Taxifolin Prevents Cisplatin Nephrotoxicity by Modulating Nrf2/HO-1 Pathway and Mitigating Oxidative Stress and Inflammation in Mice
Abstract
1. Introduction
2. Results
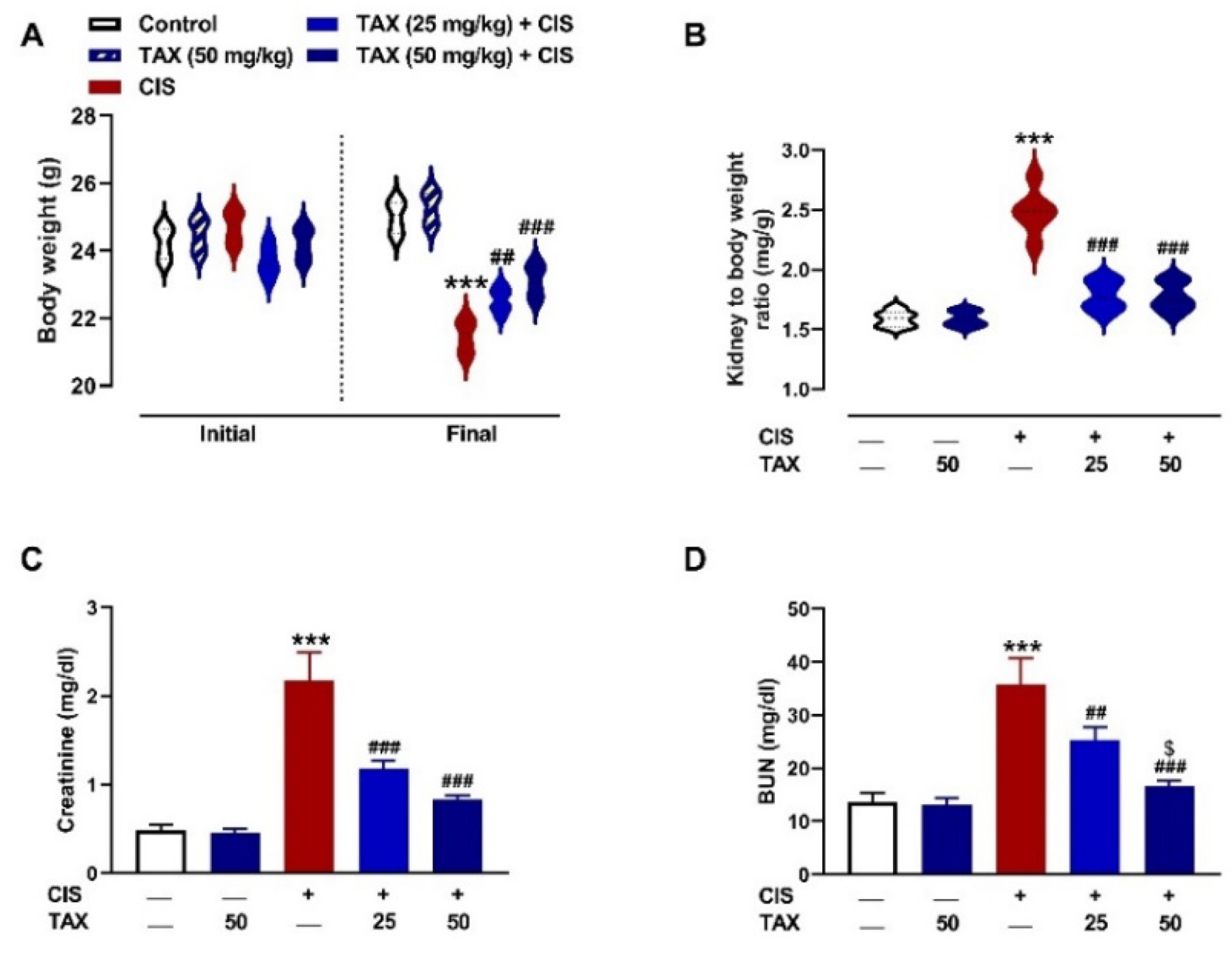
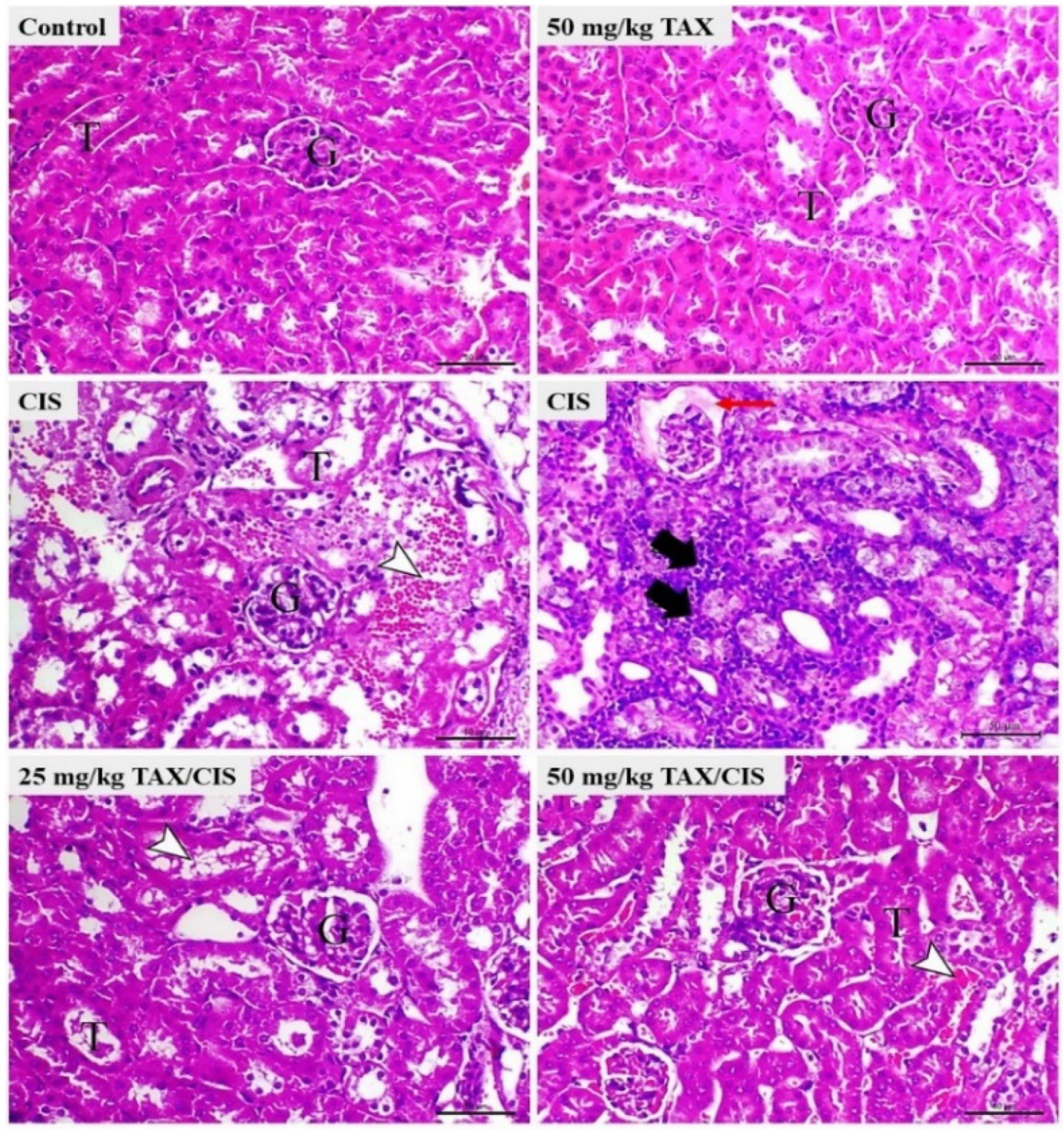
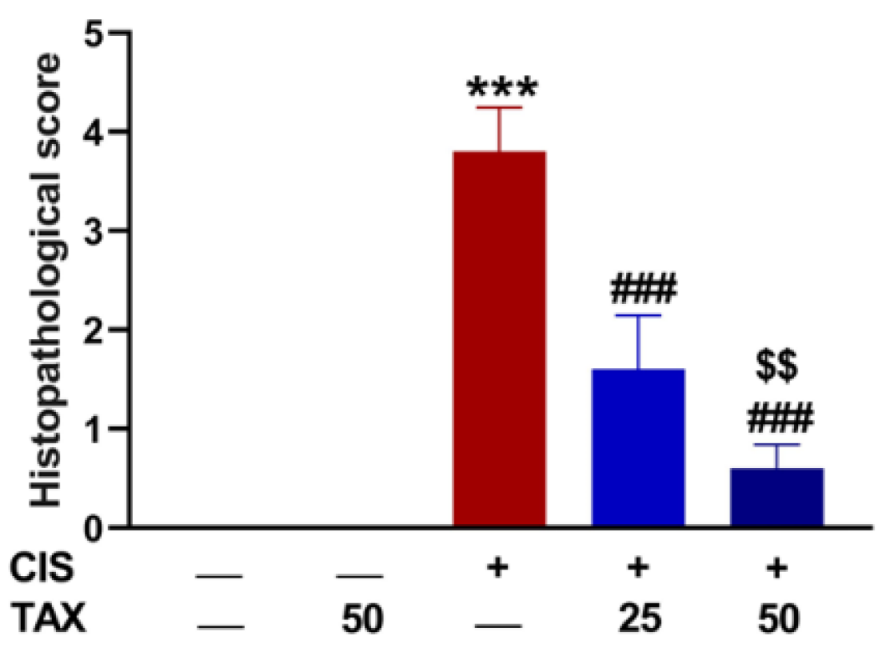
2.1. TAX Prevents CIS-Induced Body Weight Loss and Kidney Injury in Mice
2.2. TAX Attenuates CIS-Induced Renal Oxidative Stress in Mice
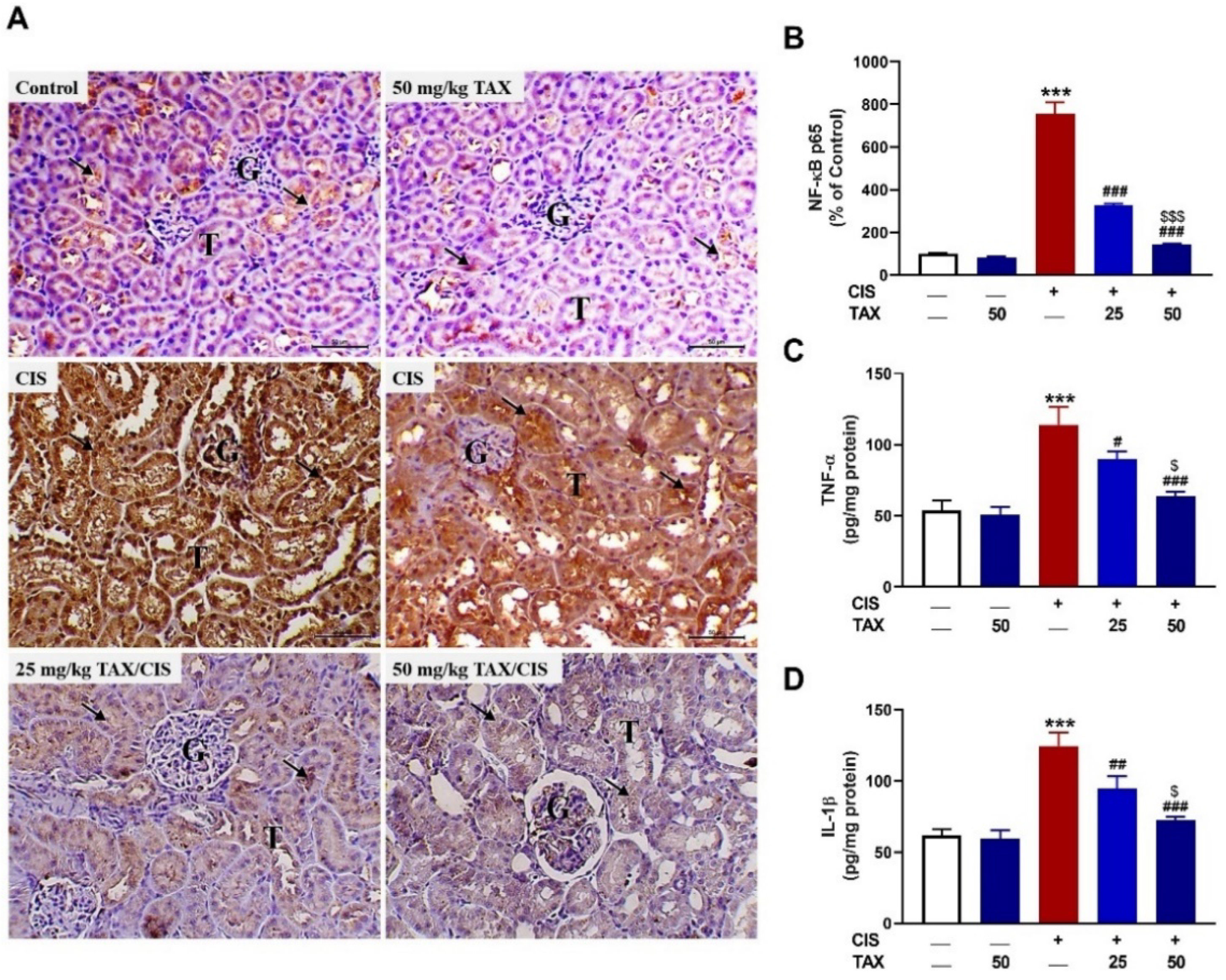
2.3. TAX mitigates Kidney Inflammation in CIS-Intoxicated Mice
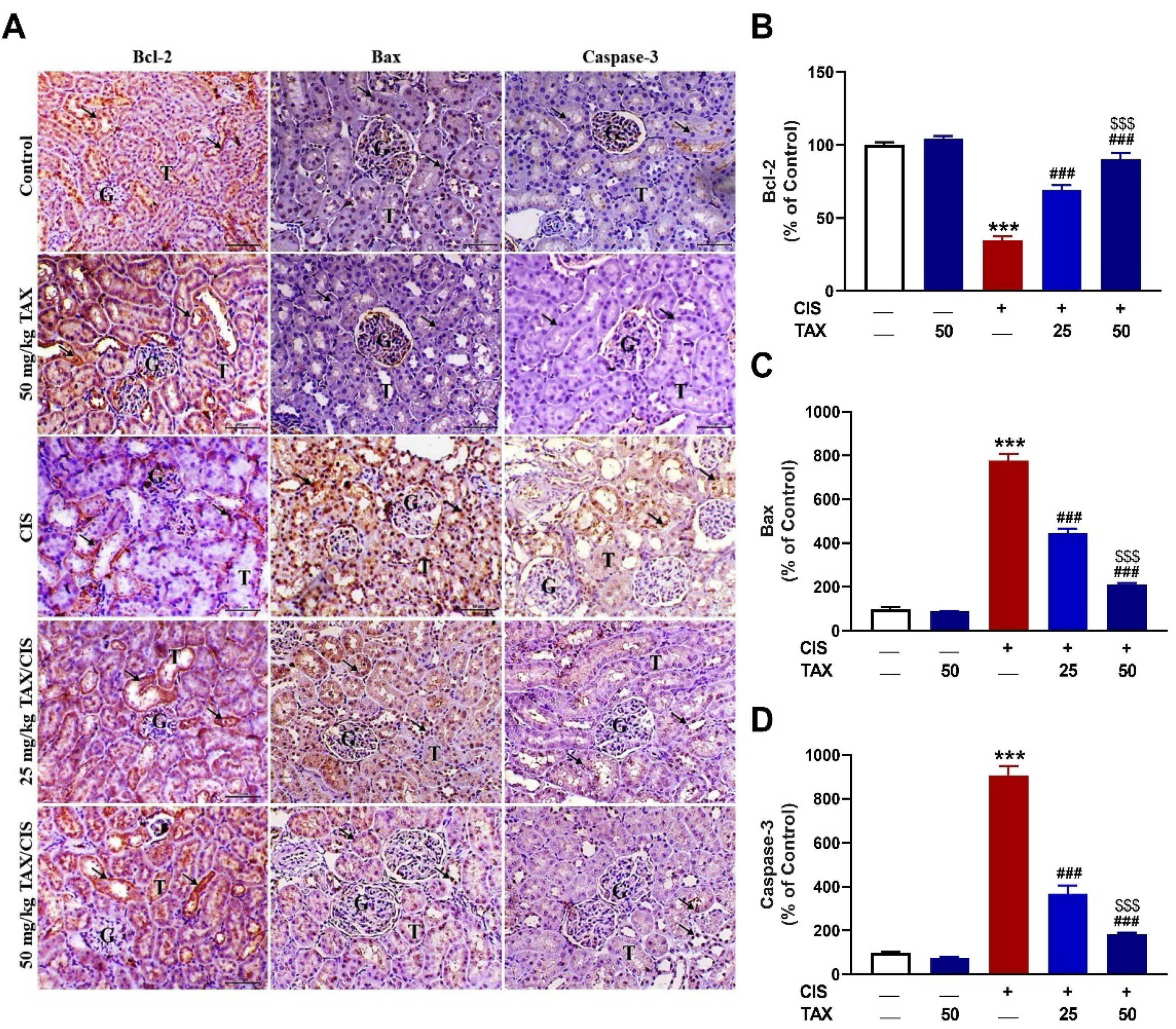
2.4. TAX Prevents CIS-Induced Renal Apoptosis
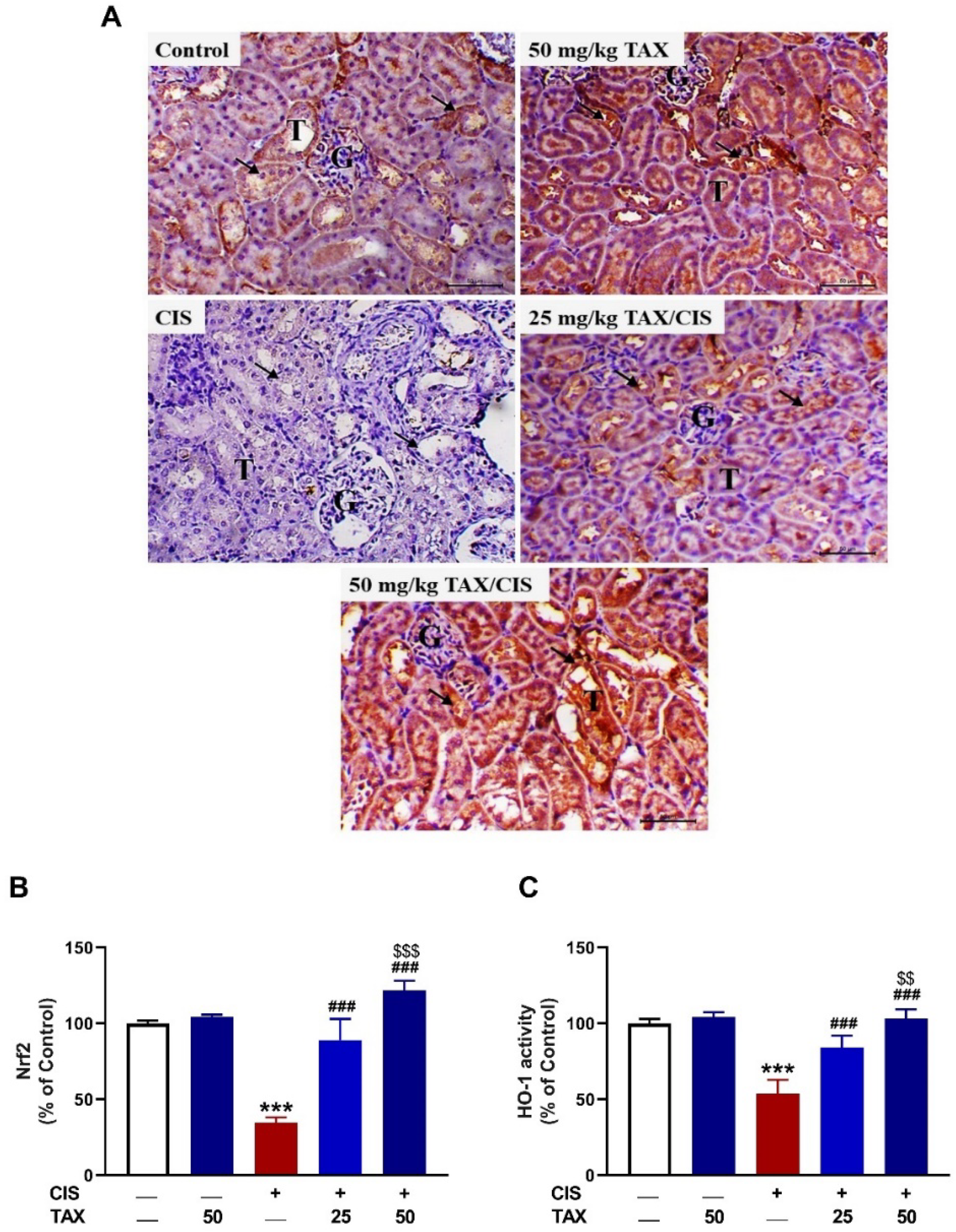
2.5. TAX Upregulates Nrf2/HO-1 Pathway in the Kidney of CIS-Intoxicated Mice
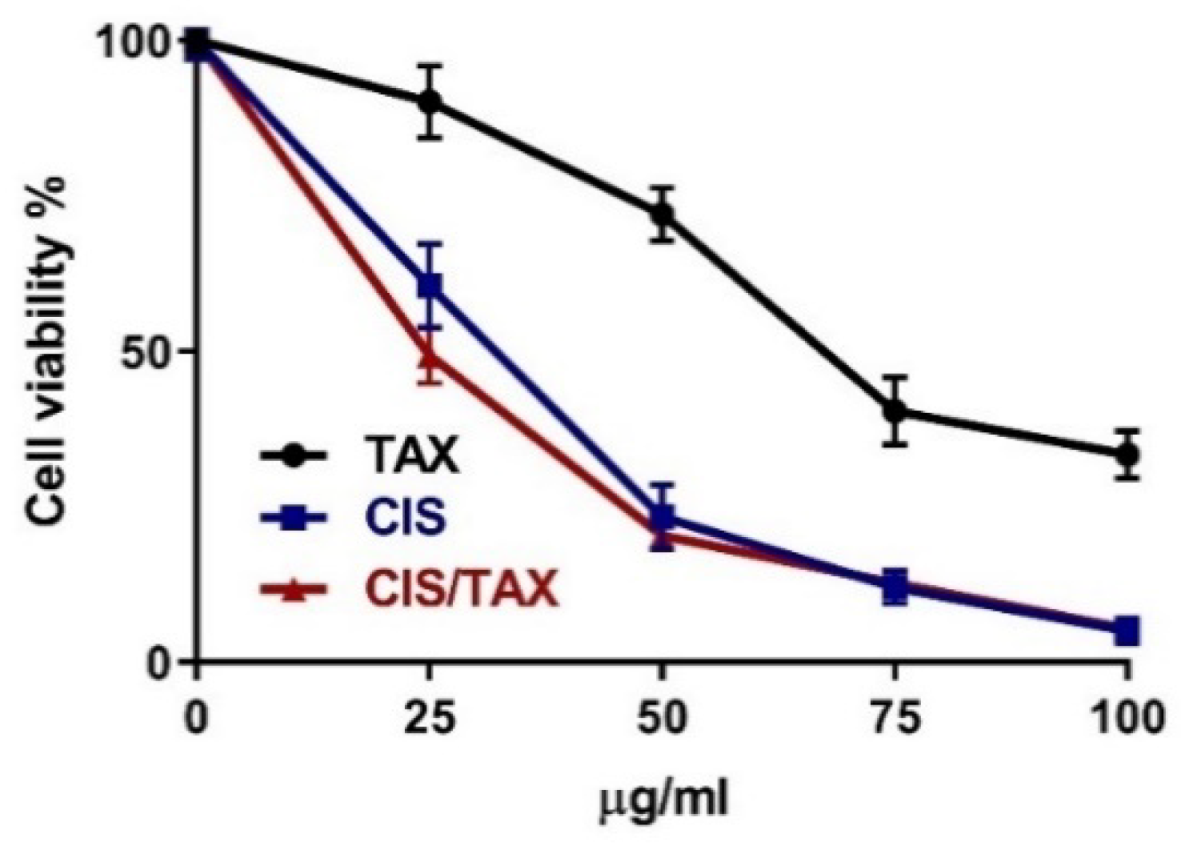
2.6. TAX Does Not Interfere with the Anti-Proliferative Activity of CIS
3. Discussion
4. Materials and Methods
4.1. Animals and Treatments
4.2. Assay of BUN, Creatinine, and Pro-Inflammatory Cytokines
4.3. Assay of MDA, NO, and Antioxidants
4.4. Histological and Immunohistochemical (IHC) Examination
4.5. Assessment of the Impact of TAX on CIS Cytotoxicity
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Manohar, S.; Leung, N. Cisplatin nephrotoxicity: A review of the literature. J. Nephrol. 2018, 31, 15–25. [Google Scholar] [CrossRef]
- Volarevic, V.; Djokovic, B.; Jankovic, M.G.; Harrell, C.R.; Fellabaum, C.; Djonov, V.; Arsenijevic, N. Molecular mechanisms of cisplatin-induced nephrotoxicity: A balance on the knife edge between renoprotection and tumor toxicity. J. Biomed. Sci. 2019, 26, 1–14. [Google Scholar] [CrossRef]
- Dasari, S.; Tchounwou, P.B. Cisplatin in cancer therapy: Molecular mechanisms of action. Eur. J. Pharmacol. 2014, 740, 364–378. [Google Scholar] [CrossRef] [PubMed]
- Tsang, R.Y.; Al-Fayea, T.; Au, H.J. Cisplatin overdose: Toxicities and management. Drug Saf. 2009, 32, 1109–1122. [Google Scholar] [CrossRef]
- Li, L.; Mok, H.; Jhaveri, P.; Bonnen, M.D.; Sikora, A.G.; Eissa, N.T.; Komaki, R.U.; Ghebre, Y.T. Anticancer therapy and lung injury: Molecular mechanisms. Expert Rev. Anticancer Ther. 2018, 18, 1041–1057. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; Panichpisal, K.; Kurtzman, N.; Nugent, K. Cisplatin nephrotoxicity: A review. Am. J. Med. Sci. 2007, 334, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Brozovic, A.; Ambriović-Ristov, A.; Osmak, M. The relationship between cisplatin-induced reactive oxygen species, glutathione, and bcl-2 and resistance to cisplatin. Crit. Rev. Toxicol. 2010, 40, 347–359. [Google Scholar] [CrossRef]
- Pabla, N.; Dong, Z. Cisplatin nephrotoxicity: Mechanisms and renoprotective strategies. Kidney Int. 2008, 73, 994–1007. [Google Scholar] [CrossRef]
- Satta, S.; Mahmoud, A.M.; Wilkinson, F.L.; Yvonne Alexander, M.; White, S.J. The role of nrf2 in cardiovascular function and disease. Oxidative Med. Cell. Longev. 2017, 2017, 9237263. [Google Scholar] [CrossRef]
- Mahmoud, A.M.; Al Dera, H.S. 18β-glycyrrhetinic acid exerts protective effects against cyclophosphamide-induced hepatotoxicity: Potential role of pparγ and nrf2 upregulation. Genes Nutr. 2015, 10, 1–13. [Google Scholar] [CrossRef]
- Abd El-Twab, S.M.; Hozayen, W.G.; Hussein, O.E.; Mahmoud, A.M. 18β-glycyrrhetinic acid protects against methotrexate-induced kidney injury by up-regulating the nrf2/are/ho-1 pathway and endogenous antioxidants. Ren Fail. 2016, 38, 1516–1527. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, A.M.; Hozayen, W.G.; Ramadan, S.M. Berberine ameliorates methotrexate-induced liver injury by activating nrf2/ho-1 pathway and ppargamma, and suppressing oxidative stress and apoptosis in rats. Biomed. Pharmacother.=Biomed. Pharmacother. 2017, 94, 280–291. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, A.M.; Germoush, M.O.; Alotaibi, M.F.; Hussein, O.E. Possible involvement of nrf2 and ppargamma up-regulation in the protective effect of umbelliferone against cyclophosphamide-induced hepatotoxicity. Biomed. Pharmacother.=Biomed. Pharmacother. 2017, 86, 297–306. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Grigoryev, D.N.; Crow, M.T.; Haas, M.; Yamamoto, M.; Reddy, S.P.; Rabb, H. Transcription factor nrf2 is protective during ischemic and nephrotoxic acute kidney injury in mice. Kidney Int. 2009, 76, 277–285. [Google Scholar] [CrossRef]
- Kamel, E.M.; Mahmoud, A.M.; Ahmed, S.A.; Lamsabhi, A.M. A phytochemical and computational study on flavonoids isolated from trifolium resupinatum l. And their novel hepatoprotective activity. Food Funct. 2016, 7, 2094–2106. [Google Scholar] [CrossRef]
- Mahmoud, A.M.; Hernández Bautista, R.J.; Sandhu, M.A.; Hussein, O.E. Beneficial effects of citrus flavonoids on cardiovascular and metabolic health. Oxid Med Cell Longev 2019, 2019, 5484138. [Google Scholar] [CrossRef]
- Mahmoud, A.M.; Sayed, A.M.; Ahmed, O.S.; Abdel-Daim, M.M.; Hassanein, E.H.M. The role of flavonoids in inhibiting il-6 and inflammatory arthritis. Curr Top Med Chem 2022, 22, 746–768. [Google Scholar] [CrossRef]
- Sayed, A.M.; Hassanein, E.H.M.; Salem, S.H.; Hussein, O.E.; Mahmoud, A.M. Flavonoids-mediated sirt1 signaling activation in hepatic disorders. Life Sci 2020, 259, 118173. [Google Scholar] [CrossRef]
- Aladaileh, S.H.; Al-Swailmi, F.K.; Abukhalil, M.H.; Ahmeda, A.F.; Mahmoud, A.M. Punicalagin prevents cisplatin-induced nephrotoxicity by attenuating oxidative stress, inflammatory response, and apoptosis in rats. Life Sci. 2021, 286, 120071. [Google Scholar] [CrossRef]
- Ma, X.; Yan, L.; Zhu, Q.; Shao, F. Puerarin attenuates cisplatin-induced rat nephrotoxicity: The involvement of tlr4/nf-κb signaling pathway. PLoS ONE 2017, 12, e0171612. [Google Scholar] [CrossRef]
- Sahin, K.; Tuzcu, M.; Gencoglu, H.; Dogukan, A.; Timurkan, M.; Sahin, N.; Aslan, A.; Kucuk, O. Epigallocatechin-3-gallate activates nrf2/ho-1 signaling pathway in cisplatin-induced nephrotoxicity in rats. Life Sci. 2010, 87, 240–245. [Google Scholar] [CrossRef]
- Das, A.; Baidya, R.; Chakraborty, T.; Samanta, A.K.; Roy, S. Pharmacological basis and new insights of taxifolin: A comprehensive review. Biomed. Pharmacother. 2021, 142, 112004. [Google Scholar] [CrossRef]
- Sunil, C.; Xu, B. An insight into the health-promoting effects of taxifolin (dihydroquercetin). Phytochemistry 2019, 166, 112066. [Google Scholar] [CrossRef]
- Xie, X.; Feng, J.; Kang, Z.; Zhang, S.; Zhang, L.; Zhang, Y.; Li, X.; Tang, Y. Taxifolin protects rpe cells against oxidative stress-induced apoptosis. Mol. Vis. 2017, 23, 520. [Google Scholar]
- Unver, E.; Tosun, M.; Olmez, H.; Kuzucu, M.; Cimen, F.K.; Suleyman, Z. The effect of taxifolin on cisplatin-induced pulmonary damage in rats: A biochemical and histopathological evaluation. Mediat. Inflamm. 2019, 2019, 3740867. [Google Scholar] [CrossRef]
- Ren, L.; Guo, H.-N.; Yang, J.; Guo, X.-Y.; Wei, Y.-S.; Yang, Z. Dissecting efficacy and metabolic characteristic mechanism of taxifolin on renal fibrosis by multivariate approach and ultra-performance liquid chromatography coupled with mass spectrometry-based metabolomics strategy. Front. Pharmacol. 2021, 11, 608511. [Google Scholar] [CrossRef]
- Ding, C.; Zhao, Y.; Chen, X.; Zheng, Y.; Liu, W.; Liu, X. Taxifolin, a novel food, attenuates acute alcohol-induced liver injury in mice through regulating the nf-κb-mediated inflammation and pi3k/akt signalling pathways. Pharm. Biol. 2021, 59, 866–877. [Google Scholar] [CrossRef]
- Chongmei, T.; Siwei, W.; Li, X. Study on the effect and mechanism of taxifolin on ameliorating cisplatin-induced acute kidney injury in mice through peroxisome proliferator-activated receptor γ coactivator-1α-mediated antioxidant pathway. Chin. J. Nephrol. 2022, 38, 39–47. [Google Scholar]
- Kara, A.V.; Aldemir, M.N.; Ozcicek, F.; Mammadov, R.; Yazici, G.N.; Sunar, M.; Gulaboglu, M. Protective effect of taxifolin on cisplatin-induced nephrotoxicity in rats. Anal. Quant. Cytopathol. Histopathol. 2019, 41, 47–54. [Google Scholar]
- Alhoshani, A.R.; Hafez, M.M.; Husain, S.; Al-Sheikh, A.M.; Alotaibi, M.R.; Al Rejaie, S.S.; Alshammari, M.A.; Almutairi, M.M.; Al-Shabanah, O.A. Protective effect of rutin supplementation against cisplatin-induced nephrotoxicity in rats. BMC Nephrol. 2017, 18, 194. [Google Scholar] [CrossRef]
- Vasaikar, N.; Mahajan, U.; Patil, K.R.; Suchal, K.; Patil, C.R.; Ojha, S.; Goyal, S.N. D-pinitol attenuates cisplatin-induced nephrotoxicity in rats: Impact on pro-inflammatory cytokines. Chem. Biol. Interact. 2018, 290, 6–11. [Google Scholar] [CrossRef] [PubMed]
- Bedir, F.; Kocatürk, H.; Yapanoğlu, T.; Gürsul, C.; Arslan, R.; Mammadov, R.; Çoban, A.; Altuner, D.; Suleyman, H. Protective effect of taxifolin against prooxidant and proinflammatory kidney damage associated with acrylamide in rats. Biomed. Pharmacother. 2021, 139, 111660. [Google Scholar] [CrossRef] [PubMed]
- Crown, O.; Ogundele, O.; Akinmoladun, A.; Famusiwa, C.; Josiah, S.; Olaleye, M.; Akindahunsi, A. Effects of catechin, quercetin and taxifolin on redox parameters and metabolites linked with renal health in rotenone-toxified rats. Niger. J. Physiol. Sci. 2019, 34, 1–10. [Google Scholar] [PubMed]
- Auten, R.L.; Davis, J.M. Oxygen toxicity and reactive oxygen species: The devil is in the details. Pediatr. Res. 2009, 66, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Pacher, P.; Beckman, J.S.; Liaudet, L. Nitric oxide and peroxynitrite in health and disease. Physiol. Rev. 2007, 87, 315–424. [Google Scholar] [CrossRef] [PubMed]
- Baeuerle, P.A.; Baichwal, V.R. Nf-kappa b as a frequent target for immunosuppressive and anti-inflammatory molecules. Adv. Immunol. 1997, 65, 111–137. [Google Scholar]
- Pan, H.; Mukhopadhyay, P.; Rajesh, M.; Patel, V.; Mukhopadhyay, B.; Gao, B.; Haskó, G.; Pacher, P. Cannabidiol attenuates cisplatin-induced nephrotoxicity by decreasing oxidative/nitrosative stress, inflammation, and cell death. J. Pharmacol. Exp. Ther. 2009, 328, 708–714. [Google Scholar] [CrossRef]
- Loverre, A.; Ditonno, P.; Crovace, A.; Gesualdo, L.; Ranieri, E.; Pontrelli, P.; Stallone, G.; Infante, B.; Schena, A.; Di Paolo, S.; et al. Ischemia-reperfusion induces glomerular and tubular activation of proinflammatory and antiapoptotic pathways: Differential modulation by rapamycin. J. Am. Soc. Nephrol. JASN 2004, 15, 2675–2686. [Google Scholar] [CrossRef]
- Sami, D.H.; Soliman, A.S.; Khowailed, A.A.; Hassanein, E.H.M.; Kamel, E.M.; Mahmoud, A.M. 7-hydroxycoumarin modulates Nrf2/HO-1 and microRNA-34a/SIRT1 signaling and prevents cisplatin-induced oxidative stress, inflammation, and kidney injury in rats. Life Sci. 2022, 310, 121104. [Google Scholar] [CrossRef]
- Kolli, V.K.; Abraham, P.; Isaac, B.; Selvakumar, D. Neutrophil infiltration and oxidative stress may play a critical role in methotrexate-induced renal damage. Chemotherapy 2009, 55, 83–90. [Google Scholar] [CrossRef]
- Ansari, M.A. Sinapic acid modulates nrf2/ho-1 signaling pathway in cisplatin-induced nephrotoxicity in rats. Biomed. Pharmacother.=Biomed. Pharmacother. 2017, 93, 646–653. [Google Scholar] [CrossRef]
- Younis, N.N.; Elsherbiny, N.M.; Shaheen, M.A.; Elseweidy, M.M. Modulation of nadph oxidase and nrf2/ho-1 pathway by vanillin in cisplatin-induced nephrotoxicity in rats. J. Pharm. Pharmacol. 2020, 72, 1546–1555. [Google Scholar] [CrossRef]
- Shi, Y.; Chen, J.; Weng, C.; Chen, R.; Zheng, Y.; Chen, Q.; Tang, H. Identification of the protein-protein contact site and interaction mode of human vdac1 with bcl-2 family proteins. Biochem. Biophys. Res. Commun. 2003, 305, 989–996. [Google Scholar] [CrossRef]
- Redza-Dutordoir, M.; Averill-Bates, D.A. Activation of apoptosis signalling pathways by reactive oxygen species. Biochim. Et Biophys. Acta (BBA)-Mol. Cell Res. 2016, 1863, 2977–2992. [Google Scholar] [CrossRef]
- Algefare, A.I. Renoprotective and oxidative stress-modulating effects of taxifolin against cadmium-induced nephrotoxicity in mice. Life 2022, 12, 1150. [Google Scholar] [CrossRef]
- Lin, J.-N.; Wang, J.-S.; Lin, C.-C.; Lin, H.-Y.; Yu, S.-H.; Wen, Y.-H.; Tseng, G.-F.; Hsu, C.-J.; Wu, H.-P. Ameliorative effect of taxifolin on gentamicin-induced ototoxicity via down-regulation of apoptotic pathways in mouse cochlear ub/oc-2 cells. J. Chin. Med. Assoc. 2022, 85, 617–626. [Google Scholar] [CrossRef]
- Kim, A.; Nam, Y.J.; Lee, C.S. Taxifolin reduces the cholesterol oxidation product-induced neuronal apoptosis by suppressing the akt and nf-κb activation-mediated cell death. Brain Res. Bull. 2017, 134, 63–71. [Google Scholar] [CrossRef]
- Nam, Y.J.; Lee, D.H.; Shin, Y.K.; Sohn, D.S.; Lee, C.S. Flavanonol taxifolin attenuates proteasome inhibition-induced apoptosis in differentiated pc12 cells by suppressing cell death process. Neurochem. Res. 2015, 40, 480–491. [Google Scholar] [CrossRef]
- Aladaileh, S.H.; Abukhalil, M.H.; Saghir, S.A.; Hanieh, H.; Alfwuaires, M.A.; Almaiman, A.A.; Bin-Jumah, M.; Mahmoud, A.M. Galangin activates nrf2 signaling and attenuates oxidative damage, inflammation, and apoptosis in a rat model of cyclophosphamide-induced hepatotoxicity. Biomolecules 2019, 9, 346. [Google Scholar] [CrossRef]
- Abd El-Twab, S.M.; Hussein, O.E.; Hozayen, W.G.; Bin-Jumah, M.; Mahmoud, A.M. Chicoric acid prevents methotrexate-induced kidney injury by suppressing nf-κb/nlrp3 inflammasome activation and up-regulating nrf2/are/ho-1 signaling. Inflamm. Res. 2019, 68, 511–523. [Google Scholar] [CrossRef]
- Liu, X.-L.; Zhao, Y.-C.; Zhu, H.-Y.; Wu, M.; Zheng, Y.-N.; Yang, M.; Cheng, Z.-Q.; Ding, C.-B.; Liu, W.-C. Taxifolin retards the d-galactose-induced aging process through inhibiting nrf2-mediated oxidative stress and regulating the gut microbiota in mice. Food Funct. 2021, 12, 12142–12158. [Google Scholar] [CrossRef]
- Wardyn, J.D.; Ponsford, A.H.; Sanderson, C.M. Dissecting molecular cross-talk between nrf2 and nf-κb response pathways. Biochem. Soc. Trans. 2015, 43, 621–626. [Google Scholar] [CrossRef]
- Sun, X.; Chen, R.C.; Yang, Z.H.; Sun, G.B.; Wang, M.; Ma, X.J.; Yang, L.J.; Sun, X.B. Taxifolin prevents diabetic cardiomyopathy in vivo and in vitro by inhibition of oxidative stress and cell apoptosis. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2014, 63, 221–232. [Google Scholar] [CrossRef]
- Ma, N.; Wei, W.; Fan, X.; Ci, X. Farrerol attenuates cisplatin-induced nephrotoxicity by inhibiting the reactive oxygen species-mediated oxidation, inflammation, and apoptotic signaling pathways. Front. Physiol. 2019, 10, 1419. [Google Scholar] [CrossRef]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef]
- Green, L.C.; Wagner, D.A.; Glogowski, J.; Skipper, P.L.; Wishnok, J.S.; Tannenbaum, S.R. Analysis of nitrate, nitrite, and [15n]nitrate in biological fluids. Anal. Biochem. 1982, 126, 131–138. [Google Scholar] [CrossRef]
- Nishikimi, M.; Rao, N.A.; Yagi, K. The occurrence of superoxide anion in the reaction of reduced phenazine methosulfate and molecular oxygen. Biochem. Biophys. Res. Commun. 1972, 46, 849–854. [Google Scholar] [CrossRef]
- Aebi, H. [13] catalase in vitro. In Methods in Enzymology; Academic Press: New York, NY, USA, 1984; Volume 105, pp. 121–126. [Google Scholar]
- Abraham, N.G.; Lutton, J.D.; Levere, R.D. Heme metabolism and erythropoiesis in abnormal iron states: Role of delta-aminolevulinic acid synthase and heme oxygenase. Exp. Hematol. 1985, 13, 838–843. [Google Scholar] [PubMed]
- Griffith, O.W. Determination of glutathione and glutathione disulfide using glutathione reductase and 2-vinylpyridine. Anal. Biochem. 1980, 106, 207–212. [Google Scholar] [CrossRef]
- Bancroft, J.D.; Gamble, M. Theory and practice of histological techniques; Elsevier health sciences: Philadelphia, PA, USA, 2008. [Google Scholar]
- Jennes, L. Chapter 7—immunohistochemical detection of neuropeptides/transmitters in mammalian brain. In Methods in Cell Biology; Conn, P.M., Ed.; Academic Press: New York, NY, USA, 2013; Volume 113, pp. 123–147. [Google Scholar]
- Aladaileh, S.H.; Hussein, O.E.; Abukhalil, M.H.; Saghir, S.A.M.; Bin-Jumah, M.; Alfwuaires, M.A.; Germoush, M.O.; Almaiman, A.A.; Mahmoud, A.M. Formononetin upregulates nrf2/ho-1 signaling and prevents oxidative stress, inflammation, and kidney injury in methotrexate-induced rats. Antioxidants 2019, 8, 430. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alanezi, A.A.; Almuqati, A.F.; Alfwuaires, M.A.; Alasmari, F.; Namazi, N.I.; Althunibat, O.Y.; Mahmoud, A.M. Taxifolin Prevents Cisplatin Nephrotoxicity by Modulating Nrf2/HO-1 Pathway and Mitigating Oxidative Stress and Inflammation in Mice. Pharmaceuticals 2022, 15, 1310. https://doi.org/10.3390/ph15111310
Alanezi AA, Almuqati AF, Alfwuaires MA, Alasmari F, Namazi NI, Althunibat OY, Mahmoud AM. Taxifolin Prevents Cisplatin Nephrotoxicity by Modulating Nrf2/HO-1 Pathway and Mitigating Oxidative Stress and Inflammation in Mice. Pharmaceuticals. 2022; 15(11):1310. https://doi.org/10.3390/ph15111310
Chicago/Turabian StyleAlanezi, Abdulkareem A., Afaf F. Almuqati, Manal A. Alfwuaires, Fawaz Alasmari, Nader I. Namazi, Osama Y. Althunibat, and Ayman M. Mahmoud. 2022. "Taxifolin Prevents Cisplatin Nephrotoxicity by Modulating Nrf2/HO-1 Pathway and Mitigating Oxidative Stress and Inflammation in Mice" Pharmaceuticals 15, no. 11: 1310. https://doi.org/10.3390/ph15111310
APA StyleAlanezi, A. A., Almuqati, A. F., Alfwuaires, M. A., Alasmari, F., Namazi, N. I., Althunibat, O. Y., & Mahmoud, A. M. (2022). Taxifolin Prevents Cisplatin Nephrotoxicity by Modulating Nrf2/HO-1 Pathway and Mitigating Oxidative Stress and Inflammation in Mice. Pharmaceuticals, 15(11), 1310. https://doi.org/10.3390/ph15111310







