.; Alle, M.; Kim, J.-C. Central Composite Design for the Development of Carvedilol-Loaded Transdermal Ethosomal Hydrogel for Extended and Enhanced Anti-Hypertensive Effect. J. Nanobiotechnol. 2021, 19, 100. [Google Scholar] [CrossRef]
- Ngan, C.L.; Basri, M.; Lye, F.F.; Fard Masoumi, H.R.; Tripathy, M.; Abedi Karjiban, R.; Abdul-Malek, E. Comparison of Box–Behnken and Central Composite Designs in Optimization of Fullerene Loaded Palm-Based Nano-Emulsions for Cosmeceutical Application. Ind. Crop. Prod. 2014, 59, 309–317. [Google Scholar] [CrossRef]
- Sarheed, O.; Dibi, M.; Ramesh, K.V.R.N.S. Studies on the Effect of Oil and Surfactant on the Formation of Alginate-Based O/W Lidocaine Nanocarriers Using Nanoemulsion Template. Pharmaceutics 2020, 12, 1223. [Google Scholar] [CrossRef]
- Xing, Q.; Song, J.; You, X.; Xu, D.; Wang, K.; Song, J.; Guo, Q.; Li, P.; Wu, C.; Hu, H. Microemulsions Containing Long Chain Oil Ethyl Oleate Improve the Oral Bioavailability of Piroxicam by Increasing Drug Solubility and Lymphatic Transportation Simultaneously. Int. J. Pharm. 2016, 511, 709–718. [Google Scholar] [CrossRef] [PubMed]
- Bahadur, S.; Pardhi, D.M.; Rautio, J.; Rosenholm, J.M.; Pathak, K. Intranasal Nanoemulsions for Direct Nose-to-Brain Delivery of Actives for CNS Disorders. Pharmaceutics 2020, 12, 1230. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.; Gull, A.; Alam, M.; Aqil, M.; Sultana, Y. Ultrasonically Tailored, Chemically Engineered and “QbD” Enabled Fabrication of Agomelatine Nanoemulsion; Optimization, Characterization, Ex-Vivo Permeation and Stability Study. Ultrason. Sonochem. 2018, 41, 213–226. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, A.R.; Sanchez-Lopez, E.; dos Santos, T.; Garcia, M.L.; Silva, A.M.; Souto, E.B. Development and Characterization of Nanoemulsions for Ophthalmic Applications: Role of Cationic Surfactants. Materials 2021, 14, 7541. [Google Scholar] [CrossRef] [PubMed]
- Javadzadeh, Y.; Adibkia, K.; Hamishehkar, H. Transcutol® (Diethylene Glycol Monoethyl Ether): A Potential Penetration Enhancer. In Percutaneous Penetration Enhancers Chemical Methods in Penetration Enhancement; Springer: Berlin/Heidelberg, Germany, 2015; pp. 195–205. ISBN 978-3-662-47038-1. [Google Scholar]
- Chuacharoen, T.; Prasongsuk, S.; Sabliov, C.M. Effect of Surfactant Concentrations on Physicochemical Properties and Functionality of Curcumin Nanoemulsions Under Conditions Relevant to Commercial Utilization. Molecules 2019, 24, 2744. [Google Scholar] [CrossRef] [PubMed]
- Danaei, M.; Dehghankhold, M.; Ataei, S.; Hasanzadeh Davarani, F.; Javanmard, R.; Dokhani, A.; Khorasani, S.; Mozafari, M.R. Impact of Particle Size and Polydispersity Index on the Clinical Applications of Lipidic Nanocarrier Systems. Pharmaceutics 2018, 10, 57. [Google Scholar] [CrossRef] [PubMed]
- Alhalmi, A.; Amin, S.; Beg, S.; Al-Salahi, R.; Mir, S.R.; Kohli, K. Formulation and Optimization of Naringin Loaded Nanostructured Lipid Carriers Using Box-Behnken Based Design: In Vitro and Ex Vivo Evaluation. J. Drug Deliv. Sci. Technol. 2022, 74, 103590. [Google Scholar] [CrossRef]
- Gupta, V.; Trivedi, P. In Vitro and in Vivo Characterization of Pharmaceutical Topical Nanocarriers Containing Anticancer Drugs for Skin Cancer Treatment. In Lipid Nanocarriers for Drug Targeting; Elsevier: Amsterdam, The Netherlands, 2018; pp. 563–627. ISBN 978-0-12-813687-4. [Google Scholar]
- Mundada, V.P.; Patel, M.H.; Mundada, P.K.; Sawant, K.K. Enhanced Bioavailability and Antihypertensive Activity of Nisoldipine Loaded Nanoemulsion: Optimization, Cytotoxicity and Uptake across Caco-2 Cell Line, Pharmacokinetic and Pharmacodynamic Studies. Drug Dev. Ind. Pharm. 2020, 46, 376–387. [Google Scholar] [CrossRef]
- Ashhar, M.U.; Kumar, S.; Ali, J.; Baboota, S. CCRD Based Development of Bromocriptine and Glutathione Nanoemulsion Tailored Ultrasonically for the Combined Anti-Parkinson Effect. Chem. Phys. Lipids 2021, 235, 105035. [Google Scholar] [CrossRef]
- Echeverri, J.D.; Alhajj, M.J.; Montero, N.; Yarce, C.J.; Barrera-Ocampo, A.; Salamanca, C.H. Study of In Vitro and In Vivo Carbamazepine Release from Coarse and Nanometric Pharmaceutical Emulsions Obtained via Ultra-High-Pressure Homogenization. Pharmaceuticals 2020, 13, E53. [Google Scholar] [CrossRef]
- Abdulla, N.A.; Balata, G.F.; El-ghamry, H.A.; Gomaa, E. Intranasal Delivery of Clozapine Using Nanoemulsion-Based in-Situ Gels: An Approach for Bioavailability Enhancement. Saudi Pharm. J. SPJ 2021, 29, 1466–1485. [Google Scholar] [CrossRef] [PubMed]
- Alhalmi, A.; Amin, S.; Khan, Z.; Beg, S.; Al Kamaly, O.; Saleh, A.; Kohli, K. Nanostructured Lipid Carrier-Based Codelivery of Raloxifene and Naringin: Formulation, Optimization, In Vitro, Ex Vivo, In Vivo Assessment, and Acute Toxicity Studies. Pharmaceutics 2022, 14, 1771. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Neupane, Y.R.; Mangla, B.; Kohli, K. Nanostructured Lipid Carriers for Oral Bioavailability Enhancement of Exemestane: Formulation Design, In Vitro, Ex Vivo, and In Vivo Studies. J. Pharm. Sci. 2019, 108, 3382–3395. [Google Scholar] [CrossRef] [PubMed]
- Qadir, A.; Aqil, M.; Ali, A.; Warsi, M.H.; Mujeeb, M.; Ahmad, F.J.; Ahmad, S.; Beg, S. Nanostructured Lipidic Carriers for Dual Drug Delivery in the Management of Psoriasis: Systematic Optimization, Dermatokinetic and Preclinical Evaluation. J. Drug Deliv. Sci. Technol. 2020, 57, 101775. [Google Scholar] [CrossRef]
- Hussain, A.; Singh, V.K.; Singh, O.P.; Shafaat, K.; Kumar, S.; Ahmad, F.J. Formulation and Optimization of Nanoemulsion Using Antifungal Lipid and Surfactant for Accentuated Topical Delivery of Amphotericin B. Drug Deliv. 2016, 23, 3101–3110. [Google Scholar] [CrossRef]
- Pandey, Y.R.; Kumar, S.; Gupta, B.K.; Ali, J.; Baboota, S. Intranasal Delivery of Paroxetine Nanoemulsion via the Olfactory Region for the Management of Depression: Formulation, Behavioural and Biochemical Estimation. Nanotechnology 2016, 27, 025102. [Google Scholar] [CrossRef]
- Singh, S.; Virmani, T.; Kohli, K. Nanoemulsion System for Improvement of Raspberry Ketone Oral Bioavailability. Indo Glob. J. Pharm. Sci. 2020, 10, 33–42. [Google Scholar] [CrossRef]
- Tarik Alhamdany, A.; Saeed, A.M.H.; Alaayedi, M. Nanoemulsion and Solid Nanoemulsion for Improving Oral Delivery of a Breast Cancer Drug: Formulation, Evaluation, and a Comparison Study. Saudi Pharm. J. 2021, 29, 1278–1288. [Google Scholar] [CrossRef]
- Ma, H.L.; Varanda, L.C.; Perussi, J.R.; Carrilho, E. Hypericin-Loaded Oil-in-Water Nanoemulsion Synthesized by Ultrasonication Process Enhances Photodynamic Therapy Efficiency. J. Photochem. Photobiol. B 2021, 223, 112303. [Google Scholar] [CrossRef]
- Singh, S.K.; Dadhania, P.; Vuddanda, P.R.; Jain, A.; Velaga, S.; Singh, S. Intranasal Delivery of Asenapine Loaded Nanostructured Lipid Carriers: Formulation, Characterization, Pharmacokinetic and Behavioural Assessment. RSC Adv. 2015, 6, 2032–2045. [Google Scholar] [CrossRef]
- Hassan, H.; Adam, S.K.; Alias, E.; Meor Mohd Affandi, M.M.R.; Shamsuddin, A.F.; Basir, R. Central Composite Design for Formulation and Optimization of Solid Lipid Nanoparticles to Enhance Oral Bioavailability of Acyclovir. Molecules 2021, 26, 5432. [Google Scholar] [CrossRef] [PubMed]
- Gul, U.; Khan, M.I.; Madni, A.; Sohail, M.F.; Rehman, M.; Rasul, A.; Peltonen, L. Olive Oil and Clove Oil-Based Nanoemulsion for Topical Delivery of Terbinafine Hydrochloride: In Vitro and Ex Vivo Evaluation. Drug Deliv. 2022, 29, 600–612. [Google Scholar] [CrossRef] [PubMed]
- Gokhale, J.P.; Mahajan, H.S.; Surana, S.J. Quercetin Loaded Nanoemulsion-Based Gel for Rheumatoid Arthritis: In Vivo and in Vitro Studies. Biomed. Pharmacother. 2019, 112, 108622. [Google Scholar] [CrossRef] [PubMed]
- Yeo, E.; Yew Chieng, C.J.; Choudhury, H.; Pandey, M.; Gorain, B. Tocotrienols-Rich Naringenin Nanoemulgel for the Management of Diabetic Wound: Fabrication, Characterization and Comparative in Vitro Evaluations. Curr. Res. Pharmacol. Drug Discov. 2021, 2, 100019. [Google Scholar] [CrossRef] [PubMed]
- Zafar, A.; Imam, S.S.; Alruwaili, N.K.; Alsaidan, O.A.; Elkomy, M.H.; Ghoneim, M.M.; Alshehri, S.; Ali, A.M.A.; Alharbi, K.S.; Yasir, M.; et al. Development of Piperine-Loaded Solid Self-Nanoemulsifying Drug Delivery System: Optimization, In-Vitro, Ex-Vivo, and In-Vivo Evaluation. Nanomaterials 2021, 11, 2920. [Google Scholar] [CrossRef] [PubMed]
- Laxmi, M.; Bhardwaj, A.; Mehta, S.; Mehta, A. Development and Characterization of Nanoemulsion as Carrier for the Enhancement of Bioavailability of Artemether. Artif. Cells Nanomed. Biotechnol. 2015, 43, 334–344. [Google Scholar] [CrossRef] [PubMed]
- Kassem, A.A.; Salama, A.; Mohsen, A.M. Formulation and Optimization of Cationic Nanoemulsions for Enhanced Ocular Delivery of Dorzolamide Hydrochloride Using Box-Behnken Design: In Vitro and in Vivo Assessments. J. Drug Deliv. Sci. Technol. 2022, 68, 103047. [Google Scholar] [CrossRef]
- Ansari, M.J.; Alnakhli, M.; Al-Otaibi, T.; Meanazel, O.A.; Anwer, M.K.; Ahmed, M.M.; Alshahrani, S.M.; Alshetaili, A.; Aldawsari, M.F.; Alalaiwe, A.S.; et al. Formulation and Evaluation of Self-Nanoemulsifying Drug Delivery System of Brigatinib: Improvement of Solubility, in Vitro Release, Ex-Vivo Permeation and Anticancer Activity. J. Drug Deliv. Sci. Technol. 2021, 61, 102204. [Google Scholar] [CrossRef]
- Singh, A.; Neupane, Y.R.; Shafi, S.; Mangla, B.; Kohli, K. PEGylated Liposomes as an Emerging Therapeutic Platform for Oral Nanomedicine in Cancer Therapy: In Vitro and in Vivo Assessment. J. Mol. Liq. 2020, 303, 112649. [Google Scholar] [CrossRef]
- Qamar, Z.; Ashhar, M.U.; Annu; Qizilibash, F.F.; Sahoo, P.K.; Ali, A.; Ali, J.; Baboota, S. Lipid Nanocarrier of Selegiline Augmented Anti-Parkinson’s Effect via P-Gp Modulation Using Quercetin. Int. J. Pharm. 2021, 609, 121131. [Google Scholar] [CrossRef]
- Altamimi, M.A.; Hussain, A.; Alshehri, S.; Imam, S.S.; Alnemer, U.A. Development and Evaluations of Transdermally Delivered Luteolin Loaded Cationic Nanoemulsion: In Vitro and Ex Vivo Evaluations. Pharmaceutics 2021, 13, 1218. [Google Scholar] [CrossRef] [PubMed]




| Oils | Codes | Surfactant | Co-Surfactant | Miscibility Outcomes |
|---|---|---|---|---|
| Nigella sativa oil | 1 | Tween 80 | Transcutol P | Clear |
| 2 | Tween 80 | PEG 300 | Phase Separation | |
| 3 | Tween 80 | PEG 400 | Phase Separation | |
| Cod liver oil | 4 | Tween 80 | Transcutol P | Phase Separation |
| 5 | Tween 80 | PEG 300 | Phase Separation | |
| 6 | Tween 80 | PEG 400 | Phase Separation | |
| Ethyl oleate | 7 | Tween 80 | Transcutol P | Clear |
| 8 | Tween 80 | PEG 300 | Phase Separation | |
| 9 | Tween 80 | PEG 400 | Phase Separation | |
| 10 | Labrasol | Transcutol P | Clear | |
| 11 | Labrasol | PEG 300 | Clear | |
| 12 | Labrasol | PEG 400 | Phase Separation | |
| Nigella sativa oil | 13 | Labrasol | Transcutol P | Phase Separation |
| 14 | Labrasol | PEG 300 | Phase Separation | |
| 15 | Labrasol | PEG 400 | Clear | |
| Cod liver oil | 16 | Labrasol | Transcutol P | Phase Separation |
| 17 | Labrasol | PEG 300 | Phase Separation |
| Formulations | Type of Point | Independent Variables | Dependent Variables | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Experimental Values | Predicted Values | |||||||||
| Run | Point Type | A: Oil (%) | B: Smix (%) | C: Sonication Time (S) | Droplet Size (nm) Y1 | %Transmittance (%) Y2 | %Drug Release (%) Y3 | Droplet Size (nm) Y1 | %Transmittance (%) Y2 | %Drug Release (%) Y3 |
| 1 | Factorial | 2.5 | 8.75 | 30 | 311.9 | 74.67 | 23.03 | 303.96 | 74.55 | 22.87 |
| 2 | Axial | 2.92612 | 13.125 | 60 | 221.92 | 83.46 | 23.08 | 227.27 | 83.31 | 22.64 |
| 3 | Factorial | 2.5 | 17.5 | 90 | 144.21 | 90.43 | 33.31 | 134.24 | 91.15 | 33.29 |
| 4 | Central | 1.875 | 13.125 | 60 | 94.83 | 91.65 | 57.23 | 96.57 | 91.81 | 58.48 |
| 5 | Factorial | 1.25 | 8.75 | 90 | 107.04 | 88.56 | 68.87 | 109.00 | 88.41 | 68.28 |
| 6 | Axial | 1.875 | 13.125 | 110.454 | 85.21 | 91.76 | 62.87 | 78.79 | 91.65 | 62.94 |
| 7 | Factorial | 1.25 | 17.5 | 90 | 80.69 | 93.89 | 91.08 | 84.91 | 94.08 | 92.33 |
| 8 | Axial | 0.823879 | 13.125 | 60 | 85.04 | 95.3 | 94.92 | 84.95 | 95.35 | 93.82 |
| 9 | Factorial | 1.25 | 8.75 | 30 | 177.78 | 86.57 | 47.38 | 184.03 | 85.92 | 48.49 |
| 10 | Axial | 1.875 | 5.76716 | 60 | 266.74 | 72.88 | 22.06 | 257.91 | 73.77 | 22.30 |
| 11 | Axial | 1.875 | 13.125 | 9.54622 | 144.38 | 87.91 | 51.52 | 156.06 | 87.92 | 49.90 |
| 12 | Factorial | 1.25 | 17.5 | 30 | 91.02 | 92.76 | 77.12 | 75.73 | 93.34 | 78.09 |
| 13 | Axial | 1.875 | 20.4828 | 60 | 95.01 | 91.85 | 56.67 | 109.10 | 90.86 | 54.89 |
| 14 | Factorial | 2.5 | 17.5 | 30 | 191.32 | 78.74 | 24.02 | 202.89 | 78.23 | 24.14 |
| 15 | Central | 1.875 | 13.125 | 60 | 100.95 | 92.12 | 60.73 | 96.57 | 91.81 | 58.48 |
| 16 | Factorial | 2.5 | 17.5 | 30 | 156.76 | 88.98 | 35.89 | 151.08 | 89.20 | 37.57 |
| 17 | Central | 1.875 | 13.125 | 60 | 94.83 | 91.65 | 57.23 | 96.57 | 91.81 | 58.48 |
| Quadratic Model | R2 Value (Coefficient of Correlation) | Adjusted R2 | Predicted R2 | Adeq. Precision | Standard Deviation | Mean | Coefficient of Variation % (C.V.%) |
|---|---|---|---|---|---|---|---|
| Y1 | 0.9853 | 0.9664 | 0.8844 | 23.4079 | 12.71 | 144.10 | 8.82 |
| Y2 | 0.9949 | 0.9883 | 0.9615 | 39.2628 | 0.7167 | 87.83 | 0.8167 |
| Y3 | 0.9974 | 0.9940 | 0.9849 | 52.1242 | 1.79 | 52.18 | 3.43 |
| Batch | Independent Variables | Dependent Variables | ||||
|---|---|---|---|---|---|---|
| A (%) | B (%) | C (Sec) | YI (nm) | Y2 (%) | Y3 (%) | |
| Predicted | 1.25 | 15.73 | 90 | 69.98 | 95.10 | 92.00 |
| Observed | 1.25 | 15.73 | 90 | 71.5 | 93.46 ± 1.13% | 90.14 ± 0.94% |
| Time (Days) | 25 ± 1 °C (Room Conditions) | 4 ± 1 °C (Refrigeration Conditions) | ||||||
|---|---|---|---|---|---|---|---|---|
| Phase Separation | Droplet Size (nm) | %Transmittance (%) | %CDR (%) | Phase Separation | Droplet Size (nm) | % Transmittance (%) | %CDR (%) | |
| 0 | No | 71.5 | 93.46 ± 1.13 | 90.14 ± 0.94 | No | 71.5 | 93.46 ± 1.13 | 90.14 ± 0.94 |
| 10 | No | 72.68 | 92.87 ± 0.29 | 89.48 ± 0.39 | No | 72.43 | 92.26 ± 0.65 | 89.53 ± 1.39 |
| 20 | No | 72.82 | 92.23 ± 0.42 | 89.41 ± 0.43 | No | 73.87 | 91.43 ± 0.87 | 88.98 ± 1.08 |
| 30 | No | 72.91 | 91.62 ± 0.34 | 88.74 ± 0.77 | No | 73.94 | 91.00 ± 0.71 | 88.78 ± 1.01 |
| Independent Variables | Levels | ||||
|---|---|---|---|---|---|
| Low (−1) | Medium (0) | High (+1) | Axial (−1.68) | Axial (+1.68) | |
| A = Oil (%) | 0.62 | 1.285 | 1.67 | 0.193879 | 2.29612 |
| B = Smix (%) | 4.36 | 8.74 | 13.12 | 1.37375 | 16.1063 |
| C = Sonication time (s) | 10 | 20 | 30 | 3.18207 | 36.8179 |
| Independent variables | Constraints | Importance | |||
| A = Oil (%) | In range | --------------- | |||
| B = Smix (%) | In range | ---------------- | |||
| C = Sonication time (Sec) | In range | ------------- | |||
| Dependent variables | Constraints | Importance | |||
| Y1 = droplet size | Minimize | +++++ | |||
| Y2 = % transmittance | Maximize | +++ | |||
| Y3 = % cumulative drug release | Maximize | +++ | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumar, G.; Virmani, T.; Pathak, K.; Kamaly, O.A.; Saleh, A. Central Composite Design Implemented Azilsartan Medoxomil Loaded Nanoemulsion to Improve Its Aqueous Solubility and Intestinal Permeability: In Vitro and Ex Vivo Evaluation. Pharmaceuticals 2022, 15, 1343. https://doi.org/10.3390/ph15111343
Kumar G, Virmani T, Pathak K, Kamaly OA, Saleh A. Central Composite Design Implemented Azilsartan Medoxomil Loaded Nanoemulsion to Improve Its Aqueous Solubility and Intestinal Permeability: In Vitro and Ex Vivo Evaluation. Pharmaceuticals. 2022; 15(11):1343. https://doi.org/10.3390/ph15111343
Chicago/Turabian StyleKumar, Girish, Tarun Virmani, Kamla Pathak, Omkulthom Al Kamaly, and Asmaa Saleh. 2022. "Central Composite Design Implemented Azilsartan Medoxomil Loaded Nanoemulsion to Improve Its Aqueous Solubility and Intestinal Permeability: In Vitro and Ex Vivo Evaluation" Pharmaceuticals 15, no. 11: 1343. https://doi.org/10.3390/ph15111343
APA StyleKumar, G., Virmani, T., Pathak, K., Kamaly, O. A., & Saleh, A. (2022). Central Composite Design Implemented Azilsartan Medoxomil Loaded Nanoemulsion to Improve Its Aqueous Solubility and Intestinal Permeability: In Vitro and Ex Vivo Evaluation. Pharmaceuticals, 15(11), 1343. https://doi.org/10.3390/ph15111343









