Enhanced Transdermal Delivery of Pranoprofen Using a Thermo-Reversible Hydrogel Loaded with Lipid Nanocarriers for the Treatment of Local Inflammation
Abstract
:1. Introduction
2. Results
2.1. Composition and Physicochemical Characterization of the PF-NLCs
2.2. Physicochemical Characterization of the PF-NLCs-F127 Hydrogel
2.2.1. Appearance, pH Evaluation
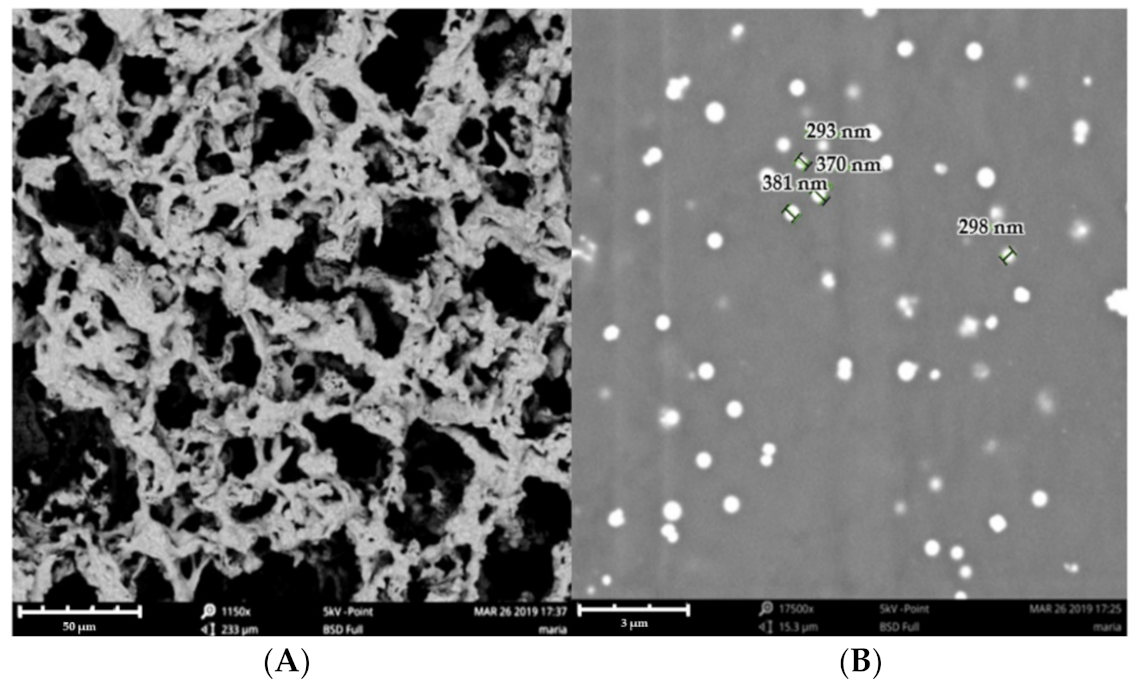
2.2.2. Morphological Studies
2.2.3. Gelling Temperature
2.2.4. Swelling, Degradation and Porosity Studies
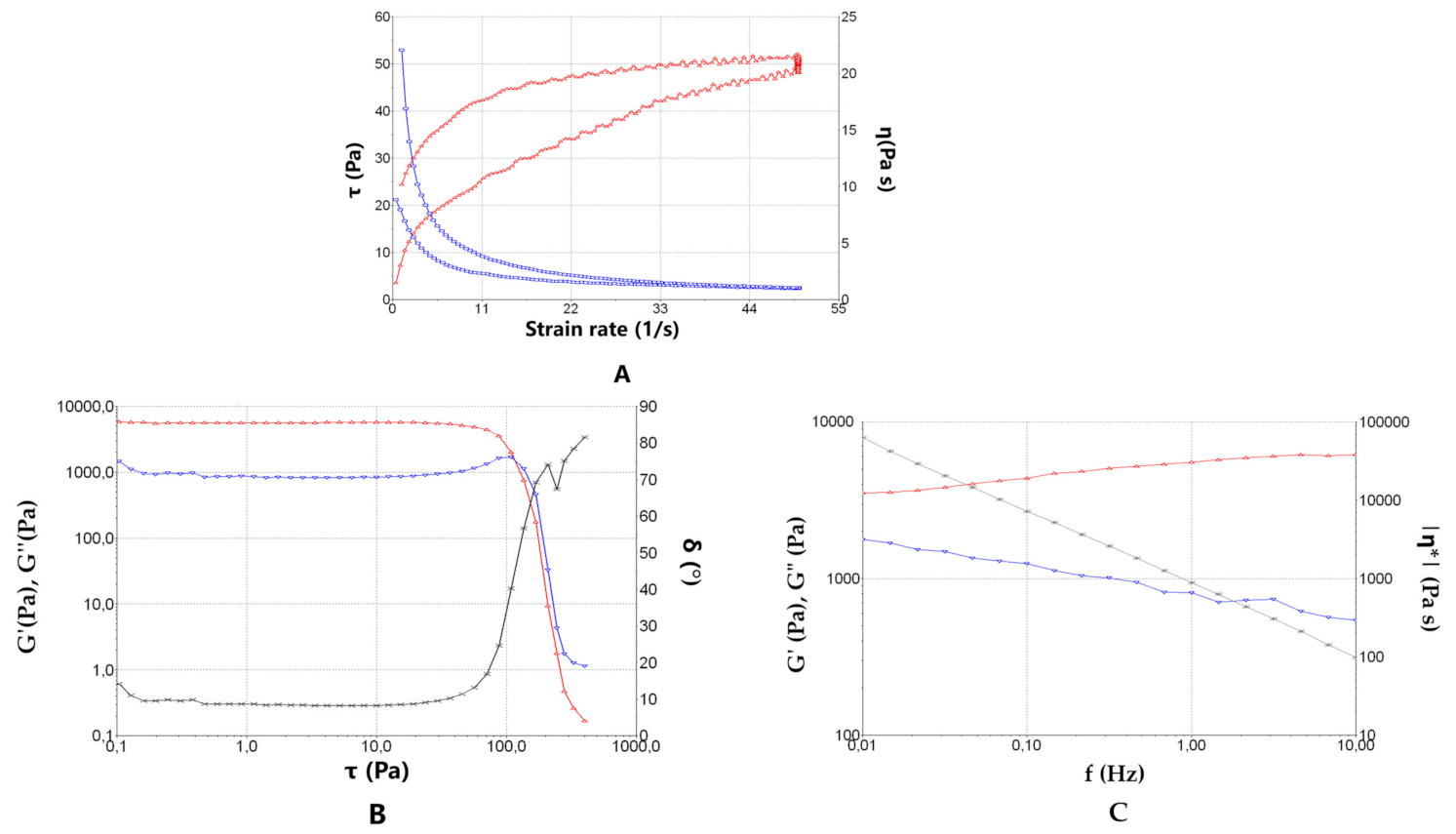
2.2.5. Rheological Behaviour
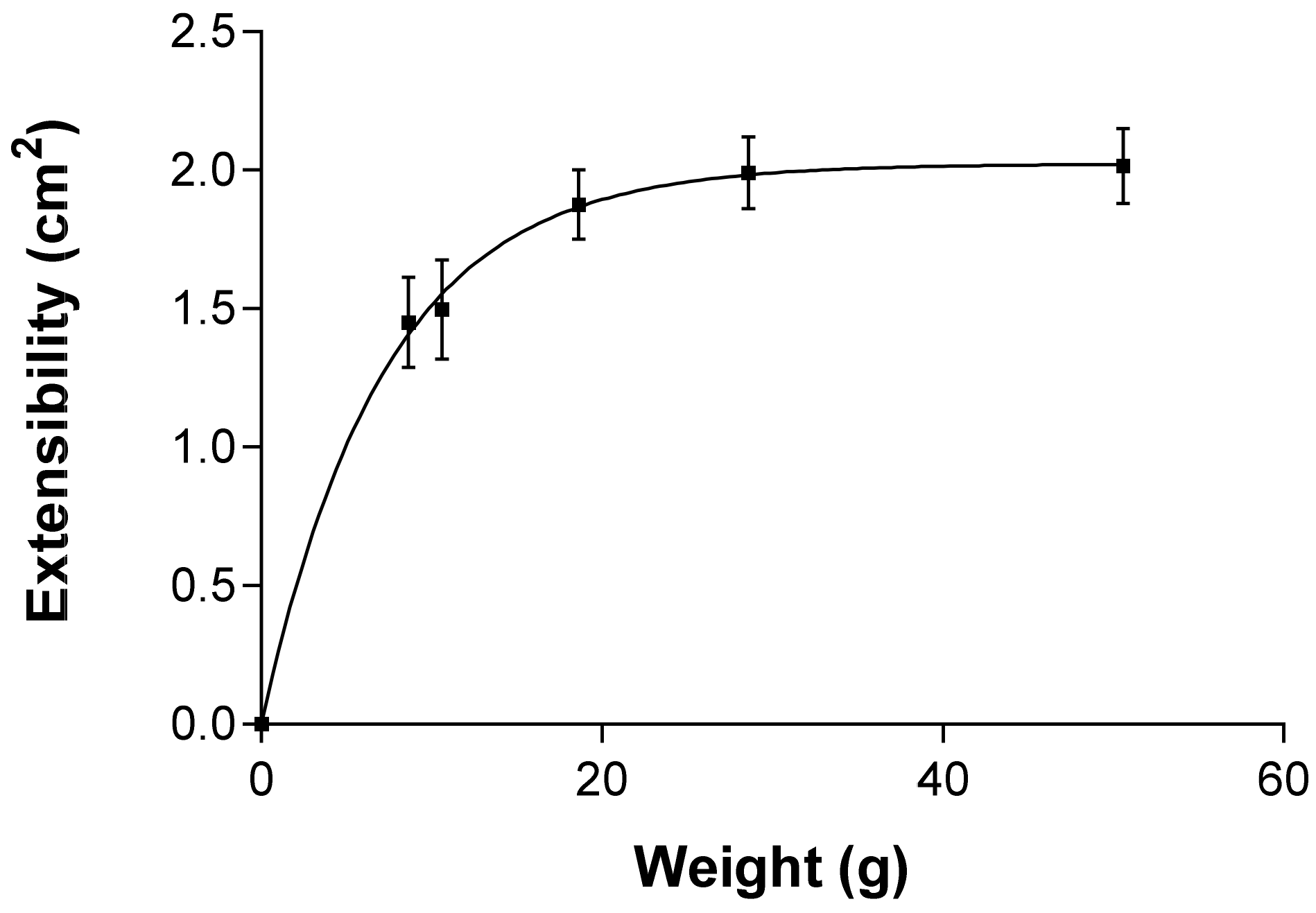
2.2.6. Extensibility (Spreadability)
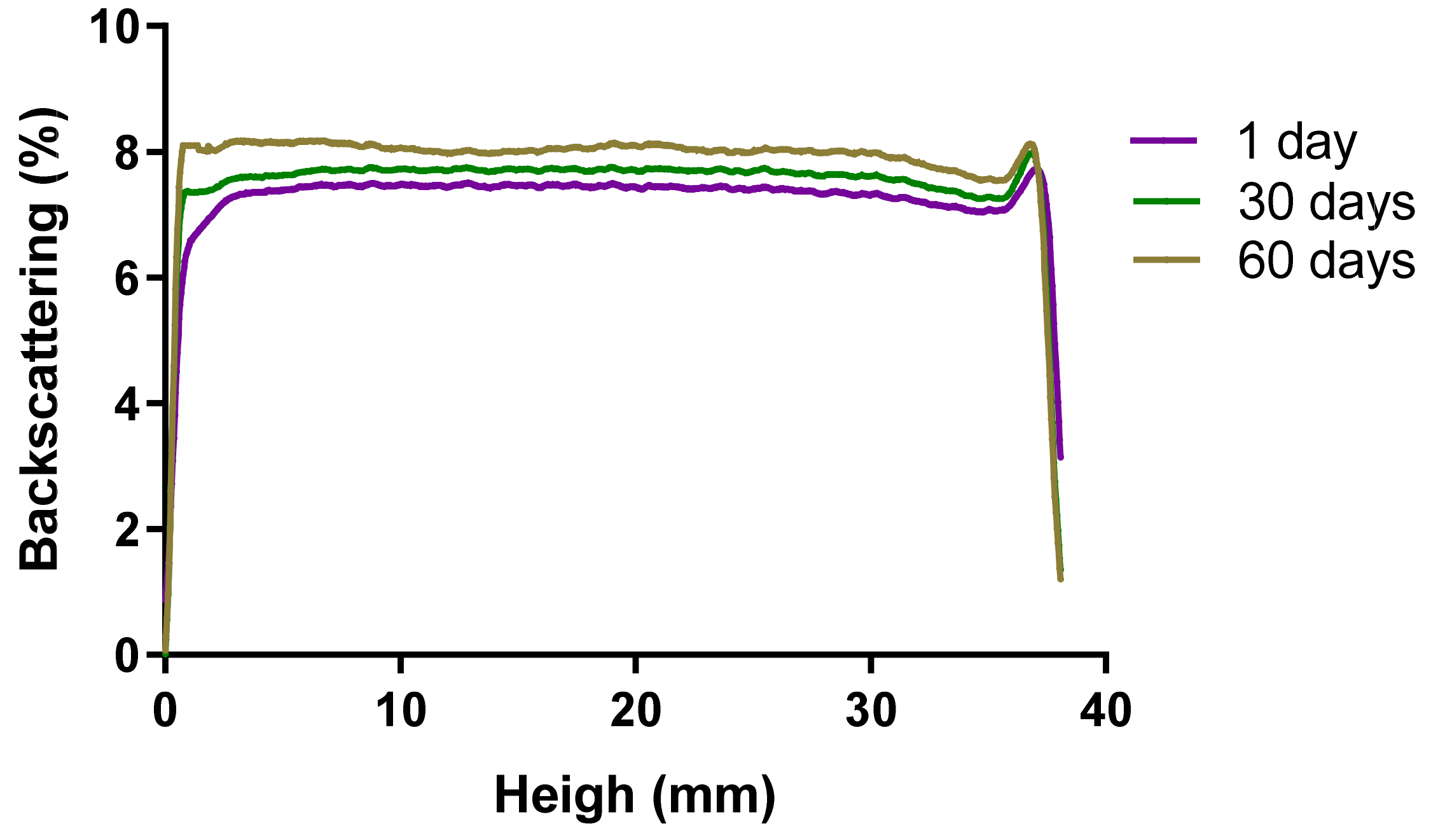
2.2.7. Short-Term Stability Studies
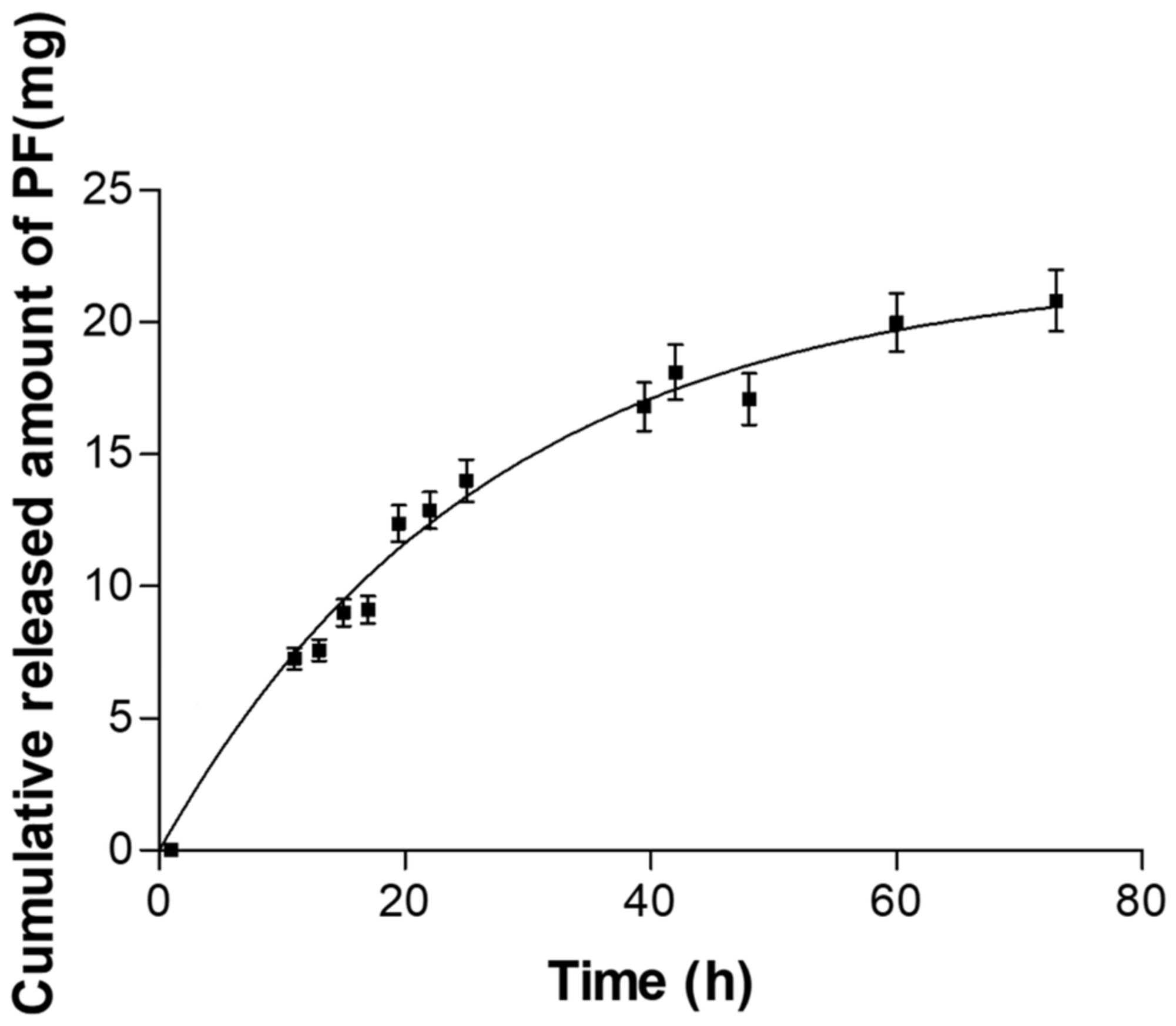
2.3. In Vitro Release Studies
2.4. Ex Vivo Permeation Studies
2.5. In Vitro Cytotoxicity Studies by MTT Assay
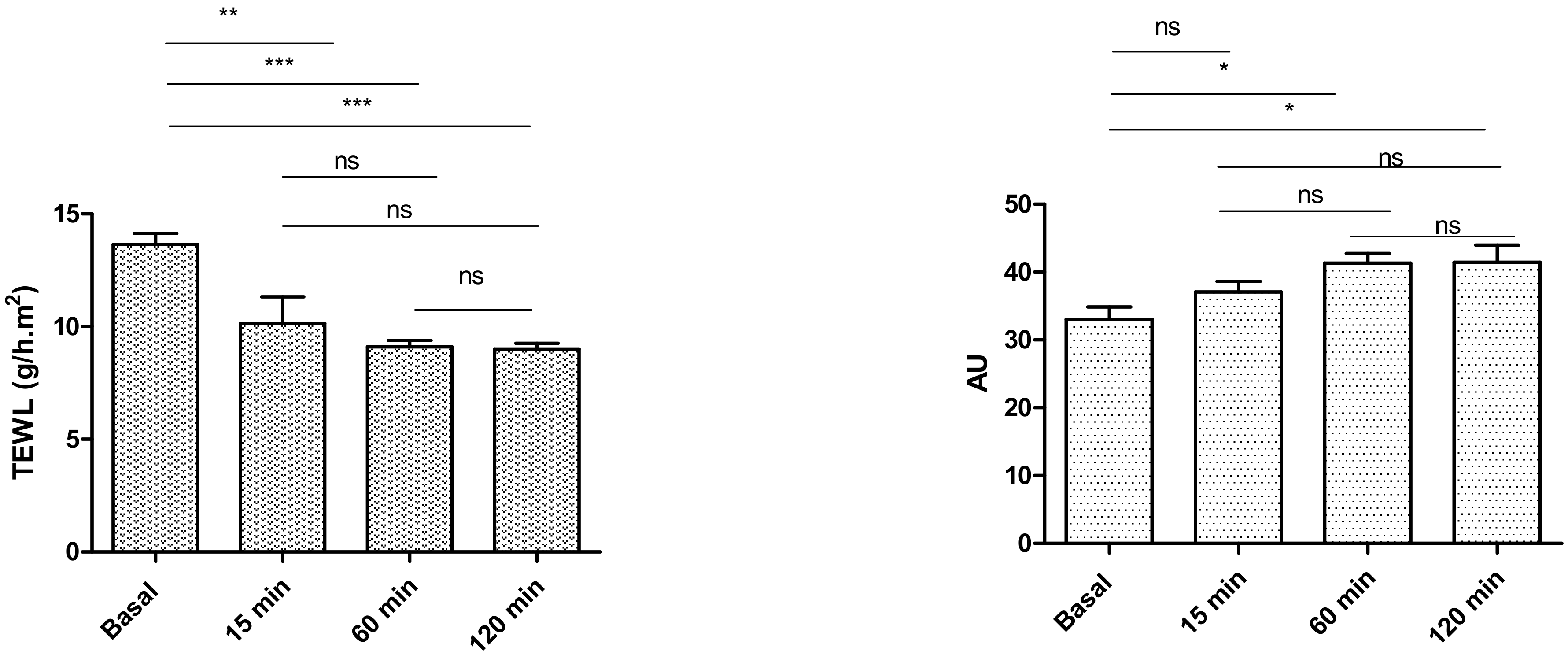
2.6. In Vivo Tolerance Study by Evaluating Biomechanical Properties of Human Skin
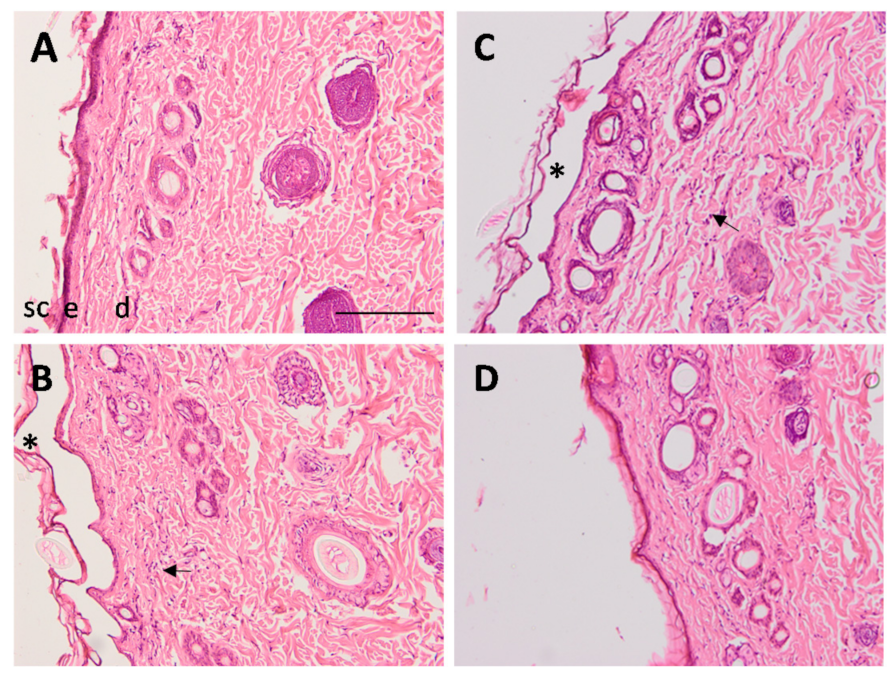
2.7. Efficacy Studies: In Vivo Assay and Histological Analysis
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Preparation of NLCs
4.3. Preparation of Pluronic® F127 Hydrogel Loaded with PF-NLCs
4.4. Physicochemical Characterization of PF-NLCs-F127 Hydrogel
4.4.1. pH and Morphological Characterization
4.4.2. Gelling Temperature
4.4.3. Swelling, Degradation, and Porosity Studies
4.4.4. Rheological Behaviour
4.4.5. Extensibility (Spreadability)
4.4.6. Stability Studies
4.5. In vitro Release Studies of PF-NLCs-F127 Hydrogel
4.6. Ex Vivo Skin Permeation Study
4.7. In Vitro Cytotoxicity Studies with the MTT Assay
4.8. In Vivo Tolerance Study by Evaluating Biomechanical Properties of Human Skin
4.9. In Vivo Assay and Histological Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Carvajal-Vidal, P.; Fábrega, M.J.; Espina, M.; Calpena, A.C.; García, M.L. Development of Halobetasol-loaded nanostructured lipid carrier for dermal administration: Optimization, physicochemical and biopharmaceutical behavior, and therapeutic efficacy. Nanomedicine 2019, 20, 102026. [Google Scholar] [CrossRef]
- National Center for Biotechnology Information. PubChem Compound Summary for CID 4888, Pran-Oprofen. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Pranoprofen (accessed on 10 October 2021).
- Yoshinaga, N.; Arimura, N.; Otsuka, H.; Kawahara, K.; Hashiguchi, T.; Maruyama, I.; Sakamoto, T. NSAIDs inhibit neovascularization of choroid through HO-1-dependent pathway. Lab. Investig. 2011, 91, 1277–1290. [Google Scholar] [CrossRef]
- Yao, B.; Wang, F.; Zhao, X.; Wang, B.; Yue, X.; Ding, Y.; Liu, G. Effect of a Topical Nonsteroidal Anti-Inflammatory Drug (0.1% Pranoprofen) on VEGF and COX-2 Expression in Primary Pterygium. Front. Pharmacol. 2021, 12, 709251. [Google Scholar] [CrossRef]
- Cañadas-Enrich, C.; Abrego, G.; Alvarado, H.L.; Calpena-Campmany, A.C.; Boix-Montañes, A. Pranoprofen quantification in ex vivo corneal and scleral permeation samples: Analytical validation. J. Pharm. Biomed. Anal. 2018, 160, 109–118. [Google Scholar] [CrossRef]
- Rincón, M.; Calpena, A.C.; Clares, B.; Espina, M.; Garduño-Ramírez, M.L.; Rodríguez-Lagunas, M.J.; García, M.L.; Abrego, G. Skin-controlled release lipid nanosystems of pranoprofen for the treatment of local inflammation and pain. Nanomedicine 2018, 13, 2397–2413. [Google Scholar] [CrossRef]
- Luo, Y.; Yang, L.; Feng, P.; Qiu, H.; Wu, X.; Lu, S.; Zhou, M.; Xu, L.; Zhu, Y. Pranoprofen Nanoparticles With Poly(L-Lactide)-b-Poly(Ethylene Glycol)-b-Poly(L-Lactide) as the Matrix Toward Improving Ocular Anti-inflammation. Front. Bioeng. Biotechnol. 2020, 8, 581621. [Google Scholar] [CrossRef]
- Abrego, G.; Alvarado, H.L.; Egea, M.A.; Gonzalez-Mira, E.; Calpena, A.C.; García, M.L. Design of nanosuspensions and freeze-dried PLGA nanoparticles as a novel approach for ophthalmic delivery of pranoprofen. J. Pharm. Sci. 2014, 103, 3153–3164. [Google Scholar] [CrossRef]
- Edgar, J.Y.C.; Wang, H. Introduction for Design of Nanoparticle Based Drug Delivery Systems. Curr. Pharm. Des. 2017, 23, 2108–2112. [Google Scholar] [CrossRef]
- Pardeike, J.; Hommoss, A.; Müller, R.H. Lipid nanoparticles (SLN, NLCS) in cosmetic and pharmaceutical dermal products. Int. J. Pharm. 2009, 366, 170–184. [Google Scholar] [CrossRef]
- Oliva, N.; Conde, J.; Wang, K.; Artzi, N. Designing Hydrogels for On-Demand Therapy. Acc. Chem. Res. 2017, 50, 669–679. [Google Scholar] [CrossRef]
- Mallandrich, M.; Fernández-Campos, F.; Clares, B.; Halbaut, L.; Alonso, C.; Coderch, L.; Garduño-Ramírez, M.L.; Andrade, B.; Del Pozo, A.; Lane, M.E.; et al. Developing Transdermal Applications of Ketorolac Tromethamine Entrapped in Stimuli Sensitive Block Copolymer Hydrogels. Pharm. Res. 2017, 34, 1728–1740. [Google Scholar] [CrossRef] [Green Version]
- European Pharmacopoiea 10.5. 07/2021:0132 Pag.5711. Available online: https://pheur.edqm.eu/ (accessed on 2 September 2021).
- Chander, V.; Gangenahalli, G. Pluronic-F127/Platelet Microvesicles nanocomplex delivers stem cells in high doses to the bone marrow and confers post-irradiation survival. Sci. Rep. 2020, 10, 156. [Google Scholar] [CrossRef] [Green Version]
- Jiménez Pardo, I. Hidrogeles Termosensibles y Fotopolimerizables Derivados de Pluronic® Para Aplicaciones Biomédicas. Ph.D. Thesis, Zaragoza University, Zaragoza, Spain, 2014; pp. 25–26. [Google Scholar]
- Gonzalez-Pizarro, R.; Carvajal-Vidal, P.; Halbault Bellowa, L.; Calpena, A.C.; Espina, M.; García, M.L. In-situ forming gels containing fluorometholone-loaded polymeric nanoparticles for ocular inflammatory conditions. Colloids Surf. B Biointerfaces 2019, 175, 365–374. [Google Scholar] [CrossRef]
- Sosa, L.; Calpena, A.C.; Silva-Abreu, M.; Espinoza, L.C.; Rincón. M.; Bozal, N.; Domenech, O.; Rodríguez-Lagunas, M.J.; Clares, B. Thermoreversible Gel-Loaded Amphotericin B for the Treatment of Dermal and Vaginal Candidiasis. Pharmaceutics 2019, 11, 312. [Google Scholar] [CrossRef] [Green Version]
- Espinoza, L.C.; Silva-Abreu, M.; Clares, B.; Rodríguez-Lagunas, M.J.; Halbaut, L.; Cañas, M.A.; Calpena, A.C. Formulation Strategies to Improve Nose-to-Brain Delivery of Donepezil. Pharmaceutics 2019, 11, 64. [Google Scholar] [CrossRef] [Green Version]
- Souto, E.B.; Baldim, I.; Oliveira, W.P.; Rao, R.; Yadav, N.; Gama, F.M.; Mahant, S. SLN and NLCS for topical, dermal, and transdermal drug delivery. Expert Opin. Drug Deliv. 2020, 17, 357–377. [Google Scholar] [CrossRef]
- Choi, J.S.; Shin, S.C. Preparation, and evaluation of pranoprofen gel for percutaneous administration. Drug Dev. Ind. Pharm. 2007, 33, 19–26. [Google Scholar] [CrossRef]
- Rincón, M.; Calpena, A.C.; Fabrega, M.-J.; Garduño-Ramírez, M.L.; Espina, M.; Rodríguez-Lagunas, M.J.; García, M.L.; Abrego, G. Development of Pranoprofen Loaded Nanostructured Lipid Carriers to Improve Its Release and Therapeutic Efficacy in Skin Inflammatory Disorders. Nanomaterials 2018, 8, 1022. [Google Scholar] [CrossRef] [Green Version]
- Ali, S.M.; Yosipovitch, G. Skin pH: From basic science to basic skin care. Acta Derm. Venereol. 2013, 93, 261–267. [Google Scholar] [CrossRef] [Green Version]
- Abrego, G.; Alvarado, H.; Souto, E.B.; Guevara, B.; Bellowa, L.H.; Garduño, M.L.; Garcia, M.L.; Calpena, A.C. Biopharmaceutical profile of hydrogels containing pranoprofen-loaded PLGA nanoparticles for skin administration: In vitro, ex vivo and in vivo characterization. Int. J. Pharm. 2016, 501, 350–361. [Google Scholar] [CrossRef]
- Espinoza, L.C.; Sosa, L.; Granda, P.C.; Bozal, N.; Díaz-Garrido, N.; Chulca-Torres, B.; Calpena, A.C. Development of a Topical Amphotericin B and Bursera graveolens Essential Oil-Loaded Gel for the Treatment of Dermal Candidiasis. Pharmaceuticals 2021, 14, 1033. [Google Scholar] [CrossRef] [PubMed]
- Roberts, M.S.; Mohammed, Y.; Pastore, M.N.; Namjoshi, S.; Yousef, S.; Alinaghi, A.; Haridass, I.N.; Abd, E.; Leite-Silva, V.R.; Benson, H.; et al. Topical and cutaneous delivery using nanosystems. J. Control Release 2017, 247, 86–105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sguizzato, M.; Esposito, E.; Cortesi, R. Lipid-Based Nanosystems as a Tool to Overcome Skin Barrier. Int. J. Mol. Sci. 2021, 22, 8319. [Google Scholar] [CrossRef] [PubMed]
- Al Khateb, K.; Ozhmukhametova, E.K.; Mussin, M.N.; Seilkhanov, S.K.; Rakhypbekov, T.K.; Lau, W.M.; Khutoryanskiy, V.V. In situ gelling systems based on Pluronic F127/Pluronic F68 formulations for ocular drug delivery. Int. J. Pharm. 2016, 502, 70–79. [Google Scholar] [CrossRef]
- Escobar-Chávez, J.J.; López-Cervantes, M.; Naïk, A.; Kalia, Y.N.; Quintanar-Guerrero, D.; Ganem-Quintanar, A. Applications of thermo-reversible pluronic F-127 gels in pharmaceutical formulations. J. Pharm. Pharm. Sci. 2006, 9, 339–358. [Google Scholar]
- Chung, C.K.; García-Couce, J.; Campos, Y.; Kralisch, D.; Bierau, K.; Chan, A.; Ossendorp, F.; Cruz, L.J. Doxorubicin Loaded Poloxamer Thermosensitive Hydrogels: Chemical, Pharmacological and Biological Evaluation. Molecules 2020, 25, 2219. [Google Scholar] [CrossRef]
- Carter, P.; Narasimhan, B.; Wang, Q. Biocompatible nanoparticles and vesicular systems in transdermal drug delivery for various skin diseases. Int. J. Pharm. 2019, 555, 49–62. [Google Scholar] [CrossRef] [PubMed]
- Sathyanarayanan, G.; Rodrigues, M.; Limón, D.; Rodriguez-Trujillo, R.; Puigmartí-Luis, J.; Pérez-García, L.; Amabilino, D.B. Drug-Loaded Supramolecular Gels Prepared in a Microfluidic Platform: Distinctive Rheology and Delivery through Controlled Far-from-Equilibrium Mixing. ACS Omega 2017, 2, 8849–8858. [Google Scholar] [CrossRef]
- Neves, J.; da Silva, M.V.; Gonçalves, M.P.; Amaral, M.H.; Bahia, M.F. Rheological properties of vaginal hydrophilic polymer gels. Curr. Drug Deliv. 2009, 6, 83–92. [Google Scholar] [CrossRef]
- Berenguer, D.; Alcover, M.M.; Sessa, M.; Halbaut, L.; Guillén, C.; Boix-Montañés, A.; Fisa, R.; Calpena-Campmany, A.C.; Riera, C.; Sosa, L. Topical Amphotericin B Semisolid Dosage Form for Cutaneous Leishmaniasis: Physicochemical Characterization, Ex Vivo Skin Permeation and Biological Activity. Pharmaceutics 2020, 12, 149. [Google Scholar] [CrossRef] [Green Version]
- Cano, A.; Ettcheto, M.; Chang, J.H.; Barroso, E.; Espina, M.; Kühne, B.A.; Barenys, M.; Auladell, C.; Folch, J.; Souto, E.B.; et al. Dual-drug loaded nanoparticles of Epigallocatechin-3-gallate (EGCG)/Ascorbic acid enhance therapeutic efficacy of EGCG in a APPswe/PS1dE9 Alzheimer’s disease mice model. J. Control Release 2019, 301, 62–75. [Google Scholar] [CrossRef]
- Folle, C.; Díaz-Garrido, N.; Sánchez-López, E.; Marqués, A.M.; Badia, J.; Baldomà, L.; Espina, M.; Calpena, A.C.; García, M.L. Surface-Modified Multifunctional Thymol-Loaded Biodegradable Nanoparticles for Topical Acne Treatment. Pharmaceutics 2021, 13, 1501. [Google Scholar] [CrossRef] [PubMed]
- Garg, N.K.; Tandel, N.; Bhadada, S.K.; Tyagi, R.K. Nanostructured Lipid Carrier-Mediated Transdermal Delivery of Aceclofenac Hydrogel Present an Effective Therapeutic Approach for Inflammatory Diseases. Front. Pharmacol. 2021, 12, 713616. [Google Scholar] [CrossRef] [PubMed]
- Bitencourt, M.A.; Dantas, G.R.; Lira, D.P.; Barbosa-Filho, J.M.; de Miranda, G.E.; Santos, B.V.; Souto, J.T. Aqueous and Methanolic Extracts of Caulerpa mexicana Suppress Cell Migration and Ear Edema Induced by Inflammatory Agents. Mar. Drugs 2011, 9, 1332–1345. [Google Scholar] [CrossRef]
- Limón, D.; Talló Domínguez, K.; Garduño-Ramírez, M.L.; Andrade, B.; Calpena, A.C.; Pérez-García, L. Nanostructured supramolecular hydrogels: Towards the topical treatment of Psoriasis and other skin diseases. Colloids Surf. B Biointerfaces 2019, 181, 657–670. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Pizarro, R.; Silva-Abreu, M.; Calpena, A.C.; Egea, M.A.; Espina, M.; García, M.L. Development of fluorometholone-loaded PLGA nanoparticles for treatment of inflammatory disorders of anterior and posterior segments of the eye. Int. J. Pharm. 2018, 547, 338–346. [Google Scholar] [CrossRef] [Green Version]
- Sarango-Granda, P.; Silva-Abreu, M.; Calpena, A.C.; Halbaut, L.; Fábrega, M.-J.; Rodríguez-Lagunas, M.J.; Díaz-Garrido, N.; Badia, J.; Espinoza, L.C. Apremilast Microemulsion as Topical Therapy for Local Inflammation: Design, Characterization and Efficacy Evaluation. Pharmaceuticals 2020, 13, 484. [Google Scholar] [CrossRef]
- World Medical Association. World medical association declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA 2013, 310, 2191–2194. [Google Scholar] [CrossRef] [Green Version]
- Dominguez-Villegas, V.; Clares Naveros, B.; García Lopez, M.L.; Calpena-Campany, A.C.; Bustos-Zagal, P.; Garduño-Ramirez, M.L. Development and charazterization of two nano-structured systems for topical application of flavanones isolated from Eysenhardtia Platycarpa. Colloids Surf. B Biointerfaces 2014, 116, 183–192. [Google Scholar] [CrossRef] [PubMed]
- Bustos-Salgado, P.; Andrade-Carrera, B.; Domínguez-Villegas, V.; Díaz-Garrido, N.; Rodríguez-Lagunas, M.J.; Badía, J.; Baldomà, L.; Mallandrich, M.; Calpena-Campmany, A.; Garduño-Ramírez, M.L. Screening Anti-Inflammatory Effects of Flavanones Solutions. Int. J. Mol. Sci. 2021, 22, 8878. [Google Scholar] [CrossRef]


| Composition of PF-NLCs | Physicochemical Characterization | |||||
|---|---|---|---|---|---|---|
| cPF (%) | cSL/L (%) | cTW (%) | Z-Ave (nm) ± SD | PI ± SD | ZP (mV) ± SD | EE (%) ± SD |
| 1.50 | 50.00 | 2.50 | 248.40 ± 6.34 | 0.22 ± 0.04 | −10.70 ± 0.44 | 99.68 ± 0.17 |
| Order Equation | Parameters | Unit Value (Mean ± SD) | ||
|---|---|---|---|---|
| Zero order | Qt = K0 t + Q∞ | K0 | mg/h | 0.26 ± 0.02 |
| Q∞ | mg | 4.98 ± 0.62 | ||
| r2 | - | 0.8395 | ||
| AIC | - | 152.98 | ||
| First order | Qt = Q∞(1 − e−kf·t) | Kf | h−1 | 0.037 ± 0.004 |
| Q∞ | mg | 22.01 ± 1.05 | ||
| r2 | - | 0.9227 | ||
| t ½ | h | 18.46 | ||
| AIC | - | 132.53 | ||
| Korsmeyer-Peppas | Qt = Kk t n | KK | h−n | 2.379 ± 0.281 |
| n | - | 0.52 ± 0.03 | ||
| r2 | - | 0.9182 | ||
| AIC | - | 134.14 | ||
| Weibull | Qt = Q∞(1 − e−(t/td)β) | td | h | 39.85 ± 19.06 |
| β | - | 0.79 ± 0.13 | ||
| Q∞ | mg | 26.36 ± 5.49 | ||
| r2 | - | 0.9262 | ||
| AIC | - | 196.84 | ||
| Jss (µg/h/cm2) | Kp (cm/h) 105 | Qret (µg/g/cm2) | Q24h (µg) | Young Subject | Elderly Subject |
|---|---|---|---|---|---|
| 1.134 (1.129–1.140) | 59.5 (59.3–59.8) | 83.33 (82.00–99.50) | 27.95 (23.10–28.02) | 84.39 (76.49–90.83) | 158.92 (144.03–171.04) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rincón, M.; Silva-Abreu, M.; Espinoza, L.C.; Sosa, L.; Calpena, A.C.; Rodríguez-Lagunas, M.J.; Colom, H. Enhanced Transdermal Delivery of Pranoprofen Using a Thermo-Reversible Hydrogel Loaded with Lipid Nanocarriers for the Treatment of Local Inflammation. Pharmaceuticals 2022, 15, 22. https://doi.org/10.3390/ph15010022
Rincón M, Silva-Abreu M, Espinoza LC, Sosa L, Calpena AC, Rodríguez-Lagunas MJ, Colom H. Enhanced Transdermal Delivery of Pranoprofen Using a Thermo-Reversible Hydrogel Loaded with Lipid Nanocarriers for the Treatment of Local Inflammation. Pharmaceuticals. 2022; 15(1):22. https://doi.org/10.3390/ph15010022
Chicago/Turabian StyleRincón, María, Marcelle Silva-Abreu, Lupe Carolina Espinoza, Lilian Sosa, Ana Cristina Calpena, María J. Rodríguez-Lagunas, and Helena Colom. 2022. "Enhanced Transdermal Delivery of Pranoprofen Using a Thermo-Reversible Hydrogel Loaded with Lipid Nanocarriers for the Treatment of Local Inflammation" Pharmaceuticals 15, no. 1: 22. https://doi.org/10.3390/ph15010022
APA StyleRincón, M., Silva-Abreu, M., Espinoza, L. C., Sosa, L., Calpena, A. C., Rodríguez-Lagunas, M. J., & Colom, H. (2022). Enhanced Transdermal Delivery of Pranoprofen Using a Thermo-Reversible Hydrogel Loaded with Lipid Nanocarriers for the Treatment of Local Inflammation. Pharmaceuticals, 15(1), 22. https://doi.org/10.3390/ph15010022










