Overcoming Challenges in Pediatric Formulation with a Patient-Centric Design Approach: A Proof-of-Concept Study on the Design of an Oral Solution of a Bitter Drug
Abstract
1. Introduction
2. Results
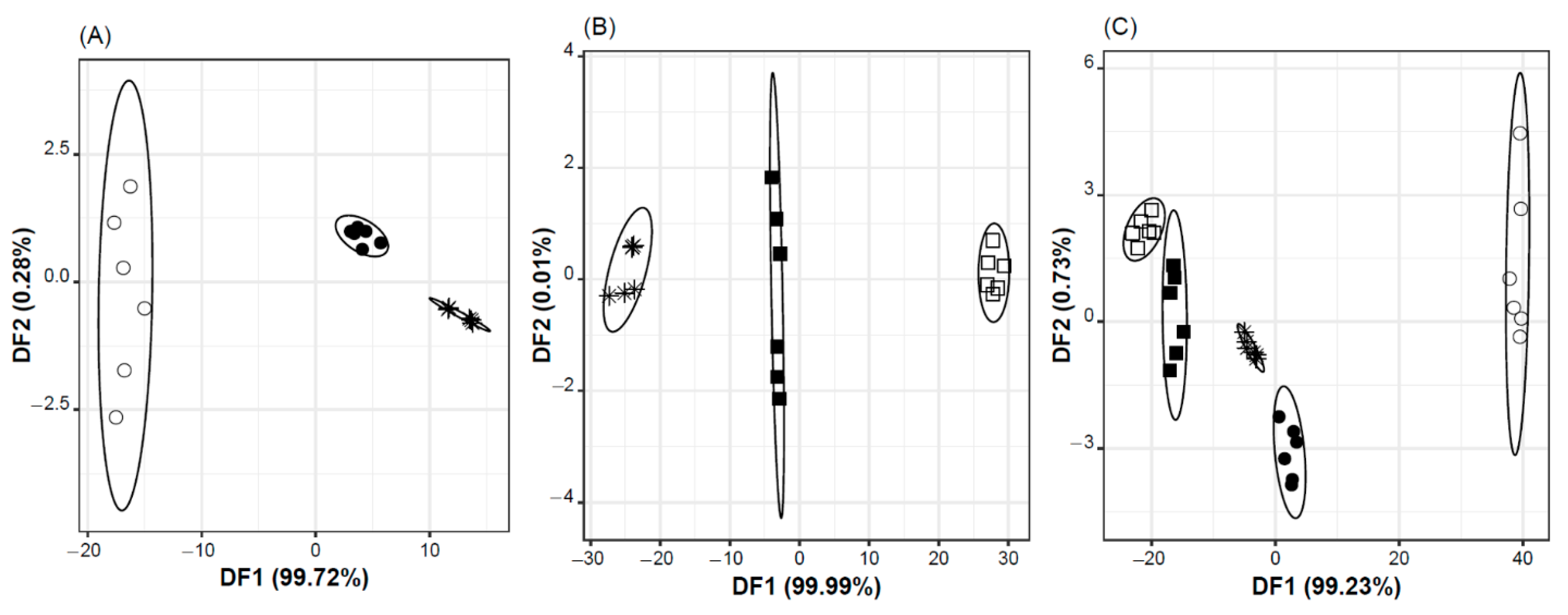
2.1. Taste Masking Effectiveness vs. Standard Vehicle
- i.
- syrup, with and without Ra-HCl, and water with Ra-HCl (Figure 1A), using a classification discriminant model with two DFs, explaining 100% of the data variability, based on the potential signals (in Volts) of two sensors: DF1 = −1066 × S2:11 + 963 × S2:12; DF2 = −56 × S2:11 − 68 × S2:12;
- ii.
- oral formulation, with or without Ra-HCl, and water with Ra-HCl (Figure 1B), using a classification discriminant model with two DFs, explaining 100% of the data variability, based on the potential signals (in Volts) of two sensors: DF1 = 3235 × S2:9 − 904 × S2:13; DF2 = −170 × S2:9 + 235 × S2:13; and,
- iii.
- the five oral solutions, using a classification discriminant model which two first DFs explained ~100% of the data variability, and were based on the potential signals (in Volts) of two sensors: DF1 = −1745 × S1:11 − 684 × S2:5 + 2511 × S2:11; DF2 = −292 × S1:11 + 226 × S2:5 + 333 × S2:11.
2.2. Chemical and Microbiological Stability
3. Discussion
Limitations
4. Materials and Methods
4.1. Chemicals and Materials
4.2. Composition of the Oral Pediatric Solution
4.3. Evaluation of Taste Masking Effectiveness vs. Standard Vehicle (Simple Syrup) Using E-Tongue
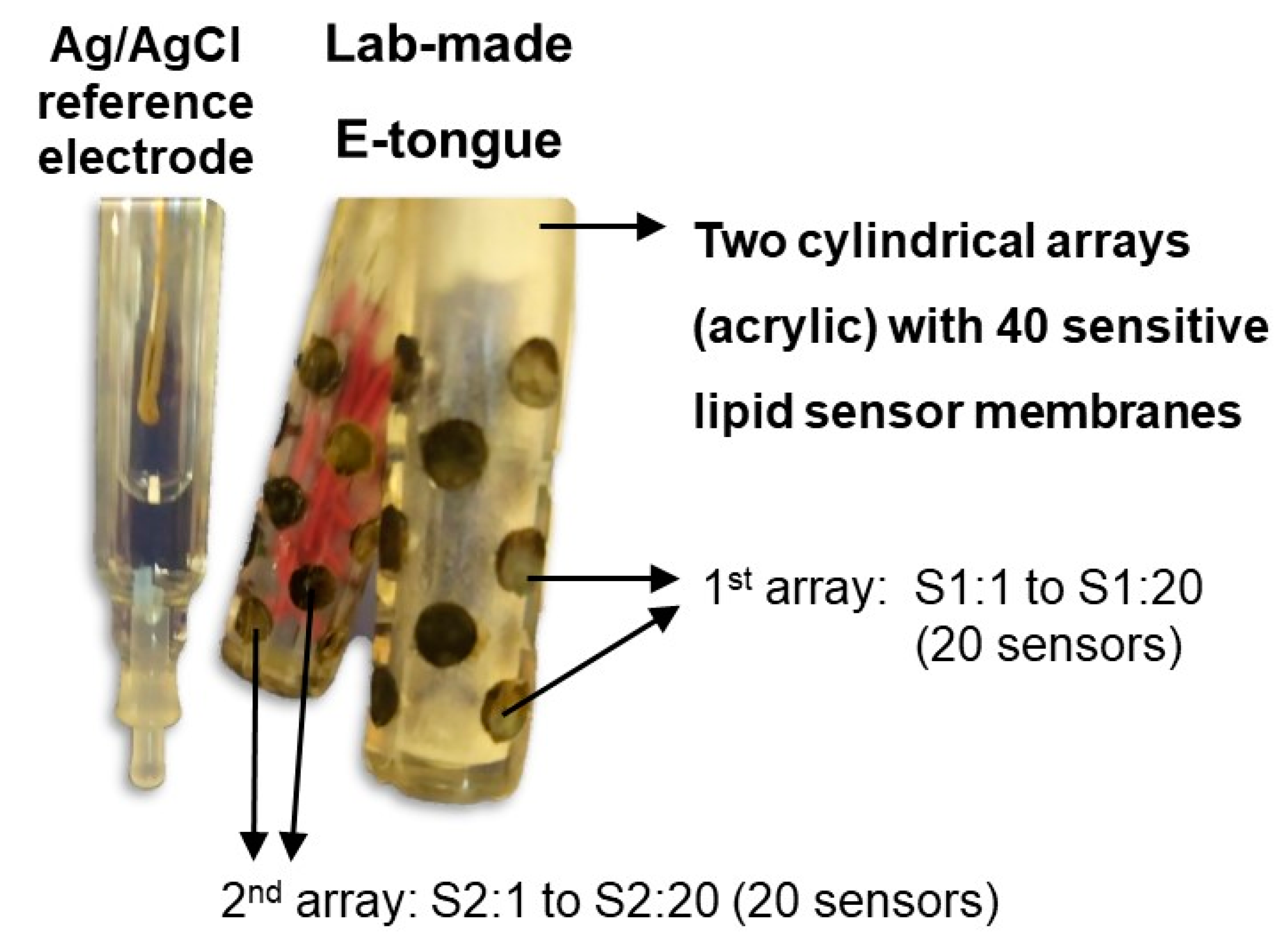
4.3.1. E-Tongue Apparatus and Potentiometric Analysis of Ra-HCl Solutions
4.3.2. Statistical Analysis
4.4. Study of the Stability of the Developed Oral Solution
4.4.1. Sample Preparation
4.4.2. Microbiological Stability
4.4.3. Chemical Stability Evaluation
pH Determination
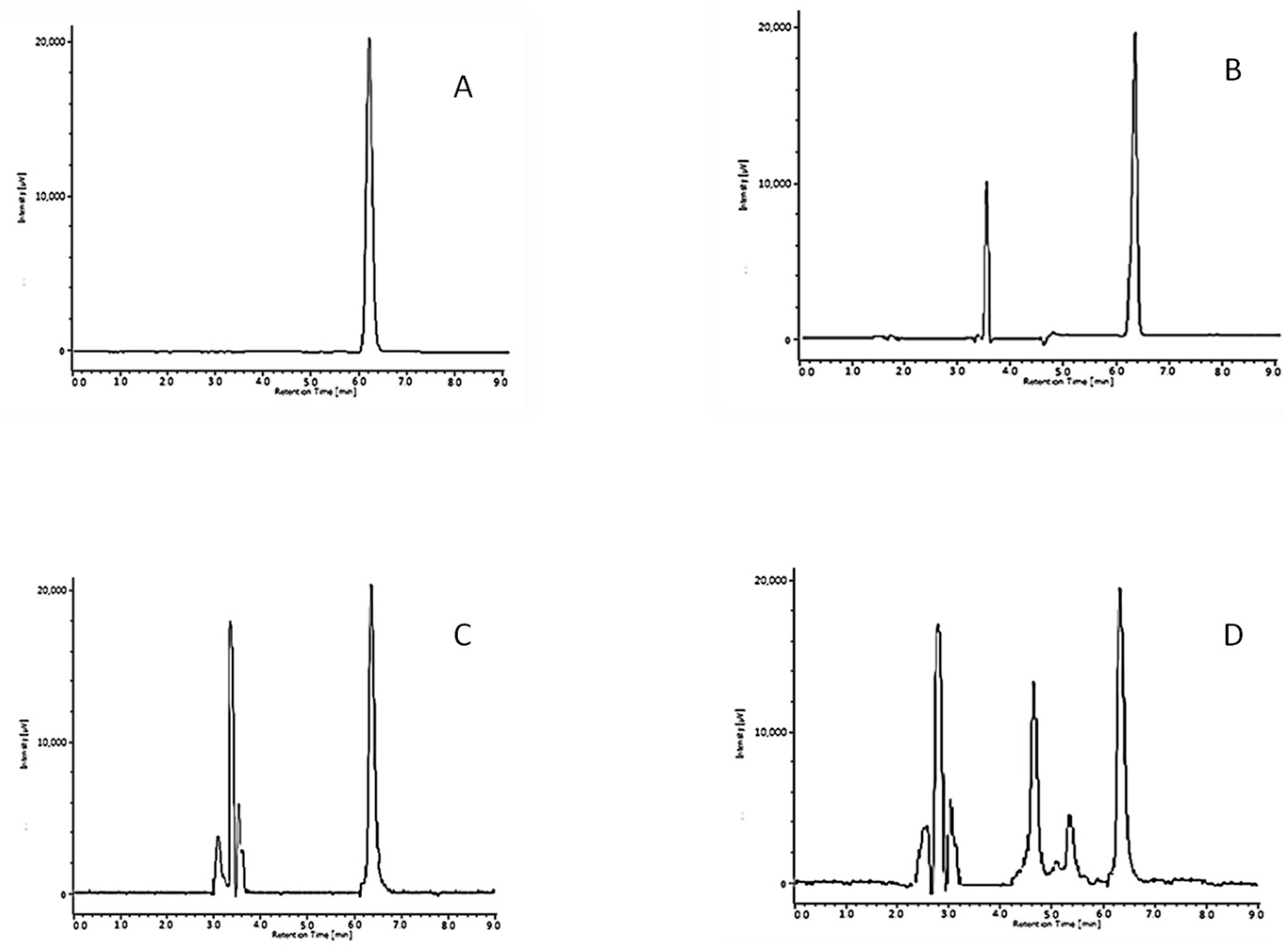
Ranitidine Assay
Chromatographic Conditions
Method Validation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cutaia, K.; Chablani, L.; Zhao, F. Basics of Compounding: Vehicles for Compounded Oral Liquid Medications: A Review. Int. J. Pharm. Compd. 2018, 22, 480–489. [Google Scholar] [PubMed]
- Nahata, M.C.; Pai, V.; Hippie, T. Pediatric Drug Formulations, 5th ed.; Harvey Whitney Books: Cincinnati, OH, USA, 2004. [Google Scholar]
- World Health Organization. Development of Paediatric Medicines: Points to Consider in Formulation; Annex 5, WHO Technical Report Series 970; World Health Organization: Geneva, Switzerland, 2012. [Google Scholar]
- Batchelor, H.K.; Marriott, J.F. Formulations for children: Problems and solutions. Br. J. Clin. Pharmacol. 2015, 79, 405–418. [Google Scholar] [CrossRef] [PubMed]
- Walsh, J.; Cram, A.; Woertz, K.; Breitkreutz, J.; Winzenburg, G.; Turner, R.; Tuleu, C.; European Formulation Initiative. Playing hide and seek with poorly tasting paediatric medicines: Do not forget the excipients. Adv. Drug Deliv. Rev. 2014, 73, 14–33. [Google Scholar] [CrossRef] [PubMed]
- Thakker, P.; Shah, J.; Mehta, T.; Agarwal, G. Taste Masking of Pharmaceutical Formulations: Review on Technologies, Recent Trends and Patents. Int. J. Life Sci. Pharma Res. 2020, 10, P88–P96. [Google Scholar] [CrossRef]
- Tang, L.; Zhang, Z.; Su, R.; He, S.; Yao, J. Advances in taste-masking technology of oral pediatric medicine. J. China Pharm. Univ. 2017, 48, 135–141. [Google Scholar]
- European Medicines Agency. Reflection Paper: Formulations of Choice for the Paediatric Population. EMEA/CHMP/PEG/194810/2005. Available online: www.ema.europa.eu/en/documents/scientific-guideline/reflection-paper-formulations-choice-paediatric-population (accessed on 28 July 2006).
- Shehab, N.; Lewis, C.L.; Streetman, D.D.; Donn, S.M. Exposure to the pharmaceutical excipients benzyl alcohol and propylene glycol among critically ill neonates. Pediatr. Crit. Care Med. 2009, 10, 256–259. [Google Scholar] [CrossRef]
- Menditto, E.; Orlando, V.; De Rosa, G.; Minghetti, P.; Musazzi, U.M.; Cahir, C.; Kurczewska-Michalak, M.; Kardas, P.; Costa, E.; Lobo, J.M.S.; et al. Patient Centric Pharmaceutical Drug Product Design—The Impact on Medication Adherence. Pharmaceutics 2020, 12, 44. [Google Scholar] [CrossRef]
- European Medicines Agency. Reflection Paper on the Pharmaceutical Development Medicines for Use in the Older Population, in Comittee for Medicinal Products for Human Use; EMA/CHMP/QWP/292439/2017; European Medicines Agency: Amsterdam, The Netherlands, 2020.
- European Medicines Agency. Concept Paper on the Need for a Reflection Paper on Quality Aspects of Medicines for Older People; EMA/165974/2013; European Medicines Agency: London, UK, 2013.
- European Medicines Agency. Guideline on Pharmaceutical Development of Medicines for Pediatric Use Guideline on Pharmaceutical Development of Medicines for Paediatric Use; EMA/CHMP/QWP/805880/2012 Rev. 2; European Medicines Agency: London, UK, 2013.
- FDA. Patient-Focused Drug Development: Collecting Comprehensive and Representative Input; Center for Drug Evaluation and Research (CDER), in Center for Biologics Evaluation and Research (CBER); FDA: Silver Spring, MD, USA, 2020. [Google Scholar]
- Park, H.J.; Kim, H.J.; Park, H.-K.; Chung, J.-H. Protective effect of histamine H2 receptor antagonist ranitidine against rotenone-induced apoptosis. Neurotoxicology 2009, 30, 1114–1119. [Google Scholar] [CrossRef]
- Atienza Fernández, M.; Álvarez del Vayo, C. Formulación en Farmacia Pediátrica; Antonio Madrid Vicente Ediciones: Madrid, Spain, 2011. [Google Scholar]
- Petit-Jean, E.; Perello, L.; Hernandez, C.; Dory, A.-C.G.; Gourieux, B.; Ubeaud-Sequier, G. Stability of an oral ranitidine suspension (15 mg/mL). Eur. J. Hosp. Pharm. 2012, 20, 46–49. [Google Scholar] [CrossRef]
- Wang, C.; Liu, Y.; Zhao, X.; Liu, B. Current Advances and Future Aspects of Sweetener Synergy: Properties, Evaluation Methods and Molecular Mechanisms. Appl. Sci. 2022, 12, 5096. [Google Scholar] [CrossRef]
- Breslin, P.A.S.; Beauchamp, G.K. Suppression of Bitterness by Sodium: Variation among Bitter Taste Stimuli. Chem. Senses 1995, 20, 609–623. [Google Scholar] [CrossRef] [PubMed]
- Fleming, K.; Donnelly, R.F. Physical Compatibility and Chemical Stability of Injectable and Oral Ranitidine Solutions. Hosp. Pharm. 2019, 54, 32–36. [Google Scholar] [CrossRef] [PubMed]
- Bruns, C.; Ober, M. Development and Preparation of Oral Suspensions for Paediatric Patients–A Challenge for Pharmacists. Pharm. Technol. Hosp. Pharm. 2018, 3, 113–119. [Google Scholar] [CrossRef]
- Keast, R.S.; Breslin, P.A.; Beauchamp, G.K. Suppression of Bitterness Using Sodium Salts. Chimia 2001, 55, 441. [Google Scholar] [CrossRef]
- Keast, R.S.; Canty, T.M.; Breslin, P.A. The Influence of Sodium Salts on Binary Mixtures of Bitter-tasting Compounds. Chem. Senses 2004, 29, 431–439. [Google Scholar] [CrossRef] [PubMed]
- Ranmal, S.R.; Cram, A.; Tuleu, C. Age-appropriate and acceptable paediatric dosage forms: Insights into end-user perceptions, preferences and practices from the Children’s Acceptability of Oral Formulations (CALF) Study. Int. J. Pharm. 2016, 514, 296–307. [Google Scholar] [CrossRef]
- Kemp, S.; Beauchamp, G. Flavor Modification by Sodium-Chloride and Monosodium Glutamate. J. Food Sci. 1994, 59, 682–686. [Google Scholar] [CrossRef]
- Ghrissi, H.; Veloso, A.C.A.; Marx, Í.M.G.; Dias, T.; Peres, A.M. A potentiometric electronic tongue as a discrimination tool of water-food indicator/contamination bacteria. Chemosensors 2021, 9, 143. [Google Scholar] [CrossRef]
- Ito, M.; Ikehama, K.; Yoshida, K.; Haraguchi, T.; Yoshida, M.; Wada, K.; Uchida, T. Bitterness prediction of H1-antihistamines and prediction of masking effects of artificial sweeteners using an electronic tongue. Int. J. Pharm. 2013, 441, 121–127. [Google Scholar] [CrossRef]
- European Medicines Agency. Reflection Paper on the Use of Methyl- and Propylparaben as Excipients in Human Medicinal Products for Oral Use. 2015: Committee for Medicinal Products for Human Use; EMA/CHMP/SWP/272921/2012; European Medicines Agency: Amsterdam, The Netherlands, 2015.
- Dias, L.; Peres, A.; Veloso, A.; Reis, F.; Vilas-Boas, M.; Machado, A. An electronic tongue taste evaluation: Identification of goat milk adulteration with bovine milk. Sens. Actuators B Chem. 2009, 136, 209–217. [Google Scholar] [CrossRef]
- Veloso, A.C.A.; Dias, L.G.; Rodrigues, N.; Pereira, J.A.; Peres, A.M. Sensory intensity assessment of olive oils using an electronic tongue. Talanta 2016, 146, 585–593. [Google Scholar] [CrossRef] [PubMed]
- Cadima, J.; Cerdeira, J.O.; Minhoto, M. Computational aspects of algorithms for variable selection in the context of principal components. Comput. Stat. Data Anal. 2004, 47, 225–236. [Google Scholar] [CrossRef]
- Bertsimas, D.; Tsitsiklis, J. Simulated annealing. Stat. Sci. 1993, 8, 10–15. [Google Scholar] [CrossRef]
- Mennella, J.A.; Roberts, R.K.; Mathew, P.S.; Reed, D.R. Children’s perceptions about medicines: Individual differences and taste. BMC Pediatr. 2015, 15, 130. [Google Scholar] [CrossRef] [PubMed]
- Belayneh, A.; Tessema, Z. A Systematic Review of the Stability of Extemporaneous Pediatric Oral Formulations. Sci. World J. 2021, 2021, 8523091. [Google Scholar] [CrossRef] [PubMed]
- European Directorate for the Quality of Medicines & HealthCare. European Pharmacopoeia, 10th ed.; Monographs, 07/2017:0946; European Directorate for the Quality of Medicines & HealthCare: Strasbourg, France, 2017; pp. 3708–3709. [Google Scholar]
| Time | Temperature | pH | Appearance | Color | Odor |
|---|---|---|---|---|---|
| 0 days | 4 °C | 5.10 ± 0.03 | clear | Colorless | Characteristic of bubble gum |
| Room Temperature | 5.12 ± 0.02 | clear | Colorless | ||
| 7 days | 4 °C | 5.10 ± 0.02 | clear | Colorless | |
| Room Temperature | 5.11 ± 0.02 | clear | Colorless | ||
| 15 days | 4 °C | 5.09 ± 0.03 | clear | Colorless | |
| Room Temperature | 5.15 ± 0.02 | clear | Colorless | ||
| 30 days | 4 °C | 5.21 ± 0.01 | clear | Colorless | |
| Room Temperature | 5.22 ± 0.01 | clear | Slightly yellow | ||
| 30 days | Unopened 4 °C | 5.20 ± 0.01 | clear | Colorless | |
| 30 days | Unopened Room Temperature | 5.29 ± 0.01 | clear | Slightly yellow |
| % of Initial Ranitidine Content | |||
|---|---|---|---|
| Day | In-Use Stability (4 °C) | In-Use Stability (RT) | Unopened Recipient (4 °C) |
| 0 | 100.0 | 100.0 | 100.0 |
| 7 | 99.8 ± 0.5 | 99.3 ± 0.4 | |
| 15 | 99.7 ± 0.2 | 99.0 ± 0.3 | |
| 30 | 99.5 ± 0.1 | 98.4 ± 0.7 | 99.7 ± 0.2 |
| Characteristic | Target | Comment |
|---|---|---|
| Concentration | 25 mg/mL | Allows a low volume administration |
| pH | 4.5–5.5 | pH of maximum stability of Ra-HCl |
| Chemical and microbiological stability | At least 30 days | Comparison between solution in closed recipient and solution with once daily sample removal (in-use stability) [13] |
| Flavor | Bubble gum | One of the preferred flavors for children |
| Taste masking strategy | Sweeteners and sodium chloride | Synergistic effect of aspartame and sodium saccharine [17] and sorbitol Sodium chloride affords bitterness masking [18,19,20] |
| Viscosity | Very fluid | Appropriated for administration with a syringe |
| Excluded excipients | Sugar-free, alcohol-free and paraben free | Parabens and alcohol are not recommended for pediatric formulations [21]. Sugar-free formulation is non-cariogenic and suitable for children with diabetes and hereditary fructose intolerance [13] |
| Preparation | Simple, with low energy consumption | Feasible in a hospital pharmacy setting |
| Sodium Saccharin | Sorbic Acid | Potassium Sorbate | Sorbitol 70% | Citric Acid | Aspartame | Sodium Chloride | Sodium Citrate |
|---|---|---|---|---|---|---|---|
| 0.6 | 0.1 | 0.1 | 5 | 0.1 | 0.3 | 0.1 | 0.5 |
| Calibration Equation | Determination Coefficient (R2) | LOD (mg/mL) | LOQ (mg/mL) | Repeatability (% RSD), n = 6 | Intermediate Precision (% RSD), n = 6 |
|---|---|---|---|---|---|
| y = 367.33x + 11.9 | 0.996 | 0.0075 | 0.0249 | 1.8 | 2.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ogbonna, J.D.N.; Cunha, E.; Attama, A.A.; Ofokansi, K.C.; Ferreira, H.; Pinto, S.; Gomes, J.; Marx, Í.M.G.; Peres, A.M.; Lobo, J.M.S.; et al. Overcoming Challenges in Pediatric Formulation with a Patient-Centric Design Approach: A Proof-of-Concept Study on the Design of an Oral Solution of a Bitter Drug. Pharmaceuticals 2022, 15, 1331. https://doi.org/10.3390/ph15111331
Ogbonna JDN, Cunha E, Attama AA, Ofokansi KC, Ferreira H, Pinto S, Gomes J, Marx ÍMG, Peres AM, Lobo JMS, et al. Overcoming Challenges in Pediatric Formulation with a Patient-Centric Design Approach: A Proof-of-Concept Study on the Design of an Oral Solution of a Bitter Drug. Pharmaceuticals. 2022; 15(11):1331. https://doi.org/10.3390/ph15111331
Chicago/Turabian StyleOgbonna, John Dike N., Edite Cunha, Anthony A. Attama, Kenneth C. Ofokansi, Helena Ferreira, Susana Pinto, Joana Gomes, Ítala M. G. Marx, António M. Peres, José Manuel Sousa Lobo, and et al. 2022. "Overcoming Challenges in Pediatric Formulation with a Patient-Centric Design Approach: A Proof-of-Concept Study on the Design of an Oral Solution of a Bitter Drug" Pharmaceuticals 15, no. 11: 1331. https://doi.org/10.3390/ph15111331
APA StyleOgbonna, J. D. N., Cunha, E., Attama, A. A., Ofokansi, K. C., Ferreira, H., Pinto, S., Gomes, J., Marx, Í. M. G., Peres, A. M., Lobo, J. M. S., & Almeida, I. F. (2022). Overcoming Challenges in Pediatric Formulation with a Patient-Centric Design Approach: A Proof-of-Concept Study on the Design of an Oral Solution of a Bitter Drug. Pharmaceuticals, 15(11), 1331. https://doi.org/10.3390/ph15111331










