1. Introduction
Pharmacotherapy is currently the most common medical intervention in the world. In chronic non-infectious diseases, pharmacotherapy, if supported by data from randomized controlled trials (RCTs) and evidence-based medicine, is the main method of improving the prognosis of the disease and prolonging the life of patients [
1]. A major concern related to pharmacotherapy is its safety issues. Data from the American (Food and Drug Administration, FDA, USA) and European (European Medicines Agency, EMA, EU) regulatory agencies for the registration and safety surveillance of medicinal products indicate a significant number of adverse events (AEs) related to pharmacotherapy [
2,
3]. A significant number of AEs are identified during the post-registration stage and in observational studies. This can be explained by the longer duration of patient follow-up, the minimal limitations of concomitant therapy in real-world clinical practice settings, and the wider prescription of drugs to the categories of patients whose proportion in the RCTs was limited by the inclusion criteria.
Therapy with oral anticoagulants (OACs) is based on finding the optimal balance of efficacy and a reduction in the incidence of thromboembolic complications and safety in relation to bleeding as the most common side effects of these drugs. Currently, the issues of the efficacy and safety of drug therapy are often studied in conjunction with the aspect of adherence to treatment [
4,
5]. Thus, Yao et al. showed that a poor adherence to OACs increases the risk of stroke in patients with atrial fibrillation (AF), but reduces the likelihood of bleeding, the frequency of which is increased in adherent patients [
6].
A prospective observational study, “ANTEY” (
Assessment of Adherence to
New Oral an
Ticoagulants in Atrial Fibrillation pati
Ents Within the Outpatient registr
Y), investigated the adherence of patients with a non-valvular form of AF to non-vitamin K antagonist oral anticoagulants (NOACs) [
7,
8]. In this study, an additional analysis was carried out, the purpose of which was to study all AEs and unfavorable outcomes reported with OACs therapy to assess their relationship with OACs administration in patients with a non-valvular form of atrial fibrillation during one year of observation.
2. Results
During one year of observation, four deaths were reported; follow-up (FU) contact occurred in 197 patients. At V1, 160 (80.0%) patients took the NOACs recommended to them during the previous visit, 33 (16.5%) patients immediately refused to take NOACs, and 7 patients began but then discontinued NOACs for various reasons. At the FU contact, 158 (80.2%) of 197 patients were adherent to the recommended therapy. The remaining 39 people did not take OACs: 15 people refused to take it (primary non-adherence), and 24 people discontinued OACs (secondary non-adherence) [
8]. These patients were considered non-adherent to treatment. Thus, during the observation period, 15 (7.5%) patients did not take OACs at all, and the remaining 186 (92.5%) patients either continued or discontinued taking OACs: dabigatran—46 patients, rivaroxaban—93 patients, apixaban—40 patients, and warfarin after V1—7 patients.
During the entire observation period, 55 cases of various AEs of pharmacotherapy were reported, 25 of which were serious adverse events (SAEs), including 4 deaths—sudden cardiac death, death from sarcoma, death from chronic heart failure, and death from an accident—and 30 AEs. All AEs, both serious and non-serious, and their relationship to OACs administration are presented in
Table 1. Deaths are presented separate from other SAEs in this table, as listed in the ClinicalTrials.gov website.
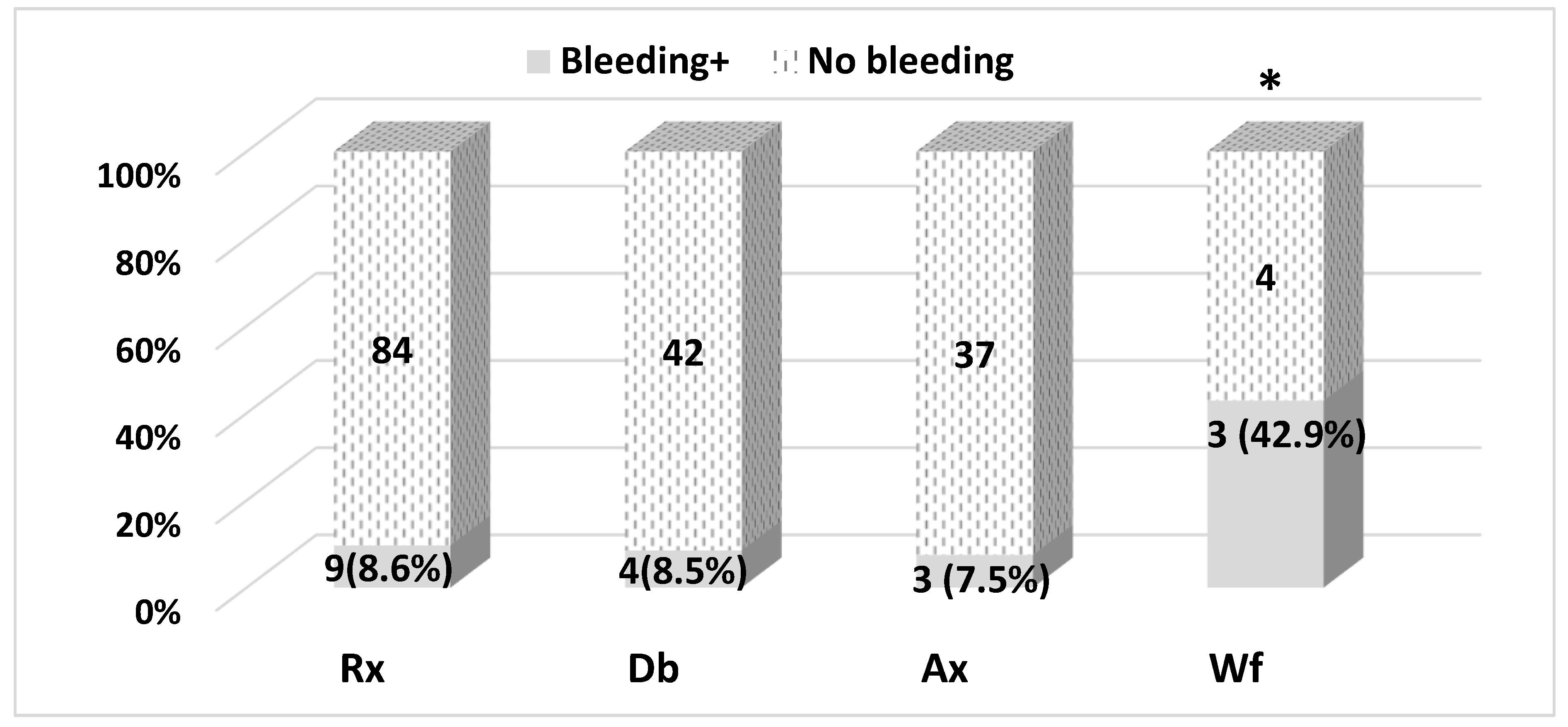
A total of 18 bleeding events were reported (
Figure 1). Eight (8.6%) of the bleeding events occurred with Rivaroxaban (93 patients initiated the drug), four (8.6%) with dabigatran (46 patients), three (7.5%) with Apixaban therapy (40 patients), and three (42.9%) with Warfarin therapy (7 patients). There were differences between the number of bleeding events.
Differences in the number of bleeding events between NOACs are statistically non-significant (p = 0.920), and the difference between NOACs and warfarin is statistically significant: OR = 7.6 (CI95%: 1.6–37.2), p = 0.025 (two-tailed Fisher’s exact probability test). However, it should be noted that a small number of study patients took warfarin; therefore, caution is necessary in interpreting the clinical significance of the results obtained.
There were no severe bleeding events reported in the study, and all bleeding events were mild to moderate in severity according to the GUSTO Bleeding Classification [
9].
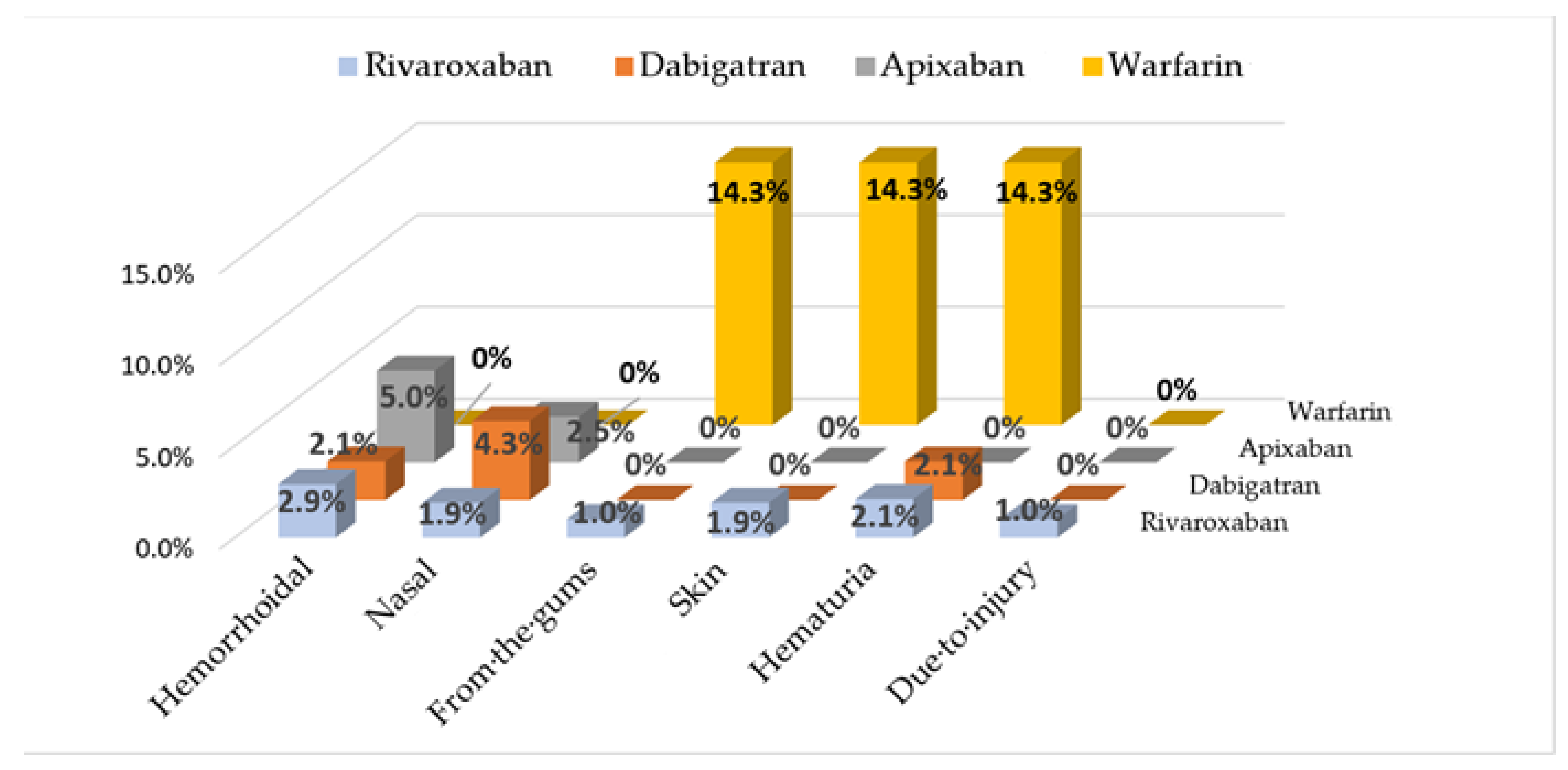
Bleeding site location information for patients who initiated one of the OACs is presented in
Figure 2. According to two-tailed Fisher’s exact probability test, the difference in the number of bleeding events between NOACs and warfarin is statistically significant: OR = 7.6 (CI95%: 1.6–37.2),
p = 0.025.
Patients’ characteristics with bleeding and without bleeding developed during OACs therapy are presented in
Table 2. Of note, among patients with developed bleeding, most had a high risk of bleeding according to the HAS-BLED scale: OR = 4.2 (CI95%: 1.4–12.3),
p = 0.006 (two-tailed Fisher’s exact probability test); almost 90% of them had CHF.
The components of the HAS-BLED scale were distributed as follows: the greatest contribution to the increased risk of bleeding was made by age: OR = 5.9 (CI95%: 0.8–45.4),
p = 0.08 (two-sided Fisher’s exact criterion); the presence of bleeding: OR = 6.0 (CI95%: 1.8–19.9),
p = 0.007; stroke history: OR = 1.8 (CI95%: 0.6–5.9),
p = 0.48; and alcohol abuse: OR = 2.6 (CI95%: 0.3–24.9),
p = 0.38. The differences between the groups of patients with developed bleeding and without this complication are presented in
Table 3.
Any pharmacotherapy AEs increased the chance of nonadherence to treatment nine-fold: OR = 9.2 (CI95%: 3.6–23.5),
p < 0.0001. In the group of patients with bleedings reported with OACs administration, 83.3% of patients refused to take these drugs and only 16.7% continued the previously recommended therapy (
p = 0.033). During the entire observation period, 80% of patients with non-valvular AF clearly followed the recommendations of the scientific center doctors regarding OACs administration [
8,
10].
3. Discussion
The issue of safety in pharmacotherapy is of great relevance in modern medicine and society. Its effective solution is complicated by the extreme difficulty of collecting complete information on pharmacotherapy AEs (adverse events). The leading sources of such information are RCT data, the results of observational studies, spontaneous reports submitted by physicians to pharmacovigilance authorities [
11]. RCTs provide the most reliable data on safety of, as a rule, new medicinal products (MPs). Unfortunately, due to a number of features, they are unable to provide complete information on the safety of the MPs being studied. This is due to the relatively small number of patients enrolled in RCTs, the frequent exclusion of some patient groups (children, pregnant, elderly people, patients with severe chronic diseases, pronounced comorbidity, etc.), and the relatively short duration of follow-up of patients in RCTs compared to the intended active treatment period. This may result in very rare AEs or SAEs being undetectable in RCTs, despite the better reporting of AEs in RCTs compared to routine clinical practice. Therefore, approximately half of the MPs require a change to the official product label in the post-marketing stage.
Due to the identified safety concerns, approximately one in five MPs receives a “black box” in the official label warning about possible serious (sometimes life-threatening) AEs identified only in the post-marketing stage. It is known that about 4% of MPs are withdrawn from circulation for safety reasons [
12,
13].
The peculiarities of the way observational studies are conducted provide them with some advantages over RCTs in detecting AEs. These features of observational studies include: (1) real-world clinical practice settings for comparison groups; (2) a rather large population allowing the investigation of rare AEs; (3) a long follow-up duration; (4) the inclusion of different groups of patients, including those who are usually excluded from RCTs; and (5) evaluation of the results of actual use of MPs in both approved indications and “off-label” prescriptions.
The FDA began establishing a national real-world clinical practice data collection system from leading US medical centers in 2008, called the Sentinel Initiative, which became fully effective in 2016. The documentation of this project emphasizes that the conduct of post-marketing studies to investigate the safety of new MPs is as important as the conduct of clinical studies before the drugs enter the pharmaceutical market. During the period of 2008–2012, 385 post-marketing studies were initiated by the FDA, resulting in changes to the labels of 65 medicinal products [
14].
The “ANTEY” study is classified as “initiated by investigators”. The AEs data obtained in it confirmed the high safety of NOACs, which caused significantly less bleedings compared to warfarin, as well as a higher adherence of patients with AF to taking these drugs [
7,
8]. This is consistent with both the results of both RCTs with NOACs [
15,
16,
17] and the data from observational studies [
18,
19,
20].
However, according to the results of the Ko YJ study, based on the Korea Institute of Drug Safety and Risk Management–Korea database, it was found that rivaroxaban was associated with the more frequent development of bleeding events than treatment with dabigatran or apixaban [
21]. The results of our study and data from a number of other studies did not identify any significant differences between the different NOACs in the incidence of bleeding [
22,
23].
The results of the study confirmed the good predictive ability of the HAS-BLED scale to predict the occurrence of bleeding in patients with AF in real clinical practice: in the presence of a high risk for HAS-BLED, the chance of bleeding increased 4-fold. The results of the study demonstrated that bleeding was more common in patients with CHF. Perhaps the reason for this was the older age of these patients. In addition, it is well-known that patients with heart failure have a high prevalence of renal dysfunction [
22]. Since all NOACs are renally eliminated to various degrees while warfarin is not, progressive renal function decline leads to an increased risk of bleeding in the treatment of NOAC [
24,
25]. However, in our study there was only one patient with renal dysfunction (according to the criteria of the HAS-BLED scale).
It should be emphasized that the results of the “ANTEY” study show a high persistence (patient adherence to long-term treatment) of patients to the administration of NOACs: 80% of patients with non-valvular AF took the recommended OACs during the 1 year of observation. The high incidence of bleedings with warfarin may be explained by failures to adhere to the rules for its administration.
The reduction in bleeding risk with NOACs treatment could be achieved by (1) adherence to clinical guidelines; (2) a risk assessment of the HAS-BLED scale and exposure to modifiable risk factors (hypertension, alcohol abuse); and (3) for avoiding the co-administration with drugs that increase the risk of bleeding, for example, NSAIDs.
According to the Institute of Safe Medicine Practices, OACs has been identified as high-risk drugs. Bleeding is the most typical AE of OACs; it is directly related to their actions and occurs in 8–19% of treated patients per year [
26,
27]. In a Norwegian study of 65,000 patients taking NOACs and 80,000 patients taking warfarin, the most common AEs were hemorrhages (48% for direct-acting oral anticoagulants and 75% for warfarin) [
28]. The results of our study are consistent with these data: twenty AEs, for which the relationship to OACs administration was determined, included 18 bleedings.
4. Material and Methods
The detailed design and protocol of the “ANTEY” study are described in earlier publications [
7,
8]. The study is registered at
www.clinicaltrials.gov (accessed on 26 September 2022) (ANTEY Trial Identifier: NCT03790917). The study was conducted in accordance with the guidelines of the Declaration of Helsinki and approved by the Institutional Ethics Committee of National Medical Research Center for Therapy and Preventive Medicine (protocol No. 05-01/17, 3 August 2017). All patients signed an informed consent form for the processing of personal data and participation in the “ANTEY” study.
The “ANTEY” study is part of the PROFILE Outpatient Prospective Registry, which was established on the basis of a specialized cardiology department. The “ANTEY” study enrolled all patients with non-valvular AF on this registry. Exclusion criteria were absolute contraindications to OACs, according to the official product labels for NOACs and warfarin. Thus, 201 people were enrolled, 83 (41.3%) of whom were women, with a mean age of 71.1 ± 8.7 years (data presented as mean with standard deviation) [
7].
There were three groups of patients included in the study: (1) patients who had never taken NOACs before (n = 55); (2) patients who had previously taken warfarin (n = 21); and (3) patients who had taken one of the NOACs (n = 125).
The study protocol included two face-to-face visits (contacts V0 and V1) and one follow-up (FU) phone contact, which were made with the patient at an interval of 6 months. At V0, all patients were recommended to take one of the non-vitamin K antagonist oral anticoagulants (NOACs): dabigatran, rivaroxaban, or apixaban. For patients of the third group (patients who took one of the NOACs before V0), the attending physician could recommend the same drug, changing its dosage and frequency of administration if necessary, or could replace it with another NOAC.
Starting from V1, warfarin could be prescribed or NOAC could be changed. Information about AEs and OACs administration was collected at V0, V1, and FU.
Information about OACs adherence and data on all AEs were obtained from the patients during contacts V0 and V1 and FU. An AE was defined as any unfavorable event happening to a patient during participation in this study. The relationship between drug intake and AEs was established by a physician according to a known scale: definite, probable, possible, unlikely, no relation. Bleeding severity was assessed using the GUSTO Bleeding Classification scale. Bleedings leading to hemodynamic impairment and requiring intervention were considered severe [
9].
Statistical processing was performed using the SPSS Statistics software package (IBM) version 23.0 (IBM, Armonk, NY, USA). Absolute values and percentages were used for descriptive statistics. The comparison of AEs with different OACs (NOACs and warfarin) was performed using Pearson’s chi-squared test with Yates’s correction for continuity and two-tailed Fisher’s exact probability test. The odds ratio (OR) and 95% confidence interval (CI95%) were calculated using Mantel–Haenszel statistics for dichotomous variables. Differences were considered statistically significant at p < 0.05.
5. Conclusions
The results of prospective observational studies provide more comprehensive information on the safety of MPs in the post-registration stage and identify AEs not reported in RCTs.
The “ANTEY” observational study showed that the most typical and common AEs in the real-world clinical practice settings treatment with OACs were bleedings, the incidence of which was approximately 8% to 9% in the treatment with NOACs. Bleedings during OACs therapy are statistically significantly associated with nonadherence to the use of these drugs in the future.
6. Strengths and Limitations
The advantage of the “ANTEY” observational study is its prospective nature, the performance of the study based on a scientific medical center with highly qualified medical personnel prescribing therapy according to clinical guidelines.
The limitations of the study are that it was a single-center study with a small number of enrolled patients; therefore, this study was likely underpowered for detecting major bleeding with a frequency of approximately 1–2%.
Author Contributions
Conceptualization, S.Y.M., Y.V.L. and N.P.K.; data curation, S.Y.M.; methodology, S.Y.M., Y.V.L. and N.P.K.; validation, S.Y.M., Y.V.L., N.P.K. and O.M.D.; formal analysis, S.Y.M., Y.V.L. and N.P.K.; investigation, Y.V.L..; resources, S.Y.M. and O.M.D.; writing—original draft preparation, S.Y.M., Y.V.L., N.P.K. and O.M.D.; writing—review and editing, S.Y.M., Y.V.L. and A.R.K.; visualization, Y.V.L. and A.R.K.; supervision, Y.V.L. and O.M.D.; project administration, S.Y.M. and N.P.K.; funding acquisition, S.Y.M. and O.M.D. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the Russian Ministry of Health as part of the scientific work “Evaluation of therapeutic and preventive care and identifying approaches to improve prognosis in patients with chronic noncommunicable diseases, new coronavirus infections and their combinations”, No. 122013100210-1, performed at the National Medical Research Center for Therapy and Preventive Medicine in 2022–2024.
Institutional Review Board Statement
The study was conducted in accordance with the guidelines of the Declaration of Helsinki, and was approved by the Institutional Ethics Committee of National Medical Research Center for Therapy and Preventive Medicine (protocol No. 05-01/17, 3 August 2017).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Institute of Medicine. Informing the Future: Critical Issues in Health, 4th ed.; The National Academies Press: Washington, DC, USA, 2007. [Google Scholar] [CrossRef]
- CDER Drug Safety Priorities. 2017. Available online: https://www.fda.gov/downloads/Drugs/DrugSafety/UCM605229.pdf (accessed on 10 August 2022).
- Bouvy, J.C.; De Bruin, M.L.; Koopmanschap, M.A. Epidemiology of Adverse Drug Reactions in Europe: A Review of Recent Observational Studies. Drug Saf. 2015, 38, 437–453. [Google Scholar] [CrossRef]
- Rodriguez, R.A.; Carrier, M.; Wells, P.S. Non-adherence to new oral anticoagulants: A reason for concern during long-term anticoagulation? J. Thromb. Haemost. 2013, 11, 390–394. [Google Scholar] [CrossRef]
- Kim, D.; Yang, P.-S.; Jang, E.; Yu, H.T.; Kim, T.-H.; Uhm, J.-S.; Kim, J.-Y.; Sung, J.-H.; Pak, H.-N.; Lee, M.-H.; et al. The optimal drug adherence to maximize the efficacy and safety of non-vitamin K antagonist oral anticoagulant in real-world atrial fibrillation patients. EP Eur. 2020, 22, 547–557. [Google Scholar] [CrossRef]
- Yao, X.; Abraham, N.S.; Alexander, G.C.; Crown, W.; Montori, V.M.; Sangaralingham, L.R.; Gersh, B.J.; Shah, N.D.; Noseworthy, P.A. Effect of Adherence to Oral Anticoagulants on Risk of Stroke and Major Bleeding Among Patients With Atrial Fibrillation. J. Am. Heart Assoc. 2016, 5, e003074. [Google Scholar] [CrossRef]
- Martsevich, S.Y.; Lukina, Y.V.; Kutishenko, N.P.; Tolpygina, S.N.; Voronina, V.P.; Dmitrieva, N.A.; Lerman, O.V.; Komkova, N.A. Adherence to Treatment with New Oral Anticoagulants in Atrial Fibrillation Patients in Real Clinical Practice (Results of the ANTEY Study). Ration. Pharmacother. Cardiol. 2019, 15, 864–872. [Google Scholar] [CrossRef]
- Martsevich, S.; Lukina, Y.; Kutishenko, N. Primary Non-adherence to Treatment with New Oral Anticoagulants: The Results of a Prospective Observational Study «ANTEY». Open Cardiovasc. Med. J. 2021, 15, 56–61. [Google Scholar] [CrossRef]
- The GUSTO Investigators. An international randomized trial comparing four thrombolytic strategies for acute myocardial infarction. N. Engl. J. Med. 1993, 329, 673–682. [Google Scholar] [CrossRef]
- Lukina, Y.V.; Kutishenko, N.P.; Tolpygina, S.N.; Voronina, V.P.; Dmitrieva, N.A.; Lerman, O.V.; Komkova, N.A.; Zagrebelny′s, A.V.; Martsevich , S.Y.; Drapkina, O.M. Main factors of adherence to new oral anticoagulants and its dynamics in outpatients with nonvalvular atrial fibrillation: Results of the ANTEY study. Cardiovasc. Ther. Prev. 2020, 19, 2680. [Google Scholar] [CrossRef]
- Phillips, R.; Hazell, L.; Sauzet, O.; Cornelius, V. Analysis and reporting of adverse events in randomised controlled trials: A review. BMJ Open 2019, 9, e024537. [Google Scholar] [CrossRef]
- Begosh, A.; Goldsmith, J.; Hass, E.; Lutter, R.; Nardinelli, C.; Vernon, J. Black Box Warnings and Drug Safety: Examining the Determinants and Timing of FDA Warning Labels; NBER Working Paper No. 12803; National Bureau of Economic Research: Cambridge, MA, USA, 2006. [Google Scholar] [CrossRef]
- Onakpoya, I.J.; Heneghan, C.J.; Aronson, J.K. Post-marketing withdrawal of 462 medicinal products because of adverse drug reactions: A systematic review of the world literature. BMC Med. 2016, 14, 10. [Google Scholar] [CrossRef]
- FDA’s Sentinel Initiative–Background. Available online: https://www.fda.gov/safety/fdas-sentinel-initiative/fdas-sentinel-initiative-background (accessed on 10 August 2022).
- Connolly, S.J.; Ezekowitz, M.D.; Yusuf, S.; Eikelboom, J.; Oldgren, J.; Parekh, A.; Pogue, J.; Reilly, P.A.; Themeles, E.; Varrone, J.; et al. Dabigatran versus Warfarin in Patients with Atrial Fibrillation. N. Engl. J. Med. 2009, 361, 1139–1151. [Google Scholar] [CrossRef]
- Patel, M.R.; Mahaffey, K.W.; Garg, J.; Pan, G.; Singer, D.E.; Hacke, W.; Breithardt, G.; Halperin, J.L.; Hankey, G.J.; Piccini, J.P.; et al. Rivaroxaban versus Warfarin in Nonvalvular Atrial Fibrillation. N. Engl. J. Med. 2011, 365, 883–891. [Google Scholar] [CrossRef]
- Granger, C.B.; Alexander, J.H.; McMurray, J.J.V.; Lopes, R.D.; Hylek, E.M.; Hanna, M.; Al-Khalidi, H.R.; Ansell, J.; Atar, D.; Ave-zum, A.; et al. Apixaban versus Warfarin in Patients with Atrial Fibrillation. N. Engl. J. Med. 2011, 365, 981–992. [Google Scholar] [CrossRef]
- Tsai, C.-T.; Liao, J.-N.; Chiang, C.-E.; Lin, Y.-J.; Chang, S.-L.; Lo, L.-W.; Hu, Y.-F.; Tuan, T.-C.; Chung, F.-P.; Chao, T.-F.; et al. Association of Ischemic Stroke, Major Bleeding, and Other Adverse Events With Warfarin Use vs Non–vitamin K Antagonist Oral Anticoagulant Use in Patients With Atrial Fibrillation With a History of Intracranial Hemorrhage. JAMA Netw. Open 2020, 3, e206424. [Google Scholar] [CrossRef]
- Southworth, M.R.; Reichman, M.E.; Unger, E.F. Dabigatran and Postmarketing Reports of Bleeding. N. Engl. J. Med. 2013, 368, 1272–1274. [Google Scholar] [CrossRef]
- Chrischilles, E.A.; Gagne, J.J.; Fireman, B.; Nelson, J.; Toh, S.; Shoaibi, A.; Reichman, M.E.; Wang, S.; Nguyen, M.; Zhang, R.; et al. Prospective surveillance pilot of rivaroxaban safety within the US Food and Drug Administration Sentinel System. Pharmacoepidemiol. Drug Saf. 2018, 27, 263–271. [Google Scholar] [CrossRef]
- Ko, Y.-J.; Kim, S.; Park, K.; Kim, M.; Yang, B.R.; Lee, J.; Kim, M.-S.; Park, B.-J. Comparison of bleeding risks among non-vitamin K antagonist oral anticoagulants using the Korea adverse event reporting system database. Ther. Adv. Drug Saf. 2019, 10, 2042098619876737. [Google Scholar] [CrossRef]
- Mihalkanin, L.; Stancak, B. The Impact of Novel Anticoagulants on the Upper Gastrointestinal Tract Mucosa. Medicina 2020, 56, 363. [Google Scholar] [CrossRef]
- Li, H.-J.; Lin, S.-Y.; Lin, F.-J.; Hung, C.-S.; Wang, C.-C. Effectiveness and safety of non-vitamin K antagonist oral anticoagulants in Asian patients with atrial fibrillation and valvular heart disease. Curr. Med. Res. Opin. 2021, 37, 535–542. [Google Scholar] [CrossRef]
- Jackevicius, C.A.; Lu, L.; Ghaznavi, Z.; Warner, A.L. Bleeding Risk of Direct Oral Anticoagulants in Patients With Heart Failure And Atrial Fibrillation. Circ. Cardiovasc. Qual. Outcomes 2021, 14, e007230. [Google Scholar] [CrossRef]
- Caturano, A.; Galiero, R.; Pafundi, P.C. Atrial Fibrillation and Stroke. A Review on the Use of Vitamin K Antagonists and Novel Oral Anticoagulants. Medicina 2019, 55, 617. [Google Scholar] [CrossRef] [PubMed]
- ISPM. QuarterWatch™ (2016 Annual Report) Part II: Oral Anticoagulants—The Nation’s Top Risk of Acute Injury from Drugs. 2017. Available online: https://www.ismp.org/resources/quarterwatchtm-2016-annual-report-part-ii-oral-anticoagulants-nations-top-risk-acute (accessed on 10 August 2022).
- Amaraneni, A.; Chippa, V.; Rettew, A.C. Anticoagulation Safety. [Updated 2022 May 24]. In StatPearls; [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2022; Available online: https://www.ncbi.nlm.nih.gov/books/NBK519025/ (accessed on 10 August 2022).
- Eek, A.K.; Strøm, B.O.; Bakkehøi, G.; Stenberg-Nilsen, H. Anticoagulant-associated adverse drug reactions in 2013-15. Tidsskr Nor Laegeforen. 2018, 138. [Google Scholar] [CrossRef]
| Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).