Comparative Effectiveness of Injection Therapies for Hemiplegic Shoulder Pain in Stroke: A Systematic Review and Network Meta-Analysis
Abstract
:1. Introduction
2. Methods
2.1. Search Strategy
2.2. Inclusion and Exclusion Criteria
2.3. Data Extraction, Quality Assessment, and Evaluation of Inconsistency
2.4. Outcomes
2.5. Statistical Analysis
3. Results
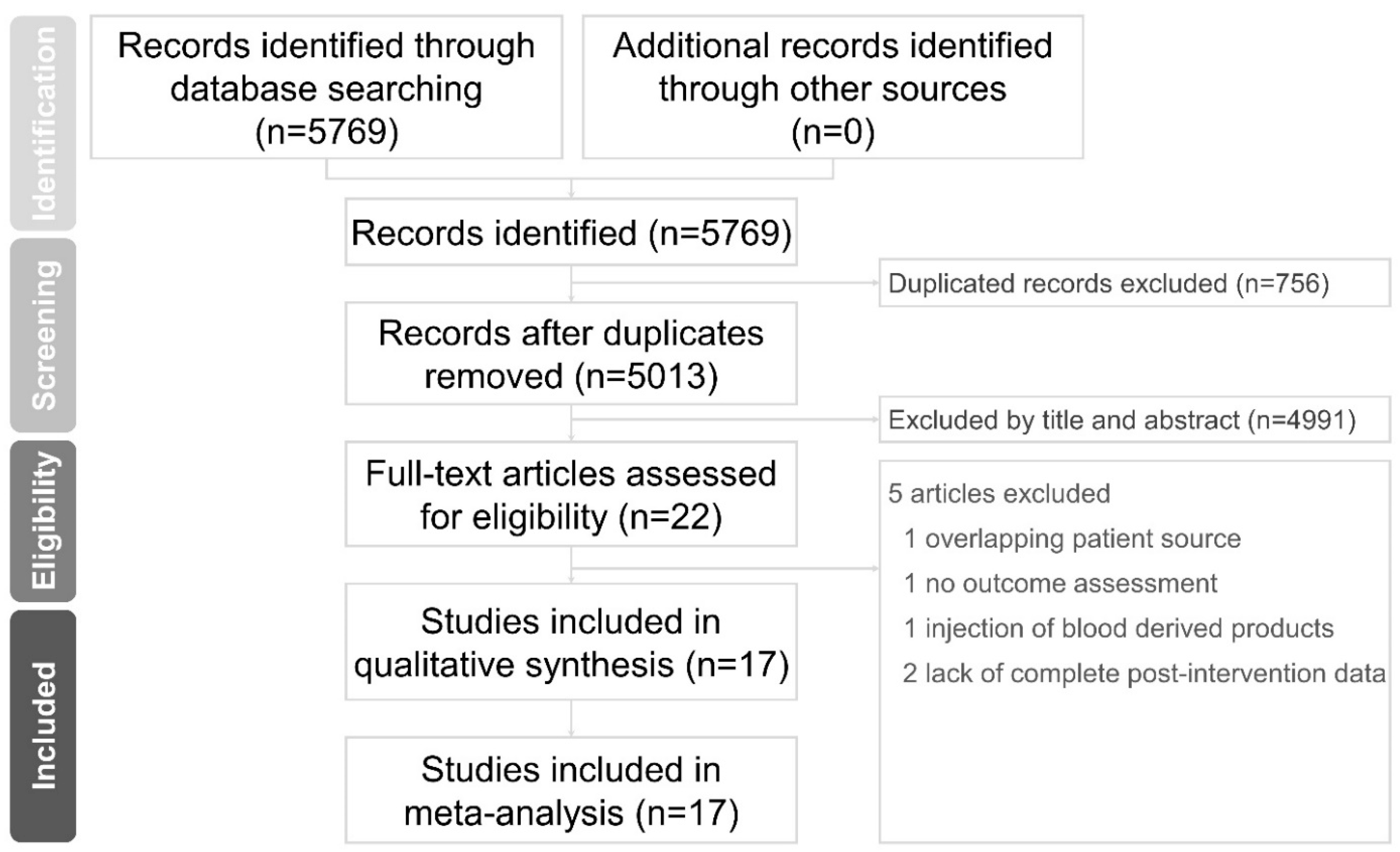
3.1. Study Selection and Characteristics
3.2. Assessment of the Study Quality
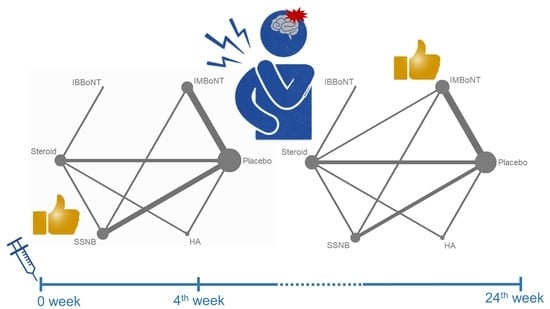
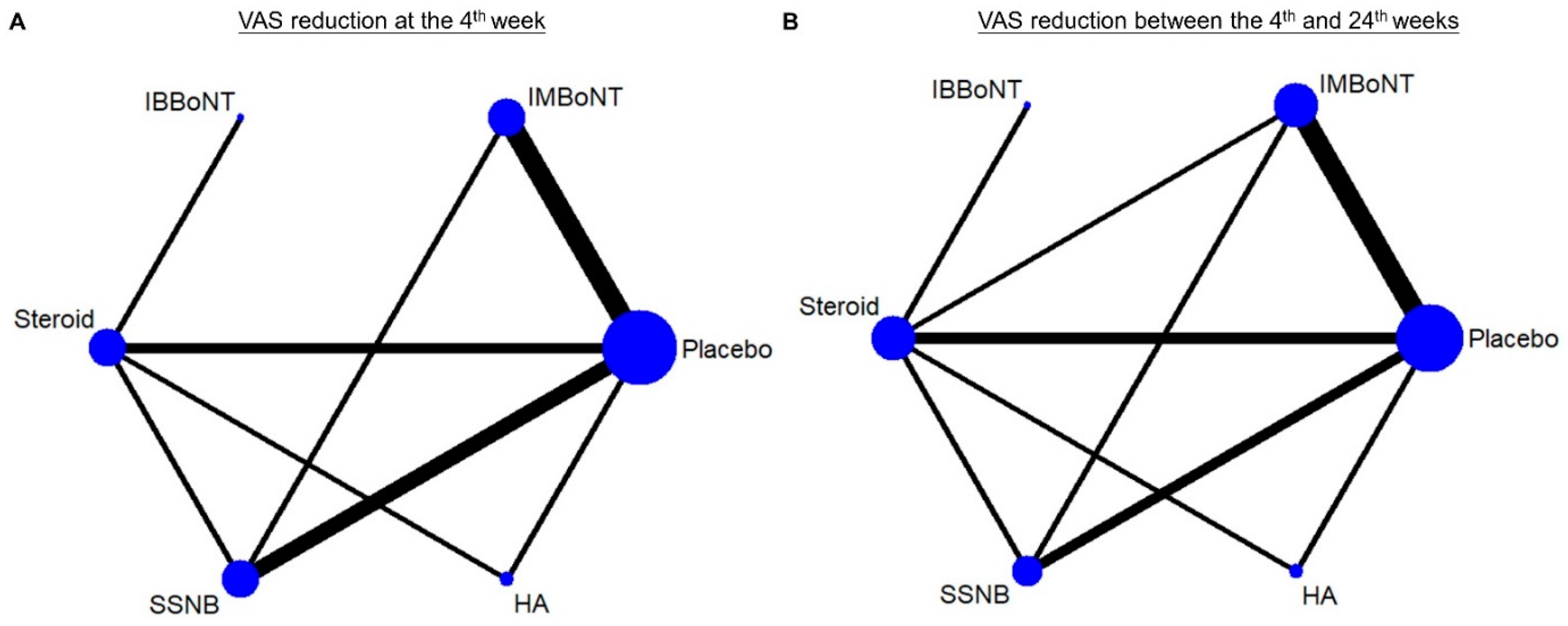
3.3. Assessment of the Inconsistency between the Direct and Indirect Evidence
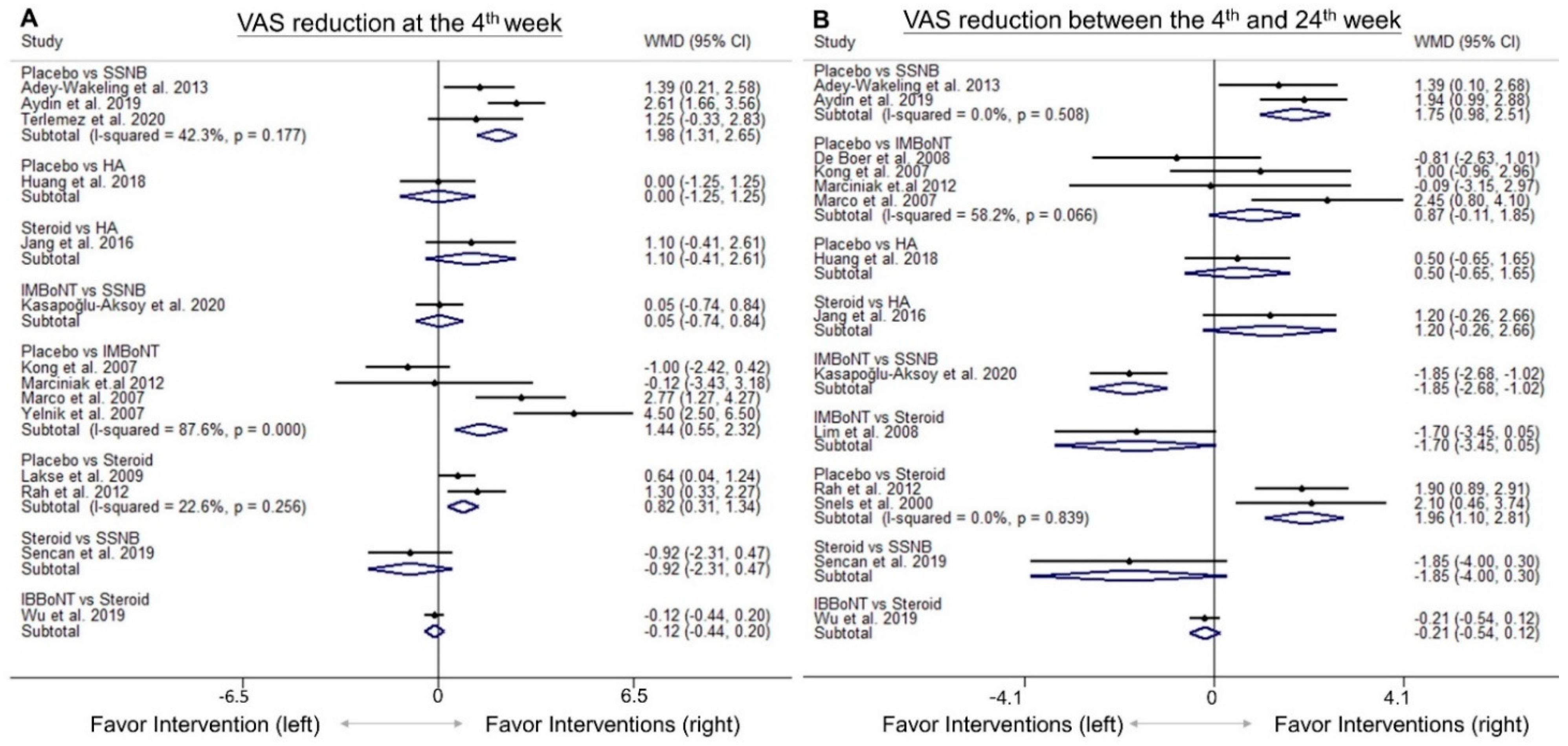
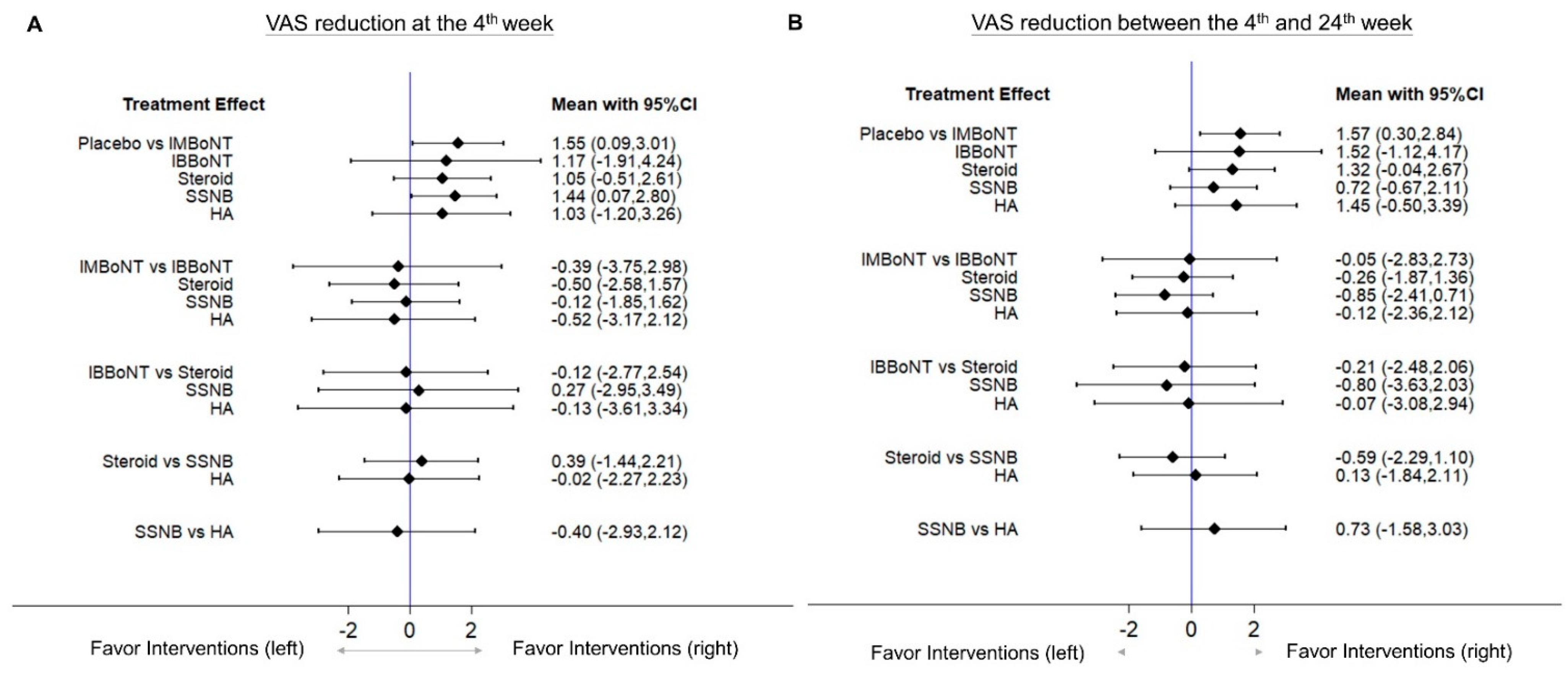
3.4. Comparison of WMD for VAS Reduction (Fourth-Week)
3.5. Comparison of WMD for VAS Reduction (4th to 24th Weeks)
3.6. Sensitivity Analysis
3.7. Publication Bias
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations and Acronyms
| BoNT | Botulinum toxin |
| HA | Hyaluronic acid |
| HSP | Hemiplegic shoulder pain |
| RCT | Randomized controlled trial |
| SSNB | Suprascapular nerve block |
| VAS | Visual analog scale |
| WMD | Weight mean difference |
| 95% CI | 95% confidence interval |
References
- McLean, D.E. Medical complications experienced by a cohort of stroke survivors during inpatient, tertiary-level stroke rehabilitation. Arch. Phys. Med. Rehabil. 2004, 85, 466–469. [Google Scholar] [CrossRef]
- Roy, C.W.; Sands, M.R.; Hill, L.D. Shoulder pain in acutely admitted hemiplegics. Clin. Rehabil. 1994, 8, 334–340. [Google Scholar] [CrossRef]
- Adey-Wakeling, Z.; Arima, H.; Crotty, M.; Leyden, J.; Kleinig, T.; Anderson, C.S.; Newbury, J.; Collaborative, S.S. Incidence and associations of hemiplegic shoulder pain poststroke: Prospective population-based study. Arch. Phys. Med. Rehabil. 2015, 96, 241–247.e1. [Google Scholar] [CrossRef] [Green Version]
- Lindgren, I.; Jonsson, A.-C.; Norrving, B.; Lindgren, A. Shoulder pain after stroke: A prospective population-based study. Stroke 2007, 38, 343–348. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.H.; Jung, S.J.; Yang, E.J.; Paik, N.J. Clinical and sonographic risk factors for hemiplegic shoulder pain: A longitudinal observational study. J. Rehabil. Med. 2014, 46, 81–87. [Google Scholar] [CrossRef] [Green Version]
- Kashi, Y.; Ratmansky, M.; Defrin, R. Deficient Pain Modulation in Patients with Chronic Hemiplegic Shoulder Pain. Pain Pract. 2018, 18, 716–728. [Google Scholar] [CrossRef]
- Klit, H.; Finnerup, N.B.; Jensen, T.S. Central post-stroke pain: Clinical characteristics, pathophysiology, and management. Lancet Neurol. 2009, 8, 857–868. [Google Scholar] [CrossRef]
- De Baets, L.; Jaspers, E.; Janssens, L.; Van Deun, S. Characteristics of neuromuscular control of the scapula after stroke: A first exploration. Front. Hum. Neurosci. 2014, 8, 933. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murie-Fernández, M.; Iragui, M.C.; Gnanakumar, V.; Meyer, M.; Foley, N.; Teasell, R. Painful hemiplegic shoulder in stroke patients: Causes and management. Neurología 2012, 27, 234–244. [Google Scholar] [CrossRef]
- Benlidayi, I.C.; Basaran, S. Hemiplegic shoulder pain: A common clinical consequence of stroke. Pract. Neurol. 2014, 14, 88–91. [Google Scholar] [CrossRef] [PubMed]
- Aydın, T.; Şen, E.İ.; Yardımcı, M.Y.; Kesiktaş, F.N.; Öneş, K.; Paker, N. Efficacy of ultrasound-guided suprascapular nerve block treatment in patients with painful hemiplegic shoulder. Neurol. Sci. 2019, 40, 985–991. [Google Scholar] [CrossRef]
- Marciniak, C.M.; Harvey, R.L.; Gagnon, C.M.; Duraski, S.A.; Denby, F.A.; McCarty, S.; Bravi, L.A.; Polo, K.M.; Fierstein, K.M. Does botulinum toxin type A decrease pain and lessen disability in hemiplegic survivors of stroke with shoulder pain and spasticity?: A randomized, double-blind, placebo-controlled trial. Am. J. Phys. Med. Rehabil. 2012, 91, 1007–1019. [Google Scholar] [CrossRef]
- Hsu, P.-C.; Wu, W.-T.; Han, D.-S.; Chang, K.-V. Comparative Effectiveness of Botulinum Toxin Injection for Chronic Shoulder Pain: A Meta-Analysis of Randomized Controlled Trials. Toxins 2020, 12, 251. [Google Scholar] [CrossRef]
- Huang, Y.-C.; Leong, C.-P.; Tso, H.-H.; Chen, M.-J.; Liaw, M.-Y.; Hsieh, H.-C.; Wang, L.-Y.; Hsu, C.-H. The long-term effects of hyaluronic acid on hemiplegic shoulder pain and injury in stroke patients: A randomized controlled study. Medicine 2018, 97, e12078. [Google Scholar] [CrossRef] [PubMed]
- Hutton, B.; Salanti, G.; Caldwell, D.M.; Chaimani, A.; Schmid, C.H.; Cameron, C.; Ioannidis, J.P.; Straus, S.; Thorlund, K.; Jansen, J.P.; et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: Checklist and explanations. Ann. Intern. Med. 2015, 162, 777–784. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Higgins, J.P.; Altman, D.G.; Gøtzsche, P.C.; Jüni, P.; Moher, D.; Oxman, A.D.; Savović, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A.; et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Veroniki, A.A.; Vasiliadis, H.S.; Higgins, J.P.T.; Salanti, G. Evaluation of inconsistency in networks of interventions. Int. J. Epidemiol. 2013, 42, 332–345. [Google Scholar] [CrossRef] [PubMed]
- Chaimani, A.; Higgins, J.P.; Mavridis, D.; Spyridonos, P.; Salanti, G. Graphical tools for network meta-analysis in STATA. PLoS ONE 2013, 8, e76654. [Google Scholar] [CrossRef]
- Olsen, M.F.; Bjerre, E.; Hansen, M.D.; Hilden, J.; Landler, N.E.; Tendal, B.; Hróbjartsson, A. Pain relief that matters to patients: Systematic review of empirical studies assessing the minimum clinically important difference in acute pain. BMC Med. 2017, 15, 35. [Google Scholar] [CrossRef] [Green Version]
- McGrath, S.; Zhao, X.; Steele, R.; Thombs, B.D.; Benedetti, A.; Levis, B.; Riehm, K.E.; Saadat, N.; Levis, A.W.; Azar, M.; et al. Estimating the sample mean and standard deviation from commonly reported quantiles in meta-analysis. Stat. Methods Med. Res. 2020, 29, 2520–2537. [Google Scholar] [CrossRef] [Green Version]
- Geisser, S. A predictive approach to the random effect model. Biometrika 1974, 61, 101–107. [Google Scholar] [CrossRef]
- Melsen, W.; Bootsma, M.; Rovers, M.; Bonten, M. The effects of clinical and statistical heterogeneity on the predictive values of results from meta-analyses. Clin. Microbiol. Infect. 2014, 20, 123–129. [Google Scholar] [CrossRef] [Green Version]
- Wolfinger, R.; O’Connell, M. Generalized linear mixed models a pseudo-likelihood approach. J. Stat. Comput. Simul. 1993, 48, 233–243. [Google Scholar] [CrossRef]
- Marotta, N.; Demeco, A.; Marinaro, C.; Moggio, L.; Pino, I.; Barletta, M.; Petraroli, A.; Ammendolia, A. Comparative Effectiveness of Orthoses for Thumb Osteoarthritis: A Systematic Review and Network Meta-analysis. Arch. Phys. Med. Rehabil. 2021, 102, 502–509. [Google Scholar] [CrossRef] [PubMed]
- Robertson, S.E. The probability ranking principle in IR. J. Doc. 1977, 33, 294–304. [Google Scholar] [CrossRef]
- Marotta, N.; Demeco, A.; Moggio, L.; Marinaro, C.; Pino, I.; Barletta, M.; Petraroli, A.; Pepe, D.; Lavano, F.; Ammendolia, A. Comparative effectiveness of breathing exercises in patients with chronic obstructive pulmonary disease. Complement. Ther. Clin. Pract. 2020, 41, 101260. [Google Scholar] [CrossRef]
- Fleiss, J. Review papers: The statistical basis of meta-analysis. Stat. Methods Med. Res. 1993, 2, 121–145. [Google Scholar] [CrossRef]
- Huang, Y.C.; Leong, C.P.; Wang, L.; Chen, M.J.; Chuang, C.Y.; Liaw, M.Y.; Wang, L.Y. The effects of hyaluronic acid on hemiplegic shoulder injury and pain in patients with subacute stroke: A randomized controlled pilot study. Medicine 2016, 95, e5547. [Google Scholar] [CrossRef]
- Allen, Z.A.; Shanahan, E.M.; Crotty, M. Does suprascapular nerve block reduce shoulder pain following stroke: A double-blind randomised controlled trial with masked outcome assessment. BMC Neurol. 2010, 10, 83. [Google Scholar] [CrossRef] [Green Version]
- Uzdu, A.; Kirazlı, Y.; Karapolat, H.; Unlu, B.; Tanıgör, G.; Çalış, F.A. Efficacy of platelet-rich plasma in the treatment of hemiplegic shoulder pain. Neurol. Sci. 2021, 42, 1977–1986. [Google Scholar] [CrossRef]
- Yasar, E.; Vural, D.; Safaz, I.; Balaban, B.; Yilmaz, B.; Goktepe, A.S.; Alaca, R. Which treatment approach is better for hemiplegic shoulder pain in stroke patients: Intra-articular steroid or suprascapular nerve block? A randomized controlled trial. Clin. Rehabil. 2011, 25, 60–68. [Google Scholar] [CrossRef]
- Jeon, W.H.; Park, G.W.; Jeong, H.J.; Sim, Y.J. The Comparison of Effects of Suprascapular Nerve Block, Intra-articular Steroid Injection, and a Combination Therapy on Hemiplegic Shoulder Pain: Pilot Study. Ann. Rehabil. Med. 2014, 38, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Jang, M.H.; Lee, C.-H.; Shin, Y.-I.; Kim, S.-Y.; Huh, S.C. Effect of intra-articular hyaluronic acid injection on hemiplegic shoulder pain after stroke. Ann. Rehabil. Med. 2016, 40, 835–844. [Google Scholar] [CrossRef] [Green Version]
- Wu, T.; Song, H.-X.; Li, Y.Z.; Ye, Y.; Li, J.-H.; Hu, X.Y. Clinical effectiveness of ultrasound guided subacromial-subdeltoid bursa injection of botulinum toxin type A in hemiplegic shoulder pain: A retrospective cohort study. Medicine 2019, 98, e17933. [Google Scholar] [CrossRef]
- De Boer, K.; Arwert, H.; De Groot, J.; Meskers, C.; Mishre, A.R.; Arendzen, J. Shoulder pain and external rotation in spastic hemiplegia do not improve by injection of botulinum toxin A into the subscapular muscle. J. Neurol. Neurosurg. Psychiatry 2008, 79, 581–583. [Google Scholar] [CrossRef]
- Kong, K.-H.; Neo, J.-J.; Chua, K.S. A randomized controlled study of botulinum toxin A in the treatment of hemiplegic shoulder pain associated with spasticity. Clin. Rehabil. 2007, 21, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Marco, E.; Duarte, E.; Vila, J.; Tejero, M.; Guillen, A.; Boza, R.; Escalada, F.; Espadaler, J.M. Is botulinum toxin type a effective in the treatment of spastic shoulder pain in patients after stroke? A double-blind randomized clinical trial. J. Rehabil. Med. 2007, 39, 440–447. [Google Scholar] [CrossRef] [Green Version]
- Yelnik, A.P.; Colle, F.M.; Bonan, I.V.; Vicaut, E. Treatment of shoulder pain in spastic hemiplegia by reducing spasticity of the subscapular muscle: A randomised, double blind, placebo controlled study of botulinum toxin A. J. Neurol. Neurosurg. Psychiatry 2007, 78, 845–848. [Google Scholar] [CrossRef]
- Kasapoğlu-Aksoy, M.; Aykurt-Karlıbel, İ.; Altan, L. Comparison of the efficacy of intramuscular botulinum toxin type-A injection into the pectoralis major and the teres major muscles and suprascapular nerve block for hemiplegic shoulder pain: A prospective, double-blind, randomized, controlled trial. Neurol. Sci. 2020, 41, 2225–2230. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.-Y.; Koh, J.-H.; Paik, N.-J. Intramuscular botulinum toxin-A reduces hemiplegic shoulder pain: A randomized, double-blind, comparative study versus intraarticular triamcinolone acetonide. Stroke 2008, 39, 126–131. [Google Scholar] [CrossRef] [Green Version]
- Adey-Wakeling, Z.; Crotty, M.; Shanahan, E.M. Suprascapular nerve block for shoulder pain in the first year after stroke: A randomized controlled trial. Stroke 2013, 44, 3136–3141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Terlemez, R.; Ciftci, S.; Topaloglu, M.; Dogu, B.; Yilmaz, F.; Kuran, B. Suprascapular nerve block in hemiplegic shoulder pain: Comparison of the effectiveness of placebo, local anesthetic, and corticosteroid injections-a randomized controlled study. Neurol. Sci. 2020, 41, 3243–3247. [Google Scholar] [CrossRef] [PubMed]
- Lakse, E.; Gunduz, B.; Erhan, B.; Celik, E.C. The effect of local injections in hemiplegic shoulder pain: A prospective, randomized, controlled study. Am. J. Phys. Med. Rehabil. 2009, 88, 805–811. [Google Scholar] [CrossRef] [PubMed]
- Rah, U.W.; Yoon, S.-H.; Moon, D.J.; Kwack, K.-S.; Hong, J.Y.; Lim, Y.C.; Joen, B. Subacromial corticosteroid injection on poststroke hemiplegic shoulder pain: A randomized, triple-blind, placebo-controlled trial. Arch. Phys. Med. Rehabil. 2012, 93, 949–956. [Google Scholar] [CrossRef]
- Snels, I.A.; Beckerman, H.; Twisk, J.W.; Dekker, J.H.; De Koning, P.; Koppe, P.A.; Lankhorst, G.J.; Bouter, L.M. Effect of triamcinolone acetonide injections on hemiplegic shoulder pain: A randomized clinical trial. Stroke 2000, 31, 2396–2401. [Google Scholar] [CrossRef]
- Sencan, S.; Celenlioglu, A.E.; Karadag-Saygı, E.; Midi, İ.; Gunduz, O.H. Effects of fluoroscopy-guıded intraartıcular injectıon, suprascapular nerve block, and combınatıon therapy ın hemıplegıc shoulder paın: A prospective double-blınd, randomızed clınıcal study. Neurol. Sci. 2019, 40, 939–946. [Google Scholar] [CrossRef]
- Achar, S.; Kundu, S. Principles of office anesthesia: Part I. Infiltrative anesthesia. Am. Fam. Phys. 2002, 66, 91. [Google Scholar]
- Barnes, P.J.; Adcock, I.; Spedding, M.; Vanhoutte, P.M. Anti-inflammatory actions of steroids: Molecular mechanisms. Trends Pharmacol. Sci. 1993, 14, 436–441. [Google Scholar] [CrossRef]
- Jankovic, J. Botulinum toxin in clinical practice. J. Neurol. Neurosurg. Psychiatry 2004, 75, 951–957. [Google Scholar] [CrossRef]
- Wissel, J.; Ward, A.B.; Erztgaard, P.; Bensmail, D.; Hecht, M.J.; Lejeune, T.M.; Schnider, P. European consensus table on the use of botulinum toxin type A in adult spasticity. J. Rehabil. Med. 2009, 41, 13–25. [Google Scholar] [CrossRef] [Green Version]
- Flynn, T.C. Botulinum toxin. Am. J. Clin. Dermatol. 2010, 11, 183–199. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.-P.; Zhang, Y.-P.; Song, Y.-F.; Zhu, C.-M.; Wang, Y.-C.; Xie, G.-L. Botulinum toxin type A inhibits rat pyloric myoelectrical activity and substance P release in vivo. Can. J. Physiol. Pharmacol. 2007, 85, 209–214. [Google Scholar] [CrossRef]
- Matak, I.; Tékus, V.; Bölcskei, K.; Lacković, Z.; Helyes, Z. Involvement of substance P in the antinociceptive effect of botulinum toxin type A: Evidence from knockout mice. Neuroscience 2017, 358, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Stephens, M.B.; Beutler, A.; O’Connor, F.G. Musculoskeletal injections: A review of the evidence. Am. Fam. Phys. 2008, 78, 971–976. [Google Scholar]
- Lee, L.-C.; Lieu, F.-K.; Lee, H.-L.; Tung, T.-H. Effectiveness of hyaluronic acid administration in treating adhesive capsulitis of the shoulder: A systematic review of randomized controlled trials. BioMed Res. Int. 2015, 2015, 314120. [Google Scholar] [CrossRef]
- Shanthanna, H.; Busse, J.; Wang, L.; Kaushal, A.; Harsha, P.; Suzumura, E.A.; Bhardwaj, V.; Zhou, E.; Couban, R.; Paul, J.; et al. Addition of corticosteroids to local anaesthetics for chronic non-cancer pain injections: A systematic review and meta-analysis of randomised controlled trials. Br. J. Anaesth. 2020, 125, 779–801. [Google Scholar] [CrossRef] [PubMed]
- Sterne, J.; Hernán, M.; McAleenan, A.; Reeves, B.; Higgins, J. Assessing risk of bias in a non-randomized study. In Cochrane Handbook for Systematic Reviews of Interventions Version 6.0; Higgins, J., Thomas, J., Eds.; John Wiley & Sons: Chichester, UK, 2019. [Google Scholar]
| Author, Year | Trial Design | Blinding | Allocation Concealment | Inclusion Criteria | Patient Characteristics | Intervention Arm | Case Number (Male/Female) | Age (Year) | Post-Stroke Follow-Up (Months) * |
|---|---|---|---|---|---|---|---|---|---|
| Kasapoğlu-Aksoy et al., 2020 | RCT | Double-blinded (lack of details) | Not mentioned | Pain ≥ 3 weeks with VAS ≥ 4; MAS 3–4 with abduction and external rotation limitation | Stroke onset for more than 6 months | IM BoNT | 30 (19/11) | 58.47 ± 14.68 1 | 11 (6 to 34) 4 |
| SSNB | 27 (16/11) | 59.89 ± 10.57 1 | 10 (6 to 28) 4 | ||||||
| Terlemez et al., 2020 | RCT | Double-blinded (patients and assessors) | Yes | VAS of pain > 3 | Stroke onset within 24 months | SSNB (local anesthetics + corticosteroid) | 10 (7/3) | 60.0 (58.0 to 75.0) 3 | 13.0 (11.0 to 15.0) 3 |
| SSNB (local anesthetics) | 10 (4/6) | 64.0 (52.0 to 65.0) 3 | 14.5 (12.0 to 24.0) 3 | ||||||
| Placebo | 10 (4/6) | 57.5 (56.0 to 66.0) 3 | 15.0 (12.0 to 18.0) 3 | ||||||
| Aydin et al., 2019 | Quasi-experimental trial | No blinding | No | Pain ≥ 3 months | Stroke with resultant hemiplegia | SSNB | 21 (8/13) | 65.1 ± 8.8 1 | 4.4 ± 1.7 1 |
| Control | 20 (10/10) | 62.7 ± 10.5 1 | 5.2 ± 2.0 1 | ||||||
| Sencan et al., 2019 | RCT | Double-blinded (patients and assessors) | Not mentioned | VAS of pain ≥ 4 | Stroke onset within 12 months | IA corticosteroid | 10 (6/4) | 61.4 ± 6.3 1 | 5.8 ± 2.0 1 |
| SSNB | 10 (5/5) | 64.5 ± 8.6 1 | 5.3 ± 1.4 1 | ||||||
| IA corticosteroid + SSNB | 10 (6/4) | 62.9 ± 9.8 1 | 5.4 ± 2.1 1 | ||||||
| Wu et al., 2019 | Retrospective cohort study | No blinding | Not mentioned | Pain ≥ 2 months, VAS of pain > 3 at rest or >5 at shoulder abduction; MAS < 2; sonographic diagnosed rotator cuff disorder or bursitis | Stroke with resultant hemiplegia | IB BoNT | 18 (10/8) | 61.4 ± 13.0 1 | 6.3 ± 4.7 1 |
| IB corticosteroid | 20 (11/9) | 66.2 ± 9.8 1 | 4.9 ± 5.6 1 | ||||||
| Huang et al., 2018 | RCT | Double-blinded (patients and assessors) | Yes | VAS of pain ≥ 3 | Stroke onset within 6 months | Hyaluronic acid | 18 (11/7) | 59.7 (10.6) 2 | 3.0 (1.3) 2 |
| Placebo | 9 (6/3) | 62.0 (9.3) 2 | 2.9 (2.4) 2 | ||||||
| Jang et al., 2016 | RCT | Single-blinded (patients) | Yes | Pain WBS score ≥ 2; passive ROM limitation of a capsular pattern | Stroke onset within 3 months | Hyaluronic acid | 21 (13/8) | 56.6 ± 11.3 1 | 1.9 ± 1.0 1 |
| IA corticosteroid | 18 (14/4) | 60.8 ± 13.7 1 | 1.8 ± 1.1 1 | ||||||
| Adey-Wakeling et al., 2013 | RCT | Double-blinded (patients and assessors) | Yes | VAS of pain ≥ 3 | Stroke onset within 12 months | SSNB | 32 (21/11) | 0 to 65 y/o: n = 15 66 to 79 y/o: n = 9 ≥80 y/o: n = 8 | 3.3 ± 2.3 1 |
| Placebo | 32 (15/17) | 0 to 65 y/o: n = 16 66 to 79 y/o: n = 13 ≥80 y/o: n = 3 | 2.8 ± 2 1 | ||||||
| Marciniak et al., 2012 | RCT | Double-blinded (patients and assessors) | Yes | VAS of pain ≥ 4; MAS ≥ 3 in shoulder adductor or internal rotator | Stroke with resultant hemiplegia or hemiparesis | IM BoNT | 10 (6/4) | 60.2 ± 7.8 1 | 28.8 ± 38.5 1 |
| Placebo | 11 (7/4) | 59.8 ± 10.3 1 | 46.5 ± 84.5 1 | ||||||
| Rah et al., 2012 | RCT | Triple-blinded (patients, physicians and assessors) | Yes | Pain ≥ 1 month and VAS ≥ 3; clinically diagnosed rotator cuff disorder; deltoid muscle power ≥ 2; MMSE ≥ 20 | Stroke with resultant hemiplegia | IB corticosteroid | 29 (21/8) | 56.6 ± 12.5 1 | 23.6 ± 16.9 1 |
| Placebo | 29 (18/11) | 54.9 ± 10.6 1 | 18.8 ± 10.7 1 | ||||||
| Lakse et al., 2009 | RCT | Not blinded | Not mentioned | Pain caused by frozen shoulder or subacromial impingement syndrome | Stroke more than 8 weeks | IA or IB corticosteroid | 21 (10/11) | 62.2 ± 9.1 1 | 10 (3 to 22) 4 |
| Placebo | 17 (8/9) | 66.3 ± 6.7 1 | 7 (2 to 64) 4 | ||||||
| De Boer et al., 2008 | RCT | Double-blinded (lack of details) | Not mentioned | Pain ≥ 1 week and VAS ≥ 4; MAS ≥ 1; passive external rotation limitation of the humerus ≥ 50% compared with the unaffected side | Stroke with spastic hemiplegia | IM BoNT | 10 (6/4) | 58.5 ± 10.3 1 | 9.3 (17.1) 2 |
| Placebo | 11 (6/5) | 56.3 ± 7.6 1 | 4.9 (5.3) 2 | ||||||
| Lim et al., 2008 | RCT | Double-blinded (patients and assessors) | Yes | Pain ≤ 12 weeks and VAS of pain ≥ 6; passive external rotation limitation ≥ 20° | Stroke within 24 months | IM BoNT | 16 (8/8) | 64.8 ± 2.1 1 | 7.7 ± 1.8 1 |
| IA corticosteroid | 13 (7/6) | 57.1 ± 3.6 1 | 10.0 ± 2.5 1 | ||||||
| Kong et al., 2007 | RCT | Double-blinded (patients and assessors) | Yes | Pain ≥ 2 weeks and VAS of pain ≥ 4; MAS ≥ 2 in shoulder adductor and elbow flexor | Stroke for more than 3 months | IM BoNT | 7 (3/4) | 46.3 ± 9.0 1 | 8.3 ± 7.0 1 |
| Placebo | 9 (8/1) | 56.0 ± 13.6 1 | 10.1 ± 6.5 1 | ||||||
| Marco et al., 2007 | RCT | Double-blinded (patients and assessors) | Yes | Pain ≥ 3 months and VAS of pain ≥ 4; MAS ≥ 3 | Stroke for more than 3 months | IM BoNT | 14 (10/4) | 63.9 ± 10.6 1 | 5.8 (3.0 to 8.8) 3 |
| Placebo | 15 (11/4) | 67.2 ± 7.4 1 | 4.4 (3.7 to 7.0) 3 | ||||||
| Yelnik et al., 2007 | RCT | Double-blinded (lack of details) | Yes | MAS ≥ 1+ in medial rotator or elbow flexor; passive external rotation limitation 10° or <30° compared to the opposite side | Stroke regardless of the stage | IM BoNT | 10 (7/3) | 53.0 ± 4.6 1 | 7.5 ± 6.2 1 |
| Placebo | 10 (8/2) | 55.2 ± 8.3 1 | 26.5 ± 35.0 1 | ||||||
| Snels et al., 2000 | RCT | Double-blinded (patients and assessors) | Yes | Pain ≥ 2 weeks and VAS of pain ≥ 4; passive external rotation limitation > 20° | Stroke with resultant hemiplegia | IA corticosteroid | 18 (12/6) | 60.6 ± 8.4 1 | <6 months: n = 12 ≥6 months: n = 6 |
| Placebo | 19 (7/12) | 62.5 ± 10.6 1 | <6 months: n = 14 ≥6 months: n = 5 |
| Author, Year | Trial Arm | Intervention Detail | Guidance | Inclusion for Meta-Analysis | Outcome for Meta-Analysis | Secondary Outcomes | Follow-Up (Week) |
|---|---|---|---|---|---|---|---|
| Kasapoğlu-Aksoy et al., 2020 | IM BoNT | Total 140 to 210 units BoNT (Botox) per person, 100 to 150 units into pectoralis major muscle and 40 to 60 units into teres major muscle | Ultrasound | Included | VAS (at rest) | ROM, FMS, MAS | 2, 6 |
| SSNB | 1 mL triamcinolone + 9 mL 2% lidocaine at suprascapular notch | Ultrasound | Included | ||||
| Terlemez et al., 2020 | SSNB (local analgesic + steroid) | 5 mL 2% lidocaine + 1 mL betamethasone at the suprascapular notch | Ultrasound | Included with data combination | VAS (during motion) | ROM | 1, 4 |
| SSNB (local analgesic) | 5 mL 2% lidocaine at the suprascapular notch | Ultrasound | |||||
| Placebo | 5 mL 2% lidocaine injected into trapezius muscles | Ultrasound | Included | ||||
| Aydin et al., 2019 | SSNB | 1 mL betamethasone + 2 mL 10% lidocaine + 2 mL physiologic serum at the suprascapular fossa | Ultrasound | Included | VAS (during motion) | ROM, MAS, Brunnstrom stage, EQ-5 D-3 L | 1, 4, 12 |
| Control | Passive and active-assistive ROM exercises (3 sets daily, 20 times in each set) | Not available | Included | ||||
| Sencan et al., 2019 | IA corticosteroid | 40 mL methylprednisolone + 1 mL 0.5% bupivacaine + 2 mL saline into the glenohumeral joint | Fluoroscopy | Included | VAS (during motion) | ROM, MAS, MBI | 2, 8 |
| SSNB | 3 mL 0.5% bupivacaine + 2 mL saline at suprascapular notch | Fluoroscopy | Included | ||||
| IA corticosteroid + SSNB | Combination of aforementioned two treatments | Fluoroscopy | Excluded | ||||
| Wu et al., 2019 | IB BoNT | 100 units BoNT (Botox) into the subacromial-subdeltoid bursa | Ultrasound | Included | VAS (at rest) | FMS | 2, 4, 8, 12 |
| IB corticosteroid | 1 mL betamethasone + 2 mL 2% lidocaine + 1 mL saline into the subacromial-subdeltoid bursa | Ultrasound | Included | ||||
| Huang et al., 2018 | Hyaluronic acid | 2.5 mL sodium hyaluronate (ARTZ Dispo) into the subdeltoid bursa; total 3 doses (1 dose per week) | Ultrasound | Included | VAS (at rest) | ROM, MAS, FMS, shoulder subluxation, soft tissue hyperemia | 4, 12 |
| Placebo | 2.5 mL saline into the subdeltoid bursa | Ultrasound | Included | ||||
| Jang et al., 2016 | Hyaluronic acid | 2 mL high molecular weight sodium hyaluronate + 4 mL 0.5% lidocaine (total 3 doses in a week) | Ultrasound | Included | Pain rating scale of WBS (0–10) | ROM | 1, 4, 8 |
| IA corticosteroid | 40 mg triamcinolone + 4 mL 0.5% lidocaine + 1 mL saline into the shoulder joint | Ultrasound | Included | ||||
| Adey-Wakeling et al., 2013 | SSNB | 40 mg methylprednisolone + 0.5% 10 mL bupivacaine into the supraspinatus fossa | Landmark | Included | VAS (not specified) | MRS, Croft Disability Index, EuroQol Health Questionnaire | 1, 4, 12 |
| Placebo | 5 mL normal saline subcutaneously to the same region of the shoulder | Landmark | Included | ||||
| Marciniak et al., 2012 | IM BoNT | Total 140 to 200 units BoNT (Botox) per person, with 100 to 150 units into pectoralis major muscles and 40 to 60 units into teres major muscles if shoulder extensors MAS ≥ 3 | Electromyography | Included | VAS (daily worst pain) | ROM, FMS, MAS, MPQ, DAS, Beck depression inventory | 2, 4, 12 |
| Placebo | 2 mL of saline into pectoralis major and teres major muscles | Electromyography | Included | ||||
| Rah et al., 2012 | IB corticosteroid | 40 mg triamcinolone + 1 mL 1% lidocaine into the subdeltoid bursa | Ultrasound | Included | VAS (at night) | ROM, MBI, SDQ | 2, 4, 8 |
| Placebo | 5 mL 1% lidocaine into the subdeltoid bursa | Ultrasound | Included | ||||
| Lakse et al., 2009 | IA or IB corticosteroid | 1 mL triamcinolone + 9 mL prilocaine into the shoulder joint in frozen shoulders, or into the subacromial bursa in impingement syndrome | Landmark | Included | VAS (at rest) | ROM, MAS, BI, Brunnstrom stage | 1, 4 |
| Placebo | Not mentioned | Landmark | Included | ||||
| De Boer et al., 2008 | IM BoNT | Total 50 units BoNT (Botox) into subscapularis muscle | Landmark | Included | VAS (not specified) | ROM | 0, 6, 12 |
| Placebo | 1 mL saline into subscapularis muscle | Landmark | Included | ||||
| Lim et al., 2008 | IM-BoNT | BoNT (Botox) into infraspinatus, subscapularis or pectoralis muscles; maximal dose: 50 units in each muscle and 100 units in each patient | Landmark | Included | NRS (during motion) | ROM, FMS, MAS Physician’s global rating scale | 2, 6, 12 |
| IA corticosteroid | 40 mg triamcinolone in shoulder joints | Landmark | Included | ||||
| Kong et al., 2007 | IM-BoNT | 250 units BoNT (Dysport) to pectoralis major muscles and 250 units BoNT (Dysport) to biceps brachii muscles | Landmark | Included | VAS (not specified) | ROM, MAS | 4, 8, 12 |
| Placebo | 2.5 mL saline into pectoralis major and biceps brachii muscles | Landmark | Included | ||||
| Marco et al., 2007 | IM BoNT | 500 units BoNT (Dysport) into pectoralis major muscles | Electromyography | Included | VAS (during motion) | ROM, MAS | 1, 4, 12, 24 |
| Placebo | 2.5 mL of saline into pectoralis major muscles | Electromyography | Included | ||||
| Yelnik et al., 2007 | IM BoNT | 500 units BoNT (Dysport) into subscapularis muscles | Electromyography | Included | VAS (not specified) | ROM, MAS | 1, 2, 4 |
| Placebo | Solvent (for BoNT) into subscapularis muscle | Electromyography | Included | ||||
| Snels et al., 2000 | IA corticosteroid | 40 mg triamcinolone into shoulder joints; total 3 doses (0, 1st, 3rd week) | Landmark | Included | VAS (not specified) | ROM, FMS, BI, Action Research Arm test, Rehabilitation Activities Profiles | 6, 12 |
| Placebo | 1 mL saline into shoulder joints, total 3 doses (0, 1st, 3rd week) | Landmark | Included |
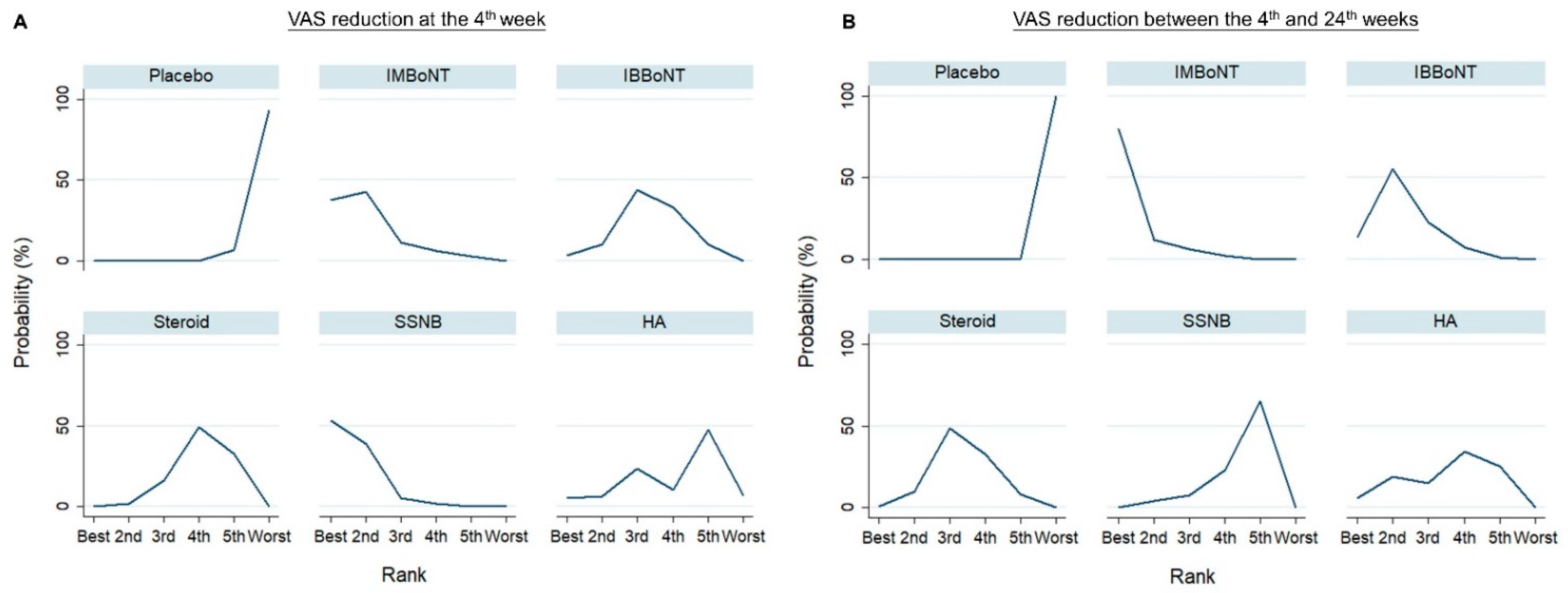
| VAS Reduction at the 4th Week | VAS Reduction between the 4th and 24th Weeks | |||
|---|---|---|---|---|
| Rank | Treatment | SUCRA | Treatment | SUCRA |
| 1 | SSNB | 88.6 | IMBoNT | 93.9 |
| 2 | IMBoNT | 81.4 | IBBoNT | 74.5 |
| 3 | IBBoNT | 52.7 | Steroid | 52.4 |
| 4 | Steroid | 37.7 | HA | 49.1 |
| 5 | HA | 37.2 | SSNB | 30.0 |
| 6 | Placebo | 1.4 | Placebo | 0.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chiu, Y.-H.; Chang, K.-V.; Wu, W.-T.; Hsu, P.-C.; Özçakar, L. Comparative Effectiveness of Injection Therapies for Hemiplegic Shoulder Pain in Stroke: A Systematic Review and Network Meta-Analysis. Pharmaceuticals 2021, 14, 788. https://doi.org/10.3390/ph14080788
Chiu Y-H, Chang K-V, Wu W-T, Hsu P-C, Özçakar L. Comparative Effectiveness of Injection Therapies for Hemiplegic Shoulder Pain in Stroke: A Systematic Review and Network Meta-Analysis. Pharmaceuticals. 2021; 14(8):788. https://doi.org/10.3390/ph14080788
Chicago/Turabian StyleChiu, Yi-Hsiang, Ke-Vin Chang, Wei-Ting Wu, Po-Cheng Hsu, and Levent Özçakar. 2021. "Comparative Effectiveness of Injection Therapies for Hemiplegic Shoulder Pain in Stroke: A Systematic Review and Network Meta-Analysis" Pharmaceuticals 14, no. 8: 788. https://doi.org/10.3390/ph14080788
APA StyleChiu, Y.-H., Chang, K.-V., Wu, W.-T., Hsu, P.-C., & Özçakar, L. (2021). Comparative Effectiveness of Injection Therapies for Hemiplegic Shoulder Pain in Stroke: A Systematic Review and Network Meta-Analysis. Pharmaceuticals, 14(8), 788. https://doi.org/10.3390/ph14080788