[177Lu]Lu-PSMA-617 (PluvictoTM): The First FDA-Approved Radiotherapeutical for Treatment of Prostate Cancer
Abstract
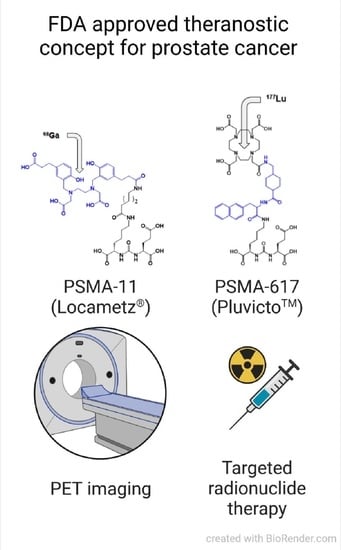
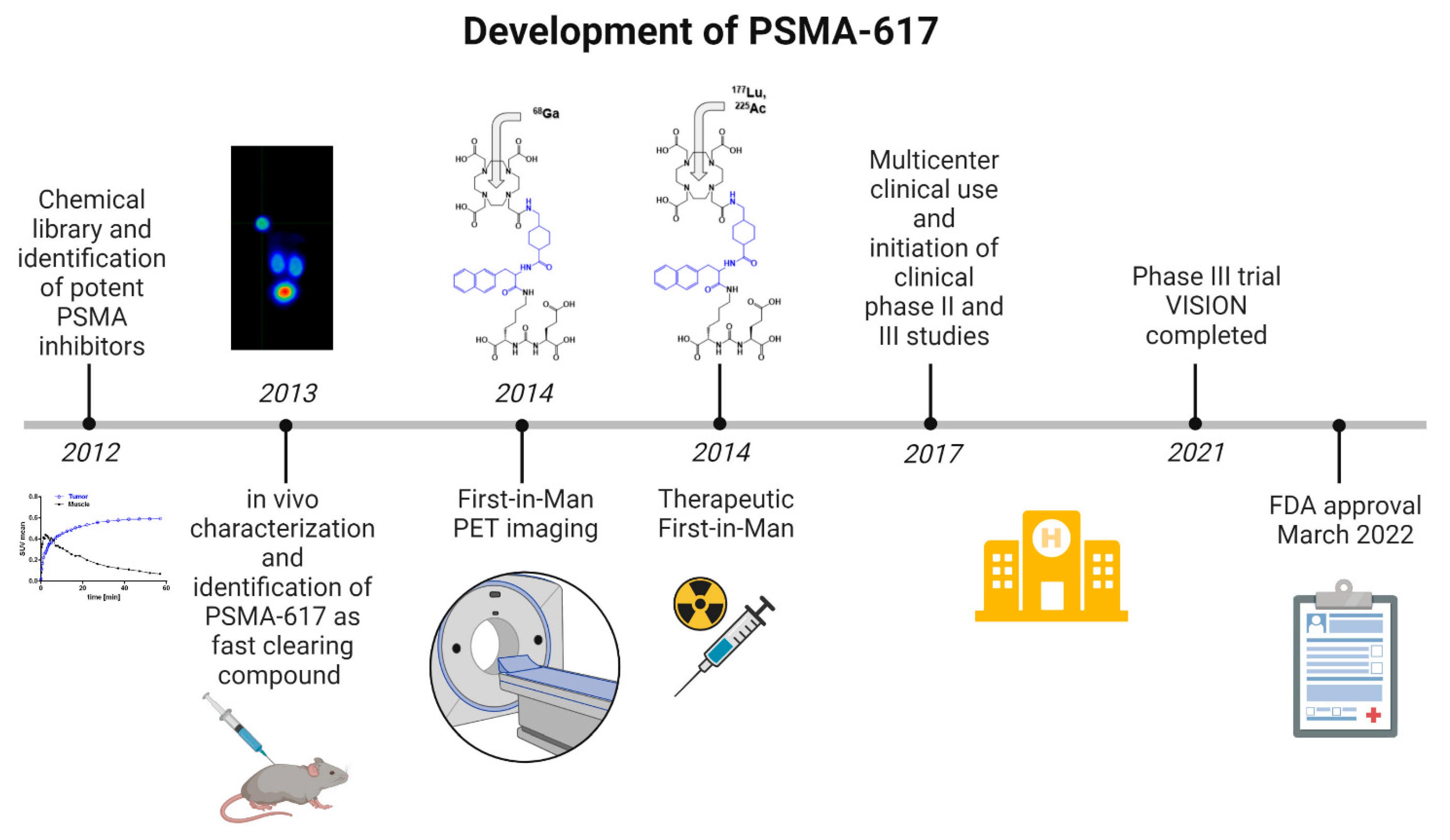
1. Introduction
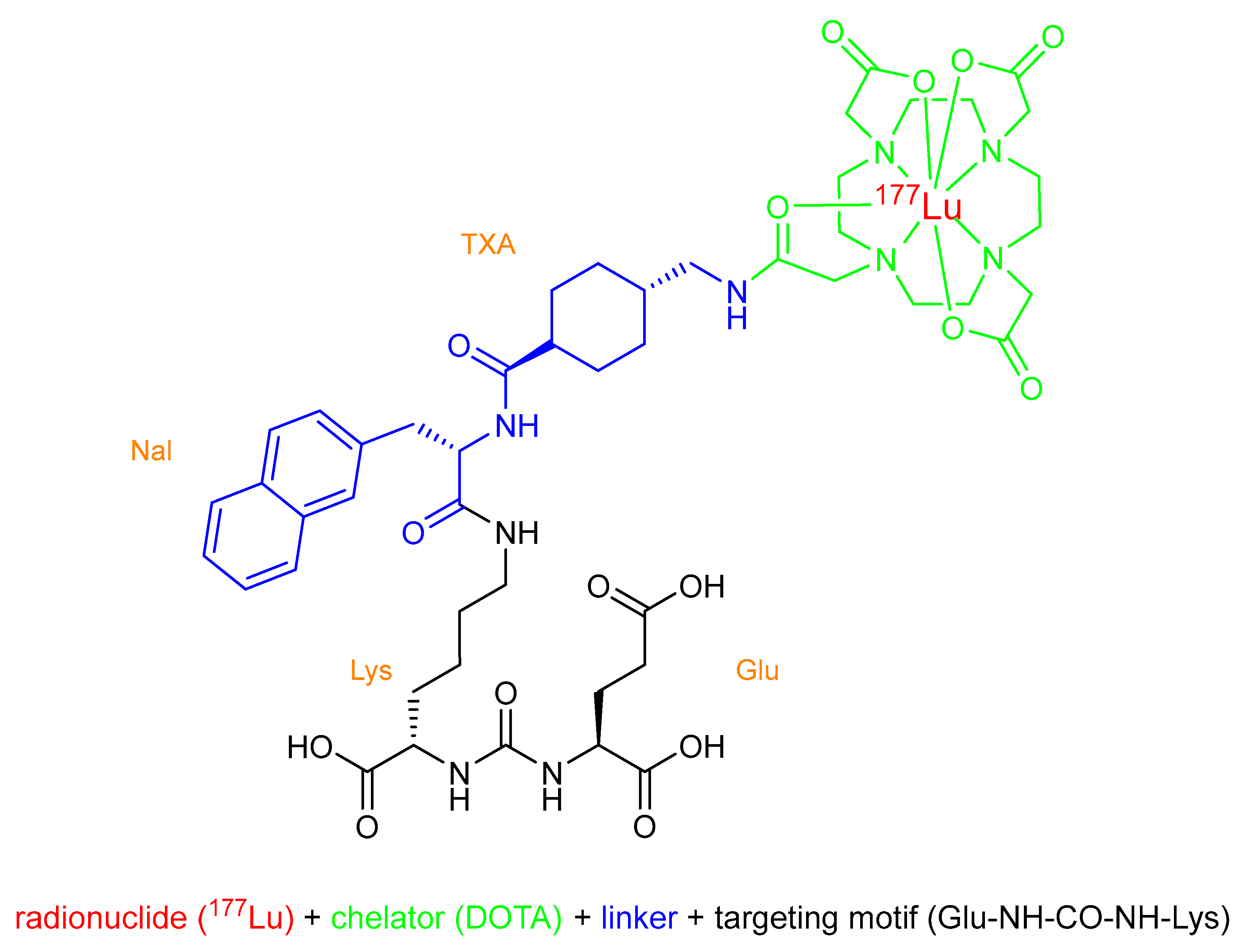
2. Chemical Overview
2.1. Names and Chemical Structure of [177Lu]Lu-PSMA-617 (PluvictoTM)
2.2. Lutetium-177
2.3. Production and Quality Control of [177Lu]Lu-PSMA-617
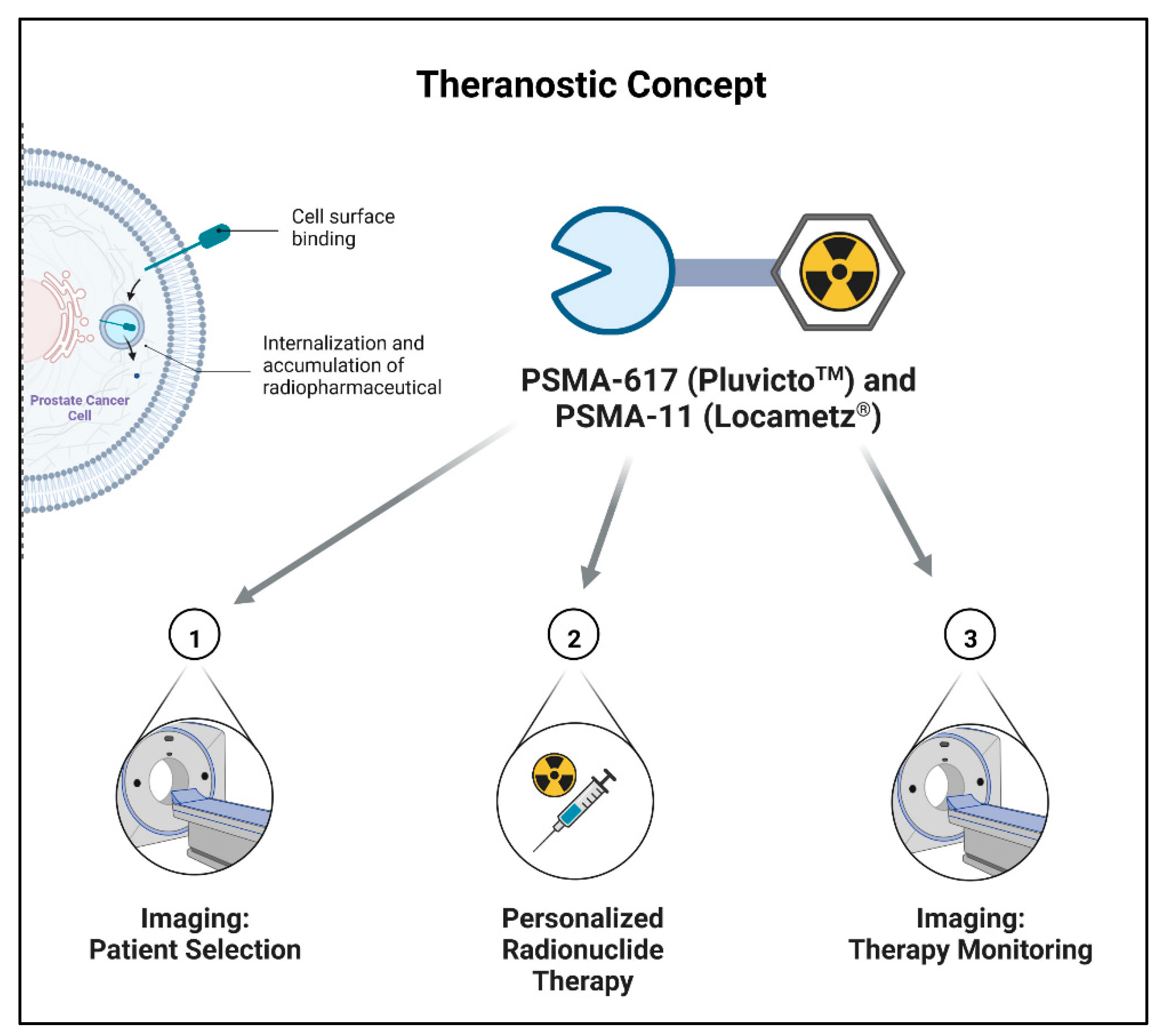
3. Medicinal and Pharmaceutical Overview
3.1. Clinical Indication
3.2. Application
3.3. Pharmacology, Pharmacokinetics, and Toxicology
4. Perspective
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sartor, O.; de Bono, J.; Chi, K.N.; Fizazi, K.; Herrmann, K.; Rahbar, K.; Tagawa, S.T.; Nordquist, L.T.; Vaishampayan, N.; El-Haddad, G.; et al. Lutetium-177-PSMA-617 for Metastatic Castration-Resistant Prostate Cancer. N. Engl. J. Med. 2021, 385, 1091–1103. [Google Scholar] [CrossRef] [PubMed]
- Horoszewicz, J.S.; Kawinski, E.; Murphy, G.P. Monoclonal antibodies to a new antigenic marker in epithelial prostatic cells and serum of prostatic cancer patients. Anticancer. Res. 1987, 7, 927–935. [Google Scholar] [PubMed]
- Label for ProstaScint® Kit. Available online: https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&ApplNo=103608 (accessed on 5 August 2022).
- Hennrich, U.; Eder, M. [68Ga]Ga-PSMA-11: The First FDA-Approved 68Ga-Radiopharmaceutical for PET Imaging of Prostate Cancer. Pharmaceuticals 2021, 14, 713. [Google Scholar] [CrossRef] [PubMed]
- Pomper, M.G.; Musachio, J.L.; Zhang, J.; Scheffel, U.; Zhou, Y.; Hilton, J.; Maini, A.; Dannals, R.F.; Wong, D.F.; Kozikowski, A.P. 11C-MCG: Synthesis, uptake selectivity, and primate PET of a probe for glutamate carboxypeptidase II (NAALADase). Mol. Imaging 2002, 1, 96–101. [Google Scholar] [CrossRef]
- Foss, C.A.; Mease, R.C.; Fan, H.; Wang, Y.; Ravert, H.T.; Dannals, R.F.; Olszewski, R.T.; Heston, W.D.; Kozikowski, A.P.; Pomper, M.G. Radiolabeled small-molecule ligands for prostate-specific membrane antigen: In vivo imaging in experimental models of prostate cancer. Clin. Cancer Res. 2005, 11, 4022–4028. [Google Scholar] [CrossRef] [PubMed]
- Kozikowski, A.P.; Nan, F.; Conti, P.; Zhang, J.; Ramadan, E.; Bzdega, T.; Wroblewska, B.; Neale, J.H.; Pshenichkin, S.; Wroblewski, J.T. Design of Remarkably Simple, Yet Potent Urea-Based Inhibitors of Glutamate Carboxypeptidase II (NAALADase). J. Med. Chem. 2001, 4, 298–301. [Google Scholar] [CrossRef] [PubMed]
- Barinka, C.; Byun, Y.; Dusich, C.L.; Banerjee, S.R.; Chen, Y.; Castanares, M.; Kozikowski, A.P.; Mease, R.C.; Pomper, M.G.; Lubkowski, J. Interactions between human glutamate carboxypeptidase II and urea-based inhibitors: Structural characterization. J. Med. Chem. 2008, 51, 7737–7743. [Google Scholar] [CrossRef] [PubMed]
- Benešová, M.; Bauder-Wüst, U.; Schäfer, M.; Klika, K.D.; Mier, W.; Haberkorn, U.; Kopka, K.; Eder, M. Linker Modification Strategies to Control the Prostate-Specific Membrane Antigen (PSMA)-Targeting and Pharmacokinetic Properties of DOTA-Conjugated PSMA Inhibitors. J. Med. Chem. 2016, 59, 1761–1775. [Google Scholar] [CrossRef] [PubMed]
- Benešová, M.; Schäfer, M.; Bauder-Wüst, U.; Afshar-Oromieh, A.; Kratochwil, C.; Mier, W.; Haberkorn, U.; Kopka, K.; Eder, M. Preclinical Evaluation of a Tailor-Made DOTA-Conjugated PSMA Inhibitor with Optimized Linker Moiety for Imaging and Endoradiotherapy of Prostate Cancer. J. Nucl. Med. 2015, 56, 914–920. [Google Scholar] [CrossRef]
- Afshar-Oromieh, A.; Hetzheim, H.; Kratochwil, C.; Benešová, M.; Eder, M.; Neels, O.C.; Eisenhut, M.; Kübler, W.; Holland-Letz, T.; Giesel, F.L.; et al. The Theranostic PSMA Ligand PSMA-617 in the Diagnosis of Prostate Cancer by PET/CT: Biodistribution in Humans, Radiation Dosimetry, and First Evaluation of Tumor Lesions. J. Nucl. Med. 2015, 56, 1697–1705. [Google Scholar] [CrossRef] [PubMed]
- Kratochwil, C.; Giesel, F.L.; Stefanova, M.; Benešová, M.; Bronzel, M.; Afshar-Oromieh, A.; Mier, W.; Eder, M.; Kopka, K.; Haberkorn, U. PSMA-Targeted Radionuclide Therapy of Metastatic Castration-Resistant Prostate Cancer with 177Lu-Labeled PSMA-617. J. Nucl. Med. 2016, 57, 1170–1176. [Google Scholar] [CrossRef] [PubMed]
- Hennrich, U.; Kopka, K. Lutathera®: The First FDA- and EMA-Approved Radiopharmaceutical for Peptide Receptor Radionuclide Therapy. Pharmaceuticals 2019, 12, 114. [Google Scholar] [CrossRef] [PubMed]
- Hennrich, U.; Benešová, M. [68Ga]Ga-DOTA-TOC: The First FDA-Approved 68Ga-Radiopharmaceutical for PET Imaging. Pharmaceuticals 2020, 13, 38. [Google Scholar] [CrossRef]
- Label for “ILLUCCIX® (Kit for the Preparation of Gallium Ga 68 Gozetotide Injection)”. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2022/214032Orig1s000TOC.cfm (accessed on 26 August 2022).
- Label for “LOCAMETZ® (Kit for the Preparation of Gallium Ga 68 Gozetotide Injection)”. Available online: https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&ApplNo=215841 (accessed on 1 September 2022).
- Lau, J.; Lee, H.; Rousseau, J.; Bénard, F.; Lin, K.-S. Application of Cleavable Linkers to Improve Therapeutic Index of Radioligand Therapies. Molecules 2022, 27, 4959. [Google Scholar] [CrossRef] [PubMed]
- Barca, C.; Griessinger, C.M.; Faust, A.; Depke, D.; Essler, M.; Windhorst, A.D.; Devoogdt, N.; Brindle, K.M.; Schäfers, M.; Zinnhardt, B.; et al. Expanding Theranostic Radiopharmaceuticals for Tumor Diagnosis and Therapy. Pharmaceuticals 2022, 15, 13. [Google Scholar] [CrossRef] [PubMed]
- Roscher, M.; Bakos, G.; Benešová, M. Atomic Nanogenerators in Targeted Alpha Therapies: Curie’s Legacy in Modern Cancer Management. Pharmaceuticals 2020, 13, 76. [Google Scholar] [CrossRef]
- Aime, S.; Barge, A.; Botta, M.; Fasano, M.; Ayala, J.D.; Bombieri, G. Crystal structure and solution dynamics of the lutetium(III) chelate of DOTA. Inorg. Chim. Acta 1996, 246, 423–426. [Google Scholar] [CrossRef]
- Decay Characteristics of 177Lu. Available online: https://www.nndc.bnl.gov/nudat3/indx_dec.jsp (accessed on 1 September 2022).
- Label for “PLUVICTOTM (Lutetium Lu 177 Vipivotide Tetraxetan) Injection”. Available online: https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&ApplNo=215833 (accessed on 1 September 2022).
- Chakravarty, R.; Chakraborty, S. A review of advances in the last decade on targeted cancer therapy using 177Lu: Focusing on 177Lu produced by the direct neutron activation route. Am. J. Nucl. Med. Mol. Imaging 2021, 11, 443–475. [Google Scholar]
- Vogel, W.V.; van der Marck, S.C.; Versleijen, M.W.J. Challenges and future options for the production of lutetium-177. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 2329–2335. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, S.; Chakravarty, R.; Shetty, P.; Vimalnath, K.V.; Sen, I.B.; Dash, A. Prospects of medium specific activity 177Lu in targeted therapy of prostate cancer using 177Lu-labeled PSMA inhibitor. J. Label. Compds. Radiopharm. 2016, 59, 364–371. [Google Scholar] [CrossRef]
- Chakraborty, S.; Vimalnath, K.V.; Chakravarty, R.; Sarma, H.D.; Dash, A. Multidose formulation of ready-to-use 177Lu-PSMA-617 in a centralized radiopharmacy set-up. Appl. Rad. Isotop. 2018, 139, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Wichmann, C.W.; Ackermann, U.; Poniger, S.; Young, K.; Nguyen, B.; Gordon, C.; Sachinidis, J.; Scott, A.M. Automated radiosynthesis of [68Ga]Ga-PSMA-11 and [177Lu]Lu-PSMA-617 on the iPHASE MultiSyn module for clinical applications. J. Label. Compds. Radiopharm. 2020, 63, 1–7. [Google Scholar] [CrossRef]
- Hofman, M.S.; Violet, J.; Hicks, R.J.; Ferdinandus, J.; Thang, S.P.; Akhurst, T.; Iravani, A.; Kong, G.; Ravi Kumar, A.; Murphy, D.G.; et al. [(177)Lu]-PSMA-617 radionuclide treatment in patients with metastatic castration-resistant prostate cancer (LuPSMA trial): A single-centre, single-arm, phase 2 study. Lancet Oncol. 2018, 19, 825–833. [Google Scholar] [CrossRef]
- Violet, J.; Sandhu, S.; Iravani, A.; Ferdinandus, J.; Thang, S.P.; Kong, G.; Kumar, A.R.; Akhurst, T.; Pattison, D.A.; Beaulieu, A.; et al. Long-Term Follow-up and Outcomes of Retreatment in an Expanded 50-Patient Single-Center Phase II Prospective Trial of (177)Lu-PSMA-617 Theranostics in Metastatic Castration-Resistant Prostate Cancer. J. Nucl. Med. 2020, 61, 857–865. [Google Scholar] [CrossRef]
- Hofman, M.S.; Emmett, L.; Sandhu, S.; Iravani, A.; Joshua, A.M.; Goh, J.C.; Pattison, D.A.; Tan, T.H.; Kirkwood, I.D.; Ng, S.; et al. [(177)Lu]Lu-PSMA-617 versus cabazitaxel in patients with metastatic castration-resistant prostate cancer (TheraP): A randomised, open-label, phase 2 trial. Lancet 2021, 397, 797–804. [Google Scholar] [CrossRef]
- Seifert, R.; Kessel, K.; Schlack, K.; Weckesser, M.; Bögemann, M.; Rahbar, K. Radioligand therapy using [177Lu]Lu-PSMA-617 in mCRPC: A pre-VISION single-center analysis. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 2106–2112. [Google Scholar] [CrossRef] [PubMed]
- Calais, J.; Gafita, A.; Eiber, M.; Armstrong, W.R.; Gartmann, J.; Thin, P.; Nguyen, K.; Lok, V.; Gosa, L.; Grogan, T.; et al. Prospective phase 2 trial of PSMA-targeted molecular RadiothErapy with (177)Lu-PSMA-617 for metastatic castration-reSISTant Prostate Cancer (RESIST-PC): Efficacy results of the UCLA cohort. J. Nucl. Med. 2021, 62, 1440–1446. [Google Scholar] [CrossRef]
- Calais, J.; Czernin, J.; Thin, P.; Gartmann, J.; Nguyen, K.; Armstrong, W.R.; Allen-Auerbach, M.; Quon, A.; Bahri, S.; Gupta, P.; et al. Safety of PSMA-Targeted Molecular Radioligand Therapy with (177)Lu-PSMA-617: Results from the Prospective Multicenter Phase 2 Trial RESIST-PC (NCT03042312). J. Nucl. Med. 2021, 62, 1447–1456. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, K.; Rahbar, K.; Eiber, M.; Krause, B.J.; Lassmann, M.; Jentzen, W.; Blumenstein, L.; Klein, P.; Basque, J.-R.; Kurth, J. Dosimetry of 177Lu-PSMA-617 for the treatment of metastatic castration-resistant prostate cancer: Results from the VISION trial sub-study. J. Clin. Oncol. 2022, 40, 97. [Google Scholar] [CrossRef]
- Kurth, J.; Krause, B.J.; Schwarzenböck, S.M.; Stegger, L.; Schäfers, M.; Rahbar, K. External radiation exposure, excretion, and effective half-life in 177Lu-PSMA-targeted therapies. EJNMMI Res. 2018, 8, 32. [Google Scholar] [CrossRef] [PubMed]
- Schuchardt, C.; Zhang, J.; Kulkarni, H.R.; Chen, X.; Müller, D.; Baum, R.P. Prostate-Specific Membrane Antigen Radioligand Therapy Using 177Lu-PSMA I&T and 177Lu-PSMA-617 in Patients with Metastatic Castration-Resistant Prostate Cancer: Comparison of Safety, Biodistribution, and Dosimetry. J. Nucl. Med. 2022, 63, 1199–1207. [Google Scholar] [CrossRef] [PubMed]
- Heynickx, N.; Herrmann, K.; Vermeulen, K.; Baatout, S.; Aerts, A. The salivary glands as a dose limiting organ of PSMA- targeted radionuclide therapy: A review of the lessons learnt so far. Nucl. Med. Biol. 2021, 98–99, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Emmett, L.; Willowson, K.; Violet, J.; Shin, J.; Blanksby, A.; Lee, J. Lutetium 177 PSMA radionuclide therapy for men with prostate cancer: A review of the current literature and discussion of practical aspects of therapy. J. Med. Radiat. Sci. 2017, 64, 52–60. [Google Scholar] [CrossRef]
- Gallyamov, M.; Meyrick, D.; Barley, J.; Lenzo, N. Renal outcomes of radioligand therapy: Experience of 177lutetium—Prostate-specific membrane antigen ligand therapy in metastatic castrate-resistant prostate cancer. Clin. Kidney J. 2020, 13, 1049–1055. [Google Scholar] [CrossRef]
- Peters, S.M.B.; Prive, B.M.; de Bakker, M.; de Lange, F.; Jentzen, W.; Eek, A.; Muselaers, C.H.J.; Mehra, N.; Witjes, J.A.; Gotthardt, M.; et al. Intra-therapeutic dosimetry of [(177)Lu]Lu-PSMA-617 in low-volume hormone-sensitive metastatic prostate cancer patients and correlation with treatment outcome. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 460–469. [Google Scholar] [CrossRef]
- Fendler, W.P.; Herrmann, K.; Eiber, M. Nuclear Medicine Beyond VISION. J. Nucl. Med. 2021, 62, 916–917. [Google Scholar] [CrossRef] [PubMed]
- Ahmadzadehfar, H.; Rahbar, K.; Baum, R.P.; Seifert, R.; Kessel, K.; Bögemann, M.; Kulkarni, H.R.; Zhang, J.; Gerke, C.; Fimmers, R.; et al. Prior therapies as prognostic factors of overall survival in metastatic castration-resistant prostate cancer patients treated with [177 Lu]Lu-PSMA-617. A WARMTH multicenter study (the 617 trial). Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 113–122. [Google Scholar] [CrossRef]
- Satapathy, S.; Mittal, B.R.; Sood, A.; Das, C.K.; Mavuduru, R.S.; Goyal, S.; Shukla, J.; Singh, S.K. 177Lu-PSMA-617 versus docetaxel in chemotherapy-naïve metastatic castration-resistant prostate cancer: A randomized, controlled, phase 2 non-inferiority trial. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 1754–1764. [Google Scholar] [CrossRef]
- Khreish, F.; Ghazal, Z.; Marlowe, R.J.; Rosar, F.; Sabet, A.; Maus, S.; Linxweiler, J.; Bartholomä, M.; Ezziddin, S. 177Lu-PSMA-617 radioligand therapy of metastatic castration-resistant prostate cancer: Initial 254-patient results from a prospective registry (REALITY Study). Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 1075–1085. [Google Scholar] [CrossRef]
- Kind, F.; Fassbender, T.F.; Andrieux, G.; Boerries, M.; Meyer, P.T.; Ruf, J. Early PSA Change after [(177)Lu]PSMA-617 Radioligand Therapy as a Predicator of Biochemical Response and Overall Survival. Cancers 2021, 14, 149. [Google Scholar] [CrossRef]
- Zippel, C.; Giesel, F.L.; Kratochwil, C.; Eiber, M.; Rahbar, K.; Albers, P.; Maurer, T.; Krause, B.J.; Bohnet-Joschko, S. PSMA radioligand therapy could pose infrastructural challenges for nuclear medicine: Results of a basic calculation for the capacity planning of nuclear medicine beds in the German hospital sector. Nuklearmedizin 2021, 60, 216–223. [Google Scholar] [CrossRef] [PubMed]
- Uijen, M.J.M.; Derks, Y.H.W.; Merkx, R.I.J.; Schilham, M.G.M.; Roosen, J.; Privé, B.M.; van Lith, S.A.M.; van Herpen, C.M.L.; Gotthardt, M.; Heskamp, S.; et al. PSMA radioligand therapy for solid tumors other than prostate cancer: Background, opportunities, challenges, and first clinical reports. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 4350–4368. [Google Scholar] [CrossRef] [PubMed]
- Schlenkhoff, C.D.; Knüpfer, E.; Essler, M.; Ahmadzadehfar, H. Metastatic Prostate Cancer With Restored Hormone-Response After Radioligand Therapy With 177Lu-PSMA-617. Clin. Nucl. Med. 2016, 41, 572–573. [Google Scholar] [CrossRef]
- Feuerecker, B.; Tauber, R.; Knorr, K.; Heck, M.; Beheshti, A.; Seidl, C.; Bruchertseifer, F.; Pickhard, A.; Gafita, A.; Kratochwil, C.; et al. Activity and Adverse Events of Actinium-225-PSMA-617 in Advanced Metastatic Castration-resistant Prostate Cancer After Failure of Lutetium-177-PSMA. Eur. Urol. 2021, 79, 343–350. [Google Scholar] [CrossRef] [PubMed]
| Organ | Absorbed Dose (Gy/GBq) Mean (±SD) n = 29 | Calculated Absorbed Dose for 6 × 7.4 GBq (Cumulated Activity 44.4 GBq) (Gy) Mean (±SD) |
|---|---|---|
| Lacrimal glands | 2.100 (±0.470) | 92 (±21) |
| Salivary glands | 0.630 (±0.360) | 28 (±16) |
| Left colon | 0.580 (±0.140) | 26 (±6.0) |
| Right colon | 0.320 (±0.078) | 14 (±3.4) |
| Kidneys | 0.430 (±0.160) | 19 (±7.3) |
| Liver | 0.090 (±0.044) | 4.0 (±2.0) |
| Red marrow | 0.040 (±0.020) | 1.5 (±0.9) |
| Spleen | 0.067 (±0.027) | 3.0 (±1.2) |
| Thyroid | 0.260 (±0.370) | 11 (±16) |
| Prostate | 0.027 (±0.026) | 1.2 (±1.1) |
| Testes | 0.023 (±0.025) | 1.0 (±1.1) |
| Rectum | 0.560 (±0.140) | 25 (±6.2) |
| Urinary bladder wall | 0.320 (±0.025) | 14 (±1.1) |
| Total body | 0.037 (±0.027) | 1.6 (±1.2) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hennrich, U.; Eder, M. [177Lu]Lu-PSMA-617 (PluvictoTM): The First FDA-Approved Radiotherapeutical for Treatment of Prostate Cancer. Pharmaceuticals 2022, 15, 1292. https://doi.org/10.3390/ph15101292
Hennrich U, Eder M. [177Lu]Lu-PSMA-617 (PluvictoTM): The First FDA-Approved Radiotherapeutical for Treatment of Prostate Cancer. Pharmaceuticals. 2022; 15(10):1292. https://doi.org/10.3390/ph15101292
Chicago/Turabian StyleHennrich, Ute, and Matthias Eder. 2022. "[177Lu]Lu-PSMA-617 (PluvictoTM): The First FDA-Approved Radiotherapeutical for Treatment of Prostate Cancer" Pharmaceuticals 15, no. 10: 1292. https://doi.org/10.3390/ph15101292
APA StyleHennrich, U., & Eder, M. (2022). [177Lu]Lu-PSMA-617 (PluvictoTM): The First FDA-Approved Radiotherapeutical for Treatment of Prostate Cancer. Pharmaceuticals, 15(10), 1292. https://doi.org/10.3390/ph15101292