4D Printing of Hydrogels: Innovation in Material Design and Emerging Smart Systems for Drug Delivery
Abstract
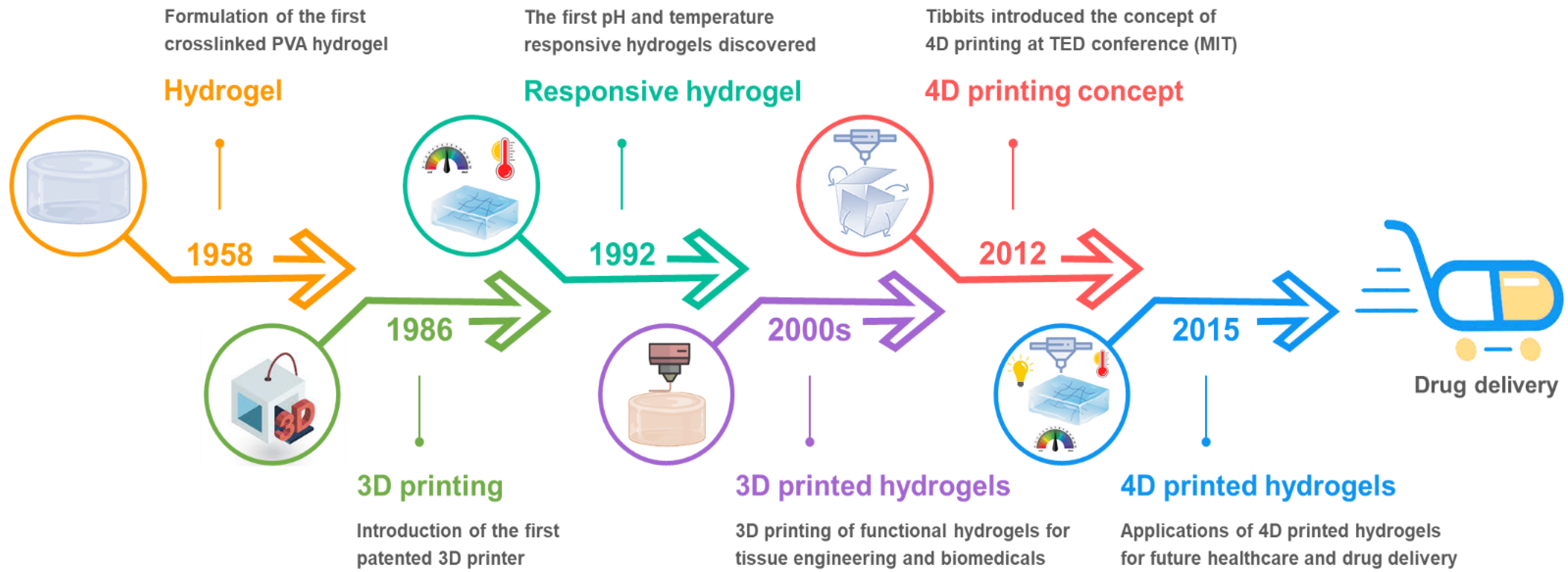
1. Introduction
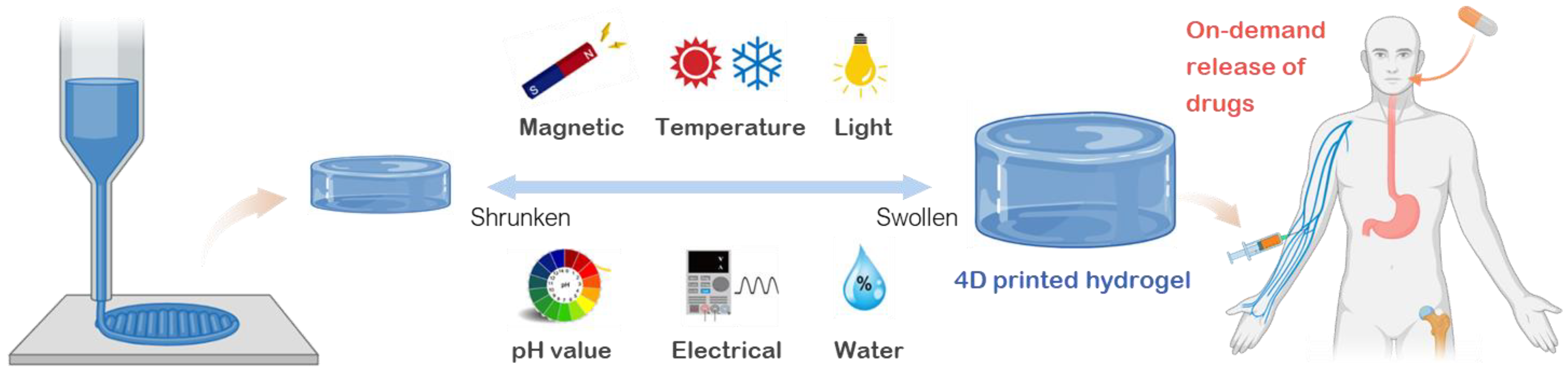
2. Material Design: Development of Smart Hydrogels for Drug Delivery
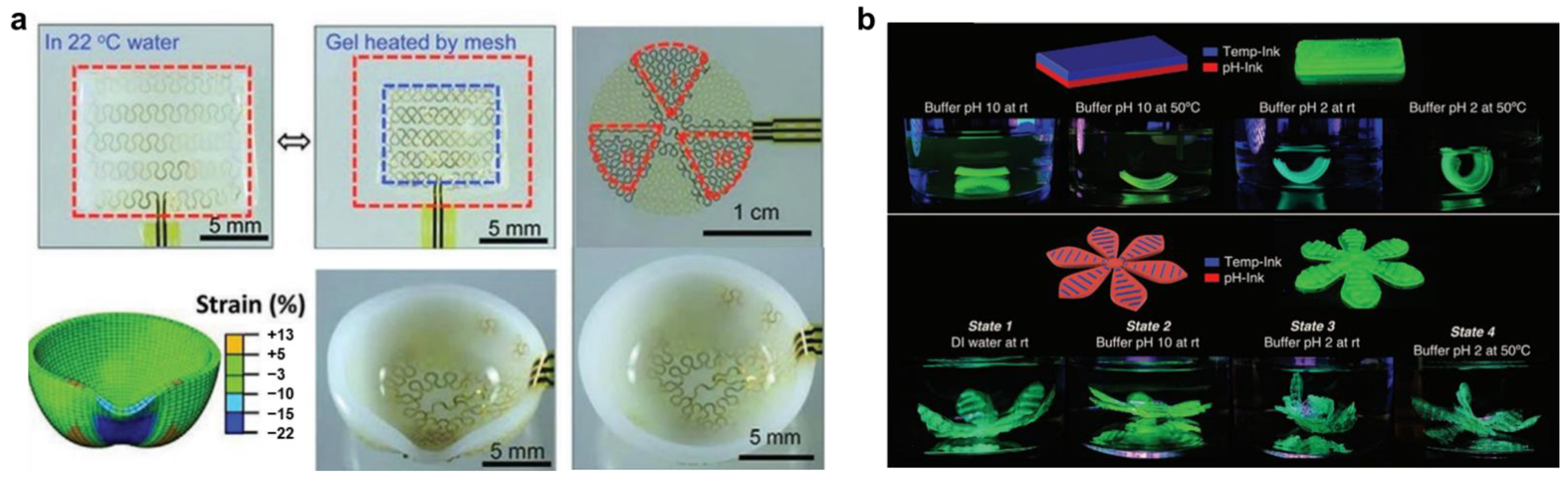
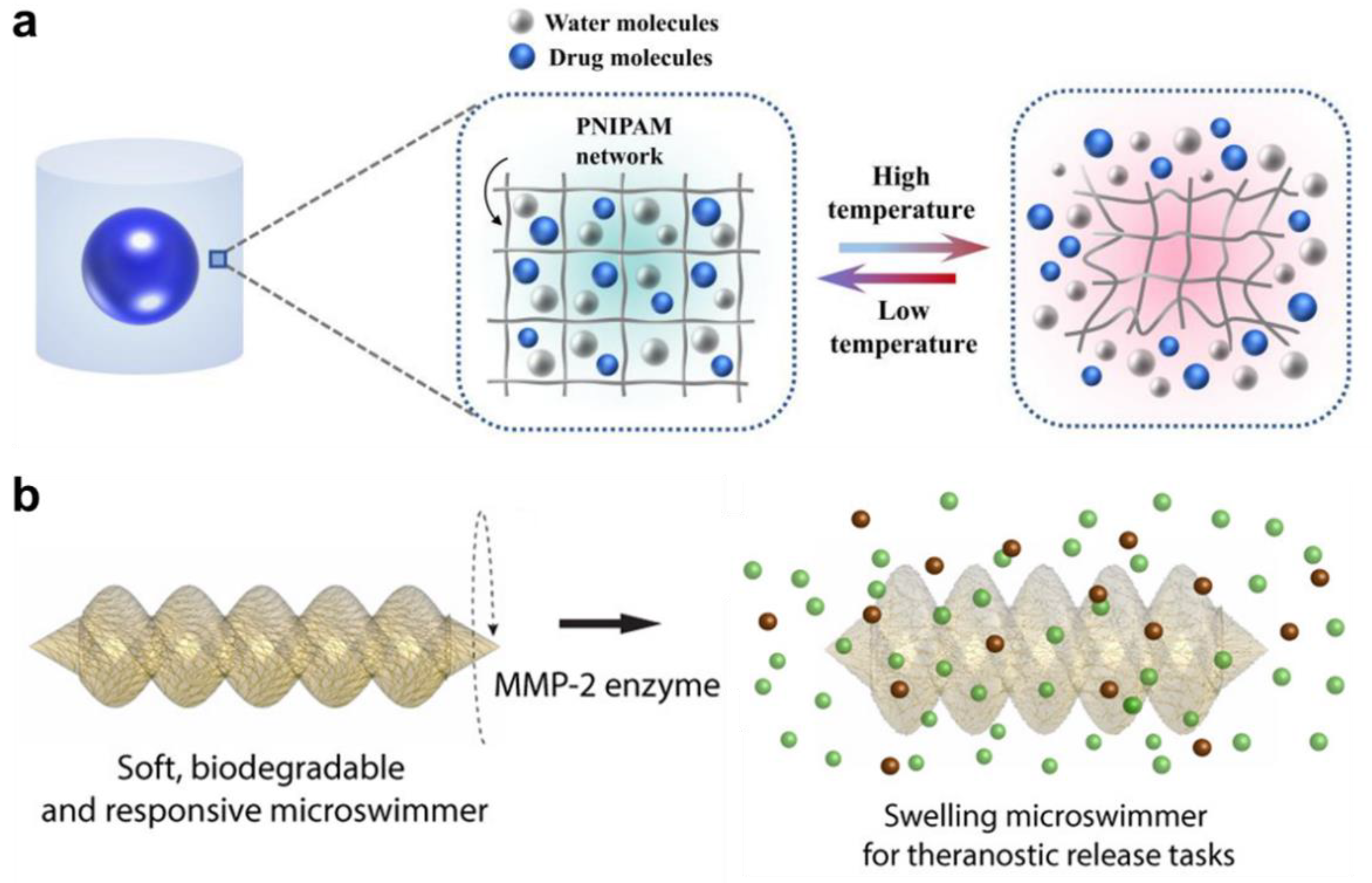
2.1. Thermo-Responsive Hydrogels
2.2. Magnetic Responsive Hydrogels
2.3. Electrical Responsive Hydrogels
2.4. Photo-Responsive Hydrogels
2.5. pH Responsive Hydrogels
2.6. Water Responsive Hydrogels
3. Technical Approaches toward 4D Printing of Hydrogels
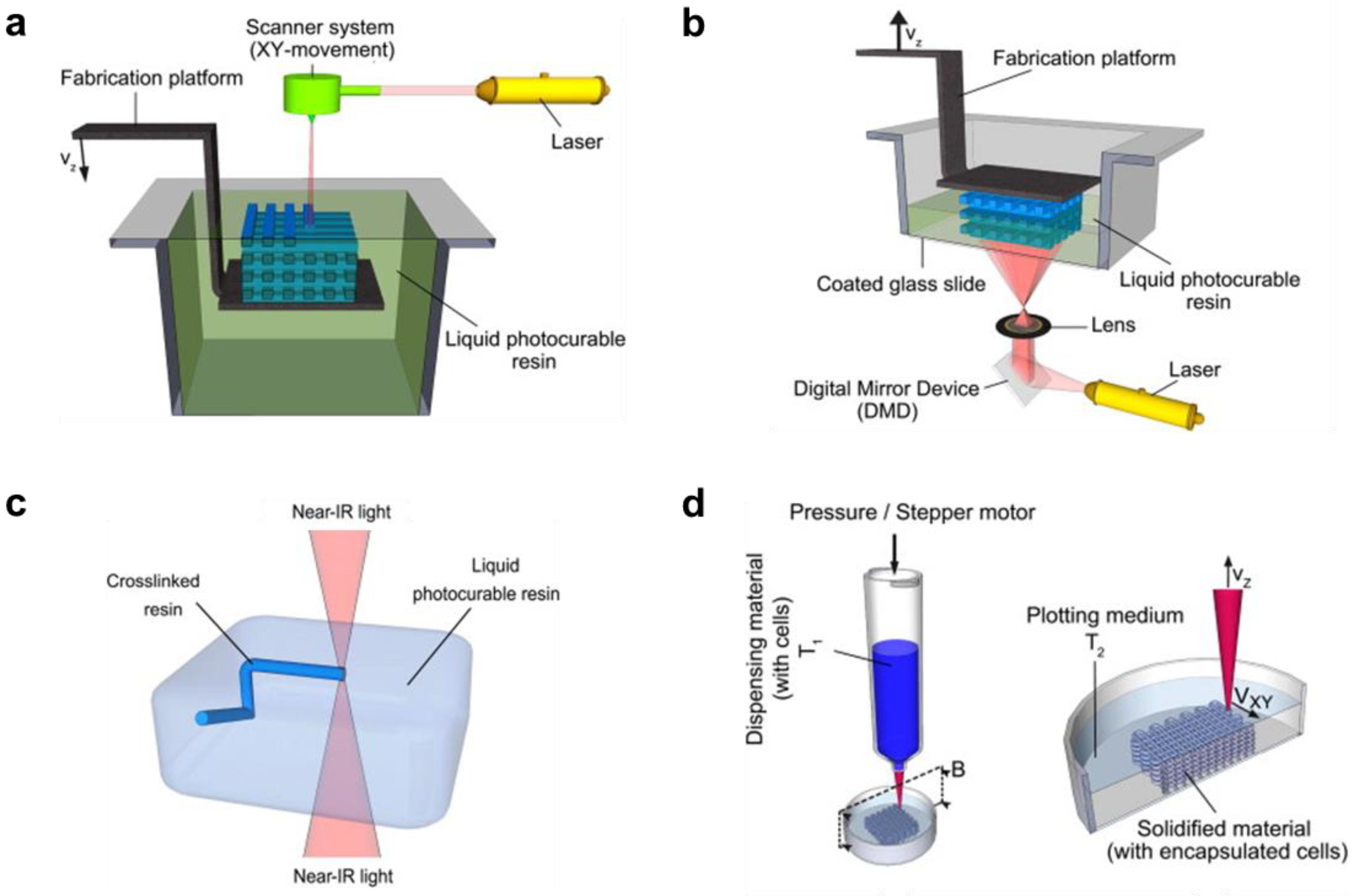
3.1. Printing Techniques for Smart Hydrogels
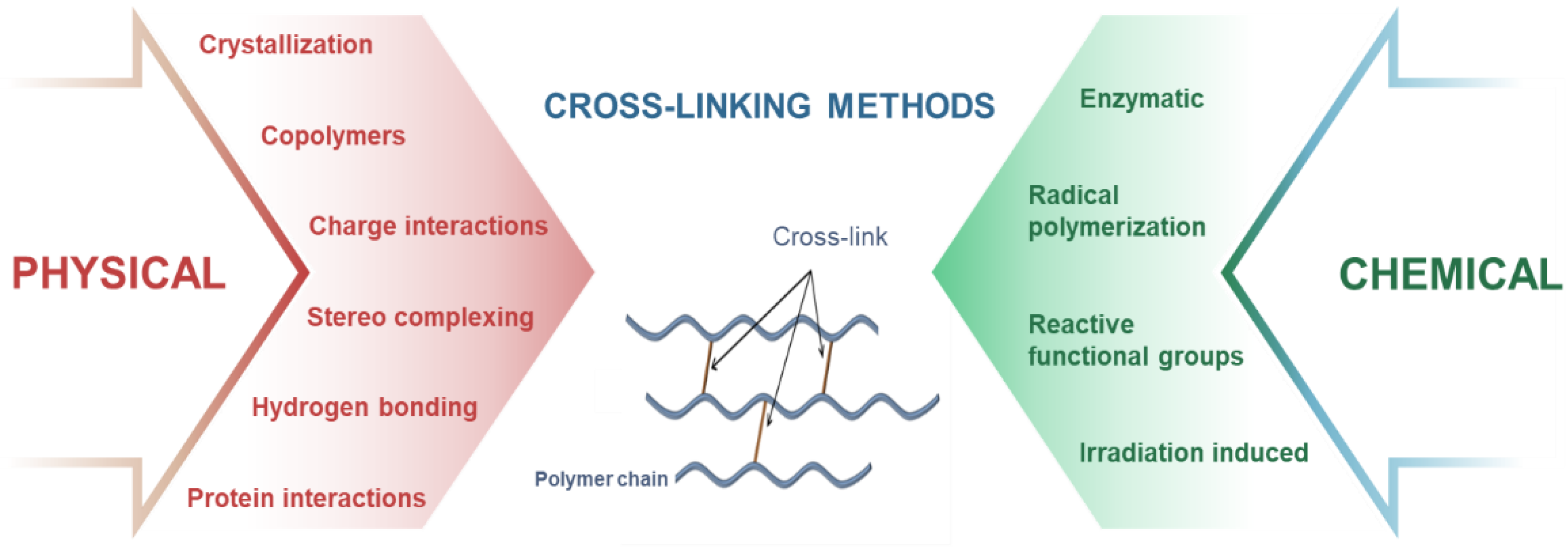
3.2. Crosslinking Strategies for the Fabricated 4D Printed Hydrogels
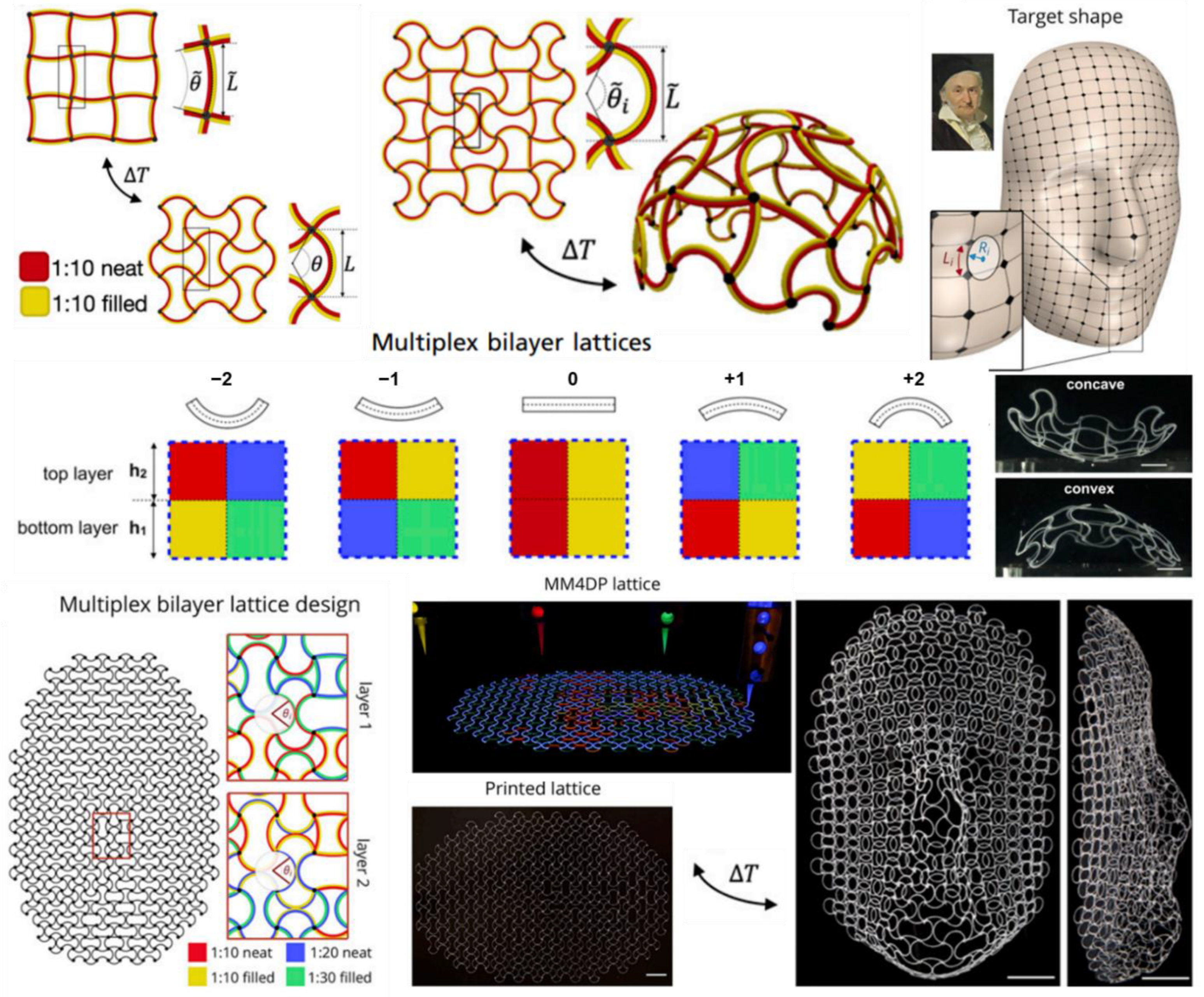
3.3. Design Considerations for 4D Printing
3.4. Multi-Materials Extrusion 4D Printing
4. Emerging 4D Printed Hydrogels for Drug Delivery
5. Conclusions and Future Perspective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ullah, F.; Othman, M.B.H.; Javed, F.; Ahmad, Z.; Akil, H.M. Classification, processing and application of hydrogels: A review. Mater. Sci. Eng. C 2015, 57, 414–433. [Google Scholar] [CrossRef]
- Shi, Q.; Liu, H.; Tang, D.; Li, Y.; Li, X.; Xu, F. Bioactuators based on stimulus-responsive hydrogels and their emerging biomedical applications. NPG Asia Mater. 2019, 11, 64. [Google Scholar] [CrossRef]
- El-Husseiny, H.M.; Mady, E.A.; Hamabe, L.; Abugomaa, A.; Shimada, K.; Yoshida, T.; Tanaka, T.; Yokoi, A.; Elbadawy, M.; Tanaka, R. Smart/stimuli-responsive hydrogels: Cutting-edge platforms for tissue engineering and other biomedical applications. Mater. Today Bio 2022, 13, 100186. [Google Scholar] [PubMed]
- Chua, C.K.; Leong, K.F. 3D Printing and Additive Manufacturing: Principles and Applications (with Companion Media Pack)-of Rapid Prototyping; World Scientific Publishing Company: Singapore, 2014. [Google Scholar]
- You, S.; Li, J.; Zhu, W.; Yu, C.; Mei, D.; Chen, S. Nanoscale 3D printing of hydrogels for cellular tissue engineering. J. Mater. Chem. B 2018, 6, 2187–2197. [Google Scholar] [CrossRef]
- He, Y.; Yang, F.; Zhao, H.; Gao, Q.; Xia, B.; Fu, J. Research on the printability of hydrogels in 3D bioprinting. Sci. Rep. 2016, 6, 29977. [Google Scholar] [CrossRef] [PubMed]
- Rivera-Tarazona, L.K.; Campbell, Z.T.; Ware, T.H. Stimuli-responsive engineered living materials. Soft Matter 2021, 17, 785–809. [Google Scholar]
- Mao, Y.; Ding, Z.; Yuan, C.; Ai, S.; Isakov, M.; Wu, J.; Wang, T.; Dunn, M.L.; Qi, H.J. 3D printed reversible shape changing components with stimuli responsive materials. Sci. Rep. 2016, 6, 24761. [Google Scholar] [CrossRef] [PubMed]
- Boydston, A.; Cao, B.; Nelson, A.; Ono, R.; Saha, A.; Schwartz, J.; Thrasher, C. Additive manufacturing with stimuli-responsive materials. J. Mater. Chem. A 2018, 6, 20621–20645. [Google Scholar] [CrossRef]
- Tibbits, S. 4D printing: Multi-material shape change. Archit. Des. 2014, 84, 116–121. [Google Scholar] [CrossRef]
- Champeau, M.; Heinze, D.A.; Viana, T.N.; de Souza, E.R.; Chinellato, A.C.; Titotto, S. 4D printing of hydrogels: A review. Adv. Funct. Mater. 2020, 30, 1910606. [Google Scholar] [CrossRef]
- Peppas, N.A.; Merrill, E.W. Crosslinked poly (vinyl alcohol) hydrogels as swollen elastic networks. J. Appl. Polym. Sci. 1977, 21, 1763–1770. [Google Scholar] [CrossRef]
- Li, J.; Mooney, D.J. Designing hydrogels for controlled drug delivery. Nat. Rev. Mater. 2016, 1, 16071. [Google Scholar] [PubMed]
- Andersson, M.; Axelsson, A.; Zacchi, G. Swelling kinetics of poly (N-isopropylacrylamide) gel. J. Control. Release 1998, 50, 273–281. [Google Scholar] [CrossRef]
- Bischofberger, I.; Trappe, V. New aspects in the phase behaviour of poly-N-isopropyl acrylamide: Systematic temperature dependent shrinking of PNiPAM assemblies well beyond the LCST. Sci. Rep. 2015, 5, 15520. [Google Scholar] [CrossRef]
- Li, Z.; Shen, J.; Ma, H.; Lu, X.; Shi, M.; Li, N.; Ye, M. Preparation and characterization of pH-and temperature-responsive nanocomposite double network hydrogels. Mater. Sci. Eng. C 2013, 33, 1951–1957. [Google Scholar] [CrossRef]
- Schild, H.G. Poly (N-isopropylacrylamide): Experiment, theory and application. Prog. Polym. Sci. 1992, 17, 163–249. [Google Scholar]
- Shimizu, T.; Yamato, M.; Kikuchi, A.; Okano, T. Cell sheet engineering for myocardial tissue reconstruction. Biomaterials 2003, 24, 2309–2316. [Google Scholar] [CrossRef]
- Cheng, Y.; Ren, K.; Yang, D.; Wei, J. Bilayer-type fluorescence hydrogels with intelligent response serve as temperature/pH driven soft actuators. Sens. Actuators B Chem. 2018, 255, 3117–3126. [Google Scholar] [CrossRef]
- Allen, A.C.; Barone, E.; Cody, O.; Crosby, K.; Suggs, L.J.; Zoldan, J. Electrospun poly (N-isopropyl acrylamide)/poly (caprolactone) fibers for the generation of anisotropic cell sheets. Biomater. Sci. 2017, 5, 1661–1669. [Google Scholar] [CrossRef]
- Porsch, C.; Hansson, S.; Nordgren, N.; Malmström, E. Thermo-responsive cellulose-based architectures: Tailoring LCST using poly (ethylene glycol) methacrylates. Polym. Chem. 2011, 2, 1114–1123. [Google Scholar] [CrossRef]
- Ma, L.; Tang, H.; Wu, P. Volume Phase Transition Mechanism of Poly [di (ethylene glycol) ethyl ether acrylate]-Based Microgels Involving a Thermosensitive Poly (ionic liquid). Langmuir 2017, 33, 12326–12335. [Google Scholar] [CrossRef] [PubMed]
- Katono, H.; Maruyama, A.; Sanui, K.; Ogata, N.; Okano, T.; Sakurai, Y. Thermo-responsive swelling and drug release switching of interpenetrating polymer networks composed of poly (acrylamide-co-butyl methacrylate) and poly (acrylic acid). J. Control. Release 1991, 16, 215–227. [Google Scholar] [CrossRef]
- Koetting, M.C.; Peters, J.T.; Steichen, S.D.; Peppas, N.A. Stimulus-responsive hydrogels: Theory, modern advances, and applications. Mater. Sci. Eng. R Rep. 2015, 93, 1–49. [Google Scholar] [PubMed]
- Dutta, N.K.; Truong, M.Y.; Mayavan, S.; Roy Choudhury, N.; Elvin, C.M.; Kim, M.; Knott, R.; Nairn, K.M.; Hill, A.J. A genetically engineered protein responsive to multiple stimuli. Angew. Chem. 2011, 123, 4520–4523. [Google Scholar] [CrossRef]
- Quiroz, F.G.; Chilkoti, A. Sequence heuristics to encode phase behaviour in intrinsically disordered protein polymers. Nat. Mater. 2015, 14, 1164–1171. [Google Scholar] [CrossRef]
- Balu, R.; Dutta, N.K.; Dutta, A.K.; Choudhury, N.R. Resilin-mimetics as a smart biomaterial platform for biomedical applications. Nat. Commun. 2021, 12, 149. [Google Scholar] [CrossRef] [PubMed]
- Balu, R.; Dutta, N.K.; Choudhury, N.R. Resilin-mimetic Polypeptides and Elastomeric Modular Protein Polymers: Amino Acid Sequence, Conformational Ensemble, and Stimuli Responsiveness. In Biomimetic Protein-Based Elastomers: Emerging Materials for the Future; Royal Society of Chemistry: London, UK, 2022; Volume 10, p. 108. [Google Scholar]
- Choudhury, N.R.; Liu, J.C.; Dutta, N.K. Biomimetic Protein-Based Elastomers: Emerging Materials for the Future; Royal Society of Chemistry: London, UK, 2022. [Google Scholar]
- Ye, H.; Owh, C.; Loh, X.J. A thixotropic polyglycerol sebacate-based supramolecular hydrogel showing UCST behavior. Rsc Adv. 2015, 5, 48720–48728. [Google Scholar] [CrossRef]
- Siegwart, D.J.; Bencherif, S.A.; Srinivasan, A.; Hollinger, J.O.; Matyjaszewski, K. Synthesis, characterization, and in vitro cell culture viability of degradable poly (N-isopropylacrylamide-co-5, 6-benzo-2-methylene-1, 3-dioxepane)-based polymers and crosslinked gels. J. Biomed. Mater. Res. Part A Off. J. Soc. Biomater. Jpn. Soc. Biomater. Aust. Soc. Biomater. Korean Soc. Biomater. 2008, 87, 345–358. [Google Scholar]
- Nelson, D.M.; Ma, Z.; Leeson, C.E.; Wagner, W.R. Extended and sequential delivery of protein from injectable thermoresponsive hydrogels. J. Biomed. Mater. Res. Part A 2012, 100, 776–785. [Google Scholar] [CrossRef]
- Kang, E.Y.; Moon, H.J.; Joo, M.K.; Jeong, B. Thermogelling chitosan-g-(PAF-PEG) aqueous solution as an injectable scaffold. Biomacromolecules 2012, 13, 1750–1757. [Google Scholar] [CrossRef]
- Cheng, Y.-H.; Yang, S.-H.; Lin, F.-H. Thermosensitive chitosan-gelatin-glycerol phosphate hydrogel as a controlled release system of ferulic acid for nucleus pulposus regeneration. Biomaterials 2011, 32, 6953–6961. [Google Scholar] [CrossRef] [PubMed]
- Ding, M.; Jing, L.; Yang, H.; Machnicki, C.; Fu, X.; Li, K.; Wong, I.; Chen, P.-Y. Multifunctional soft machines based on stimuli-responsive hydrogels: From freestanding hydrogels to smart integrated systems. Mater. Today Adv. 2020, 8, 100088. [Google Scholar] [CrossRef]
- Shankar, A.; Safronov, A.P.; Mikhnevich, E.A.; Beketov, I.V. Multidomain iron nanoparticles for the preparation of polyacrylamide ferrogels. J. Magn. Magn. Mater. 2017, 431, 134–137. [Google Scholar] [CrossRef]
- Zrínyi, M.; Barsi, L.; Büki, A. Deformation of ferrogels induced by nonuniform magnetic fields. J. Chem. Phys. 1996, 104, 8750–8756. [Google Scholar] [CrossRef]
- Messing, R.; Frickel, N.; Belkoura, L.; Strey, R.; Rahn, H.; Odenbach, S.; Schmidt, A.M. Cobalt ferrite nanoparticles as multifunctional crosslinkers in PAAm ferrohydrogels. Macromolecules 2011, 44, 2990–2999. [Google Scholar] [CrossRef]
- Ghadban, A.; Ahmed, A.S.; Ping, Y.; Ramos, R.; Arfin, N.; Cantaert, B.; Ramanujan, R.V.; Miserez, A. Bioinspired pH and magnetic responsive catechol-functionalized chitosan hydrogels with tunable elastic properties. Chem. Commun. 2016, 52, 697–700. [Google Scholar] [CrossRef]
- Li, Z.; Li, Y.; Chen, C.; Cheng, Y. Magnetic-responsive hydrogels: From strategic design to biomedical applications. J. Control. Release 2021, 335, 541–556. [Google Scholar] [CrossRef]
- Liao, J.; Huang, H. Review on magnetic natural polymer constructed hydrogels as vehicles for drug delivery. Biomacromolecules 2020, 21, 2574–2594. [Google Scholar] [CrossRef]
- Barbucci, R.; Pasqui, D.; Giani, G.; De Cagna, M.; Fini, M.; Giardino, R.; Atrei, A. A novel strategy for engineering hydrogels with ferromagnetic nanoparticles as crosslinkers of the polymer chains. Potential applications as a targeted drug delivery system. Soft Matter 2011, 7, 5558–5565. [Google Scholar] [CrossRef]
- Roeder, L.; Reckenthaler, M.; Belkoura, L.; Roitsch, S.; Strey, R.; Schmidt, A. Covalent ferrohydrogels based on elongated particulate crosslinkers. Macromolecules 2014, 47, 7200–7207. [Google Scholar] [CrossRef]
- Mañas-Torres, M.C.; Gila-Vilchez, C.; Vazquez-Perez, F.J.; Kuzhir, P.; Momier, D.; Scimeca, J.-C.; Borderie, A.; Goracci, M.; Burel-Vandenbos, F.; Blanco-Elices, C. Injectable Magnetic-Responsive Short-Peptide Supramolecular Hydrogels: Ex Vivo and In Vivo Evaluation. ACS Appl. Mater. Interfaces 2021, 13, 49692–49704. [Google Scholar] [CrossRef] [PubMed]
- Xie, W.; Gao, Q.; Guo, Z.; Wang, D.; Gao, F.; Wang, X.; Wei, Y.; Zhao, L. Injectable and self-healing thermosensitive magnetic hydrogel for asynchronous control release of doxorubicin and docetaxel to treat triple-negative breast cancer. ACS Appl. Mater. Interfaces 2017, 9, 33660–33673. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, K.; Sakikawa, N.; Miyata, T. Thermo-responsive gels that absorb moisture and ooze water. Nat. Commun. 2018, 9, 2315. [Google Scholar] [CrossRef] [PubMed]
- Merino, S.; Martin, C.; Kostarelos, K.; Prato, M.; Vazquez, E. Nanocomposite hydrogels: 3D polymer–nanoparticle synergies for on-demand drug delivery. ACS Nano 2015, 9, 4686–4697. [Google Scholar] [CrossRef]
- Li, L.; Scheiger, J.M.; Levkin, P.A. Design and applications of photoresponsive hydrogels. Adv. Mater. 2019, 31, 1807333. [Google Scholar] [CrossRef]
- Zixuan, C.; Bin, Z.; Liyang, J.; Yunyi, L.; Guohe, X.; Jingjun, M. Intelligent-Responsive Hydrogels-Based Controlled Drug Release Systems and Its Applications. Prog. Chem. 2019, 31, 1653. [Google Scholar]
- Lee, H.; Wong, H.; Buenfeld, N. Self-sealing of cracks in concrete using superabsorbent polymers. Cem. Concr. Res. 2016, 79, 194–208. [Google Scholar] [CrossRef]
- Sun, S.; Mak, A.F. The dynamical response of a hydrogel fiber to electrochemical stimulation. J. Polym. Sci. Part B Polym. Phys. 2001, 39, 236–246. [Google Scholar] [CrossRef]
- Hirai, T.; Nemoto, H.; Hirai, M.; Hayashi, S. Electrostriction of highly swollen polymer gel: Possible application for gel actuator. J. Appl. Polym. Sci. 1994, 53, 79–84. [Google Scholar] [CrossRef]
- Kim, S.Y.; Lee, Y.M. Drug release behavior of electrical responsive poly (vinyl alcohol)/poly (acrylic acid) IPN hydrogels under an electric stimulus. J. Appl. Polym. Sci. 1999, 74, 1752–1761. [Google Scholar] [CrossRef]
- Guo, B.; Finne-Wistrand, A.; Albertsson, A.-C. Degradable and electroactive hydrogels with tunable electrical conductivity and swelling behavior. Chem. Mater. 2011, 23, 1254–1262. [Google Scholar] [CrossRef]
- Shiga, T.; Hirose, Y.; Okada, A.; Kurauchi, T. Bending of poly (vinyl alcohol)–poly (sodium acrylate) composite hydrogel in electric fields. J. Appl. Polym. Sci. 1992, 44, 249–253. [Google Scholar] [CrossRef]
- Wanasingha, N.; Dorishetty, P.; Dutta, N.K.; Choudhury, N.R. Polyelectrolyte gels: Fundamentals, fabrication and applications. Gels 2021, 7, 148. [Google Scholar] [CrossRef] [PubMed]
- Hirai, T.; Nemoto, H.; Suzuki, T.; Hayashi, S.; Hirai, M. Actuation of poly (vinyl alcohol) gel by electric field. J. Intell. Mater. Syst. Struct. 1993, 4, 277–279. [Google Scholar] [CrossRef]
- Gao, Y.; Xu, S.; Wu, R.; Wang, J.; Wei, J. Preparation and characteristic of electric stimuli responsive hydrogel composed of polyvinyl alcohol/poly (sodium maleate-co-sodium acrylate). J. Appl. Polym. Sci. 2008, 107, 391–395. [Google Scholar]
- Li, L.; Hsieh, Y.-L. Ultra-fine polyelectrolyte hydrogel fibres from poly (acrylic acid)/poly (vinyl alcohol). Nanotechnology 2005, 16, 2852. [Google Scholar] [CrossRef]
- Jin, S.; Gu, J.; Shi, Y.; Shao, K.; Yu, X.; Yue, G. Preparation and electrical sensitive behavior of poly (N-vinylpyrrolidone-co-acrylic acid) hydrogel with flexible chain nature. Eur. Polym. J. 2013, 49, 1871–1880. [Google Scholar]
- Yao, L.; Krause, S. Electromechanical responses of strong acid polymer gels in DC electric fields. Macromolecules 2003, 36, 2055–2065. [Google Scholar]
- Tang, Y.; Zhang, X.; Li, X.; Ma, C.; Chu, X.; Wang, L.; Xu, W. A review on recent advances of Protein-Polymer hydrogels. Eur. Polym. J. 2022, 162, 110881. [Google Scholar]
- Liu, J.; Ge, X.; Liu, L.; Xu, W.; Shao, R. Challenges and opportunities of silk protein hydrogels in biomedical applications. Mater. Adv. 2022, 3, 2291–2308. [Google Scholar]
- Zhang, Y.; Dong, L.; Liu, L.; Wu, Z.; Pan, D.; Liu, L. Recent Advances of Stimuli-Responsive Polysaccharide Hydrogels in Delivery Systems: A Review. J. Agric. Food Chem. 2022, 70, 6300–6316. [Google Scholar] [CrossRef] [PubMed]
- Franco García, M.L.; Valle Mendoza, L.J.d.; Puiggalí Bellalta, J. Smart systems related to polypeptide sequences. AIMS Mater. Sci. 2016, 3, 289–323. [Google Scholar] [CrossRef]
- Dorishetty, P.; Dutta, N.K.; Choudhury, N.R. Bioprintable tough hydrogels for tissue engineering applications. Adv. Colloid Interface Sci. 2020, 281, 102163. [Google Scholar] [CrossRef] [PubMed]
- Athukorala, S.S.; Tran, T.S.; Balu, R.; Truong, V.K.; Chapman, J.; Dutta, N.K.; Roy Choudhury, N. 3D printable electrically conductive hydrogel scaffolds for biomedical applications: A review. Polymers 2021, 13, 474. [Google Scholar] [CrossRef]
- Dorishetty, P.; Balu, R.; Athukoralalage, S.S.; Greaves, T.L.; Mata, J.; De Campo, L.; Saha, N.; Zannettino, A.C.; Dutta, N.K.; Choudhury, N.R. Tunable biomimetic hydrogels from silk fibroin and nanocellulose. ACS Sustain. Chem. Eng. 2020, 8, 2375–2389. [Google Scholar] [CrossRef]
- Rahimi, N.; Molin, D.G.; Cleij, T.J.; van Zandvoort, M.A.; Post, M.J. Electrosensitive polyacrylic acid/fibrin hydrogel facilitates cell seeding and alignment. Biomacromolecules 2012, 13, 1448–1457. [Google Scholar] [CrossRef]
- Sarmad, S.; Yenici, G.; Gürkan, K.; Keçeli, G.; Gürdağ, G. Electric field responsive chitosan–poly (N, N-dimethyl acrylamide) semi-IPN gel films and their dielectric, thermal and swelling characterization. Smart Mater. Struct. 2013, 22, 055010. [Google Scholar] [CrossRef]
- Kim, S.J.; Yoon, S.G.; Lee, Y.H.; Kim, S.I. Bending behavior of hydrogels composed of poly (methacrylic acid) and alginate by electrical stimulus. Polym. Int. 2004, 53, 1456–1460. [Google Scholar] [CrossRef]
- Wu, Y.; Wu, S.; Tian, X.; Wang, X.; Wu, W.; Zou, G.; Zhang, Q. Photoinduced reversible gel–sol transitions of dicholesterol-linked azobenzene derivatives through breaking and reforming of van der Waals interactions. Soft Matter 2011, 7, 716–721. [Google Scholar] [CrossRef]
- Kloxin, A.M.; Kasko, A.M.; Salinas, C.N.; Anseth, K.S. Photodegradable hydrogels for dynamic tuning of physical and chemical properties. Science 2009, 324, 59–63. [Google Scholar] [CrossRef]
- Deng, L.; Xu, Y.; Sun, C.; Yun, B.; Sun, Q.; Zhao, C.; Li, Z. Functionalization of small black phosphorus nanoparticles for targeted imaging and photothermal therapy of cancer. Sci. Bull. 2018, 63, 917–924. [Google Scholar] [CrossRef]
- Fujigaya, T.; Morimoto, T.; Niidome, Y.; Nakashima, N. NIR Laser-Driven Reversible Volume Phase Transition of Single-Walled Carbon Nanotube/Poly (N-isopropylacrylamide) Composite Gels. Adv. Mater. 2008, 20, 3610–3614. [Google Scholar]
- Sershen, S.R.; Mensing, G.A.; Ng, M.; Halas, N.J.; Beebe, D.J.; West, J.L. Independent optical control of microfluidic valves formed from optomechanically responsive nanocomposite hydrogels. Adv. Mater. 2005, 17, 1366–1368. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Yuan, Z.; Liu, J.; Fang, Z.; Fang, L.; Yu, D.; Li, Q. Photoresponsive Actuators Built from Carbon-Based Soft Materials. Adv. Opt. Mater. 2019, 7, 1900069. [Google Scholar] [CrossRef]
- Shiotani, A.; Mori, T.; Niidome, T.; Niidome, Y.; Katayama, Y. Stable incorporation of gold nanorods into N-isopropylacrylamide hydrogels and their rapid shrinkage induced by near-infrared laser irradiation. Langmuir 2007, 23, 4012–4018. [Google Scholar]
- Miyako, E.; Nagata, H.; Hirano, K.; Hirotsu, T. Photodynamic thermoresponsive nanocarbon–polymer gel hybrids. Small 2008, 4, 1711–1715. [Google Scholar] [CrossRef]
- Qian, X.; Zhao, Y.; Alsaid, Y.; Wang, X.; Hua, M.; Galy, T.; Gopalakrishna, H.; Yang, Y.; Cui, J.; Liu, N. Artificial phototropism for omnidirectional tracking and harvesting of light. Nat. Nanotechnol. 2019, 14, 1048–1055. [Google Scholar] [CrossRef]
- Gupta, P.; Vermani, K.; Garg, S. Hydrogels: From controlled release to pH-responsive drug delivery. Drug Discov. Today 2002, 7, 569–579. [Google Scholar]
- Schmaljohann, D. Thermo-and pH-responsive polymers in drug delivery. Adv. Drug Deliv. Rev. 2006, 58, 1655–1670. [Google Scholar] [CrossRef]
- Brannon-Peppas, L.; Peppas, N.A. Solute and penetrant diffusion in swellable polymers. IX. The mechanisms of drug release from pH-sensitive swelling-controlled systems. J. Control. Release 1989, 8, 267–274. [Google Scholar]
- De, S.K.; Aluru, N.; Johnson, B.; Crone, W.; Beebe, D.J.; Moore, J. Equilibrium swelling and kinetics of pH-responsive hydrogels: Models, experiments, and simulations. J. Microelectromech. Syst. 2002, 11, 544–555. [Google Scholar] [CrossRef]
- Huang, Y.; Yu, H.; Xiao, C. pH-sensitive cationic guar gum/poly (acrylic acid) polyelectrolyte hydrogels: Swelling and in vitro drug release. Carbohydr. Polym. 2007, 69, 774–783. [Google Scholar] [CrossRef]
- Kamei, N.; Morishita, M.; Chiba, H.; Kavimandan, N.J.; Peppas, N.A.; Takayama, K. Complexation hydrogels for intestinal delivery of interferon β and calcitonin. J. Control. Release 2009, 134, 98–102. [Google Scholar] [CrossRef] [PubMed]
- Khan, H.; Chaudhary, J.P.; Meena, R. Anionic carboxymethylagarose-based pH-responsive smart superabsorbent hydrogels for controlled release of anticancer drug. Int. J. Biol. Macromol. 2019, 124, 1220–1229. [Google Scholar] [CrossRef]
- Zhang, J.; Chu, L.-Y.; Li, Y.-K.; Lee, Y.M. Dual thermo-and pH-sensitive poly (N-isopropylacrylamide-co-acrylic acid) hydrogels with rapid response behaviors. Polymer 2007, 48, 1718–1728. [Google Scholar] [CrossRef]
- Turan, E.; Caykara, T. Swelling and network parameters of pH-sensitive poly (acrylamide-co-acrylic acid) hydrogels. J. Appl. Polym. Sci. 2007, 106, 2000–2007. [Google Scholar] [CrossRef]
- Yanfeng, C.; Min, Y. Swelling kinetics and stimuli-responsiveness of poly (DMAEMA) hydrogels prepared by UV-irradiation. Radiat. Phys. Chem. 2001, 61, 65–68. [Google Scholar] [CrossRef]
- Wu, W.; Liu, J.; Cao, S.; Tan, H.; Li, J.; Xu, F.; Zhang, X. Drug release behaviors of a pH sensitive semi-interpenetrating polymer network hydrogel composed of poly (vinyl alcohol) and star poly [2-(dimethylamino) ethyl methacrylate]. Int. J. Pharm. 2011, 416, 104–109. [Google Scholar] [CrossRef]
- Xu, F.-J.; Kang, E.-T.; Neoh, K.-G. pH-and temperature-responsive hydrogels from crosslinked triblock copolymers prepared via consecutive atom transfer radical polymerizations. Biomaterials 2006, 27, 2787–2797. [Google Scholar] [CrossRef]
- Abd El-Ghaffar, M.; Hashem, M.; El-Awady, M.; Rabie, A. pH-sensitive sodium alginate hydrogels for riboflavin controlled release. Carbohydr. Polym. 2012, 89, 667–675. [Google Scholar] [CrossRef]
- Yoon, S.; Chen, B. Elastomeric and pH-responsive hydrogels based on direct crosslinking of the poly (glycerol sebacate) pre-polymer and gelatin. Polym. Chem. 2018, 9, 3727–3740. [Google Scholar] [CrossRef]
- Che, Y.; Li, D.; Liu, Y.; Ma, Q.; Tan, Y.; Yue, Q.; Meng, F. Physically crosslinked pH-responsive chitosan-based hydrogels with enhanced mechanical performance for controlled drug delivery. RSC Adv. 2016, 6, 106035–106045. [Google Scholar] [CrossRef]
- Raja, S.; Thiruselvi, T.; Mandal, A.B.; Gnanamani, A. pH and redox sensitive albumin hydrogel: A self-derived biomaterial. Sci. Rep. 2015, 5, 15977. [Google Scholar] [CrossRef] [PubMed]
- Balu, R.; Reeder, S.; Knott, R.; Mata, J.; de Campo, L.; Dutta, N.K.; Choudhury, N.R. Tough photocrosslinked silk fibroin/graphene oxide nanocomposite hydrogels. Langmuir 2018, 34, 9238–9251. [Google Scholar] [CrossRef]
- Dorishetty, P.; Balu, R.; Gelmi, A.; Mata, J.P.; Dutta, N.K.; Choudhury, N.R. 3D printable soy/silk hybrid hydrogels for tissue engineering applications. Biomacromolecules 2021, 22, 3668–3678. [Google Scholar] [CrossRef]
- Bashari, A.; Rouhani Shirvan, A.; Shakeri, M. Cellulose-based hydrogels for personal care products. Polym. Adv. Technol. 2018, 29, 2853–2867. [Google Scholar]
- Cipriano, B.H.; Banik, S.J.; Sharma, R.; Rumore, D.; Hwang, W.; Briber, R.M.; Raghavan, S.R. Superabsorbent hydrogels that are robust and highly stretchable. Macromolecules 2014, 47, 4445–4452. [Google Scholar] [CrossRef]
- Sharma, K.; Kumar, V.; Chaudhary, B.; Kaith, B.; Kalia, S.; Swart, H. Application of biodegradable superabsorbent hydrogel composite based on Gum ghatti-co-poly (acrylic acid-aniline) for controlled drug delivery. Polym. Degrad. Stab. 2016, 124, 101–111. [Google Scholar]
- Sadeghi, M.; Soleimani, F. Synthesis and characterization of superabsorbent hydrogels for oral drug delivery systems. Int. J. Chem. Eng. Appl. 2011, 2, 314–316. [Google Scholar]
- Karadağ, E.; Saraydın, D.; Güven, O. Radiation induced superabsorbent hydrogels. Acrylamide/itaconic acid copolymers. Macromol. Mater. Eng. 2001, 286, 34–42. [Google Scholar]
- Zhu, Z.-Q.; Sun, H.-X.; Qin, X.-J.; Jiang, L.; Pei, C.-J.; Wang, L.; Zeng, Y.-Q.; Wen, S.-H.; La, P.-Q.; Li, A. Preparation of poly (acrylic acid)–graphite oxide superabsorbent nanocomposites. J. Mater. Chem. 2012, 22, 4811–4817. [Google Scholar] [CrossRef]
- Liu, Z.; Rempel, G. Preparation of superabsorbent polymers by crosslinking acrylic acid and acrylamide copolymers. J. Appl. Polym. Sci. 1997, 64, 1345–1353. [Google Scholar] [CrossRef]
- Ma, J.; Li, X.; Bao, Y. Advances in cellulose-based superabsorbent hydrogels. RSC Adv. 2015, 5, 59745–59757. [Google Scholar] [CrossRef]
- Athukoralalage, S.S.; Balu, R.; Dutta, N.K.; Roy Choudhury, N. 3D bioprinted nanocellulose-based hydrogels for tissue engineering applications: A brief review. Polymers 2019, 11, 898. [Google Scholar] [CrossRef]
- Cuadri, A.; Bengoechea, C.; Romero, A.; Guerrero, A. A natural-based polymeric hydrogel based on functionalized soy protein. Eur. Polym. J. 2016, 85, 164–174. [Google Scholar] [CrossRef]
- Dorishetty, P.; Balu, R.; Sreekumar, A.; de Campo, L.; Mata, J.P.; Choudhury, N.R.; Dutta, N.K. Robust and tunable hybrid hydrogels from photo-crosslinked soy protein isolate and regenerated silk fibroin. ACS Sustain. Chem. Eng. 2019, 7, 9257–9271. [Google Scholar] [CrossRef]
- Zain, G.; Nada, A.A.; El-Sheikh, M.A.; Attaby, F.A.; Waly, A.I. Superabsorbent hydrogel based on sulfonated-starch for improving water and saline absorbency. Int. J. Biol. Macromol. 2018, 115, 61–68. [Google Scholar] [CrossRef]
- Warkar, S.G.; Kumar, A. Synthesis and assessment of carboxymethyl tamarind kernel gum based novel superabsorbent hydrogels for agricultural applications. Polymer 2019, 182, 121823. [Google Scholar]
- Yoshimura, T.; Uchikoshi, I.; Yoshiura, Y.; Fujioka, R. Synthesis and characterization of novel biodegradable superabsorbent hydrogels based on chitin and succinic anhydride. Carbohydr. Polym. 2005, 61, 322–326. [Google Scholar] [CrossRef]
- Li, J.; Wu, C.; Chu, P.K.; Gelinsky, M. 3D printing of hydrogels: Rational design strategies and emerging biomedical applications. Mater. Sci. Eng. R Rep. 2020, 140, 100543. [Google Scholar] [CrossRef]
- Mondschein, R.J.; Kanitkar, A.; Williams, C.B.; Verbridge, S.S.; Long, T.E. Polymer structure-property requirements for stereolithographic 3D printing of soft tissue engineering scaffolds. Biomaterials 2017, 140, 170–188. [Google Scholar] [CrossRef] [PubMed]
- Xing, J.-F.; Zheng, M.-L.; Duan, X.-M. Two-photon polymerization microfabrication of hydrogels: An advanced 3D printing technology for tissue engineering and drug delivery. Chem. Soc. Rev. 2015, 44, 5031–5039. [Google Scholar] [CrossRef] [PubMed]
- Kirchmajer, D.M.; Gorkin Iii, R. An overview of the suitability of hydrogel-forming polymers for extrusion-based 3D-printing. J. Mater. Chem. B 2015, 3, 4105–4117. [Google Scholar] [CrossRef] [PubMed]
- Narayan, R.; Yoo, J.; Atala, A. 3D bioprinting: Physical and chemical processes. Appl. Phys. Rev. 2021, 8, 030401. [Google Scholar] [CrossRef]
- Billiet, T.; Vandenhaute, M.; Schelfhout, J.; Van Vlierberghe, S.; Dubruel, P. A review of trends and limitations in hydrogel-rapid prototyping for tissue engineering. Biomaterials 2012, 33, 6020–6041. [Google Scholar] [CrossRef] [PubMed]
- Bustamante-Torres, M.; Romero-Fierro, D.; Arcentales-Vera, B.; Palomino, K.; Magaña, H.; Bucio, E. Hydrogels Classification According to the Physical or Chemical Interactions and as Stimuli-Sensitive Materials. Gels 2021, 7, 182. [Google Scholar] [CrossRef]
- Yokoyama, F.; Masada, I.; Shimamura, K.; Ikawa, T.; Monobe, K. Morphology and structure of highly elastic poly (vinyl alcohol) hydrogel prepared by repeated freezing-and-melting. Colloid Polym. Sci. 1986, 264, 595–601. [Google Scholar] [CrossRef]
- Kim, S.Y.; Cho, S.M.; Lee, Y.M.; Kim, S.J. Thermo-and pH-responsive behaviors of graft copolymer and blend based on chitosan and N-isopropylacrylamide. J. Appl. Polym. Sci. 2000, 78, 1381–1391. [Google Scholar] [CrossRef]
- Prado, H.J.; Matulewicz, M.C.; Bonelli, P.R.; Cukierman, A.L. Preparation and characterization of a novel starch-based interpolyelectrolyte complex as matrix for controlled drug release. Carbohydr. Res. 2009, 344, 1325–1331. [Google Scholar] [CrossRef]
- You, Y.; Yang, J.; Zheng, Q.; Wu, N.; Lv, Z.; Jiang, Z. Ultra-stretchable hydrogels with hierarchical hydrogen bonds. Sci. Rep. 2020, 10, 11727. [Google Scholar] [CrossRef]
- De Jong, S.; Van Eerdenbrugh, B.; van Nostrum, C.F.; Kettenes-Van Den Bosch, J.; Hennink, W. Physically crosslinked dextran hydrogels by stereocomplex formation of lactic acid oligomers: Degradation and protein release behavior. J. Control. Release 2001, 71, 261–275. [Google Scholar] [CrossRef]
- Petka, W.A.; Harden, J.L.; McGrath, K.P.; Wirtz, D.; Tirrell, D.A. Reversible hydrogels from self-assembling artificial proteins. Science 1998, 281, 389–392. [Google Scholar] [CrossRef] [PubMed]
- Amin, M.C.I.M.; Ahmad, N.; Halib, N.; Ahmad, I. Synthesis and characterization of thermo-and pH-responsive bacterial cellulose/acrylic acid hydrogels for drug delivery. Carbohydr. Polym. 2012, 88, 465–473. [Google Scholar] [CrossRef]
- Tortora, M.; Cavalieri, F.; Chiessi, E.; Paradossi, G. Michael-type addition reactions for the in situ formation of poly (vinyl alcohol)-based hydrogels. Biomacromolecules 2007, 8, 209–214. [Google Scholar] [CrossRef]
- Chen, T.; Embree, H.D.; Brown, E.M.; Taylor, M.M.; Payne, G.F. Enzyme-catalyzed gel formation of gelatin and chitosan: Potential for in situ applications. Biomaterials 2003, 24, 2831–2841. [Google Scholar] [CrossRef]
- Lee, J.M.; Yeong, W.Y. Design and printing strategies in 3D bioprinting of cell-hydrogels: A review. Adv. Healthc. Mater. 2016, 5, 2856–2865. [Google Scholar] [CrossRef]
- Groll, J.; Burdick, J.A.; Cho, D.-W.; Derby, B.; Gelinsky, M.; Heilshorn, S.C.; Juengst, T.; Malda, J.; Mironov, V.A.; Nakayama, K. A definition of bioinks and their distinction from biomaterial inks. Biofabrication 2018, 11, 013001. [Google Scholar] [CrossRef]
- Bom, S.; Ribeiro, R.; Ribeiro, H.M.; Santos, C.; Marto, J. On the progress of hydrogel-based 3D printing: Correlating rheological properties with printing behaviour. Int. J. Pharm. 2022, 212, 121506. [Google Scholar] [CrossRef]
- Boley, J.W.; Van Rees, W.M.; Lissandrello, C.; Horenstein, M.N.; Truby, R.L.; Kotikian, A.; Lewis, J.A.; Mahadevan, L. Shape-shifting structured lattices via multimaterial 4D printing. Proc. Natl. Acad. Sci. USA 2019, 116, 20856–20862. [Google Scholar] [CrossRef]
- Kolesky, D.B.; Homan, K.A.; Skylar-Scott, M.; Lewis, J.A. In vitro human tissues via multi-material 3-D bioprinting. Altern. Lab. Anim. 2018, 46, 209–215. [Google Scholar] [CrossRef]
- Lewis, J.A.; Uzel, S.; Eriksson, M. Multinozzle Printhead with an Adaptable Profile for 3d-Printing. U.S. Patent US20200147873A1, 14 May 2020. [Google Scholar]
- Uzel, S.G.; Weeks, R.D.; Eriksson, M.; Kokkinis, D.; Lewis, J.A. Multimaterial Multinozzle Adaptive 3D Printing of Soft Materials. Adv. Mater. Technol. 2022, 7, 2101710. [Google Scholar] [CrossRef]
- McCracken, J.M.; Rauzan, B.M.; Kjellman, J.C.; Su, H.; Rogers, S.A.; Nuzzo, R.G. Ionic hydrogels with biomimetic 4D-printed mechanical gradients: Models for soft-bodied aquatic organisms. Adv. Funct. Mater. 2019, 29, 1806723. [Google Scholar] [CrossRef]
- Taylor, J.M.; Luan, H.; Lewis, J.A.; Rogers, J.A.; Nuzzo, R.G.; Braun, P.V. Biomimetic and Biologically Compliant Soft Architectures via 3D and 4D Assembly Methods: A Perspective. Adv. Mater. 2022, 34, 2108391. [Google Scholar] [CrossRef] [PubMed]
- Sydney Gladman, A.; Matsumoto, E.A.; Nuzzo, R.G.; Mahadevan, L.; Lewis, J.A. Biomimetic 4D printing. Nat. Mater. 2016, 15, 413–418. [Google Scholar] [CrossRef]
- Yu, C.; Duan, Z.; Yuan, P.; Li, Y.; Su, Y.; Zhang, X.; Pan, Y.; Dai, L.L.; Nuzzo, R.G.; Huang, Y. Electronically programmable, reversible shape change in two-and three-dimensional hydrogel structures. Adv. Mater. 2013, 25, 1541–1546. [Google Scholar] [CrossRef]
- Narupai, B.; Smith, P.T.; Nelson, A. 4D printing of multi-stimuli responsive protein-based hydrogels for autonomous shape transformations. Adv. Funct. Mater. 2021, 31, 2011012. [Google Scholar] [CrossRef]
- Kolambkar, Y.M.; Dupont, K.M.; Boerckel, J.D.; Huebsch, N.; Mooney, D.J.; Hutmacher, D.W.; Guldberg, R.E. An alginate-based hybrid system for growth factor delivery in the functional repair of large bone defects. Biomaterials 2011, 32, 65–74. [Google Scholar] [CrossRef]
- Jensen, B.E.; Dávila, I.; Zelikin, A.N. Poly (vinyl alcohol) physical hydrogels: Matrix-mediated drug delivery using spontaneously eroding substrate. J. Phys. Chem. B 2016, 120, 5916–5926. [Google Scholar] [CrossRef]
- Ashley, G.W.; Henise, J.; Reid, R.; Santi, D.V. Hydrogel drug delivery system with predictable and tunable drug release and degradation rates. Proc. Natl. Acad. Sci. USA 2013, 110, 2318–2323. [Google Scholar] [CrossRef]
- Young, M.; Carroad, P.; Bell, R. Estimation of diffusion coefficients of proteins. Biotechnol. Bioeng. 1980, 22, 947–955. [Google Scholar] [CrossRef]
- Brazel, C.S.; Peppas, N.A. Modeling of drug release from swellable polymers. Eur. J. Pharm. Biopharm. 2000, 49, 47–58. [Google Scholar] [CrossRef]
- Ritger, P.L.; Peppas, N.A. A simple equation for description of solute release I. Fickian and non-fickian release from non-swellable devices in the form of slabs, spheres, cylinders or discs. J. Control. Release 1987, 5, 23–36. [Google Scholar] [CrossRef]
- Lin, C.-C.; Metters, A.T. Hydrogels in controlled release formulations: Network design and mathematical modeling. Adv. Drug Deliv. Rev. 2006, 58, 1379–1408. [Google Scholar] [CrossRef]
- Wang, Y.; Miao, Y.; Zhang, J.; Wu, J.P.; Kirk, T.B.; Xu, J.; Ma, D.; Xue, W. Three-dimensional printing of shape memory hydrogels with internal structure for drug delivery. Mater. Sci. Eng. C 2018, 84, 44–51. [Google Scholar] [CrossRef]
- Zhao, Y.-D.; Lai, J.-H.; Wang, M. 4D Printing of Self-Folding Hydrogel Tubes for Potential Tissue Engineering Applications. Nano Life 2021, 11, 2141001. [Google Scholar] [CrossRef]
- Zu, S.; Wang, Z.; Zhang, S.; Guo, Y.; Chen, C.; Zhang, Q.; Liu, T.; Liu, Q.; Zhang, Z. A bioinspired 4D printed hydrogel capsule for smart controlled drug release. Mater. Today Chem. 2022, 24, 100789. [Google Scholar] [CrossRef]
- Melocchi, A.; Inverardi, N.; Uboldi, M.; Baldi, F.; Maroni, A.; Pandini, S.; Briatico-Vangosa, F.; Zema, L.; Gazzaniga, A. Retentive device for intravesical drug delivery based on water-induced shape memory response of poly (vinyl alcohol): Design concept and 4D printing feasibility. Int. J. Pharm. 2019, 559, 299–311. [Google Scholar] [CrossRef]
- Bozuyuk, U.; Yasa, O.; Yasa, I.C.; Ceylan, H.; Kizilel, S.; Sitti, M. Light-triggered drug release from 3D-printed magnetic chitosan microswimmers. ACS Nano 2018, 12, 9617–9625. [Google Scholar] [CrossRef]
- Ceylan, H.; Yasa, I.C.; Yasa, O.; Tabak, A.F.; Giltinan, J.; Sitti, M. 3D-printed biodegradable microswimmer for theranostic cargo delivery and release. ACS Nano 2019, 13, 3353–3362. [Google Scholar] [CrossRef]
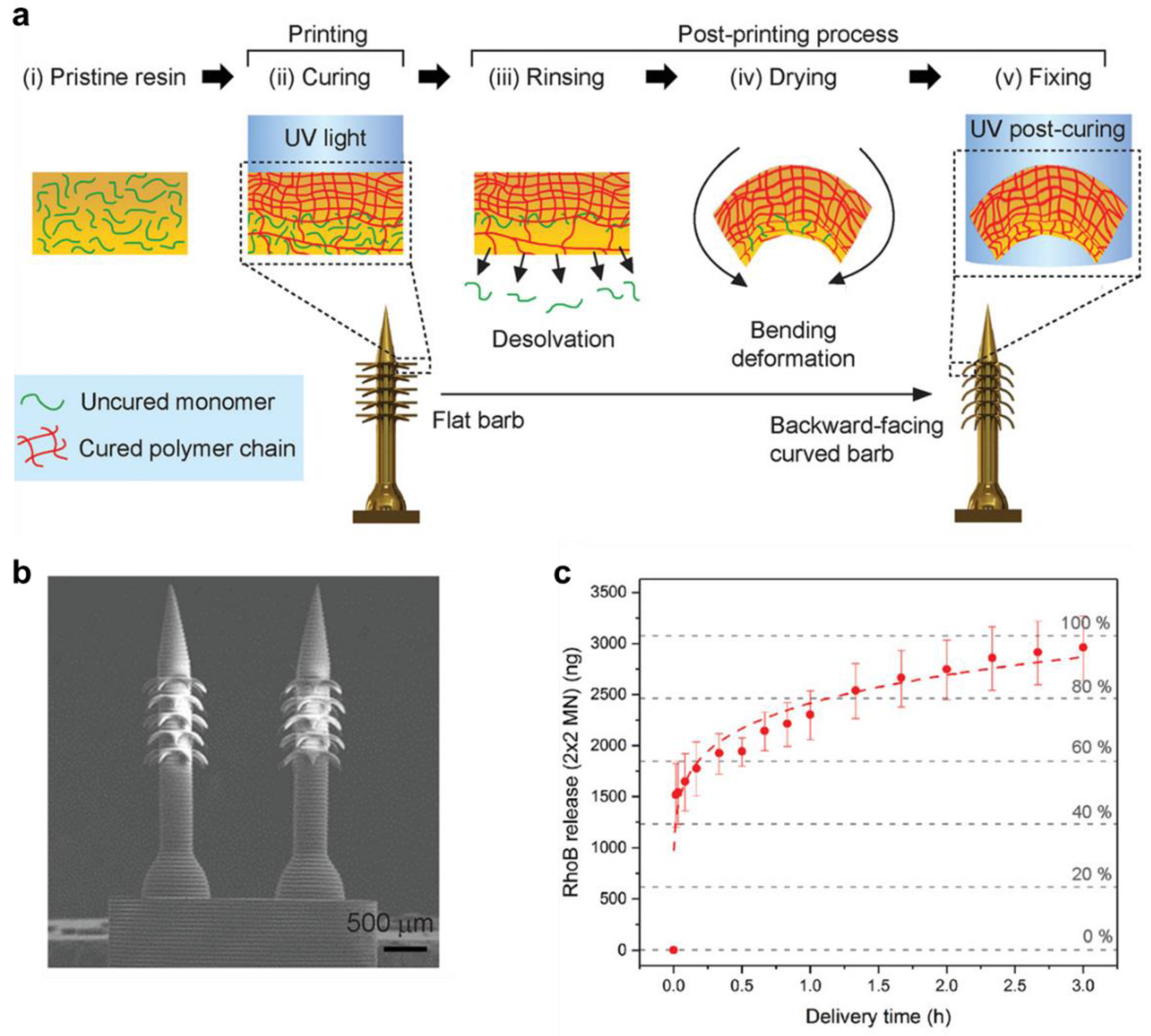
- Han, D.; Morde, R.S.; Mariani, S.; La Mattina, A.A.; Vignali, E.; Yang, C.; Barillaro, G.; Lee, H. 4D printing of a bioinspired microneedle array with backward-facing barbs for enhanced tissue adhesion. Adv. Funct. Mater. 2020, 30, 1909197. [Google Scholar] [CrossRef]
- Fang, J.-H.; Hsu, H.-H.; Hsu, R.-S.; Peng, C.-K.; Lu, Y.-J.; Chen, Y.-Y.; Chen, S.-Y.; Hu, S.-H. 4D printing of stretchable nanocookie@ conduit material hosting biocues and magnetoelectric stimulation for neurite sprouting. NPG Asia Mater. 2020, 12, 61. [Google Scholar] [CrossRef]



| Tissue/Cellular Compartment | pH |
|---|---|
| Stomach | 1.0–3.0 |
| Vagina | 3.8–4.5 |
| Late endosome | 4.5–5.0 |
| Upper small intestine | 4.8–8.2 |
| Inflamed tissue/wound | 5.4–7.4 |
| Early endosome | 6.0–6.5 |
| Tumor, extracellular | 6.5–7.2 |
| Colon | 7.0–7.5 |
| Blood | 7.3–7.5 |
| Materials | Printing Parameters | Crosslinking | Printed Shape Transformation | Drug & Loading Method | External Stimulus | Drug Release Profile | Ref. |
|---|---|---|---|---|---|---|---|
| Pluronic diacrylate macromer, and alginate | DIW: Nozzle diameter—400 µm; nozzle temperature—25 °C; print speed—15 mm/s; pressure—0.04 kPa; bed temperature—60 °C | UV curing | Square mesh to folded mesh | Methotrexate—Co-mixing | Ion (Calcium chloride) | Fast release up to 6 h followed by steady release up to 12 h | [148] |
| Gelatin methacryloyl | DIW: Nozzle diameter—210 µm; nozzle temperature—26 °C; print speed—20 mm/s | UV curing | Sheet to tubular | Heparin—Co-mixing | Solvent (Water) | Fast release up to 8 h followed by steady release up to 28 h | [149] |
| Poly(N-isopropylacrylamide) | DIW: Nozzle diameter—340 µm | UV curing | Expanding core–shell capsules | Brilliant blue and lemon yellow—Injection | Temperature (22 °C) | Fast release up to 15 h followed by medium release up to 48 h | [150] |
| Poly(vinyl alcohol) | FDM: Nozzle diameter—400 µm; nozzle temperature—180 °C; print speed—23 mm/s | - | I to U, U to I, and helix to extended conformation | Caffeine—Co-mixing | Solvent (Water) | Fast release up to 2 h followed by steady release up to 6 h | [151] |
| Methacrylamide Chitosan | 2PP: Sub-micron resolution | - | Expandable microswimmers | Doxorubicin—Immersion | Light (UV 365 nm) | Fast release up to 1 min (light ON) followed by steady release up to 6 min (light OFF) | [152] |
| Gelatin methacryloyl | 2PP: Sub-micron resolution | - | Expandable microswimmers | Fluorescein isothiocyanate—Immersion | Enzyme (Metallo-proteinase 2) | Fast release up to 2 h followed by medium release up to 48 h | [153] |
| Poly(ethylene glycol) diacrylate | µSLA: Layer thicknesss—50 µm | UV curing | Straight to backward-facing curved barbs in microneedle array | Rhodamine B—Immersion | Ion (Phosphate buffered saline) | Fast release in 1 min followed by medium release up to 2.5 h, and slow releases up to 3 h | [154] |
| 4-hydroxybutyl acrylate, and urethanepolyethylene glycol-polypropylene glycol | DLP: Layer thicknesss—100 µm | UV curing | Stretchable nerve guide tubing | Doxorubicin, and ovalbumin—Immersion | Magnetic field (1 MHz) | Fast release in 4 h (doxorubicin) or 30 min (ovalbumin) followed by medium release up to 50 h, and steady releases up to 75 h | [155] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tran, T.S.; Balu, R.; Mettu, S.; Roy Choudhury, N.; Dutta, N.K. 4D Printing of Hydrogels: Innovation in Material Design and Emerging Smart Systems for Drug Delivery. Pharmaceuticals 2022, 15, 1282. https://doi.org/10.3390/ph15101282
Tran TS, Balu R, Mettu S, Roy Choudhury N, Dutta NK. 4D Printing of Hydrogels: Innovation in Material Design and Emerging Smart Systems for Drug Delivery. Pharmaceuticals. 2022; 15(10):1282. https://doi.org/10.3390/ph15101282
Chicago/Turabian StyleTran, Tuan Sang, Rajkamal Balu, Srinivas Mettu, Namita Roy Choudhury, and Naba Kumar Dutta. 2022. "4D Printing of Hydrogels: Innovation in Material Design and Emerging Smart Systems for Drug Delivery" Pharmaceuticals 15, no. 10: 1282. https://doi.org/10.3390/ph15101282
APA StyleTran, T. S., Balu, R., Mettu, S., Roy Choudhury, N., & Dutta, N. K. (2022). 4D Printing of Hydrogels: Innovation in Material Design and Emerging Smart Systems for Drug Delivery. Pharmaceuticals, 15(10), 1282. https://doi.org/10.3390/ph15101282






