Monocrotaline-Induced Pulmonary Arterial Hypertension and Bosentan Treatment in Rats: Focus on Plasma and Erythrocyte Parameters
Abstract
1. Introduction
2. Results
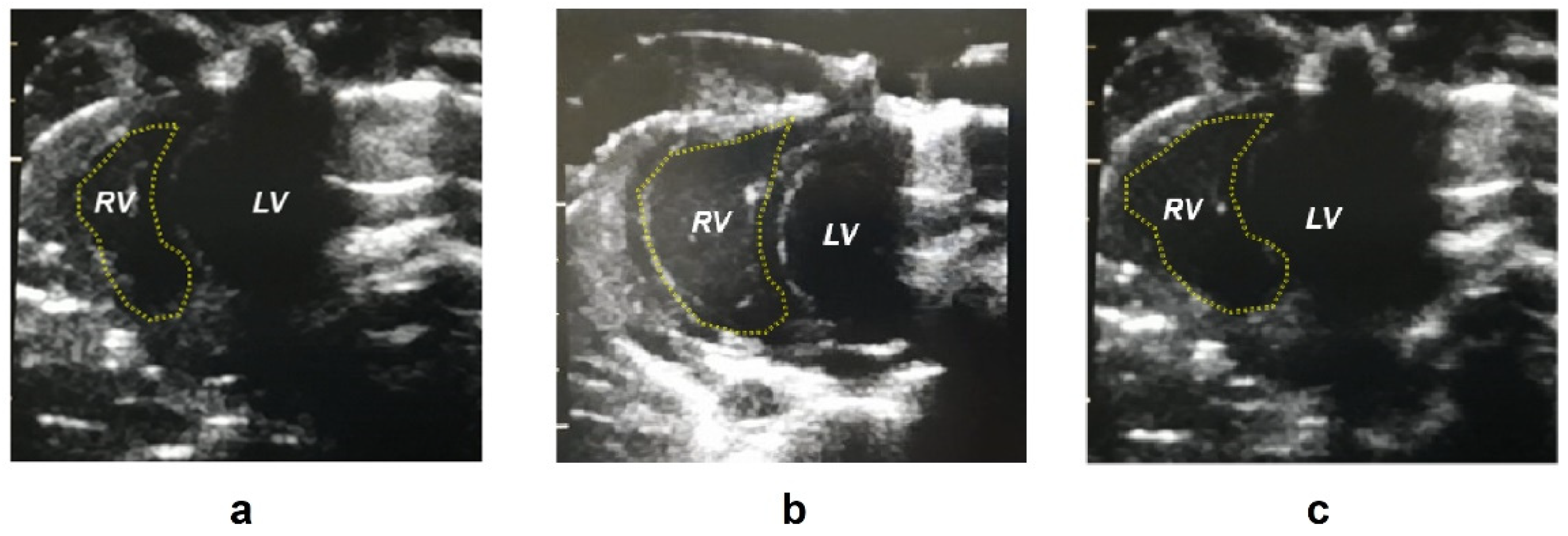
2.1. Biometric Parameters
2.2. Angiotensin Peptide and Aldosterone Concentrations in Blood Plasma
2.3. Parameters of Oxidative and Carbonyl Stress in Blood Plasma
2.4. Parameters of Antioxidative Status in Blood Plasma
2.5. MMP-2, MMP-9 Activity, and TIMP-1 Concentrations in Blood Plasma
2.6. Erythrocyte Parameters
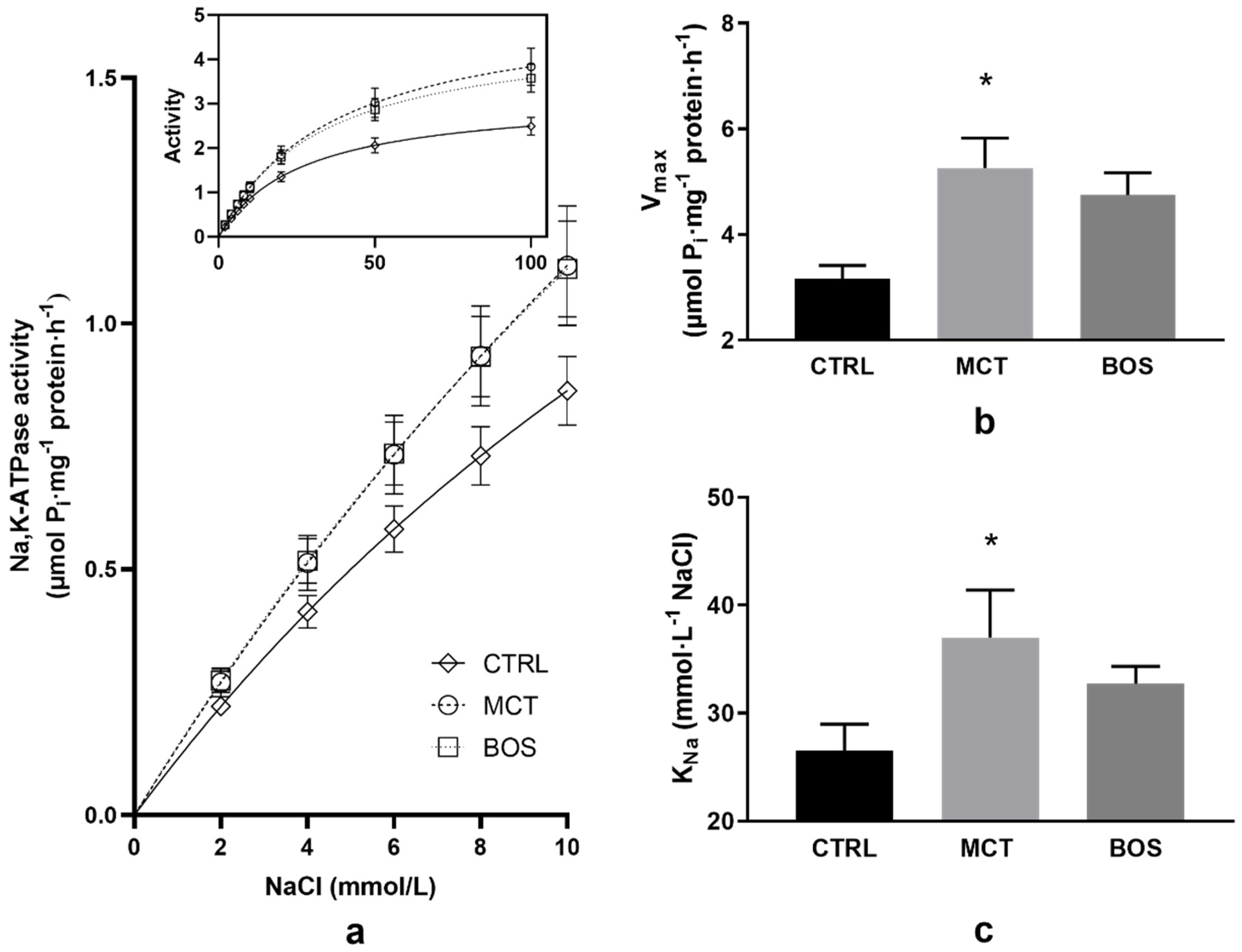
2.7. Na,K-ATPase Kinetics
3. Discussion
3.1. Biometry
3.2. Renin–Angiotensin System Peptides and Aldosterone Plasma Concentration
3.3. Oxidative Stress
3.4. MMPs
3.5. Erythrocyte Characteristics
4. Materials and Methods
4.1. Study Design
4.2. Pulmonary Artery Blood Pressure Measurements
4.3. Angiotensin Peptide Concentration Assessment
4.4. Parameters of Oxidative Stress and Antioxidant Status in Blood Plasma
4.5. MMP-2 and MMP-9 Activity and TIMP-1 Level in Blood Plasma
4.6. Erythrocyte Deformability, Nnitric Oxide Production, and Osmotic Resistance
4.7. Kinetic Measurements of Na,K-ATPase in Erythrocyte Membranes
4.8. Statistical Analyses
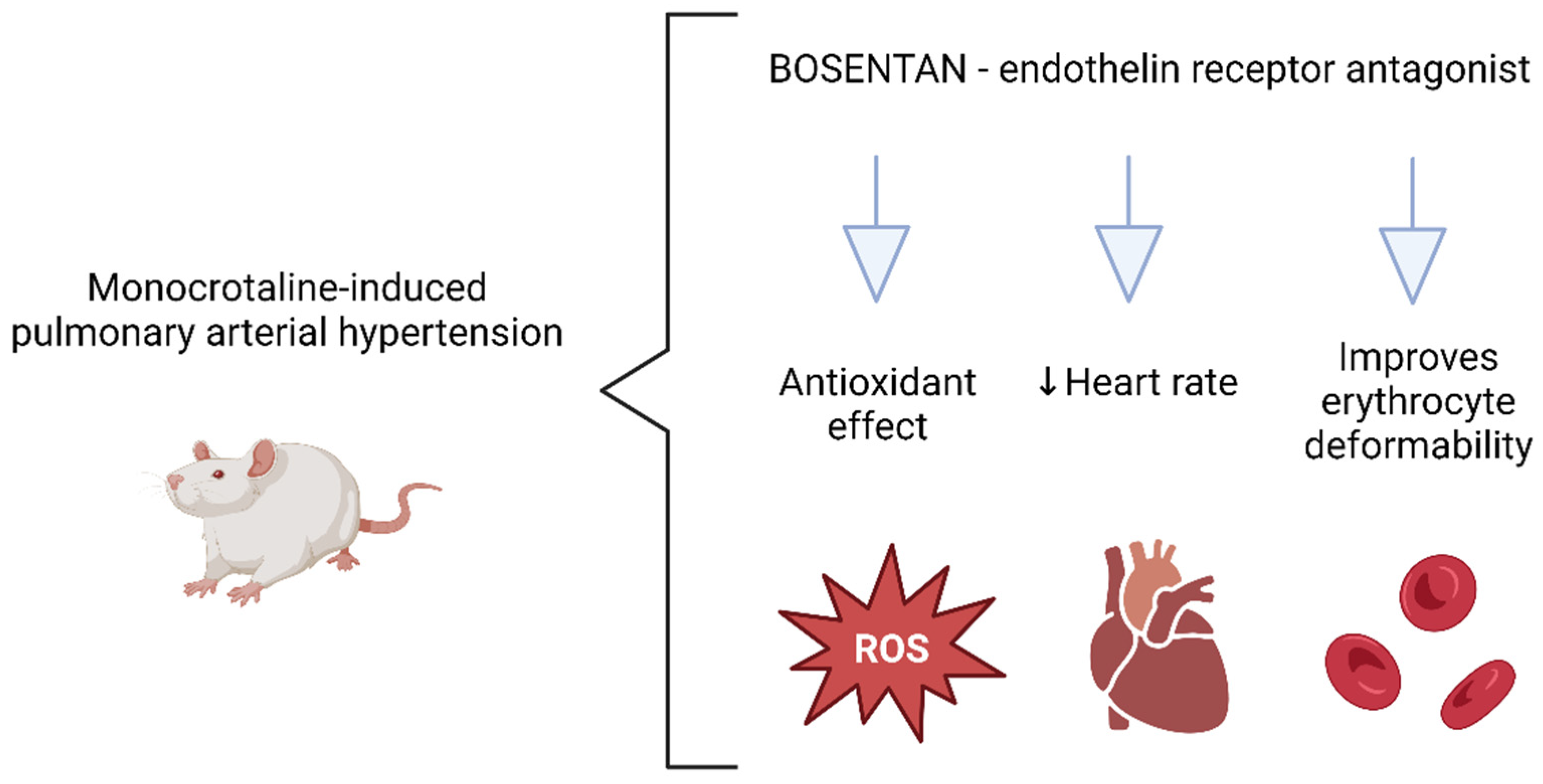
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gelzinis, T.A. Pulmonary Hypertension in 2021: Part I—Definition, Classification, Pathophysiology, and Presentation. J. Cardiothorac. Vasc. Anesthesia 2022, 36, 1552–1564. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.J.; Vorla, M.; Kalra, D.K. Molecular Pathways in Pulmonary Arterial Hypertension. Int. J. Mol. Sci. 2022, 23, 10001. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.-Q.; Zhu, S.-K.; Wang, M.; Wang, X.-A.; Tong, X.-H.; Wan, J.-Q.; Ding, J.-W. New progress in diagnosis and treatment of pulmonary arterial hypertension. J. Cardiothorac. Surg. 2022, 17, 216. [Google Scholar] [CrossRef] [PubMed]
- Nogueira-Ferreira, R.; Vitorino, R.; Henriques-Coelho, T. Exploring the monocrotaline animal model for the study of pulmonary arterial hypertension: A network approach. Pulm. Pharmacol. Ther. 2015, 35, 8–16. [Google Scholar] [CrossRef]
- Li, S.; Han, D.; Zhang, Y.; Xie, X.; Ke, R.; Zhu, Y.; Liu, L.; Song, Y.; Yang, L.; Li, M. Activation of AMPK Prevents Monocrotaline-Induced Extracellular Matrix Remodeling of Pulmonary Artery. Med. Sci. Monit. Basic Res. 2016, 22, 27–33. [Google Scholar] [CrossRef]
- Wu, Y.; Cai, C.; Yang, L.; Xiang, Y.; Zhao, H.; Zeng, C. Inhibitory effects of formononetin on the monocrotaline-induced pulmonary arterial hypertension in rats. Mol. Med. Rep. 2020, 21, 1192–1200. [Google Scholar] [CrossRef]
- Wetzl, V.; Tiede, S.L.; Faerber, L.; Weissmann, N.; Schermuly, R.T.; Ghofrani, H.A.; Gall, H. Plasma MMP2/TIMP4 Ratio at Follow-up Assessment Predicts Disease Progression of Idiopathic Pulmonary Arterial Hypertension. Lung 2017, 195, 489–496. [Google Scholar] [CrossRef]
- Hemnes, A.R.; Rathinasabapathy, A.; Austin, E.A.; Brittain, E.L.; Carrier, E.J.; Chen, X.; Fessel, J.P.; Fike, C.D.; Fong, P.; Fortune, N.; et al. A potential therapeutic role for angiotensin-converting enzyme 2 in human pulmonary arterial hypertension. Eur. Respir. J. 2018, 51, 1702638. [Google Scholar] [CrossRef]
- Radosinska, J.; Vrbjar, N. Erythrocyte Deformability and Na,K-ATPase Activity in Various Pathophysiological Situations and Their Protection by Selected Nutritional Antioxidants in Humans. Int. J. Mol. Sci. 2021, 22, 11924. [Google Scholar] [CrossRef]
- Yaylali, Y.T.; Kilic-Toprak, E.; Ozdemir, Y.; Senol, H.; Bor-Kucukatay, M. Impaired Blood Rheology in Pulmonary Arterial Hypertension. Heart Lung Circ. 2019, 28, 1067–1073. [Google Scholar] [CrossRef]
- Shubat, P.J.; Bowers, R.J.; Huxtable, R.J. Na+/K(+)-adenosine triphosphatase activity of pulmonary arteries after intoxication with the pyrrolizidine alkaloid, monocrotaline. J. Pharmacol. Exp. Ther. 1990, 252, 70–76. [Google Scholar] [PubMed]
- Tawa, M.; Furukawa, T.; Tongu, H.; Sugihara, M.; Taguwa, S.; Yamanaka, M.; Yano, Y.; Matsumori, H.; Kitada, R.; Sawano, T.; et al. Stimulation of nitric oxide-sensitive soluble guanylate cyclase in monocrotaline-induced pulmonary hypertensive rats. Life Sci. 2018, 203, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Basarici, I.; Özen, N.; Kilavuz, E.; Kısak, F.; Basrali, F.; Yaras, N.; Koksoy, S.; Celik, M.L.; Ulker, P. Concealed role of red blood cells in pathogenesis of pulmonary arterial hypertension: Decreased red blood cell nitric oxide generation and effect of Rho-Kinase inhibitor fasudil. Clin. Hemorheol. Microcirc. 2020, 76, 535–548. [Google Scholar] [CrossRef]
- Chen, X.; Zhai, Z.; Huang, K.; Xie, W.; Wan, J.; Wang, C. Bosentan therapy for pulmonary arterial hypertension and chronic thromboembolic pulmonary hypertension: A systemic review and meta-analysis. Clin. Respir. J. 2018, 12, 2065–2074. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.M.; Kwon, J.H.; Choi, S.; Kim, K.C. Apoptosis and Inflammation Associated Gene Expressions in Monocrotaline-Induced Pulmonary Hypertensive Rats after Bosentan Treatment. Korean Circ. J. 2014, 44, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Clozel, M.; Hess, P.; Rey, M.; Iglarz, M.; Binkert, C.; Qiu, C. Bosentan, sildenafil, and their combination in the monocrotaline model of pulmonary hypertension in rats. Exp. Biol. Med. 2006, 231, 967–973. [Google Scholar]
- Packer, M.; McMurray, J.; Massie, B.M.; Caspi, A.; Charlon, V.; Cohen-Solal, A.; Kiowski, W.; Kostuk, W.; Krum, H.; Levine, B.; et al. Clinical effects of endothelin receptor antagonism with bosentan in patients with severe chronic heart failure: Results of a pilot study. J. Card. Fail. 2005, 11, 12–20. [Google Scholar] [CrossRef]
- Trammell, A.W.; Hemnes, A.R.; Tseng, V.; Shah, A.J.; Phillips, L.S.; Hart, C.M. Influence of Body Weight and Diabetes Mellitus in Patients with Pulmonary Hypertension. Am. J. Cardiol. 2020, 134, 130–137. [Google Scholar] [CrossRef]
- Ahn, B.; Empinado, H.M.; Al-Rajhi, M.; Judge, A.R.; Ferreira, L.F. Diaphragm Atrophy and Contractile Dysfunction in a Murine Model of Pulmonary Hypertension. PLoS ONE 2013, 8, e62702. [Google Scholar] [CrossRef]
- Cao, Y.; Liu, M.; Wu, S.; Xu, J.; Wang, W.; Qi, X.; Ren, J.; Sun, J.; Chen, J.; Gong, L. Kupffer cells play a crucial role in monocrotaline-induced liver injury by producing TNF-α. Toxicology 2022, 468, 153101. [Google Scholar] [CrossRef]
- Huang, Z.; Zhao, Q.; Chen, M.; Zhang, J.; Ji, L. Liquiritigenin and liquiritin alleviated monocrotaline-induced hepatic sinusoidal obstruction syndrome via inhibiting HSP60-induced inflammatory injury. Toxicology 2019, 428, 152307. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Ruan, J.; Fu, P.P.; Lin, G. Cytotoxicity of pyrrolizidine alkaloid in human hepatic parenchymal and sinusoidal endothelial cells: Firm evidence for the reactive metabolites mediated pyrrolizidine alkaloid-induced hepatotoxicity. Chem. Interact. 2016, 243, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Gewehr, D.M.; Giovanini, A.F.; Mattar, B.A.; Agulham, A.P.; Bertoldi, A.D.S.; Nagashima, S.; Kubrusly, F.B.; Kubrusly, L.F. Congestive Hepatopathy Secondary to Right Ventricular Hypertrophy Related to Monocrotaline-Induced Pulmonary Arterial Hypertension. Int. J. Mol. Sci. 2021, 22, 11891. [Google Scholar] [CrossRef] [PubMed]
- Maki, H.; Hara, T.; Tsuji, M.; Saito, A.; Minatsuki, S.; Inaba, T.; Amiya, E.; Hosoya, Y.; Hatano, M.; Morita, H.; et al. The Clinical Efficacy of Endothelin Receptor Antagonists in Patients with Pulmonary Arterial Hypertension. Int. Heart J. 2020, 61, 799–805. [Google Scholar] [CrossRef]
- Pehlivan, Y.; Dokuyucu, R.; Demir, T.; Kaplan, D.S.; Koç, I.; Orkmez, M.; Turkbeyler, I.H.; Çeríbaşi, A.O.; Tutar, E.; Taysi, S.; et al. Palosuran Treatment Effective as Bosentan in the Treatment Model of Pulmonary Arterial Hypertension. Inflammation 2014, 37, 1280–1288. [Google Scholar] [CrossRef]
- An, X.; Li, S.; Weng, X.; Wang, X.; Wu, H.; Zhang, X.; Gao, J.; Yang, R.; Peng, B. Maxingxiongting mixture attenuates hypoxia pulmonary arterial hypertension to improve right ventricular hypertrophy by inhibiting the rho-kinase signaling pathway. J. Tradit. Chin. Med. 2020, 40, 992–998. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, J.; Xiao, M.; Wang, J.; Yao, F.; Zeng, W.; Yu, L.; Guan, Y.; Wei, W.; Peng, Z.; et al. Mesenchymal stem cell–derived microvesicles alleviate pulmonary arterial hypertension by regulating renin-angiotensin system. J. Am. Soc. Hypertens. 2018, 12, 470–478. [Google Scholar] [CrossRef]
- Wilson, D.N.; Schacht, S.E.; Al-Nakkash, L.; Babu, J.R.; Broderick, T.L. Resveratrol prevents pulmonary trunk remodeling but not right ventricular hypertrophy in monocrotaline-induced pulmonary hypertension. Pathophysiology 2016, 23, 243–250. [Google Scholar] [CrossRef]
- de Man, F.S.; Tu, L.; Handoko, M.L.; Rain, S.; Ruiter, G.; François, C.; Schalij, I.; Dorfmüller, P.; Simonneau, G.; Fadel, E.; et al. Dysregulated Renin–Angiotensin–Aldosterone System Contributes to Pulmonary Arterial Hypertension. Am. J. Respir. Crit. Care Med. 2012, 186, 780–789. [Google Scholar] [CrossRef]
- Falcão-Pires, I.; Gonçalves, N.; Henriques-Coelho, T.; Moreira-Gonçalves, D.; Jr, R.R.-A.; Leite-Moreira, A.F. Apelin decreases myocardial injury and improves right ventricular function in monocrotaline-induced pulmonary hypertension. Am. J. Physiol. Circ. Physiol. 2009, 296, H2007–H2014. [Google Scholar] [CrossRef]
- Sandoval, J.; Del Valle-Mondragón, L.; Masso, F.; Zayas, N.; Pulido, T.; Teijeiro, R.; Gonzalez-Pacheco, H.; Olmedo-Ocampo, R.; Sisniega, C.; Paez-Arenas, A.; et al. Angiotensin converting enzyme 2 and angiotensin (1–7) axis in pulmonary arterial hypertension. Eur. Respir. J. 2020, 56, 1902416. [Google Scholar] [CrossRef] [PubMed]
- Breitling, S.; Krauszman, A.; Parihar, R.; Walther, T.; Friedberg, M.K.; Kuebler, W.M. Dose-Dependent, Therapeutic Potential of Angiotensin-(1–7) for the Treatment of Pulmonary Arterial Hypertension. Pulm. Circ. 2015, 5, 649–657. [Google Scholar] [CrossRef]
- Wang, Y.; Zhong, B.; Wu, Q.; Zhu, T.; Wang, Y.; Zhang, M. Aldosterone Contributed to Pulmonary Arterial Hypertension Development via Stimulating Aquaporin Expression and Pulmonary Arterial Smooth Muscle Cells Proliferation. Pharmacology 2020, 105, 405–415. [Google Scholar] [CrossRef] [PubMed]
- Broderick, T.L.; Wang, Y.; Gutkowska, J.; Wang, D.; Jankowski, M. Downregulation of oxytocin receptors in right ventricle of rats with monocrotaline-induced pulmonary hypertension. Acta Physiol. 2010, 200, 147–158. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Jiang, X.-M.; Zhang, J.; Li, B.; Li, J.; Xie, D.-J.; Hu, Z.-Y. Pulmonary artery denervation improves pulmonary arterial hypertension induced right ventricular dysfunction by modulating the local renin-angiotensin-aldosterone system. BMC Cardiovasc. Disord. 2016, 16, 192. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Wang, Y.; Lu, S.; Hu, J.; Zeng, X.; Liu, W.; Wang, Z. Sacubitril/valsartan treatment relieved the progression of established pulmonary hypertension in rat model and its mechanism. Life Sci. 2021, 266, 118877. [Google Scholar] [CrossRef]
- Rampa, D.R.; Murugesan, P.; Chao, H.; Feng, H.; Dai, W.; Lee, D.; Pekcec, A.; Doods, H.; Wu, D. Reversal of pulmonary arterial hypertension and neointimal formation by kinin B1 receptor blockade. Respir. Res. 2021, 22, 281. [Google Scholar] [CrossRef]
- Rademaker, M.T.; AScott, N.J.; Koh, C.Y.; Kini, R.M.; Richards, A.M. Natriuretic peptide analogues with distinct vasodilatory or renal activity: Integrated effects in health and experimental heart failure. Cardiovasc. Res. 2021, 117, 508–519. [Google Scholar] [CrossRef]
- Chen, Y.; Li, F.; Luo, J.; Chen, J.; Luo, P.; Li, J. Comparative Efficacy and Safety of Targeted Therapies for Chronic Thromboembolic Pulmonary Hypertension: A Systematic Review and Network Meta-Analysis. Can. Respir. J. 2021, 2021, 1626971. [Google Scholar] [CrossRef]
- Vlachogeorgos, G.S.; Daskalopoulos, N.; Blatsiotis, P.; Kourbeti, I.S.; Mantas, I.; Stathopoulos, G.T. Bosentan for patients with echocardiographic evidence of pulmonary hypertension due to long-standing rheumatic mitral stenosis. Hell. J. Cardiol. 2015, 56, 36–43. [Google Scholar]
- Dianat, M.; Radan, M.; Mard, S.A.; Sohrabi, F.; Saryazdi, S.S.N. Contribution of reactive oxygen species via the OXR1 signaling pathway in the pathogenesis of monocrotaline-induced pulmonary arterial hypertension: The protective role of Crocin. Life Sci. 2020, 256, 117848. [Google Scholar] [CrossRef]
- Furtado, R.G.; Rassi, D.D.C.; Melato, L.H.; de Oliveira, A.C.R.; Nunes, P.M.; Baccelli, P.E.; Santos, S.C.d.O.; Santos, V.E.; Rassi, L.; Nunes, C.G. Segurança do Agente de Contraste SF6 (SonoVue®) no Ecocardiograma sob Estresse Farmacológico. Arq. Bras. Cardiol. 2021, 117, 1170–1178. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Hanaoka, M.; Droma, Y.; Chen, P.; Voelkel, N.F.; Kubo, K. Endothelin-1 receptor antagonists prevent the development of pulmonary emphysema in rats. Eur. Respir. J. 2010, 35, 904–912. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.K.; Saxena, A.; Singh, U.; Arya, D.S. Bosentan, the mixed ETA–ETB endothelin receptor antagonist, attenuated oxidative stress after experimental myocardial ischemia and reperfusion. Mol. Cell. Biochem. 2005, 275, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Lepetit, H.; Eddahibi, S.; Fadel, E.; Frisdal, E.; Munaut, C.; Noel, A.; Humbert, M.; Adnot, S.; D’Ortho, M.-P.; Lafuma, C. Smooth muscle cell matrix metalloproteinases in idiopathic pulmonary arterial hypertension. Eur. Respir. J. 2005, 25, 834–842. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Wang, D.; Wang, H.; Chen, L.; Sun, X.; Wan, Y. Inhibition of HDAC1 alleviates monocrotaline-induced pulmonary arterial remodeling through up-regulation of miR-34a. Respir. Res. 2021, 22, 239. [Google Scholar] [CrossRef]
- Koo, H.S.; Kim, K.C.; Hong, Y.M. Gene Expressions of Nitric Oxide Synthase and Matrix Metalloproteinase-2 in Monocrotaline-Induced Pulmonary Hypertension in Rats After Bosentan Treatment. Korean Circ. J. 2011, 41, 83–90. [Google Scholar] [CrossRef]
- Buehler, P.W.; Baek, J.H.; Lisk, C.; Connor, I.; Sullivan, T.; Kominsky, D.; Majka, S.; Stenmark, K.R.; Nozik-Grayck, E.; Bonaventura, J.; et al. Free hemoglobin induction of pulmonary vascular disease: Evidence for an inflammatory mechanism. Am. J. Physiol. Cell. Mol. Physiol. 2012, 303, L312–L326. [Google Scholar] [CrossRef]
- Rafikova, O.; Williams, E.R.; McBride, M.L.; Zemskova, M.; Srivastava, A.; Nair, V.; Desai, A.; Langlais, P.R.; Zemskov, E.; Simon, M.; et al. Hemolysis-induced Lung Vascular Leakage Contributes to the Development of Pulmonary Hypertension. Am. J. Respir. Cell Mol. Biol. 2018, 59, 334–345. [Google Scholar] [CrossRef]
- Chesney, C.; Allen, J.; Hsu, I. Right ventricular hypertrophy in monocrotaline pyrrole treated rats. Exp. Mol. Pathol. 1974, 20, 257–268. [Google Scholar] [CrossRef]
- Yavuz, T.; Uzun, O.; Macit, A.; Comunoglu, C.; Yavuz, O.; Silan, C.; Yuksel, H.; Yildirim, H.A. Pyrrolidine dithiocarbamate attenuates the development of monocrotaline-induced pulmonary arterial hypertension. Pathol.-Res. Pract. 2013, 209, 302–308. [Google Scholar] [CrossRef] [PubMed]
- Hammad, M.A.; Abdel-Bakky, M.S.; Walker, L.A.; Ashfaq, M.K. Tissue factor antisense deoxyoligonucleotide prevents monocrotaline/LPS hepatotoxicity in mice. J. Appl. Toxicol. 2013, 33, 774–783. [Google Scholar] [CrossRef] [PubMed]
- Smukowska-Gorynia, A.; Tomaszewska, I.; Malaczynska-Rajpold, K.; Marcinkowska, J.; Komosa, A.; Janus, M.; Olasinska-Wisniewska, A.; Slawek, S.; Araszkiewicz, A.; Jankiewicz, S.; et al. Red Blood Cells Distribution Width as a Potential Prognostic Biomarker in Patients with Pulmonary Arterial Hypertension and Chronic Thromboembolic Pulmonary Hypertension. Heart Lung Circ. 2018, 27, 842–848. [Google Scholar] [CrossRef] [PubMed]
- Baron, M.D.M.; Yu, B.; Lei, M.C.; Bagchi, A.; Beloiartsev, M.A.; Stowell, M.C.P.; Steinbicker, M.A.U.; Malhotra, R.; Bloch, M.K.D.; Zapol, M.W.M. Pulmonary Hypertension in Lambs Transfused with Stored Blood Is Prevented by Breathing Nitric Oxide. Anesthesiology 2012, 116, 637–647. [Google Scholar] [CrossRef] [PubMed]
- Berra, L.; Pinciroli, R.; Stowell, C.P.; Wang, L.; Yu, B.; Fernandez, B.O.; Feelisch, M.; Mietto, C.; Hod, E.A.; Chipman, D.; et al. Autologous Transfusion of Stored Red Blood Cells Increases Pulmonary Artery Pressure. Am. J. Respir. Crit. Care Med. 2014, 190, 800–807. [Google Scholar] [CrossRef] [PubMed]
- Sabaa, N.; de Franceschi, L.; Bonnin, P.; Castier, Y.; Malpeli, G.; Debbabi, H.; Galaup, A.; Maier-Redelsperger, M.; Vandermeersch, S.; Scarpa, A.; et al. Endothelin receptor antagonism prevents hypoxia-induced mortality and morbidity in a mouse model of sickle-cell disease. J. Clin. Investig. 2008, 118, 1924–1933. [Google Scholar] [CrossRef]
- Tang, B.; Kang, P.; Zhu, L.; Xuan, L.; Wang, H.; Zhang, H.; Wang, X.; Xu, J. Simvastatin protects heart function and myocardial energy metabolism in pulmonary arterial hypertension induced right heart failure. J. Bioenerg. Biomembr. 2021, 53, 1–12. [Google Scholar] [CrossRef]
- Urboniene, D.; Haber, I.; Fang, Y.-H.; Thenappan, T.; Archer, S.L. Validation of high-resolution echocardiography and magnetic resonance imaging vs. high-fidelity catheterization in experimental pulmonary hypertension. Am. J. Physiol. Cell. Mol. Physiol. 2010, 299, L401–L412. [Google Scholar] [CrossRef]
- Basu, R.; Poglitsch, M.; Yogasundaram, H.; Thomas, J.; Rowe, B.H.; Oudit, G.Y. Roles of Angiotensin Peptides and Recombinant Human ACE2 in Heart Failure. J. Am. Coll. Cardiol. 2017, 69, 805–819. [Google Scholar] [CrossRef]
- Kollarova, M.; Puzserova, A.; Balis, P.; Radosinska, D.; Tothova, L.; Bartekova, M.; Barancik, M.; Radosinska, J. Age- and Phenotype-Dependent Changes in Circulating MMP-2 and MMP-9 Activities in Normotensive and Hypertensive Rats. Int. J. Mol. Sci. 2020, 21, 7286. [Google Scholar] [CrossRef]
- Jasenovec, T.; Radosinska, D.; Kollarova, M.; Balis, P.; Dayar, E.; Bernatova, I.; Zorad, S.; Vrbjar, N.; Cacanyova, S.; Radosinska, J. Angiotensin System Modulations in Spontaneously Hypertensive Rats and Consequences on Erythrocyte Properties; Action of MLN-4760 and Zofenopril. Biomedicines 2021, 9, 1902. [Google Scholar] [CrossRef] [PubMed]
- Jasenovec, T.; Radosinska, D.; Kollarova, M.; Balis, P.; Ferenczyova, K.; Kalocayova, B.; Bartekova, M.; Tothova, L.; Radosinska, J. Beneficial Effect of Quercetin on Erythrocyte Properties in Type 2 Diabetic Rats. Molecules 2021, 26, 4868. [Google Scholar] [CrossRef] [PubMed]
- Radosinska, J.; Mezesova, L.; Okruhlicova, L.; Frimmel, K.; Breierova, E.; Bartekova, M.; Vrbjar, N. Effect of yeast biomass with high content of carotenoids on erythrocyte deformability, NO production and Na,K-ATPase activity in healthy and LPS treated rats. Clin. Hemorheol. Microcirc. 2016, 64, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Radosinska, J.; Jasenovec, T.; Puzserova, A.; Krajcir, J.; Lacekova, J.; Kucerova, K.; Kalnovicova, T.; Tothova, L.; Kovacicova, I.; Vrbjar, N. Promotion of whole blood rheology after vitamin C supplementation: Focus on red blood cells. Can. J. Physiol. Pharmacol. 2019, 97, 837–843. [Google Scholar] [CrossRef]

| CTRL (n = 11) | MCT (n = 10) | BOS (n = 9) | |
|---|---|---|---|
| Body weight (g) | 431 ± 17.4 * | 408 ± 21.5 | 412 ± 17.6 |
| Blood pressure (mmHg) | 139 ± 5.61 | 143 ± 7.05 | 139 ± 7.65 |
| Heart rate (min−1) | 380 ± 29.1 | 408 ± 36.3 | 347 ± 39.3 ** |
| Right ventricle area d (mm2) | 28.0 ± 5.26 **** | 48.3 ± 5.77 | 44.3 ± 7.55 |
| Right ventricle wall thickness (mm) | 1.34 ± 0.39 * | 1.70 ± 0.14 | 1.34 ± 0.15 * |
| Right ventricle/body weight (g/kg) | 0.46 ± 0.11 | 0.65 ± 0.22 | 0.63 ± 0.27 |
| PASP (mmHg) | 12.9 ± 8.87 **** | 60.4 ± 11.7 | 58.5 ± 9.99 |
| mPAP (mmHg) | 13.1 ± 3.25 **** | 30.6 ± 4.33 | 29.8 ± 3.67 |
| Lungs/body weight (g/kg) | 4.27 ± 0.36 *** | 5.20 ± 0.56 | 5.31 ± 0.59 |
| Liver/body weight (g/kg) | 36.5 ± 1.89 * | 39.1 ± 1.39 | 38.6 ± 3.04 |
| CTRL (n = 11) | MCT (n = 10) | BOS (n = 9) | |
|---|---|---|---|
| Ang I (1–10) (pmol/L) | 398 ± 142 ** | 738 ± 265 | 576 ± 266 |
| Ang II (1–8) (nmol/L) | 0.80 ± 0.26 | 1.08 ± 0.43 | 1.04 ± 0.24 |
| Ang 1–7 (pmol/L) | 11.9 ± 2.92 ** | 26.3 ± 11.3 | 19.1 ± 7.25 |
| Ang 1–5 (pmol/L) | 34.4 ± 11.7 * | 58.6 ± 26.6 | 42.4 ± 15.6 |
| Ang III (2–8) (pmol/L) | 38.5 ± 12.6 | 51.6 ± 20.8 | 50.9 ± 12.0 |
| Ang IV (3–8) (pmol/L) | 44.6 ± 12.3 | 60.7 ± 20.4 | 55.0 ± 13.0 |
| Aldosterone (nmol/L) | 0.51 (0.31; 1.22) ** | 0.10 (0.08; 0.20) | 0.51 (0.16; 0.92) * |
| CTRL (n = 11) | MCT (n = 10) | BOS (n = 9) | |
|---|---|---|---|
| AGEs (g/g protein) | 0.19 ± 0.06 | 0.20 ± 0.07 | 0.15 ± 0.04 |
| AOPP (µmol/g protein) | 8.53 ± 4.93 | 12.4 ± 9.80 | 4.51 ± 1.99 * |
| Fructosamine (mmol/g protein) | 0.56 ± 0.06 | 0.66 ± 0.14 | 0.36 ± 0.12 **** |
| TBARS (µmol/L) | 74.1 ± 17.5 | 101 ± 19.5 | 97.7 ± 43.4 |
| FRAP (µmol/L) | 201 ± 42.6 | 204 ± 44.1 | 187 ± 16.2 |
| TAC (mmol/L) | 4.43 ± 0.42 | 4.58 ± 0.26 | 4.20 ± 0.38 |
| GSH/GSSG | 58.8 ± 22.1 | 62.1 ± 38.3 | 118 ± 75.7 * |
| CTRL (n = 11) | MCT (n = 10) | BOS (n = 9) | |
|---|---|---|---|
| MMP-2 (a.u.) | 7.97 ± 1.09 | 8.11 ± 2.26 | 6.52 ± 1.37 |
| MMP-9 (a.u.) | 3.09 ± 0.93 | 2.81 ± 0.92 | 2.56 ± 1.34 |
| TIMP-1 (ng/mL) | 1.98 ± 0.26 | 1.89 ± 0.19 | 1.88 ± 0.15 |
| CTRL (n = 11) | MCT (n = 10) | BOS (n = 9) | |
|---|---|---|---|
| Count (109/mL) | 7.49 ± 0.42 | 7.25 ± 0.47 | 7.17 ± 0.28 |
| Hematocrit (%) | 43.8 ± 3.99 * | 39.6 ± 1.74 | 39.5 ± 2.34 |
| Mean Cell Volume (fL) | 56.0 ± 2.10 | 55.5 ± 1.12 | 56.0 ± 0.70 |
| RDW-SD (fL) | 27.7 ± 1.50 | 26.8 ± 1.01 | 27.1 ± 1.11 |
| Deformability (%) | 37.0 ± 3.65 | 32.1 ± 7.08 | 40.5 ± 5.24 * |
| IC50 (% NaCl) | 0.46 ± 0.02 | 0.46 ± 0.01 | 0.46 ± 0.02 |
| Nitric Oxide Production (a.u.) | 9.93 ± 2.22 | 8.58 ± 2.68 | 9.33 ± 2.29 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jasenovec, T.; Radosinska, D.; Kollarova, M.; Vrbjar, N.; Balis, P.; Trubacova, S.; Paulis, L.; Tothova, L.; Shawkatova, I.; Radosinska, J. Monocrotaline-Induced Pulmonary Arterial Hypertension and Bosentan Treatment in Rats: Focus on Plasma and Erythrocyte Parameters. Pharmaceuticals 2022, 15, 1227. https://doi.org/10.3390/ph15101227
Jasenovec T, Radosinska D, Kollarova M, Vrbjar N, Balis P, Trubacova S, Paulis L, Tothova L, Shawkatova I, Radosinska J. Monocrotaline-Induced Pulmonary Arterial Hypertension and Bosentan Treatment in Rats: Focus on Plasma and Erythrocyte Parameters. Pharmaceuticals. 2022; 15(10):1227. https://doi.org/10.3390/ph15101227
Chicago/Turabian StyleJasenovec, Tomas, Dominika Radosinska, Marta Kollarova, Norbert Vrbjar, Peter Balis, Simona Trubacova, Ludovit Paulis, Lubomira Tothova, Ivana Shawkatova, and Jana Radosinska. 2022. "Monocrotaline-Induced Pulmonary Arterial Hypertension and Bosentan Treatment in Rats: Focus on Plasma and Erythrocyte Parameters" Pharmaceuticals 15, no. 10: 1227. https://doi.org/10.3390/ph15101227
APA StyleJasenovec, T., Radosinska, D., Kollarova, M., Vrbjar, N., Balis, P., Trubacova, S., Paulis, L., Tothova, L., Shawkatova, I., & Radosinska, J. (2022). Monocrotaline-Induced Pulmonary Arterial Hypertension and Bosentan Treatment in Rats: Focus on Plasma and Erythrocyte Parameters. Pharmaceuticals, 15(10), 1227. https://doi.org/10.3390/ph15101227





