The GPR18 Agonist PSB-KD-107 Exerts Endothelium-Dependent Vasorelaxant Effects
Abstract
:1. Introduction
2. Results
2.1. Effect Induced by PSB-KD-107 and PSB-CB-92 in Rat Aorta Precontracted with Phenylephrine
2.2. Effect of PSB-KD-107 on Guinea-Pig Ileum Contraction Induced by Carbachol
2.3. Effect of PSB-KD-107 and PSB-CB-92 on Platelet Aggregation
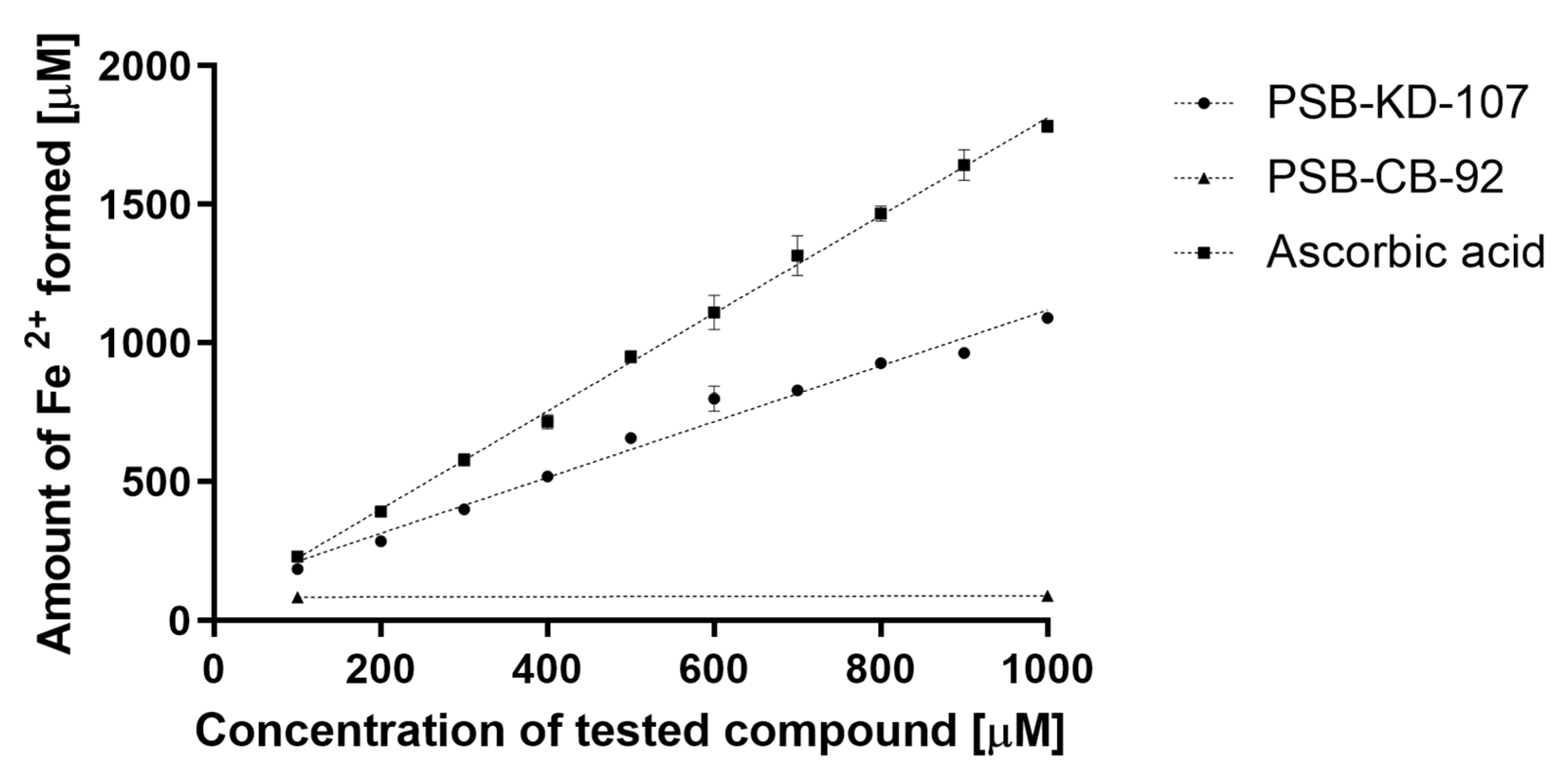
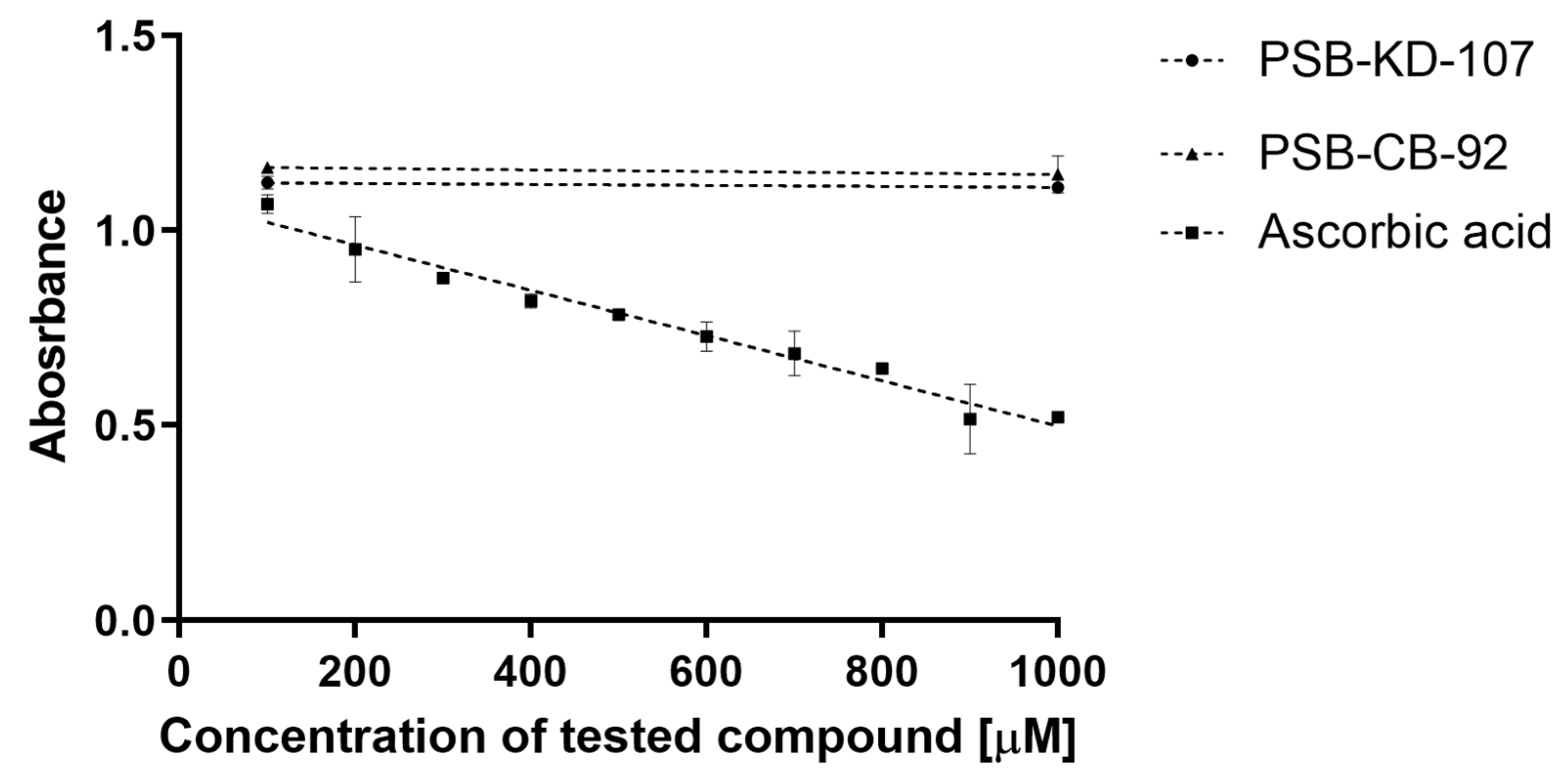
2.4. Antioxidant Activity of PSB-KD-107 and PSB-CB-92
2.4.1. Ferric Reducing Antioxidant Power (FRAP) Assay
2.4.2. 2,2-Diphenyl-1-picryl-hydrazyl-hydrate Free Radical (DPPH) Assay
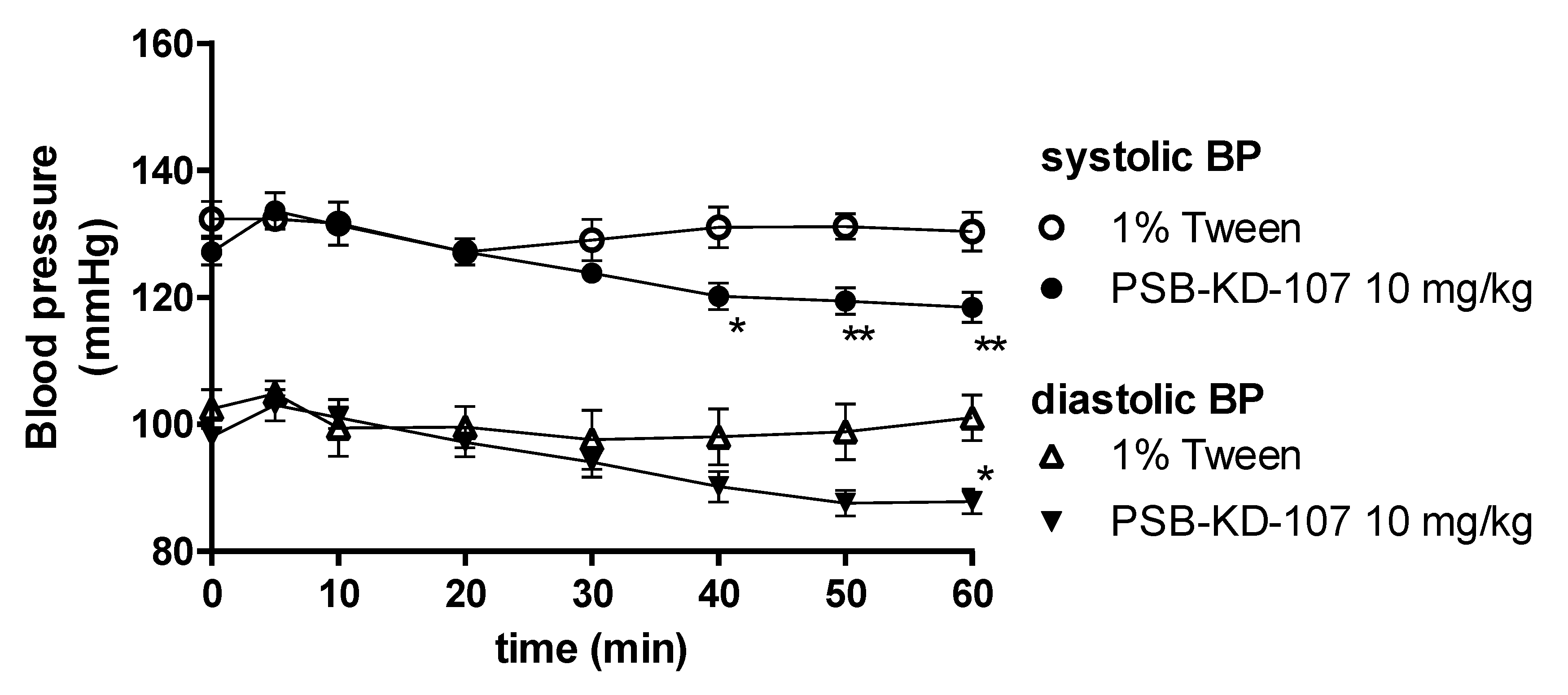
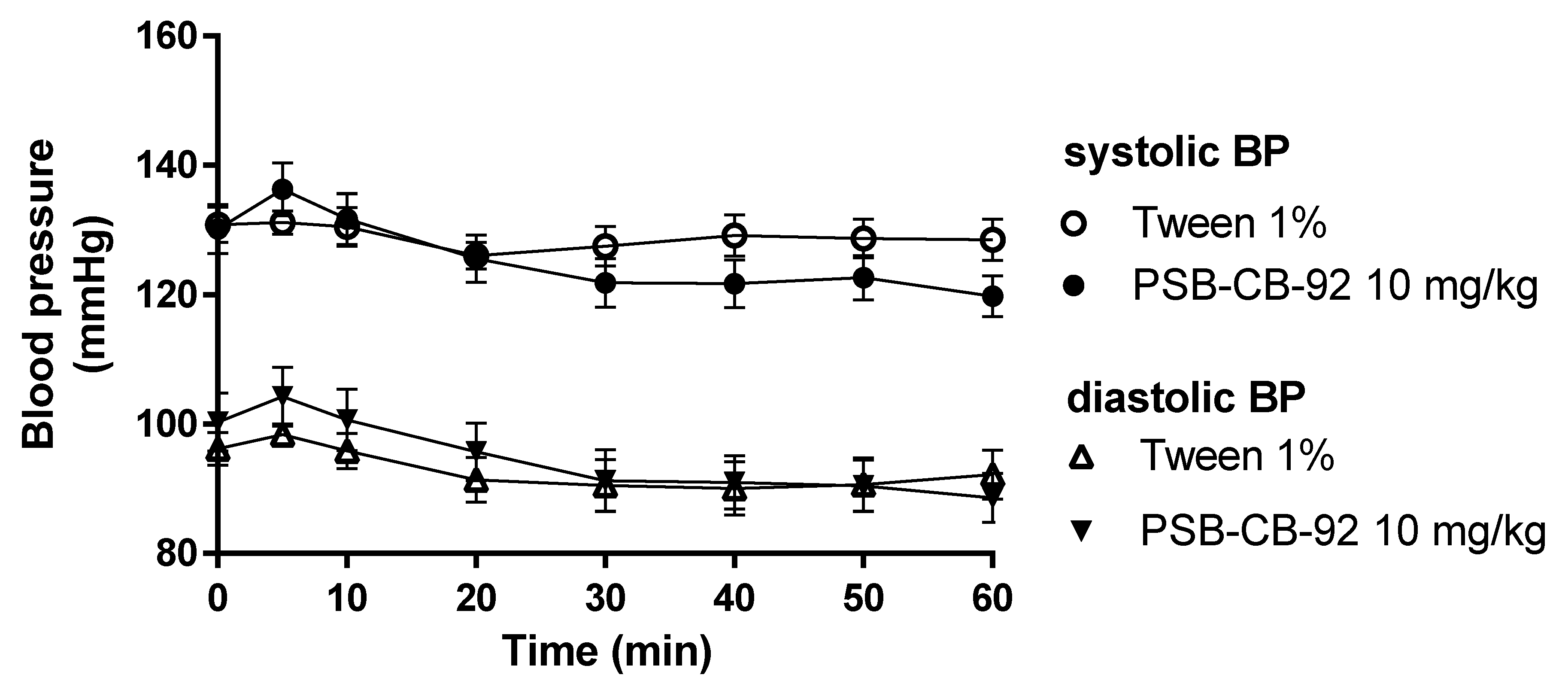
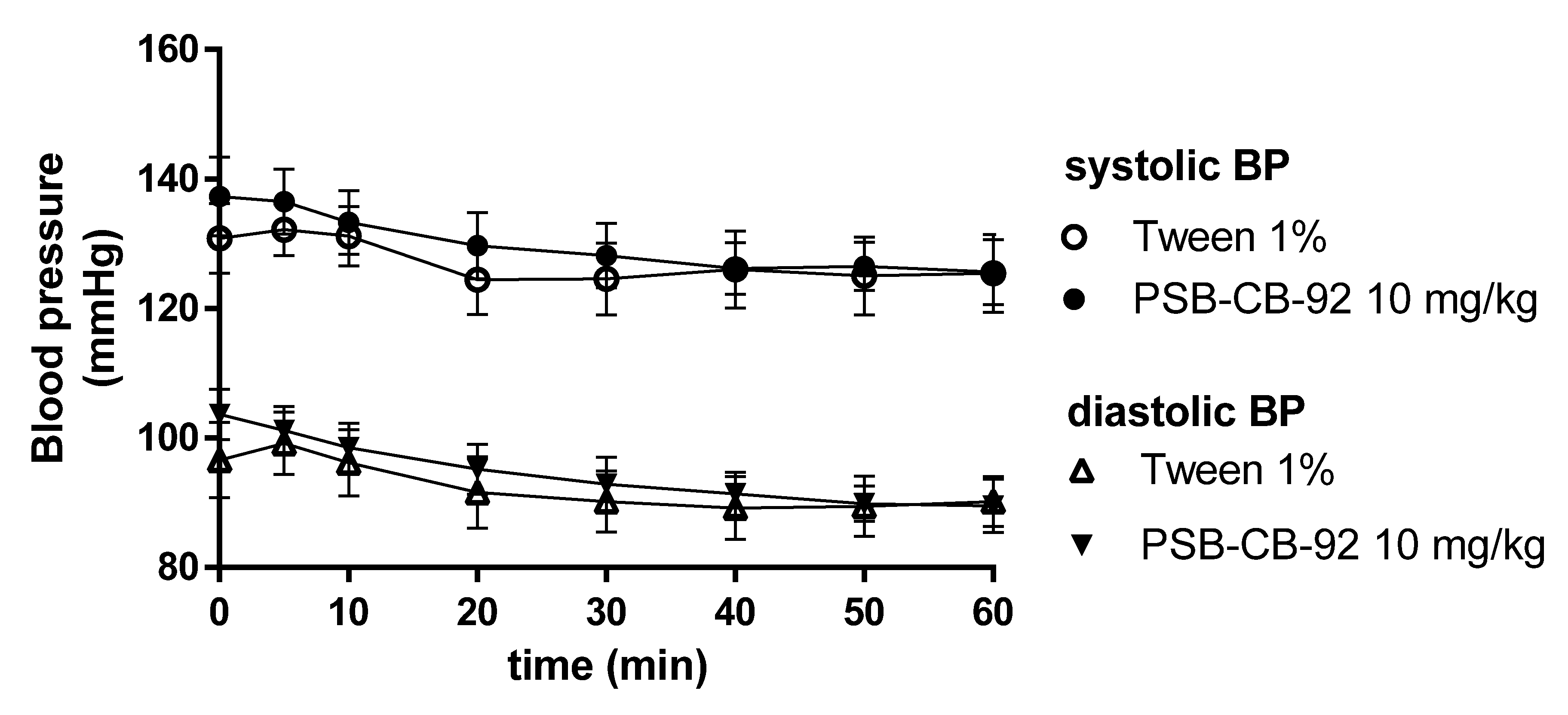
2.5. Influence of PSB-KD-107 and PSB-CB-92 on Blood Pressure in Normotensive Rats
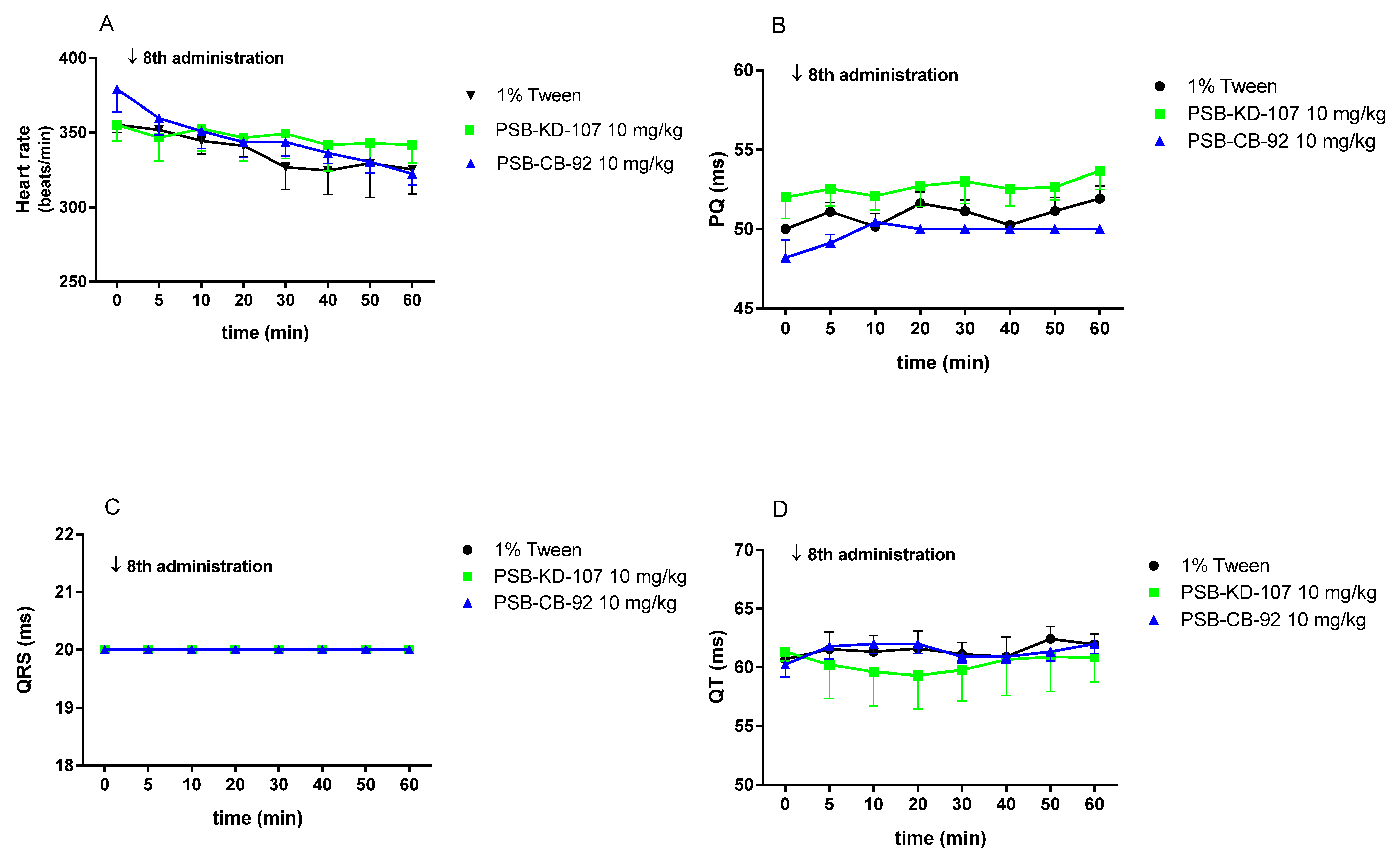
2.6. The Effects of PSB-KD-107 and PSB-CB-92 on the Normal Electrocardiogram (ECG)
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Drugs and Chemicals
4.3. In Vitro Studies
4.3.1. Influence of PSB-KD-107 and PSB-CB-92 on Rat Aorta Precontracted with Phenylephrine
4.3.2. Influence of PSB-KD-107 and PSB-CB-92 on the Guinea Pig Ileum Contraction Induced by Carbachol
4.3.3. In Vitro Aggregation Test
4.3.4. Antioxidant Assays
DPPH Assay
FRAP Assay
4.4. In Vivo Studies
4.4.1. Influence of PSB-KD-107 and PSB-CB-92 on Blood Pressure in Rats
4.4.2. The Effect of PSB-KD-107 and PSB-CB-92 on Normal Electrocardiogram
4.5. Data Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pertwee, R.G. Targeting the endocannabinoid system with cannabinoid receptor agonists: Pharmacological strategies and therapeutic possibilities. Phil. Trans. R. Soc. B 2012, 367, 3353–3363. [Google Scholar] [CrossRef]
- Huang, W.J.; Chen, W.W.; Zhang, X. Endocannabinoid system: Role in depression, reward and pain control (Revier). Mol. Med. Rep. 2016, 14, 2899–2903. [Google Scholar] [CrossRef] [Green Version]
- Pacher, P.; Bátkai, S.; Kunos, G. The endocannabinoid system as an emerging target of pharmacotherapy. Pharmacol. Rev. 2006, 58, 389–462. [Google Scholar] [CrossRef] [Green Version]
- Kotańska, M.; Mika, K.; Szafarz, M.; Kubacka, M.; Müller, C.E.; Sapa, J.; Kieć-Kononowicz, K. Effects of gpr18 ligands on body weight and metabolic parameters in a female rat model of excessive eating. Pharmaceuticals 2021, 14, 270. [Google Scholar] [CrossRef] [PubMed]
- Rempel, V.; Atzler, K.; Behrenswerth, A.; Karcz, T.; Schoeder, C.; Hinz, S.; Kaleta, M.; Thimm, D.; Kie´c-Kononowicz, K.; Müller, C.E. Bicyclic imidazole-4-one derivatives: A new class of antagonists for the orphan G Protein-coupled receptors GPR18 and GPR55. MedChemComm 2014, 5, 632–649. [Google Scholar] [CrossRef]
- Piomelli, D.; Giuffrida, A.; Calignano, A.; de Fonseca, F.R. The endocannabinoid system as a target for therapeutic drugs. Trends Pharmacol. Sci. 2000, 21, 218–224. [Google Scholar] [CrossRef] [Green Version]
- Adel, Y.; Alexander, S.P.H. Neuromolecular Mechanisms of Cannabis Action. Adv. Exp. Med. Biol. 2021, 1264, 15–28. [Google Scholar]
- Poleszak, E.; Wośko, S.; Sławińska, K.; Szopa, A.; Wróbel, A.; Serefko, A. Cannabinoids in depressive disorders. Life Sci. 2018, 213, 18–24. [Google Scholar] [CrossRef]
- Irving, A.; Abdulrazzaq, G.; Chan, S.L.F.; Penman, J.; Harvey, J.; Alexander, S.P.H. Cannabinoid receptor-related orphan G protein-coupled receptors. Adv. Pharmacol. 2017, 80, 223–247. [Google Scholar]
- Penumarti, A.; Abdel-Rahman, A.A. The novel endocannabinoid receptor GPR18 is expressed in the rostral ventrolateral medulla and exerts tonic restraining influence on blood pressure. J. Pharmacol. Exp. Ther. 2014, 349, 29–38. [Google Scholar] [CrossRef] [Green Version]
- Schoeder, C.T.; Mahardhika, A.B.; Drabczyńska, A.; Kieć-Kononowicz, K.; Müller, C.E. Discovery of Tricyclic Xanthines as Agonists of the Cannabinoid-Activated Orphan G-Protein-Coupled Receptor GPR18. ACS Med. Chem. Lett. 2020, 11, 2024–2031. [Google Scholar] [CrossRef]
- Schoeder, C.T.; Kaleta, M.; Mahardhika, A.B.; Olejarz-Maciej, A.; Łażewska, D.; Kieć-Kononowicz, K.; Müller, C.E. Structure-activity relationships of imidazothiazinones and analogs as antagonists of the cannabinoid-activated orphan G protein-coupled receptor GPR18. Eur. J. Med. Chem. 2018, 155, 381–397. [Google Scholar] [CrossRef]
- Rajaraman, G.; Simcocks, A.; Hryciw, D.H.; Hutchinson, D.S.; McAinch, A.J. G protein coupled receptor 18: A potential role for endocannaninoid signaling in metabolic dysfunction. Mol. Nutr. Food Res. 2016, 60, 92–102. [Google Scholar] [CrossRef]
- Fabisiak, A.; Fabisiak, N.; Małecka-Panas, A.M.E.; Jacenik, D.; Kordek, R.; Zielińska, M.; Fichna, K.K.J. Novel selective agonist of GPR18, PSB-KK-1415 exerts potent anti-inflammatory and anti-nociceptive activities in animal models of intestinal inflammation and inflammatory pain. Neurogastroenterol. Motil. 2021, 33, e14003. [Google Scholar] [CrossRef] [PubMed]
- Neumann, A.; Engel, V.; Mahardhika, A.B.; Schoeder, C.T.; Namasivayam, V.; Kieć-Kononowicz, K.; Müller, C.E. Computational Investigations on the Binding Mode of Ligands for the Cannabinoid-Activated G Protein-Coupled Receptor GPR18. Biomolecules 2020, 10, 686. [Google Scholar] [CrossRef] [PubMed]
- Matouk, A.I.; Taye, A.; El-Moselhy, M.A.; Heeba, G.H.; Abdel-Rahman, A.A. The Effect of Chronic Activation of the Novel Endocannabinoid Receptor GPR18 on Myocardial Function and Blood Pressure in Conscious Rats. J. Cardiovasc. Pharmacol. 2017, 69, 23–33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jarai, Z.; Wagner, J.A.; Varga, K.; Lake, K.D.; Compton, D.R.; Martin, B.R.; Zimmer, A.M.; Bonner, T.I.; Buckley, N.E.; Mezey, E.; et al. Cannabinoid-induced mesenteric vasodilation through an endothelial site distinct from CB1 or CB2 receptors. Proc. Natl. Acad. Sci. USA 1999, 96, 14136–14141. [Google Scholar] [CrossRef] [Green Version]
- Offertaler, L.; Mo, F.M.; Batkai, S.; Liu, J.; Begg, M.; Razdan, R.K.; Martin, B.R.; Bukoski, R.D.; Kunos, G. Selective ligands and cellular effectors of a G protein-coupled endothelial cannabinoid receptor. Mol. Pharmacol. 2003, 63, 699–705. [Google Scholar] [CrossRef] [Green Version]
- Al Suleimani, Y.M.; Al Mahruqi, A.S. The endogenous lipid N-arachidonyl glycine is hypotensive and nitric oxide-cGMP-dependent vasorelaxant. Eur. J. Pharmacol. 2017, 794, 209–215. [Google Scholar] [CrossRef]
- Penumarti, A.; Abdel-Rahman, A.A. Role of central atypical cannabinoid receptor GPR18 in modulating cardiovascular funtion. FASEB J. 2012, 26, 663.10. [Google Scholar] [CrossRef]
- Finlay, D.B.; Joseph, W.R.; Grimsey, N.L.; Glass, M. GPR18 undergoes a high degree of con-stitutive trafficking but is unresponsive to N-Arachidonoyl Glycine. Peer J. 2016, 4, e1835. [Google Scholar] [CrossRef] [Green Version]
- Tomioka, H.; Hattori, Y.; Fukao, M.; Sato, A.; Liu, M.; Sakuma, I.; Kitabatake, A.; Kanno, M. Relaxation in different-sized rat blood vessels mediated by endothelium-derived hyperpolarizing factor: Importance of processes mediating precontractions. J. Vasc. Res. 1999, 36, 311–320. [Google Scholar] [CrossRef]
- Ko, E.A.; Han, J.; Jung, I.D.; Park, W.S. Physiological roles of K+ channels in vascular smooth muscle cells. J. Smooth. Muscle Res. 2008, 44, 65–81. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parmar, N.; Ho, W.S. N-arachidonoyl glycine, an endogenous lipid that acts as a vasorelaxant via nitric oxide and large conductance calcium-activated potassium channels. Br. J. Pharmacol. 2010, 160, 594–603. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kenakin, T.; Jenkinson, S.; Watson, C.J. Determining the potency and molecular mechanism of action of insurmountable antagonists. Pharmacol. Exp. Ther. 2006, 319, 710–723. [Google Scholar] [CrossRef] [Green Version]
- Haspula, D.; Clark, M.A. Cannabinoid Receptors: An Update on Cell Signaling, Pathophysiological Roles and Therapeutic Opportunities in Neurological, Cardiovascular, and Inflammatory Diseases. Int. J. Mol. Sci. 2020, 21, 7693. [Google Scholar] [CrossRef] [PubMed]
- Pajouhesh, H.; Lenz, G.R. Medicinal chemical properties of successful central nervous system drugs. NeuroRx 2005, 2, 541–553. [Google Scholar] [CrossRef] [Green Version]
- Drabczyńska, A.; Müller, C.E.; Schiedel, A.; Schumacher, B.; Karolak-Wojciechowska, J.; Fruziński, A.; Zobnina, W.; Yuzlenko, O.; Kieć-Kononowicz, K. Phenylethyl-substituted pyrimido [2, 1-f] purinediones and related compounds: Structure–activity relationships as adenosine A1 and A2A receptor ligands. Bioorg. Med. Chem. 2007, 15, 6956–6974. [Google Scholar] [CrossRef]
- Bahreyni, A.; Avan, A.; Shabani, M.; Ryzhikov, M.; Fiuji, H.; Soleimanpour, S.; Khazaei, M.; Hassanian, S.M. Therapeutic potential of A2 adenosine receptor pharmacological regulators in the treatment of cardiovascular diseases, recent progress, and prospective. J. Cell. Physiol. 2019, 234, 1295–1299. [Google Scholar] [CrossRef]
- Wolska, N.; Rozalski, M. Blood Platelet Adenosine Receptors as Potential Targets for Anti-Platelet Therapy. Int. J. Mol. Sci. 2019, 20, 5475. [Google Scholar] [CrossRef] [Green Version]
- Atalay, S.; Jarocka-karpowicz, I.; Skrzydlewskas, E. Antioxidative and anti-inflammatory properties of cannabidiol. Antioxidants 2019, 9, 21. [Google Scholar] [CrossRef] [Green Version]
- Juknat, A.; Pietr, M.; Kozela, E.; Rimmerman, N.; Levy, R.; Gao, F.; Coppola, G.; Geschwind, D.; Vogel, Z. Microarray and Pathway Analysis Reveal Distinct Mechanisms Underlying Cannabinoid-Mediated Modulation of LPS-Induced Activation of BV-2 Microglial Cells. PLoS ONE 2013, 8, e61462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamelink, C.; Hampson, A.; Wink, D.A.; Eiden, L.E.; Eskay, R.L. Comparison of cannabidiol, antioxidants, and diuretics in reversing binge ethanol-induced neurotoxicity. J. Pharmacol. Exp. Ther. 2005, 314, 780–788. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, H.Y.; Goble, K.; Mecha, M.; Wang, C.C.; Huang, C.H.; Guaza, C.; Jan, T.R. Cannabidiol-induced apoptosis in murine microglial cells through lipid raft. Glia 2012, 60, 1182–1190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gugliandolo, A.; Pollastro, F.; Grassi, G.; Bramanti, P.; Mazzon, E. In vitro model of neuroinflammation: Efficacy of cannabigerol, a non-psychoactive cannabinoid. Int. J. Mol. Sci. 2018, 19, 1992. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valvassori, S.S.; Elias, G.; De Souza, B.; Petronilho, F.; Dal-Pizzo, F.; Kapczinski, F.; Trzesniak, C.; Tumas, V.; Dursun, S.; Nisihara Chagas, M.H.; et al. Effects of cannabidiol on amphetamine-induced oxidative stress generation in an animal model of mania. J. Psychopharmacol. 2011, 25, 274–280. [Google Scholar] [CrossRef]
- Wang, Y.; Mukhopadhyay, P.; Cao, Z.; Wang, H.; Feng, D.; Haskó, G.; Mechoulam, R.; Gao, B.; Pacher, P. Cannabidiol attenuates alcohol-induced liver steatosis, metabolic dysregulation, inflammation and neutrophil-mediated injury. Sci. Rep. 2017, 7, 12064. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, D.; Low, J.K.; Logge, W.; Garner, B.; Karl, T. Chronic cannabidiol treatment improves social and object recognition in double transgenic APPswe/PS1ΔE9 mice. Psychopharmacology 2014, 231, 3009–3017. [Google Scholar] [CrossRef]
- McPartland, J.M.; Duncan, M.; Di Marzo, V.; Pertwee, R.G. Are cannabidiol and Δ9-tetrahydrocannabivarin negative modulators of the endocannabinoid system? A systematic review. Br. J. Pharmacol. 2015, 172, 737–753. [Google Scholar] [CrossRef] [Green Version]
- Kubacka, M.; Kotańska, M.; Kazek, G.; Waszkielewicz, A.M.; Marona, H.; Filipek, B.; Mogilski, S. Involvement of the NO/sGC/cGMP/K+ channels pathway in vascular relaxation evoked by two non-quinazoline α1-adrenoceptor antagonists. Biomed. Pharmacother. 2018, 103, 157–166. [Google Scholar] [CrossRef]
- Mogilski, S.; Kubacka, M.; Redzicka, A.; Kazek, G.; Dudek, M.; Malinka, W.; Filipek, B. Antinociceptive, anti-inflammatory and smooth muscle relaxant activities of the pyrrolo [3,4-d] pyridazinone derivatives: Possible mechanisms of action. Pharmacol. Biochem. Behav. 2015, 133, 99–110. [Google Scholar] [CrossRef]
- Dziubina, A.; Szkatuła, D.; Gdula-Argasińska, J.; Kotańska, M.; Filipek, B. Antinociceptive, antiedematous, and antiallodynic activity of 1H-pyrrolo[3,4-c] pyridine-1,3(2H)-dione derivatives in experimental models of pain. Naunyn Schmiedebergs Arch. Pharmacol. 2019, 393, 813–827. [Google Scholar] [CrossRef] [PubMed]
- Kotańska, M.; Mika, K.; Szafarz, M.; Dziubina, A.; Bednarski, M.; Müller, C.E.; Sapa, J.; Kieć-Kononowicz, K. PSB 603—A known selective adenosine A2B receptor antagonist–has anti-inflammatory activity in mice. Biomed. Pharmacother. 2021, 135, 111164. [Google Scholar] [CrossRef]
- Kuder, K.J.; Kotańska, M.; Szczepańska, K.; Mika, K.; Reiner-Link, D.; Stark, H.; Kieć-Kononowicz, K. Discovery of Potential, Dual-Active Histamine H3 Receptor Ligands with Combined Antioxidant Properties. Molecules 2021, 26, 2300. [Google Scholar] [CrossRef] [PubMed]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. Ferric reducing/antioxidant power assay: Direct measure of total antioxidant activity of biological fluids and modified version for simultaneous measurement of total antioxidant power and ascorbic acid concentration. Methods Enzymol. 1999, 299, 15–27. [Google Scholar] [CrossRef] [PubMed]
- Kotańska, M.; Kulig, K.; Marcinkowska, M.; Bednarski, M.; Malawska, K.; Zaręba, P. Metabolic benefits of 1-(3-(4-(o-tolyl) piperazin-1-yl) propyl) pyrrolidin-2-one: A non-selective α-adrenoceptor antagonist. J. Endocrinol. Investig. 2018, 41, 609–619. [Google Scholar] [CrossRef] [Green Version]
- De Clerck, F.; Van de Water, A.; D’Aubioul, J.; Lu, H.R.; Van Rossem, K.; Hermans, A.; Van Ammel, K. In vivo measurement of QT prolongation, dispersion and arrhythmogenesis: Application to the preclinical cardiovascular safety pharmacology of a new chemical entity. Fundam. Clin. Pharmacol. 2002, 16, 125–140. [Google Scholar] [CrossRef]
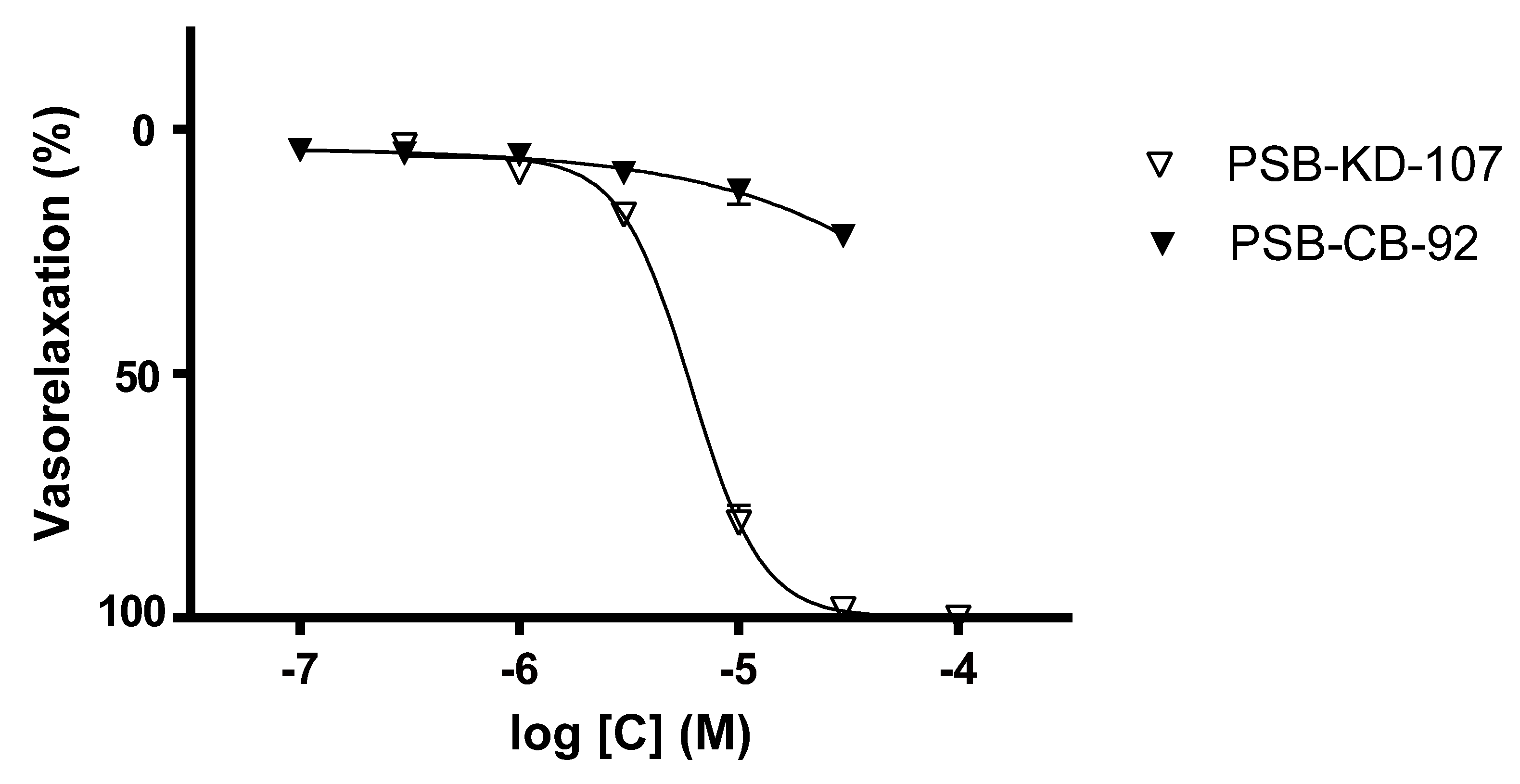
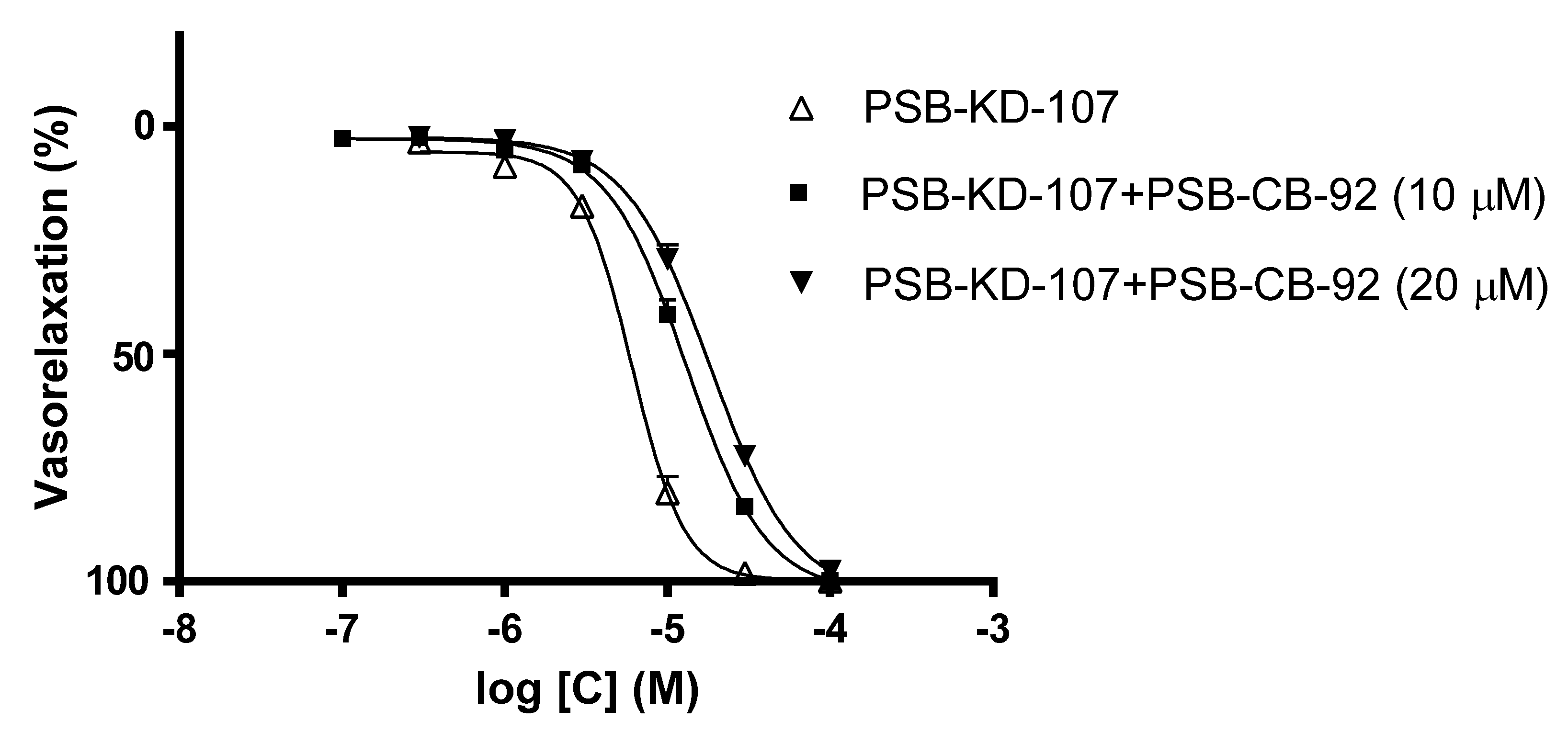
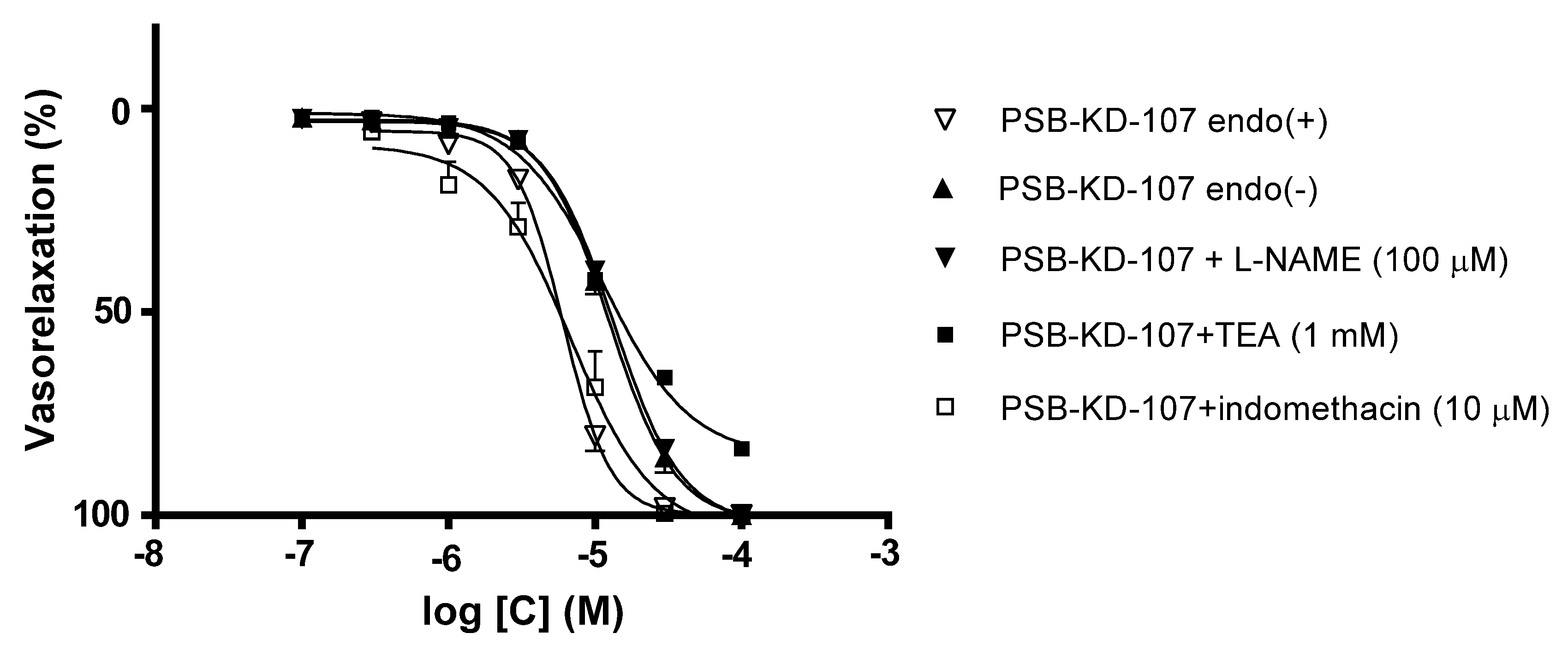


| Compound | Pretreatment | pIC50 | Emax (%) |
|---|---|---|---|
| PSB-KD-107 | none | 5.217 ± 0.020 | 100.0 ± 0.0 |
| PSB-CB-92 (10 µM) | 4.884 ± 0.020 **** | 100.0 ± 0.0 | |
| PSB-CB-92 (20 µM) | 4.735 ± 0.023 **** | 97.8 ± 0.8 | |
| denudation | 4.904 ± 0.024 **** | 100.0 ± 0.0 | |
| L-NAME (100 µM) | 4.873 ± 0.015 **** | 100.0 ± 0.0 | |
| Indomethacin (10 µM) | 5.165 ± 0.075 | 100.0 ± 0.0 | |
| TEA (1 mM) | 4.940 ± 0.030 **** | 83.5 ± 1.5 | |
| PSB-CB-92 | none | none | 21.8 ± 1.60 |
| Parameter | Time (min) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 | 5 | 10 | 20 | 30 | 40 | 50 | 60 | ||
| PSB-KD-107 10 mg/kg | Heart rate (beats/min) | 334.0 ± 73.2 | 330.0 ± 77.2 | 331.3 ± 75.8 | 328.7 ± 78.6 | 311.3 ± 67.6 | 304.3 ± 60.2 * | 316.0 ± 46.4 | 339.0 ± 27.4 |
| PQ (ms) | 52.2 ± 1.7 | 51.1 ± 1.7 | 51.3 ± 1.0 | 50.9 ± 1.3 | 52.2 ± 1.7 | 52.6 ± 0.7 | 52.7 ± 0.8 | 52.9 ± 2.0 | |
| QRS (ms) | 20.0 ± 0.0 | 20.0 ± 0.0 | 20.0 ± 0.0 | 20.0 ± 0.0 | 20.0 ± 0.0 | 20.0 ± 0.0 | 20.0 ± 0.0 | 20.0 ± 0.0 | |
| QT (ms) | 63.3 ± 5.1 | 65.5 ± 3.4 | 65.5 ± 6.1 | 65.3 ± 3.7 | 64.4 ± 4.5 | 66.7 ± 2.9 ** | 65.5 ± 3.4 | 64.4 ± 1.7 | |
| QTc (ms) | 132.8 ± 21.7 | 132.4 ± 24.4 | 129.7 ± 25.1 | 127.5 ± 25.6 | 131.1 ± 25.1 | 132.2± 24.5 | 132.9 ± 26.5 | 134.6 ± 19.6 | |
| PSB-CB-92 10 mg/kg | Heart rate (beats/min) | 393.0 ± 27.4 | 376.7 ± 40.8 *** | 385.7 ± 34.3 | 385.7 ± 34.3 | 384.7 ± 29.0 | 393.0 ± 27.4 | 393.0 ± 27.4 | 393.0 ±27.4 |
| PQ (ms) | 48.9 ± 6.1 | 48.9 ± 4.5 | 49.8 ± 7.9 | 51.1 ± 7.4 | 48.9 ± 6.1 | 48.9 ± 6.1 | 51.1 ± 4.5 | 50.0 ± 8.8 | |
| QRS (ms) | 22.7 ± 5.4 | 23.6 ± 5.1 | 22.7 ± 5.4 | 22.7 ± 5.4 | 22.7 ± 5.4 | 22.7 ± 5.4 | 22.7 ± 5.4 | 22.7 ± 5.4 | |
| QT (ms) | 62.2 ± 3.4 | 64.4 ± 6.7 | 63.3 ± 5.1 | 63.3 ± 2.9 | 62.2 ± 3.4 | 62.2 ±3.4 | 63.3 ± 5.1 | 63.3 ± 2.9 | |
| QTc (ms) | 159.1 ± 6.9 | 162.4 ± 13.4 | 159.2 ± 8.7 | 159.4 ± 1.0 | 157.7 ± 5.6 | 157.8 ± 6.4 | 160.0 ± 8.8 | 160.0 ± 8.0 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kotańska, M.; Kubacka, M.; Bednarski, M.; Nicosia, N.; Szafarz, M.; Jawień, W.; Müller, C.E.; Kieć-Kononowicz, K. The GPR18 Agonist PSB-KD-107 Exerts Endothelium-Dependent Vasorelaxant Effects. Pharmaceuticals 2021, 14, 799. https://doi.org/10.3390/ph14080799
Kotańska M, Kubacka M, Bednarski M, Nicosia N, Szafarz M, Jawień W, Müller CE, Kieć-Kononowicz K. The GPR18 Agonist PSB-KD-107 Exerts Endothelium-Dependent Vasorelaxant Effects. Pharmaceuticals. 2021; 14(8):799. https://doi.org/10.3390/ph14080799
Chicago/Turabian StyleKotańska, Magdalena, Monika Kubacka, Marek Bednarski, Noemi Nicosia, Małgorzata Szafarz, Wojciech Jawień, Christa E. Müller, and Katarzyna Kieć-Kononowicz. 2021. "The GPR18 Agonist PSB-KD-107 Exerts Endothelium-Dependent Vasorelaxant Effects" Pharmaceuticals 14, no. 8: 799. https://doi.org/10.3390/ph14080799
APA StyleKotańska, M., Kubacka, M., Bednarski, M., Nicosia, N., Szafarz, M., Jawień, W., Müller, C. E., & Kieć-Kononowicz, K. (2021). The GPR18 Agonist PSB-KD-107 Exerts Endothelium-Dependent Vasorelaxant Effects. Pharmaceuticals, 14(8), 799. https://doi.org/10.3390/ph14080799






