Antimicrobial and Cytotoxic Effects of Cannabinoids: An Updated Review with Future Perspectives and Current Challenges
Abstract
1. Introduction
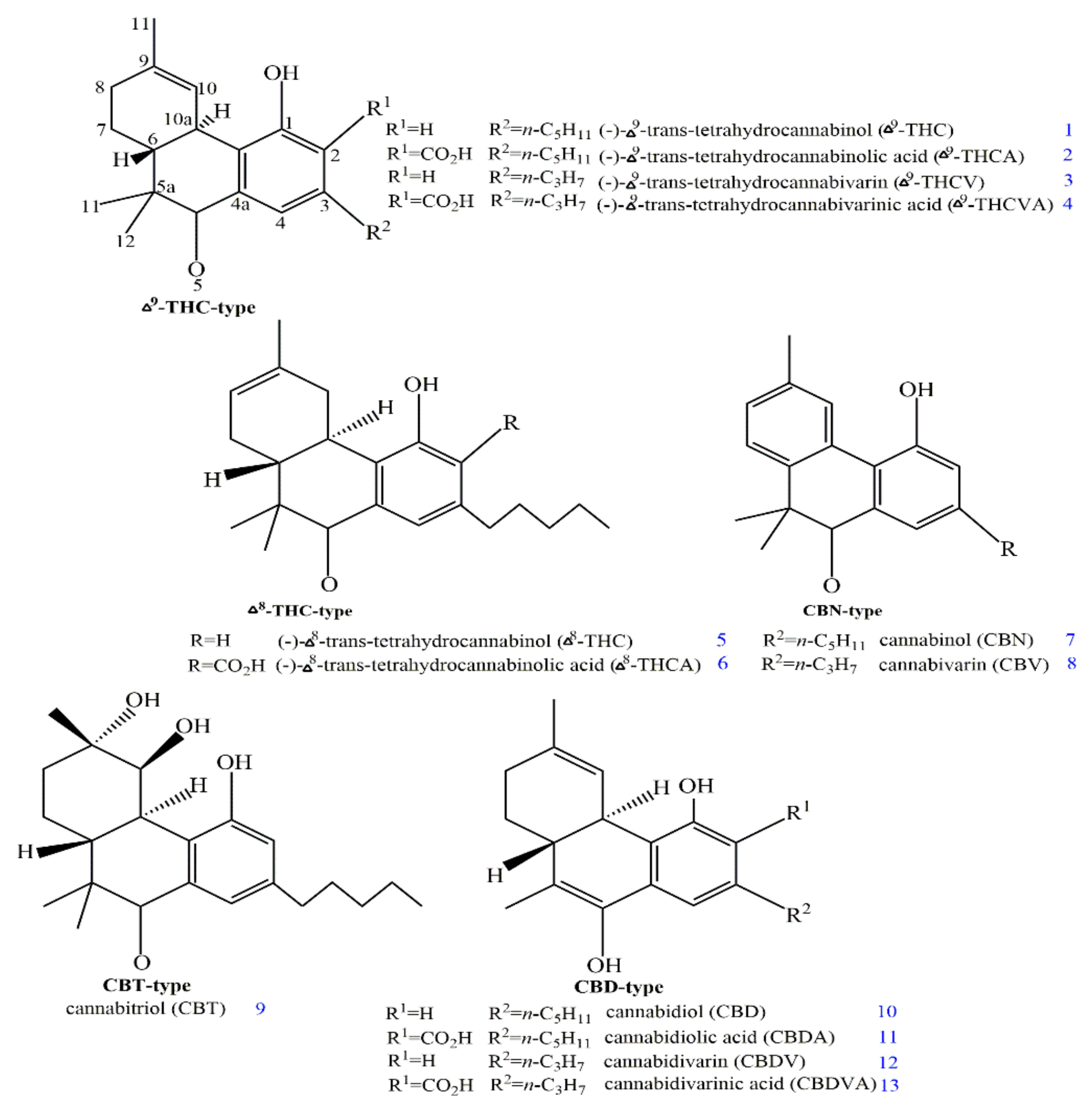
2. Structures and Origin of Natural Cannabinoids from Cannabis sativa
3. Structure–Activity Associations of Cannabinoids
| Bacterial Strains | Compound/Extract/Essential Oils | Activity | Reference Antibiotic | Outcomes | Ref. |
|---|---|---|---|---|---|
| P. aeruginosa | Aqueous extract | MIC 7.14 mg/mL | Ciprofloxacin | A higher anti-inflammatory and antioxidant profile was shown by the water extract, along with a significant inhibition on the selected pathogen. | [53] |
| Plant extract | MIC 12.5 µg/mL | - | The plant extracts show considerable antibacterial activities against P. aeruginosa. | [54] | |
| N. gonorrhoeae | CBD (10) | MIC 1–2 µg/mLMIC 0.03–16.0 µg/mL | Vancomycin, Levofloxacin, Meropenem, Gentamicin, Mupirocin, Colistin | The findings show that cannabidiol has superior anti-biofilm activities, limited tendency to cause resistance, and topical in vivo efficacy. Various investigations on the mechanisms of action of cannabidiol point to membrane disruption as the main mechanism. | [55] |
| Staph aureus, Lactobacillus | Seed extract | MIC 2.5 mg/mL | - | The results of the study revealed that C. sativa extracts can effectively treat pathogenic strains. It also did not affect the growth of beneficial bacteria. | [56] |
| P. aeruginosa, E. coli | Essential oil | MIC 1.2 mg/mL | - | The use of C. sativa essential oil as a potential source of antimicrobials and natural antioxidants could offer a promising strategy to treat various infectious diseases. | [57] |
| E. coli, Salmonella typhimurium | Seed extract | Growth inhibition at MIC 1 mg/mL | - | It has been observed that C. sativa extracts had selective antimicrobial action against pathogenic strains and had no negative effects on the growth of probiotic strains. | [56] |
| E. coli | Seed extract | MIC 25 µg/mL | - | The plant extracts show higher antibacterial activities against pathogens. | [54] |
| N-p-trans-coumaroyl-tyramine | IC50 0.8 µg/mL | Ciprofloxacin | The compound displayed strong antibacterial activities against bacteria. | [58] | |
| Aqueous extract | MIC 7.14 mg/mL | Ciprofloxacin | A higher anti-inflammatory and antioxidant profile was shown by the water extract, along with a significant inhibition on the selected pathogen. | [53] | |
| Vancomycin-resistant Enterococci | CBCA (20) | MIC 7.8 µM | - | It was observed that CBCA (20) demonstrated faster and more potent bactericidal activity than vancomycin. Microscopical analysis reveals that CBCA (20) may work by altering the bacterial nucleoid and degrading the lipid membrane of the bacterial cell. | [59] |
| S. pneumoniae | CBD (10) | MIC 1–4 µg/mL | Vancomycin, Daptomycin, Trimethoprim, Mupirocin, Clindamycin | The findings show that cannabidiol has superior anti-biofilm activity, limited tendency to cause resistance, and topical in vivo efficiency. | [55] |
| MRSA, E. faecium | CBD (10) | MIC 1–2 µg/mL | Vancomycin, Daptomycin, Trimethoprim, Mupirocin, Clindamycin | Various investigations on the mechanisms of action of cannabidiol point to membrane disruption as the main mechanism. Moreover, cannabidiol has superior anti-biofilm activity, limited tendency to cause resistance, and topical in vivo efficacy. | [55] |
| EMRSA 15, EMRSA 16 | CBD (10), ∆1 & 9-THC (1), CBG (17), CBC (19), CBND (16) | MIC 0.5–2.0 µg/mL | - | The compounds demonstrated strong antimicrobial activity against various MRSA strains with contemporary clinical significance. | [46] |
| CBD (10), ∆1 & 9-THC (1), CBG (17), CBC (19), CBND (16) | MIC 1–4 µg/mL | Ciprofloxacin | The results of the study showed that five of the hemp essential oils inhibited the growth of pathogens. This suggests that these can help reduce bacterial populations in the environment. | [60] | |
| E. faecium | Essential oil, α-humulene, α-pinene, β-pinene, myrcene | MIC 0.75–1.87 (%, v/v) MBC 1.39–2.83 (%. v/v) | - | Essential oils extracted from industrial hemp can help prevent the growth of harmful microbes. This benefit can be achieved depending on the variety and sowing time. | [61] |
| Essential oil | IC50 0.82–4.22 µg/mL | - | The essential oil showed potent and selective antibacterial activity against selected bacteria. | [62] | |
| CBG (17) | MIC 2 µg/mL and MBEC 4 µg/mL | - | The study shows that the drug can target the membrane of Gram-positive bacteria. It also shows that the drug can be effective in treating an infection caused by MRSA in a mouse model. | [51] | |
| CBDA (11) | MIC 4 µg/mL | Tobramycin, Meropenem, Ofloxacin | The compound had strong antibacterial activities towards bacterial strains and may be used as a substitute drug to treat MRSA. | [47] | |
| MRSA | CBD (10), CBND (16), CBC (19), CBDV (12) and ∆1 & 9-THC (1) | IC50 5.8–10.6 µM | Ciprofloxacin | All compounds showed antimicrobial properties when tested for antibacterial activity against a panel of pathogens. | [63] |
| CBD analogs | MIC 0.25–64.0 µg/mL | Vancomycin, Daptomycin, Mupirocin | The findings show that cannabidiol has superior anti-biofilm activity, limited tendency to cause resistance, and topical in vivo efficacy. | [55] | |
| CBD (10) | MIC 1 µg/mL | Tobramycin, Meropenem, Ofloxacin | CBD (10) had a potent antibacterial activity against Gram-positive strains and may be used as a substitute drug to treat MRSA. | [47] | |
| CBCA (20) | MIC 3.9 µM | - | Microscopical analysis reveals that CBCA (20) may work by altering the bacterial nucleoid and degrading the lipid membrane of the bacterial cell. | [59] | |
| 4-acetoxy-2-geranyl-5-hydroxy-3- n-pentylphenol | IC50 6.7 µM | Ciprofloxacin | Compounds displayed significant antibacterial activities towards MRSA. | [64] |
4. Antimicrobial Activity of Cannabis sativa
5. Antibacterial Mechanism of Action
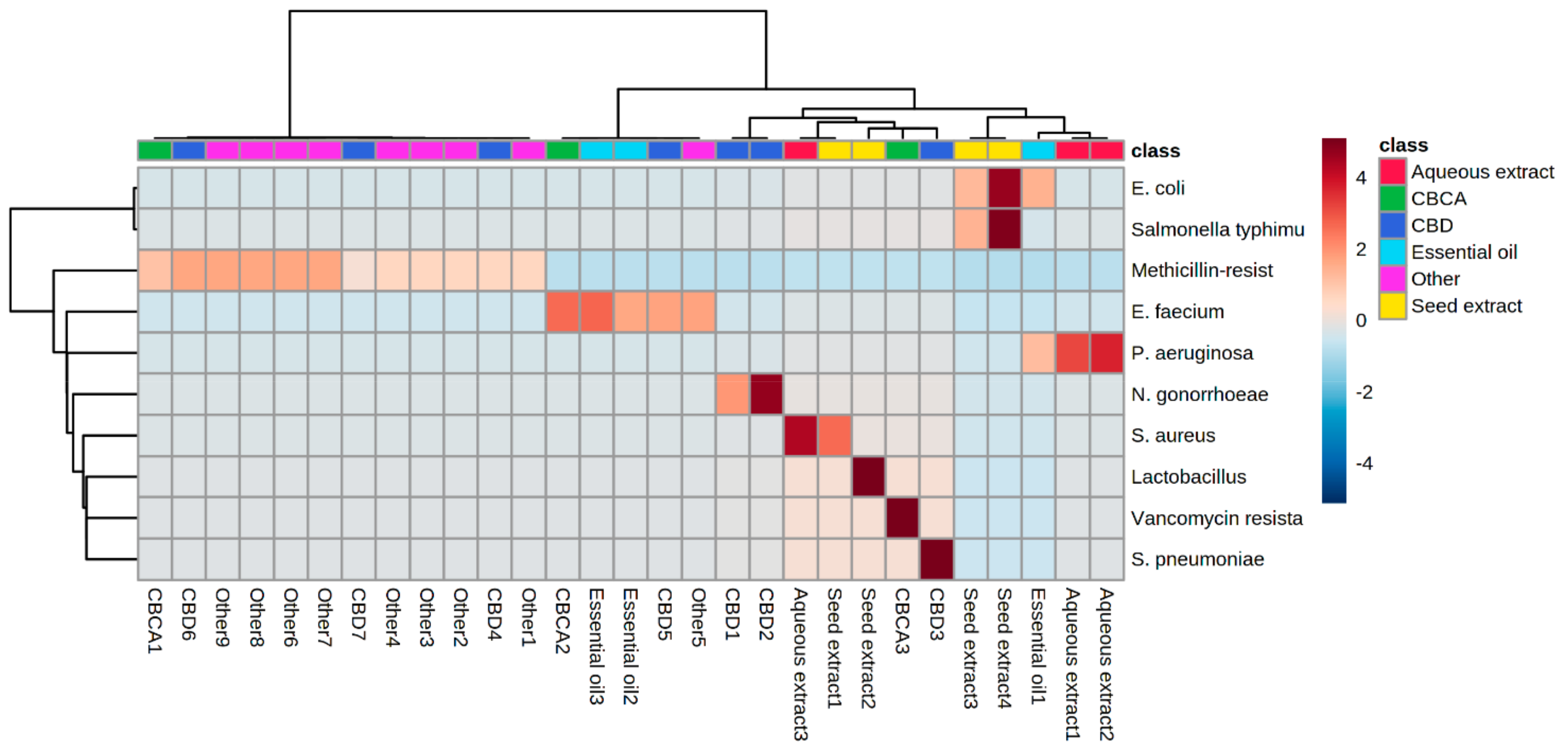
6. Heat Map Clusters of Cannabinoids
7. Cytotoxic Effects of Cannabinoids
7.1. Colon Cancer
7.2. Breast Cancer
7.3. Lung Cancer
7.4. Prostate Cancer
7.5. Neuroblastoma and Glioma
7.6. Other Cancers
8. Current Challenges and Future Perspectives
9. Conclusions and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zaman, S.B.; Hussain, M.A.; Nye, R.; Mehta, V.; Mamun, K.T.; Hossain, N. A review on antibiotic resistance: Alarm bells are ringing. Cureus 2017, 9, 1403. [Google Scholar] [CrossRef] [PubMed]
- Tyers, M.; Wright, G.D. Drug combinations: A strategy to extend the life of antibiotics in the 21st century. Nat. Rev. Microbiol. 2019, 17, 141–155. [Google Scholar] [CrossRef] [PubMed]
- Douafer, H.; Andrieu, V.; Phanstiel, O., IV; Brunel, J.M. Antibiotic adjuvants: Make antibiotics great again! J. Med. Chem. 2019, 62, 8665–8681. [Google Scholar] [CrossRef] [PubMed]
- González-Bello, C. Antibiotic adjuvants–A strategy to unlock bacterial resistance to antibiotics. Bioorg. Med. Chem. Lett. 2017, 27, 4221–4228. [Google Scholar] [CrossRef]
- Christian, T.V.; Morten, W.; Oliver, H.; Jean-Marie, P.; Jette, K. Population Dynamics Approach for the Study of Synergetic Coupling between Antibiotic and Helper Compounds. Comput. Mol. Biosci. 2012, 2, 18268. [Google Scholar]
- Lodato, E.M.; Kaplan, W. Background Paper 6.1 Antimicrobial Resistance; World Health Organization: Geneva, Switzerland, 2013. [Google Scholar]
- Jim, O.N. Antimicrobial Resistance: Tackling a crisis for the health and wealth of nations. Rev. Antimicrob. Resist. Lond. Engl. 2014, 20, 1–16. [Google Scholar]
- Naveed, M.; Khan, T.A.; Ali, I.; Hassan, A.; Ali, H.; Ud, Z.; Din, Z.H.; Tabassum, S.; Saqib, A.M.; Rehman, M.U. In vitro antibacterial activity of Cannabis sativa leaf extracts to some selective pathogenicbacterial strains. Int. J. Biosci. 2014, 4, 65–70. [Google Scholar]
- ElSohly, M.A.; Slade, D. Chemical constituents of marijuana: The complex mixture of natural cannabinoids. Life Sci. 2005, 78, 539–548. [Google Scholar] [CrossRef]
- Klahn, P. Cannabinoids-Promising Antimicrobial Drugs or Intoxicants with Benefits? Antibiotics 2020, 9, 297. [Google Scholar] [CrossRef]
- Mackie, K. Cannabinoid receptors: Where they are and what they do. J. Neuroendocrinol. 2008, 20, 10–14. [Google Scholar] [CrossRef]
- Mechoulam, R.; Gaoni, Y. Recent advances in the chemistry of hashish. Fortschr. Chem. Org. Nat. Prog. Chem. Org. Nat. Prod. Progrès Dans Chim. Subst. Org. Nat. 1967, 25, 175–213. [Google Scholar]
- Pacher, P.; Bátkai, S.; Kunos, G. The endocannabinoid system as an emerging target of pharmacotherapy. Pharmacol. Rev. 2006, 58, 389–462. [Google Scholar] [CrossRef] [PubMed]
- Howard, P.; Twycross, R.; Shuster, J.; Mihalyo, M.; Wilcock, A. Cannabinoids. J. Pain Symptom Manag. 2013, 46, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Weiss, L.; Zeira, M.; Reich, S.; Har-Noy, M.; Mechoulam, R.; Slavin, S.; Gallily, R. Cannabidiol lowers incidence of diabetes in non-obese diabetic mice. Autoimmunity 2006, 39, 143–151. [Google Scholar] [CrossRef]
- Massi, P.; Solinas, M.; Cinquina, V.; Parolaro, D. Cannabidiol as potential anticancer drug. Br. J. Clin. Pharmacol. 2013, 75, 303–312. [Google Scholar] [CrossRef]
- Lastres-Becker, I.; Molina-Holgado, F.; Ramos, J.A.; Mechoulam, R.; Fernández-Ruiz, J. Cannabinoids provide neuroprotection against 6-hydroxydopamine toxicity in vivo and in vitro: Relevance to Parkinson’s disease. Neurobiol. Dis. 2005, 19, 96–107. [Google Scholar] [CrossRef]
- Hall, W.; Solowij, N.; Lemon, J. The Health and Psychological Effects of Cannabis; National Drug Strategy Monograph No. 25; Australian Government Publishing Service: Canberra, Australia, 1994. [Google Scholar]
- Curran, V.H.; Brignell, C.; Fletcher, S.; Middleton, P.; Henry, J. Cognitive and subjective dose-response effects of acute oral Δ9-tetrahydrocannabinol (THC) in infrequent cannabis users. Psychopharmacology 2002, 164, 61–70. [Google Scholar] [CrossRef]
- Dorard, G.; Berthoz, S.; Phan, O.; Corcos, M.; Bungener, C. Affect dysregulation in cannabis abusers. Eur. Child Adolesc. Psychiatry 2008, 17, 274–282. [Google Scholar] [CrossRef]
- Crean, R.D.; Tapert, S.F.; Minassian, A.; MacDonald, K.; Crane, N.A.; Mason, B.J. Effects of chronic, heavy cannabis use on executive functions. J. Addict. Med. 2011, 5, 9. [Google Scholar] [CrossRef]
- Hall, W. Health and Social Effects of Nonmedical Cannabis Use; The World Health Organization: Geneva, Switzerland, 2016. [Google Scholar]
- Pacher, P.; Steffens, S.; Haskó, G.; Schindler, T.H.; Kunos, G. Cardiovascular effects of marijuana and synthetic cannabinoids: The good, the bad, and the ugly. Nat. Rev. Cardiol. 2018, 15, 151–166. [Google Scholar] [CrossRef]
- El Sohly, M.A.; Radwan, M.M.; Gul, W.; Chandra, S.; Galal, A. Phytochemistry of Cannabis sativa L. Prog. Chem. Org. Nat. Prod. 2017, 103, 1–36. [Google Scholar]
- Izzo, A.A.; Borrelli, F.; Capasso, R.; Di Marzo, V.; Mechoulam, R. Non-psychotropic plant cannabinoids: New therapeutic opportunities from an ancient herb. Trends Pharmacol. Sci. 2009, 30, 515–527. [Google Scholar] [CrossRef] [PubMed]
- Kögel, C.C.; López-Pelayo, H.; Balcells-Olivero, M.M.; Colom, J.; Gual, A. Psychoactive constituents of cannabis and their clinical implications: A systematic review. Adicciones 2018, 30, 140–151. [Google Scholar]
- Thomas, F.J.; Kayser, O. Minor Cannabinoids of Cannabis sativa L. J. Med. Sci. 2019, 88, 141–149. [Google Scholar] [CrossRef]
- Morales, P.; Reggio, P.H.; Jagerovic, N. An overview on medicinal chemistry of synthetic and natural derivatives of cannabidiol. Front. Pharmacol. 2017, 8, 422. [Google Scholar] [CrossRef] [PubMed]
- Gaoni, Y.; Mechoulam, R. Hashish—VII: The isomerization of cannabidiol to tetrahydrocannabinols. Tetrahedron 1966, 22, 1481–1488. [Google Scholar] [CrossRef]
- Küppers, F.; Bercht, C.; Salemink, C.; Lousberg, R.C.; Terlouw, J.; Heerma, W. Cannabis—XV: Pyrolysis of cannabidiol. Structure elucidation of four pyrolytic products. Tetrahedron 1975, 31, 1513–1516. [Google Scholar] [CrossRef]
- Shani, A.; Mechoulam, R. Cannabielsoic acids: Isolation and synthesis by a novel oxidative cyclization. Tetrahedron 1974, 30, 2437–2446. [Google Scholar] [CrossRef]
- Aizpurua-Olaizola, O.; Soydaner, U.; Öztürk, E.; Schibano, D.; Simsir, Y.; Navarro, P.; Etxebarria, N.; Usobiaga, A. Evolution of the cannabinoid and terpene content during the growth of Cannabis sativa plants from different chemotypes. J. Nat. Prod. 2016, 79, 324–331. [Google Scholar] [CrossRef]
- El Sohly, M.; Gul, W. Constituents of Cannabis sativa. Handb. Cannabis 2014, 3, 1093. [Google Scholar]
- Yeom, H.-S.; Li, H.; Tang, Y.; Hsung, R.P. Total syntheses of cannabicyclol, clusiacyclol A and B, iso-eriobrucinol A and B, and eriobrucinol. Org. Lett. 2013, 15, 3130–3133. [Google Scholar] [CrossRef] [PubMed]
- Sirikantaramas, S.; Taura, F. Cannabinoids: Biosynthesis and biotechnological applications. In Cannabis sativa L.-Botany and Biotechnology; Springer: Cham, Switzerland, 2017; pp. 183–206. [Google Scholar]
- Luo, X.; Reiter, M.A.; d’Espaux, L.; Wong, J.; Denby, C.M.; Lechner, A.; Zhang, Y.; Grzybowski, A.T.; Harth, S.; Lin, W. Complete biosynthesis of cannabinoids and their unnatural analogues in yeast. Nature 2019, 567, 123–126. [Google Scholar] [CrossRef] [PubMed]
- Degenhardt, F.; Stehle, F.; Kayser, O. Chapter 2: The biosynthesis of cannabinoids. In Handbook of Cannabis and Related Pathologies; Preedy, V.R., Ed.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 12–23. [Google Scholar]
- Taura, F.; Tanaka, S.; Taguchi, C.; Fukamizu, T.; Tanaka, H.; Shoyama, Y.; Morimoto, S. Characterization of olivetol synthase, a polyketide synthase putatively involved in cannabinoid biosynthetic pathway. FEBS Lett. 2009, 583, 2061–2066. [Google Scholar] [CrossRef] [PubMed]
- Gagne, S.J.; Stout, J.M.; Liu, E.; Boubakir, Z.; Clark, S.M.; Page, J.E. Identification of olivetolic acid cyclase from Cannabis sativa reveals a unique catalytic route to plant polyketides. Proc. Natl. Acad. Sci. USA 2012, 109, 12811–12816. [Google Scholar] [CrossRef]
- Page, J.E.; Boubakir, Z. Aromatic prenyltransferase from Cannabis. Google Patents 13/389815, 6 July 2012. [Google Scholar]
- Taura, F.; Sirikantaramas, S.; Shoyama, Y.; Yoshikai, K.; Shoyama, Y.; Morimoto, S. Cannabidiolic-acid synthase, the chemotype-determining enzyme in the fiber-type Cannabis sativa. FEBS Lett. 2007, 581, 2929–2934. [Google Scholar] [CrossRef]
- Morimoto, S.; Komatsu, K.; Taura, F.; Shoyama, Y. Purification and characterization of cannabichromenic acid synthase from Cannabis sativa. Phytochemistry 1998, 49, 1525–1529. [Google Scholar] [CrossRef]
- Moreno-Sanz, G. Can you pass the acid test? critical review and novel therapeutic perspectives of Δ9-tetrahydrocannabinolic acid A. Cannabis Cannabinoid Res. 2016, 1, 124–130. [Google Scholar] [CrossRef]
- Van Klingeren, B.; Ten Ham, M. Antibacterial activity of Δ9-tetrahydrocannabinol and cannabidiol. Antonie Leeuwenhoek 1976, 42, 9–12. [Google Scholar] [CrossRef]
- Turner, C.E.; Elsohly, M.A. Biological activity of cannabichromene, its homologs and isomers. J. Clin. Pharmacol. 1981, 21, 283S–291S. [Google Scholar] [CrossRef]
- Appendino, G.; Gibbons, S.; Giana, A.; Pagani, A.; Grassi, G.; Stavri, M.; Smith, E.; Rahman, M.M. Antibacterial cannabinoids from Cannabis sativa: A structure−activity study. J. Nat. Prod. 2008, 71, 1427–1430. [Google Scholar] [CrossRef]
- Martinenghi, L.D.; Jønsson, R.; Lund, T.; Jenssen, H. Isolation, Purification, and antimicrobial characterization of cannabidiolic acid and cannabidiol from Cannabis sativa L. Biomolecules 2020, 10, 900. [Google Scholar] [CrossRef]
- Feldman, M.; Smoum, R.; Mechoulam, R.; Steinberg, D. Antimicrobial potential of endocannabinoid and endocannabinoid-like compounds against methicillin-resistant Staphylococcus aureus. Sci. Rep. 2018, 8, 17696. [Google Scholar] [CrossRef] [PubMed]
- Feldman, M.; Smoum, R.; Mechoulam, R.; Steinberg, D. Potential combinations of endocannabinoid/endocannabinoid-like compounds and antibiotics against methicillin-resistant Staphylococcus aureus. PLoS ONE 2020, 15, e0231583. [Google Scholar] [CrossRef] [PubMed]
- Wassmann, C.S.; Højrup, P.; Klitgaard, J.K. Cannabidiol is an effective helper compound in combination with bacitracin to kill Gram-positive bacteria. Sci. Rep. 2020, 10, 4112. [Google Scholar] [CrossRef]
- Farha, M.A.; El-Halfawy, O.M.; Gale, R.T.; MacNair, C.R.; Carfrae, L.A.; Zhang, X.; Jentsch, N.G.; Magolan, J.; Brown, E.D. Uncovering the hidden antibiotic potential of cannabis. ACS Infect. Dis. 2020, 6, 338–346. [Google Scholar] [CrossRef] [PubMed]
- Kosgodage, U.S.; Matewele, P.; Awamaria, B.; Kraev, I.; Warde, P.; Mastroianni, G.; Nunn, A.V.; Guy, G.W.; Bell, J.D.; Inal, J.M. Cannabidiol is a novel modulator of bacterial membrane vesicles. Front. Cell. Infect. Microbiol. 2019, 9, 324. [Google Scholar] [CrossRef] [PubMed]
- Ferrante, C.; Recinella, L.; Ronci, M.; Menghini, L.; Brunetti, L.; Chiavaroli, A.; Leone, S.; Di Iorio, L.; Carradori, S.; Tirillini, B. Multiple pharmacognostic characterization on hemp commercial cultivars: Focus on inflorescence water extract activity. Food Chem. Toxicol. 2019, 125, 452–461. [Google Scholar] [CrossRef] [PubMed]
- Ali, E.M.; Almagboul, A.Z.; Khogali, S.M.; Gergeir, U.M. Antimicrobial activity of Cannabis sativa L. Chin. Med. 2012, 3, 61–64. [Google Scholar] [CrossRef]
- Blaskovich, M.A.; Kavanagh, A.M.; Elliott, A.G.; Zhang, B.; Ramu, S.; Amado, M.; Lowe, G.J.; Hinton, A.O.; Pham, D.M.T.; Zuegg, J. The antimicrobial potential of cannabidiol. Commun. Biol. 2021, 4, 7. [Google Scholar] [CrossRef]
- Frassinetti, S.; Gabriele, M.; Moccia, E.; Longo, V.; Di Gioia, D. Antimicrobial and antibiofilm activity of Cannabis sativa L. seeds extract against Staphylococcus aureus and growth effects on probiotic Lactobacillus spp. LWT 2020, 124, 109149. [Google Scholar] [CrossRef]
- Nafis, A.; Kasrati, A.; Jamali, C.A.; Mezrioui, N.; Setzer, W.; Abbad, A.; Hassani, L. Antioxidant activity and evidence for synergism of Cannabis sativa (L.) essential oil with antimicrobial standards. Ind. Crops Prod. 2019, 137, 396–400. [Google Scholar] [CrossRef]
- Elhendawy, M.A.; Wanas, A.S.; Radwan, M.M.; Azzaz, N.A.; Toson, E.S.; El Sohly, M.A. Chemical and biological studies of Cannabis sativa roots. Med. Cannabis Cannabinoids 2018, 1, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Galletta, M.; Reekie, T.A.; Nagalingam, G.; Bottomley, A.L.; Harry, E.J.; Kassiou, M.; Triccas, J.A. Rapid Antibacterial activity of cannabichromenic acid against methicillin-resistant Staphylococcus aureus. Antibiotics 2020, 9, 523. [Google Scholar] [CrossRef] [PubMed]
- Iseppi, R.; Brighenti, V.; Licata, M.; Lambertini, A.; Sabia, C.; Messi, P.; Pellati, F.; Benvenuti, S. Chemical characterization and evaluation of the antibacterial activity of essential oils from fibre-type Cannabis sativa L.(Hemp). Molecules 2019, 24, 2302. [Google Scholar] [CrossRef] [PubMed]
- Nissen, L.; Zatta, A.; Stefanini, I.; Grandi, S.; Sgorbati, B.; Biavati, B.; Monti, A. Characterization and antimicrobial activity of essential oils of industrial hemp varieties (Cannabis sativa L.). Fitoterapia 2010, 81, 413–419. [Google Scholar] [CrossRef] [PubMed]
- Wanas, A.S.; Radwan, M.M.; Mehmedic, Z.; Jacob, M.; Khan, I.A.; Elsohly, M.A. Antifungal activity of the volatiles of high potency Cannabis sativa L. against Cryptococcus neoformans. Rec. Nat. Prod. 2016, 10, 214. [Google Scholar]
- Nalli, Y.; Arora, P.; Riyaz-Ul-Hassan, S.; Ali, A. Chemical investigation of Cannabis sativa leading to the discovery of a prenylspirodinone with anti-microbial potential. Tetrahedron Lett. 2018, 59, 2470–2472. [Google Scholar] [CrossRef]
- Radwan, M.M.; El Sohly, M.A.; Slade, D.; Ahmed, S.A.; Khan, I.A.; Ross, S.A. Biologically active cannabinoids from high-potency Cannabis sativa. J. Nat. Prod. 2009, 72, 906–911. [Google Scholar] [CrossRef]
- As, R.; Blu, A.; Si, Z. Isolation and investigation of antibacterial properties of preparations from wild hemp (Cannabis ruderalis) growing in the Ukraine. Mikrobiolohichnyi Zhurnal 1959, 21, 40–48. [Google Scholar]
- Krejci, Z. Hemp (Cannabis sativa)---Antibiotic drugs. II. Method & results of bacteriological experiments & preliminary clinical experience. Pharmazie 1958, 13, 155–166. [Google Scholar]
- Fathordoobady, F.; Singh, A.; Kitts, D.D.; Singh, A.P. Hemp (Cannabis Sativa L.) extract: Anti-microbial properties, methods of extraction, and potential oral delivery. Food Rev. Int. 2019, 35, 664–684. [Google Scholar] [CrossRef]
- Novak, J.; Zitterl-Eglseer, K.; Deans, S.G.; Franz, C.M. Essential oils of different cultivars of Cannabis sativa L. and their antimicrobial activity. Flavour Fragr. J. 2001, 16, 259–262. [Google Scholar] [CrossRef]
- Lone, T.A.; Lone, R.A. Extraction of cannabinoids from Cannabis sativa L. plant and its potential antimicrobial activity. Univers. J. Med. Dent 2012, 1, 51–55. [Google Scholar]
- Sarmadyan, H.; Solhi, H.; Najarian-Araghi, N.; Ghaznavi-Rad, E. Determination of the Antimicrobial Effects of Hydro-Alcoholic Extract of Cannabis Sativa on Multiple Drug Resistant Bacteria Isolated from Nosocomial Infections. Iran. J. Toxicol. 2014, 7, 967–972. [Google Scholar]
- Vu, T.T.; Kim, H.; Tran, V.K.; Le Dang, Q.; Nguyen, H.T.; Kim, H.; Kim, I.S.; Choi, G.J.; Kim, J.-C. In vitro antibacterial activity of selected medicinal plants traditionally used in Vietnam against human pathogenic bacteria. BMC Complement. Altern. Med. 2015, 16, 32. [Google Scholar] [CrossRef] [PubMed]
- Lelario, F.; Scrano, L.; De Franchi, S.; Bonomo, M.; Salzano, G.; Milan, S.; Milella, L.; Bufo, S. Identification and antimicrobial activity of most representative secondary metabolites from different plant species. Chem. Biol. Technol. Agric. 2018, 5, 13. [Google Scholar] [CrossRef]
- Mikulcová, V.; Kašpárková, V.; Humpolíček, P.; Buňková, L. Formulation, characterization and properties of hemp seed oil and its emulsions. Molecules 2017, 22, 700. [Google Scholar] [CrossRef]
- Chakraborty, S.; Afaq, N.; Singh, N.; Majumdar, S. Antimicrobial activity of Cannabis sativa, Thuja orientalis and Psidium guajava leaf extracts against methicillin-resistant Staphylococcus aureus. J. Integr. Med. 2018, 16, 350–357. [Google Scholar] [CrossRef]
- Stahl, V.; Vasudevan, K. Comparison of efficacy of cannabinoids versus commercial oral care products in reducing bacterial content from dental plaque: A preliminary observation. Cureus 2020, 12, 6809. [Google Scholar] [CrossRef]
- Zengin, G.; Menghini, L.; Di Sotto, A.; Mancinelli, R.; Sisto, F.; Carradori, S.; Cesa, S.; Fraschetti, C.; Filippi, A.; Angiolella, L. Chromatographic analyses, in vitro biological activities, and cytotoxicity of Cannabis sativa L. essential oil: A multidisciplinary study. Molecules 2018, 23, 3266. [Google Scholar] [CrossRef]
- Jin, S.; Lee, M.-Y. The ameliorative effect of hemp seed hexane extracts on the Propionibacterium acnes-induced inflammation and lipogenesis in sebocytes. PLoS ONE 2018, 13, e0202933. [Google Scholar] [CrossRef] [PubMed]
- Nadir, I.; Rana, N.F.; Ahmad, N.M.; Tanweer, T.; Batool, A.; Taimoor, Z.; Riaz, S.; Ali, S.M. Cannabinoids and terpenes as an antibacterial and antibiofouling promotor for PES water filtration membranes. Molecules 2020, 25, 691. [Google Scholar] [CrossRef] [PubMed]
- Borges, A.; Abreu, A.C.; Dias, C.; Saavedra, M.J.; Borges, F.; Simões, M. New perspectives on the use of phytochemicals as an emergent strategy to control bacterial infections including biofilms. Molecules 2016, 21, 877. [Google Scholar] [CrossRef] [PubMed]
- Moo, C.-L.; Yang, S.-K.; Osman, M.-A.; Yuswan, M.H.; Loh, J.-Y.; Lim, W.-M.; Swee-Hua-Erin, L.; Lai, K.-S. Antibacterial Activity and Mode of Action of β-caryophyllene on. Pol. J. Microbiol. 2020, 69, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Feldman, M.; Sionov, R.; Smoum, R.; Mechoulam, R.; Ginsburg, I.; Steinberg, D. Comparative evaluation of combinatory interaction between endocannabinoid system compounds and Poly-L-lysine against Streptococcus mutans growth and biofilm formation. BioMed Res. Int. 2020, 2020, 7258380. [Google Scholar] [CrossRef]
- Al-Sadi, A.M.; Al-Oweisi, F.A.; Edwards, S.G.; Al-Nadabi, H.; Al-Fahdi, A.M. Genetic analysis reveals diversity and genetic relationship among Trichoderma isolates from potting media, cultivated soil and uncultivated soil. BMC Microbiol. 2015, 15, 147. [Google Scholar] [CrossRef]
- Saleemi, M.A.; Lim, V. Overview of antimicrobial polyurethane-based nanocomposite materials and associated signalling pathways. Eur. Polym. J. 2022, 167, 111087. [Google Scholar] [CrossRef]
- Quach, D.; Sakoulas, G.; Nizet, V.; Pogliano, J.; Pogliano, K. Bacterial cytological profiling (BCP) as a rapid and accurate antimicrobial susceptibility testing method for Staphylococcus aureus. EBioMedicine 2016, 4, 95–103. [Google Scholar] [CrossRef]
- Lamsa, A.; Liu, W.T.; Dorrestein, P.C.; Pogliano, K. The Bacillus subtilis cannibalism toxin SDP collapses the proton motive force and induces autolysis. Mol. Microbiol. 2012, 84, 486–500. [Google Scholar] [CrossRef]
- Afrin, F.; Chi, M.; Eamens, A.L.; Duchatel, R.J.; Douglas, A.M.; Schneider, J.; Gedye, C.; Woldu, A.S.; Dun, M.D. Can hemp help? Low-THC cannabis and non-THC cannabinoids for the treatment of cancer. Cancers 2020, 12, 1033. [Google Scholar] [CrossRef]
- Kis, B.; Ifrim, F.C.; Buda, V.; Avram, S.; Pavel, I.Z.; Antal, D.; Paunescu, V.; Dehelean, C.A.; Ardelean, F.; Diaconeasa, Z. Cannabidiol—From plant to human body: A promising bioactive molecule with multi-target effects in cancer. Int. J. Mol. Sci. 2019, 20, 5905. [Google Scholar] [CrossRef] [PubMed]
- Mangal, N.; Erridge, S.; Habib, N.; Sadanandam, A.; Reebye, V.; Sodergren, M.H. Cannabinoids in the landscape of cancer. J. Cancer Res. Clin. Oncol. 2021, 147, 2507–2534. [Google Scholar] [CrossRef] [PubMed]
- Hirao-Suzuki, M.; Takeda, S.; Koga, T.; Takiguchi, M.; Toda, A. Cannabidiolic acid dampens the expression of cyclooxygenase-2 in MDA-MB-231 breast cancer cells: Possible implication of the peroxisome proliferator-activated receptor β/δ abrogation. J. Toxicol. Sci. 2020, 45, 227–236. [Google Scholar] [CrossRef]
- Lin, Y.; Xu, J.; Lan, H. Tumor-associated macrophages in tumor metastasis: Biological roles and clinical therapeutic applications. J. Hematol. Oncol. 2019, 12, 76. [Google Scholar] [CrossRef] [PubMed]
- Nallathambi, R.; Mazuz, M.; Namdar, D.; Shik, M.; Namintzer, D.; Vinayaka, A.C.; Ion, A.; Faigenboim, A.; Nasser, A.; Laish, I.; et al. Identification of synergistic interaction between cannabis-derived compounds for cytotoxic activity in colorectal cancer cell lines and colon polyps that induces apoptosis-related cell death and distinct gene expression. Cannabis Cannabinoid Res. 2018, 3, 120–135. [Google Scholar] [CrossRef] [PubMed]
- Mohammadpour, F.; Ostad, S.N.; Aliebrahimi, S.; Daman, Z. Anti-invasion effects of cannabinoids agonist and antagonist on human breast cancer stem cells. Iran. J. Pharm. Res. 2017, 16, 1479. [Google Scholar] [PubMed]
- Gazzerro, P.; Malfitano, A.M.; Proto, M.C.; Santoro, A.; Pisanti, S.; Caruso, M.G.; Notarnicola, M.; Messa, C.; Laezza, C.; Misso, G.; et al. Synergistic inhibition of human colon cancer cell growth by the cannabinoid CB1 receptor antagonist rimonabant and oxaliplatin. Oncol. Rep. 2010, 23, 171–175. [Google Scholar]
- Fraguas-Sánchez, A.; Fernández-Carballido, A.; Simancas-Herbada, R.; Martin-Sabroso, C.; Torres-Suárez, A. CBD loaded microparticles as a potential formulation to improve paclitaxel and doxorubicin-based chemotherapy in breast cancer. Int. J. Pharm. 2020, 574, 118916. [Google Scholar] [CrossRef]
- López-Valero, I.; Saiz-Ladera, C.; Torres, S.; Hernández-Tiedra, S.; García-Taboada, E.; Rodríguez-Fornés, F.; Barba, M.; Dávila, D.; Salvador-Tormo, N.; Guzmán, M.; et al. Targeting Glioma Initiating Cells with A combined therapy of cannabinoids and temozolomide. Biochem. Pharmacol. 2018, 157, 266–274. [Google Scholar] [CrossRef]
- Singer, E.; Judkins, J.; Salomonis, N.; Matlaf, L.; Soteropoulos, P.; McAllister, S.; Soroceanu, L. Reactive oxygen species-mediated therapeutic response and resistance in glioblastoma. Cell Death Dis. 2015, 6, e1601. [Google Scholar] [CrossRef]
- Fisher, T.; Golan, H.; Schiby, G.; PriChen, S.; Smoum, R.; Moshe, I.; Peshes-Yaloz, N.; Castiel, A.; Waldman, D.; Gallily, R.; et al. In vitro and in vivo efficacy of non-psychoactive cannabidiol in neuroblastoma. Curr. Oncol. 2016, 23, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Ellert-Miklaszewska, A.; Ciechomska, I.A.; Kaminska, B. Synthetic cannabinoids induce autophagy and mitochondrial apoptotic pathways in human glioblastoma cells independently of deficiency in TP53 or PTEN tumor suppressors. Cancers 2021, 13, 419. [Google Scholar] [CrossRef] [PubMed]
- Ferro, R.; Adamska, A.; Lattanzio, R.; Mavrommati, I.; Edling, C.; Arifin, S.; Fyffe, C.; Sala, G.; Sacchetto, L.; Chiorino, G.; et al. GPR55 signalling promotes proliferation of pancreatic cancer cells and tumour growth in mice, and its inhibition increases effects of gemcitabine. Oncogene 2018, 37, 6368–6382. [Google Scholar] [CrossRef] [PubMed]
- Proto, M.C.; Fiore, D.; Piscopo, C.; Franceschelli, S.; Bizzarro, V.; Laezza, C.; Lauro, G.; Feoli, A.; Tosco, A.; Bifulco, G.; et al. Inhibition of Wnt/β-Catenin pathway and Histone acetyltransferase activity by Rimonabant: A therapeutic target for colon cancer. Sci. Rep. 2017, 7, 11678. [Google Scholar] [CrossRef] [PubMed]
- Fiore, D.; Ramesh, P.; Proto, M.C.; Piscopo, C.; Franceschelli, S.; Anzelmo, S.; Medema, J.P.; Bifulco, M.; Gazzerro, P. Rimonabant kills colon cancer stem cells without inducing toxicity in normal colon organoids. Front. Pharmacol. 2018, 8, 949. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Wang, J.; Zhou, Z.; He, Z.; Zhao, Q. Cannabinoid WIN55, 212–2 induces cell cycle arrest and inhibits the proliferation and migration of human BEL7402 hepatocellular carcinoma cells Corrigendum in/mmr/13/1/1054. Mol. Med. Rep. 2015, 12, 7963–7970. [Google Scholar] [CrossRef]
- Ortega, A.; García-Hernández, V.; Ruiz-García, E.; Meneses-García, A.; Herrera-Gómez, A.; Aguilar-Ponce, J.; Montes-Servín, E.; Prospero-García, O.; Del Angel, S. Comparing the effects of endogenous and synthetic cannabinoid receptor agonists on survival of gastric cancer cells. Life Sci. 2016, 165, 56–62. [Google Scholar] [CrossRef]
- Xian, X.; Huang, L.; Zhang, B.; Wu, C.; Cui, J.; Wang, Z. WIN 55,212-2 inhibits the epithelial mesenchymal transition of gastric cancer cells via COX-2 signals. Cell. Physiol. Biochem. 2016, 39, 2149–2157. [Google Scholar] [CrossRef]
- Orellana-Serradell, O.; Poblete, C.; Sanchez, C.; Castellón, E.; Gallegos, I.; Huidobro, C.; Llanos, M.; Contreras, H. Proapoptotic effect of endocannabinoids in prostate cancer cells. Oncol. Rep. 2015, 33, 1599–1608. [Google Scholar] [CrossRef]
- Morell, C.; Bort, A.; Vara, D.; Ramos-Torres, A.; Rodríguez-Henche, N.; Díaz-Laviada, I. The cannabinoid WIN 55,212-2 prevents neuroendocrine differentiation of LNCaP prostate cancer cells. Prostate Cancer Prostatic Dis. 2016, 19, 248–257. [Google Scholar] [CrossRef]
- Ravi, J.; Elbaz, M.; Wani, N.A.; Nasser, M.W.; Ganju, R.K. Cannabinoid receptor-2 agonist inhibits macrophage induced EMT in non-small cell lung cancer by downregulation of EGFR pathway. Mol. Carcinog. 2016, 55, 2063–2076. [Google Scholar] [CrossRef] [PubMed]
- Müller, L.; Radtke, A.; Decker, J.; Koch, M.; Belge, G. The synthetic cannabinoid WIN 55,212-2 elicits death in human cancer cell lines. Anticancer Res. 2017, 37, 6341–6345. [Google Scholar] [PubMed]
- Barbado, M.V.; Medrano, M.; Caballero-Velázquez, T.; Álvarez-Laderas, I.; Sánchez-Abarca, L.I.; García-Guerrero, E.; Martín-Sánchez, J.; Rosado, I.V.; Piruat, J.I.; Gonzalez-Naranjo, P.; et al. Cannabinoid derivatives exert a potent anti-myeloma activity both in vitro and in vivo. Int. J. Cancer 2017, 140, 674–685. [Google Scholar] [CrossRef] [PubMed]
- Kalenderoglou, N.; Macpherson, T.; Wright, K.L. Cannabidiol reduces leukemic cell size–but is it important? Front. Pharmacol. 2017, 8, 144. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, J.L.; Hill, D.S.; McKee, C.S.; Hernandez-Tiedra, S.; Lorente, M.; Lopez-Valero, I.; Anagnostou, M.E.; Babatunde, F.; Corazzari, M.; Redfern, C.P. Exploiting cannabinoid-induced cytotoxic autophagy to drive melanoma cell death. J. Investig. Dermatol. 2015, 135, 1629–1637. [Google Scholar] [CrossRef] [PubMed]
- Soliman, E.; Henderson, K.L.; Danell, A.S.; Van Dross, R. Arachidonoyl-ethanolamide activates endoplasmic reticulum stress-apoptosis in tumorigenic keratinocytes: Role of cyclooxygenase-2 and novel J-series prostamides. Mol. Carcinog. 2016, 55, 117–130. [Google Scholar] [CrossRef]
- Khan, M.I.; Sobocińska, A.A.; Brodaczewska, K.K.; Zielniok, K.; Gajewska, M.; Kieda, C.; Czarnecka, A.M.; Szczylik, C. Involvement of the CB2 cannabinoid receptor in cell growth inhibition and G0/G1 cell cycle arrest via the cannabinoid agonist WIN 55,212–2 in renal cell carcinoma. BMC Cancer 2018, 18, 583. [Google Scholar] [CrossRef]
- Akimov, M.G.; Gamisonia, A.M.; Dudina, P.V.; Gretskaya, N.M.; Gaydaryova, A.A.; Kuznetsov, A.S.; Zinchenko, G.N.; Bezuglov, V.V. GPR55 receptor activation by the N-acyl dopamine family lipids induces apoptosis in cancer cells via the nitric oxide synthase (nNOS) over-stimulation. Int. J. Mol. Sci. 2021, 22, 622. [Google Scholar] [CrossRef]
- Aviello, G.; Romano, B.; Borrelli, F.; Capasso, R.; Gallo, L.; Piscitelli, F.; Di Marzo, V.; Izzo, A.A. Chemopreventive effect of the non-psychotropic phytocannabinoid cannabidiol on experimental colon cancer. J. Mol. Med. 2012, 90, 925–934. [Google Scholar] [CrossRef]
- Borrelli, F.; Pagano, E.; Romano, B.; Panzera, S.; Maiello, F.; Coppola, D.; De Petrocellis, L.; Buono, L.; Orlando, P.; Izzo, A.A. Colon carcinogenesis is inhibited by the TRPM8 antagonist cannabigerol, a Cannabis-derived non-psychotropic cannabinoid. Carcinogenesis 2014, 35, 2787–2797. [Google Scholar] [CrossRef] [PubMed]
- Honarmand, M.; Namazi, F.; Mohammadi, A.; Nazifi, S. Can cannabidiol inhibit angiogenesis in colon cancer? Comp. Clin. Pathol. 2019, 28, 165–172. [Google Scholar] [CrossRef]
- Kargl, J.; Andersen, L.; Hasenöhrl, C.; Feuersinger, D.; Stančić, A.; Fauland, A.; Magnes, C.; El-Heliebi, A.; Lax, S.; Uranitsch, S.; et al. GPR55 promotes migration and adhesion of colon cancer cells indicating a role in metastasis. Br. J. Pharmacol. 2016, 173, 142–154. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.; Yun, H.K.; Jeong, Y.A.; Jo, M.J.; Kang, S.H.; Kim, J.L.; Kim, D.Y.; Park, S.H.; Kim, B.R.; Na, Y.J.; et al. Cannabidiol-induced apoptosis is mediated by activation of Noxa in human colorectal cancer cells. Cancer Lett. 2019, 447, 12–23. [Google Scholar] [CrossRef] [PubMed]
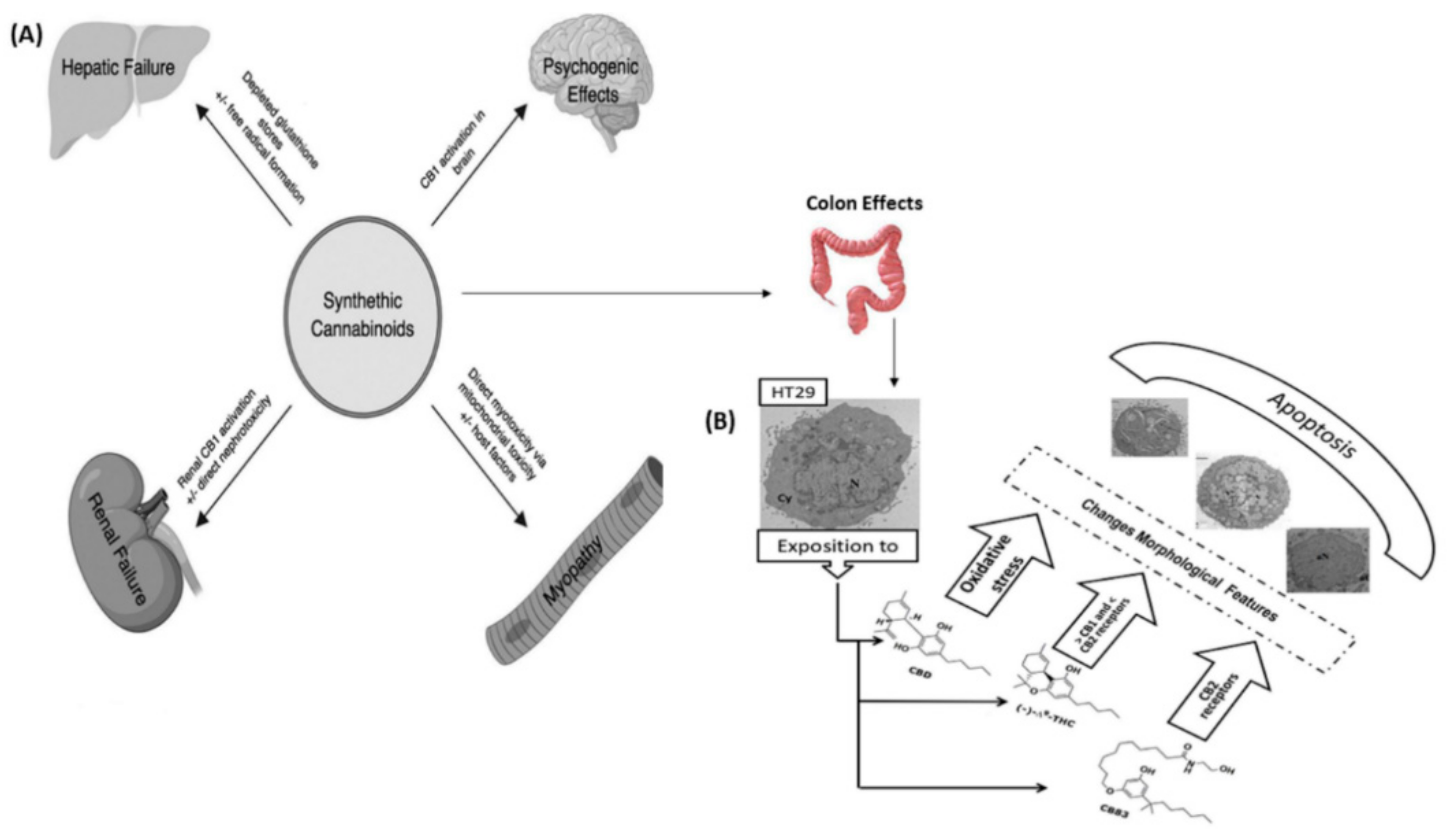
- Armstrong, F.; McCurdy, M.T.; Heavner, M.S. Synthetic cannabinoid-associated multiple organ failure: Case series and literature review. Pharmacother. J. Hum. Pharmacol. Drug Ther. 2019, 39, 508–513. [Google Scholar] [CrossRef] [PubMed]
- Cerretani, D.; Collodel, G.; Brizzi, A.; Fiaschi, A.I.; Menchiari, A.; Moretti, E.; Moltoni, L.; Micheli, L. Cytotoxic effects of cannabinoids on human HT-29 colorectal adenocarcinoma cells: Different mechanisms of THC, CBD, and CB83. Int. J. Mol. Sci. 2020, 21, 5533. [Google Scholar] [CrossRef] [PubMed]
- Ligresti, A.; Moriello, A.S.; Starowicz, K.; Matias, I.; Pisanti, S.; De Petrocellis, L.; Laezza, C.; Portella, G.; Bifulco, M.; Di Marzo, V. Antitumor activity of plant cannabinoids with emphasis on the effect of cannabidiol on human breast carcinoma. J. Pharmacol. Exp. Ther. 2006, 318, 1375–1387. [Google Scholar] [CrossRef]
- Shrivastava, A.; Kuzontkoski, P.M.; Groopman, J.E.; Prasad, A. Cannabidiol Induces Programmed Cell Death in Breast Cancer Cells by Coordinating the Cross-talk between Apoptosis and AutophagyCBD Induces Programmed Cell Death in Breast Cancer Cells. Mol. Cancer Ther. 2011, 10, 1161–1172. [Google Scholar] [CrossRef]
- Elbaz, M.; Nasser, M.W.; Ravi, J.; Wani, N.A.; Ahirwar, D.K.; Zhao, H.; Oghumu, S.; Satoskar, A.R.; Shilo, K.; Carson, W.E., III; et al. Modulation of the tumor microenvironment and inhibition of EGF/EGFR pathway: Novel anti-tumor mechanisms of Cannabidiol in breast cancer. Mol. Oncol. 2015, 9, 906–919. [Google Scholar] [CrossRef]
- García-Morales, L.; Castillo, A.M.; Ramírez, J.T.; Zamudio-Meza, H.; Domínguez-Robles, M.D.C.; Meza, I. CBD reverts the mesenchymal invasive phenotype of breast cancer cells induced by the inflammatory cytokine IL-1β. Int. J. Mol. Sci. 2020, 21, 2429. [Google Scholar] [CrossRef]
- Murase, R.; Kawamura, R.; Singer, E.; Pakdel, A.; Sarma, P.; Judkins, J.; Elwakeel, E.; Dayal, S.; Martinez-Martinez, E.; Amere, M.; et al. Targeting multiple cannabinoid anti-tumour pathways with a resorcinol derivative leads to inhibition of advanced stages of breast cancer. Br. J. Pharmacol. 2014, 171, 4464–4477. [Google Scholar] [CrossRef]
- McAllister, S.D.; Christian, R.T.; Horowitz, M.P.; Garcia, A.; Desprez, P.-Y. Cannabidiol as a novel inhibitor of Id-1 gene expression in aggressive breast cancer cells. Mol. Cancer Ther. 2007, 6, 2921–2927. [Google Scholar] [CrossRef] [PubMed]
- Ramer, R.; Heinemann, K.; Merkord, J.; Rohde, H.; Salamon, A.; Linnebacher, M.; Hinz, B. COX-2 and PPAR-γ Confer Cannabidiol-Induced Apoptosis of Human Lung Cancer CellsInduction of Cancer Cell Apoptosis by Cannabidiol. Mol. Cancer Ther. 2013, 12, 69–82. [Google Scholar] [CrossRef] [PubMed]
- Ramer, R.; Rohde, A.; Merkord, J.; Rohde, H.; Hinz, B. Decrease of plasminogen activator inhibitor-1 may contribute to the anti-invasive action of cannabidiol on human lung cancer cells. Pharm. Res. 2010, 27, 2162–2174. [Google Scholar] [CrossRef]
- McMahon, G.A.; Petitclerc, E.; Stefansson, S.; Smith, E.; Wong, M.K.; Westrick, R.J.; Ginsburg, D.; Brooks, P.C.; Lawrence, D.A. Plasminogen activator inhibitor-1 regulates tumor growth and angiogenesis. J. Biol. Chem. 2001, 276, 33964–33968. [Google Scholar] [CrossRef] [PubMed]
- Haustein, M.; Ramer, R.; Linnebacher, M.; Manda, K.; Hinz, B. Cannabinoids increase lung cancer cell lysis by lymphokine-activated killer cells via upregulation of ICAM-1. Biochem. Pharmacol. 2014, 92, 312–325. [Google Scholar] [CrossRef]
- Milian, L.; Mata, M.; Alcacer, J.; Oliver, M.; Sancho-Tello, M.; de Llano, J.J.M.; Camps, C.; Galbis, J.; Carretero, J.; Carda, C. Cannabinoid receptor expression in non-small cell lung cancer. Effectiveness of tetrahydrocannabinol and cannabidiol inhibiting cell proliferation and epithelial-mesenchymal transition in vitro. PLoS ONE 2020, 15, e0228909. [Google Scholar] [CrossRef] [PubMed]
- De Petrocellis, L.; Ligresti, A.; Moriello, A.S.; Iappelli, M.; Verde, R.; Stott, C.G.; Cristino, L.; Orlando, P.; Di Marzo, V. Non-THC cannabinoids inhibit prostate carcinoma growth in vitro and in vivo: Pro-apoptotic effects and underlying mechanisms. Br. J. Pharmacol. 2013, 168, 79–102. [Google Scholar] [CrossRef] [PubMed]
- Alharris, E.; Singh, N.P.; Nagarkatti, P.S.; Nagarkatti, M. Role of miRNA in the regulation of cannabidiol-mediated apoptosis in neuroblastoma cells. Oncotarget 2019, 10, 45. [Google Scholar] [CrossRef]
- Scott, K.A.; Dalgleish, A.G.; Liu, W.M. The combination of cannabidiol and Δ9-tetrahydrocannabinol enhances the anticancer effects of radiation in an orthotopic murine glioma model. Mol. Cancer Ther. 2014, 13, 2955–2967. [Google Scholar] [CrossRef]
- Scott, K.A.; Dennis, J.L.; Dalgleish, A.G.; Liu, W.M. Inhibiting heat shock proteins can potentiate the cytotoxic effect of cannabidiol in human glioma cells. Anticancer Res. 2015, 35, 5827–5837. [Google Scholar]
- López-Valero, I.; Torres, S.; Salazar-Roa, M.; García-Taboada, E.; Hernández-Tiedra, S.; Guzmán, M.; Sepúlveda, J.M.; Velasco, G.; Lorente, M. Optimization of a preclinical therapy of cannabinoids in combination with temozolomide against glioma. Biochem. Pharmacol. 2018, 157, 275–284. [Google Scholar] [CrossRef] [PubMed]
- De la Ossa, D.H.P.; Lorente, M.; Gil-Alegre, M.E.; Torres, S.; Garcia-Taboada, E.; Aberturas, M.D.R.; Molpeceres, J.; Velasco, G.; Torres-Suarez, A.I. Local delivery of cannabinoid-loaded microparticles inhibits tumor growth in a murine xenograft model of glioblastoma multiforme. PLoS ONE 2013, 8, e54795. [Google Scholar]
- Simmerman, E.; Qin, X.; Jack, C.Y.; Baban, B. Cannabinoids as a potential new and novel treatment for melanoma: A pilot study in a murine model. J. Surg. Res. 2019, 235, 210–215. [Google Scholar] [CrossRef] [PubMed]
- McKallip, R.J.; Jia, W.; Schlomer, J.; Warren, J.W.; Nagarkatti, P.S.; Nagarkatti, M. Cannabidiol-induced apoptosis in human leukemia cells: A novel role of cannabidiol in the regulation of p22phox and Nox4 expression. Mol. Pharmacol. 2006, 70, 897–908. [Google Scholar] [CrossRef] [PubMed]
- Holland, M.; Panetta, J.; Hoskins, J.; Bebawy, M.; Roufogalis, B.; Allen, J.; Arnold, J. The effects of cannabinoids on P-glycoprotein transport and expression in multidrug resistant cells. Biochem. Pharmacol. 2006, 71, 1146–1154. [Google Scholar] [CrossRef] [PubMed]
- Lukhele, S.T.; Motadi, L.R. Cannabidiol rather than Cannabis sativa extracts inhibit cell growth and induce apoptosis in cervical cancer cells. BMC Complement. Altern. Med. 2016, 16, 335. [Google Scholar] [CrossRef] [PubMed]
- Holland, M.L.; Allen, J.D.; Arnold, J.C. Interaction of plant cannabinoids with the multidrug transporter ABCC1 (MRP1). Eur. J. Pharmacol. 2008, 591, 128–131. [Google Scholar] [CrossRef]
- Morelli, M.B.; Offidani, M.; Alesiani, F.; Discepoli, G.; Liberati, S.; Olivieri, A.; Santoni, M.; Santoni, G.; Leoni, P.; Nabissi, M. The effects of cannabidiol and its synergism with bortezomib in multiple myeloma cell lines. A role for transient receptor potential vanilloid type-2. Int. J. Cancer 2014, 134, 2534–2546. [Google Scholar] [CrossRef]
- Fonseca, B.M.; Correia-da-Silva, G.; Teixeira, N. Cannabinoid-induced cell death in endometrial cancer cells: Involvement of TRPV1 receptors in apoptosis. J. Physiol. Biochem. 2018, 74, 261–272. [Google Scholar] [CrossRef]
- Vickers, N.J. Animal communication: When I’m calling you, will you answer too? Curr. Biol. 2017, 27, R713–R715. [Google Scholar] [CrossRef]
- NCCIH. Cannabis (Marijuana) and Cannabinoids: What You Need to Know. Available online: https://www.nccih.nih.gov/health/cannabis-marijuana-and-cannabinoids-what-you-need-to-know (accessed on 7 May 2020).
- Kamel, R. Transdermal drug delivery: Benefits and challenges. J. Appl. Pharm. 2015, 8, e103. [Google Scholar] [CrossRef]
- Tanwar, H.; Sachdeva, R. Transdermal drug delivery system: A review. Int. J. Pharm. Sci. Res. 2016, 7, 2274. [Google Scholar]
- Schofs, L.; Sparo, M.D.; Bruni, S.F.S. The antimicrobial effect behind Cannabis sativa. Pharmacol. Res. Perspect. 2021, 9, e00761. [Google Scholar] [CrossRef] [PubMed]
- Zou, S.; Kumar, U. Cannabinoid receptors and the endocannabinoid system: Signaling and function in the central nervous system. Int. J. Mol. Sci. 2018, 19, 833. [Google Scholar] [CrossRef]
- Turner, S.E.; Williams, C.M.; Iversen, L.; Whalley, B.J. Molecular pharmacology of phytocannabinoids. Phytocannabinoids 2017, 103, 61–101. [Google Scholar]
- Morales, P.; Hurst, D.P.; Reggio, P.H. Molecular targets of the phytocannabinoids: A complex picture. Phytocannabinoids 2017, 103, 103–131. [Google Scholar]
- Brierley, D.I.; Samuels, J.; Duncan, M.; Whalley, B.J.; Williams, C.M. Cannabigerol is a novel, well-tolerated appetite stimulant in pre-satiated rats. Psychopharmacology 2016, 233, 3603–3613. [Google Scholar] [CrossRef]
- Fraguas-Sánchez, A.I.; Torres-Suárez, A.I. Medical use of cannabinoids. Drugs 2018, 78, 1665–1703. [Google Scholar] [CrossRef]
- Gonçalves, J.; Rosado, T.; Soares, S.; Simão, A.Y.; Caramelo, D.; Luís, Â.; Fernández, N.; Barroso, M.; Gallardo, E.; Duarte, A.P. Cannabis and its secondary metabolites: Their use as therapeutic drugs, toxicological aspects, and analytical determination. Medicines 2019, 6, 31. [Google Scholar] [CrossRef]
- Sharma, P.; Murthy, P.; Bharath, M.S. Chemistry, metabolism, and toxicology of cannabis: Clinical implications. Iran. J. Psychiatry 2012, 7, 149. [Google Scholar]
- Schwilke, E.W.; Schwope, D.M.; Karschner, E.L.; Lowe, R.H.; Darwin, W.D.; Kelly, D.L.; Goodwin, R.S.; Gorelick, D.A.; Huestis, M.A. Δ9-tetrahydrocannabinol (THC), 11-hydroxy-THC, and 11-nor-9-carboxy-THC plasma pharmacokinetics during and after continuous high-dose oral THC. Clin. Chem. 2009, 55, 2180–2189. [Google Scholar] [CrossRef] [PubMed]


| Cancer Cell Lines | Cannabinoid (s) | Inhibitory Concentrations | In Vitro Activity | Ref. |
|---|---|---|---|---|
| WIN 55, JWH-133, AM251, SR144528 | 0–10 µM | Both CB1 and CB2 receptors are expressed by all cell lines. COX-2 signalling and apoptosis-mediated inhibition of cell migration and proliferation | [89] | |
| CBD (10), Capazepine, AM251, AM630 | 0–10 µM | Reduced cell viability, ER stress-induced autophagy and apoptosis, suppression of Akt, and mTOR signalling | [90] | |
| Human breast adenocarcinoma | CBD (10) | 1.5 µM | Inhibition of cell growth and invasion is achieved via modifying ERK and ROS, downregulating Id-1 expression, and upregulating Id-2 expression. | [91] |
| AEA, AM251 | 0–0.5 µM | Decrease in the invasiveness of CD44+/CD24−/low/ESA+ cancer stem cell | [92] | |
| CBDA (11), ST-247, GSK0660, GW501516 | 1–50 µM | CBDA (11) prevents transcriptional activation of PPARβ/δ | [93] | |
| CBD (10) | 1–50 µM | A synergistic effect observed after co-administration of CBDsol and paclitaxel or docetaxel | [94] | |
| Human glioblastoma | Δ9-THC (1), CBD (10) | 0–5 µM | The substantial apoptotic induction and GIC population reduction | [95] |
| CBD (10) | 0–5 µM | Downregulation of key stem cell regulators including Sox2 and p-STAT3 and activation of p-p38 pathway | [96] | |
| CBD (10), SR141716, SR144528 | 5–40 µM | Effects on apoptosis induction and antiproliferative activity | [96] | |
| Human neuroblastoma | Δ9-THC (1), CBD (10) | 0–50 µg/mL | Cell viability reduction and apoptosis | [97] |
| Human glioblastoma multiforme, Human GBM cultures | Δ9-THC (1), WIN 55,212–2 | 0.1 nM–2 µM | Increase in apoptosis and antiproliferative effects | [98] |
| Pancreatic cancer | CBD (10) | 0–10 µM | GPR55-mediated antiproliferative effects | [99] |
| Human colon cancer | SR141716 | 0–20 µM | Cell growth inhibition, a rise in caspase-3, and the cleavage of PARP | [100] |
| SR141716 | 0.1–20 µM | Reduction in the growth of colon CSCs and tumour-derived cells | [101] | |
| Human hepatocellular carcinoma | WIN 55, AM630, JWH-015 | 0, 5 or 10 µM | ERK1/2 phosphorylation is downregulated by CB2 | [102] |
| Human gastric adenocarcinoma | AEA, Meth-AEA (R-(+)), CP 55,940 | 0.5–5 µM | Effects of concentrations on changes in cell morphology | [103] |
| WIN 55, 212–2 | 5 µM | Prevention of cell invasion, migration, and EMT | [104] | |
| Human prostate adenocarcinoma | AEA, 2-AG, Methanandamide (AM-356), SR141716 | 2.5, 5 and 10 µM | Induction of apoptosis and cell cycle arrest | [105] |
| WIN 55, 212–2, SR141716, SR144528 | 0–10 µM | By inhibiting PI3K/Akt/mTOR signalling, WIN suppresses neuroendocrine differentiation | [106] | |
| Human NSCLC; A549 (epithelial), CALU1 (mesenchymal) | JWH-015, SR144528 | 0–5 µM | Decreased ability to migrate and invade through reductions in FAK, VCAM1, and MMP2 | [107] |
| Human lung cancer | WIN 55, 212–2 | 5–20 µM | Reduction in viability of cell due to apoptosis | [108] |
| Human myeloma | WIN 55, 212–2 | 5–50 µM | Apoptosis | [109] |
| Human T acute lymphoblastic leukaemia, Jurkat | CBD (10) | 0.01–10 µM | Decreased in viability of cell and cell cycle arrest | [110] |
| Human melanoma | Δ9-THC (1), CBD (10) | 0–10 µM | Decreased in viability of cell | [111] |
| Murine squamous, non-melanoma skin cancer | AEA, AMG9810, AM251, AM630 | 2.5–40 µM | Reduction in viability of cell due to apoptosis | [112] |
| Human renal carcinoma | WIN 55, 212–2, JWH-133, SR141716A, AM630 | 0–25 µM | Induction of apoptosis and reduction in cell proliferation | [113] |
| Human ovarian cancer | CBD (10) | 10–50 µM | Inhibition of proliferation of cell | [94] |
| Rat adrenal gland | DHA-DA, AEA | 0–80 µM | NOS activation, enhanced Ca2+ signalling, and GPR55 activation cause apoptosis | [114] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saleemi, M.A.; Yahaya, N.; Zain, N.N.M.; Raoov, M.; Yong, Y.K.; Noor, N.S.; Lim, V. Antimicrobial and Cytotoxic Effects of Cannabinoids: An Updated Review with Future Perspectives and Current Challenges. Pharmaceuticals 2022, 15, 1228. https://doi.org/10.3390/ph15101228
Saleemi MA, Yahaya N, Zain NNM, Raoov M, Yong YK, Noor NS, Lim V. Antimicrobial and Cytotoxic Effects of Cannabinoids: An Updated Review with Future Perspectives and Current Challenges. Pharmaceuticals. 2022; 15(10):1228. https://doi.org/10.3390/ph15101228
Chicago/Turabian StyleSaleemi, Mansab Ali, Noorfatimah Yahaya, Nur Nadhirah Mohamad Zain, Muggundha Raoov, Yoke Keong Yong, Nurul Shahfiza Noor, and Vuanghao Lim. 2022. "Antimicrobial and Cytotoxic Effects of Cannabinoids: An Updated Review with Future Perspectives and Current Challenges" Pharmaceuticals 15, no. 10: 1228. https://doi.org/10.3390/ph15101228
APA StyleSaleemi, M. A., Yahaya, N., Zain, N. N. M., Raoov, M., Yong, Y. K., Noor, N. S., & Lim, V. (2022). Antimicrobial and Cytotoxic Effects of Cannabinoids: An Updated Review with Future Perspectives and Current Challenges. Pharmaceuticals, 15(10), 1228. https://doi.org/10.3390/ph15101228








