Presence of TRPA1 Modifies CD4+/CD8+ T Lymphocyte Ratio and Activation
Abstract
:1. Introduction
2. Results
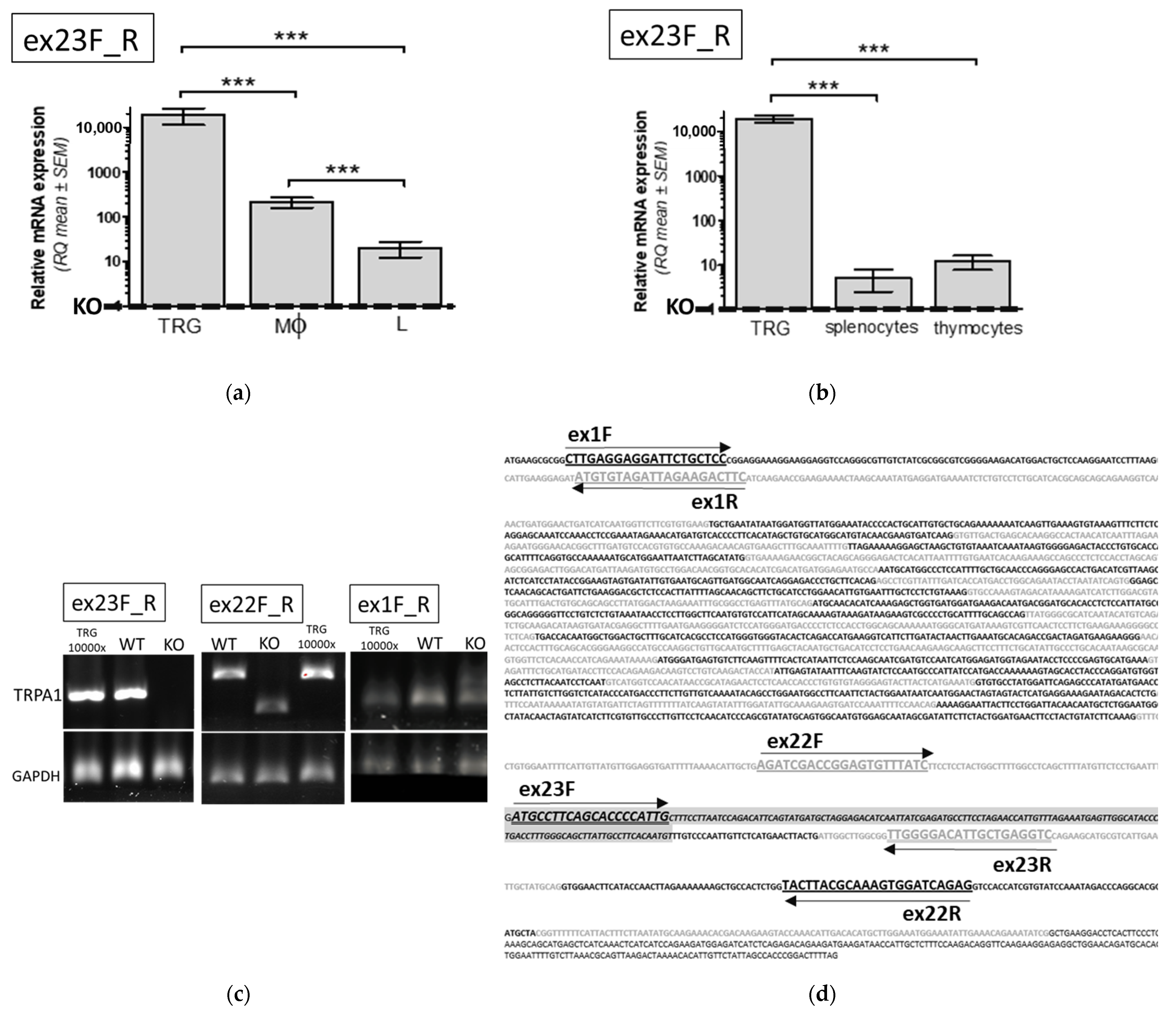
2.1. TRPA1 mRNA and Protein Expression in WT and Functional KO Mice
2.1.1. Evaluation of TRPA1 mRNA Expression in Mononuclear Immune Cells of WT Animals
2.1.2. Comparison of TRPA1 mRNA Expression in Immune Cells of WT and KO Mice
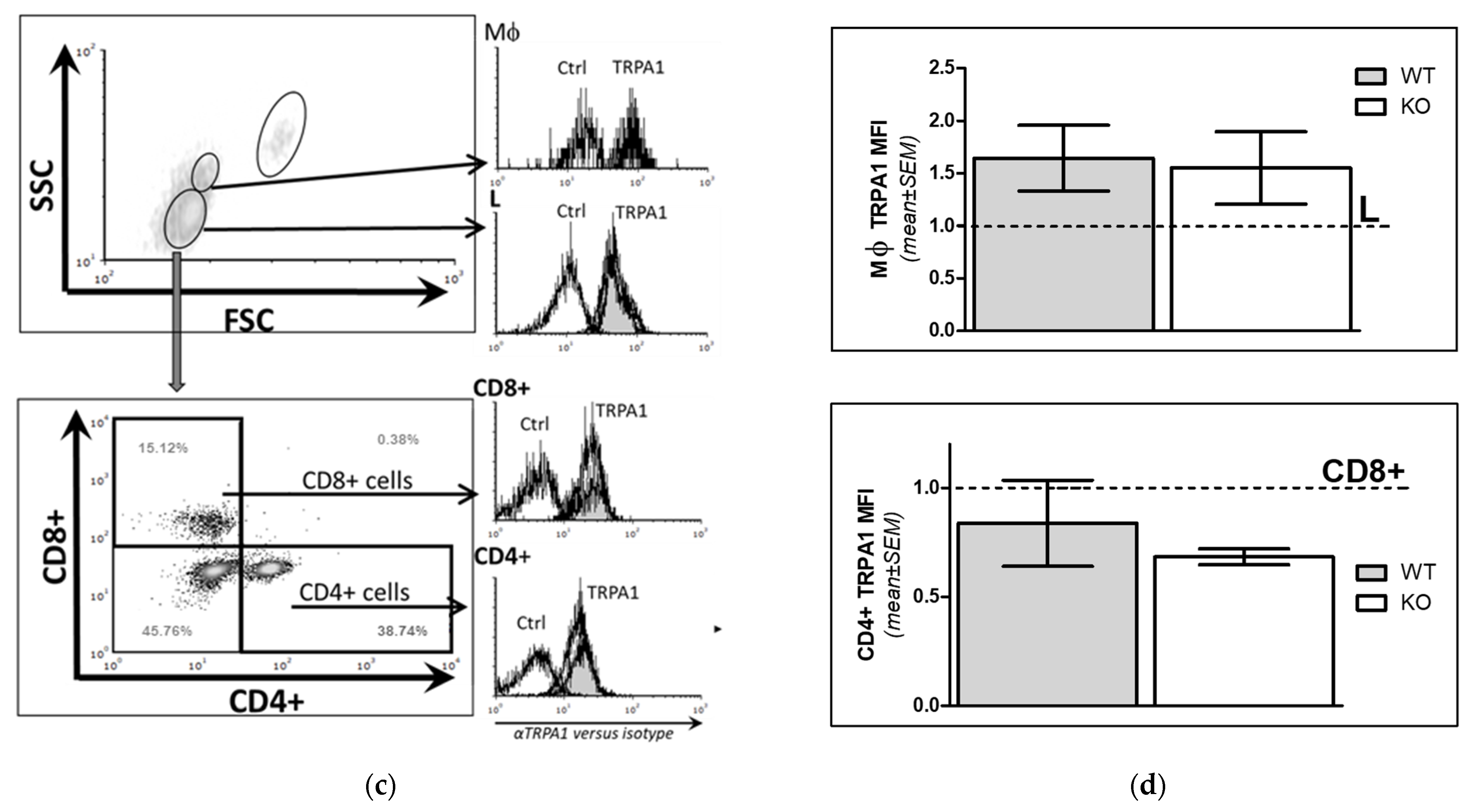
2.1.3. Comparison of TRPA1 Protein Expression Level in WT and KO Mice
2.2. Phenotypic Analyses of WT and KO Lymphocytes
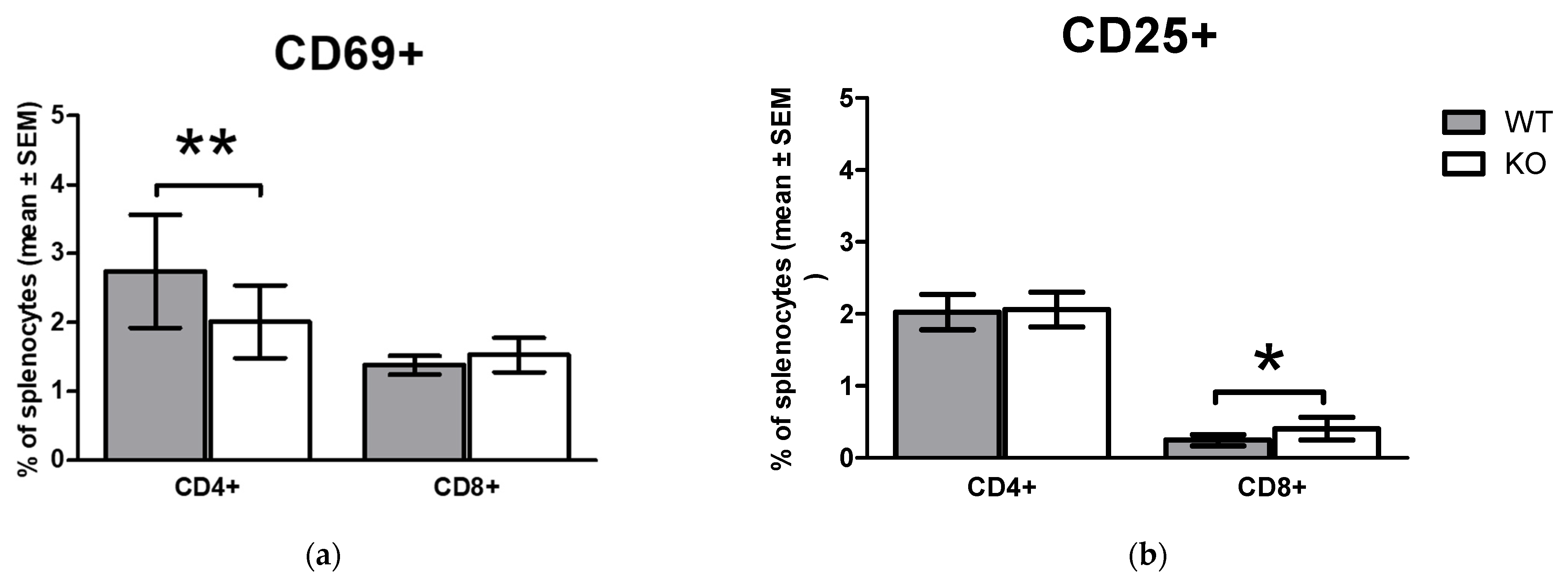
2.3. Comparison of Early Activation Markers on CD4+ T Cells and CD8+ T Cells In Vivo in WT and KO Animals
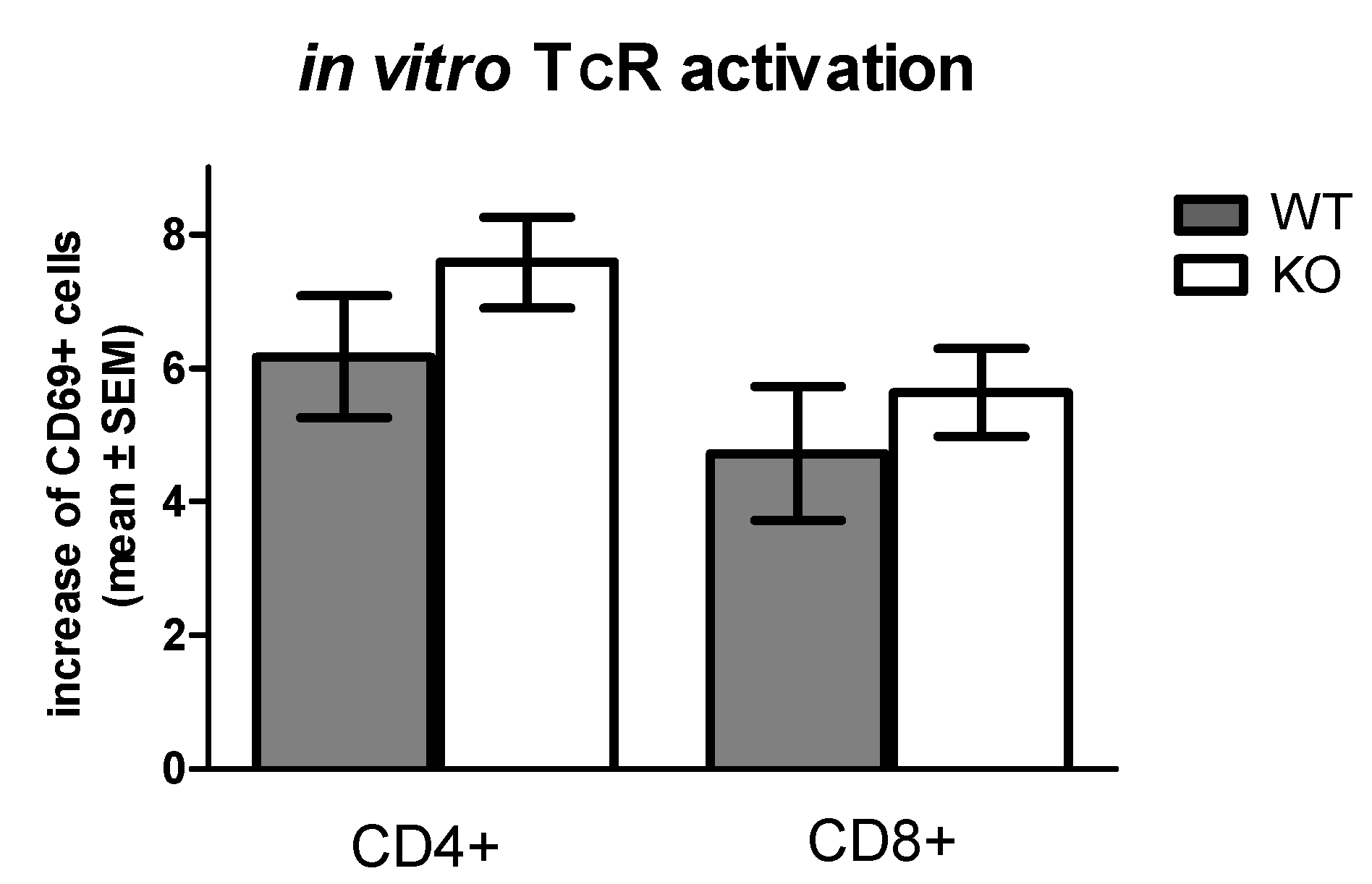
2.4. Comparison of In Vitro TcR (CD3/CD28) Mediated Early Activation Marker CD69 Expression on T Cells of WT and KO Mice
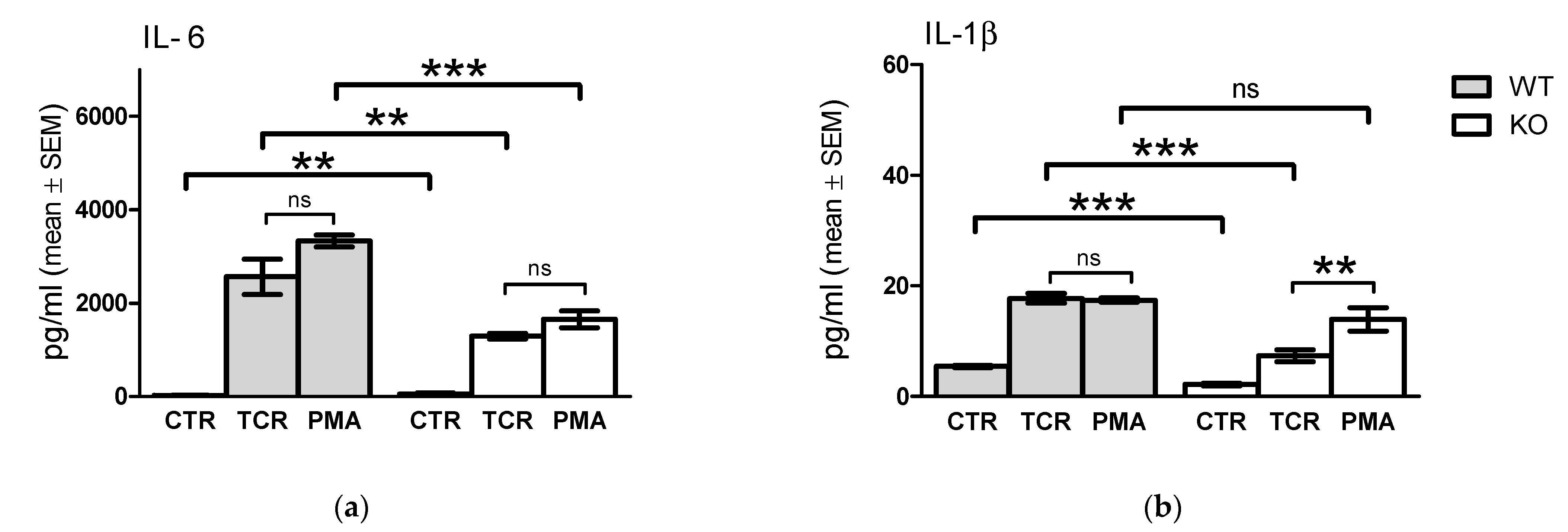
2.5. Comparison of In Vitro TcR and PMA/Ionomycin Stimulation-Induced Cytokine Release Profile of WT and KO Cells
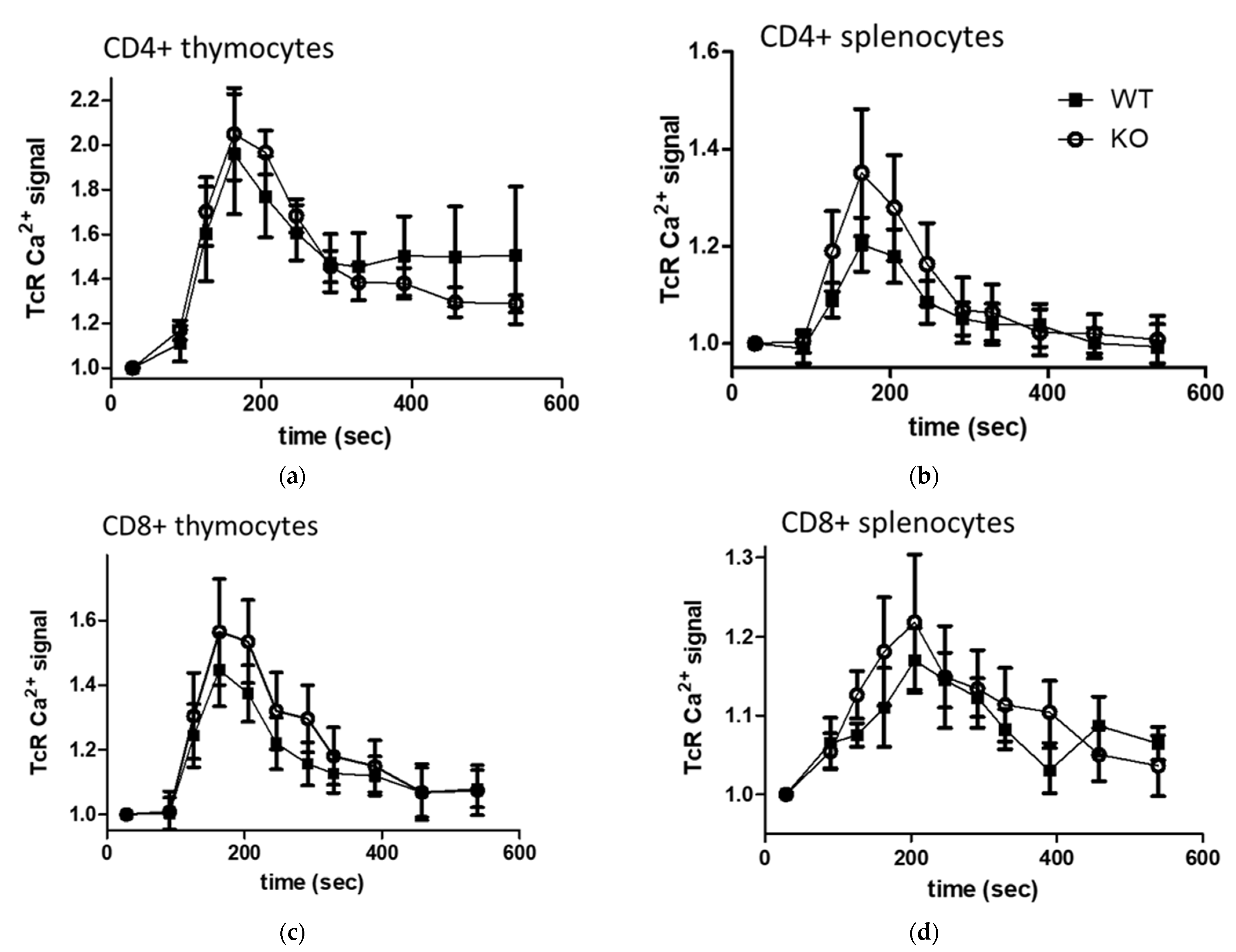
2.6. In Vitro TcR (anti-CD3) Stimulated Calcium Signal and Basal Intracellular Ca2+ Level in Lymphocytes and Thymocyte Subpopulations of WT and KO Mice
2.7. Comparison of IMQ Induced Prolonged Ca2+ Signal in WT and KO Lymphocyte Subpopulations
3. Discussion
- Functional TRPA1 monomers may form heterotetramers with other TRP channels (e.g., TRPV1)
- Functional TRPA1 may modulate through changes in Ca2+ concentration in microdomains, and thereby:
4. Materials and Methods
4.1. Mice, Mononuclear Cell Isolation and Separation
4.2. Cell Stimulation, In Vitro Cell Culture
4.3. Cytokine Detection
4.4. RNA Isolation, cDNA Synthesis, and qPCR
4.5. Flow Cytometry
4.6. Measurement of Intracellular Calcium Signaling
4.7. Statistical Methods
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Talavera, K.; Startek, J.B.; Alvarez-Collazo, J.; Boonen, B.; Alpizar, Y.A.; Sanchez, A.; Naert, R.; Nilius, B. Mammalian Transient Receptor Potential TRPA1 Channels: From Structure to Disease. Physiol. Rev. 2020, 100, 725–803. [Google Scholar] [CrossRef]
- Brazzini, B.; Ghersetich, I.; Hercogova, J.; Lotti, T. The neuro-immuno-cutaneous-endocrine network: Relationship between mind and skin. Dermatol. Ther. 2003, 16, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Grace, P.M.; Hutchinson, M.R.; Maier, S.F.; Watkins, L.R. Pathological pain and the neuroimmune interface. Nat. Rev. Immunol. 2014, 14, 217–231. [Google Scholar] [CrossRef] [Green Version]
- Imamura, F.; Hasegawa-Ishii, S. Environmental Toxicants-Induced Immune Responses in the Olfactory Mucosa. Front. Immunol. 2016, 7, 475. [Google Scholar] [CrossRef] [Green Version]
- Hewitt, R.J.; Lloyd, C.M. Regulation of immune responses by the airway epithelial cell landscape. Nat. Rev. Immunol. 2021, 21, 347–362. [Google Scholar] [CrossRef] [PubMed]
- Maglie, R.; Souza Monteiro de Araujo, D.; Antiga, E.; Geppetti, P.; Nassini, R.; De Logu, F. The Role of TRPA1 in Skin Physiology and Pathology. Int. J. Mol. Sci. 2021, 22, 3065. [Google Scholar] [CrossRef]
- Bertin, S.; Aoki-Nonaka, Y.; Lee, J.; de Jong, P.R.; Kim, P.; Han, T.; Yu, T.; To, K.; Takahashi, N.; Boland, B.S.; et al. The TRPA1 ion channel is expressed in CD4+ T cells and restrains T-cell-mediated colitis through inhibition of TRPV1. Gut 2017, 66, 1584–1596. [Google Scholar] [CrossRef] [PubMed]
- Froghi, S.; Grant, C.R.; Tandon, R.; Quaglia, A.; Davidson, B.; Fuller, B. New Insights on the Role of TRP Channels in Calcium Signalling and Immunomodulation: Review of Pathways and Implications for Clinical Practice. Clin. Rev. Allergy Immunol. 2021, 60, 271–292. [Google Scholar] [CrossRef] [PubMed]
- Koivisto, A.P.; Belvisi, M.G.; Gaudet, R.; Szallasi, A. Advances in TRP channel drug discovery: From target validation to clinical studies. Nat. Rev. Drug Discov. 2021, 15, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Gouin, O.; L’Herondelle, K.; Lebonvallet, N.; Le Gall-Ianotto, C.; Sakka, M.; Buhé, V.; Plée-Gautier, E.; Carré, J.L.; Lefeuvre, L.; Misery, L.; et al. TRPV1 and TRPA1 in cutaneous neurogenic and chronic inflammation: Pro-inflammatory response induced by their activation and their sensitization. Protein Cell 2017, 8, 644–661. [Google Scholar] [CrossRef] [Green Version]
- Fernandes, E.S.; Fernandes, M.A.; Keeble, J.E. The functions of TRPA1 and TRPV1: Moving away from sensory nerves. Br. J. Pharmacol. 2012, 166, 510–521. [Google Scholar] [CrossRef] [Green Version]
- Nilius, B.; Owsianik, G.; Voets, T.; Peters, J.A. Transient receptor potential cation channels in disease. Physiol. Rev. 2007, 87, 165–217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benhadou, F.; Mintoff, D.; Del Marmol, V. Psoriasis: Keratinocytes or Immune Cells–Which Is the Trigger? Dermatology 2019, 235, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Pereira, I.; Mendes, S.J.; Pereira, D.M.; Muniz, T.F.; Colares, V.L.; Monteiro, C.R.; Martins, M.M.; Grisotto, M.A.; Monteiro-Neto, V.; Monteiro, S.G.; et al. Transient Receptor Potential Ankyrin 1 Channel Expression on Peripheral Blood Leukocytes from Rheumatoid Arthritic Patients and Correlation with Pain and Disability. Front Pharmacol. 2017, 8, 53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ho, J.C.; Lee, C.H. TRP channels in skin: From physiological implications to clinical significances. Biophysics 2015, 11, 17–24. [Google Scholar] [CrossRef] [Green Version]
- Nattkemper, L.A.; Tey, H.L.; Valdes-Rodriguez, R.; Lee, H.; Mollanazar, N.K.; Albornoz, C.; Sanders, K.M.; Yosipovitch, G. The Genetics of Chronic Itch: Gene Expression in the Skin of Patients with Atopic Dermatitis and Psoriasis with Severe Itch. J. Investig. Dermatol. 2018, 138, 1311–1317. [Google Scholar] [CrossRef] [Green Version]
- Oh, M.H.; Oh, S.Y.; Lu, J.; Lou, H.; Myers, A.C.; Zhu, Z.; Zheng, T. TRPA1-dependent pruritus in IL-13-induced chronic atopic dermatitis. J. Immunol. 2013, 191, 5371–5382. [Google Scholar] [CrossRef] [Green Version]
- Zeng, D.; Chen, C.; Zhou, W.; Ma, X.; Pu, X.; Zeng, Y.; Lv, F. TRPA1 deficiency alleviates inflammation of atopic dermatitis by reducing macrophage infiltration. Life Sci. 2021, 266, 118906. [Google Scholar] [CrossRef]
- Dalenogare, D.P.; Ritter, C.; Bellinaso, F.R.A.; Kudsi, S.Q.; Pereira, G.C.; Fialho, M.F.P.; Lückemeyer, D.D.; Antoniazzi, C.T.D.; Landini, L.; Ferreira, J.; et al. Periorbital Nociception in a Progressive Multiple Sclerosis Mouse Model Is Dependent on TRPA1 Channel Activation. Pharmaceuticals 2021, 14, 831. [Google Scholar] [CrossRef]
- Peres, D.S.; Theisen, M.C.; Fialho, M.F.P.; Dalenogare, D.P.; Rodrigues, P.; Kudsi, S.Q.; Bernardes, L.B.; Ruviaro da Silva, N.A.; Lückemeyer, D.D.; Sampaio, T.B.; et al. TRPA1 involvement in depression- and anxiety-like behaviors in a progressive multiple sclerosis model in mice. Brain Res. Bull. 2021, 175, 1–15. [Google Scholar] [CrossRef]
- Mihai, D.P.; Nitulescu, G.M.; Ion, G.N.D.; Ciotu, C.I.; Chirita, C.; Negres, S. Computational Drug Repurposing Algorithm Targeting TRPA1 Calcium Channel as a Potential Therapeutic Solution for Multiple Sclerosis. Pharmaceutics 2019, 11, 446. [Google Scholar] [CrossRef] [Green Version]
- Sághy, É.; Sipos, É.; Ács, P.; Bölcskei, K.; Pohóczky, K.; Kemény, Á.; Sándor, Z.; Szőke, É.; Sétáló, G.; Komoly, S.; et al. TRPA1 deficiency is protective in cuprizone-induced demyelination-A new target against oligodendrocyte apoptosis. Glia 2016, 64, 2166–2180. [Google Scholar] [CrossRef] [PubMed]
- Bölcskei, K.; Kriszta, G.; Sághy, É.; Payrits, M.; Sipos, É.; Vranesics, A.; Berente, Z.; Ábrahám, H.; Ács, P.; Komoly, S.; et al. Behavioural alterations and morphological changes are attenuated by the lack of TRPA1 receptors in the cuprizone-induced demyelination model in mice. J. Neuroimmunol. 2018, 320, 1–10. [Google Scholar] [CrossRef]
- Kriszta, G.; Nemes, B.; Sándor, Z.; Ács, P.; Komoly, S.; Berente, Z.; Bölcskei, K.; Pintér, E. Investigation of Cuprizone-Induced Demyelination in mGFAP-Driven Conditional Transient Receptor Potential Ankyrin 1 (TRPA1) Receptor Knockout Mice. Cells 2019, 9, 81. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, X.; Tai, Y.; He, D.; Liu, B.; Wang, C.; Shao, X.; Jordt, S.E. ETAR and protein kinase A pathway mediate ET-1 sensitization of TRPA1 channel: A molecular mechanism of ET-1-induced mechanical hyperalgesia. Mol. Pain 2019, 15, 1744806919842473. [Google Scholar] [CrossRef] [Green Version]
- Koivisto, A.; Jalava, N.; Bratty, R.; Pertovaara, A. TRPA1 Antagonists for Pain Relief. Pharmaceuticals 2018, 11, 117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.J.; Park, G.H.; Kim, D.; Lee, J.; Min, H.; Wall, E.; Lee, C.J.; Simon, M.I.; Lee, S.J.; Han, S.K. Analysis of cellular and behavioral responses to imiquimod reveals a unique itch pathway in transient receptor potential vanilloid 1 (TRPV1)-expressing neurons. Proc. Natl. Acad. Sci. USA 2011, 108, 3371–3376. [Google Scholar] [CrossRef] [Green Version]
- Nassini, R.; Pedretti, P.; Moretto, N.; Fusi, C.; Carnini, C.; Facchinetti, F.; Viscomi, A.R.; Pisano, A.R.; Stokesberry, S.; Brunmark, C.; et al. Transient receptor potential ankyrin 1 channel localized to non-neuronal airway cells promotes non-neurogenic inflammation. PLoS ONE 2012, 7, e42454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hajna, Z.; Csekő, K.; Kemény, Á.; Kereskai, L.; Kiss, T.; Perkecz, A.; Szitter, I.; Kocsis, B.; Pintér, E.; Helyes, Z. Complex Regulatory Role of the TRPA1 Receptor in Acute and Chronic Airway Inflammation Mouse Models. Int. J. Mol. Sci. 2020, 21, 4109. [Google Scholar] [CrossRef] [PubMed]
- Bousquet, J.; Czarlewski, W.; Zuberbier, T.; Mullol, J.; Blain, H.; Cristol, J.P.; De La Torre, R.; Pizarro Lozano, N.; Le Moing, V.; Bedbrook, A.; et al. Potential Interplay between Nrf2, TRPA1, and TRPV1 in Nutrients for the Control of COVID-19. Int. Arch. Allergy Immunol. 2021, 182, 324–338. [Google Scholar] [CrossRef]
- Jaffal, S.M.; Abbas, M.A. TRP channels in COVID-19 disease: Potential targets for prevention and treatment. Chem. Biol. Interact. 2021, 345, 109567. [Google Scholar] [CrossRef]
- Andersson, D.A.; Gentry, C.; Moss, S.; Bevan, S. Transient receptor potential A1 is a sensory receptor for multiple products of oxidative stress. J. Neurosci. 2008, 28, 2485–2494. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takahashi, N.; Mizuno, Y.; Kozai, D.; Yamamoto, S.; Kiyonaka, S.; Shibata, T.; Uchida, K.; Mori, Y. Molecular characterization of TRPA1 channel activation by cysteine-reactive inflammatory mediators. Channels 2008, 2, 287–298. [Google Scholar] [CrossRef] [Green Version]
- Chiu, I.M.; von Hehn, C.A.; Woolf, C.J. Neurogenic inflammation and the peripheral nervous system in host defense and immunopathology. Nat. Neurosci. 2012, 15, 1063–1067. [Google Scholar] [CrossRef]
- Angelopoulou, A.; Alexandris, N.; Konstantinou, E.; Mesiakaris, K.; Zanidis, C.; Farsalinos, K.; Poulas, K. Imiquimod–A toll like receptor 7 agonist–Is an ideal option for management of COVID 19. Environ. Res. 2020, 188, 109858. [Google Scholar] [CrossRef] [PubMed]
- Alexander, S.P.; Benson, H.E.; Faccenda, E.; Pawson, A.J.; Sharman, J.L.; Catterall, W.A.; Spedding, M.; Peters, J.A.; Harmar, A.J.; Collaborators, C. The Concise Guide to PHARMACOLOGY 2013/14: Ion channels. Br. J. Pharmacol. 2013, 170, 1607–1651. [Google Scholar] [CrossRef] [Green Version]
- Engel, M.A.; Leffler, A.; Niedermirtl, F.; Babes, A.; Zimmermann, K.; Filipović, M.R.; Izydorczyk, I.; Eberhardt, M.; Kichko, T.I.; Mueller-Tribbensee, S.M.; et al. TRPA1 and substance P mediate colitis in mice. Gastroenterology 2011, 141, 1346–1358. [Google Scholar] [CrossRef] [PubMed]
- Kádková, A.; Synytsya, V.; Krusek, J.; Zímová, L.; Vlachová, V. Molecular basis of TRPA1 regulation in nociceptive neurons. A review. Physiol. Res. 2017, 66, 425–439. [Google Scholar] [CrossRef]
- Taylor-Clark, T.E.; Undem, B.J.; Macglashan, D.W.; Ghatta, S.; Carr, M.J.; McAlexander, M.A. Prostaglandin-induced activation of nociceptive neurons via direct interaction with transient receptor potential A1 (TRPA1). Mol. Pharmacol. 2008, 73, 274–281. [Google Scholar] [CrossRef] [Green Version]
- Bautista, D.M.; Pellegrino, M.; Tsunozaki, M. TRPA1: A gatekeeper for inflammation. Annu. Rev. Physiol. 2013, 75, 181–200. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Dai, Y.; Fukuoka, T.; Yamanaka, H.; Kobayashi, K.; Obata, K.; Cui, X.; Tominaga, M.; Noguchi, K. Phospholipase C and protein kinase A mediate bradykinin sensitization of TRPA1: A molecular mechanism of inflammatory pain. Brain 2008, 131, 1241–1251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soares, A.G.; Muscara, M.N.; Costa, S.K.P. Molecular mechanism and health effects of 1,2-Naphtoquinone. EXCLI J. 2020, 19, 707–717. [Google Scholar] [CrossRef]
- Stokes, A.; Wakano, C.; Koblan-Huberson, M.; Adra, C.N.; Fleig, A.; Turner, H. TRPA1 is a substrate for de-ubiquitination by the tumor suppressor CYLD. Cell Signal 2006, 18, 1584–1594. [Google Scholar] [CrossRef]
- Meng, J.; Li, Y.; Fischer, M.J.M.; Steinhoff, M.; Chen, W.; Wang, J. Th2 Modulation of Transient Receptor Potential Channels: An Unmet Therapeutic Intervention for Atopic Dermatitis. Front Immunol. 2021, 12, 696784. [Google Scholar] [CrossRef] [PubMed]
- Alvarado, M.G.; Thakore, P.; Earley, S. Transient Receptor Potential Channel Ankyrin 1: A Unique Regulator of Vascular Function. Cells 2021, 10, 1167. [Google Scholar] [CrossRef]
- Silverman, H.A.; Chen, A.; Kravatz, N.L.; Chavan, S.S.; Chang, E.H. Involvement of Neural Transient Receptor Potential Channels in Peripheral Inflammation. Front Immunol. 2020, 11, 590261. [Google Scholar] [CrossRef]
- Nozawa, K.; Kawabata-Shoda, E.; Doihara, H.; Kojima, R.; Okada, H.; Mochizuki, S.; Sano, Y.; Inamura, K.; Matsushime, H.; Koizumi, T.; et al. TRPA1 regulates gastrointestinal motility through serotonin release from enterochromaffin cells. Proc. Natl. Acad. Sci. USA 2009, 106, 3408–3413. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Atoyan, R.; Shander, D.; Botchkareva, N.V. Non-neuronal expression of transient receptor potential type A1 (TRPA1) in human skin. J. Investig. Dermatol. 2009, 129, 2312–2315. [Google Scholar] [CrossRef] [Green Version]
- Bellono, N.W.; Kammel, L.G.; Zimmerman, A.L.; Oancea, E. UV light phototransduction activates transient receptor potential A1 ion channels in human melanocytes. Proc. Natl. Acad. Sci. USA 2013, 110, 2383–2388. [Google Scholar] [CrossRef] [Green Version]
- Majhi, R.K.; Sahoo, S.S.; Yadav, M.; Pratheek, B.M.; Chattopadhyay, S.; Goswami, C. Functional expression of TRPV channels in T cells and their implications in immune regulation. FEBS J. 2015, 282, 2661–2681. [Google Scholar] [CrossRef]
- Billeter, A.T.; Galbraith, N.; Walker, S.; Lawson, C.; Gardner, S.A.; Sarojini, H.; Galandiuk, S.; Polk, H.C. TRPA1 mediates the effects of hypothermia on the monocyte inflammatory response. Surgery 2015, 158, 646–654. [Google Scholar] [CrossRef] [PubMed]
- Tian, C.; Huang, R.; Tang, F.; Lin, Z.; Cheng, N.; Han, X.; Li, S.; Zhou, P.; Deng, S.; Huang, H.; et al. Transient Receptor Potential Ankyrin 1 Contributes to Lysophosphatidylcholine-Induced Intracellular Calcium Regulation and THP-1-Derived Macrophage Activation. J. Membr. Biol. 2020, 253, 43–55. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, S.S.; Majhi, R.K.; Tiwari, A.; Acharya, T.; Kumar, P.S.; Saha, S.; Kumar, A.; Goswami, C.; Chattopadhyay, S. Transient receptor potential ankyrin1 channel is endogenously expressed in T cells and is involved in immune functions. Biosci. Rep. 2019, 39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Virk, H.S.; Rekas, M.Z.; Biddle, M.S.; Wright, A.K.A.; Sousa, J.; Weston, C.A.; Chachi, L.; Roach, K.M.; Bradding, P. Validation of antibodies for the specific detection of human TRPA1. Sci. Rep. 2019, 9, 18500. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, Y.; Suzuki, Y.; Uchida, K.; Tominaga, M. Identification of a splice variant of mouse TRPA1 that regulates TRPA1 activity. Nat. Commun. 2013, 4, 2399. [Google Scholar] [CrossRef] [Green Version]
- Huang, H.; Tay, S.H.; Ng, W.; Ng, S.Y.; Soong, T.W. Targeting novel human transient receptor potential ankyrin 1 splice variation with splice-switching antisense oligonucleotides. Pain 2021, 162, 2097–2109. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, V.; Swain, S.; Murray, K.; Reardon, C. Neural Immune Communication in the Control of Host-Bacterial Pathogen Interactions in the Gastrointestinal Tract. Infect Immun. 2020, 88, e00928-19. [Google Scholar] [CrossRef]
- Bautista, D.M.; Jordt, S.E.; Nikai, T.; Tsuruda, P.R.; Read, A.J.; Poblete, J.; Yamoah, E.N.; Basbaum, A.I.; Julius, D. TRPA1 mediates the inflammatory actions of environmental irritants and proalgesic agents. Cell 2006, 124, 1269–1282. [Google Scholar] [CrossRef] [Green Version]
- Kun, J.; Szitter, I.; Kemény, A.; Perkecz, A.; Kereskai, L.; Pohóczky, K.; Vincze, A.; Gódi, S.; Szabó, I.; Szolcsányi, J.; et al. Upregulation of the transient receptor potential ankyrin 1 ion channel in the inflamed human and mouse colon and its protective roles. PLoS ONE 2014, 9, e108164. [Google Scholar] [CrossRef]
- Paulsen, C.E.; Armache, J.P.; Gao, Y.; Cheng, Y.; Julius, D. Structure of the TRPA1 ion channel suggests regulatory mechanisms. Nature 2015, 525, 552. [Google Scholar] [CrossRef] [Green Version]
- Hinman, A.; Chuang, H.H.; Bautista, D.M.; Julius, D. TRP channel activation by reversible covalent modification. Proc. Natl. Acad. Sci. USA 2006, 103, 19564–19568. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zappia, K.J.; Garrison, S.R.; Palygin, O.; Weyer, A.D.; Barabas, M.E.; Lawlor, M.W.; Staruschenko, A.; Stucky, C.L. Mechanosensory and ATP Release Deficits following Keratin14-Cre-Mediated TRPA1 Deletion Despite Absence of TRPA1 in Murine Keratinocytes. PLoS ONE 2016, 11, e0151602. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Forni, M.F.; Domínguez-Amorocho, O.A.; de Assis, L.V.M.; Kinker, G.S.; Moraes, M.N.; Castrucci, A.M.L.; Câmara, N.O.S. An Immunometabolic Shift Modulates Cytotoxic Lymphocyte Activation During Melanoma Progression in TRPA1 Channel Null Mice. Front Oncol. 2021, 11, 667715. [Google Scholar] [CrossRef]
- Story, G.M.; Peier, A.M.; Reeve, A.J.; Eid, S.R.; Mosbacher, J.; Hricik, T.R.; Earley, T.J.; Hergarden, A.C.; Andersson, D.A.; Hwang, S.W.; et al. ANKTM1, a TRP-like channel expressed in nociceptive neurons, is activated by cold temperatures. Cell 2003, 112, 819–829. [Google Scholar] [CrossRef] [Green Version]
- Marsakova, L.; Barvik, I.; Zima, V.; Zimova, L.; Vlachova, V. The First Extracellular Linker Is Important for Several Aspects of the Gating Mechanism of Human TRPA1 Channel. Front Mol. Neurosci. 2017, 10, 16. [Google Scholar] [CrossRef] [PubMed]
- Zygmunt, P.M.; Högestätt, E.D. TRPA1. Handb. Exp. Pharmacol. 2014, 222, 583–630. [Google Scholar] [CrossRef]
- Garland, S.M. Imiquimod. Curr. Opin. Infect. Dis. 2003, 16, 85–89. [Google Scholar] [CrossRef] [PubMed]
- Stanley, M.A. Imiquimod and the imidazoquinolones: Mechanism of action and therapeutic potential. Clin. Exp. Dermatol. 2002, 27, 571–577. [Google Scholar] [CrossRef]
- Schön, M.P.; Schön, M. Imiquimod: Mode of action. Br. J. Dermatol. 2007, 157 (Suppl. S2), 8–13. [Google Scholar] [CrossRef]
- Vidal, D. Topical imiquimod: Mechanism of action and clinical applications. Mini Rev. Med. Chem. 2006, 6, 499–503. [Google Scholar] [CrossRef]
- Yoon, H.K.; Shim, Y.S.; Kim, P.H.; Park, S.R. The TLR7 agonist imiquimod selectively inhibits IL-4-induced IgE production by suppressing IgG1/IgE class switching and germline ε transcription through the induction of BCL6 expression in B cells. Cell Immunol. 2019, 338, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Nyberg, W.A.; Espinosa, A. Imiquimod induces ER stress and Ca(2+) influx independently of TLR7 and TLR8. Biochem. Biophys. Res. Commun. 2016, 473, 789–794. [Google Scholar] [CrossRef] [Green Version]
- Kemény, Á.; Kodji, X.; Horváth, S.; Komlódi, R.; Szőke, É.; Sándor, Z.; Perkecz, A.; Gyömörei, C.; Sétáló, G.; Kelemen, B.; et al. TRPA1 Acts in a Protective Manner in Imiquimod-Induced Psoriasiform Dermatitis in Mice. J. Investig. Dermatol. 2018, 138, 1774–1784. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wülfing, C.; Schuran, F.A.; Urban, J.; Oehlmann, J.; Günther, H.S. Neural architecture in lymphoid organs: Hard-wired antigen presenting cells and neurite networks in antigen entrance areas. Immun. Inflamm. Dis. 2018, 6, 354–370. [Google Scholar] [CrossRef] [Green Version]
- Chavan, S.S.; Tracey, K.J. Essential Neuroscience in Immunology. J. Immunol. 2017, 198, 3389–3397. [Google Scholar] [CrossRef]
- Yang, E.V.; Donovan, E.L.; Benson, D.M.; Glaser, R. VEGF is differentially regulated in multiple myeloma-derived cell lines by norepinephrine. Brain Behav. Immun. 2008, 22, 318–323. [Google Scholar] [CrossRef] [Green Version]
- Hu, D.; Al-Shalan, H.A.M.; Shi, Z.; Wang, P.; Wu, Y.; Nicholls, P.K.; Greene, W.K.; Ma, B. Distribution of nerve fibers and nerve-immune cell association in mouse spleen revealed by immunofluorescent staining. Sci. Rep. 2020, 10, 9850. [Google Scholar] [CrossRef]
- Kerage, D.; Sloan, E.K.; Mattarollo, S.R.; McCombe, P.A. Interaction of neurotransmitters and neurochemicals with lymphocytes. J. Neuroimmunol. 2019, 332, 99–111. [Google Scholar] [CrossRef] [Green Version]
- Thakore, P.; Alvarado, M.G.; Ali, S.; Mughal, A.; Pires, P.W.; Yamasaki, E.; Pritchard, H.A.; Isakson, B.E.; Tran, C.H.T.; Earley, S. Brain endothelial cell TRPA1 channels initiate neurovascular coupling. Elife 2021, 10, e63040. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Cano, R.; Montilla-García, Á.; Perazzoli, G.; Torres, J.M.; Cañizares, F.J.; Fernández-Segura, E.; Costigan, M.; Baeyens, J.M.; Cobos, E.J. Intracolonic Mustard Oil Induces Visceral Pain in Mice by TRPA1-Dependent and -Independent Mechanisms: Role of Tissue Injury and P2X Receptors. Front Pharmacol. 2020, 11, 613068. [Google Scholar] [CrossRef]
- Bertin, S.; de Jong, P.R.; Jefferies, W.A.; Raz, E. Novel immune function for the TRPV1 channel in T lymphocytes. Channels 2014, 8, 479–480. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fischer, M.J.; Balasuriya, D.; Jeggle, P.; Goetze, T.A.; McNaughton, P.A.; Reeh, P.W.; Edwardson, J.M. Direct evidence for functional TRPV1/TRPA1 heteromers. Pflugers Arch. 2014, 466, 2229–2241. [Google Scholar] [CrossRef]
- Schilling, W.P.; Goel, M. Mammalian TRPC channel subunit assembly. Novartis Found Symp. 2004, 258, 18–30. [Google Scholar] [PubMed]
- Dietrich, A.; Mederos y Schnitzler, M.; Kalwa, H.; Storch, U.; Gudermann, T. Functional characterization and physiological relevance of the TRPC3/6/7 subfamily of cation channels. Naunyn. Schmiedebergs Arch. Pharmacol. 2005, 371, 257–265. [Google Scholar] [CrossRef] [Green Version]
- Bai, C.X.; Giamarchi, A.; Rodat-Despoix, L.; Padilla, F.; Downs, T.; Tsiokas, L.; Delmas, P. Formation of a new receptor-operated channel by heteromeric assembly of TRPP2 and TRPC1 subunits. EMBO Rep. 2008, 9, 472–479. [Google Scholar] [CrossRef] [Green Version]
- Park, J.Y.; Hwang, E.M.; Yarishkin, O.; Seo, J.H.; Kim, E.; Yoo, J.; Yi, G.S.; Kim, D.G.; Park, N.; Ha, C.M.; et al. TRPM4b channel suppresses store-operated Ca2+ entry by a novel protein-protein interaction with the TRPC3 channel. Biochem. Biophys. Res. Commun. 2008, 368, 677–683. [Google Scholar] [CrossRef]
- Zhang, X.; He, Y. The Role of Nociceptive Neurons in the Pathogenesis of Psoriasis. Front Immunol. 2020, 11, 1984. [Google Scholar] [CrossRef] [PubMed]
- Spahn, V.; Stein, C.; Zöllner, C. Modulation of transient receptor vanilloid 1 activity by transient receptor potential ankyrin 1. Mol. Pharmacol. 2014, 85, 335–344. [Google Scholar] [CrossRef] [PubMed]
- Inada, H.; Iida, T.; Tominaga, M. Different expression patterns of TRP genes in murine B and T lymphocytes. Biochem. Biophys. Res. Commun. 2006, 350, 762–767. [Google Scholar] [CrossRef]
- Nummenmaa, E.; Hämäläinen, M.; Pemmari, A.; Moilanen, L.J.; Tuure, L.; Nieminen, R.M.; Moilanen, T.; Vuolteenaho, K.; Moilanen, E. Transient Receptor Potential Ankyrin 1 (TRPA1) Is Involved in Upregulating Interleukin-6 Expression in Osteoarthritic Chondrocyte Models. Int. J. Mol. Sci. 2020, 22, 87. [Google Scholar] [CrossRef]
- Yap, J.M.G.; Ueda, T.; Takeda, N.; Fukumitsu, K.; Fukuda, S.; Uemura, T.; Tajiri, T.; Ohkubo, H.; Maeno, K.; Ito, Y.; et al. An inflammatory stimulus sensitizes TRPA1 channel to increase cytokine release in human lung fibroblasts. Cytokine 2020, 129, 155027. [Google Scholar] [CrossRef]
- Kamali, A.N.; Noorbakhsh, S.M.; Hamedifar, H.; Jadidi-Niaragh, F.; Yazdani, R.; Bautista, J.M.; Azizi, G. A role for Th1-like Th17 cells in the pathogenesis of inflammatory and autoimmune disorders. Mol. Immunol. 2019, 105, 107–115. [Google Scholar] [CrossRef]
- Choy, E.H.; Panayi, G.S. Cytokine pathways and joint inflammation in rheumatoid arthritis. N. Engl. J. Med. 2001, 344, 907–916. [Google Scholar] [CrossRef] [PubMed]
- Hawkes, J.E.; Chan, T.C.; Krueger, J.G. Psoriasis pathogenesis and the development of novel targeted immune therapies. J. Allergy Clin. Immunol. 2017, 140, 645–653. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ni, X.; Lai, Y. Keratinocyte: A trigger or an executor of psoriasis? J. Leukoc. Biol. 2020, 108, 485–491. [Google Scholar] [CrossRef] [PubMed]
- Pasparakis, M.; Courtois, G.; Hafner, M.; Schmidt-Supprian, M.; Nenci, A.; Toksoy, A.; Krampert, M.; Goebeler, M.; Gillitzer, R.; Israel, A.; et al. TNF-mediated inflammatory skin disease in mice with epidermis-specific deletion of IKK2. Nature 2002, 417, 861–866. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.C. NF-κB signaling in inflammation. Signal Transduct. Target Ther. 2017, 2, 17023. [Google Scholar] [CrossRef] [Green Version]
- Schön, M.P.; Schön, M.; Klotz, K.N. The small antitumoral immune response modifier imiquimod interacts with adenosine receptor signaling in a TLR7- and TLR8-independent fashion. J. Investig. Dermatol. 2006, 126, 1338–1347. [Google Scholar] [CrossRef] [Green Version]
- Wagstaff, A.J.; Perry, C.M. Topical imiquimod: A review of its use in the management of anogenital warts, actinic keratoses, basal cell carcinoma and other skin lesions. Drugs 2007, 67, 2187–2210. [Google Scholar] [CrossRef]
- Hwang, H.; Min, H.; Kim, D.; Yu, S.W.; Jung, S.J.; Choi, S.Y.; Lee, S.J. Imiquimod induces a Toll-like receptor 7-independent increase in intracellular calcium via IP(3) receptor activation. Biochem. Biophys. Res. Commun. 2014, 450, 875–879. [Google Scholar] [CrossRef]
- Huang, A.Y.; Wu, S.Y. The effect of imiquimod on taste bud calcium transients and transmitter secretion. Br. J. Pharmacol. 2016, 173, 3121–3133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Knowlton, W.M.; Bifolck-Fisher, A.; Bautista, D.M.; McKemy, D.D. TRPM8, but not TRPA1, is required for neural and behavioral responses to acute noxious cold temperatures and cold-mimetics in vivo. Pain 2010, 150, 340–350. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kugyelka, R.; Prenek, L.; Olasz, K.; Kohl, Z.; Botz, B.; Glant, T.T.; Berki, T.; Boldizsár, F. ZAP-70 Regulates Autoimmune Arthritis via Alterations in T Cell Activation and Apoptosis. Cells 2019, 8, 504. [Google Scholar] [CrossRef] [Green Version]
- Jia, X.; Gábris, F.; Jacobsen, Ó.; Bedics, G.; Botz, B.; Helyes, Z.; Kellermayer, Z.; Vojkovics, D.; Berta, G.; Nagy, N.; et al. Foliate Lymphoid Aggregates as Novel Forms of Serous Lymphocyte Entry Sites of Peritoneal B Cells and High-Grade B Cell Lymphomas. J. Immunol. 2020, 204, 23–36. [Google Scholar] [CrossRef] [PubMed]
- Khanfar, E.; Olasz, K.; Gábris, F.; Gajdócsi, E.; Botz, B.; Kiss, T.; Kugyelka, R.; Berki, T.; Balogh, P.; Boldizsár, F. Ameliorated Autoimmune Arthritis and Impaired B Cell Receptor-Mediated Ca. Int. J. Mol. Sci. 2020, 21, 6162. [Google Scholar] [CrossRef] [PubMed]
- Minta, A.; Kao, J.P.; Tsien, R.Y. Fluorescent indicators for cytosolic calcium based on rhodamine and fluorescein chromophores. J. Biol. Chem. 1989, 264, 8171–8178. [Google Scholar] [CrossRef]
- Boldizsár, F.; Berki, T.; Miseta, A.; Németh, P. Effect of hyperglycemia on the basal cytosolic free calcium level, calcium signal and tyrosine-phosphorylation in human T-cells. Immunol. Lett. 2002, 82, 159–164. [Google Scholar] [CrossRef]
- Takaishi, M.; Fujita, F.; Uchida, K.; Yamamoto, S.; Sawada Shimizu, M.; Hatai Uotsu, C.; Shimizu, M.; Tominaga, M. 1,8-cineole, a TRPM8 agonist, is a novel natural antagonist of human TRPA1. Mol. Pain 2012, 8, 86. [Google Scholar] [CrossRef] [Green Version]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szabó, K.; Kemény, Á.; Balázs, N.; Khanfar, E.; Sándor, Z.; Boldizsár, F.; Gyulai, R.; Najbauer, J.; Pintér, E.; Berki, T. Presence of TRPA1 Modifies CD4+/CD8+ T Lymphocyte Ratio and Activation. Pharmaceuticals 2022, 15, 57. https://doi.org/10.3390/ph15010057
Szabó K, Kemény Á, Balázs N, Khanfar E, Sándor Z, Boldizsár F, Gyulai R, Najbauer J, Pintér E, Berki T. Presence of TRPA1 Modifies CD4+/CD8+ T Lymphocyte Ratio and Activation. Pharmaceuticals. 2022; 15(1):57. https://doi.org/10.3390/ph15010057
Chicago/Turabian StyleSzabó, Katalin, Ágnes Kemény, Noémi Balázs, Esam Khanfar, Zoltán Sándor, Ferenc Boldizsár, Rolland Gyulai, József Najbauer, Erika Pintér, and Tímea Berki. 2022. "Presence of TRPA1 Modifies CD4+/CD8+ T Lymphocyte Ratio and Activation" Pharmaceuticals 15, no. 1: 57. https://doi.org/10.3390/ph15010057
APA StyleSzabó, K., Kemény, Á., Balázs, N., Khanfar, E., Sándor, Z., Boldizsár, F., Gyulai, R., Najbauer, J., Pintér, E., & Berki, T. (2022). Presence of TRPA1 Modifies CD4+/CD8+ T Lymphocyte Ratio and Activation. Pharmaceuticals, 15(1), 57. https://doi.org/10.3390/ph15010057







